Fatigue Behavior of an AM50 Die-Casting Alloy Anodized by Plasma Electrolytic Oxidation
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Conclusions
- (1)
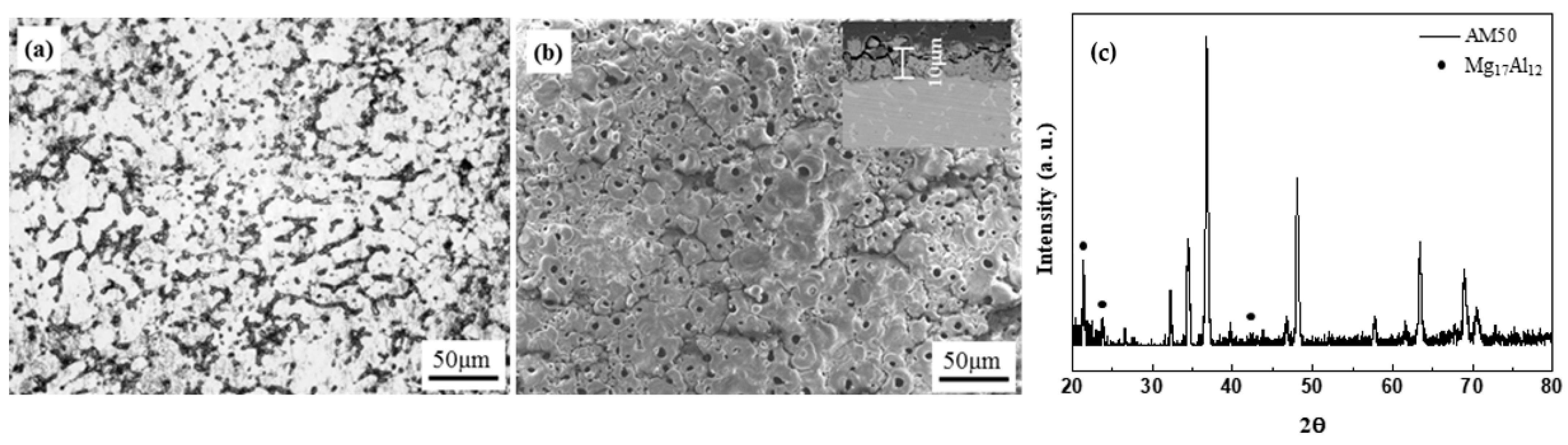
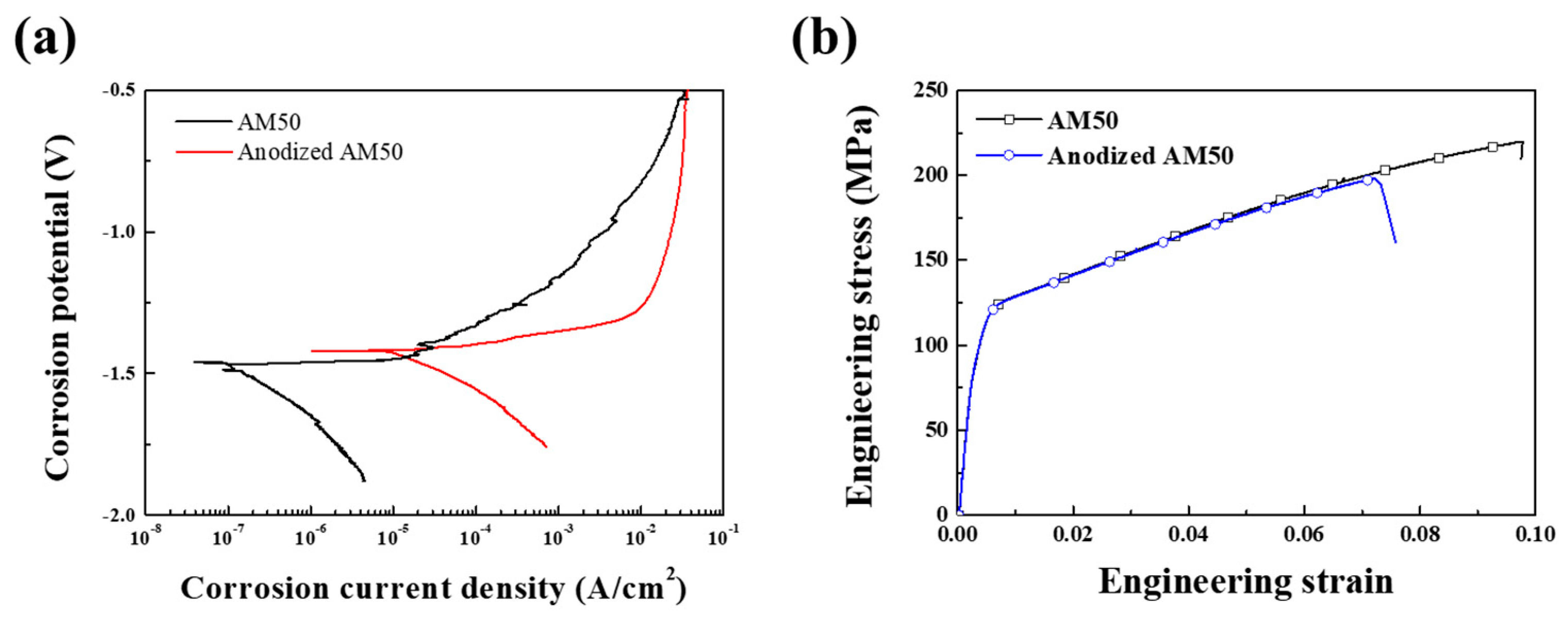
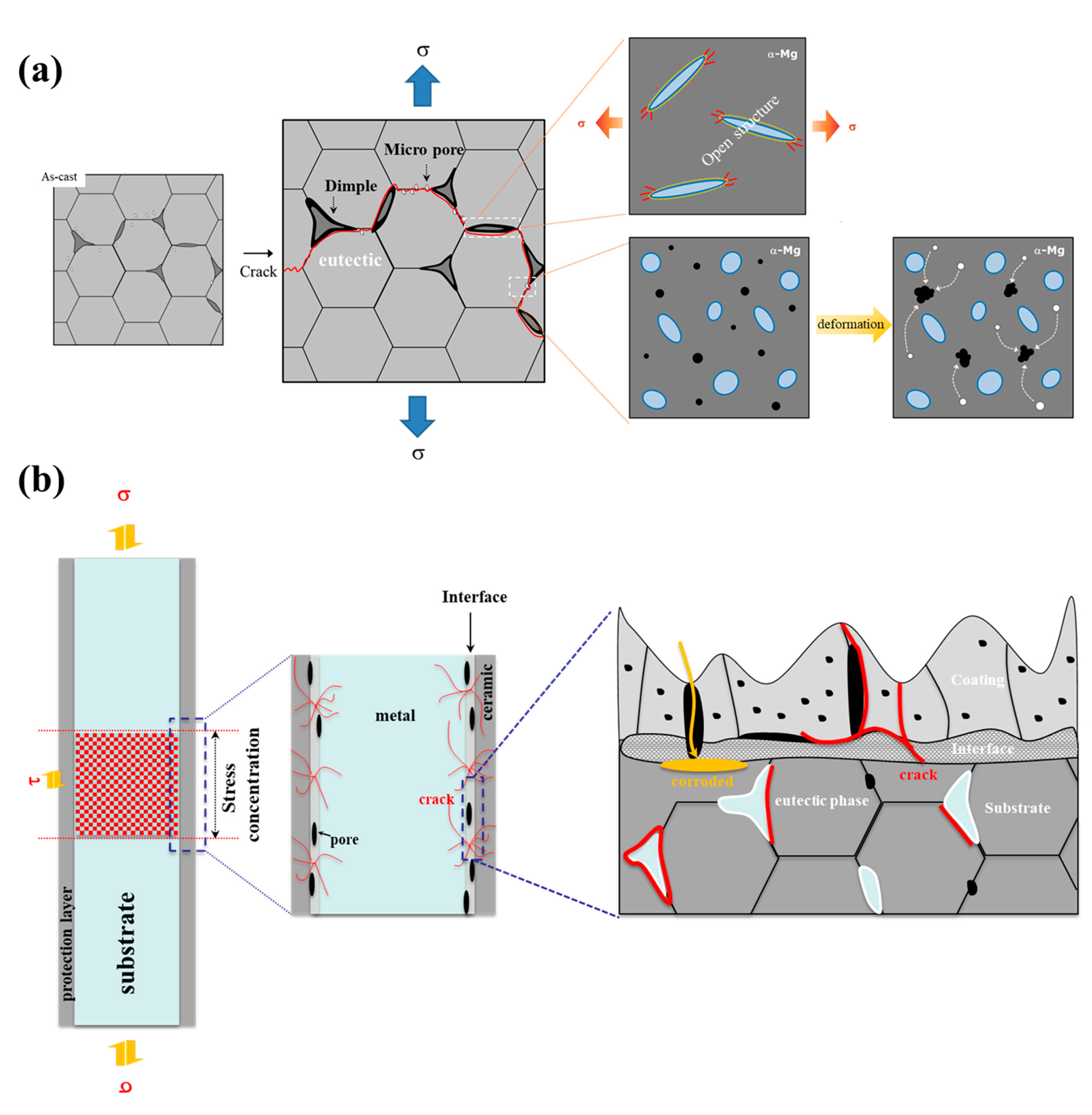
- The die-casted AM50 alloy is anodized using the PEO method and a crater-like microstructure with some round shrinkage pores in the PEO-anodized alloy shown. The PEO-anodized AM50 Mg alloys with 10 μm in anodizing thickness exhibited ~110% enhanced strength and ~100 times enhanced corrosion current density. The corrosion potential of the PEO-anodized specimen is slightly increased, and the corrosion current density is 100 times increased compared to the unanodized specimen.
- (2)
- The yield stress of the PEO-anodized specimens is 11 MPa higher than the unanodized alloy. The calculated toughness value of the unanodized alloy is higher than that of the PEO-anodized alloy at 13.6 MPam0.5 due to the decrease in the elongation to a failure caused by the thick anodized layer.
- (3)
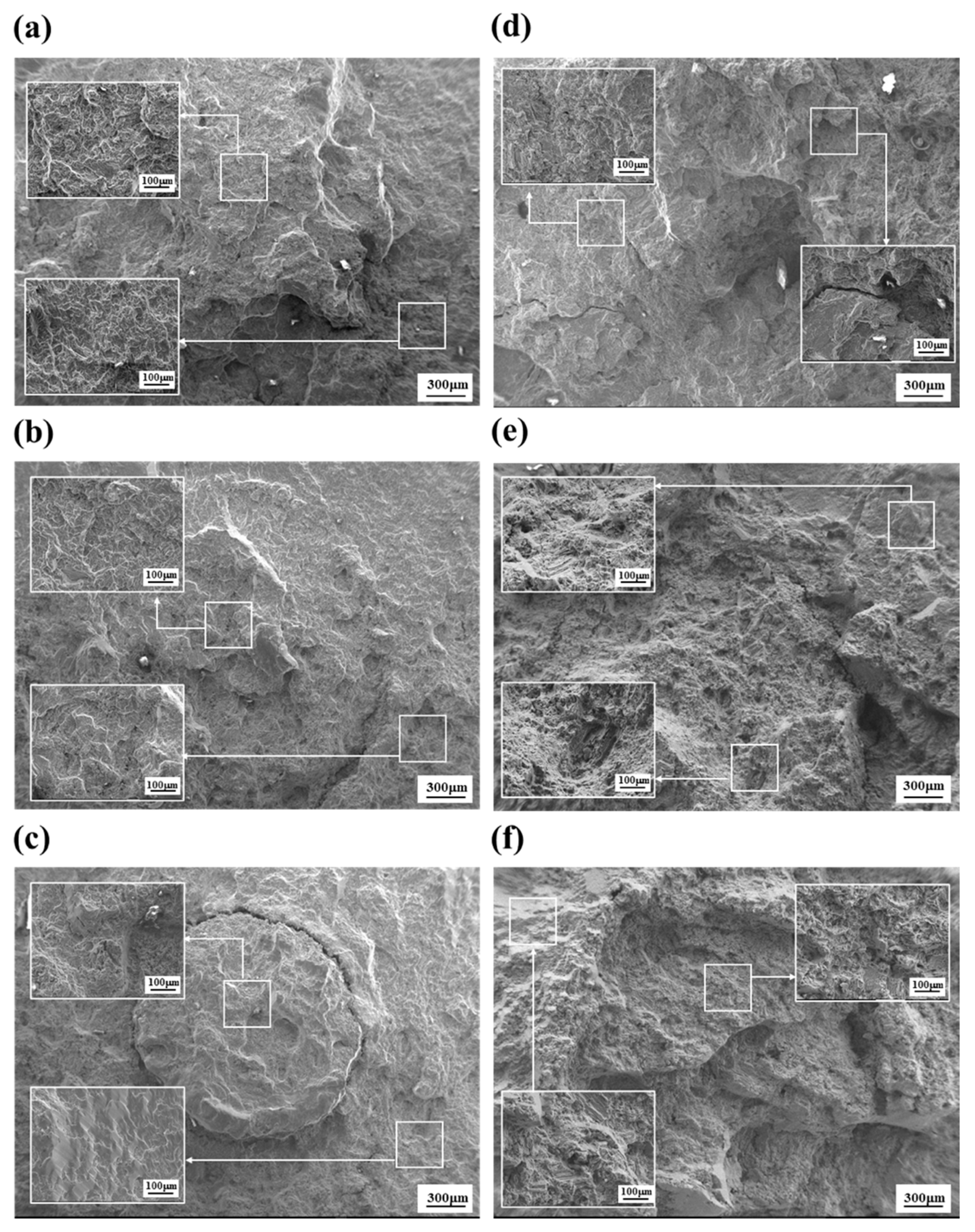
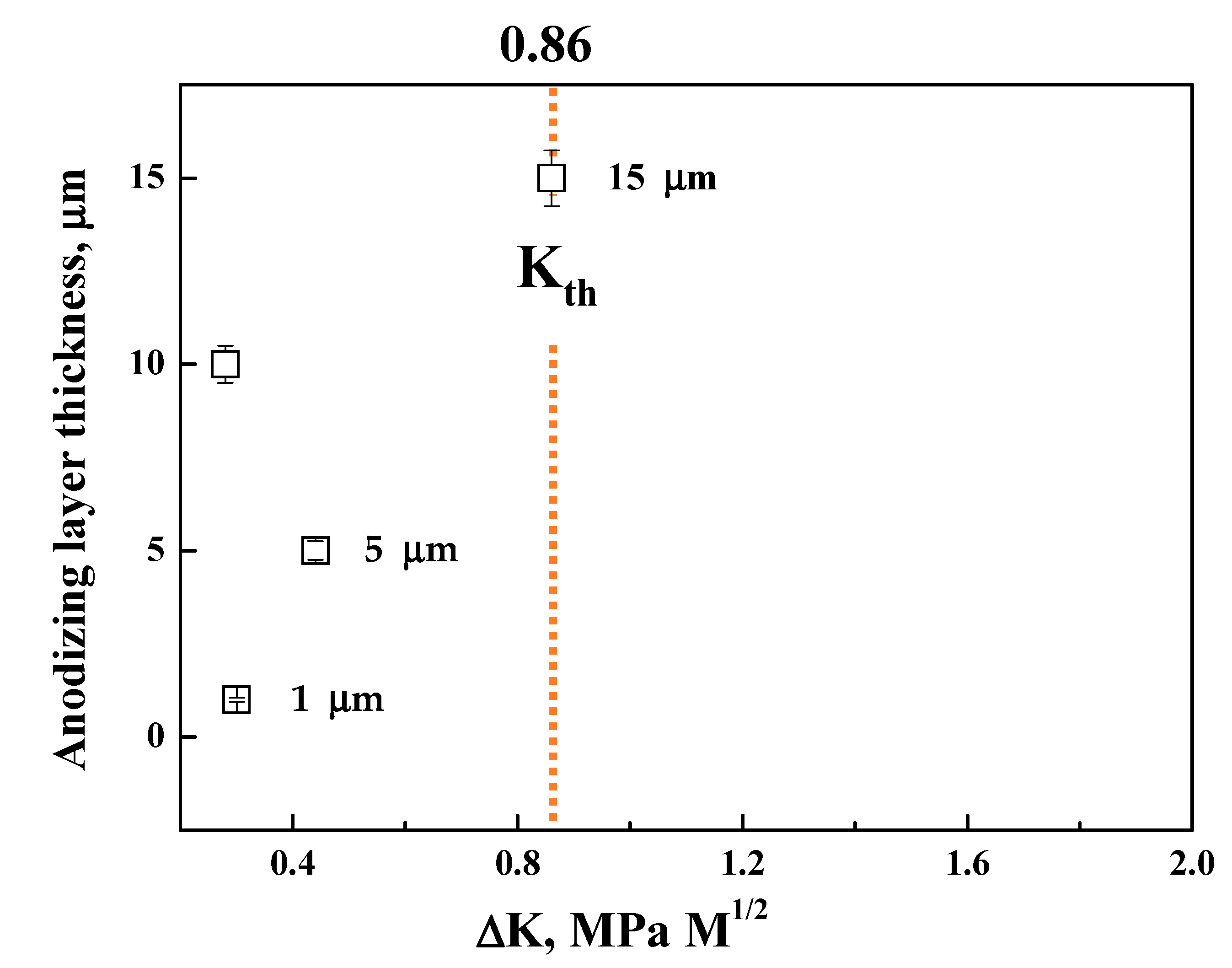
- The PEO-anodized AM 50 Mg alloys show good fatigue properties (i.e., ΔK1 value almost close to ΔKth (0.86 MPa√m) and Δσth of 74 MPa), the level of which corresponds to those of thin coating specimens.
- (4)
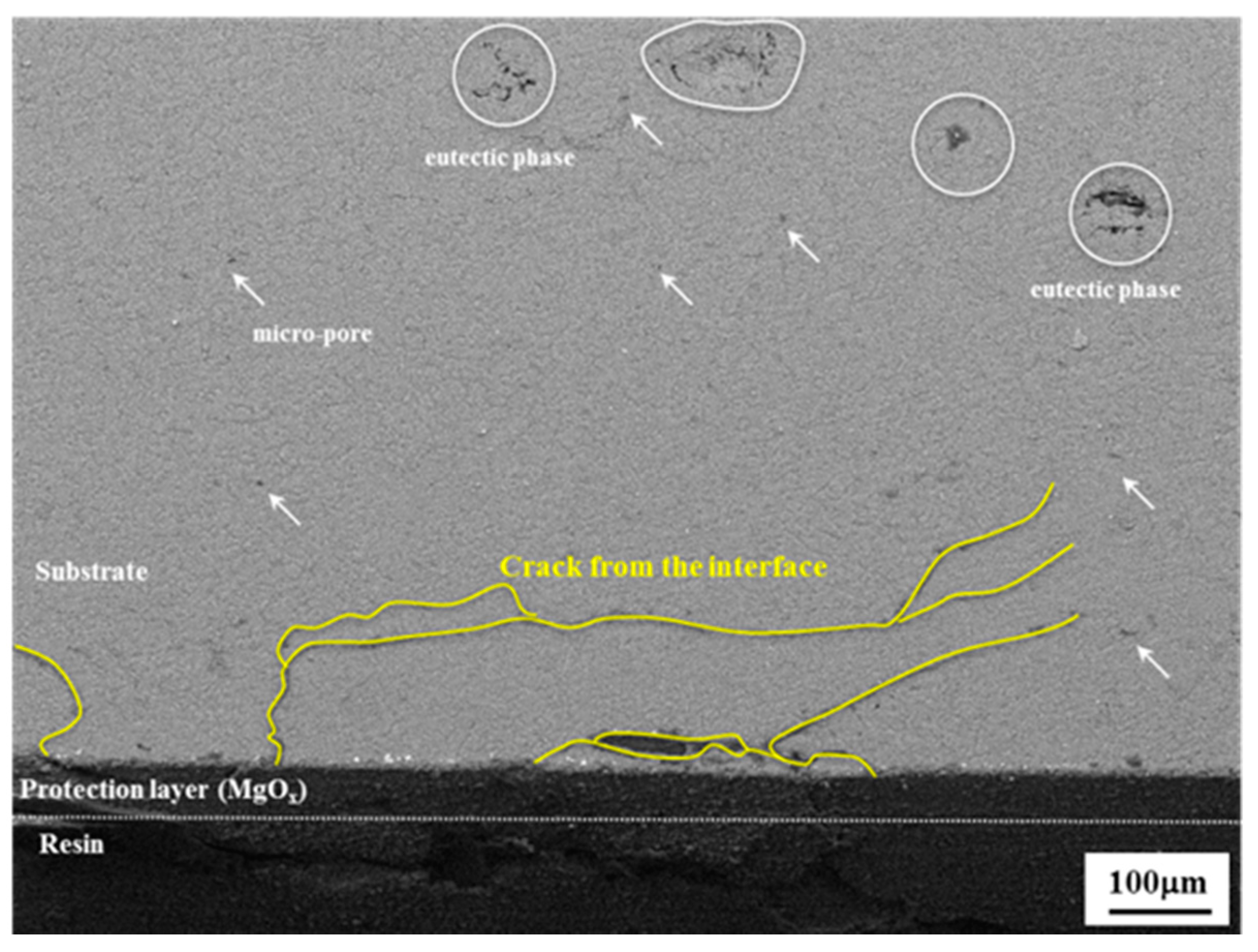
- Since the anodizing layer of the PEO-anodized specimen is densely constructed, the 74 MPa of the Δσth value of the PEO-anodized alloy is close to the value of the thin layer anodized alloy. The cracks in the PEO-anodized alloy are predominantly generated at the interface between the substrate and the anodizing layer, resulting in a relatively long fatigue life.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Friedrich, H.; Schumann, S. Research for a “new age of magnesium” in the automotive industry. J. Mater. Process. Technol. 2001, 117, 276–281. [Google Scholar] [CrossRef]
- Furuya, H.; Kogiso, N.; Matunaga, S.; Senda, K. Applications of magnesium alloys for aerospace structure systems. Mat. Sci. Forum 2000, 350–351, 341–348. [Google Scholar] [CrossRef]
- Kojima, Y. Platform science and technology for advanced magnesium alloys. Mat. Sci. Forum 2000, 350–351, 3–18. [Google Scholar] [CrossRef]
- Luo, A.A. Magnesium casting technology for structural applications. J. Magnes. Alloy 2013, 1, 2–22. [Google Scholar] [CrossRef] [Green Version]
- Makar, G.L.; Kruger, J. Corrosion studies of rapidly solidified magnesium alloys. J. Electrochem. Soc. 1990, 137, 414. [Google Scholar] [CrossRef]
- Song, G.L.; Atrens, A. Corrosion mechanisms of magnesium alloys. Adv. Eng. Mater. 2000, 1, 11–33. [Google Scholar] [CrossRef]
- Atrens, A.; Song, G.L.; Cao, F.; Shi, Z.; Bowen, P.K. Advanced in Mg corrosion and research suggestions. J. Magnes. Alloy. 2013, 1, 177–200. [Google Scholar] [CrossRef] [Green Version]
- Khaselev, O.; Weiss, D.; Yahalom, J. Anodizing of pure magnesium in KOH-aluminate solutions under sparking. J. Electrochem. Soc. 1999, 146, 1757–1761. [Google Scholar] [CrossRef]
- Song, Y.W.; Shan, D.Y.; Han, E.H. High corrosion resistance of electroless composite plating coatings on AZ91D magnesium alloys. Electrochimica Acta 2008, 53, 2135–2143. [Google Scholar] [CrossRef]
- Hwang, D.Y.; Kim, Y.M.; Park, D.-Y.; Yoo, B.; Shin, D.H. Corrosion resistance of oxide layers formed on AZ91 Mg alloy in KMnO4 electrolyte by plasma electrolytic oxidation. Electrochimica Acta 2009, 54, 5479–5485. [Google Scholar] [CrossRef]
- Barchiche, C.E.; Rocca, E.; Hazan, J. Corrosion behaviour of Sn-containing oxide layer on AZ91D alloy formed by plasma electrolytic oxidation. Surf. Coat. Technol. 2008, 202, 4145–4152. [Google Scholar] [CrossRef]
- Namgung, S.; Ko, Y.G.; Shin, K.R.; Shin, D.H. Influence of ZrO2 Particulates on Corrosion Resistance of Magnesium Alloy Coated by Plasma Electrolytic Oxidation. Korean J. Met. Mater. 2010, 48, 813–818. [Google Scholar]
- Chang, S.Y.; Lee, D.H.; Kim, B.S.; Kim, T.S.; Song, Y.-S.; Kim, S.H.; Lee, C.B. Characteristics of plasma electrolytic oxide coatings on Mg-Al-Zn alloy prepared by powder metallurgy. Met. Mater. Int. 2009, 15, 759–764. [Google Scholar] [CrossRef]
- Khan, S.A.; Miyashita, Y.; Mutoh, Y.; Koike, T. Effect of anodized layer thickness on fatigue behavior of magnesium alloy. Mater. Sci. Eng. A 2008, 474, 261–269. [Google Scholar] [CrossRef]
- Khan, S.A.; Miyashita, Y.; Mutoh, Y.; Koike, T. Fatigue behavior of anodized AM60 magnesium alloy under humid environment. Mater. Sci. Eng. 2008, 498, 377–383. [Google Scholar] [CrossRef]
- de Oliveira, L.A.; dos Santos, S.L.; de Oliveira, V.A.; Antunes, R.A. Influence of Anodization on the Fatigue and Corrosion-Fatigue Behaviors of the AZ31B Magnesium Alloy. Metals 2021, 11, 1573. [Google Scholar] [CrossRef]
- Wang, X.; Fan, J. An evaluation on the growth rate of small fatigue cracks in cast AM50 magnesium alloy at different temperatures in vacuum conditions. Int. J. Fatigue 2006, 28, 79–86. [Google Scholar] [CrossRef]
- Tang, T.; Kim, S.; Horstemeyer, M.F. Fatigue crack growth in magnesium single crystals under cyclic loading: Molecular dynamics simulation. Comput. Mater. Sci. 2010, 48, 426–439. [Google Scholar] [CrossRef]
- Uematsu, Y.; Kakiuchi, T.; Teratani, T.; Harada, Y.; Tokaji, K. Improvement of corrosion fatigue strength of magnesium alloy by multilayer diamond-like carbon coatings. Surf. Coatings Technol. 2011, 205, 2778–2784. [Google Scholar] [CrossRef]
- Fontana, M.G.; Greene, N.D. Corrosion Engineering, 2nd ed.; McGraw-Hill: New York, NY, USA, 2018. [Google Scholar]
- Anderson, T.L. Fracture Mechanics: Fundamentals and Applications, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Avedesian, M.M.; Baker, H. (Eds.) ASM Specialty Handbook: Magnesium and Magnesium Alloys; ASM International: Novelty, OH, USA, 1999; ISBN 978-0-87170-657-7. [Google Scholar]
- Evangelides, H.A. Method of Electrolytically Coating Magnesium and Electrolyte Therefore. U.S. Patent 2723952, 15 November 1955. [Google Scholar]
- El Mahallawy, N.A.; Shoeib, M.A.; Abouelenain, M.H. AZ91 magnesium alloys: Anodizing of using environmental friendly electrolytes. J. Surf. Eng. Mater. Adv. Technol. 2011, 1, 62. [Google Scholar] [CrossRef]
- Haruyama, S. Proceedings of Tenth World Congress on Metal Finishing, Kyoto, Japan, 12–17 October 1980; Metal Finishing Society of Japan: Tokyo, Japan, 1980. [Google Scholar]
- Ibrahim, M.F.E.; Miller, K.J. Determination of Fatigue Crack Initiation Life. Fatigue Fract. Eng. Mater. Struct. 1979, 2, 351–360. [Google Scholar] [CrossRef]
- Dowling, N. Notched member fatigue life predictions combining crack initiation and propagation. Fatigue Fract. Eng. Mater. Struct. 1979, 2, 129–138. [Google Scholar] [CrossRef]
- Van der Giessen, E.; Tvergaard, V. Effect of random variations in microstructure on the development of final creep failure in polycrystalline aggregates. Model. Simul. Mat. Sci. Eng. 1994, 2, 721–738. [Google Scholar] [CrossRef]
- Khan, S.A.; Miyashita, Y.; Mutoh, Y. Corrosion fatigue behavior of AM60 magnesium alloy with anodizing layer and chemical-conversion-coating layer. Mater. Corros. 2014, 66, 940–948. [Google Scholar] [CrossRef]
- Sajuri, Z.; Miyashita, Y.; Mutoh, Y. Effect of Stress Ratio on Fatigue Crack Growth Behavior of Magnesium Alloys. Mater. Sci. Forum 2003, 419-422, 81–86. [Google Scholar] [CrossRef]
- El Haddad, M.; Topper, T.; Smith, K. Prediction of non propagating cracks. Eng. Fract. Mech. 1979, 11, 573–584. [Google Scholar] [CrossRef]
- Bhuiyan, M.S.; Ostuka, Y.; Mutoh, Y.; Murai, T.; Iwakami, S. Corrosion fatigue behavior of conversion coated AZ61 magnesium alloy. Mater. Sci. Eng. 2010, 527, 4978–4984. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, C.; Wang, F.; Lou, H.; Cao, C. Study on the environmentally friendly anodizing of AZ91D magnesium alloy. Surf. Coat. Technol. 2002, 161, 36–43. [Google Scholar] [CrossRef]


| Mechanical Properties | Corrosion Properties | |||||
|---|---|---|---|---|---|---|
| Yield Strength (MPa) | Ultimate Tensile Strength (MPa) | Elongation to Failure (%) | Fracture Toughness (MPam1/2) | Corrosion Potential (V) | Current Density (mA/mm2) | |
| UnanodizedAM50 | 119 | 219 | 9.7 | 15.9 | −1.41 | 7.9 |
| Anodized AM50 | 130 | 197 | 7.2 | 14.4 | −1.47 | 0.08 |
| 50 MPa | 60 MPa | 70 MPa | 80 MPa | ||
|---|---|---|---|---|---|
| Total Fatigue life (cycle, Nf) | Unanodized | 181,211 | 34,584 | 17,318 | 13,711 |
| Anodized (depth: 10 mm) | 169,758 | 25,993 | 7564 | 4868 | |
| HAE (depth: 1 μm) | 124,586 | 7846 | 1543 | 523 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, K.; Kang, S.; Kang, H. Fatigue Behavior of an AM50 Die-Casting Alloy Anodized by Plasma Electrolytic Oxidation. Materials 2021, 14, 7795. https://doi.org/10.3390/ma14247795
Choi K, Kang S, Kang H. Fatigue Behavior of an AM50 Die-Casting Alloy Anodized by Plasma Electrolytic Oxidation. Materials. 2021; 14(24):7795. https://doi.org/10.3390/ma14247795
Chicago/Turabian StyleChoi, Kwangmin, Seungwon Kang, and Heon Kang. 2021. "Fatigue Behavior of an AM50 Die-Casting Alloy Anodized by Plasma Electrolytic Oxidation" Materials 14, no. 24: 7795. https://doi.org/10.3390/ma14247795
APA StyleChoi, K., Kang, S., & Kang, H. (2021). Fatigue Behavior of an AM50 Die-Casting Alloy Anodized by Plasma Electrolytic Oxidation. Materials, 14(24), 7795. https://doi.org/10.3390/ma14247795





