1. Introduction
Molecules in confinement experience different physico-chemical properties from those in bulk [
1,
2,
3]. In some cases, molecules located in confined space do not form crystals and thus can demonstrate improved dissolution and bioavailability [
4]. Moreover, molecules in confinement can experience shifted temperatures of phase transitions, e.g., freezing and melting temperatures [
5]. Most of the studies focused on an introduction to pores of different host material chemicals that are liquid in ambient conditions, and their freezing, melting, and glass transitions were examined [
5,
6,
7,
8,
9,
10,
11,
12,
13,
14]. Many organic and inorganic guest molecules have been used, with water being probably the most widely studied [
7,
9,
10,
12,
13,
14].
Ibuprofen is a propionic acid derivative belonging to the class of nonsteroidal anti-inflammatory drugs. It has been widely studied in different drug delivery systems, being introduced in vessels based on silica [
15,
16,
17,
18,
19,
20,
21,
22,
23], polymers [
24,
25,
26], and carbons [
27,
28], among others. Bulk ibuprofen is a crystalline solid in ambient conditions, but it exists in a different state when it is introduced into mesopores. It experiences a specific confinement effect as well. It exhibits high mobility, a glassy-like or even liquid-like state when it is introduced into a mesoporous MCM-41 (Mobil Composition of Mater no. 41) material [
29]. It was shown that ibuprofen exhibits higher mobility in larger pores (116 Å), in comparison to smaller ones (35 Å). Due to this specific dynamic behaviour of ibuprofen in confinement, it was possible to use NMR (nuclear magnetic resonance) techniques characteristic for liquid-state NMR (like INEPT (insensitive nuclei enhanced by polarization transfer)) to characterize solid samples.
The importance of water in the ibuprofen–mesoporous silica system has been suggested in the literature. By the means of DFT (density functional theory) calculations it was showed that water plays an important role in the mobility of ibuprofen introduced into microsolvated MCM-41 material. It was proven that water interacts with ibuprofen by “dynamical” H-bonds: the organic molecules desorb and readsorb on the microsolvated surface of the silica, therefore obtaining high mobility [
30]. In ref. [
31], it was stated that apart from the confinement effect, a chemical interaction between the organic molecule and the surface of the host also impacts the dynamical behavior of the guest molecules. By means of variable temperature NMR, it was proven that a fast chemical exchange between the COOH group of the drug molecules and water molecules is taking place. Using a
1H-
29Si-
1H “double back” CP (Cross Polarization) experiment, the signal from water H-bonded to silanols was identified. Potrzebowski et al. [
32] showed that water vapour can push the ibuprofen out of mesopores. Recently, SBA-15 (Santa Barbara Amorphous-15) samples with ibuprofen introduced into mesopores using the melting method were examined [
33]. SBA-15 is a highly stable mesoporous silica-based material with ordered hexagonal cylindrical pores and narrow pore size distribution. The samples have been examined in the ambient (hydrated) state and in a dehydrated state after using an oven or a vacuum treatment (both at 80 °C for 20 h). For a more complete dehydration, samples prepared under a N
2 atmosphere after complete dehydration of the host in vacuum (at 300 °C in 20 h) have been prepared. It was evident that water was crucial for the existence of high mobility ibuprofen, while it exhibited decreased mobility in a dehydrated state. The presence of water also changed the location of ibuprofen molecules (inside or outside mesopores). The kinetics of the release of ibuprofen to simulated body fluid (SBF) showed a correlation with a higher mobility of introduced phase inside mesopores, exhibiting the faster release of hydrated samples, in comparison to non-hydrated ones.
Zeolites are aluminosilicates that come with more than 200 different topologies, i.e., different sizes, shapes of pores (in the range on micropores), and spatial connections between them. This creates a wide range of spatially different materials with very high surface areas. In the last years, a growing interest in the field of hierarchical zeolites can be noticed. These are zeolites that apart from the standard micropores also possess a second type of pores in the range between 2 and 50 nm (mesopores). These two generations of pores are interconnected. It is one of the strategies to fight with limited diffusion in zeolites and make the acid sites located in zeolites accessible to bulky molecules. There are many strategies to obtain mesoporous zeolites. One of the most widely used zeolites in the industry, ultra-stable zeolite Y used in fluid catalytic cracking (FCC), has mesoporosity generated by steaming and acid treatment. It is easily accessible or synthesized and thoroughly investigated in the literature, and therefore makes a perfect choice for the study of the use of zeolites as hosts for medically active compounds.
Zeolites are preferably used as sorbents [
34] and catalysts [
35,
36,
37], but also in biomedical applications [
38,
39,
40,
41]. They have been used as drug delivery systems of many drugs (ibuprofen, fluorouracil, sulfadiazine, and others). Ibuprofen molecule dimensions (ca. 11 Å × 8 Å × 5 Å) make it more preferential to be introduced into mesoporous materials with pore widths from 5 to 50 nm, rather than solely microporous materials, like standard zeolites. Horcajada et al. used mesoporous zeolites Y with different Al content to introduce ibuprofen molecules [
42]. These materials exhibited mesoporosity created by a dealumination process. They discovered that the amount of ibuprofen adsorbed into the material is related to the volume of pores and is comparable to this obtained for pure-silica MCM-41 material. The ibuprofen release rate was connected with Al atoms in the structure: the presence of extra-framework Aluminum and the Si/Al ratio of framework atoms.
Zeolites are known to be perfect sorbents for water, which is directly connected with framework aluminum [
43]. It is also known that mesoporous zeolites exhibit enhanced water diffusivity in comparison to standard zeolites [
44,
45]. Therefore, mesoporous zeolites are perfect candidates to deepen insights concerning the impact of water on the dynamic behaviour of ibuprofen molecules in confinement. Here, the study on the incorporation of ibuprofen molecules into mesoporous ultra-stable zeolite Y is presented. The aim of this study was to verify if the mobile, liquid-like behavior of ibuprofen molecules observed for mesoporous silicate materials is also observed for mesoporous zeolites, as well as to check how water naturally occurring in zeolites changes the characteristics of ibuprofen in confinement. To do that, samples under atmospheric humidity, additional hydration, and in dehydrated states were studied. Nitrogen adsorption was used to study the changes in porosity after the introduction of ibuprofen. X-ray diffraction (XRD) was applied to check if ibuprofen crystallizes in this system, while thermogravimetry (TG) and differential scanning calorimetry (DSC) showed the content of ibuprofen in the samples, and the phase changes of the organic molecules under confinement. Magic angle spinning nuclear magnetic resonance (MAS NMR) was applied to characterize the samples under different states of hydration. This is probably the first study to utilize the solid-state NMR technique to study the effect of water on the dynamic behaviour of ibuprofen introduced into mesoporous aluminosilicates. The results could have a significant impact on the future use of this system in drug delivery applications.
2. Materials and Methods
Dealuminated zeolite denoted as FAU31 was purchased from Zeolyst (Kansas City, MO, USA) (CBV760). Ibuprofen (98%) was purchased from Sigma-Aldrich (Darmstadt, Germany).
Samples denoted as FAU31-12IBU, FAU31-24IBU, and FAU31-38IBU were synthesized by mixing 300 mg of zeolite powder with 30 mg, 90 mg, and 150 mg of ibuprofen, respectively. Afterwards, samples were kept in an oven heated to 85 °C for 1 h. After at least 20 min or more of equilibration at room temperature, they were transported to further examination.
For studies of hydration and dehydration, the samples were kept in a desiccator with 49% of humidity over a saturated solution of Mg(NO3)2 for 19 h or in an oven heated to 85 °C for the same time, respectively.
The samples synthesized in a water-free environment were prepared using a degassed zeolite: 0.15 mg of a zeolite sample was dehydrated in a vacuum line at 300 °C or 500 °C for 19 h, after which a glass tube with the sample was sealed. It was transported to a glove box with N2 gas. Inside, a glass tube with the degassed sample was opened and mixed with ibuprofen. After that, a rotor with the sample was transported to a vacuum line using a glass device with a Teflon valve. In the Schlenk line, a thermal treatment was applied (85 °C for 1 h). After this, a glass tube was opened in a glove box, where a rotor was closed and transported to NMR spectrometer (Bruker BioSpin GMBH, Rheinstetten, Germany).
X-ray diffractometry (XRD) results were obtained using a PANalyticalCubixX’Pert Pro diffractometer (PANanalytical, Almelo, The Netherlands), with CuKa radiation (lambda = 1.5418 Å) in the 2theta angle range 5–50°. The N2 sorption at 77 K was performed on ASAP 2420 Micromeritics apparatus (Micromeritics, Norcross, GA, USA). Prior to the adsorption, samples were degassed in a vacuum at 298 K for 18 h. The specific surface area has been determined using the nitrogen adsorption method. Pore size distribution has been calculated using a BJH method from a desorption branch. Thermogravimetric analysis (TG) and differential scanning calorimetry (DSC) curves have been recorded using Netzsch STA 409 PC Luxx (Netzsch, Selb, Germany) in the temperature range from 30 to 1000 °C (10 °C min−1).
1H and 13C MAS (magic angle spinning) NMR spectra were recorded using Bruker Avance III 500 MHz spectrometer (Bruker BioSpin GMBH, Rheinstetten, Germany) with frequencies 500.1 and 125.8 MHz, respectively, and RF fields of 60 kHz. Typically, for 1H MAS NMR spectra a single pulse π/2 excitation and recycle delay of 10 s were used. Typically, 32 scans were collected. For 13C CP MAS spectra, 2 ms of contact time, 70–100% of camp amplitude, and SPINAL64 decoupling were used. The same decoupling sequence was used for 13C HPDEC (high power decoupling) MAS NMR experiments. Typically, 256 scans were collected for 13C CP and 410 scans for 13C HPDEC MAS NMR spectra. All chemical shifts were referenced to TMS (tetramethylsilane). If not stated otherwise, 4 mm rotors were used to spin the samples to 10 kHz.
1H MAS NMR spectra in a temperature range from −50 to 45 °C were performed on Bruker Avance III 400 WB spectrometer (Bruker BioSpin GMBH, Rheinstetten, Germany) (B0 = 9.4 T) with a 4 mm probe and a radio frequency field 60 kHz in the Laboratory of Radiospectroscopy of University of Szczecin. Samples were spun at the magic angle using ZrO2 rotors and ZrO2 caps at MAS frequency of 10 kHz. For temperatures below 270 K, we used a heat exchanger to produce the cooling gas. 1H MAS NMR spectra were recorded by a single pulse π/2 excitation and the recycle delay of 5 s by 10 scans.
3. Results
3.1. Synthesis and Characterization under Ambient Conditions
Table 1 shows the content and porosimetric characteristics of synthesized samples. While the content of ibuprofen increases, the content of water decreases. The specific surface area (S
BET) and pore volume decreases as well, from 716 to 33 m
2/g and 0.53 to 0.01 cm
3/g, respectively. Diameters of pores do not change. Pore size distributions depicted in
Figure S1 (Supplementary Information) show these trends graphically. In the sample FAU31-38IBU, there is no detectable volume of pores indicating that all pores are filled with guest molecules. Another possibility is that ibuprofen located outside mesopore clogged the entrance to the pores hindering the penetration of the pores by N
2 molecules.
Figure 1 shows XRD patterns of the samples under study. The diffraction pattern characteristic for faujasite is preserved after the introduction of organic molecules [
46]. There are no detectible diffraction peaks for ibuprofen in most of the samples. Only the sample with the highest content of ibuprofen exhibits very low intensity diffraction peaks characteristic for the guest phase. It means that only in this sample, a small part of ibuprofen forms crystals but the majority of it, as in other samples, is present in a non-crystalline form.
Thermogravimetric analysis (TG) and differential scanning calorimetry (DSC) provide an insight into temperatures of phase changes that ibuprofen experience upon confinement. TG curves are showed in
Supplementary Information (Figure S2). Differential thermogravimetric (DTG) and DSC curves depicted in
Figure 2 show that there is a shift of the evaporation temperature of ibuprofen located in mesopores of FAU31 with respect to bulk molecules. It shifts from ca. 280 °C to 210 °C (∆T = 70 °C), and therefore the shift is higher than for SBA-15 (∆T = 55 °C) [
33]. The melting temperature of ibuprofen can be detected as an endothermic peak in the DSC curve. For bulk ibuprofen, it is 85 °C, which is in agreement with the literature [
47]. In most of the samples, this signal overlaps with the signal from water that is present at 100 °C and is exclusively seen in the host FAU31. Only in the sample FAU31-38IBU is there a small peak at 85 °C that could be discerned from a wider signal from water. Broad exothermic peaks in the region from 400 to 600 °C probably come from the oxidation of organic molecules.
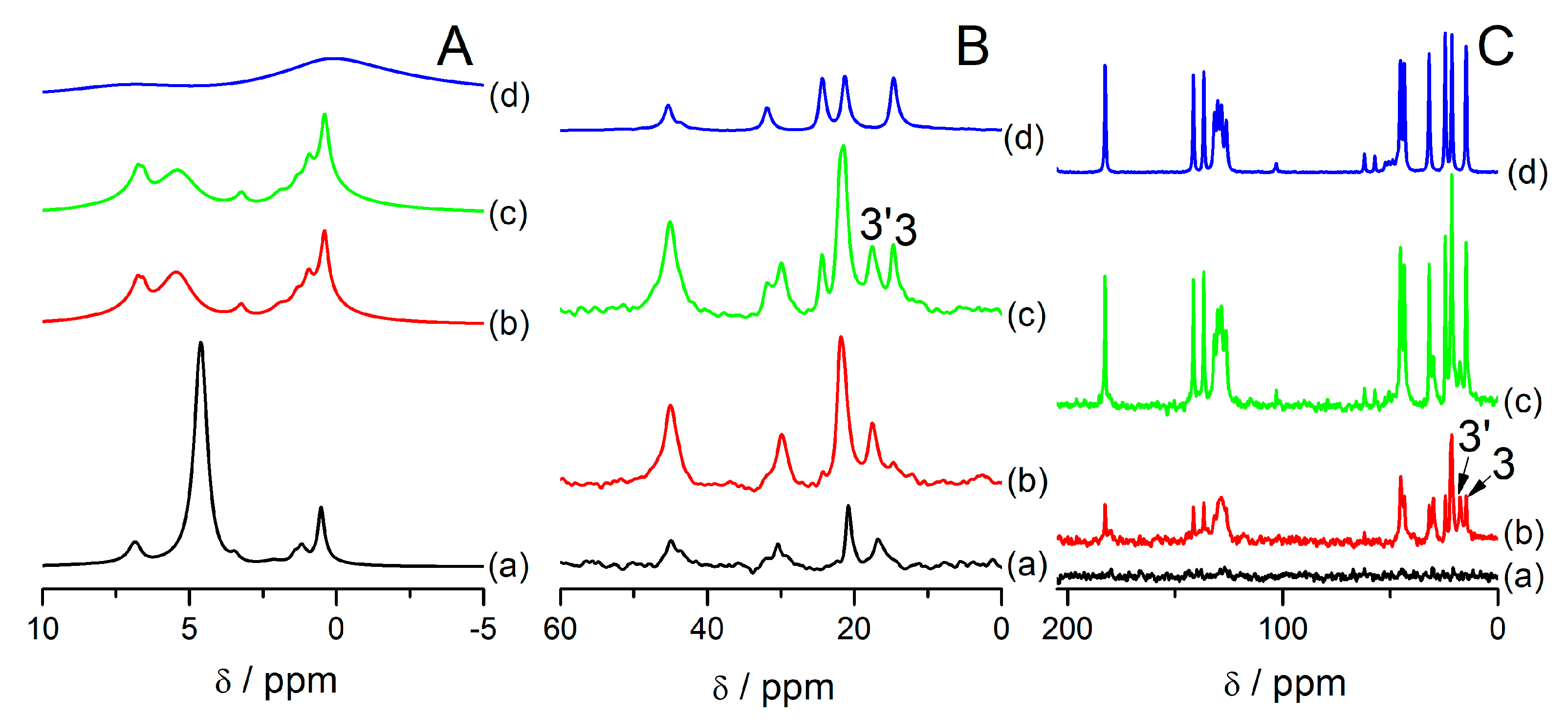
Figure 3 shows MAS NMR spectra of the samples under study. In
Figure 3A,
1H MAS NMR spectra are presented. Signals are assigned according to the convention presented in
Figure S3 (Supplementary Information). All the samples exhibit a higher resolution in comparison to bulk ibuprofen (
Figure 3A(d)), but the resolution is not as high as in the samples with ibuprofen introduced to SBA-15 [
33] or MCM-41 [
29]. In the latter, the linewidths in
1H MAS NMR spectra was ca. 30 Hz, for SBA-15 ca. 100 Hz. Here, we cannot differentiate all the signals from protons. Specifically, the signals from protons 2, 10, 11, and 3 are not present as discernible separate peaks but overlap each other. It could be due to the fact that a part of the introduced ibuprofen is probably strongly bonded with the zeolite surface, which makes that part of the introduced phase less mobile. The signal from water is also present and its intensity is higher with the lower content of ibuprofen in the samples.
13C HPDEC (
Figure 3B) and
13C CP (
Figure 3C) MAS NMR spectra of the samples under study also show distinct changes in reference to bulk ibuprofen. They present signals characteristic for ibuprofen located in mesopores, which are highlighted with apostrophes in
Figure 3B,C. These signals are used in this article as “fingerprints” of ibuprofen located in mesopores that exhibits high mobility. Namely:
The signal 3′ which is the signal from carbon 3 shifted to lower field,
The signal 11′ which is the signal from carbon 11 shifted to higher field,
Signal 12′13′ which is due to overlapping the signals from carbons 12 and 13
Signal 2′10′ which is due to overlapping the signals from carbons 2 and 10.
The overlaying of the signals is due to motional averaging due to the high mobility of confined species. These signals are the same as what was observed in the literature for ibuprofen confined in silica mesoporous materials [
29,
33].
13C NMR spectra of ibuprofen in solution show the same signals [
48]. Based on this assignment, it can be said that samples FAU31-12IBU and FAU31-24IBU exhibit signals from mobile ibuprofen located only in mesopores, while the sample FAU31-38IBU signals from ibuprofen located both inside and outside mesopores.
3.2. Hydration and Dehydration under Atmospheric Pressure
Owing to a known impact of water on the mobility of ibuprofen molecules in confinement, the experiments of hydration and dehydration on SBA-15 with introduced ibuprofen have been performed.
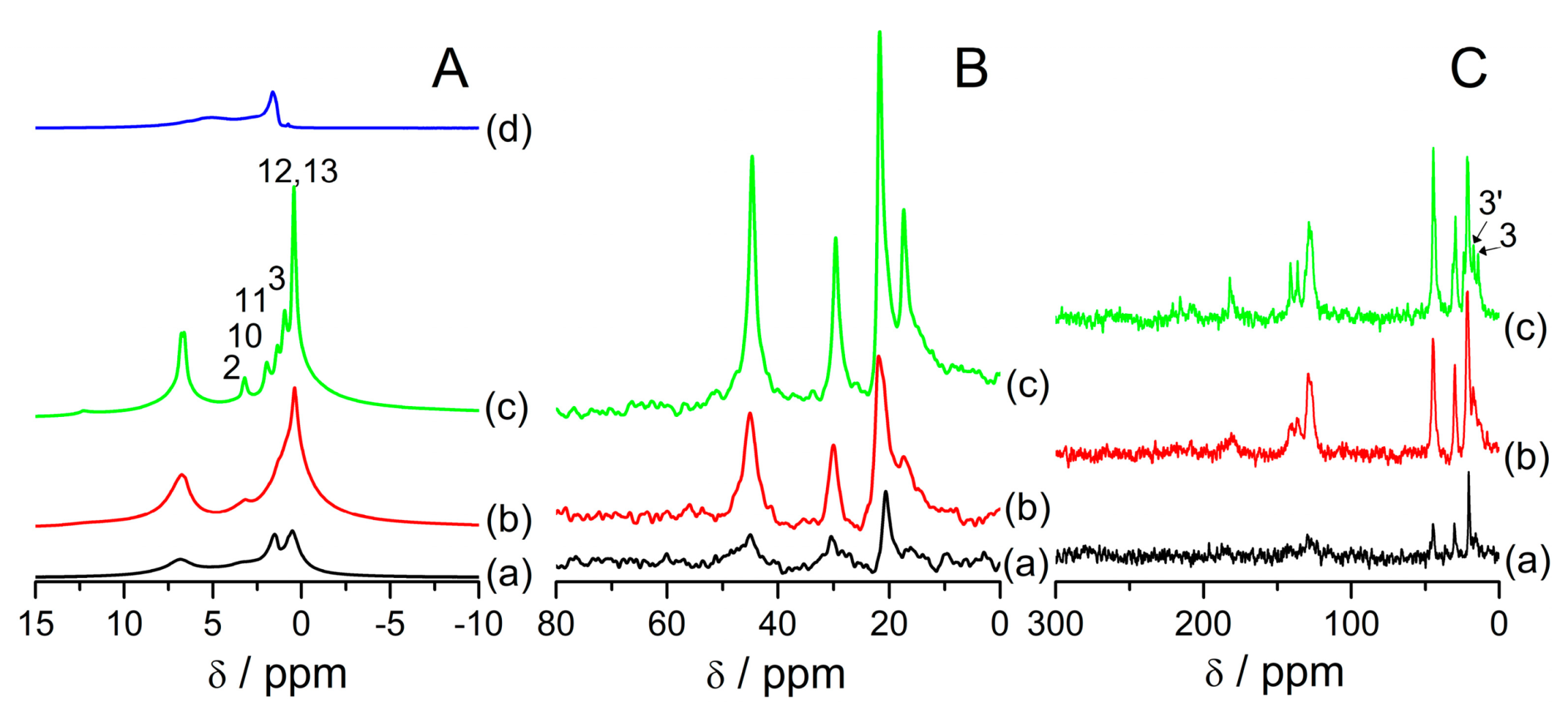
Figure 4 shows
1H (A),
13C HPDEC (B), and
13C CP (C) MAS NMR spectra of the samples after hydration for 19 h in a desiccator over a saturated solution of Mg(NO
3)
2. Crystalline ibuprofen was also hydrated this way, but its NMR spectra are not changed in respect to the same sample under ambient conditions (
Figure 4A(d),B(d),C(d)).
1H MAS NMR spectra show higher signals from water and more distinctive peaks from protons 3, 10, and 11 in comparison to samples measured in ambient conditions (
Figure 4A). This agrees with a previous study in that water increases the mobility of ibuprofen located in mesopores of SBA-15. It was shown that dehydrated or prepared in a water-free environment samples exhibited more broadened signals from ibuprofen in the
1H MAS NMR spectra with respect to the samples measured under atmospheric humidity, indicating the much lower mobility of the guest molecules [
33].
13C MAS NMR spectra show no changes in comparison to samples in ambient conditions. Only the
13C CP MAS NMR spectrum of FAU31-24IBU exhibits a signal from carbon 3 that was absent in the ambient condition (
Figure 4C), which indicates that there are ibuprofen molecules located outside mesopores in this sample. According to other studies, the sample with the lowest amount of ibuprofen should have the highest mobility of drug molecules [
29,
33]. Indeed, the sample FAU31-12IBU has no detected (
Figure 4C(a))
13C CP signals, which could be due to the high mobility of those species. On the other hand, only this sample shows a lack of the signal from protons 3 in
1H MAS NMR spectra. This could be due to the interaction of ibuprofen molecules with the zeolite framework. Ibuprofen interacts with a zeolite host via hydrogen bonds of carboxylic groups with zeolite’s hydroxyl groups and water molecules, provided a hydrated state is present. There could also be another interaction, namely strong coordinative bonds between carboxylate species and extra-framework aluminum [
42]. It was shown that in ultra-stable zeolite Y with a high content of extra-framework Al, the carbonyl group is deprotonated and FT-IR spectra show bands characteristic for monodentate carboxylic species. This interaction could immobilize at least the part of the molecule that is close to the carboxylic group, like a methyl group that protons 3 are a part of.
Figure 5 shows MAS NMR spectra of the samples after dehydration in an oven at 85 °C for 19 h. Samples FAU31-12IBU and FAU31-24IBU exhibit almost no changes in reference to samples in ambient conditions in all the spectra, except the lack of water signals in
1H MAS NMR spectra. NMR spectra of FAU31-38IBU change distinctly.
13C HPDEC MAS NMR spectrum in
Figure 5B shows no signal from carbon 3, so ibuprofen was located outside mesopores with low mobility. This signal is present in
13C CP MAS NMR (
Figure 5C(c)), but the relative intensities of signals from carbons 3 and 3′ is similar, contrarily to samples examined in ambient condition, when the signal from ibuprofen located outside mesopores (carbon 3) was much more intense than the signal from carbon 3′.
The most resounding for the dehydrated sample FAU31-38IBU was the 1H MAS NMR spectrum, which showed a clear increase of the resolution with distinct peaks for protons 10, 11, and 3.
3.3. Synthesis in a Water-Free Environment
To further examine this issue, a series of experiments in a dehydrated state have been done. For the samples synthesized under ambient conditions, water was present in the parent material, as well as in the samples with ibuprofen (
Table 1). Samples dehydrated in an oven probably also contained some water, because the parent zeolite FAU31 exhibits signals from water in
1H MAS NMR (
Figure 5A(d)). Here, the host material was dehydrated in a vacuum, ibuprofen was introduced in a water-free environment, and the heating treatment was performed in a vacuum, so that no water was present in the samples examined in NMR spectroscopy.
Figure 6 shows
1H MAS NMR spectra of the samples prepared in a water-free environment with varying ibuprofen content, from 3% to 66% of ibuprofen in the samples. When the content of ibuprofen was 34% or more, there is a visible increase in the resolution of ibuprofen signals.
When a 4 mm NMR probe was used, an even higher resolution can be found, like this presented in
Figure 7A(a). The high resolution of
1H MAS NMR spectra in a dehydrated state was maintained even after 3 h after the thermal treatment in vacuum (
Figure S4, Supplementary Information). Moreover, the signals got even narrower this time. This sample exhibited
13C signals exclusively from ibuprofen of high mobility (
Figure 7B(a),C(a)).
After that, a closed rotor was kept in a dehydrated state in a desiccator filled with silica for 2 days, and after this time, the spectrum of the sample changed. There is no high resolution of
1H MAS NMR spectrum.
13C MAS NMR spectra showed the presence of ibuprofen located outside mesopores (carbon 3) (
Figure 7B(b),C(b)). Then, a rotor was opened for 4 h and measured in a partially hydrated state, which made small changes in the
1H MAS NMR spectrum (
Figure 7A(c)), making signals a little narrower and presenting a small shoulder at ca. 6 ppm due to water molecules.
13C MAS NMR spectra in
Figure 7B,C also only slightly changed.
3.4. High Mobility and the Effect of the Temperature
What is the reason for the observed temporary higher mobility in a dehydrated state? At first, it was assessed whether it is connected with a possible presence of residual water in the sample. The host material for the introduction of ibuprofen was dehydrated in vacuum at 300 °C, while 450 °C or more is used in many articles for complete dehydration of zeolites [
49], and after activation at 350 °C, strongly bonded water is detected in hierarchical zeolite Y [
50]. Indeed, there are some differences in
1H MAS NMR spectra of the samples dehydrated at 300 °C and 500 °C. Specifically, there are signals at ca. 6 ppm and a broad resonance at ca. 2.5 ppm that is present in the sample dehydrated using a lower temperature (
Figure S5, Supplementary Information). These signals can be connected with the presence of strongly bonded water, the kind of water that is not desorbed at 300 °C. The signal at 2.5 ppm is connected with water interacting with SiOH groups [
51], while the peak at ca. 6 ppm is connected to water bonding with defect sites [
52,
53].
1H MAS NMR spectrum of the sample with ibuprofen prepared in a water-free environment with the host dehydrated at 500 °C is presented in
Figure S6 (Supplementary Information). Narrow signals from mobile ibuprofen are still present, so this phenomenon is not connected directly with the presence of water in a zeolite sample.
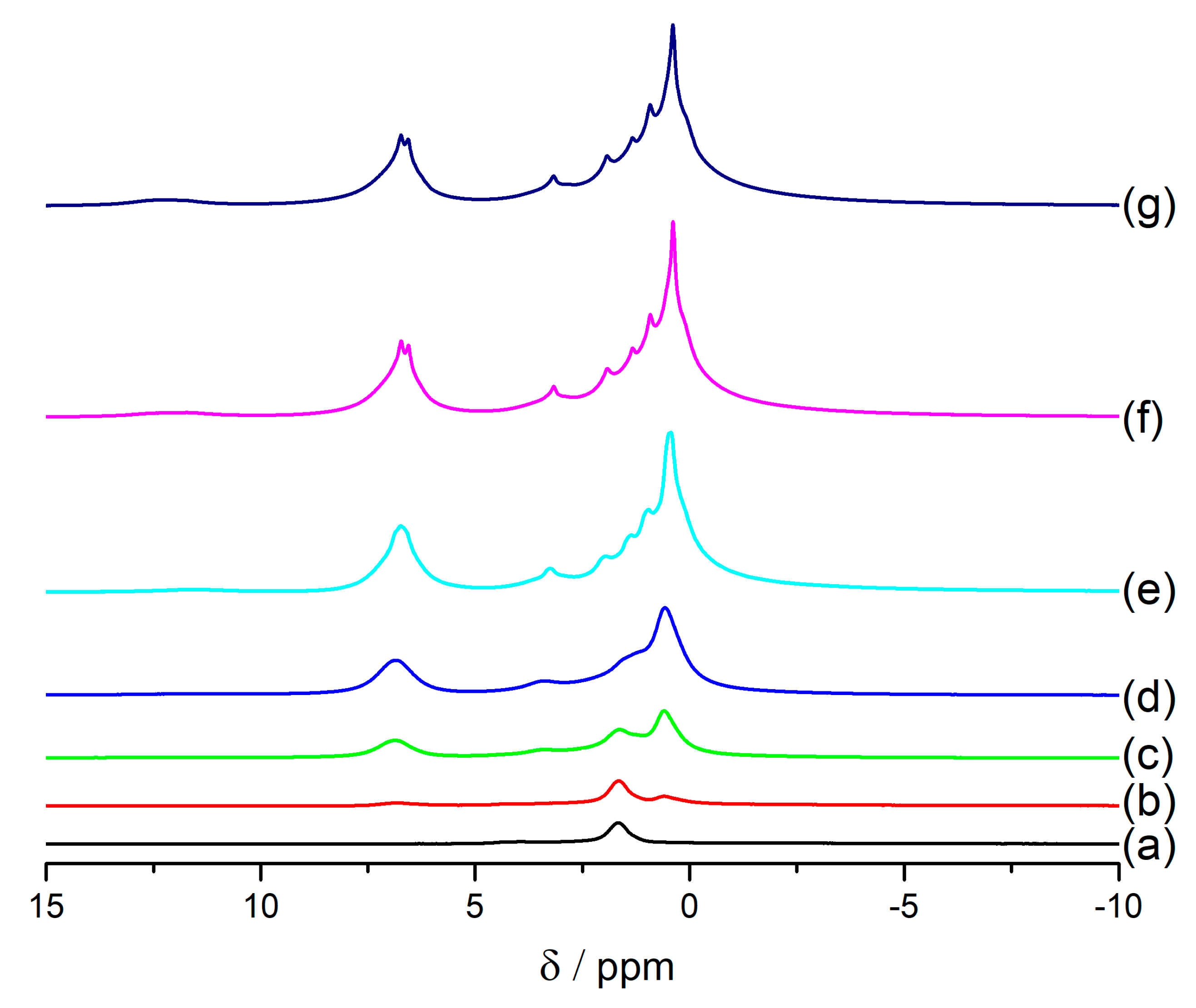
The second hypothesis was connected to a long time that ibuprofen needs to cool down in the sample and to equilibrate in the complicated porous architecture of a mesoporous zeolite. NMR experiments performed at different temperatures from −50 °C to 45 °C show that the lines in
1H MAS NMR spectra are getting narrower with an increase of the temperature for the sample FAU31-24IBU (
Figure 8). This shows that ibuprofen is very mobile in this sample when it is heated to 45 °C.
4. Discussion
High mobility in a dehydrated state was observed in the samples treated in an oven for an overnight treatment (
Figure 5A(c),B(c),C(c)) and in the samples prepared in a dehydrated state (
Figure 6 and
Figure 7A(a),B(a),C(a)). Special care was taken that the rotors were not transported to NMR spectrometer right after thermal treatments so that a sample had the time to cool down and equilibrate in a water-free desiccator for at least 20 min (for an oven treatment) and 30 min in a glove box (for the water-free synthesis). Despite that, increased mobility was observed even after 3 h after thermal treatments. What is more, this phenomenon is probably not connected with molecules located in mesopores. On the contrary, when the ibuprofen content was up to 24%, for the samples without water, this phenomenon was not observed (
Figure 5A(a,b) and
Figure 6a–c). Therefore, this feature is ascribed to ibuprofen molecules located in intercrystalline voids. That is why in a 4 mm rotor these liquid-like signals from ibuprofen were much more intense (
Figure 7) than in a smaller rotor that contained a smaller amount of sample (
Figure 6). Probably, ibuprofen in a closed rotor needs much more time to cool down and crystallize. For at least few hours after the heating treatment, ibuprofen stays in a liquid phase in between the voids between crystals. Only after a prolonged time, the signals characteristic for ibuprofen with low mobility located outside mesopores is present (carbon 3 in
Figure 7B(b),C(b)). It is also probable that a high rotation speed (10 kHz) and high power decoupling used in
13C CP MAS NMR experiments may influence the temperature of the sample and, in effect, elongate the effect of liquification of ibuprofen in the samples.
5. Conclusions
Ibuprofen introduced into mesopores of dealuminated zeolite Y exists in a similar state as in other mesoporous materials. It does not form crystals but exists in a mobile phase with considerable shifts of the melting and evaporation temperatures. Solid-state NMR characterization of the zeolite samples with ibuprofen in ambient conditions, after hydration and dehydration proved that water increases the resolution of 1H MAS NMR spectra, and thus the mobility of the introduced phase. Surprisingly, the increased mobility of ibuprofen was also observed in a dehydrated state. This was ascribed to organic species located outside mesopores that were still very mobile after the thermal treatment. It took more than 3 h for ibuprofen to equilibrate in water-free conditions in a closed rotor. These observations are important for the preparation of guest–host systems using the melting method and the use of zeolites in drug delivery. Possibly, the impact of water on the mobility of the pharmaceuticals in drug delivery systems could lead to the modification of the delivery methods by the regulation of the humidity or temperature of the systems.
Supplementary Materials
The following are available online at
https://www.mdpi.com/article/10.3390/ma14247823/s1, Figure S1. Pore size distributions from BJH desorption branches recorded for Nitrogen Adsorption for the samples under study., Figure S2. TG curves for the samples under study. There are two regions of interest: from 30 to 120 °C (I) that is used for quantification of the water content, and from 121 to 1000 °C (II) that is used for the calculation of the ibuprofen content in the samples., Figure S3: Molecular structure of ibuprofen with labelled positions of atoms. The convention of numbering for protons and carbons is the same. There is no proton in positions 4, 9, and 1. The signals in
1H MAS NMR spectra labelled 1 comes from OH from the carboxyl group., Figure S4:
1H MAS NMR spectra of the sample prepared in a water-free environment. Colors denote the time that passed after the heating treatment in a closed rotor. The signals from ibuprofen molecules are very narrow and are getting even narrower with the time. The inset in a chosen region of chemical shifts between 5.5 and 8 ppm show clearly this tendency., Figure S5:
1H MAS NMR spectra of the parent zeolite FAU31 degassed in vacuum in 300 °C and in 500 °C. Arrows indicate signals from bonded water, at ca. 2.5 ppm and 6.2 ppm., Figure S6:
1H MAS NMR spectrum of the sample FAU31 degassed in 500
oC with introduced ibuprofen molecules in a water-free environment.
Author Contributions
Conceptualization, M.G.; methodology, M.G. and M.P.; validation, M.G. and M.P; formal analysis, M.G. and M.P; investigation, M.G. and M.P; resources, M.G.; data curation, M.G. and M.P; writing—original draft preparation, M.G. and M.P; writing—review and editing, M.G. and M.P; visualization, M.G.; supervision, M.G.; project administration, M.G.; funding acquisition, M.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the young scientists project no. 118/2018—H/MŁ. NAUK/18 and partly within statutory funds from the Jerzy Haber Institute of Catalysis and Surface Chemistry, Polish Academy of Sciences.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Radhakrishnan, R.; Gubbins, K.E.; Sliwinska-Bartkowiak, M. Effect of the fluid-wall interaction on freezing of confined fluids: Toward the development of a global phase diagram. J. Chem. Phys. 2000, 112, 11048–11057. [Google Scholar] [CrossRef] [Green Version]
- Klein, J.; Kumacheva, E. Confinement-Induced Phase Transitions in Simple Liquids. Science 1995, 269, 816–819. [Google Scholar] [CrossRef]
- Heuberger, M.; Zäch, M.; Spencer, N.D. Density fluctuations under confinement: When is a fluid not a fluid? Science 2001, 292, 905–908. [Google Scholar] [CrossRef] [PubMed]
- Hancock, B.C.; Shamblin, S.L.; Zografi, G. Molecular Mobility of Amorphous Pharmaceutical Solids Below Their Glass Transition Temperatures. Pharm. Res. 1995, 12, 799–806. [Google Scholar]
- Sliwinska-Bartkowiak, M.; Gras, J.; Sikorski, R.; Radhakrishnan, R.; Gelb, L.; Gubbins, K.E. Phase transitions in pores: Experimental and simulation studies of melting and freezing. Langmuir 1999, 15, 6060–6069. [Google Scholar] [CrossRef]
- Dosseh, G.; Xia, Y.; Alba-Simionesco, C. Cyclohexane and benzene confined in MCM-41 and SBA-15: Confinement effects on freezing and melting. J. Phys. Chem. B 2003, 107, 6445–6453. [Google Scholar] [CrossRef]
- Sterczyńska, A.; Deryło-Marczewska, A.; Zienkiewicz-Strzałka, M.; Śliwińska-Bartkowiak, M.; Domin, K. Surface Properties of Al-Functionalized Mesoporous MCM-41 and the Melting Behavior of Water in Al-MCM-41 Nanopores. Langmuir 2017, 33, 11203–11216. [Google Scholar] [CrossRef]
- Gubbins, K.E.; Long, Y.; Śliwinska-Bartkowiak, M. Thermodynamics of confined nano-phases. J. Chem. Thermodyn. 2014, 74, 169–183. [Google Scholar] [CrossRef]
- Domin, K.; Chan, K.Y.; Yung, H.; Gubbins, K.E.; Jarek, M.; Sterczynska, A.; Sliwinska-Bartkowiak, M. Structure of ice in confinement: Water in mesoporous carbons. J. Chem. Eng. Data 2016, 61, 4252–4260. [Google Scholar] [CrossRef]
- Jazdzewska, M.; Śliwinska-Bartkowiak, M.M.; Beskrovnyy, A.I.; Vasilovskiy, S.G.; Ting, S.W.; Chan, K.Y.; Huang, L.; Gubbins, K.E. Novel ice structures in carbon nanopores: Pressure enhancement effect of confinement. Phys. Chem. Chem. Phys. 2011, 13, 9008–9013. [Google Scholar] [CrossRef]
- Morishige, K.; Kawano, K. Freezing and melting of methanol in a single cylindrical pore: Vitrification of methanol. J. Chem. Phys. 2000, 112, 11023–11029. [Google Scholar] [CrossRef]
- Morishige, K.; Yasunaga, H.; Matsutani, Y. Effect of pore shape on freezing and melting temperatures of water. J. Phys. Chem. C 2010, 114, 4028–4035. [Google Scholar] [CrossRef]
- Morishige, K.; Yasunaga, H.; Uematsu, H. Stability of cubic ice in mesopores. J. Phys. Chem. C 2009, 113, 3056–3061. [Google Scholar] [CrossRef]
- Suzuki, Y.; Steinhart, M.; Butt, H.J.; Floudas, G. Kinetics of Ice Nucleation Confined in Nanoporous Alumina. J. Phys. Chem. B 2015, 119, 11960–11966. [Google Scholar] [CrossRef]
- Vallet-Regi, M.; Rámila, A.; Del Real, R.P.; Pérez-Pariente, J. A new property of MCM-41: Drug delivery system. Chem. Mater. 2001, 13, 308–311. [Google Scholar] [CrossRef]
- Charnay, C.; Bégu, S.; Tourné-Péteilh, C.; Nicole, L.; Lerner, D.A.; Devoisselle, J.M. Inclusion of ibuprofen in mesoporous templated silica: Drug loading and release property. Eur. J. Pharm. Biopharm. 2004, 57, 533–540. [Google Scholar] [CrossRef]
- Qu, F.; Zhu, G.; Lin, H.; Zhang, W.; Sun, J.; Li, S.; Qiu, S. A controlled release of ibuprofen by systematically tailoring the morphology of mesoporous silica materials. J. Solid State Chem. 2006, 179, 2027–2035. [Google Scholar] [CrossRef]
- Horcajada, P.; Rámila, A.; Pérez-Pariente, J.; Vallet-Regí, M. Influence of pore size of MCM-41 matrices on drug delivery rate. Microporous Mesoporous Mater. 2004, 68, 105–109. [Google Scholar] [CrossRef]
- Inocêncio, S.; Cordeiro, T.; Matos, I.; Danède, F.; Sotomayor, J.C.; Fonseca, I.M.; Correia, N.T.; Corvo, M.C.; Dionísio, M. Ibuprofen incorporated into unmodified and modified mesoporous silica: From matrix synthesis to drug release. Microporous Mesoporous Mater. 2021, 310, 110541. [Google Scholar] [CrossRef]
- Brás, A.R.; Merino, E.G.; Neves, P.D.; Fonseca, I.M.; Dionísio, M.; Schönhals, A.; Correia, N.T. Amorphous ibuprofen confined in nanostructured silica materials: A dynamical approach. J. Phys. Chem. C 2011, 115, 4616–4623. [Google Scholar] [CrossRef]
- Song, S.W.; Hidajat, K.; Kawi, S. Functionalized SBA-15 materials as carriers for controlled drug delivery: Influence of surface properties on matrix-drug interactions. Langmuir 2005, 21, 9568–9575. [Google Scholar] [CrossRef]
- Ahmadi, E.; Dehghannejad, N.; Hashemikia, S.; Ghasemnejad, M.; Tabebordbar, H. Synthesis and surface modification of mesoporous silica nanoparticles and its application as carriers for sustained drug delivery. Drug Deliv. 2014, 21, 164–172. [Google Scholar] [CrossRef]
- Malfait, B.; Correia, N.T.; Mussi, A.; Paccou, L.; Guinet, Y.; Hédoux, A. Solid-state loading of organic molecular materials within mesoporous silica matrix: Application to ibuprofen. Microporous Mesoporous Mater. 2019, 277, 203–207. [Google Scholar] [CrossRef]
- Ferro, M.; Castiglione, F.; Pastori, N.; Punta, C.; Melone, L.; Panzeri, W.; Rossi, B.; Trotta, F.; Mele, A. Dynamics and interactions of ibuprofen in cyclodextrin nanosponges by solid-state NMR spectroscopy. Beilstein J. Org. Chem. 2017, 13, 182–194. [Google Scholar] [CrossRef]
- Venuti, V.; Rossi, B.; Mele, A.; Melone, L.; Punta, C.; Majolino, D.; Masciovecchio, C.; Caldera, F.; Trotta, F. Tuning structural parameters for the optimization of drug delivery performance of cyclodextrin-based nanosponges. Expert Opin. Drug Deliv. 2017, 14, 331–340. [Google Scholar] [CrossRef]
- Kierys, A.; Grochowicz, M.; Kosik, P. The release of ibuprofen sodium salt from permanently porous poly(hydroxyethyl methacrylate-co-trimethylolpropane trimethacrylate) resins. Microporous Mesoporous Mater. 2015, 217, 133–140. [Google Scholar] [CrossRef]
- Wang, X.; Liu, P.; Tian, Y.; Zang, L. Novel synthesis of Fe-containing mesoporous carbons and their release of ibuprofen. Microporous Mesoporous Mater. 2011, 145, 98–103. [Google Scholar] [CrossRef]
- Wang, X.; Liu, P.; Tian, Y. Ordered mesoporous carbons for ibuprofen drug loading and release behavior. Microporous Mesoporous Mater. 2011, 142, 334–340. [Google Scholar] [CrossRef]
- Azaïs, T.; Tourné-Péteilh, C.; Aussenac, F.; Baccile, N.; Coelho, C.; Devoisselle, J.M.; Babonneau, F. Solid-state NMR study of ibuprofen confined in MCM-41 material. Chem. Mater. 2006, 18, 6382–6390. [Google Scholar] [CrossRef]
- Tielens, F.; Folliet, N.; Bondaz, L.; Etemovic, S.; Babonneau, F.; Gervais, C.; Azaïs, T. Molecular Picture of the Adsorption of Ibuprofen and Benzoic Acid on Hydrated Amorphous Silica through DFT-D Calculations Combined with Solid-State NMR Experiments. J. Phys. Chem. C 2017, 121, 17339–17347. [Google Scholar] [CrossRef] [Green Version]
- Azaïs, T.; Laurent, G.; Panesar, K.; Nossov, A.; Guenneau, F.; Sanfeliu Cano, C.; Tourné-Péteilh, C.; Devoisselle, J.M.; Babonneau, F. Implication of Water Molecules at the Silica-Ibuprofen Interface in Silica-Based Drug Delivery Systems Obtained through Incipient Wetness Impregnation. J. Phys. Chem. C 2017, 121, 26833–26839. [Google Scholar] [CrossRef]
- Skorupska, E.; Jeziorna, A.; Paluch, P.; Potrzebowski, M.J. Ibuprofen in mesopores of mobil crystalline material 41 (MCM-41): A deeper understanding. Mol. Pharm. 2014, 11, 1512–1519. [Google Scholar] [CrossRef]
- Gackowski, M.; Ruggiero-Mikołajczyk, M.; Duraczyńska, D.; Hinz, A.; Bzowska, M.; Szczepanowicz, K. The role of water in the confinement of ibuprofen in SBA-15. J. Mater. Chem. B 2021, 9, 7482–7491. [Google Scholar] [CrossRef]
- Misaelides, P. Application of natural zeolites in environmental remediation: A short review. Microporous Mesoporous Mater. 2011, 144, 15–18. [Google Scholar] [CrossRef]
- Onik, K.; Gackowski, M.; Derewinski, M.A.; Sulikowski, B. Mesoporous Layered Aluminosilicates Prepared from Protozeolitic Nanoclusters: Synthesis and Physicochemical and Catalytic Properties. J. Phys. Chem. C 2018, 122, 25983–25991. [Google Scholar] [CrossRef]
- Gackowski, M.; Kuterasiński, Ł.; Podobiński, J.; Korzeniowska, A.; Sulikowski, B.; Datka, J. Hierarchical zeolite mazzite: Physicochemical properties and A-pinene isomerization. Appl. Catal. A Gen. 2019, 578, 53–62. [Google Scholar] [CrossRef]
- Gackowski, M.; Tarach, K.; Kuterasiński, Ł.; Podobiński, J.; Jarczewski, S.; Kuśtrowski, P.; Datka, J. Hierarchical zeolites Y obtained by desilication: Porosity, acidity and catalytic properties. Microporous Mesoporous Mater. 2018, 263, 282–288. [Google Scholar] [CrossRef]
- Tosheva, L.; Belkhair, S.; Gackowski, M.; Malic, S.; Al-Shanti, N.; Verran, J. Rapid screening of the antimicrobial efficacy of Ag zeolites. Colloids Surf. B Biointerfaces 2017, 157, 254–260. [Google Scholar] [CrossRef]
- Datt, A.; Burns, E.A.; Dhuna, N.A.; Larsen, S.C. Loading and release of 5-fluorouracil from HY zeolites with varying SiO2/Al2O3 ratios. Microporous Mesoporous Mater. 2013, 167, 182–187. [Google Scholar] [CrossRef]
- Al-Thawabeia, R.A.; Hodali, H.A. Use of Zeolite ZSM-5 for Loading and Release of 5-Fluorouracil. J. Chem. 2015, 2015, 403597. [Google Scholar] [CrossRef] [Green Version]
- Szegedi, Á.; Popova, M.; Trendafilova, I.; Trif, L.; Mihály, J.; Makk, J.; Mavrodinova, V. Bicomponent drug formulation for simultaneous release of Ag and sulfadiazine supported on nanosized zeolite Beta. Nano-Struct. Nano-Objects 2020, 24, 100562. [Google Scholar] [CrossRef]
- Horcajada, P.; Márquez-Alvarez, C.; Rámila, A.; Pérez-Pariente, J.; Vallet-Regí, M. Controlled release of Ibuprofen from dealuminated faujasites. Solid State Sci. 2006, 8, 1459–1465. [Google Scholar] [CrossRef]
- Szostak, R. Molecular Sieves: Principles of Synthesis and Identification; Springer Science+Business: New York, NY, USA, 1989; ISBN 9789401095310. [Google Scholar]
- Katoh, M.; Kimura, M.; Sugino, M.; Horikawa, T.; Nakagawa, K.; Sugiyama, S. Modification of commercial NaY zeolite to give high water diffusivity and adsorb a large amount of water. J. Colloid Interface Sci. 2015, 455, 220–225. [Google Scholar] [CrossRef]
- Valiullin, R.; Kärger, J.; Cho, K.; Choi, M.; Ryoo, R. Dynamics of water diffusion in mesoporous zeolites. Microporous Mesoporous Mater. 2011, 142, 236–244. [Google Scholar] [CrossRef]
- Treacy, M.M.J.; Higgins, J.B. Collection of Simulated XRD Powder Patterns for Zeolites, 5th ed.; Elsevier: Amsterdam, The Natherlands, 2007. [Google Scholar]
- Xu, F.; Sun, L.X.; Tan, Z.C.; Liang, J.G.; Li, R.L. Thermodynamic study of ibuprofen by adiabatic calorimetry and thermal analysis. Thermochim. Acta 2004, 412, 33–57. [Google Scholar] [CrossRef]
- Azais, T.; Hartmeyer, G.; Quignard, S.; Laurent, G.; Tourne-Peteilh, C.; Devoisselle, J.-M.; Babonneau, F. Solid-state NMR characterization of drug-model molecules encapsulated in MCM-41 silica. Pure Appl. Chem. 2009, 81, 1345–1355. [Google Scholar] [CrossRef]
- Chen, K.; Kelsey, J.; White, J.L.; Zhang, L.; Resasco, D. Water Interactions in Zeolite Catalysts and Their Hydrophobically Modified Analogues. ACS Catal. 2015, 5, 7480–7487. [Google Scholar] [CrossRef]
- Van Aelst, J.; Haouas, M.; Gobechiya, E.; Houthoofd, K.; Philippaerts, A.; Sree, S.P.; Kirschhock, C.E.A.; Jacobs, P.; Martens, J.A.; Sels, B.F.; et al. Hierarchization of USY zeolite by NH4OH. A postsynthetic process investigated by NMR and XRD. J. Phys. Chem. C 2014, 118, 22573–22582. [Google Scholar] [CrossRef] [Green Version]
- Grünberg, B.; Emmler, T.; Gedat, E.; Shenderovich, I.; Findenegg, G.H.; Limbach, H.H.; Buntkowsky, G. Hydrogen bonding of water confined in mesoporous silica MCM-41 and SBA-15 studied by 1H solid-state NMR. Chemistry 2004, 10, 5689–5696. [Google Scholar] [CrossRef]
- Li, Z.; Rieg, C.; Beurer, A.-K.K.; Benz, M.; Bender, J.; Schneck, C.; Traa, Y.; Dyballa, M.; Hunger, M. Effect of aluminum and sodium on the sorption of water and methanol in microporous MFI-type zeolites and mesoporous SBA-15 materials. Adsorption 2021, 27, 49–68. [Google Scholar] [CrossRef]
- Hunger, M.; Freude, D.; Pfeifer, H. Magic-angle Spinning Nuclear Magnetic Resonance Studies of Water Molecules adsorbed on Bronsted and Lewis-acid Sites in Zeolites and Amorphous Silica-Aluminas. J. Chem. Soc. Faraday Trans. 1991, 87, 657–662. [Google Scholar] [CrossRef]
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).