Evaluation of Newly Introduced Bioactive Materials in Terms of Cavity Floor Adaptation: OCT Study
Abstract
:1. Introduction
2. Material and Methods
2.1. Materials Used
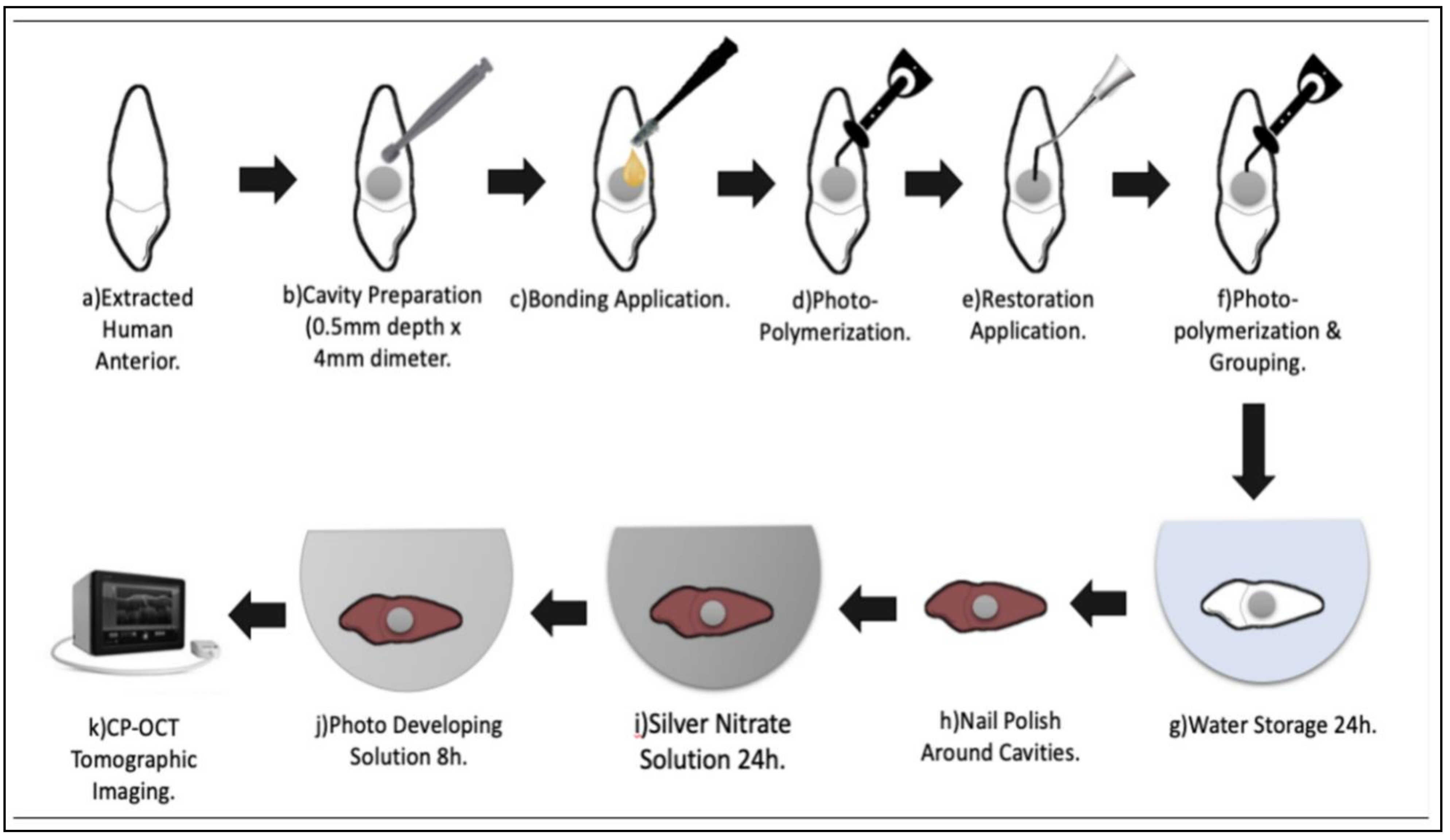
2.2. Sample Preparation
2.3. Contrasting Medium Preparation
2.4. CP-OCT System
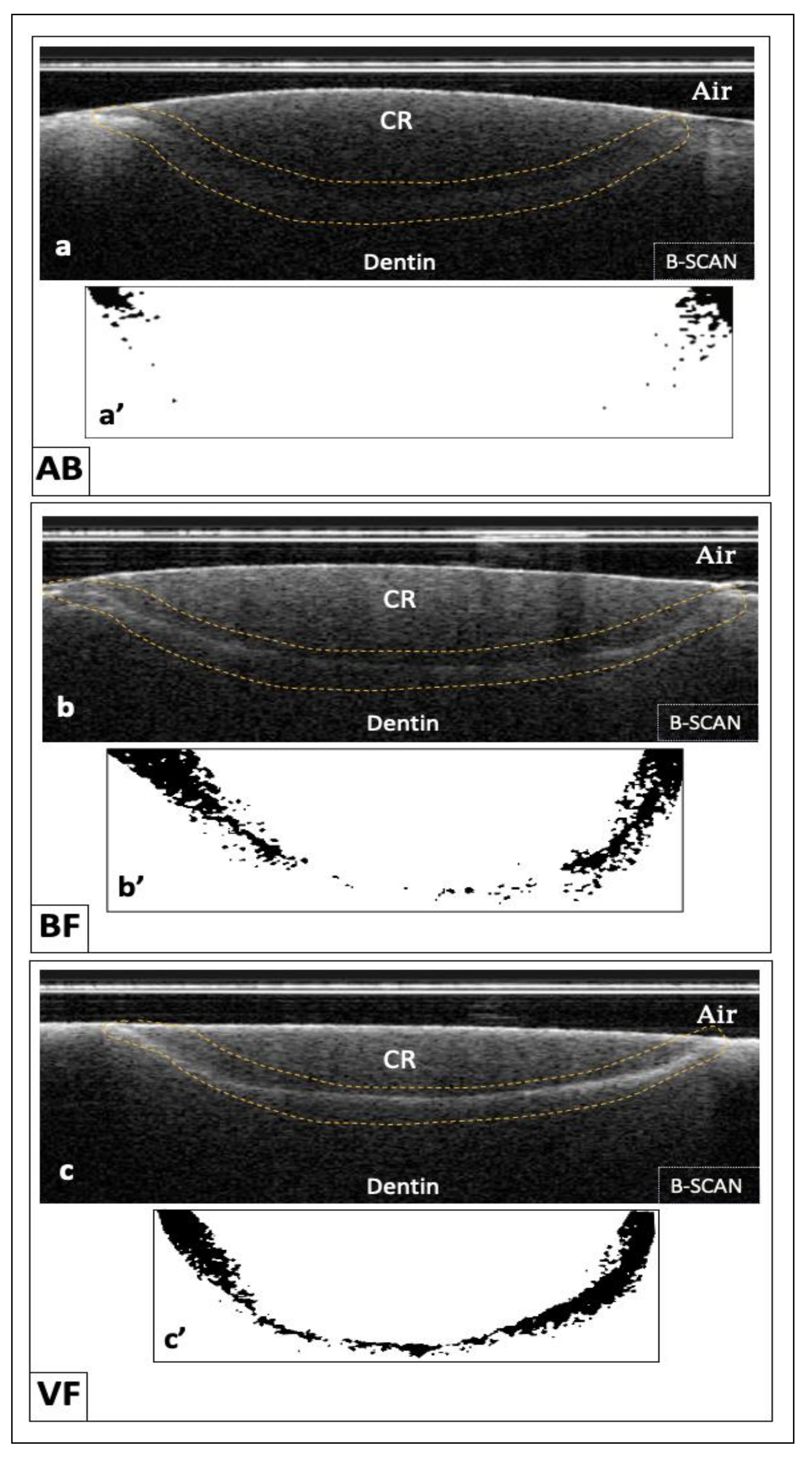
2.5. Tomographic Imaging and Image Analysis
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
6. Clinical Recommendations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gong, H.; Zhang, X.; Guo, X.; Gao, P.; Zhao, Q.; Xu, T.; Shi, X.; Zhu, S.; Cui, Z. Advances in reducing microleakage in dental composites. Rev. Roum. Chim. 2019, 64, 519–527. [Google Scholar] [CrossRef]
- Bahgat, H.; Farouk, H. Effect of different beverages on microhardness of Zirconia reinforced Glass Ionomer and micro-hybrid Resin composite. Egypt. Dent. J. 2019, 65, 523–530. [Google Scholar] [CrossRef]
- Opdam, N.J.M.; Roeters, F.J.M.; Verdonschot, E.H. Adaptation and radiographic evaluation of four adhesive systems. J. Dent. 1997, 25, 391–397. [Google Scholar] [CrossRef]
- Haak, R.; Wicht, M.J.; Hellmich, M.; Noack, M.J. Detection of marginal defects of composite restorations with conventional and digital radiographs. Eur. J. Oral Sci. 2002, 110, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Bakhsh, T.A.; Alrabghi, K.K.; Kawther, S.W.; Mirdad, F.S.; Mirdad, L.Y.; Jamleh, A.O. Adaptation Assessment of Three Bonded Resin Restorations at the Cavity Floor Using Cross-Polarization Optical Coherence Tomography. Photobiomodul. Photomed. Laser Surg. 2018, 37, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Mandurah, M.M.; Sadr, A.; Bakhsh, T.A.; Shimada, Y.; Sumi, Y.; Tagami, J. Characterization of transparent dentin in attrited teeth using optical coherence tomography. Lasers Med. Sci. 2015, 30, 1189–1196. [Google Scholar] [CrossRef] [PubMed]
- Lenth, R.V. Statistical power calculations. J. Anim. Sci. 2007, 85 (Suppl. S13), E24–E29. [Google Scholar] [CrossRef]
- Kemp-Scholte, C.M.; Davidson, C.L. Complete Marginal Seal of Class V Resin Composite Restorations Effected by Increased Flexibility. J. Dent. Res. 1990, 69, 1240–1243. [Google Scholar] [CrossRef] [PubMed]
- Oglakci, B.; Kazak, M.; Donmez, N.; Dalkilic, E.E.; Koymen, S.S. The use of a liner under different bulk-fill resin composites: 3D GAP formation analysis by x-ray micro-computed tomography. J. Appl. Oral Sci. 2020, 28, e20190042. [Google Scholar] [CrossRef]
- Rengo, C.; Spagnuolo, G.; Ametrano, G.; Goracci, C.; Nappo, A.; Rengo, S.; Ferrari, M. Marginal leakage of bulk fill composites in Class II restorations: A microCT and digital microscope analysis. Int. J. Adhes. Adhes. 2015, 60, 123–129. [Google Scholar] [CrossRef]
- Hayes, M.; Burke, F.; Allen, P.F. Incidence, Prevalence and Global Distribution of Root Caries. Monogr. Oral Sci. 2017, 26, 1–8. [Google Scholar]
- Shimada, Y.; Sadr, A.; Sumi, Y.; Tagami, J. Application of Optical Coherence Tomography (OCT) for Diagnosis of Caries, Cracks, and Defects of Restorations. Curr. Oral Health Rep. 2015, 2, 73–80. [Google Scholar] [CrossRef]
- Bakhsh, T.A.; Altouki, N.H.; Baeesa, L.S.; Baamer, R.A.; Alshebany, R.M.; Natto, Z.; Nasir, A.; Turkistani, A.; Hasanain, F.; Naguib, G.H. Effect of self-etch adhesives on the internal adaptation of composite restoration: A CP-OCT Study. Odontology 2019, 107, 165–173. [Google Scholar] [CrossRef]
- Benetti, A.R.; Michou, S.; Larsen, L.; Peutzfeldt, A.; Pallesen, U.; van Dijken, J.W.V. Adhesion and marginal adaptation of a claimed bioactive, restorative material. Biomater. Investig. Dent. 2019, 6, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Owens, B.M.; Phebus, J.G.; Johnson, W.W. Evaluation of the marginal integrity of a bioactive restorative material. Gen. Dent. 2018, 66, 32–36. [Google Scholar] [PubMed]
- Omidi, B.R.; Naeini, F.F.; Dehghan, H. Microleakage of an enhanced resin-modified glassionomer restorative material in primary molars. J. Dent. 2018, 15, 205–213. [Google Scholar]
- Kaushik, M.; Yadav, M. Marginal microleakage properties of activa bioactive restorative and nanohybridcomposite resin using two different adhesives non-carious cervical lesions—An in vitro study. J. West Afr. Coll. Surg. 2017, 7, 1–14. [Google Scholar] [PubMed]
- Hughes, K.O.; Powell, K.J.; Hill, A.E. Delayed photoactivation of dual-cure composites: Effect on cuspal flexure, depth-of-cure, and mechanical properties. Oper. Dent. 2019, 44, E97–E104. [Google Scholar] [CrossRef] [PubMed]
- Kurokawa, H.; Takamizawa, T.; Akitomo, R.; Keishi, T.; Masashi, M. Three-year clinical evaluation of posterior composite restorations placed with a single-step self-etch adhesive. J. Oral Sci. 2015, 57, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Peterson, J.; Rizk, M.; Hoch, M. Bonding performance of self-adhesive flowable composites to enamel, dentin and a nano-hybrid composite. Odontology 2018, 106, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Brueckner, C.; Schneider, H.; Haak, R. Shear bond strength and tooth-composite interaction with self-adhering flowable composites. Oper Dent. 2017, 42, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Silikas, N.; Zhang, Z.; Watts, D. Hygroscopic dimen- sional changes of self-adhering and new resin-matrix com- posites during water sorption/desorption cycles. Dent. Mater. 2011, 27, 259–266. [Google Scholar] [CrossRef]
- Bektas, O.O.; Eren, D.; Akin, E.G.; Akin, H. Evaluation of a self- adhering flowable composite in terms of micro-shear bond strength and microleakage. Acta Odontol. Scand. 2013, 71, 541–546. [Google Scholar] [CrossRef]
- Memarpour, M.; Shafiei, F.; Razmjoei, F.; Kianimanesh, N. Effect of laser preparation on adhesion of a self-adhesive flowable composite resin to primary teeth. Microsc. Res. Tech. 2016, 79, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Juloski, J.; Goracci, C.; Rengo, C.; Giovannetti, A.; Vichi, A.; Vulicevic, Z.R.; Ferrari, M. Enamel and dentin bond strength of new simplified adhesive materials with and without preliminary phosphoric acid-etching. Am. J. Dent. 2012, 25, 239–243. [Google Scholar] [PubMed]
- Wang, R.; Shi, Y.; Li, T.; Pan, Y.; Cui, Y.; Xia, W. Adhesive interfacial characteristics and the related bonding performance of four self- etching adhesives with different functional monomers applied to dentin. J. Dent. 2017, 62, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Vichi, A.; Goracci, C.; Papacchini, F.; Ferrari, M. Bonding and sealing ability of a new self-adhering flowable composite resin in class I restorations. Clin. Oral Investig. 2013, 17, 1497–1506. [Google Scholar] [CrossRef] [PubMed]
- Mine, A.; de Munck, J.; Ende, A.V.a.; Poitevin, A.; Matsumoto, M.; Yoshida, Y. Limited interaction of a self-adhesive flowable composite with dentin/enamel characterized by TEM. Dent. Mater. 2017, 33, 209–217. [Google Scholar] [CrossRef]
- Bakhsh, T.A.; Sadr, A.; Shimada, Y.; Tagami, J.; Sumi, Y. Non- invasive quantification of resin-dentin interfacial gaps using optical coherence tomography: Validation against confocal microscopy. Dent. Mater. 2011, 27, 915–925. [Google Scholar] [CrossRef] [PubMed]
- Benetti, A.R.; Havndrup-Pedersen, C.; Honor, D. Bulk-fill resin composites: Polymerization contraction, depth of cure, and gap formation. Oper. Dent. 2015, 40, 190–200. [Google Scholar] [CrossRef]
- Kaga, M.; Kakuda, S.; Ida, Y.; Toshima, H.; Hashimoto, M.; Endo, K. Inhibition of enamel demineralization by buffering effect of S-PRG filler-containing dental sealant. Eur. J. Oral Sci. 2014, 122, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Ferrini, F.; Sannino, G.; Chiola, C.; Capparé, P.; Gastaldi, G.; Gherlone, E.F. Influence of intra-oral scanner (IOS) on the marginal accuracy of CAD/CAM single crowns. Int. J. Environ. Res. Public Health 2019, 16, 544. [Google Scholar] [CrossRef] [PubMed]
- Ciancaglini, R.; Gherlone, E.F.; Radaelli, G. Association between loss of occlusal support and symptoms of functional disturbances of the masticatory system. J. Oral Rehabil. 1999, 26, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Tetè, G.; D’Orto, B.; Nagni, M.; Agostinacchio, M.; Polizzi, E.; Agliardi, E. Role of induced pluripotent stem cells (IPSCS) in bone tissue regeneration in dentistry: A narrative review. J. Biol. Regul. Homeost. Agents 2020, 34 (Suppl. S3), 1–10. [Google Scholar]
| Material (CODE) Manufacturer | Composition * | Lot NO. |
|---|---|---|
| Vertise flow (VF) Kerr, Orange, CA | Matrix: GPDM, HEMA. Fillers: prepolymerized filler, nanosized ytterbium fluoride, 1 mm barium glass filler, 10–40 nm nanosized colloidal silica. Uncured methacrylate ester monomers. (70% by weight) | 7063158 |
| Beautifil II (BF) Shofu Inc., Koyoto, Japan | Bis-GMA, TEGDMA, S_PRG filler based on fluoroboroaluminosilicate glass, polymerization initiator, pigments, and others. Filler wight 83.3% Filler volume 68.6% | 051951 |
| ACTIVA BioACTIVE Restorative (AB) Pulpdent Corporation, USA | No bisphenol A, No bis-GMA, No BPA derivatives Filler by wight 56% | 190513 |
| Tetric N-Bond Universal (TN) Ivoclar/Vivadent | Bis-acrylamide derivative, Bis-GMA, amino acid acrylamide, hydroxyl alkyl methacrylamide diphenyl phosphine oxide, nano-fillers (SiO2), initiators, water, stabilizers | W91986 |
| Parameter | Specification |
|---|---|
| Wavelength | 1330 ± 30 nm |
| Scan rate | 30 ± 0.1 kHz |
| Axial resolution | ≤12 μm (in air) |
| Lateral resolution (based on spot size) | 30 ± 7 μm (in air) |
| System sensitivity | >95 dB |
| Lateral scan area | ≥5 × 5 mm |
| Imaging depth | 3 mm |
| Maximum output power | ≥1 mW (near-infrared class 1 laser) |
| Groups | Gap (%) | St. Err | |
|---|---|---|---|
| Mean | SD | ||
| VF | 86.65% a | 18.67 | 0.04 |
| AB | 58.18% c | 24.62 | 0.05 |
| BF | 84.41% b | 17.06 | 0.03 |
| p-value | <0.001 * | - | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdel-Maksoud, H.B.; Bahanan, A.W.; Alkhattabi, L.J.; Bakhsh, T.A. Evaluation of Newly Introduced Bioactive Materials in Terms of Cavity Floor Adaptation: OCT Study. Materials 2021, 14, 7668. https://doi.org/10.3390/ma14247668
Abdel-Maksoud HB, Bahanan AW, Alkhattabi LJ, Bakhsh TA. Evaluation of Newly Introduced Bioactive Materials in Terms of Cavity Floor Adaptation: OCT Study. Materials. 2021; 14(24):7668. https://doi.org/10.3390/ma14247668
Chicago/Turabian StyleAbdel-Maksoud, Heba B., Aziza W. Bahanan, Lujain J. Alkhattabi, and Turki A. Bakhsh. 2021. "Evaluation of Newly Introduced Bioactive Materials in Terms of Cavity Floor Adaptation: OCT Study" Materials 14, no. 24: 7668. https://doi.org/10.3390/ma14247668
APA StyleAbdel-Maksoud, H. B., Bahanan, A. W., Alkhattabi, L. J., & Bakhsh, T. A. (2021). Evaluation of Newly Introduced Bioactive Materials in Terms of Cavity Floor Adaptation: OCT Study. Materials, 14(24), 7668. https://doi.org/10.3390/ma14247668







