Heavy Metal Adsorption Using Magnetic Nanoparticles for Water Purification: A Critical Review
Abstract
1. Introduction
2. Adsorption Method
2.1. Synthesis and Characterization of Magnetic Nanoparticles
2.2. Adsorption Evaluation Strategies
3. Main Findings during the Last Decade
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Olvera, R.C.; Silva, S.L.; Robles-Belmont, E.; Lau, E.Z. Review of Nanotechnology Value Chain for Water Treatment Applications in Mexico. Resour. Technol. 2017, 3, 1–11. [Google Scholar] [CrossRef]
- Jiang, L.; Jiapaer, G.; Bao, A.; Yuan, Y.; Zheng, G.; Guo, H.; Yu, T.; De Maeyer, P. The Effects of Water Stress on Croplands in the Aral Sea Basin. J. Clean. Prod. 2020, 254, 120114. [Google Scholar] [CrossRef]
- Lee, U.; Xu, H.; Daystar, J.; Elgowainy, A.; Wang, M. AWARE-US: Quantifying Water Stress Impacts of Energy Systems in the United States. Sci. Total Environ. 2019, 648, 1313–1322. [Google Scholar] [CrossRef] [PubMed]
- Ward, F.A.; Mayer, A.S.; Garnica, L.A.; Townsend, N.T.; Gutzler, D.S. The Economics of Aquifer Protection Plans under Climate Water Stress: New Insights from Hydroeconomic Modeling. J. Hydrol. 2019, 576, 667–684. [Google Scholar] [CrossRef]
- Dinar, A.; Tieu, A.; Huynh, H. Water Scarcity Impacts on Global Food Production. Glob. Food Sec. 2019, 23, 212–226. [Google Scholar] [CrossRef]
- Zhao, H.; Qu, S.; Guo, S.; Zhao, H.; Liang, S.; Xu, M. Virtual Water Scarcity Risk to Global Trade under Climate Change. J. Clean. Prod. 2019, 230, 1013–1026. [Google Scholar] [CrossRef]
- Jia, X.; Klemeš, J.J.; Alwi, S.R.W.; Varbanov, P.S. Regional Water Resources Assessment Using Water Scarcity Pinch Analysis. Resour. Conserv. Recycl. 2020, 157, 104749. [Google Scholar] [CrossRef]
- Moursi, H.; Kim, D.; Kaluarachchi, J.J. A Probabilistic Assessment of Agricultural Water Scarcity in a Semi-Arid and Snowmelt-Dominated River Basin under Climate Change. Agric. Water Manag. 2017, 193, 142–152. [Google Scholar] [CrossRef]
- Defining Water Scarcity, Water Stress, and Water Risk. Available online: https://pacinst.org/water-definitions/ (accessed on 14 January 2019).
- McNabb, D. The Population Growth Barrier. In Global Pathways to Water Sustainability, 1st ed.; Springer International Publishing: Berlin/Heidelberg, Germany, 2019; pp. 67–81. [Google Scholar] [CrossRef]
- McNabb, D. Alternative Sources of Water Supply. In Global Pathways to Water Sustainability, 1st ed.; Springer International Publishing: Berlin/Heidelberg, Germany, 2019; pp. 251–262. [Google Scholar] [CrossRef]
- Omer, A.; Elagib, N.A.; Zhuguo, M.; Saleem, F.; Mohammed, A. Water Scarcity in the Yellow River Basin under Future Climate Change and Human Activities. Sci. Total Environ. 2020, 749, 141446. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Sanghi, R.; Mudhoo, A. Green Practices to Save Our Precious “Water Resource.” In Advances in Water Treatment and Pollution Prevention; Sharma, S., Ed.; Springer Netherlands: Dordrecht, The Netherlands, 2012; pp. 1–36. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Q.; Yang, H. Assessing Water Scarcity by Simultaneously Considering Environmental Flow Requirements, Water Quantity, and Water Quality. Ecol. Indic. 2016, 60, 434–441. [Google Scholar] [CrossRef]
- Boholm, Å.; Prutzer, M. Experts’ Understandings of Drinking Water Risk Management in a Climate Change Scenario. Clim. Risk Manag. 2017, 16, 133–144. [Google Scholar] [CrossRef]
- Joseph, L.; Jun, B.-M.; Flora, J.R.V.; Park, C.M.; Yoon, Y. Removal of Heavy Metals from Water Sources in the Developing World Using Low-Cost Materials: A Review. Chemosphere 2019, 229, 142–159. [Google Scholar] [CrossRef]
- Hao, X.; Chen, G.; Yuan, Z. Water in China. Water Res. 2020, 169, 115256. [Google Scholar] [CrossRef] [PubMed]
- Mo, W.; Zhang, Q. Energy-Nutrients-Water Nexus: Integrated Resource Recovery in Municipal Wastewater Treatment Plants. J. Environ. Manag. 2013, 127, 255–267. [Google Scholar] [CrossRef]
- Xia, J.; Duan, Q.-Y.; Luo, Y.; Xie, Z.-H.; Liu, Z.-Y.; Mo, X.-G. Climate Change and Water Resources: Case Study of Eastern Monsoon Region of China. Adv. Clim. Chang. Res. 2017, 8, 63–67. [Google Scholar] [CrossRef]
- Peirce, J.J.; Weiner, R.F.; Vesilind, P.A.; Peirce, J.J.; Weiner, R.F.; Vesilind, P.A. Water Pollution. Environ. Pollut. Control. 1998, 31–55. [Google Scholar] [CrossRef]
- Wasewar, K.L.; Singh, S.; Kansal, S.K. Process intensification of treatment of inorganic water pollutants. In Inorganic Pollutants in Water; Devi, P., Singh, P., Kansal, S.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 245–271. [Google Scholar] [CrossRef]
- Orubu, C.O.; Omotor, D.G. Environmental Quality and Economic Growth: Searching for Environmental Kuznets Curves for Air and Water Pollutants in Africa. Energy Policy 2011, 39, 4178–4188. [Google Scholar] [CrossRef]
- Liu, H.; Feng, S.; Du, X.; Zhang, N.; Liu, Y. Comparison of Three Sorbents for Organic Pollutant Removal in Drinking Water. Energy Procedia 2011, 5, 985–990. [Google Scholar] [CrossRef][Green Version]
- Ahmed, M.J.; Hameed, B.H.; Hummadi, E.H. Review on Recent Progress in Chitosan/Chitin-Carbonaceous Material Composites for the Adsorption of Water Pollutants. Carbohydr. Polym. 2020, 247, 116690. [Google Scholar] [CrossRef]
- Kim, K.H.; Keller, A.A.; Yang, J.K. Removal of Heavy Metals from Aqueous Solution Using a Novel Composite of Recycled Materials. Colloids Surfaces A Physicochem. Eng. Asp. 2013, 425, 6–14. [Google Scholar] [CrossRef]
- Hu, G.; Bakhtavar, E.; Hewage, K.; Mohseni, M.; Sadiq, R. Heavy Metals Risk Assessment in Drinking Water: An Integrated Probabilistic-Fuzzy Approach. J. Environ. Manag. 2019, 250, 109514. [Google Scholar] [CrossRef]
- Khan, M.U.; Muhammad, S.; Malik, R.N.; Khan, S.A.; Tariq, M. Heavy Metals Potential Health Risk Assessment through Consumption of Wastewater Irrigated Wild Plants: A Case Study. Hum. Ecol. Risk Assess. 2016, 22, 141–152. [Google Scholar] [CrossRef]
- Fu, F.; Wang, Q. Removal of Heavy Metal Ions from Wastewaters: A Review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef]
- Sarkar, A.; Paul, B. The Global Menace of Arsenic and Its Conventional Remediation-A Critical Review. Chemosphere 2016, 158, 37–49. [Google Scholar] [CrossRef]
- Barakat, M.A. New Trends in Removing Heavy Metals from Industrial Wastewater. Arab. J. Chem. 2011, 4, 361–377. [Google Scholar] [CrossRef]
- Babel, S.; Kurniawan, T.A. Low-Cost Adsorbents for Heavy Metals Uptake from Contaminated Water: A Review. J. Hazard. Mater. 2003, 97, 219–243. [Google Scholar] [CrossRef]
- Stohs, S.J.; Bagchi, D. Oxidative Mechanisms in the Toxicity of Metal Ions. Free. Radic. Biol. Med. 1995, 18, 321–336. [Google Scholar] [CrossRef]
- Parmar, M.; Thakur, L.S. Heavy Metal Cu, Ni and Zn: Toxicity, Health Hazards and Their Removal. Int. J. Plant Sci. 2013, 3, 143–157. [Google Scholar]
- Vardhan, K.H.; Kumar, P.S.; Panda, R.C. A Review on Heavy Metal Pollution, Toxicity and Remedial Measures: Current Trends and Future Perspectives. J. Mol. Liq. 2019, 290, 111197. [Google Scholar] [CrossRef]
- Kim, J.-J.; Kim, Y.-S.; Kumar, V. Heavy Metal Toxicity: An Update of Chelating Therapeutic Strategies. J. Trace Elem. Med. Biol. 2019, 54, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, M.; Jafari, O.; Mohammdifar, A. Intensification of Liquid-Liquid Mass Transfer in Micromixer Assisted by Ultrasound Irradiation and Fe3O4 Nanoparticles. Chem. Eng. Process. Process Intensif. 2017, 111, 79–88. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, K.; Zhao, Q.; Huang, M.; Ouyang, X. Comparative Adsorption of Heavy Metal Ions in Wastewater on Monolayer Molybdenum Disulfide. Green Energy Env. 2020, 6, 751–758. [Google Scholar] [CrossRef]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Molecular, Clinical and Environmental Toxicicology Volume 3: Environmental Toxicology. Mol. Clin. Environ. Toxicol. 2012, 101, 133–164. [Google Scholar] [CrossRef]
- Qiao, Y.; Yang, Y.; Gu, J.; Zhao, J. Distribution and Geochemical Speciation of Heavy Metals in Sediments from Coastal Area Suffered Rapid Urbanization, a Case Study of Shantou Bay, China. Mar. Pollut. Bull. 2013, 68, 140–146. [Google Scholar] [CrossRef]
- Singh, R.; Singh, S.; Parihar, P.; Singh, V.P.; Prasad, S.M. Arsenic Contamination, Consequences and Remediation Techniques: A Review. Ecotoxicol. Environ. Saf. 2015, 112, 247–270. [Google Scholar] [CrossRef] [PubMed]
- Gautam, R.K.; Sharma, S.K.; Mahiya, S.; Chattopadhyaya, M.C. Contamination of Heavy Metals in Aquatic Media: Transport, Toxicity and Technologies for Remediation. In Heavy Metals In Water: Presence, Removal and Safety Heavy; Sharma, S.K., Ed.; Royal Society of Chemistry: London, UK, 2014; pp. 1–24. [Google Scholar] [CrossRef]
- Valko, M.; Morris, H.; Cronin, T.D.M. Metals, Toxicity and Oxidative Stress Metals. Curr. Med. Chem. 2005, 12, 1161–1208. [Google Scholar] [CrossRef]
- Schroeder, H.; Duester, L.; Fabricius, A.L.; Ecker, D.; Breitung, V.; Ternes, T. ASediment Water (Interface) Mobility of Metal(Loid)s and Nutrients under Undisturbed Conditions and during Resuspension. J. Hazard. Mater. 2020, 394, 122543. [Google Scholar] [CrossRef] [PubMed]
- Bhateria, R.; Singh, R. A Review on Nanotechnological Application of Magnetic Iron Oxides for Heavy Metal Removal. J. Water Process Eng. 2019, 31, 100845. [Google Scholar] [CrossRef]
- Sonone, S.S.; Jadhav, S.V.; Sankhla, M.S.; Kumar, R. Water Contamination by Heavy Metals and Their Toxic Effect on Aquaculture and Human Health through Food Chain. Lett. Appl. NanoBioScience. 2020, 10, 2148–2166. [Google Scholar] [CrossRef]
- Bradl, H.B. Sources and Origins of Heavy Metals. In Interface Science and Technology; Bradl, H.B., Ed.; Elsevier: Amsterdam, The Netherlands, 2005; pp. 1–27. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Bomis, G.; Kosheleva, R.I.; Efthimiadou, E.K.; Favvas, E.P.; Kostoglou, M.; Mitropoulos, A.C. Nanobubbles Effect on Heavy Metal Ions Adsorption by Activated Carbon. Chem. Eng. J. 2019, 356, 91–97. [Google Scholar] [CrossRef]
- Liosis, C.; Karvelas, E.G.; Karakasidis, T.; Sarris, I.E. Numerical Study of Magnetic Particles Mixing in Waste Water under an External Magnetic Field. J. Water Supply Res. Technol. 2020, 69, 266–275. [Google Scholar] [CrossRef]
- Parvin, F.; Rikta, S.Y.; Tareq, S.M. Application of Nanomaterials for the Removal of Heavy Metal From Wastewater. In Nanotechnology in Water and Wastewater Treatment Theory and Applications Micro and Nano Technologies; Ahsan, A., Ismail, A.F., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 137–157. [Google Scholar] [CrossRef]
- Karvelas, E.; Liosis, C.; Karakasidis, T.; Sarris, I. Mixing of Particles in Micromixers under Different Angles and Velocities of the Incoming Water. Proceedings 2018, 2, 577. [Google Scholar] [CrossRef]
- Karvelas, E.; Liosis, C.; Benos, L.; Karakasidis, T.; Sarris, I. Micromixing Efficiency of Particles in Heavy Metal Removal Processes under Various Inlet Conditions. Water 2019, 11, 1135. [Google Scholar] [CrossRef]
- Wu, Y.; Pang, H.; Liu, Y.; Wang, X.; Yu, S.; Fu, D.; Chen, J.; Wang, X. Environmental Remediation of Heavy Metal Ions by Novel-Nanomaterials: A Review. Environ. Pollut. 2019, 246, 608–620. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Li, C.; Wang, J.; Zhang, H.; Zhang, J.; Shen, Y.; Li, C.; Wang, C.; Xie, A. A Simple Method to Synthesize Modified Fe3O4 for the Removal of Organic Pollutants on Water Surface. Appl. Surf. Sci. 2012, 258, 6326–6330. [Google Scholar] [CrossRef]
- Turan, N.B.; Erkan, H.S.; Engin, G.O.; Bilgili, M.S. Nanoparticles in the Aquatic Environment: Usage, Properties, Transformation and Toxicity—A Review. Process. Saf. Environ. Prot. 2019, 130, 238–249. [Google Scholar] [CrossRef]
- Nowack, B.; Bucheli, T.D. Occurrence, Behavior and Effects of Nanoparticles in the Environment. Environ. Pollut. 2007, 150, 5–22. [Google Scholar] [CrossRef]
- Gatoo, M.A.; Naseem, S.; Arfat, M.Y.; Mahmood, D.A.; Qasim, K.; Zubair, S. Physicochemical Properties of Nanomaterials: Implication in Associated Toxic Manifestations. Biomed Res. Int. 2014, 2014. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, L.; Chan, H.K.; Watanabe, W. Formation, Characterization, and Fate of Inhaled Drug Nanoparticles. Adv. Drug Deliv. Rev. 2011, 63, 441–455. [Google Scholar] [CrossRef] [PubMed]
- Wadhawan, S.; Jain, A.; Nayyar, J.; Mehta, S.K. Role of Nanomaterials as Adsorbents in Heavy Metal Ion Removal from Waste Water: A Review. J. Water Process Eng. 2020, 33, 101038. [Google Scholar] [CrossRef]
- Karvelas, E.; Liosis, C.; Karakasidis, T.; Sarris, I. Micromixing Nanoparticles and Contaminated Water Under Different Velocities for Optimum Heavy Metal Ions Adsorption. Env. Sci. Proc. 2020, 2, 65. [Google Scholar] [CrossRef]
- Du, X.; He, J.; Zhu, J.; Sun, L.; An, S. Ag-Deposited Silica-Coated Fe3O4 Magnetic Nanoparticles Catalyzed Reduction of p-Nitrophenol. Appl. Surf. Sci. 2012, 258, 2717–2723. [Google Scholar] [CrossRef]
- Mohammed, L.; Gomaa, H.G.; Ragab, D.; Zhu, J. Magnetic Nanoparticles for Environmental and Biomedical Applications: A Review. Particuology. 2017, 30, 1–14. [Google Scholar] [CrossRef]
- Faraji, M.; Yamini, Y.; Saleh, A.; Rezaee, M.; Ghambarian, M.; Hassani, R. A Nanoparticle-Based Solid-Phase Extraction Procedure Followed by Flow Injection Inductively Coupled Plasma-Optical Emission Spectrometry to Determine Some Heavy Metal Ions in Water Samples. Anal. Chim. Acta 2010, 659, 172–177. [Google Scholar] [CrossRef]
- Al-Ghouti, M.A.; Da’ana, D.A. Guidelines for the Use and Interpretation of Adsorption Isotherm Models: A Review. J. Hazard. Mater. 2020, 393, 122383. [Google Scholar] [CrossRef] [PubMed]
- Kurniawan, T.A.; Chan, G.Y.S.; Lo, W.H.; Babel, S. Physico-Chemical Treatment Techniques for Wastewater Laden with Heavy Metals. Chem. Eng. J. 2006, 118, 83–98. [Google Scholar] [CrossRef]
- Wang, J.; Zhuang, S. Removal of Various Pollutants from Water and Wastewater by Modified Chitosan Adsorbents. Crit. Rev. Environ. Sci. Technol. 2017, 47, 2331–2386. [Google Scholar] [CrossRef]
- Qu, X.; Alvarez, P.J.J.; Li, Q. Applications of Nanotechnology in Water and Wastewater Treatment. Water Res. 2013, 47, 3931–3946. [Google Scholar] [CrossRef]
- Hu, H.; Xu, K. Physicochemical Technologies for HRPs and Risk Control. In High-Risk Pollutants in Wastewater; Hongqiang, R., Zhang, X., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 169–207. [Google Scholar] [CrossRef]
- Zhang, K.; Yong, F.; McCarthy, D.T.; Deletic, A. Predicting Long Term Removal of Heavy Metals from Porous Pavements for Stormwater Treatment. Water Res. 2018, 142, 236–245. [Google Scholar] [CrossRef]
- Laurent, S.; Forge, D.; Port, M.; Roch, A.; Robic, C.; Vander Elst, L.; Muller, R.N. Magnetic Iron Oxide Nanoparticles: Synthesis, Stabilization, Vectorization, Physicochemical Characterizations and Biological Applications. Chem. Rev. 2008, 108, 2064–2110. [Google Scholar] [CrossRef]
- Zia, M.; Phull, A.R.; Ali, J.S. Challenges of Iron Oxide Nanoparticles. Powder Technol. 2016, 7, 49–67. [Google Scholar]
- Drmota, A.; Drofenik, M.; Koselj, J.; Žnidarši, A. Microemulsion Method for Synthesis of Magnetic Oxide Nanoparticles. In Microemulsions-An Introduction to Properties and Applications; Najjar, R., Ed.; InTech: Rijeka, Croatia, 2012; pp. 192–214. [Google Scholar]
- Ling, X.; Wei, L.; Hong, G.; Qiong, M. Synthesis and Characterization of Magnetic and Luminescent Fe3O4/CdTe Nanocomposites Using Aspartic Acid as Linker. Chinese Chem. Lett. 2011, 22, 233–236. [Google Scholar] [CrossRef]
- Takai, Z.; Mustafa, M.K.; Asman, S. Preparation and Characterization of Magnetite (Fe3O4) Nanoparticles By Sol- Gel Method Preparation and Characterization of Magnetite (Fe3O4) Nanoparticles By Sol-Gel Method. Asian J. Chem. 2018, 30, 2625–2630. [Google Scholar] [CrossRef]
- Wang, Y.; Nkurikiyimfura, I.; Pan, Z. Sonochemical Synthesis of Magnetic Nanoparticles. Chem. Eng. Commun. 2014, 202, 616–621. [Google Scholar] [CrossRef]
- Ge, S.; Shi, X.; Sun, K.; Li, C.; Uher, C.; Baker, J.R.; Holl, M.M.B.; Orr, B.G. Facile Hydrothermal Synthesis of Iron Oxide Nanoparticles with Tunable Magnetic Properties. J. Phys. Chem. 2009, 113, 13593–13599. [Google Scholar] [CrossRef]
- Oćwieja, M.; Węgrzynowicz, A.; Maciejewska-Prończuk, J.; Michorczyk, P.; Adamczyk, Z.; Roman, M.; Bielańska, E. Preparation of Iron Oxide Nanoparticles Doped by Chromium for Application in Water–Gas Shift Reaction. Colloids Surf. A Physicochem. Eng. Asp. 2017, 523, 71–80. [Google Scholar] [CrossRef]
- Compeán-Jasso, M.E.; Ruiz, F.; Martínez, J.R.; Herrera-Gómez, A. Magnetic Properties of Magnetite Nanoparticles Synthesized by Forced Hydrolysis. Mater. Lett. 2008, 62, 4248–4250. [Google Scholar] [CrossRef]
- Stefanescu, M.; Stefanescu, O.; Stoia, M.; Lazau, C. Thermal Decomposition of Some Metal-Organic Precursors. J. Anal Calorim. 2007, 88, 27–32. [Google Scholar] [CrossRef]
- Salazar-Alvarez, G.; Muhammed, M.; Zagorodni, A.A. Novel Flow Injection Synthesis of Iron Oxide Nanoparticles with Narrow Size Distribution. Chem. Eng. Sci. 2006, 61, 4625–4633. [Google Scholar] [CrossRef]
- El-amin, M.F.; Saad, A.M.; Salama, A.; Sun, S. Modeling and Analysis of Magnetic Nanoparticles Injection in Water-Oil Two-Phase Flow in Porous Media under Magnetic Field Effect. Geofluids 2017, 2017, 12. [Google Scholar] [CrossRef]
- Agostini, P.; Meffre, A.; Damien, L.L.; Benjamin, R. Electrospray Deposition of Isolated Chemically Synthesized Magnetic Nanoparticles. J. Nanopart Res. 2016, 18, 11. [Google Scholar] [CrossRef]
- Atabaev, T.S.; Kim, H.K.; Hwang, Y.H. Fabrication of Bifunctional Core-Shell Fe3O4 Particles Coated with Ultrathin Phosphor Layer. Nanoscale Res. Lett. 2013, 8, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Chaverra, M.J.; Restrepo-Parra, E.; Acosta-Medina, C.D.; Mello, A.; Ospina, R. Synthesis of Oxide Iron Nanoparticles Using Laser Ablation for Possible Hyperthermia Applications. Nanomaterials 2020, 10, 2099. [Google Scholar] [CrossRef]
- Park, J.Y.; Lee, Y.J.; Khanna, P.K.; Jun, K.W.; Bae, J.W.; Kim, Y.H. Alumina-Supported Iron Oxide Nanoparticles as Fischer-Tropsch Catalysts: Effect of Particle Size of Iron Oxide. J. Mol. Catal. A Chem. 2010, 323, 84–90. [Google Scholar] [CrossRef]
- Bokuniaeva, A.O.; Vorokh, A.S. Estimation of Particle Size Using the Debye Equation and the Scherrer Formula for Polyphasic TiO2 Powder. J. Phys. Conf. Ser. 2019, 1410, 12057. [Google Scholar] [CrossRef]
- Mahdavi, M.; Ahmad, M.B.; Haron, M.J.; Namvar, F.; Nadi, B.; Ab Rahman, M.Z.; Amin, J. Synthesis, Surface Modification and Characterisation of Biocompatible Magnetic Iron Oxide Nanoparticles for Biomedical Applications. Molecules 2013, 18, 7533–7548. [Google Scholar] [CrossRef]
- Alexander, L.; Klug, H.P. Determination of Crystallite Size with the X-Ray Spectrometer. J. Appl. Phys. 1950, 21, 137–142. [Google Scholar] [CrossRef]
- Holzwarth, U.; Gibson, N. The Scherrer Equation versus the “Debye-Scherrer Equation”. Nat. Nanotechnol. 2011, 6, 534. [Google Scholar] [CrossRef]
- Zhu, N.; Ji, H.; Yu, P.; Niu, J.; Farooq, M.U.; Akram, M.W.; Udego, I.O.; Li, H.; Niu, X. Surface Modification of Magnetic Iron Oxide Nanoparticles. Nanomaterials 2018, 8, 810. [Google Scholar] [CrossRef] [PubMed]
- Abu-dief, A.M.; Abdel-fatah, S.M. Development and Functionalization of Magnetic Nanoparticles as Powerful and Green Catalysts for Organic Synthesis. Beni-Suef Univ. J. Basic Appl. Sci. 2018, 7, 55–67. [Google Scholar] [CrossRef]
- Jadhav, S.A.; Brunella, V.; Scalarone, D. Polymerizable Ligands as Stabilizers for Nanoparticles. Part. Part. Syst. Charact. 2014, 32, 417–428. [Google Scholar] [CrossRef]
- Cîrcu, M.; Nan, A.; Borodi, G.; Liebscher, J.; Turcu, R. Refinement of Magnetite Nanoparticles by Coating with Organic Stabilizers. Nanomaterials 2016, 6, 228. [Google Scholar] [CrossRef]
- Sanchez, L.M.; Martin, D.A.; Alvarez, V.A.; Gonzalez, J.S. Polyacrylic Acid-Coated Iron Oxide Magnetic Nanoparticles: The Polymer Molecular Weight in Fl Uence. Colloids Surf. A 2018, 543, 28–37. [Google Scholar] [CrossRef]
- Gholami, A.; Moghadassi, A.R.; Hosseini, S.M.; Shabani, S.; Gholami, F. Preparation and Characterization of Polyvinyl Chloride Based Nanocomposite Nanofiltration-Membrane Modified by Iron Oxide Nanoparticles for Lead Removal from Water. J. Ind. Eng. Chem. 2014, 20, 1517–1522. [Google Scholar] [CrossRef]
- de Dios, A.S.; Díaz-García, M.E. Multifunctional Nanoparticles: Analytical Prospects. Anal. Chim. Acta. 2010, 666, 1–22. [Google Scholar] [CrossRef]
- Karatapanis, A.E.; Petrakis, D.E.; Stalikas, C.D. A Layered Magnetic Iron/Iron Oxide Nanoscavenger for the Analytical Enrichment of ng-L−1 Concentration Levels of Heavy Metals from Water. Anal. Chim. Acta 2012, 726, 22–27. [Google Scholar] [CrossRef]
- Ji, Y. Ions Removal by Iron Nanoparticles: A Study on Solid-Water Interface with Zeta Potential. Colloids Surf. A Physicochem. Eng. Asp. 2014, 444, 1–8. [Google Scholar] [CrossRef]
- Kosmulski, M. PH-Dependent Surface Charging and Points of Zero Charge. IV. Update and New Approach. J. Colloid Interface Sci. 2009, 337, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Chavda, V.P. Nanobased Nano Drug Delivery: A Comprehensive Review. In Micro and Nano Technologies, Applications of Targeted Nano Drugs and Delivery Systems; Mohapatra, S., Ranjan, S., Dasgupta, N., Mishra, R.K., Thomas, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 69–92. [Google Scholar] [CrossRef]
- Crawford, R.J.; Harding, I.H.; Mainwaring, D.E. The Zeta Potential of Iron and Chromium Hydrous Oxides during Adsorption and Coprecipitation of Aqueous Heavy Metals. J. Colloid Interface Sci. 1996, 181, 561–570. [Google Scholar] [CrossRef]
- Jiuhui, Q. Research progress of novel adsorption processes in water purification: A review. J. Environ. Sci. 2008, 20, 1–13. [Google Scholar] [CrossRef]
- Petracic, O. Superparamagnetic Nanoparticle Ensembles. Superlattices Microstruct. 2010, 47, 569–578. [Google Scholar] [CrossRef]
- Wu, W.; He, Q.; Jiang, C. Magnetic Iron Oxide Nanoparticles: Synthesis and Surface Functionalization Strategies. Nanoscale Res. Lett. 2008, 3, 397–415. [Google Scholar] [CrossRef]
- Zhang, F.; Lan, J.; Zhao, Z.; Yang, Y.; Tan, R.; Song, W. Removal of Heavy Metal Ions from Aqueous Solution Using Fe3O4–SiO2-Poly(1,2-Diaminobenzene) Core–Shell Sub-Micron Particles. J. Colloid Interface Sci. 2012, 387, 205–212. [Google Scholar] [CrossRef]
- Fan, C.; Li, K.; Li, J.; Ying, D.; Wang, Y.; Jia, J. Comparative and Competitive Adsorption of Pb (II) and Cu (II) Using Tetraethylenepentamine Modified Chitosan/CoFe2O4 Particles. J. Hazard. Mater. 2017, 326, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.M.; Khodabakhshi, A. Removal of Cr (VI) From Simulated Electroplating. Environ. Eng. Manag. J. 2010, 9, 921–927. [Google Scholar]
- Zhao, Y.G.; Shen, H.Y.; Pan, S.D.; Hu, M.Q. Synthesis, Characterization and Properties of Ethylenediamine-Functionalized Fe3O4 Magnetic Polymers for Removal of Cr (VI) in Wastewater. J. Hazard. Mater. 2010, 182, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Kargin, D.B.; Konyukhov, Y.V.; Biseken, A.B.; Lileev, A.S.; Karpenkov, D.Y. Structure, Morphology and Magnetic Properties of Hematite and Maghemite Nanopowders Produced from Rolling Mill Scale. Steel Transl. 2020, 50, 151–158. [Google Scholar] [CrossRef]
- Liu, Y.; Jia, S.; Wu, Q.; Ran, J.; Zhang, W.; Wu, S. Studies of Fe3O4-Chitosan Nanoparticles Prepared by Co-Precipitation under the Magnetic Field for Lipase Immobilization. Catal. Commun. 2011, 12, 717–720. [Google Scholar] [CrossRef]
- Qiang, C.; Xu, J.; Zhang, Z.; Tian, L.; Xiao, S.; Liu, Y.; Xu, P. Magnetic Properties and Microwave Absorption Properties of Carbon Fibers Coated by Fe3O4 Nanoparticles. J. Alloy. Compd. 2010, 506, 93–97. [Google Scholar] [CrossRef]
- Aredes, S.; Klein, B.; Pawlik, M. The Removal of Arsenic from Water Using Natural Iron Oxide Minerals. J. Clean. Prod. 2012, 29–30, 208–213. [Google Scholar] [CrossRef]
- Rahim, P.S.; Abdul, R.A.A.; Wan, D.W.M.A. Review on the Application of Modified Iron Oxides as Heterogeneous Catalysts in Fenton Reactions. J. Clean. Prod. 2014, 64, 24–35. [Google Scholar] [CrossRef]
- Xue, X.Y.; Cheng, R.; Shi, L.; Ma, Z.; Zheng, X. Nanomaterials for Water Pollution Monitoring and Remediation. Env. Chem. Lett. 2017, 15, 23–27. [Google Scholar] [CrossRef]
- Tang, S.C.N.; Lo, I.M.C. Magnetic Nanoparticles: Essential Factors for Sustainable Environmental Applications. Water Res. 2013, 47, 2613–2632. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S.R.; Yanful, E.K. Arsenic and Chromium Removal by Mixed Magnetite-Maghemite Nanoparticles and the Effect of Phosphate on Removal. J. Env. Manag. 2010, 91, 2238–2247. [Google Scholar] [CrossRef] [PubMed]
- Teja, A.S.; Koh, P.-Y. Synthesis, Properties, and Applications of Magnetic Iron Oxide Nanoparticles. Prog. Cryst. Growth Charact. Mater. 2009, 55, 22–45. [Google Scholar] [CrossRef]
- Tadic, M.; Trpkov, D.; Kopanja, L.; Vojnovic, S.; Panjan, M. Hydrothermal Synthesis of Hematite (α-Fe2O3) Nanoparticle Forms: Synthesis Conditions, Structure, Particle Shape Analysis, Cytotoxicity and Magnetic Properties. J. Alloy. Compd. 2019, 792, 599–609. [Google Scholar] [CrossRef]
- Gurmen, S.; Ebin, B. Production and Characterization of the Nanostructured Hollow Iron Oxide Spheres and Nanoparticles by Aerosol Route. J. Alloy. Compd. 2010, 492, 585–589. [Google Scholar] [CrossRef]
- Tadic, M.; Kusigerski, V.; Markovic, D.; Milosevic, I.; Spasojevic, V. High Concentration of Hematite Nanoparticles in a Silica Matrix: Structural and Magnetic Properties. J. Magn. Magn. Mater. 2009, 321, 12–16. [Google Scholar] [CrossRef]
- Estelrich, J.; Escribano, E.; Queralt, J.; Busquets, M.A. Iron Oxide Nanoparticles for Magnetically-Guided and Magnetically-Responsive Drug Delivery. Int. J. Mol. Sci. 2015, 16, 8070–8101. [Google Scholar] [CrossRef]
- Ling, D.; Lee, N.; Hyeon, T. Chemical Synthesis and Assembly of Uniformly Sized Iron Oxide Nanoparticles for Medical Applications. Acc. Chem. Res. 2015, 48, 1276–1285. [Google Scholar] [CrossRef] [PubMed]
- Gholizadeh, A. A Comparative Study of Physical Properties in Fe3O4 Nanoparticles Prepared by Coprecipitation and Citrate Methods. J. Am. Ceram. Soc. 2017, 100, 3577–3588. [Google Scholar] [CrossRef]
- Roto, R.; Yusran, Y.; Kuncaka, A. Magnetic Adsorbent of Fe3O4@SiO2 Core-Shell Nanoparticles Modified with Thiol Group for Chloroauric Ion Adsorption. Appl. Surf. Sci. 2016, 377, 30–36. [Google Scholar] [CrossRef]
- Wu, S.; Sun, A.; Zhai, F.; Wang, J.; Xu, W.; Zhang, Q.; Volinsky, A.A. Fe3O4 Magnetic Nanoparticles Synthesis from Tailings by Ultrasonic Chemical Co-Precipitation. Mater. Lett. 2011, 65, 1882–1884. [Google Scholar] [CrossRef]
- Can, M.M.; Coşkun, M.; Firat, T. A Comparative Study of Nanosized Iron Oxide Particles; Magnetite (Fe3O4), Maghemite (γ-Fe2O3) and Hematite (α-Fe2O3), Using Ferromagnetic Resonance. J. Alloys Compd. 2012, 542, 241–247. [Google Scholar] [CrossRef]
- Liang, X.; Xi, B.; Xiong, S.; Zhu, Y.; Xue, F.; Qian, Y. Porous Soft Magnetic Material: The Maghemite Microsphere with Hierarchical Nanoarchitecture and Its Application in Water Purification. Mater. Res. Bull. 2009, 44, 2233–2239. [Google Scholar] [CrossRef]
- Mursalat, M.; Hastings, D.L.; Schoenitz, M.; Dreizin, E.L. Microspheres with Diverse Material Compositions Can Be Prepared by Mechanical Milling. Adv. Eng. Mater. 2020, 22, 1–4. [Google Scholar] [CrossRef]
- Arbain, R.; Othman, M.; Palaniandy, S. Preparation of Iron Oxide Nanoparticles by Mechanical Milling. Miner. Eng. 2011, 24, 1–9. [Google Scholar] [CrossRef]
- Nassar, N.N.; Hassan, A.; Carbognani, L.; Lopez-Linares, F.; Pereira-Almao, P. Iron Oxide Nanoparticles for Rapid Adsorption and Enhanced Catalytic Oxidation of Thermally Cracked Asphaltenes. Fuel 2012, 95, 257–262. [Google Scholar] [CrossRef]
- Mohseni-Bandpi, A.; Al-Musawi, T.J.; Ghahramani, E.; Zarrabi, M.; Mohebi, S.; Vahed, S.A. Improvement of Zeolite Adsorption Capacity for Cephalexin by Coating with Magnetic Fe3O4 Nanoparticles. J. Mol. Liq. 2016, 218, 615–624. [Google Scholar] [CrossRef]
- Wu, Y.W.; Zhang, J.; Liu, J.F.; Chen, L.; Deng, Z.L.; Han, M.X.; Wei, X.S.; Yu, A.M.; Zhang, H.L. Fe3O4 @ZrO2 Nanoparticles Magnetic Solid Phase Extraction Coupled with Flame Atomic Absorption Spectrometry for Chromium (III) Speciation in Environmental and Biological Samples. Appl. Surf. Sci. 2012, 258, 6772–6776. [Google Scholar] [CrossRef]
- Mandel, K.; Hutter, F. The Magnetic Nanoparticle Separation Problem. Nano Today 2012, 7, 485–487. [Google Scholar] [CrossRef]
- MacCuspie, R.I. Characterization of Nanomaterials for NanoEHS Studies. In Nanotechnology Environmental Health and Safety Risks, Regulation, and Management Micro and Nano Technologies, 3rd ed.; Hull, M.S., Bowman, D.M., Eds.; William Andrew Publishing: New York, NY, USA, 2018; pp. 59–82. [Google Scholar] [CrossRef]
- Mourdikoudis, S.; Pallares, R.M.; Thanh, N.T.K. Characterization Techniques for Nanoparticles: Comparison and Complementarity upon Studying Nanoparticle Properties. Nanoscale 2018, 10, 12871–12934. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, E.P.; Bazaka, K.; Crawford, R.J. Metallic Biomaterials: Types and Advanced Applications. In New Functional Biomaterials for Medicine and Healthcare; Ivanova, E.P., Bazaka, K., Crawford, R.J., Eds.; Woodhead Publishing Limited: Sawston, UK, 2014; pp. 121–147. [Google Scholar] [CrossRef]
- Fathy, M.M.; Fahmy, H.M.; Saad, O.A.; Elshemey, W.M. Silica-Coated Iron Oxide Nanoparticles as a Novel Nano-Radiosensitizer for Electron Therapy. Life Sci. 2019, 234, 116756. [Google Scholar] [CrossRef] [PubMed]
- Koutsikou, T.S.; Krokidis, M.G.; Boukos, N.; Mitrikas, G.; Efthimiadou, E. Journal of Drug Delivery Science and Technology Synthesis, Characterization and Evaluation of Multi Sensitive Nanocarriers by Using the Layer by Layer Method. J. Drug Deliv. Sci. Technol. 2019, 53, 101142. [Google Scholar] [CrossRef]
- Mitra, R.; Bhattacharya, S. Inhibition in Binding between Fullerene and a Bisporphyrin in Presence of Silver Nanoparticles in Solution: UV-Vis, DLS, SEM and TEM Studies. Spectrochim. Acta-Part A Mol. Biomol. Spectrosc. 2013, 114, 11–18. [Google Scholar] [CrossRef]
- de la Calle, I.; Menta, M.; Klein, M.; Séby, F. Screening of TiO2 and Au Nanoparticles in Cosmetics and Determination of Elemental Impurities by Multiple Techniques (DLS, SP-ICP-MS, ICP-MS and ICP-OES). Talanta 2017, 171, 291–306. [Google Scholar] [CrossRef]
- Yang, S.C.; Paik, S.Y.R.; Ryu, J.; Choi, K.O.; Kang, T.S.; Lee, J.K.; Song, C.W.; Ko, S. Dynamic Light Scattering-Based Method to Determine Primary Particle Size of Iron Oxide Nanoparticles in Simulated Gastrointestinal Fluid. Food Chem 2014, 161, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Zatsepin, A.F.; Kuznetsova, Y.A.; Sokolov, V.I. UV Absorption and Effects of Local Atomic Disordering in the Nickel Oxide Nanoparticles. J. Lumin. 2017, 183, 135–142. [Google Scholar] [CrossRef]
- Tan, M.I.S.M.H.; Omar, A.F.; Rashid, M.; Hashim, U. VIS-NIR Spectral and Particles Distribution of Au, Ag, Cu, Al and Ni Nanoparticles Synthesized in Distilled Water Using Laser Ablation. Results Phys. 2019, 14, 102497. [Google Scholar] [CrossRef]
- Kumar, A.; Dixit, C.K. Methods for Characterization of Nanoparticles. In Advances in Nanomedicine for the Delivery of Therapeutic Nucleic Acids; Nimesh, S., Chandra, R., Gupta, N., Eds.; Woodhead Publishing: Sawston, UK, 2017; pp. 43–48. [Google Scholar] [CrossRef]
- Rouchota, M.; Loudos, G.; Efthimiadou, E.; Kordas, G.C.; Kagadis, G.C. Assessment of Low Energy X-Ray Imaging for Magnetic and Gold Nanoparticles. Phys. Medica 2016, 32, 257. [Google Scholar] [CrossRef]
- Singh, A.K. Experimental Methodologies for the Characterization of Nanoparticles. In Engineered Nanoparticles; Hill-Parks, E., Ed.; Academic Press: San Diego, CA, USA, 2016; pp. 125–170. [Google Scholar] [CrossRef]
- Li, X.; Li, Z.; Pan, D.; Xiang, Z.; Yoshimura, S.; Saito, H. Magnetization Behaviors of Fe3O4 Nanoparticles Studied by Frequency-Modulated Magnetic Force Microscopy. Mater. Lett. 2014, 125, 36–39. [Google Scholar] [CrossRef]
- Qiu, H.; Lv, L.; Pan, B.C.; Zhang, Q.J.; Zhang, W.M.; Zhang, Q.X. Critical Review in Adsorption Kinetic Models. J. Zhejiang Univ. Sci. A 2009, 10, 716–724. [Google Scholar] [CrossRef]
- Ashrafi, A.; Rahbar-Kelishami, A.; Shayesteh, H. Highly Efficient Simultaneous Ultrasonic Assisted Adsorption of Pb (II) by Fe3O4@MnO2 Core-Shell Magnetic Nanoparticles: Synthesis and Characterization, Kinetic, Equilibrium, and Thermodynamic Studies. J. Mol. Struct. 2017, 1147, 40–47. [Google Scholar] [CrossRef]
- Limousin, G.; Gaudet, J.P.; Charlet, L.; Szenknect, S.; Barthès, V.; Krimissa, M. Sorption Isotherms: A Review on Physical Bases, Modeling and Measurement. Appl. Geochem. 2007, 22, 249–275. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Insights into the Modeling of Adsorption Isotherm Systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Xu, Q.; Wang, Y.; Jin, L.; Wang, Y.; Qin, M. Adsorption of Cu (II), Pb (II) and Cr (VI) from Aqueous Solutions Using Black Wattle Tannin-Immobilized Nanocellulose. J. Hazard. Mater. 2017, 339, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Sheet, I.; Kabbani, A.; Holail, H. Removal of Heavy Metals Using Nanostructured Graphite Oxide, Silica Nanoparticles and Silica/Graphite Oxide Composite. Energy Procedia 2014, 50, 130–138. [Google Scholar] [CrossRef]
- Wang, X.; Cai, J.; Zhang, Y.; Li, L.; Jiang, L.; Wang, C. Heavy Metal Sorption Properties of Magnesium Titanate Mesoporous Nanorods. J. Mater. Chem. A 2015, 3, 11796–11800. [Google Scholar] [CrossRef]
- Singh, A.K. Nanoparticle Ecotoxicology. In Engineered Nanoparticles; Hill-Parks, E., Ed.; Academic Press: San Diego, CA, USA, 2016; pp. 343–450. [Google Scholar] [CrossRef]
- Altin, O.; Özbelge, H.Ö.; Doǧu, T. Use of General Purpose Adsorption Isotherms for Heavy Metal-Clay Mineral Interactions. J. Colloid Interface Sci. 1998, 198, 130–140. [Google Scholar] [CrossRef]
- Zhang, Y.; Ni, S.; Wang, X.; Zhang, W.; Lagerquist, L.; Qin, M.; Willför, S.; Xu, C.; Fatehi, P. Ultrafast Adsorption of Heavy Metal Ions onto Functionalized Lignin-Based Hybrid Magnetic Nanoparticles. Chem. Eng. J. 2019, 372, 82–91. [Google Scholar] [CrossRef]
- Song, J.; Kong, H.; Jang, J. Adsorption of Heavy Metal Ions from Aqueous Solution by Polyrhodanine-Encapsulated Magnetic Nanoparticles. J. Colloid Interface Sci. 2011, 359, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Wang, J. A General Kinetic Model for Adsorption: Theoretical Analysis and Modeling. J. Mol. Liq. 2019, 288, 111100. [Google Scholar] [CrossRef]
- Dehghan, M.A.; Ghazanfari, M.H.; Jamialahmadi, M.; Helalizadeh, A. Adsorption of Silica Nanoparticles onto Calcite: Equilibrium, Kinetic, Thermodynamic and DLVO Analysis. Chem. Eng. J. 2015, 281, 334–344. [Google Scholar] [CrossRef]
- Singh, D.; Verma, S.; Gautam, R.K.; Krishna, V. Copper Adsorption onto Synthesized Nitrilotriacetic Acid Functionalized Fe3O4 Nanoparticles: Kinetic, Equilibrium and Thermodynamic Studies. J. Environ. Chem. Eng. 2016, 3, 2161–2171. [Google Scholar] [CrossRef]
- Shaker, M.A. Adsorption of Co (II), Ni (II) and Cu (II) Ions onto Chitosan-Modified Poly (Methacrylate) Nanoparticles: Dynamics, Equilibrium and Thermodynamics Studies. J. Taiwan Inst. Chem. Eng. 2015, 57, 111–122. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X. Adsorption Kinetic Models: Physical Meanings, Applications, and Solving Methods. J. Hazard. Mater. 2020, 390, 122156. [Google Scholar] [CrossRef]
- Tanhaei, B.; Ayati, A.; Lahtinen, M.; Sillanpää, M. Preparation and Characterization of a Novel Chitosan/Al2O3/Magnetite Nanoparticles Composite Adsorbent for Kinetic, Thermodynamic and Isotherm Studies of Methyl Orange Adsorption. Chem. Eng. J. 2015, 259, 1–10. [Google Scholar] [CrossRef]
- Atar, N.; Olgun, A. Removal of Basic and Acid Dyes from Aqueous Solutions by a Waste Containing Boron Impurity. Desalination 2009, 249, 109–115. [Google Scholar] [CrossRef]
- Mostafa, M.G.; Chen, Y.H.; Jean, J.S.; Liu, C.C.; Lee, Y.C. Kinetics and Mechanism of Arsenate Removal by Nanosized Iron Oxide-Coated Perlite. J. Hazard. Mater. 2011, 187, 89–95. [Google Scholar] [CrossRef]
- Shaikh, M.N.; Helal, A.; Kalanthoden, A.N.; Najjar, B.; Abdul Aziz, M.; Mohamed, H.D. Sub-Nanometric Rh Decorated Magnetic Nanoparticles as Reusable Catalysts for Nitroarene Reduction in Water. Catal. Commun. 2019, 119, 134–138. [Google Scholar] [CrossRef]
- Ain, Q.-U.; Farooq, M.U.; Jalees, M.I. Application of Magnetic Graphene Oxide for Water Purification: Heavy Metals Removal and Disinfection. J. Water Process Eng. 2020, 33, 101044. [Google Scholar] [CrossRef]
- Nassar, N.N. Rapid Removal and Recovery of Pb (II) from Wastewater by Magnetic Nanoadsorbents. J. Hazard. Mater. 2010, 184, 538–546. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Niu, H.; Cai, Y.; Zhao, X.; Shi, Y. Arsenite and Arsenate Adsorption on Coprecipitated Bimetal Oxide Magnetic Nanomaterials: MnFe2O4 and CoFe2O4. Chem. Eng. J. 2010, 158, 599–607. [Google Scholar] [CrossRef]
- Kalantari, K.; Ahmad, M.B.; Fard Masoumi, H.R.; Shameli, K.; Basri, M.; Khandanlou, R. Rapid and High Capacity Adsorption of Heavy Metals by Fe3O4/Montmorillonite Nanocomposite Using Response Surface Methodology: Preparation, Characterization, Optimization, Equilibrium Isotherms, and Adsorption Kinetics Study. J. Taiwan Inst. Chem. Eng. 2015, 49, 192–198. [Google Scholar] [CrossRef]
- Hao, Y.M.; Man, C.; Hu, Z.B. Effective Removal of Cu (II) Ions from Aqueous Solution by Amino-Functionalized Magnetic Nanoparticles. J. Hazard. Mater. 2010, 184, 392–399. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, S.; Shao, Y.; Liu, J.; Xu, Z.; Zhu, D. Amino-Functionalized Fe3O4@SiO2 Core–Shell Magnetic Nanomaterial as a Novel Adsorbent for Aqueous Heavy Metals Removal. J. Colloid Interface Sci. 2010, 349, 293–299. [Google Scholar] [CrossRef]
- Ozmen, M.; Can, K.; Arslan, G.; Tor, A.; Cengeloglu, Y.; Ersoz, M. Adsorption of Cu (II) from Aqueous Solution by Using Modified Fe3O4 Magnetic Nanoparticles. Desalination 2010, 254, 162–169. [Google Scholar] [CrossRef]
- Tran, H.V.; Tran, L.D.; Nguyen, T.N. Preparation of Chitosan/Magnetite Composite Beads and Their Application for Removal of Pb(II) and Ni(II) from Aqueous Solution. Mater. Sci. Eng. C 2010, 30, 304–310. [Google Scholar] [CrossRef]
- Hu, H.; Wang, Z.; Pan, L. Synthesis of Monodisperse Fe3O4@silica Core–Shell Microspheres and Their Application for Removal of Heavy Metal Ions from Water. J. Alloys Compd. 2010, 492, 656–661. [Google Scholar] [CrossRef]
- Sharma, Y.C.; Srivastava, V. Separation of Ni (II) Ions from Aqueous Solutions by Magnetic Nanoparticles. J. Chem. Eng. Data 2010, 55, 1441–1442. [Google Scholar] [CrossRef]
- Zhao, Y.G.; Shen, H.Y.; Pan, S.D.; Hu, M.Q.; Xia, Q.H. Preparation and Characterization of Amino-Functionalized Nano-Fe3O4 Magnetic Polymer Adsorbents for Removal of Chromium (VI) Ions. J. Mater. Sci. 2010, 45, 5291–5301. [Google Scholar] [CrossRef]
- Chowdhury, S.R.; Yanful, E.K.; Pratt, A.R. Arsenic Removal from Aqueous Solutions by Mixed Magnetite-Maghemite Nanoparticles. Environ. Earth Sci. 2011, 64, 411–423. [Google Scholar] [CrossRef]
- Nguyen Thanh, D.; Singh, M.; Ulbrich, P.; Strnadova, N.; Štěpánek, F. Perlite Incorporating γ-Fe2O3 and α-MnO2 Nanomaterials: Preparation and Evaluation of a New Adsorbent for As (V) Removal. Sep. Purif. Technol. 2011, 82, 93–101. [Google Scholar] [CrossRef]
- Hou, X.; Feng, J.; Liu, X.; Ren, Y.; Fan, Z.; Wei, T.; Meng, J.; Zhang, M. Synthesis of 3D Porous Ferromagnetic NiFe2O4 and Using as Novel Adsorbent to Treat Wastewater. J. Colloid Interface Sci. 2011, 362, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Zhang, Y.; Zhang, Z.; Shen, Y.; Zhao, M.; Pan, G. Study on the Adsorption of Ca2+, Cd2+ and Pb2+ by Magnetic Fe3O4 Yeast Treated with EDTA Dianhydride. Chem. Eng. J. 2011, 168, 737–745. [Google Scholar] [CrossRef]
- Luo, X.; Luo, S.; Zhan, Y.; Shu, H.; Huang, Y.; Tu, X. Novel Cu (II) Magnetic Ion Imprinted Materials Prepared by Surface Imprinted Technique Combined with a Sol–Gel Process. J. Hazard. Mater. 2011, 192, 949–955. [Google Scholar] [CrossRef] [PubMed]
- Panneerselvam, P.; Morad, N.; Tan, K.A. Magnetic Nanoparticle (Fe3O4) Impregnated onto Tea Waste for the Removal of Nickel (II) from Aqueous Solution. J. Hazard. Mater. 2011, 186, 160–168. [Google Scholar] [CrossRef]
- Pang, Y.; Zeng, G.; Tang, L.; Zhang, Y.; Liu, Y.; Lei, X.; Li, Z.; Zhang, J.; Liu, Z.; Xiong, Y. Preparation and Application of Stability Enhanced Magnetic Nanoparticles for Rapid Removal of Cr (VI). Chem. Eng. J. 2011, 175, 222–227. [Google Scholar] [CrossRef]
- Bhaumik, M.; Maity, A.; Srinivasu, V.V.; Onyango, M.S. Enhanced Removal of Cr (VI) from Aqueous Solution Using Polypyrrole/Fe3O4 Magnetic Nanocomposite. J. Hazard. Mater. 2011, 190, 381–390. [Google Scholar] [CrossRef]
- Phuengprasop, T.; Sittiwong, J.; Unob, F. Removal of Heavy Metal Ions by Iron Oxide Coated Sewage Sludge. J. Hazard. Mater. 2011, 186, 502–507. [Google Scholar] [CrossRef]
- Li, G.; Zhao, Z.; Liu, J.; Jiang, G. Effective Heavy Metal Removal from Aqueous Systems by Thiol Functionalized Magnetic Mesoporous Silica. J. Hazard. Mater. 2011, 192, 277–283. [Google Scholar] [CrossRef]
- Sun, L.; Li, Y.; Sun, M.; Wang, H.; Xu, S.; Zhang, C.; Yang, Q. Porphyrin-Functionalized Fe3O4@SiO2 Core/Shell Magnetic Colorimetric Material for Detection, Adsorption and Removal of Hg2+ in Aqueous Solution. New J. Chem. 2011, 35, 2697–2704. [Google Scholar] [CrossRef]
- Hu, J.; Yang, S.; Wang, X. Adsorption of Cu (II) on β-Cyclodextrin Modified Multiwall Carbon Nanotube/Iron Oxides in the Absence/Presence of Fulvic Acid. J. Chem. Technol. Biotechnol. 2012, 87, 673–681. [Google Scholar] [CrossRef]
- Paulino, A.T.; Belfiore, L.A.; Kubota, L.T.; Muniz, E.C.; Almeida, V.C.; Tambourgi, E.B. Effect of Magnetite on the Adsorption Behavior of Pb (II), Cd (II), and Cu (II) in Chitosan-Based Hydrogels. Desalination 2011, 275, 187–196. [Google Scholar] [CrossRef]
- Gupta, V.K.; Agarwal, S.; Saleh, T.A. Chromium Removal by Combining the Magnetic Properties of Iron Oxide with Adsorption Properties of Carbon Nanotubes. Water Res. 2011, 45, 2207–2212. [Google Scholar] [CrossRef]
- Wang, X.S.; Zhu, L.; Lu, H.J. Surface Chemical Properties and Adsorption of Cu (II) on Nanoscale Magnetite in Aqueous Solutions. Desalination 2011, 276, 154–160. [Google Scholar] [CrossRef]
- Pang, Y.; Zeng, G.; Tang, L.; Zhang, Y.; Liu, Y.; Lei, X.; Li, Z.; Zhang, J.; Xie, G. PEI-Grafted Magnetic Porous Powder for Highly Effective Adsorption of Heavy Metal Ions. Desalination 2011, 281, 278–284. [Google Scholar] [CrossRef]
- Chen, R.; Zhi, C.; Yang, H.; Bando, Y.; Zhang, Z.; Sugiur, N.; Golberg, D. Arsenic (V) Adsorption on Fe3O4 Nanoparticle-Coated Boron Nitride Nanotubes. J. Colloid Interface Sci. 2011, 359, 261–268. [Google Scholar] [CrossRef]
- Gong, J.; Wang, X.; Shao, X.; Yuan, S.; Yang, C.; Hu, X. Adsorption of Heavy Metal Ions by Hierarchically Structured Magnetite-Carbonaceous Spheres. Talanta 2012, 101, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.L.; Wang, X.Y.; Zeng, G.M.; Chen, L.; Deng, J.H.; Zhang, X.R.; Niu, Q.Y. Copper (II) Removal by Pectin-Iron Oxide Magnetic Nanocomposite Adsorbent. Chem. Eng. J. 2012, 185–186, 100–107. [Google Scholar] [CrossRef]
- Xu, P.; Zeng, G.M.; Huang, D.L.; Lai, C.; Zhao, M.H.; Wei, Z.; Li, N.J.; Huang, C.; Xie, G.X. Adsorption of Pb (II) by Iron Oxide Nanoparticles Immobilized Phanerochaete Chrysosporium: Equilibrium, Kinetic, Thermodynamic and Mechanisms Analysis. Chem. Eng. J. 2012, 203, 423–431. [Google Scholar] [CrossRef]
- Xin, X.; Wei, Q.; Yang, J.; Yan, L.; Feng, R.; Chen, G.; Du, B.; Li, H. Highly Efficient Removal of Heavy Metal Ions by Amine-Functionalized Mesoporous Fe3O4 Nanoparticles. Chem. Eng. J. 2012, 184, 132–140. [Google Scholar] [CrossRef]
- Ge, F.; Li, M.M.; Ye, H.; Zhao, B.X. Effective Removal of Heavy Metal Ions Cd2+, Zn2+, Pb2+, Cu2+ from Aqueous Solution by Polymer-Modified Magnetic Nanoparticles. J. Hazard. Mater. 2012, 211–212, 366–372. [Google Scholar] [CrossRef]
- Feng, L.; Cao, M.; Ma, X.; Zhu, Y.; Hu, C. Superparamagnetic High-Surface-Area Fe3O4 Nanoparticles as Adsorbents for Arsenic Removal. J. Hazard. Mater. 2012, 217–218, 439–446. [Google Scholar] [CrossRef]
- Ngomsik, A.F.; Bee, A.; Talbot, D.; Cote, G. Magnetic Solid–Liquid Extraction of Eu (III), La (III), Ni (II) and Co (II) with Maghemite Nanoparticles. Sep. Purif. Technol. 2012, 86, 1–8. [Google Scholar] [CrossRef]
- Jin, Y.; Liu, F.; Tong, M.; Hou, Y. Removal of Arsenate by Cetyltrimethylammonium Bromide Modified Magnetic Nanoparticles. J. Hazard. Mater. 2012, 227–228, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Larraza, I.; López-Gónzalez, M.; Corrales, T.; Marcelo, G. Hybrid Materials: Magnetite–Polyethylenimine–Montmorillonite, as Magnetic Adsorbents for Cr (VI) Water Treatment. J. Colloid Interface Sci. 2012, 385, 24–33. [Google Scholar] [CrossRef]
- Zhang, C.; Sui, J.; Li, J.; Tang, Y.; Cai, W. Efficient Removal of Heavy Metal Ions by Thiol-Functionalized Superparamagnetic Carbon Nanotubes. Chem. Eng. J. 2012, 210, 45–52. [Google Scholar] [CrossRef]
- Bhunia, P.; Kim, G.; Baik, C.; Lee, H. A Strategically Designed Porous Iron–Iron Oxide Matrix on Graphene for Heavy Metal Adsorption. Chem. Commun. 2012, 48, 9888–9890. [Google Scholar] [CrossRef] [PubMed]
- Cho, D.W.; Jeon, B.H.; Chon, C.M.; Kim, Y.; Schwartz, F.W.; Lee, E.S.; Song, H. A Novel Chitosan/Clay/Magnetite Composite for Adsorption of Cu (II) and As (V). Chem. Eng. J. 2012, 200–202, 654–662. [Google Scholar] [CrossRef]
- Wang, L.; Li, J.; Jiang, Q.; Zhao, L. Water-Soluble Fe3O4 Nanoparticles with High Solubility for Removal of Heavy-Metal Ions from Waste Water. Dalt. Trans. 2012, 41, 4544–4551. [Google Scholar] [CrossRef]
- Hakami, O.; Zhang, Y.; Banks, C.J. Thiol-Functionalised Mesoporous Silica-Coated Magnetite Nanoparticles for High Efficiency Removal and Recovery of Hg from Water. Water Res. 2012, 46, 3913–3922. [Google Scholar] [CrossRef]
- Parham, H.; Zargar, B.; Shiralipour, R. Fast and Efficient Removal of Mercury from Water Samples Using Magnetic Iron Oxide Nanoparticles Modified with 2-Mercaptobenzothiazole. J. Hazard. Mater. 2012, 205–206, 94–100. [Google Scholar] [CrossRef]
- Luo, X.; Wang, C.; Luo, S.; Dong, R.; Tu, X.; Zeng, G. Adsorption of As(III) and As (V) from Water Using Magnetite Fe3O4-Reduced Graphite Oxide–MnO2 Nanocomposites. Chem. Eng. J. 2012, 187, 45–52. [Google Scholar] [CrossRef]
- Ji, L.; Zhou, L.; Bai, X.; Shao, Y.; Zhao, G.; Qu, Y.; Wang, C.; Li, Y. Facile Synthesis of Multiwall Carbon Nanotubes/Iron Oxides for Removal of Tetrabromobisphenol A and Pb (II). J. Mater. Chem. 2012, 22, 15853–15862. [Google Scholar] [CrossRef]
- Roy, A.; Bhattacharya, J. Removal of Cu (II), Zn (II) and Pb (II) from Water Using Microwave-Assisted Synthesized Maghemite Nanotubes. Chem. Eng. J. 2012, 211–212, 493–500. [Google Scholar] [CrossRef]
- Zhang, S.; Zhou, Y.F.; Nie, W.Y.; Song, L.Y. Preparation of Fe3O4/Chitosan/Poly(Acrylic Acid) Composite Particles and Its Application in Adsorbing Copper Ion (II). Cellulose 2012, 19, 2081–2091. [Google Scholar] [CrossRef]
- Mahdavi, M.; Ahmad, M.B.; Haron, M.J.; Gharayebi, Y.; Shameli, K.; Nadi, B. Fabrication and Characterization of SiO2/(3-Aminopropyl)Triethoxysilane-Coated Magnetite Nanoparticles for Lead(II) Removal from Aqueous Solution. J. Inorg. Organomet. Polym. Mater. 2013, 23, 599–607. [Google Scholar] [CrossRef]
- Mahapatra, A.; Mishra, B.G.; Hota, G. Electrospun Fe2O3-Al2O3 Nanocomposite Fibers as Efficient Adsorbent for Removal of Heavy Metal Ions from Aqueous Solution. J. Hazard. Mater. 2013, 258–259, 116–123. [Google Scholar] [CrossRef]
- Ahmed, M.A.; Ali, S.M.; El-Dek, S.I.; Galal, A. Magnetite–Hematite Nanoparticles Prepared by Green Methods for Heavy Metal Ions Removal from Water. Mater. Sci. Eng. B 2013, 178, 744–751. [Google Scholar] [CrossRef]
- Xiao, Y.; Liang, H.; Chen, W.; Wang, Z. Synthesis and Adsorption Behavior of Chitosan-Coated MnFe2O4 Nanoparticles for Trace Heavy Metal Ions Removal. Appl. Surf. Sci. 2013, 285, 498–504. [Google Scholar] [CrossRef]
- Chang, J.; Zhong, Z.; Xu, H.; Yao, Z.; Chen, R. Fabrication of Poly (γ-Glutamic Acid)-Coated Fe3O4 Magnetic Nanoparticles and Their Application in Heavy Metal Removal. Chinese J. Chem. Eng. 2013, 21, 1244–1250. [Google Scholar] [CrossRef]
- Badruddoza, A.Z.M.; Shawon, Z.B.Z.; Tay, W.J.D.; Hidajat, K.; Uddin, M.S. Fe3O4/Cyclodextrin Polymer Nanocomposites for Selective Heavy Metals Removal from Industrial Wastewater. Carbohydr. Polym. 2013, 91, 322–332. [Google Scholar] [CrossRef]
- Liang, H.; Xu, B.; Wang, Z. Self-Assembled 3D Flower-like α-Fe2O3 Microstructures and Their Superior Capability for Heavy Metal Ion Removal. Mater. Chem. Phys. 2013, 141, 727–734. [Google Scholar] [CrossRef]
- Karami, H. Heavy Metal Removal from Water by Magnetite Nanorods. Chem. Eng. J. 2013, 219, 209–216. [Google Scholar] [CrossRef]
- Ren, Y.; Abbood, H.A.; He, F.; Peng, H.; Huang, K. Magnetic EDTA-Modified Chitosan/SiO2/Fe3O4 Adsorbent: Preparation, Characterization, and Application in Heavy Metal Adsorption. Chem. Eng. J. 2013, 226, 300–311. [Google Scholar] [CrossRef]
- Tang, Y.; Liang, S.; Wang, J.; Yu, S.; Wang, Y. Amino-Functionalized Core-Shell Magnetic Mesoporous Composite Microspheres for Pb (II) and Cd (II) Removal. J. Environ. Sci. 2013, 25, 830–837. [Google Scholar] [CrossRef]
- Liu, M.; Wen, T.; Wu, X.; Chen, C.; Hu, J.; Li, J.; Wang, X. Synthesis of Porous Fe3O4 Hollow Microspheres/ Graphene Oxide Composite for Cr(vi) Removal. Dalt. Trans. 2013, 42, 14710–14717. [Google Scholar] [CrossRef]
- Wei, Z.; Xing, R.; Zhang, X.; Liu, S.; Yu, H.; Li, P. Facile Template-Free Fabrication of Hollow Nestlike α-Fe2O3 Nanostructures for Water Treatment. ACS Appl. Mater. Interfaces 2013, 5, 598–604. [Google Scholar] [CrossRef]
- Ren, T.; He, P.; Niu, W.; Wu, Y.; Ai, L.; Gou, X. Synthesis of α-Fe2O3 Nanofibers for Applications in Removal and Recovery of Cr (VI) from Wastewater. Env. Sci. Pollut. Res. 2013, 20, 155–162. [Google Scholar] [CrossRef]
- Zhang, J.; Zhai, S.; Li, S.; Xiao, Z.; Song, Y.; An, Q.; Tian, G. Pb (II) Removal of Fe3O4@SiO2–NH2 Core–Shell Nanomaterials Prepared via a Controllable Sol–Gel Process. Chem. Eng. J. 2013, 215–216, 461–471. [Google Scholar] [CrossRef]
- Burks, T.; Uheida, A.; Saleemi, M.; Eita, M.; Toprak, M.S.; Muhammed, M. Removal of Chromium (VI) Using Surface Modified Superparamagnetic Iron Oxide Nanoparticles. Sep. Sci. Technol. 2013, 48, 1243–1251. [Google Scholar] [CrossRef]
- Zhao, L.; Chang, X.L.; Liao, R.; Zhang, X.; Xie, J.; Yu, B.; Wu, R.; Wang, R.; Yang, S.T. Facile Hydrothermal Preparation of S-Doped Fe3O4@C Nanoparticles for Cu2+ Removal. Mater. Lett. 2014, 135, 154–157. [Google Scholar] [CrossRef]
- Xu, Y.; Zhou, Y.; Li, R. Simultaneous Fluorescence Response and Adsorption of Functionalized Fe3O4@SiO2 Nanoparticles to Cd2+, Zn2+ and Cu2+. Colloids Surf. A Physicochem. Eng. Asp. 2014, 459, 240–246. [Google Scholar] [CrossRef]
- Feitoza, N.C.; Gonçalves, T.D.; Mesquita, J.J.; Menegucci, J.S.; Santos, M.K.M.S.; Chaker, J.A.; Cunha, R.B.; Medeiros, A.M.M.; Rubim, J.C.; Sousa, M.H. Fabrication of Glycine-Functionalized Maghemite Nanoparticles for Magnetic Removal of Copper from Wastewater. J. Hazard. Mater. 2014, 264, 153–160. [Google Scholar] [CrossRef]
- Ahmadi, A.; Heidarzadeh, S.; Mokhtari, A.R.; Darezereshki, E.; Harouni, H.A. Optimization of Heavy Metal Removal from Aqueous Solutions by Maghemite (γ-Fe2O3) Nanoparticles Using Response Surface Methodology. J. Geochem. Explor. 2014, 147, 151–158. [Google Scholar] [CrossRef]
- Hao, T.; Yang, C.; Rao, X.; Wang, J.; Niu, C.; Su, X. Facile Additive-Free Synthesis of Iron Oxide Nanoparticles for Efficient Adsorptive Removal of Congo Red and Cr (VI). Appl. Surf. Sci. 2014, 292, 174–180. [Google Scholar] [CrossRef]
- Burks, T.; Avila, M.; Akhtar, F.; Göthelid, M.; Lansåker, P.C.; Toprak, M.S.; Muhammed, M.; Uheida, A. Studies on the Adsorption of Chromium (VI) onto 3-Mercaptopropionic Acid Coated Superparamagnetic Iron Oxide Nanoparticles. J. Colloid Interface Sci. 2014, 425, 36–43. [Google Scholar] [CrossRef]
- Fang, X.B.; Fang, Z.Q.; Tsang, P.K.E.; Cheng, W.; Yan, X.M.; Zheng, L.C. Selective Adsorption of Cr (VI) from Aqueous Solution by EDA-Fe3O4 Nanoparticles Prepared from Steel Pickling Waste Liquor. Appl. Surf. Sci. 2014, 314, 655–662. [Google Scholar] [CrossRef]
- Türk, T.; Alp, I. Arsenic Removal from Aqueous Solutions with Fe-Hydrotalcite Supported Magnetite Nanoparticle. J. Ind. Eng. Chem. 2014, 20, 732–738. [Google Scholar] [CrossRef]
- Guo, X.; Du, B.; Wei, Q.; Yang, J.; Hu, L.; Yan, L.; Xu, W. Synthesis of Amino Functionalized Magnetic Graphenes Composite Material and Its Application to Remove Cr (VI), Pb (II), Hg (II), Cd (II) and Ni (II) from Contaminated Water. J. Hazard. Mater. 2014, 278, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Lan, S.; Leng, Z.; Guo, N.; Wu, X.; Gan, S. Sesbania Gum-Based Magnetic Carbonaceous Nanocomposites: Facile Fabrication and Adsorption Behavior. Colloids Surf. A Physicochem. Eng. Asp. 2014, 446, 163–171. [Google Scholar] [CrossRef]
- Zhou, L.; Ji, L.; Ma, P.C.; Shao, Y.; Zhang, H.; Gao, W.; Li, Y. Development of Carbon Nanotubes/CoFe2O4 Magnetic Hybrid Material for Removal of Tetrabromobisphenol A and Pb (II). J. Hazard. Mater. 2014, 265, 104–114. [Google Scholar] [CrossRef]
- Kalantari, K.; Ahmad, M.B.; Masoumi, H.R.F.; Shameli, K.; Basri, M.; Khandanlou, R. Rapid Adsorption of Heavy Metals by Fe3O4/Talc Nanocomposite and Optimization Study Using Response Surface Methodology. Int. J. Mol. Sci. 2014, 15, 12913–12927. [Google Scholar] [CrossRef]
- Lasheen, M.R.; El-Sherif, I.Y.; Sabry, D.Y.; El-Wakeel, S.T.; El-Shahat, M.F. Removal and Recovery of Cr (VI) by Magnetite Nanoparticles. Desalin. Water Treat. 2014, 52, 6464–6473. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, L.; Xu, W.; Guo, X.; Cui, L.; Gao, L.; Wei, Q.; Du, B. Adsorption of Pb (II) and Hg (II) from Aqueous Solution Using Magnetic CoFe2O4-Reduced Graphene Oxide. J. Mol. Liq. 2014, 191, 177–182. [Google Scholar] [CrossRef]
- Kumar, S.; Nair, R.R.; Pillai, P.B.; Gupta, S.N.; Iyengar, M.A.R.; Sood, A.K. Graphene Oxide-MnFe2O4 Magnetic Nanohybrids for Efficient Removal of Lead and Arsenic from Water. ACS Appl. Mater. Interfaces 2014, 6, 17426–17436. [Google Scholar] [CrossRef]
- Qi, X.; Li, N.; Xu, Q.; Chen, D.; Li, H.; Lu, J. Water-Soluble Fe3O4 Superparamagnetic Nanocomposites for the Removal of Low Concentration Mercury (II) Ions from Water. RSC Adv. 2014, 4, 47643–47648. [Google Scholar] [CrossRef]
- Chen, Z.; Geng, Z.; Zhang, Z.; Ren, L.; Tao, T.; Yang, R.; Guo, Z. Synthesis of Magnetic Fe3O4@C Nanoparticles Modified with -SO3H and -COOH Groups for Fast Removal of Pb2+, Hg2+, and Cd2+ Ions. Eur. J. Inorg. Chem. 2015, 2014, 3172–3177. [Google Scholar] [CrossRef]
- Peng, X.; Xu, F.; Zhang, W.; Wang, J.; Zeng, C.; Niu, M.; Chmielewská, E. Magnetic Fe3O4 @ Silica–Xanthan Gum Composites for Aqueous Removal and Recovery of Pb2+. Colloids Surf. A Physicochem. Eng. Asp. 2014, 443, 27–36. [Google Scholar] [CrossRef]
- Luo, X.; Zeng, J.; Liu, S.; Zhang, L. An Effective and Recyclable Adsorbent for the Removal of Heavy Metal Ions from Aqueous System: Magnetic Chitosan/Cellulose Microspheres. Bioresour. Technol. 2015, 194, 403–406. [Google Scholar] [CrossRef] [PubMed]
- Culita, D.C.; Simonescu, C.M.; Dragne, M.; Stanica, N.; Munteanu, C.; Preda, S.; Oprea, O. Effect of Surfactant Concentration on Textural, Morphological and Magnetic Properties of CoFe2O4 Nanoparticles and Evaluation of Their Adsorptive Capacity for Pb (II) Ions. Ceram. Int. 2015, 41, 13553–13560. [Google Scholar] [CrossRef]
- Madrakian, T.; Afkhami, A.; Zadpour, B.; Ahmadi, M. New Synthetic Mercaptoethylamino Homopolymer-Modified Maghemite Nanoparticles for Effective Removal of Some Heavy Metal Ions from Aqueous Solution. J. Ind. Eng. Chem. 2015, 21, 1160–1166. [Google Scholar] [CrossRef]
- Tadjarodi, A.; Abbaszadeh, A.; Taghizadeh, M.; Shekari, N.; Asgharinezhad, A.A. Solid Phase Extraction of Cd (II) and Pb (II) Ions Based on a Novel Functionalized Fe3O4@SiO2 Core-Shell Nanoparticles with the Aid of Multivariate Optimization Methodology. Mater. Sci. Eng. C. 2015, 49, 416–421. [Google Scholar] [CrossRef] [PubMed]
- Gautam, R.K.; Gautam, P.K.; Banerjee, S.; Soni, S.; Singh, S.K.; Chattopadhyaya, M.C. Removal of Ni (II) by Magnetic Nanoparticles. J. Mol. Liq. 2015, 204, 60–69. [Google Scholar] [CrossRef]
- Souda, P.; Sreejith, L. Magnetic Hydrogel for Better Adsorption of Heavy Metals from Aqueous Solutions. J. Environ. Chem. Eng. 2015, 3, 1882–1891. [Google Scholar] [CrossRef]
- Kumari, M.; Pittman, C.U.; Mohan, D. Heavy Metals [Chromium (VI) and Lead (II)] Removal from Water Using Mesoporous Magnetite (Fe3O4) Nanospheres. J. Colloid Interface Sci. 2015, 442, 120–132. [Google Scholar] [CrossRef]
- Morillo, D.; Uheida, A.; Pérez, G.; Muhammed, M.; Valiente, M. Arsenate Removal with 3-Mercaptopropanoic Acid-Coated Superparamagnetic Iron Oxide Nanoparticles. J. Colloid Interface Sci. 2015, 438, 227–234. [Google Scholar] [CrossRef][Green Version]
- Shan, C.; Ma, Z.; Tong, M. Efficient Removal of Free and Nitrilotriacetic Acid Complexed Cd (II) from Water by Poly(1-Vinylimidazole)-Grafted Fe3O4@SiO2 Magnetic Nanoparticles. J. Hazard. Mater. 2015, 299, 479–485. [Google Scholar] [CrossRef]
- Chávez-Guajardo, A.E.; Medina-Llamas, J.C.; Maqueira, L.; Andrade, C.A.S.; Alves, K.G.B.; de Melo, C.P. Efficient Removal of Cr (VI) and Cu (II) Ions from Aqueous Media by Use of Polypyrrole/Maghemite and Polyaniline/Maghemite Magnetic Nanocomposites. Chem. Eng. J. 2015, 281, 826–836. [Google Scholar] [CrossRef]
- Cui, L.; Wang, Y.; Gao, L.; Hu, L.; Yan, L.; Wei, Q.; Du, B. EDTA Functionalized Magnetic Graphene Oxide for Removal of Pb (II), Hg (II) and Cu (II) in Water Treatment: Adsorption Mechanism and Separation Property. Chem. Eng. J. 2015, 281, 1–10. [Google Scholar] [CrossRef]
- Moradinasab, S.; Behzad, M. Removal of Heavy Metals from Aqueous Solution Using Fe3O4 Nanoparticles Coated with Schiff Base Ligand. Desalin. Water Treat. 2016, 57, 4028–4036. [Google Scholar] [CrossRef]
- Mittal, A.; Ahmad, R.; Hasan, I. Poly (Methyl Methacrylate)-Grafted Alginate/Fe3O4 Nanocomposite: Synthesis and Its Application for the Removal of Heavy Metal Ions. Desalin. Water Treat. 2016, 57, 19820–19833. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, T.; Zhang, Y.; Ng, D.H.L.; Zhao, H.; Wang, G. Adsorption of Hg2+ by Thiol Functionalized Hollow Mesoporous Silica Microspheres with Magnetic Cores. RSC Adv. 2015, 5, 51446–51453. [Google Scholar] [CrossRef]
- Wang, H.; Yuan, X.; Wu, Y.; Chen, X.; Leng, L.; Wang, H.; Li, H.; Zeng, G. Facile Synthesis of Polypyrrole Decorated Reduced Graphene Oxide–Fe3O4 Magnetic Composites and Its Application for the Cr (VI) Removal. Chem. Eng. J. 2015, 262, 597–606. [Google Scholar] [CrossRef]
- Duan, S.; Tang, R.; Xue, Z.; Zhang, X.; Zhao, Y.; Zhang, W.; Zhang, J.; Wang, B.; Zeng, S.; Sun, D. Effective Removal of Pb (II) Using Magnetic Co0.6Fe2.4O4 Micro-Particles as the Adsorbent: Synthesis and Study on the Kinetic and Thermodynamic Behaviors for Its Adsorption. Colloids Surf. A Physicochem. Eng. Asp. 2015, 469, 211–223. [Google Scholar] [CrossRef]
- Shan, R.R.; Yan, L.G.; Yang, K.; Hao, Y.F.; Du, B. Adsorption of Cd (II) by Mg–Al–CO3- and Magnetic Fe3O4/Mg–Al–CO3-Layered Double Hydroxides: Kinetic, Isothermal, Thermodynamic and Mechanistic Studies. J. Hazard. Mater 2015, 299, 42–49. [Google Scholar] [CrossRef]
- Lasheen, M.R.; El-Sherif, I.Y.; Tawfik, M.E.; El-Wakeel, S.T.; El-Shahat, M.F. Preparation and Adsorption Properties of Nano Magnetite Chitosan Films for Heavy Metal Ions from Aqueous Solution. Mater. Res. Bull. 2016, 80, 344–350. [Google Scholar] [CrossRef]
- Bao, S.; Tang, L.; Li, K.; Ning, P.; Peng, J.; Guo, H.; Zhu, T.; Liu, Y. Highly Selective Removal of Zn (II) Ion from Hot-Dip Galvanizing Pickling Waste with Amino-Functionalized Fe3O4@SiO2 Magnetic Nano-Adsorbent. J. Colloid Interface Sci. 2016, 462, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Tamez, C.; Hernandez, R.; Parsons, J.G. Removal of Cu (II) and Pb (II) from Aqueous Solution Using Engineered Iron Oxide Nanoparticles. Microchem. J. 2016, 125, 97–104. [Google Scholar] [CrossRef]
- Babu, C.M.; Vinodh, R.; Sundaravel, B.; Abidov, A.; Peng, M.M.; Cha, W.S.; Jang, H.T. Characterization of Reduced Graphene Oxide Supported Mesoporous Fe2O3/TiO2 Nanoparticles and Adsorption of As (III) and As (V) from Potable Water. J. Taiwan Inst. Chem. Eng. 2016, 62, 199–208. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, J.; Hu, Y.; Zhao, H.; Zhou, J.; Liu, Y.; Lou, Z.; Xu, X. Functional Nanomaterials: Study on Aqueous Hg (II) Adsorption by Magnetic Fe3O4@SiO2-SH Nanoparticles. J. Taiwan Inst. Chem. Eng. 2016, 60, 394–402. [Google Scholar] [CrossRef]
- Singh, D.; Singh, S.K.; Atar, N.; Krishna, V. Amino Acid Functionalized Magnetic Nanoparticles for Removal of Ni (II) from Aqueous Solution. J. Taiwan Inst. Chem. Eng. 2016, 67, 148–160. [Google Scholar] [CrossRef]
- Davarnejad, R.; Panahi, P. Cu (II) Removal from Aqueous Wastewaters by Adsorption on the Modified Henna with Fe3O4 Nanoparticles Using Response Surface Methodology. Sep. Purif. Technol. 2016, 158, 286–292. [Google Scholar] [CrossRef]
- Yan, L.; Li, S.; Yu, H.; Shan, R.; Du, B.; Liu, T. Facile Solvothermal Synthesis of Fe3O4/Bentonite for Efficient Removal of Heavy Metals from Aqueous Solution. Powder Technol. 2016, 301, 632–640. [Google Scholar] [CrossRef]
- Bagbi, Y.; Sarswat, A.; Mohan, D.; Pandey, A.; Solanki, P.R. Lead (Pb2+) Adsorption by Monodispersed Magnetite Nanoparticles: Surface Analysis and Effects of Solution Chemistry. J. Environ. Chem. Eng. 2016, 4, 4237–4247. [Google Scholar] [CrossRef]
- Rajput, S.; Pittman, C.U.; Mohan, D. Magnetic Magnetite (Fe3O4) Nanoparticle Synthesis and Applications for Lead (Pb2+) and Chromium (Cr6+) Removal from Water. J. Colloid Interface Sci. 2016, 468, 334–346. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Song, T.; Lv, Z.; Ji, G. High-Efficiency and Low-Cost α-Fe2O3 Nanoparticles-Coated Volcanic Rock for Cd (II) Removal from Wastewater. Process. Saf. Environ. Prot. 2016, 104, 373–381. [Google Scholar] [CrossRef]
- Danesh, N.; Hosseini, M.; Ghorbani, M.; Marjani, A. Fabrication, Characterization and Physical Properties of a Novel Magnetite Graphene Oxide/Lauric Acid Nanoparticles Modified by Ethylenediaminetetraacetic Acid and Its Applications as an Adsorbent for the Removal of Pb (II) Ions. Synth. Met. 2016, 220, 508–523. [Google Scholar] [CrossRef]
- Gao, J.; He, Y.; Zhao, X.; Ran, X.; Wu, Y.; Su, Y.; Dai, J. Single Step Synthesis of Amine-Functionalized Mesoporous Magnetite Nanoparticles and Their Application for Copper Ions Removal from Aqueous Solution. J. Colloid Interface Sci. 2016, 481, 220–228. [Google Scholar] [CrossRef]
- Habila, M.A.; Alothman, Z.A.; El-Toni, A.M.; Labis, J.P.; Soylak, M. Synthesis and Application of Fe3O4@SiO2@TiO2 for Photocatalytic Decomposition of Organic Matrix Simultaneously with Magnetic Solid Phase Extraction of Heavy Metals Prior to ICP-MS Analysis. Talanta 2016, 154, 539–547. [Google Scholar] [CrossRef]
- Khandanlou, R.; Fard Masoumi, H.R.; Ahmad, M.B.; Shameli, K.; Basri, M.; Kalantari, K. Enhancement of Heavy Metals Sorption via Nanocomposites of Rice Straw and Fe3O4 Nanoparticles Using Artificial Neural Network (ANN). Ecol. Eng. 2016, 91, 249–256. [Google Scholar] [CrossRef]
- Fan, H.L.; Li, L.; Zhou, S.F.; Liu, Y.Z. Continuous Preparation of Fe3O4 Nanoparticles Combined with Surface Modification by L-Cysteine and Their Application in Heavy Metal Adsorption. Ceram. Int. 2016, 42, 4228–4237. [Google Scholar] [CrossRef]
- Mahmoud, M.E.; Amira, M.F.; Zaghloul, A.A.; Ibrahim, G.A.A. High Performance Microwave-Enforced Solid Phase Extraction of Heavy Metals from Aqueous Solutions Using Magnetic Iron Oxide Nanoparticles-Protected-Nanosilica. Sep. Purif. Technol. 2016, 163, 169–172. [Google Scholar] [CrossRef]
- Mukherjee, D.; Ghosh, S.; Majumdar, S.; Annapurna, K. Green Synthesis of α-Fe2O3 Nanoparticles for Arsenic (V) Remediation with a Novel Aspect for Sludge Management. J. Environ. Chem. Eng. 2016, 4, 639–650. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, J.; Li, N.; Wang, W.; Nan, J.; Zhao, Z.; Cui, F. Highly Efficient Removal of Bivalent Heavy Metals from Aqueous Systems by Magnetic Porous Fe3O4-MnO2: Adsorption Behavior and Process Study. Chem. Eng. J. 2016, 304, 737–746. [Google Scholar] [CrossRef]
- Liu, Y.; Fu, R.; Sun, Y.; Zhou, X.; Baig, S.A.; Xu, X. Multifunctional Nanocomposites Fe3O4@SiO2-EDTA for Pb (II) and Cu (II) Removal from Aqueous Solutions. Appl. Surf. Sci. 2016, 369, 267–276. [Google Scholar] [CrossRef]
- Shen, W.; Mu, Y.; Xiao, T.; Ai, Z. Magnetic Fe3O4–FeB Nanocomposites with Promoted Cr (VI) Removal Performance. Chem. Eng. J. 2016, 285, 57–68. [Google Scholar] [CrossRef]
- Beduk, F. Superparamagnetic Nanomaterial Fe3O4-TiO2 for the Removal of As (V) and As (III) from Aqueous Solutions. Env. Technol. 2016, 37, 1790–1801. [Google Scholar] [CrossRef] [PubMed]
- Beheshti, H.; Irani, M.; Hosseini, L.; Rahimi, A.; Aliabadi, M. Removal of Cr (VI) from Aqueous Solutions Using Chitosan/MWCNT/Fe3O4 Composite Nanofibers-Batch and Column Studies. Chem. Eng. J. 2016, 284, 557–564. [Google Scholar] [CrossRef]
- Zhao, D.; Gao, X.; Wu, C.; Xie, R.; Feng, S.; Chen, C. Facile Preparation of Amino Functionalized Graphene Oxide Decorated with Fe3O4 Nanoparticles for the Adsorption of Cr (VI). Appl. Surf. Sci. 2016, 384, 1–9. [Google Scholar] [CrossRef]
- Chen, K.; He, J.; Li, Y.; Cai, X.; Zhang, K.; Liu, T.; Hu, Y.; Lin, D.; Kong, L.; Liu, J. Removal of Cadmium and Lead Ions from Water by Sulfonated Magnetic Nanoparticle Adsorbents. J. Colloid Interface Sci. 2017, 494, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Hasanzadeh, R.; Moghadam, P.N.; Bahri-Laleh, N.; Sillanpää, M. Effective Removal of Toxic Metal Ions from Aqueous Solutions: 2-Bifunctional Magnetic Nanocomposite Base on Novel Reactive PGMA-MAn Copolymer@Fe3O4 Nanoparticles. J. Colloid Interface Sci. 2017, 490, 727–746. [Google Scholar] [CrossRef] [PubMed]
- Elfeky, S.A.; Mahmoud, S.E.; Youssef, A.F. Applications of CTAB Modified Magnetic Nanoparticles for Removal of Chromium (VI) from Contaminated Water. J. Adv. Res. 2017, 8, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Ke, F.; Jiang, J.; Li, Y.; Liang, J.; Wan, X.; Ko, S. Highly Selective Removal of Hg2+ and Pb2+ by Thiol-Functionalized Fe3O4@metal-Organic Framework Core-Shell Magnetic Microspheres. Appl. Surf. Sci. 2017, 413, 266–274. [Google Scholar] [CrossRef]
- Guo, S.; Jiao, P.; Dan, Z.; Duan, N.; Zhang, J.; Chen, G.; Gao, W. Synthesis of Magnetic Bioadsorbent for Adsorption of Zn (II), Cd (II) and Pb (II) Ions from Aqueous Solution. Chem. Eng. Res. Des. 2017, 126, 217–231. [Google Scholar] [CrossRef]
- Jin, S.; Park, B.C.; Ham, W.S.; Pan, L.; Kim, Y.K. Effect of the Magnetic Core Size of Amino-Functionalized Fe3O4-Mesoporous SiO2 Core-Shell Nanoparticles on the Removal of Heavy Metal Ions. Colloids Surfaces A Physicochem. Eng. Asp. 2017, 531, 133–140. [Google Scholar] [CrossRef]
- Ahmadi, M.; Hazrati Niari, M.; Kakavandi, B. Development of Maghemite Nanoparticles Supported on Cross-Linked Chitosan (γ-Fe2O3@CS) as a Recoverable Mesoporous Magnetic Composite for Effective Heavy Metals Removal. J. Mol. Liq. 2017, 248, 184–196. [Google Scholar] [CrossRef]
- Bagbi, Y.; Sarswat, A.; Mohan, D.; Pandey, A.; Solanki, P.R. Lead and Chromium Adsorption from Water Using L-Cysteine Functionalized Magnetite (Fe3O4) Nanoparticles. Sci. Rep. 2017, 7, 1–15. [Google Scholar] [CrossRef]
- Jiryaei Sharahi, F.; Shahbazi, A. Melamine-Based Dendrimer Amine-Modified Magnetic Nanoparticles as an Efficient Pb (II) Adsorbent for Wastewater Treatment: Adsorption Optimization by Response Surface Methodology. Chemosphere 2017, 189, 291–300. [Google Scholar] [CrossRef]
- Sun, T.; Zhao, Z.; Liang, Z.; Liu, J.; Shi, W.; Cui, F. Efficient Removal of Arsenite through Photocatalytic Oxidation and Adsorption by ZrO2-Fe3O4 Magnetic Nanoparticles. Appl. Surf. Sci. 2017, 416, 656–665. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, L.; Wang, Y.; Liu, X.; Rohani, S.; Lu, J. Fe3O4@SiO2@CS-TETA Functionalized Graphene Oxide for the Adsorption of Methylene Blue (MB) and Cu (II). Appl. Surf. Sci. 2017, 420, 970–981. [Google Scholar] [CrossRef]
- Zhu, H.; Shen, Y.; Wang, Q.; Chen, K.; Wang, X.; Zhang, G.; Yang, J.; Guo, Y.; Bai, R. Highly Promoted Removal of Hg (II) with Magnetic CoFe2O4@SiO2 Core-Shell Nanoparticles Modified by Thiol Groups. RSC Adv. 2017, 7, 39204–39215. [Google Scholar] [CrossRef]
- Zou, Z.; Shi, Z.; Deng, L. Highly Efficient Removal of Cu (II) from Aqueous Solution Using a Novel Magnetic EDTA Functionalized CoFe2O4. RSC Adv. 2017, 7, 5195–5205. [Google Scholar] [CrossRef]
- Lalhmunsiama; Pawar, R.R.; Hong, S.M.; Jin, K.J.; Lee, S.M. Iron-Oxide Modified Sericite Alginate Beads: A Sustainable Adsorbent for the Removal of As (V) and Pb (II) from Aqueous Solutions. J. Mol. Liq. 2017, 240, 497–503. [Google Scholar] [CrossRef]
- Ren, C.; Ding, X.; Fu, H.; Li, W.; Wu, H.; Yang, H. Core-Shell Superparamagnetic Monodisperse Nanospheres Based on Amino-Functionalized CoFe2O4@SiO2 for Removal of Heavy Metals from Aqueous Solutions. RSC Adv. 2017, 7, 6911–6921. [Google Scholar] [CrossRef]
- Nithya, K.; Sathish, A.; Senthil Kumar, P.; Ramachandran, T. Fast Kinetics and High Adsorption Capacity of Green Extract Capped Superparamagnetic Iron Oxide Nanoparticles for the Adsorption of Ni (II) Ions. J. Ind. Eng. Chem. 2018, 59, 230–241. [Google Scholar] [CrossRef]
- Silva-Yumi, J.; Escudey, M.; Gacitua, M.; Pizarro, C. Kinetics, Adsorption and Desorption of Cd (II) and Cu (II) on Natural Allophane: Effect of Iron Oxide Coating. Geoderma 2018, 319, 70–79. [Google Scholar] [CrossRef]
- Guo, T.; Bulin, C.; Li, B.; Zhao, Z.; Yu, H.; Sun, H.; Ge, X.; Xing, R.; Zhang, B. Efficient Removal of Aqueous Pb (II) Using Partially Reduced Graphene Oxide-Fe3O4. Adsorpt. Sci. Technol. 2018, 36, 1031–1048. [Google Scholar] [CrossRef]
- Mirzaeinejad, M.; Mansoori, Y.; Amiri, M. Amino Functionalized ATRP-Prepared Polyacrylamide-g-Magnetite Nanoparticles for the Effective Removal of Cu (II) Ions: Kinetics Investigations. Mater. Chem. Phys. 2018, 205, 195–205. [Google Scholar] [CrossRef]
- Sun, J.; Chen, Y.; Yu, H.; Yan, L.; Du, B.; Pei, Z. Removal of Cu2+, Cd2+ and Pb2+ from Aqueous Solutions by Magnetic Alginate Microsphere Based on Fe3O4/MgAl-Layered Double Hydroxide. J. Colloid Interface Sci. 2018, 532, 474–484. [Google Scholar] [CrossRef]
- Xu, W.; Song, Y.; Dai, K.; Sun, S.; Liu, G.; Yao, J. Novel Ternary Nanohybrids of Tetraethylenepentamine and Graphene Oxide Decorated with MnFe2O4 Magnetic Nanoparticles for the Adsorption of Pb (II). J. Hazard. Mater. 2018, 358, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Y.; Ji, H.Y.; Lu, H.H.; Liu, Y.X.; Yang, R.Q.; He, L.L.; Yang, S.M. Simultaneous Removal of Sb (III) and Cd (II) in Water by Adsorption onto a MnFe2O4-Biochar Nanocomposite. RSC Adv. 2018, 8, 3264–3273. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Z.; Zhao, Y.; Xia, K.; Guo, Y.; Qu, Z.; Bai, R. A Mild and Facile Synthesis of Amino Functionalized CoFe2O4@SiO2 for Hg (II) Removal. Nanomaterials 2018, 8, 673. [Google Scholar] [CrossRef] [PubMed]
- Song, T.; Yu, C.; He, X.; Lin, J.; Liu, Z.; Yang, X.; Zhang, Y.; Huang, Y.; Tang, C. Synthesis of Magnetically Separable Porous BN Microrods@Fe3O4 Nanocomposites for Pb (II) Adsorption. Colloids Surfaces A Physicochem. Eng. Asp. 2018, 537, 508–515. [Google Scholar] [CrossRef]
- Koushkbaghi, S.; Zakialamdari, A.; Pishnamazi, M.; Ramandi, H.F.; Aliabadi, M.; Irani, M. Aminated-Fe3O4 Nanoparticles Filled Chitosan/PVA/PES Dual Layers Nanofibrous Membrane for the Removal of Cr (VI) and Pb (II) Ions from Aqueous Solutions in Adsorption and Membrane Processes. Chem. Eng. J. 2018, 337, 169–182. [Google Scholar] [CrossRef]
- Fu, W.; Huang, Z. One-Pot Synthesis of a Two-Dimensional Porous Fe3O4/Poly(C3N3S3) Network Nanocomposite for the Selective Removal of Pb (II) and Hg (II) from Synthetic Wastewater. ACS Sustain. Chem. Eng. 2018, 6, 14785–14794. [Google Scholar] [CrossRef]
- Fu, W.; Huang, Z. Magnetic Dithiocarbamate Functionalized Reduced Graphene Oxide for the Removal of Cu (II), Cd (II), Pb (II), and Hg (II) Ions from Aqueous Solution: Synthesis, Adsorption, and Regeneration. Chemosphere 2018, 209, 449–456. [Google Scholar] [CrossRef]
- Song, Y.; Lu, M.; Huang, B.; Wang, D.; Wang, G.; Zhou, L. Decoration of Defective MoS2 Nanosheets with Fe3O4 Nanoparticles as Superior Magnetic Adsorbent for Highly Selective and Efficient Mercury Ions (Hg2+) Removal. J. Alloys Compd. 2018, 737, 113–121. [Google Scholar] [CrossRef]
- Facchi, D.P.; Cazetta, A.L.; Canesin, E.A.; Almeida, V.C.; Bonafé, E.G.; Kipper, M.J.; Martins, A.F. New Magnetic Chitosan/Alginate/Fe3O4@SiO2 Hydrogel Composites Applied for Removal of Pb (II) Ions from Aqueous Systems. Chem. Eng. J. 2018, 337, 595–608. [Google Scholar] [CrossRef]
- Huang, X.; Zhan, X.; Wen, C.; Xu, F.; Luo, L. Amino-Functionalized Magnetic Bacterial Cellulose/Activated Carbon Composite for Pb2+ and Methyl Orange Sorption from Aqueous Solution. J. Mater. Sci. Technol. 2018, 34, 855–863. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, C.; Zhang, L.; Liu, H.; Cao, B.; Liu, L.; Gong, W. Adsorption Studies of Cadmium onto Magnetic Fe3O4@FePO4 and Its Preconcentration with Detection by Electrothermal Atomic Absorption Spectrometry. Talanta 2018, 181, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Kumar, N.P.; Srinivas, A.; Roy, S. Core-Shell Fe3O4@Au Nanocomposite as Dual-Functional Optical Probe and Potential Removal System for Arsenic (III) from Water. J. Hazard. Mater. 2019, 375, 216–223. [Google Scholar] [CrossRef]
- Fu, W.; Wang, X.; Huang, Z. Remarkable Reusability of Magnetic Fe3O4-Encapsulated C3N3S3 Polymer/Reduced Graphene Oxide Composite: A Highly Effective Adsorbent for Pb and Hg Ions. Sci. Total Environ. 2019, 659, 895–904. [Google Scholar] [CrossRef]
- Rowley, J.; Abu-Zahra, N.H. Synthesis and Characterization of Polyethersulfone Membranes Impregnated with (3-Aminopropyltriethoxysilane) APTES-Fe3O4 Nanoparticles for As (V) Removal from Water. J. Environ. Chem. Eng. 2019, 7, 102875. [Google Scholar] [CrossRef]
- Ramutshatsha-Makhwedzha, D.; Ngila, J.C.; Ndungu, P.G.; Nomngongo, P.N. Ultrasound Assisted Adsorptive Removal of Cr, Cu, Al, Ba, Zn, Ni, Mn, Co and Ti from Seawater Using Fe2O3-SiO2-PAN Nanocomposite: Equilibrium Kinetics. J. Mar. Sci. Eng. 2019, 7, 133. [Google Scholar] [CrossRef]
- Zhang, L.; Guo, J.; Huang, X.; Wang, W.; Sun, P.; Li, Y.; Han, J. Functionalized Biochar-Supported Magnetic MnFe2O4 Nanocomposite for the Removal of Pb (II) and Cd (II). RSC Adv. 2019, 9, 365–376. [Google Scholar] [CrossRef]
- Xia, K.; Guo, Y.; Shao, Q.; Zan, Q.; Bai, R. Removal of Mercury (II) by EDTA-Functionalized Magnetic CoFe2O4@SiO2 Nanomaterial with Core-Shell Structure. Nanomaterials 2019, 9, 1532. [Google Scholar] [CrossRef]
- Feng, G.; Ma, J.; Zhang, X.; Zhang, Q.; Xiao, Y.; Ma, Q.; Wang, S. Magnetic Natural Composite Fe3O4-Chitosan@bentonite for Removal of Heavy Metals from Acid Mine Drainage. J. Colloid Interface Sci. 2019, 538, 132–141. [Google Scholar] [CrossRef]
- Wu, Z.; Deng, W.; Zhou, W.; Luo, J. Novel Magnetic Polysaccharide/Graphene Oxide @Fe3O4 Gel Beads for Adsorbing Heavy Metal Ions. Carbohydr. Polym. 2019, 216, 119–128. [Google Scholar] [CrossRef]
- Zendehdel, M.; Ramezani, M.; Shoshtari-yeganeh, B.; Salmani, A. Simultaneous Removal of Pb (II), Cd (II) and Bacteria from Aqueous Solution Using Amino-Functionalized Fe3O 4 / NaP Zeolite Nanocomposite. Environ. Technol. 2019, 40, 3689–3704. [Google Scholar] [CrossRef] [PubMed]
- Anush, S.M.; Vishalakshi, B. Modified Chitosan Gel Incorporated with Magnetic Nanoparticle for Removal of Cu (II) and Cr (VI) from Aqueous Solution. Int. J. Biol. Macromol. 2019, 133, 1051–1062. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Yuan, H.; Yu, J.; Lin, S. Study on the Competitive Adsorption and Correlational Mechanism for Heavy Metal Ions Using the Carboxylated Magnetic Iron Oxide Nanoparticles (MNPs-COOH) as Efficient Adsorbents. Appl. Surf. Sci. 2019, 473, 960–966. [Google Scholar] [CrossRef]
- Fan, H.; Ma, X.; Zhou, S.; Huang, J.; Liu, Y.; Liu, Y. Highly Efficient Removal of Heavy Metal Ions by Carboxymethyl Cellulose-Immobilized Fe3O4 Nanoparticles Prepared via High-Gravity Technology. Carbohydr. Polym. 2019, 213, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Tan, X.; Qiu, T.; Zhou, L.; Li, R.; Deng, Z. A Novel and Biocompatible Fe3O4 Loaded Chitosan Polyelectrolyte Nanoparticles for the Removal of Cd2+ Ion. Int. J. Biol. Macromol. 2019, 141, 1165–1174. [Google Scholar] [CrossRef]
- Al Yaqoob, K.; Bououdina, M.; Akhter, M.S.; Al Najar, B.; Vijaya, J.J. Selectivity and Efficient Pb and Cd Ions Removal by Magnetic MFe2O4 (M=Co, Ni, Cu and Zn) Nanoparticles. Mater. Chem. Phys. 2019, 232, 254–264. [Google Scholar] [CrossRef]
- Tahir, M.U.; Su, X.; Zhao, M.; Liao, Y.; Wu, R.; Chen, D. Preparation of Hydroxypropyl-Cyclodextrin-Graphene/Fe3O4 and Its Adsorption Properties for Heavy Metals. Surf. Interfaces 2019, 16, 43–49. [Google Scholar] [CrossRef]
- Zhou, G.; Wang, Y.; Zhou, R.; Wang, C.; Jin, Y.; Qiu, J.; Hua, C.; Cao, Y. Synthesis of Amino-Functionalized Bentonite/CoFe2O4@MnO2 Magnetic Recoverable Nanoparticles for Aqueous Cd2+ Removal. Sci. Total Environ. 2019, 682, 505–513. [Google Scholar] [CrossRef]
- Masjedi, A.; Askarizadeh, E.; Baniyaghoob, S. Magnetic Nanoparticles Surface-Modified with Tridentate Ligands for Removal of Heavy Metal Ions from Water. Mater. Chem. Phys. 2020, 249, 122917. [Google Scholar] [CrossRef]
- Shi, Y.; Xing, Y.; Deng, S.; Zhao, B.; Fu, Y.; Liu, Z. Synthesis of Proanthocyanidins-Functionalized Fe3O4 Magnetic Nanoparticles with High Solubility for Removal of Heavy-Metal Ions. Chem. Phys. Lett. 2020, 753, 137600. [Google Scholar] [CrossRef]
- Kulal, P.; Badalamoole, V. Efficient Removal of Dyes and Heavy Metal Ions from Wastewater Using Gum Ghatti–Graft–Poly(4-Acryloylmorpholine) Hydrogel Incorporated with Magnetite Nanoparticles. J. Environ. Chem. Eng. 2020, 8, 104207. [Google Scholar] [CrossRef]
- Yoo, J.; Kim, H.S.; Park, S.Y.; Kwon, S.; Lee, J.; Koo, J.; Seo, Y.S. Instantaneous Integration of Magnetite Nanoparticles on Graphene Oxide Assisted by Ultrasound for Efficient Heavy Metal Ion Retrieval. Ultrason. Sonochem. 2020, 64, 104962. [Google Scholar] [CrossRef] [PubMed]
- El-Dib, F.I.; Mohamed, D.E.; El-Shamy, O.A.A.; Mishrif, M.R. Study the Adsorption Properties of Magnetite Nanoparticles in the Presence of Different Synthesized Surfactants for Heavy Metal Ions Removal. Egypt. J. Pet. 2020, 29, 1–7. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, Y.; He, X.; Lin, J.; Yang, X.; Li, D.; Yu, M.; Yu, C.; Tang, C. Synergistic Adsorption of Pb (II) Ions by Fe3O4 Nanoparticles-Decorated Porous BN Nanofibers. Colloids Surf. A Physicochem. Eng. Asp. 2020, 589, 124400. [Google Scholar] [CrossRef]
- Kamari, S.; Shahbazi, A. Biocompatible Fe3O4@SiO2-NH2 Nanocomposite as a Green Nanofiller Embedded in PES–Nanofiltration Membrane Matrix for Salts, Heavy Metal Ion and Dye Removal: Long–Term Operation and Reusability Tests. Chemosphere 2020, 243, 125282. [Google Scholar] [CrossRef] [PubMed]
- Pillai, P.; Kakadiya, N.; Timaniya, Z.; Dharaskar, S.; Sillanpaa, M. Removal of Arsenic Using Iron Oxide Amended with Rice Husk Nanoparticles from Aqueous Solution. Mater. Today Proc. 2020, 28, 830–835. [Google Scholar] [CrossRef]
- Asadi, R.; Abdollahi, H.; Gharabaghi, M.; Boroumand, Z. Effective Removal of Zn (II) Ions from Aqueous Solution by the Magnetic MnFe2O4 and CoFe2O4 Spinel Ferrite Nanoparticles with Focuses on Synthesis, Characterization, Adsorption, and Desorption. Adv. Powder Technol. 2020, 31, 1480–1489. [Google Scholar] [CrossRef]
- Abu Taleb, M.; Kumar, R.; Al-Rashdi, A.A.; Seliem, M.K.; Barakat, M.A. Fabrication of SiO2/CuFe2O4/Polyaniline Composite: A Highly Efficient Adsorbent for Heavy Metals Removal from Aquatic Environment. Arab. J. Chem. 2020, 13, 7533–7543. [Google Scholar] [CrossRef]
- You, J.; Zhao, Y.; Wang, L.; Bao, W.; He, Y. Atomic Layer Deposition of γ-Fe2O3 Nanoparticles on Modified MWCNT for Efficient Adsorption of Cr (VI) Ions from Aqueous Solution. J. Phys. Chem. Solids 2020, 142, 109441. [Google Scholar] [CrossRef]
- Zhou, Z.; Liu, X.; Zhang, M.; Jiao, J.; Zhang, H.; Du, J.; Zhang, B.; Ren, Z. Preparation of Highly Efficient Ion-Imprinted Polymers with Fe3O4 Nanoparticles as Carrier for Removal of Cr (VI) from Aqueous Solution. Sci. Total Environ. 2020, 699, 134334. [Google Scholar] [CrossRef]
- Jafari, Z.; Avargani, V.M.; Rahimi, M.R.; Mosleh, S. Magnetic Nanoparticles-Embedded Nitrogen-Doped Carbon Nanotube/Porous Carbon Hybrid Derived from a Metal-Organic Framework as a Highly Efficient Adsorbent for Selective Removal of Pb (II) Ions from Aqueous Solution. J. Mol. Liq. 2020, 318, 113987. [Google Scholar] [CrossRef]
- Xiao, J.; Hu, R.; Chen, G.; Xing, B. Facile Synthesis of Multifunctional Bone Biochar Composites Decorated with Fe/Mn Oxide Micro-Nanoparticles: Physicochemical Properties, Heavy Metals Sorption Behavior and Mechanism. J. Hazard. Mater. 2020, 399, 123067. [Google Scholar] [CrossRef] [PubMed]
- Subana, P.S.; Manjunatha, C.; Manmadha, R.B.; Venkateswarlu, B.; Nagaraju, G.; Suresh, R. Surface Functionalized Magnetic α-Fe2O3 Nanoparticles: Synthesis, Characterization and Hg2+ Ion Removal in Water. Surf. Interfaces 2020, 21, 100680. [Google Scholar] [CrossRef]
- Devatha, C.P.; Shivani, S. Novel Application of Maghemite Nanoparticles Coated Bacteria for the Removal of Cadmium from Aqueous Solution. J. Environ. Manag. 2020, 258, 110038. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, Z.; Yue, R.; Gao, F.; Ren, R.; Wei, J.; Wang, X.; Kong, Z. Functional Group-Rich Hyperbranched Magnetic Material for Simultaneous Efficient Removal of Heavy Metal Ions from Aqueous Solution. J. Hazard. Mater. 2020, 384, 121288. [Google Scholar] [CrossRef] [PubMed]
- Mahanty, S.; Chatterjee, S.; Ghosh, S.; Tudu, P.; Gaine, T.; Bakshi, M.; Das, S.; Das, P.; Bhattacharyya, S.; Bandyopadhyay, S.; et al. Synergistic Approach towards the Sustainable Management of Heavy Metals in Wastewater Using Mycosynthesized Iron Oxide Nanoparticles: Biofabrication, Adsorptive Dynamics and Chemometric Modeling Study. J. Water Process Eng. 2020, 37, 101426. [Google Scholar] [CrossRef]
- Kulal, P.; Badalamoole, V. Magnetite Nanoparticle Embedded Pectin-Graft-Poly(N-Hydroxyethylacrylamide) Hydrogel: Evaluation as Adsorbent for Dyes and Heavy Metal Ions from Wastewater. Int. J. Biol. Macromol. 2020, 156, 1408–1417. [Google Scholar] [CrossRef]
- Shi, S.; Xu, C.; Wang, X.; Xie, Y.; Wang, Y.; Dong, Q.; Zhu, L.; Zhang, G.; Xu, D. Electrospinning Fabrication of Flexible Fe3O4 Fibers by Sol-Gel Method with High Saturation Magnetization for Heavy Metal Adsorption. Mater. Des. 2020, 186, 108298. [Google Scholar] [CrossRef]
- Almomani, F.; Bhosale, R.; Khraisheh, M.; Kumar, A.; Almomani, T. Heavy Metal Ions Removal from Industrial Wastewater Using Magnetic Nanoparticles (MNP). Appl. Surf. Sci. 2020, 506, 144924. [Google Scholar] [CrossRef]
- Hosain, A.N.A.; El Nemr, A.; El Sikaily, A.; Mahmoud, M.E.; Amira, M.F. Surface Modifications of Nanochitosan Coated Magnetic Nanoparticles and Their Applications in Pb (II), Cu (II) and Cd (II) Removal. J. Environ. Chem. Eng. 2020, 8, 104316. [Google Scholar] [CrossRef]
- Hou, L.; Liang, Q.; Wang, F. Mechanisms That Control the Adsorption-Desorption Behavior of Phosphate on Magnetite Nanoparticles: The Role of Particle Size and Surface Chemistry Characteristics. RSC Adv. 2020, 10, 2378–2388. [Google Scholar] [CrossRef]
- Karvelas, E.G.; Lampropoulos, N.K.; Karakasidis, T.E.; Sarris, I.E. A computational tool for the estimation of the optimum gradient magnetic field for the magnetic driving of the spherical particles in the process of cleaning water. Desalination Water Treat. 2017, 99, 27–33. [Google Scholar] [CrossRef]
- Peng, W.; Li, H.; Liu, Y.; Song, S. A Review on Heavy Metal Ions Adsorption from Water by Graphene Oxide and Its Composites. J. Mol. Liq. 2017, 496–504. [Google Scholar] [CrossRef]
- Gautam, P.K.; Shivalkar, S.; Banerjee, S. Synthesis of M. Oleifera Leaf Extract Capped Magnetic Nanoparticles for Effective Lead [Pb(II)] Removal from Solution: Kinetics, Isotherm and Reusability Study. J. Mol. Liq. 2020, 305, 112811. [Google Scholar] [CrossRef]
- Jadidi, M.; Etesami, N.; Nasr, E.M. Adsorption and Desorption Process of Chromium Ions Using Magnetic Iron Oxide Nanoparticles and Its Relevant Mechanism. Iran. J. Chem. Eng. 2017, 14, 31–40. [Google Scholar]
- Lata, S.; Singh, P.K.; Samadder, S.R. Regeneration of Adsorbents and Recovery of Heavy Metals: A Review. Int. J. Environ. Sci. Technol. 2015, 12, 1461–1478. [Google Scholar] [CrossRef]
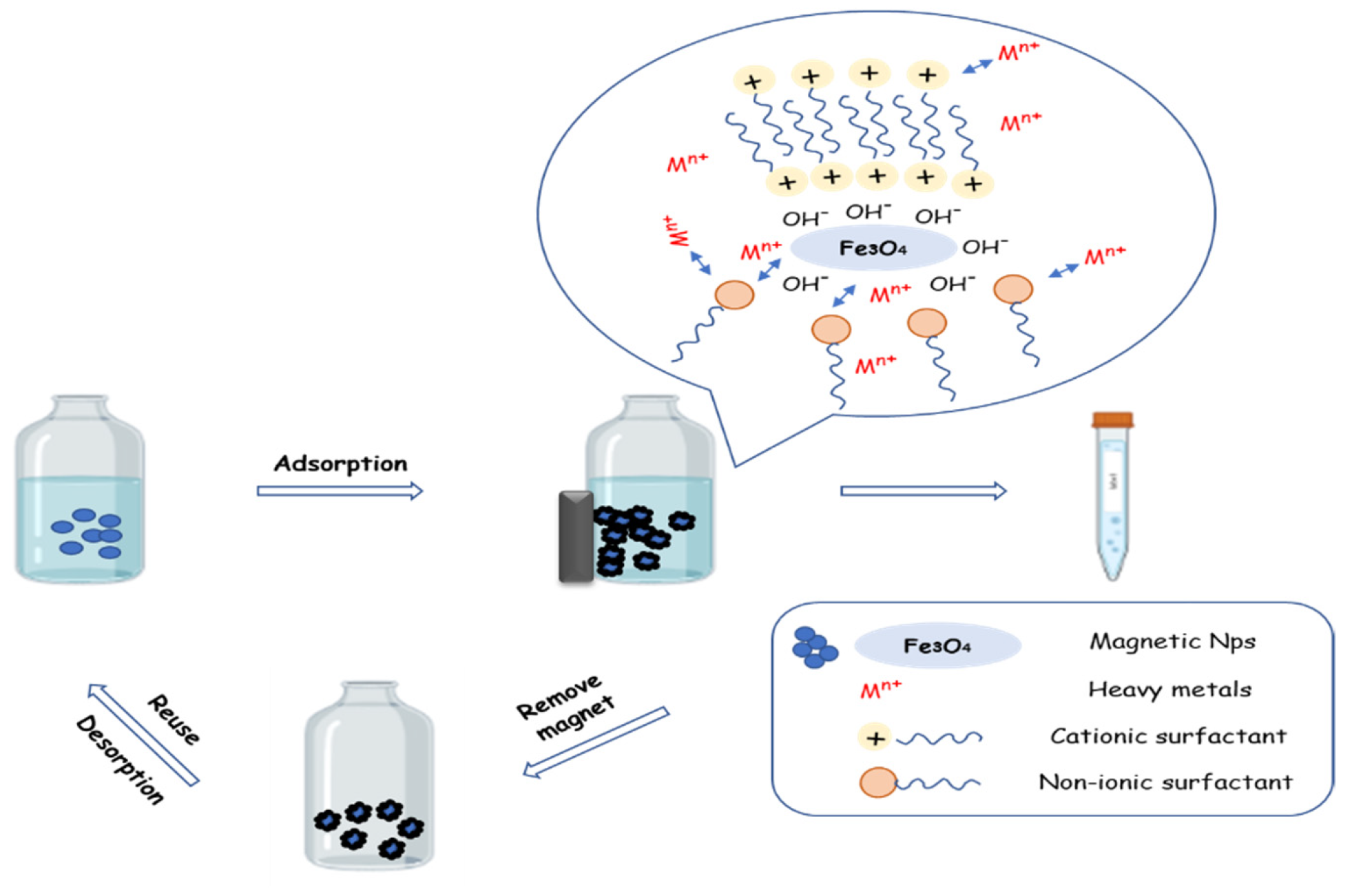
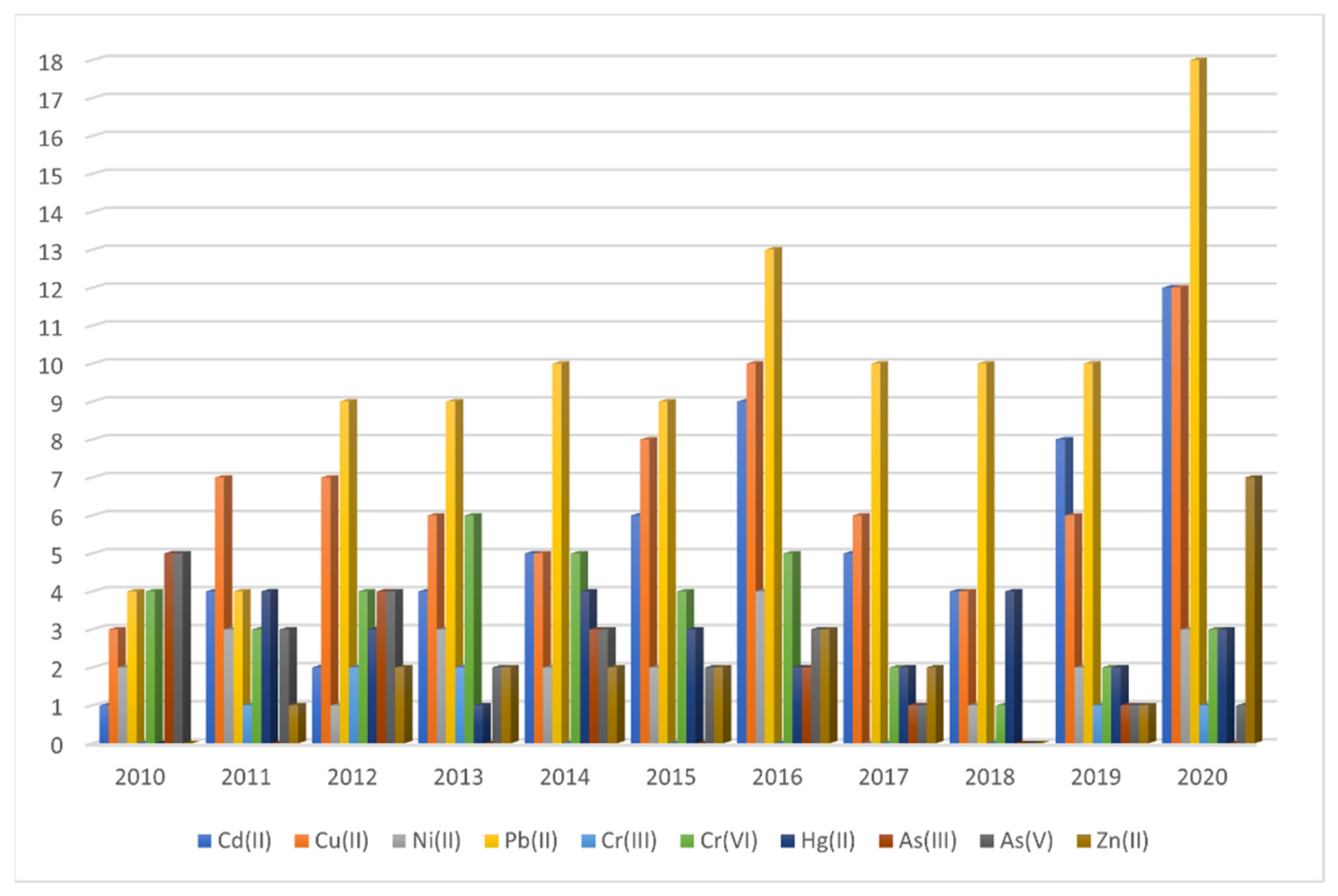
| Heavy Metals | MCL (mg/L) × 102 |
|---|---|
| Zinc (Zn) | 80 |
| Nickel (Ni) | 20 |
| Copper (Cu) | 25 |
| Chromium (Cr) | 5 |
| Arsenic (As) | 5 |
| Cadmium (Cd) | 1 |
| Lead (Pb) | 0.06 |
| Mercury (Hg) | 0.003 |
| Heavy Metal | Human Health Impacts | Common Sources |
|---|---|---|
| Arsenic | Skin damage, circulatory system issues, protein coagulation, nerve inflammation, muscle weakness, carcinogenicity | Naturally occurring, electronic production, agricultural applications, nonferrous smelters, metallurgy, coal-fired and geothermal electrical generation, tanning, pigments, antifouling paints, light filters, fireworks, veterinary medicine |
| Cadmium | Kidney damage, carcinogenicity, DNA damage, gastrointestinal irritation, hyperactivity, renal failure | Naturally occurring, various chemical industries, agricultural applications (phosphatic fertilizers), pigments, anticorrosive metal coatings, plastic stabilizers, alloys, coal combustion |
| Chromium | Allergic dermatitis, diarrhoea, nausea, vomiting, headache, neurotoxicity, kidney and liver damage | Naturally occurring, steel manufacturing metallurgy, refractory, chemical industries, plating, pigments, textile and leather tanning, passivation of corrosion of cooling circuits, wood treatment |
| Copper | Gastrointestinal issues, liver and kidney damage, anorexia, Wilson’s disease | Household plumbing systems, naturally occurring, chemical and pharmaceutical equipment, pigments, alloys |
| Lead | Kidney damage, reduced neural development, carcinogenicity, high blood pressure | Lead-based products (batteries), household plumbing systems, antiknock agents, pigments, glassware, ceramics, plastic, alloys, sheets, cable sheathings, solder |
| Mercury | Kidney damage, nervous system damage, carcinogenicity, gingivitis, stomatitis, gastrointestinal issues, abortions | Fossil fuel combustion, electronic industries, fluorescent light bulbs, electrical and measuring apparatus, catalysts, pharmaceuticals, dental fillings, scientific instruments, rectifiers, oscillators, solders |
| Nickel | Allergic dermatitis, nausea, chronic asthma, coughing, carcinogenicity, hair loss | Paper products, fertilizer plating, electroplating, batteries, arc welding, rods, pigments for paints and ceramics, surgical and dental prostheses, moulds for ceramic and glass containers, computer components, catalysts |
| Zinc | Depression, lethargy, neurological signs, increased thirst, hyperactivity, physical dysfunction | Mining, coal, waste combustion, steel processing, agricultural applications (phosphatic fertilizers), anticorrosion coating, batteries, cans, PVC stabilizers, medicines and chemicals, rubber industry, paints, soldering and welding fluxes |
| Molecular Formula | α-Fe2O3 | Fe3O4 | γ-Fe2O3 |
|---|---|---|---|
| Density (g/cm3) | 5.26 | 5.18 | 4.87 |
| Melting point (°C) Hardness | 1350 | 1583–1597 | - |
| 6.5 | 5.5 | 5 | |
| Type of magnetism | Weakly ferromagnetic, antiferromagnetic | Ferromagnetic | Ferromagnetic |
| Curie temperature (K) | - | 850 | 948 |
| Point of zero charge (pHpzc) | - | - | 7.5 |
| Morin temperature (K) | 263 | - | - |
| Neel temperature (K) | 948 < TN < 963 | - | - |
| a | - Rhombohedral | 0.8394 Cubic | 0.8346 Cubic |
| Method | Iron Oxide | Nanoparticle Size (nm) | pH | Temperature (°C) | Reference |
|---|---|---|---|---|---|
| Coprecipitation | Fe3O4 | 8.3 | 11 | 45 | [86] |
| Sonochemical coprecipitation (ultrasound assistance) | Fe3O4 | 13 | [74] | ||
| Sonochemical coprecipitation | Fe3O4 | 17 | [74] | ||
| Coprecipitation | Fe3O4 | 10–20 | 9 | 80 | [123] |
| Ultrasonic-assisted chemical coprecipitation | Fe3O4 | 15 | 7 | 60 | [124] |
| Coprecipitation | Fe3O4 | 13.2 | 11 | 85 | [86] |
| Sol–gel | Fe3O4 | 2.02 | 200 | [73] | |
| Sol–gel | Fe3O4 | 5.58 | 400 | [73] | |
| Sol–gel | Fe3O4 | 8.35 | 600 | [73] | |
| Fe3O4 | 23 | [125] | |||
| γ-Fe2O3 | 2 | 130 | [84] | ||
| γ-Fe2O3 | 4.5 | 180 | [84] | ||
| γ-Fe2O3 | 6.1 | 200 | [84] | ||
| γ-Fe2O3 | 9 | 230 | [84] | ||
| γ-Fe2O3 | 12 | 250 | [84] | ||
| γ-Fe2O3 | 25.5 | 250 | [125] | ||
| Solvothermal | γ-Fe2O3 | 3000 | 400 | [126] | |
| Mechanochemical milling | Fe2O3 | 10,000 | [127] | ||
| Mechanochemical milling | α-Fe2O3 | 17.1 | [128] | ||
| Ultrasonic spray pyrolysis | α-Fe2O3 | 18 | 400 | [118] | |
| Ultrasonic spray pyrolysis | α-Fe2O3 | 33 | 600 | [118] | |
| α-Fe2O3 | 53.7 | 500 | [125] |
| Year of Publish | Magnetic Nanoparticle | Heavy Metal Ion | Adsorption Capacity (mg/g) 1 | Removal Efficiency (%) | Time (min) | pH | Tempe-rature (°C) | Number of Cycles | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| 2010 | Amino-functionalized Fe3O4 | Cu(II) | 25.77 | 5 | 6 | 25 | 15 | [171] | |
| Fe3O4 | As(V) | 44.1 | 3 | 25 | [169] | ||||
| Fe3O4 | As(III) | 49.8 | 7 | 25 | [169] | ||||
| MnFe2O4 | As(V) | 90.4 | 3 | 25 | [169] | ||||
| MnFe2O4 | As(III) | 93.8 | 7 | 25 | [169] | ||||
| CoFe2O4 | As(V) | 73.8 | 3 | 25 | [169] | ||||
| CoFe2O4 | As(III) | 100.3 | 7 | 25 | [169] | ||||
| Amino-functionalized Fe3O4@SiO2 | Cu(II) | 0.69 mmol/g | 45 | 4 | [172] | ||||
| Amino-functionalized Fe3O4@SiO2 | Cu(II) | 0.60 mmol/g | 35 | 4 | [172] | ||||
| Amino-functionalized Fe3O4@SiO2 | Cu(II) | 0.47 mmol/g | 25 | 4 | [172] | ||||
| Amino-functionalized Fe3O4@SiO2 | Pb(II) | 0.54 mmol/g | 45 | 4 | [172] | ||||
| Amino-functionalized Fe3O4@SiO2 | Pb(II) | 0.45 mmol/g | 35 | 4 | [172] | ||||
| 2010 | Amino-functionalized Fe3O4@SiO2 | Pb(II) | 0.37 mmol/g | 25 | 4 | [172] | |||
| Amino-functionalized Fe3O4@SiO2 | Cd(II) | 0.33 mmol/g | 45 | 4 | [172] | ||||
| Amino-functionalized Fe3O4@SiO2 | Cd(II) | 0.27 mmol/g | 35 | 4 | [172] | ||||
| Amino-functionalized Fe3O4@SiO2 | Cd(II) | 0.20 mmol/g | 25 | 4 | [172] | ||||
| GA–APTES-NPs | Cu(II) | 61.07 | 75.3 | 15 | 4–5.3 | 20 ± 0.1 | 3 | [173] | |
| Fe3O4 | Pb(II) | 36 | 30 | 5.5 | 5 | [168] | |||
| EDA-MPs-10 | Cr(VI) | 61.35 | 60 | 2.5 | 35 | [107] | |||
| EDA-MPs-8 | Cr(VI) | 60.98 | 60 | 2.5 | 35 | [107] | |||
| EDA-MPs-6 | Cr(VI) | 49.5 | 60 | 2.5 | 35 | [107] | |||
| EDA-MPs-4 | Cr(VI) | 36.63 | 60 | 2.5 | 35 | [107] | |||
| EDA-MPs-2 | Cr(VI) | 32.15 | 60 | 2.5 | 35 | [107] | |||
| Fe3O4-γ-Fe2O3 | As(III) | 3.69 | 96 | 2 | [115] | ||||
| Fe3O4-γ-Fe2O3 | As(V) | 3.71 | 96 | 2 | [115] | ||||
| Fe3O4-γ-Fe2O3 | Cr(VI) | 2.4 | 99 | 2 | [115] | ||||
| Fe3O4 | Pb(II) | 63.33 | 6 | [174] | |||||
| Fe3O4 | Ni(II) | 52.55 | 6 | [174] | |||||
| Fe3O4@SiO2 | Pb(II) | 97.34 | 6 | 5 | [175] | ||||
| Nanoiron | Ni(II) | 11.53 | 5 | 25 | [176] | ||||
| EDA-NMPs | Cr(VI) | 136.98 | 30 | 2.5 | 35 | [177] | |||
| DEDA-NMPs | Cr(VI) | 149.25 | 30 | 2.5 | 35 | [177] | |||
| TETA-NMPs | Cr(VI) | 204.08 | 30 | 2.5 | 35 | [177] | |||
| TEPA-NMPs | Cr(VI) | 370.37 | 30 | 2 | 35 | [177] | |||
| Magnetite NPs | Cr(VI) | 82 | 20 | 2 | [106] | ||||
| Fe3O4-γFe2O3 | As(ΙΙΙ) | 4.75 | 91 | 180 | 6.5 | [178] | |||
| Fe3O4-γFe2O3 | As(V) | 4.85 | 92 | 180 | 6.5 | [178] | |||
| 2011 | γ-Fe2O3 | Hg(ΙΙ) | 140 | 8 | [157] | ||||
| Iron oxide-coated perlite (IOCP) | As(V) | 0.39 | 5 | 6.5–7 | [165] | ||||
| γ-Fe2O3 onto ball-milled expanded perlite carrier | As(V) | 4.64 | 7 | [179] | |||||
| NiFe2O4 | Cu(II) | 55.83 | [180] | ||||||
| NiFe2O4 | Cr(VI) | 36.95 | [180] | ||||||
| NiFe2O4 | Ni(II) | 37.02 | [180] | ||||||
| EDTAD-treated Fe3O4 | Pb(II) | 99.26 | 5.5 | 30 | [181] | ||||
| EDTAD-treated Fe3O4 | Cd(II) | 48.70 | 6 | 30 | [181] | ||||
| Fe3O4@SiO2-MIIP | Cu(II) | 24.2 | 5 | [182] | |||||
| Fe3O4@SiO2-NIP | Cu(II) | 5.2 | [182] | ||||||
| Fe3O4–TW | Ni(II) | 38.3 | 5 | [183] | |||||
| γ-Fe2O3@Fe3O4 | Cr(VI) | 74.07 | 30 | 35 | [184] | ||||
| γ-Fe2O3@Fe3O4 | Cr(VI) | 78.13 | 30 | 25 | [184] | ||||
| γ-Fe2O3@Fe3O4 | Cr(VI) | 83.33 | 30 | 15 | [184] | ||||
| Polyrhodanine-coated γ-Fe2O3 | Hg(II) | 179 | 5 | [157] | |||||
| Polypyrrole/F3O4 | Cr(VI) | 169.49 | 30–180 | 2 | 25 | 3 | [185] | ||
| Polypyrrole/F3O4 | Cr(VI) | 204.08 | 30–180 | 2 | 35 | [185] | |||
| 2011 | Polypyrrole/F3O4 | Cr(VI) | 238.09 | 30–180 | 2 | 45 | [185] | ||
| Iron oxide-modified sewage sludge | Pb(II) | 42.4 | 6 | 25 ± 0.1 | [186] | ||||
| Iron oxide-modified sewage sludge | Cu(II) | 17.3 | 6 | 25 ± 0.1 | [186] | ||||
| Iron oxide-modified sewage sludge | Cd(II) | 14.7 | 7 | 25 ± 0.1 | [186] | ||||
| Iron oxide-modified sewage sludge | Ni(II) | 7.8 | 7 | 25 ± 0.1 | [186] | ||||
| SH-mSi@Fe3O4 | Hg(II) | 260 | 6.5 | 25 | 6 | [187] | |||
| SH-mSi@Fe3O4 | Pb(II) | 91.5 | 6.5 | 25 | 6 | [187] | |||
| Fe3O4@SiO2 | Hg(II) | 98 | [188] | ||||||
| MWCNT/IO/CD | Cu(II) | 59 | 5.5 | 25.15 | [189] | ||||
| CS-co-MMB-co-PAA hydrogel | Pb(II) | 163.90 | 5.5 | 25 | [190] | ||||
| CS-co-MMB-co-PAA hydrogel | Cd(II) | 135.51 | 5.5 | 25 | [190] | ||||
| CS-co-MMB-co-PAA hydrogel | Cu(II) | 152.42 | 5.5 | 25 | [190] | ||||
| MWCNT/nano-iron oxide | Cr(III) | 82 | 5 | [191] | |||||
| MWCNT/nano-iron oxide | Cr(III) | 88 | 6 | [191] | |||||
| Nano-Fe3O4 | Cu(II) | 8.90 | 5 | 25 | [192] | ||||
| PEI-grafted magnetic porous | Cu(II) | 157.8 | 10 | 6–7.5 | 4 | [193] | |||
| PEI-grafted magnetic porous | Zn(II) | 138.8 | 10 | 6–7.5 | 4 | [193] | |||
| PEI-grafted magnetic porous | Cd(II) | 105.2 | 10 | 6–7.5 | 4 | [193] | |||
| Fe3O4-coated boron nitride nanotubes | As(V) | 0.96 | 720 | 9 | 25 | 4 | [194] | ||
| 2012 | Fe3O4-C | Pb(II) | 126 | 6 | [195] | ||||
| Pectin-coated iron oxide | Cu(II) | 48.9 | [196] | ||||||
| Fe3O4@ZrO2 | Cr(III) | 24.5 | 8–9 | [131] | |||||
| MNPs–Ca-alginate immobilized P. chrysosporium | Pb(II) | 176.33 | 35 | 5 | [197] | ||||
| AF-Fe3O4 | Cu(II) | 523.6 | 120 | 7 | [198] | ||||
| AF-Fe3O4 | Cd(II) | 446.4 | 120 | 7 | [198] | ||||
| AF-Fe3O4 | Pb(II) | 369.0 | 120 | 7 | [198] | ||||
| Fe3O4@APS@AA-co-CA | Cd(II) | 29.6 | 45 | 5.5 | 25 | 4 | [199] | ||
| Fe3O4@APS@AA-co-CA | Zn(II) | 43.4 | 45 | 5.5 | 25 | 4 | [199] | ||
| Fe3O4@APS@AA-co-CA | Pb(II) | 166.1 | 45 | 5.5 | 25 | 4 | [199] | ||
| Fe3O4@APS@AA-co-CA | Cu(II) | 126.9 | 45 | 5.5 | 25 | 4 | [199] | ||
| Fe3O4–SiO2-poly | As(III) | 84±5 | 120 | 6 | 30 | [104] | |||
| Fe3O4–SiO2-poly | Cu(II) | 65±3 | 120 | 6 | 30 | [104] | |||
| Fe3O4–SiO2-poly | Cr(III) | 77±3 | 120 | 5.3 | 30 | [104] | |||
| Acid-coated Fe3O4 | As(V) | 16.56 | [200] | ||||||
| Acid-coated Fe3O4 | As(III) | 46.06 | [200] | ||||||
| γ-Fe2O3 functionalized with citrate ions | Ni(II) | 0.57 mmol/g | 15 | 6 ± 0.5 | [201] | ||||
| Fe3O4@CTAB | As(V) | 23.07 | 2 | 6 | 5 | [202] | |||
| Fe3O4-γ-Fe2O3 | Cr(VI) | 6 | 4 | 10 | [202] | ||||
| 2012 | Fe3O4-γ-Fe2O3 | Cr(VI) | 6.9 | 4 | 22 | [202] | |||
| Fe3O4-γ-Fe2O3 | Cr(VI) | 7 | 4 | 50 | [202] | ||||
| Fe3O4–PEI800–MMT | Cr(VI) | 8.77 | [203] | ||||||
| Fe3O4–PEI25000–MMT | Cr(VI) | 7.69 | [203] | ||||||
| MPTS-CNTs/Fe3O4 | Hg(II) | 65.52 | 6.5 | 25 ± 0.2 | [204] | ||||
| MPTS-CNTs/Fe3O4 | Pb(II) | 65.40 | 6.5 | 25 ± 0.2 | [204] | ||||
| rGO–Fe(0)–Fe3O4 | As(III) | 44 | 60 | 7 | 25 | [205] | |||
| rGO–Fe3O4 | As(III) | 21 | 60 | 7 | 25 | [205] | |||
| Nanomagnetite (NMT) | Cu(II) | 14.3 | 70 | 45 | [206] | ||||
| Nanomagnetite (NMT) | As(V) | 6.5 | 120 | 45 | [206] | ||||
| Water-soluble Fe3O4 nanoparticles | Pb(II) | 96.8 | 60 | 7 | 18 | [207] | |||
| Water-soluble Fe3O4 nanoparticles | Cr(VI) | 41.5 | 90 | 7 | 18 | [207] | |||
| TF-SCMNPs | Hg(II) | 207 | 93.76 | 15 | 6 | 22.5 | [208] | ||
| M-MIONPs | Hg(II) | 98.6 | 4 | 9 | 25 | [209] | |||
| Fe3O4-RGO–MnO2 | As(III) | 14.04 | 7 | 25 | [210] | ||||
| Fe3O4-RGO–MnO2 | As(V) | 12.22 | 7 | 25 | [210] | ||||
| MWCNTs/Fe3O4 | Pb(II) | 41.77 | 5.3 | 5 | [211] | ||||
| MWCNTs/Fe3O4-NH2 | Pb(II) | 75.02 | 5.3 | 5 | [211] | ||||
| γ-Fe2O3 | Cu(II) | 71.42 | 6 ± 0.1 | 25 ± 1 | [212] | ||||
| γ-Fe2O3 | Zn(II) | 111.11 | 6 ± 0.1 | 25 ± 1 | [212] | ||||
| γ-Fe2O3 | Pb(II) | 84.95 | 6 ± 0.1 | 25 ± 1 | [212] | ||||
| Fe3O4/CS/PAA | Cu(II) | 193 | [213] | ||||||
| 2013 | Fe3O4/SiO2 | Pb(II) | 14.65 | 4 | 27 | [214] | |||
| Fe3O4/SiO2 | Pb(II) | 16.83 | 4 | 50 | [214] | ||||
| Fe3O4/SiO2 | Pb(II) | 17.65 | 4 | 70 | [214] | ||||
| Fe2O3–Al2O3 | Cu(II) | 4.98 | 60 | 6 | 4 | [215] | |||
| Fe2O3–Al2O3 | Pb(II) | 23.75 | 60 | 6 | 4 | [215] | |||
| Fe2O3–Al2O3 | Ni(II) | 32.36 | 60 | 6 | 4 | [215] | |||
| Fe2O3–Al2O3 | Hg(II) | 63.69 | 60 | 6 | 4 | [215] | |||
| Mixed magnetite– hematite | Pb(II) | 617.3 | 60 | 7 | 25 | [216] | |||
| Mixed magnetite– hematite | Cr(III) | 277.0 | 120 | 7 | 25 | [216] | |||
| Mixed magnetite– hematite | Cd(II) | 223.7 | 1440 | 7 | 25 | [216] | |||
| Chitosan-coated MnFe2O4 (CCMNPs) | Cu(II) | 22.6 | 6 | 5 | [217] | ||||
| Chitosan-coated MnFe2O4 (CCMNPs) | Cr(VI) | 15.4 | 6 | 5 | [217] | ||||
| γ-PGA/Fe3O4 MNPs | Cr(III) | 162.6 | 99.66 | 120 | 6 | 30 | [218] | ||
| CDpoly-MNPs | Pb(II) | 64.5 | 45 | 5.5–6 | 25 | 4 | [219] | ||
| CDpoly-MNPs | Cd(II) | 27.7 | 45 | 5.5–6 | 25 | [219] | |||
| CDpoly-MNPs | Ni(II) | 13.2 | 45 | 5.5–6 | 25 | [219] | |||
| 3D flowerlike a-Fe2O3 | As(V) | 41.46 | 120 | [220] | |||||
| 3D flowerlike a-Fe2O3 | Cr(VI) | 33.82 | 120 | [220] | |||||
| Magnetite nanorods | Pb(II) | 112.86 | 60 | 5–6 | 25 | [221] | |||
| Magnetite nanorods | Zn(II) | 107.27 | 60 | 5–6 | 25 | [221] | |||
| 2013 | Magnetite nanorods | Ni(II) | 95.42 | 60 | 5–6 | 25 | [221] | ||
| Magnetite nanorods | Cd(II) | 88.39 | 60 | 5–6 | 25 | [221] | |||
| Magnetite nanorods | Cu(II) | 76.10 | 60 | 5–6 | 25 | [221] | |||
| Maghemite nanotubes | Pb(II) | 71.42 | [221] | ||||||
| Maghemite nanotubes | Zn(II) | 86.95 | [221] | ||||||
| Maghemite nanotubes | Cu(II) | 111.11 | [221] | ||||||
| EDTA-modified chitosan/SiO2/Fe3O4 | Cu(II) | 0.495 mmol/g | 720 | 5 | 25 | 12 | [222] | ||
| EDTA-modified chitosan/SiO2/Fe3O4 | Pb(II) | 0.045 mmol/g | 720 | 5 | 25 | 12 | [222] | ||
| EDTA-modified chitosan/SiO2/Fe3O4 | Cd(II) | 0.040 mmol/g | 720 | 5 | 25 | 12 | [222] | ||
| Fe3O4@mesoporous SiO2 core-shell | Pb(II) | 128.21 | 6 | [223] | |||||
| Fe3O4@mesoporous SiO2 core-shell | Cu(II) | 51.81 | 6 | [223] | |||||
| Fe3O4/GO | Cr(VI) | 32.33 | 4.5 | 20 | [224] | ||||
| Hollow nestlike α-Fe2O3 spheres | As(V) | 75.3 | 88 | 120 | [225] | ||||
| Hollow nestlike α-Fe2O3 spheres | Cr(VI) | 58.6 | 67 | 120 | [225] | ||||
| α-Fe2O3 nanofibers | Cr(VI) | 16.17 | 25 | 4 | [226] | ||||
| Fe3O4@SiO2–NH2 | Pb(II) | 243.9 | 180 | 5.2 | 25 | 5 | [227] | ||
| Cyanex-301-coated SPION | Cr(VI) | 30.8 | 2 | 23 | [228] | ||||
| 2014 | S-doped Fe3O4@C | Cu(II) | 54.7 | 5 | 35 | 4 | [229] | ||
| Fe3O4@SiO2-QTPA | Cd(II) | 95.29 | 720 | 25 | [230] | ||||
| Fe3O4@SiO2-QTPA | Zn(II) | 92.37 | 720 | 25 | [230] | ||||
| Fe3O4@SiO2-QTPA | Cu(II) | 91.06 | 720 | 25 | [230] | ||||
| Glycine-functionalized maghemite nanoparticles | Cu(II) | 625 | 6.5 | 25 | [231] | ||||
| Maghemite (γ-Fe2O3) | Pb(II) | 10.55 | 7.5 | 45 | [232] | ||||
| Maghemite (γ-Fe2O3) | Zn(II) | 4.79 | 7.5 | 45 | [232] | ||||
| Maghemite (γ-Fe2O3) | Cd(II) | 1.75 | 7.5 | 45 | [232] | ||||
| α-Fe2O3 | Cr(VI) | 17 | 300 | [233] | |||||
| 3-MPA-coated SPION | Cr(VI) | 45 | 1 | 25 | [234] | ||||
| EDA-Fe3O4 NPs | Cr(VI) | 98 | 120 | 2 | 6 | [235] | |||
| M-FeHT | As(V) | 1.2813 | 15 | 9 | 25 | [236] | |||
| M-FeHT | As(III) | 0.1213 | 15 | 9 | 25 | [236] | |||
| Fe3O4-GS | Cr(VI) | 17.29 | 240 | 1–3.5 | 5 | [237] | |||
| Fe3O4-GS | Pb(II) | 27.95 | 120 | 6–7 | 5 | [237] | |||
| Fe3O4-GS | Hg(II) | 23.03 | 120 | 6–7 | [237] | ||||
| Fe3O4-GS | Cd(II) | 27.83 | 120 | 6–7 | [237] | ||||
| Fe3O4-GS | Ni(II) | 22.07 | 120 | 6–7 | [237] | ||||
| Fe3O4@CPS | Cu(II) | 53.6 | 5 | 25 | 3 | [238] | |||
| Fe3O4@CPS | Cd(II) | 87.1 | 6 | 25 | 3 | [238] | |||
| Fe3O4@CPS | Pb(II) | 25.2 | 6 | 25 | 3 | [238] | |||
| MWCNT CoFe2O4–NH2 | Pb(II) | 140.1 | 6 | 5 | [239] | ||||
| Fe3O4/talc | Cu(II) | 72.15 | [240] | ||||||
| Fe3O4/talc | Ni(II) | 50.23 | [240] | ||||||
| 2014 | Fe3O4/talc | Pb(II) | 91.35 | [240] | |||||
| Magnetite nanoparticles | Cr(VI) | 121.9 | 60 | 5.5 | [241] | ||||
| CoFe2O4-rGO | Pb(II) | 299.4 | 80 | 5.3 | 25 | [242] | |||
| CoFe2O4-rGO | Pb(II) | 274.7 | 35 | [242] | |||||
| CoFe2O4-rGO | Pb(II) | 253.2 | 45 | [242] | |||||
| CoFe2O4-rGO | Hg(II) | 157.9 | 60 | 4.6 | 25 | [242] | |||
| CoFe2O4-rGO | Hg(II) | 105.1 | 35 | [242] | |||||
| CoFe2O4-rGO | Hg(II) | 90.49 | 45 | [242] | |||||
| Graphene Oxide−MnFe2O4 | Pb(II) | 673 | 5 | [243] | |||||
| MnFe2O4 | Pb(II) | 488 | 5 | 60 | [243] | ||||
| Graphene Oxide−MnFe2O4 | As(III) | 146 | 6.5 | 60 | [243] | ||||
| MnFe2O4 | As(III) | 97 | 6.5 | 60 | [243] | ||||
| Graphene Oxide−MnFe2O4 | As(V) | 207 | 4 | 60 | [243] | ||||
| MnFe2O4 | As(V) | 136 | 4 | 60 | [243] | ||||
| Water-soluble Fe3O4 | Hg(II) | >99 | 10 | 7 | 25 | 3 | [244] | ||
| Fe3O4@C | Pb(II) | 90.7 | 96.3 | 10 | [245] | ||||
| Fe3O4@C | Hg(II) | 83.1 | 98.1 | 10 | [245] | ||||
| Fe3O4@C | Cd(II) | 39.7 | 93.8 | 10 | [245] | ||||
| Fe3O4@silica xanthan gum | Pb(II) | 21.32 | 6 | 20 | 22 | [246] | |||
| 2015 | Fe3O4-NTA | Cu(II) | 40.24 | 35 | 5 | [160] | |||
| Magnetic chitosan/cellulose hybrid microspheres by embedding γ-Fe2O3 | Cu(II) | 88.21 | 30 | [247] | |||||
| Magnetic chitosan/cellulose hybrid microspheres by embedding γ-Fe2O3 | Cd(II) | 61.1 | 30 | [247] | |||||
| Magnetic chitosan/cellulose hybrid microspheres by embedding γ-Fe2O3 | Pb(II) | 45.86 | 30 | [247] | |||||
| Mesoporous CoFe2O4 | Pb(II) | 32.11 | 480 | 5 | [248] | ||||
| MAMNPs | Cd(II) | 91.5 | 100 | 6 | 3 | [249] | |||
| MAMNPs | Hg(II) | 237.6 | 100 | 6 | 3 | [249] | |||
| MAMNPs | Pb(II) | 118.5 | 100 | 6 | 3 | [249] | |||
| Fe3O4/MMT NC | Pb(II) | 263.15 | 2 | [170] | |||||
| Fe3O4/MMT NC | Cu(II) | 70.92 | 2 | [170] | |||||
| Fe3O4/MMT NC | Ni(II) | 65.78 | 2 | [170] | |||||
| Fe3O4@SiO2 core-shell nanoparticles | Cd(II) | 179 | [250] | ||||||
| Fe3O4@SiO2 core-shell nanoparticles | Pb(II) | 156 | [250] | ||||||
| Fe3O4 | Ni(II) | 362.318 | 8 | 5 | [251] | ||||
| Magnetic PAAAM/ PAMPS | Zn(II) | 289.12 | 25 | [252] | |||||
| Magnetic PAAAM/ PAMPS | Cd(II) | 385.2 | 25 | [252] | |||||
| Hollow magnetite nanospheres | Cr(VI) | 6.64 | 4 | 25 | [253] | ||||
| Hollow magnetite nanospheres | Cr(VI) | 7.31 | 4 | 35 | [253] | ||||
| Hollow magnetite nanospheres | Cr(VI) | 8.90 | 4 | 45 | [253] | ||||
| Hollow magnetite nanospheres | Pb(II) | 13.40 | 5 | 25 | [253] | ||||
| 2015 | Hollow magnetite nanospheres | Pb(II) | 14.11 | 5 | 35 | [253] | |||
| Hollow magnetite nanospheres | Pb(II) | 18.47 | 5 | 45 | [253] | ||||
| SPION | As(V) | 0.91 mmol/g | 60 | 3.8 | [254] | ||||
| SPION surface-coated with 3-mercaptopropionic acid (3-MPA) | As(V) | 1.92 mmol/g | 60 | 3.6 | [254] | ||||
| Fe3O4@SiO2 | Cd(II) | 24.8 | 180 | 7 | 25 | 5 | [255] | ||
| PPY/γ-Fe2O3 | Cr(VI) | 209 | 15 | 2 | 4 | [256] | |||
| PANI/γ-Fe2O3 | Cr(VI) | 196 | 35 | 2 | 4 | [256] | |||
| PPY/γ-Fe2O3 | Cu(II) | 171 | 15 | 5.5 | 4 | [256] | |||
| PANI/γ-Fe2O3 | Cu(II) | 107 | 35 | 5.5 | 4 | [256] | |||
| EDTA-Fe3O4 | Pb(II) | 508.4 | 40 | 4.2 | 45 | [257] | |||
| EDTA-Fe3O4 | Hg(II) | 268.4 | 50 | 4.1 | 45 | [257] | |||
| EDTA-Fe3O4 | Cu(II) | 301.2 | 90 | 5.1 | 45 | [257] | |||
| EDTA-Fe3O4 | Pb(II) | 548.1 | 35 | [257] | |||||
| EDTA-Fe3O4 | Hg(II) | 242.2 | 35 | [257] | |||||
| EDTA-Fe3O4 | Cu(II) | 289.4 | 35 | [257] | |||||
| EDTA-Fe3O4 | Pb(II) | 481.2 | 25 | 5 | [257] | ||||
| EDTA-Fe3O4 | Hg(II) | 203.1 | 25 | 5 | [257] | ||||
| EDTA-Fe3O4 | Cu(II) | 246.1 | 25 | 5 | [257] | ||||
| Fe3O4@SiO2/Schiff | Cu(II) | 97.2 | 60 | 5 | [258] | ||||
| Fe3O4@SiO2/Schiff | Zn(II) | 81.6 | 60 | 5 | [258] | ||||
| PMMA-gft-Alg/Fe3O4 | Pb(II) | 62.5 | 5 | 50 | [259] | ||||
| PMMA-gft-Alg/Fe3O4 | Cu(II) | 35.71 | 5 | 50 | [259] | ||||
| SH-HMSMCS | Hg(II) | 95 | 6.2 | 5 | [260] | ||||
| Ppy–Fe3O4/rGO | Cr(VI) | 293.3 | 3 | 45 | [261] | ||||
| Ppy–Fe3O4/rGO | Cr(VI) | 226.8 | 3 | 30 | [261] | ||||
| Ppy–Fe3O4/rGO | Cr(VI) | 180.8 | 3 | 20 | [261] | ||||
| Co0.6Fe2.4O4 | Pb(II) | 80.32 | 30 | 45.18 | [262] | ||||
| Co0.6Fe2.4O4 | Pb(II) | 70.22 | 35.15 | [262] | |||||
| Co0.6Fe2.4O4 | Pb(II) | 44.58 | 25.15 | [262] | |||||
| Fe3O4/Mg–Al–CO3 | Cd(II) | 54.7 | 50 | [263] | |||||
| Fe3O4/Mg–Al–CO3 | Cd(II) | 50.5 | 40 | [263] | |||||
| Fe3O4/Mg–Al–CO3 | Cd(II) | 45.6 | 30 | [263] | |||||
| 2016 | NMag–CS | Cu(II) | 123.4 | 120 | 5.5 | [264] | |||
| NMag–CS | Pb(II) | 114.9 | 120 | 5.5 | [264] | ||||
| NMag–CS | Cr(VI) | 116.2 | 120 | 5.5 | [264] | ||||
| NMag–CS | Cd(II) | 112.3 | 120 | 5.5 | [264] | ||||
| NMag–CS | Ni(II) | 109.8 | 120 | 5.5 | [264] | ||||
| Amino-functionalized Fe3O4@SiO2 | Zn(II) | 169.5 | 120 | 5 ± 0.1 | 25 | [265] | |||
| Fe3O4 | Cu(II) | 37.04 | 4–6 | [266] | |||||
| Fe3O4 | Pb(II) | 166.67 | 4–6 | [266] | |||||
| Fe2O3 | Cu(II) | 19.61 | 5–6 | 4 | [266] | ||||
| Fe2O3 | Pb(II) | 47.62 | 3–4 | 4 | [266] | ||||
| Fe2O3@SiO2 | As(III) | 77.7 | 180 | 7 | 30 | [267] | |||
| 2016 | Fe2O3@TiO2 | As(V) | 99.5 | 180 | 6 | 30 | [267] | ||
| Fe3O4@SiO2-SH | Hg(II) | 132 | 6 | 25 | 5 | [268] | |||
| Fe3O4-Aspa | Ni(II) | 87.183 | 70 | 6 | 40 | [269] | |||
| Fe3O4-Aspa | Ni(II) | 58.582 | 70 | 6 | 35 | [269] | |||
| Fe3O4-Aspa | Ni(II) | 34.602 | 70 | 6 | 30 | 6 | [269] | ||
| Henna-Fe3O4 | Cu(II) | 28.90 | 20 | 5.2 | [270] | ||||
| Fe3O4/Bent-2.0 | Pb(II) | 81.5 | 30 | [271] | |||||
| Fe3O4/Bent-2.0 | Cd(II) | 21.7 | 30 | [271] | |||||
| Fe3O4/Bent-2.0 | Cu(II) | 19.6 | 30 | [271] | |||||
| Fe3O4 | Pb(II) | 100 | 30 | 5 | 25 | [272] | |||
| Fe3O4 | Cr(VI) | 34.9 | 2 | 45 | [273] | ||||
| Fe3O4 | Pb(II) | 53.1 | 5 | 45 | [273] | ||||
| Fe3O4 | Cr(VI) | 26.8 | 2 | 35 | [273] | ||||
| Fe3O4 | Pb(II) | 52.8 | 5 | 35 | [273] | ||||
| Fe3O4 | Cr(VI) | 20.2 | 2 | 25 | 2 | [273] | |||
| Fe3O4 | Pb(II) | 52.9 | 5 | 25 | 2 | [273] | |||
| a-Fe2O3 | Cd(II) | 127.23 | 6 | 20 | [274] | ||||
| a-Fe2O3 | Cd(II) | 146.41 | 6 | 30 | [274] | ||||
| a-Fe2O3 | Cd(II) | 158.48 | 6 | 40 | [274] | ||||
| GO/Fe3O4 | Pb(II) | 65.96 | 180 | 3 | 20 | [275] | |||
| GO/Fe3O4/LA | Pb(II) | 53.06 | 180 | 3 | 20 | [275] | |||
| GO/Fe3O4/LA/EDTA | Pb(II) | 161.80 | 180 | 3 | 20 | [275] | |||
| Amine-functionalized Fe3O4 | Cu(II) | 85 | 30 | 7 | [276] | ||||
| Fe3O4@SiO2@TiO2 | Cu(II) | 125 | 30 | [277] | |||||
| Fe3O4@SiO2@TiO2 | Zn(II) | 137 | 30 | [277] | |||||
| Fe3O4@SiO2@TiO2 | Cd(II) | 148 | 30 | [277] | |||||
| Fe3O4@SiO2@TiO2 | Pb(II) | 160 | 30 | [277] | |||||
| Rice straw/Fe3O4 NCs | Pb(II) | 91.18 | [278] | ||||||
| Rice straw/Fe3O4 NCs | Cu(II) | 75.54 | [278] | ||||||
| Cys-Fe3O4 | Pb(II) | 183.5 | 120 | 6 | 5 | [279] | |||
| Cys-Fe3O4 | Cd(II) | 64.35 | 120 | 6 | 5 | [279] | |||
| Nano-Fe3O4@Nano-SiO2 | Pb(II) | 1100 μmol/g | [280] | ||||||
| Nano-Fe3O4@Nano-SiO2 | Cu(II) | 300 μmol/g | [280] | ||||||
| Nano-Fe3O4@Nano-SiO2 | Cd(II) | 150 μmol/g | [280] | ||||||
| Nano-Fe3O4@Nano-SiO2 | Hg(II) | 100 μmol/g | [280] | ||||||
| a-Fe2O3 | As(V) | 38.48 | 20 | [281] | |||||
| Fe3O4-MnO2 | Pb(II) | 208.17 | 25 | [282] | |||||
| Fe3O4-MnO2 | Cu(II) | 111.90 | 25 | [282] | |||||
| Fe3O4-MnO2 | Cd(II) | 169.90 | 25 | [282] | |||||
| Fe3O4-MnO2 | Zn(II) | 100.24 | 25 | [282] | |||||
| Fe3O4-MnO2 | Ni(II) | 55.63 | 25 | [282] | |||||
| Fe3O4@SiO2-EDTA | Cu(II) | 37.59 | 10 | 5.3 | 30 | [283] | |||
| Fe3O4@SiO2-EDTA | Pb(II) | 114.94 | 10 | 5.3 | 30 | [283] | |||
| Fe3O4@SiO2-EDTA | Ni(II) | 32.15 | 10 | 5.3 | 30 | [283] | |||
| Fe3O4@SiO2-EDTA | Cd(II) | 50.25 | 10 | 5.3 | 30 | [283] | |||
| Fe3O4–FeB | Cr(VI) | 38.9 | 6.3 | [284] | |||||
| Fe3O4–TiO2 | As(V) | 11.434 μg/g | 40 | 6.3 | 25 | [285] | |||
| Fe3O4–TiO2 | As(III) | 93 | 11 | 25 | [285] | ||||
| Chitosan MWCNT/Fe3O4 | Cr(VI) | 360.1 | 30 | 2 | 45 | [286] | |||
| 2016 | Chitosan MWCNT/Fe3O4 | Cr(VI) | 348.2 | 35 | [286] | ||||
| Chitosan MWCNT/Fe3O4 | Cr(VI) | 335.6 | 25 | [286] | |||||
| AMGO | Cr(VI) | 123.4 | 2 | 5 | [287] | ||||
| 2017 | Fe3O4@MnO2 | Pb(II) | 666.67 | 120 | 25 | [148] | |||
| Fe3O4-SO3H | Pb(II) | 108.93 | 7 | 25 | [288] | ||||
| Fe3O4-SO3H | Cd(II) | 80.9 | 7 | 25 | [288] | ||||
| PGMA-MAn Copolymer@Fe3O4 | Pb(II) | 53.33 | 20 | 5 | 25 | [289] | |||
| PGMA-MAn Copolymer@Fe3O4 | Cu(II) | 48.53 | 5 | [289] | |||||
| Fe3O4/CTAB | Cr(VI) | 95.77 | 720 | 4 | 25 ± 0.1 | [290] | |||
| Fe3O4@Cu3(btc)2 | Pb(II) | 215.05 | 4 | [291] | |||||
| Fe3O4@Cu3(btc)2 | Hg(II) | 348.43 | 4 | [291] | |||||
| Fe3O4-CS-L | Zn(II) | 256.41 | 45 | 6 | 25 | 5 | [292] | ||
| Fe3O4-CS-L | Cd(II) | 156.99 | 45 | 6 | 25 | 5 | [292] | ||
| Fe3O4-CS-L | Pb(II) | 128.63 | 45 | 6 | 25 | 5 | [292] | ||
| Fe3O4-mSiO2 | Cu(II) | 84.4 | [293] | ||||||
| Fe3O4-mSiO2 | Cd(II) | 80.5 | [293] | ||||||
| Fe3O4-mSiO2 | Zn (II) | 72.6 | [293] | ||||||
| γ-Fe2O3@CS | Cd(II) | 15.2 | 60 | 5 | 20 ± 0.1 | 5 | [294] | ||
| L-Cyst-Fe3O4 | Pb(II) | 18.8 | 2 | 45 | 5 | [295] | |||
| L-Cyst-Fe3O4 | Cr(VI) | 34.5 | 6 | 45 | 5 | [295] | |||
| MDA-Fe3O4 | Pb(II) | 333.3 | 5 | 30 | [296] | ||||
| ZrO2-Fe3O4 | As(III) | 113.48 | 7 | 5 | [297] | ||||
| Fe3O4@SiO2@CS-TETA-GO | Cu(II) | 324.7 | 16 | 6 | 30 | 6 | [298] | ||
| CoFe2O4@SiO2–SH | Hg(II) | 641.0 | 8 | 25 | 5 | [299] | |||
| CoFe2O4@SiO2–SH | Hg(II) | 628.9 | 8 | 35 | [299] | ||||
| CoFe2O4@SiO2–SH | Hg(II) | 591.7 | 8 | 45 | [299] | ||||
| EDTA-functionalized CoFe2O4 (EDTA-MNP) | Cu(II) | 73.26 | 6 | 50 | [300] | ||||
| IMSA | Pb(II) | 133.73 | [301] | ||||||
| IMSA | As(V) | 21.61 | [301] | ||||||
| CoFe2O4@SiO2 | Cd(II) | 199.9 | 7 | 5 | [302] | ||||
| CoFe2O4@SiO2 | Cu(II) | 177.8 | 7 | 5 | [302] | ||||
| CoFe2O4@SiO2 | Pb(II) | 181.6 | 30 | 7 | 5 | [302] | |||
| TEPA chitosan/CoFe2O4 | Cu(II) | 168.067 | 50 | 5 | 30 | [105] | |||
| TEPA chitosan/CoFe2O4 | Pb(II) | 228.311 | 50 | 5 | 30 | [105] | |||
| 2018 | Lantana camara capped iron nanoparticles | Ni(II) | 227.2 | 6 | 60 | [303] | |||
| NA-FeOx | Cd(II) | 11.3 | 60 | [304] | |||||
| NA-FeOx | Cu(II) | 12.3 | 60 | [304] | |||||
| Graphene oxide-Fe3O4 | Pb(II) | 373.14 | 10 | 6 | [305] | ||||
| PAE-AAm-g-MNPs | Cu(II) | 30.34 | 6 | 7 | [306] | ||||
| Fe3O4/LDH-AM | Cu(II) | 64.66 | 240 | 5 | [307] | ||||
| Fe3O4/LDH-AM | Cd(II) | 74.06 | 240 | 5 | [307] | ||||
| Fe3O4/LDH-AM | Pb(II) | 266.6 | 180 | 5 | [307] | ||||
| TEPA-GO/MnFe2O4 | Pb(II) | 263.2 | 5.5 | 30 | 4 | [308] | |||
| GO/MnFe2O4 | Pb(II) | 133.3 | [308] | ||||||
| MnFe2O4–BC | Cd(II) | 181.49 | 7 | 25 | 5 | [309] | |||
| 2018 | CoFe2O4@SiO2 | Hg(II) | 149.3 | 7 | 25 | 5 | [310] | ||
| CoFe2O4@SiO2 | Hg(II) | 144.9 | 7 | 35 | [310] | ||||
| CoFe2O4@SiO2 | Hg(II) | 131.6 | 7 | 45 | [310] | ||||
| p-BNMR@Fe3O4 | Pb(II) | 249.5 | 5.5 | 25 | [311] | ||||
| Aminated-Fe3O4 | Cr(VI) | 19.5 | 3.5 | 3 | [312] | ||||
| Aminated-Fe3O4 | Pb(II) | 21.2 | 3.5 | 3 | [312] | ||||
| Fe3O4/poly(C3N3S3) | Pb(II) | 232.6 | 6 | 25 | 7 | [313] | |||
| Fe3O4/poly(C3N3S3) | Hg(II) | 344.8 | 6 | 25 | 7 | [313] | |||
| rGO-PDTC/Fe3O4 | Cu(II) | 113.64 | 5 | 25 | 5 | [314] | |||
| rGO-PDTC/Fe3O4 | Cd(II) | 116.28 | 6 | 25 | 5 | [314] | |||
| rGO-PDTC/Fe3O4 | Pb(II) | 147.06 | 6 | 25 | 5 | [314] | |||
| rGO-PDTC/Fe3O4 | Hg(II) | 181.82 | 6 | 25 | 5 | [314] | |||
| d-MoS2/Fe3O4 | Hg(II) | 425.5 | 20 | 5 | [315] | ||||
| CHT/ALG/Fe3O4@SiO2 (8 beads) | Pb(II) | 243.77 | 4.2 | 20 | [316] | ||||
| CHT/ALG/Fe3O4@SiO2 (1 bead) | Pb(II) | 228.73 | 4.2 | 20 | [316] | ||||
| 2019 | Fe3O4/BC/AC | Pb(II) | 169.78 | 5 | 25 | 5 | [317] | ||
| Fe3O4@FePO4 | Cd(II) | 13.51 | 15 | 7 | [318] | ||||
| Fe3O4@SiO2-NH-MFL | Pb(II) | 150.33 | 0.5 | 5 | [156] | ||||
| Fe3O4@SiO2-NH-MFL | Cu(II) | 70.7 | 0.5 | 5 | [156] | ||||
| DTT-Fe3O4@Au | As(III) | 68.8 | 5 | [319] | |||||
| rGO-poly(C3N3S3)/Fe3O4 | Pb(II) | 270.3 | 60 | 6 | 25 | 15 | [320] | ||
| rGO-poly(C3N3S3)/Fe3O4 | Hg(II) | 400 | 60 | 6 | 25 | 15 | [320] | ||
| APTES-Fe3O4 (3 wt%) | As(V) | 14.6 | 210 | 2 | 25 | [321] | |||
| Fe2O3-SiO2-PAN | Cr(III) | 4.36 | [322] | ||||||
| Fe2O3-SiO2-PAN | Cu(II) | 7.20 | [322] | ||||||
| Fe2O3-SiO2-PAN | Zn(II) | 5.06 | [322] | ||||||
| Fe2O3-SiO2-PAN | Ni(II) | 2.60 | [322] | ||||||
| biochar-MnFe2O4 | Pb(II) | 154.94 | 5 | 25 | [323] | ||||
| biochar-MnFe2O4 | Cd(II) | 127.83 | 5 | 25 | [323] | ||||
| CoFe2O4@SiO2-EDTA | Hg(II) | 103.3 | 360 | 7 | 25 | 5 | [324] | ||
| Fe3O4-CS@BT | Cr(VI) | 62.1 | 2 | 25 | 5 | [325] | |||
| Fe3O4-CS@BT | Cr(VI) | 48.3 | 4 | 25 | [325] | ||||
| Fe3O4-CS@BT | Cr(VI) | 36.4 | 6 | 25 | [325] | ||||
| CMC/SA/graphene oxide@Fe3O4 | Cu(II) | 55.96 | 5 | 30 | [326] | ||||
| CMC/SA/graphene oxide@Fe3O4 | Cd(II) | 86.28 | 6 | 30 | [326] | ||||
| CMC/SA/graphene oxide@Fe3O4 | Pb(II) | 189.04 | 5 | 30 | [326] | ||||
| Fe3O4/NaP/NH2 | Pb(II) | 181.81 | 480 | 5–6 | 60 | 10 | [327] | ||
| Fe3O4/NaP/NH2 | Cd(II) | 50.25 | 240 | 5–6 | 70 | 10 | [327] | ||
| Fe3O4 ECSBNC | Cu(II) | 90.90 | [328] | ||||||
| Fe3O4 ECSBNC | Cr(VI) | 83.33 | [328] | ||||||
| MNPs-COOH | Pb(II) | 0.855 mmol/g | 6 | 25 | [329] | ||||
| MNPs-COOH | Cu(II) | 0.660 mmol/g | 6 | 25 | [329] | ||||
| 2019 | MNPs-COOH | Cd(II) | 0.518 mmol/g | 6 | 25 | [329] | |||
| MNPs-COOH | Ni(II) | 0.441 mmol/g | 6 | 25 | [329] | ||||
| CMC-Fe3O4 | Pb(II) | 152.0 | 6 | [330] | |||||
| Fe3O4-loaded CS NPs | Cd(II) | 97.76 | 5 | 5 | [331] | ||||
| CuFe2O4 | Cd(II) | 157.7 | 2 | [332] | |||||
| CoFe2O4 | Pb(II) | 63.1 | 12 | [332] | |||||
| HP-β-CD-GO/Fe3O4 | Cu(II) | 17.91 | 6 | 5 | [333] | ||||
| HP-β-CD-GO/Fe3O4 | Pb(II) | 50.39 | 5 | 5 | [333] | ||||
| Bentonite/CoFe2O4@MnO2-NH2 | Cd(II) | 115.79 | 98.88 | [334] | |||||
| 2020 | Fe3O4@SiO2@GLYMO(S) | Cd(II) | 80.64 | 55 | 7 | 5 | [335] | ||
| Fe3O4@SiO2@GLYMO(S) | Pb(II) | 93.5 | 55 | 7 | 5 | [335] | |||
| MGO | Pb(II) | 200 | 30 | 5 | [167] | ||||
| MGO | Cr(III) | 24.33 | 30 | 6 | [167] | ||||
| MGO | Cu(II) | 62.89 | 30 | 6 | [167] | ||||
| MGO | Zn(II) | 63.69 | 30 | 7 | [167] | ||||
| MGO | Ni(II) | 51.02 | 30 | 8 | [167] | ||||
| Proanthocyanidin- functionalized Fe3O4 | Cu(II) | 18.8 | 30 | 8 | 5 | [336] | |||
| Proanthocyanidin- functionalized Fe3O4 | Cd(II) | 20.9 | 30 | 8 | [336] | ||||
| Proanthocyanidin-functionalized Fe3O4 | Pb(II) | 21.5 | 30 | 8 | [336] | ||||
| Ggh-g-PAcM/Fe3O4 | Cu(II) | 224.8 | 30 | [337] | |||||
| Ggh-g-PAcM/Fe3O4 | Hg(II) | 213.8 | 30 | [337] | |||||
| Fe3O4-GO hybrid (9:1) | Pb(II) | 107.56 | 6 | [338] | |||||
| Fe3O4-GO hybrid (5:1) | Pb(II) | 151.22 | 6 | [338] | |||||
| M-45 OA | Pb(II) | 42.553 | 6 | [338] | |||||
| M-45 OA | Zn(II) | 42.919 | 6 | [339] | |||||
| M-45 OA | Cd(II) | 42.373 | 7 | [339] | |||||
| M-55 OA | Pb(II) | 41.841 | 6 | [339] | |||||
| M-55 OA | Zn(II) | 42.735 | 6 | [339] | |||||
| M-55 OA | Cd(II) | 42.017 | 7 | [339] | |||||
| M-55+ | Pb(II) | 40.816 | 6 | [339] | |||||
| M-55+ | Zn(II) | 40.816 | 6 | [339] | |||||
| M-55+ | Cd(II) | 39.216 | 7 | [339] | |||||
| BNNF@Fe3O4 | Pb(II) | 203.75 | 50 | 25 | [340] | ||||
| Fe3O4@SiO2-NH2 | Cd(II) | 93 | 5 | [341] | |||||
| RH + iron oxide NPs | As(V) | 82 | 60 | 5 | [342] | ||||
| MnFe2O4 | Zn(II) | 454.4 | 120 | 6 | 25 | 3 | [343] | ||
| CoFe2O4 | Zn(II) | 384.6 | 120 | 6 | 25 | 3 | [343] | ||
| SiO2/CuFe2O4/PANI | Cu(II) | 285.71 | 300 | 5.3 | 30 | 4 | [344] | ||
| MWCNT/γ-Fe2O3 | Cr(VI) | 208.1 | 150 | 4 | [345] | ||||
| MWCNT-PEI/γ-Fe2O3 | Cr(VI) | 352.3 | 150 | 4 | [345] | ||||
| Fe3O4 | Cr(VI) | 201.55 | 50 | 6 | [346] | ||||
| Fe3O4@Z-NCNT/PC | Pb(II) | 789.87 | 20 | 5.5 | 10 | [347] | |||
| FO-BC-450 | Cd(II) | 151.3 | 2 | 25 | [348] | ||||
| 2020 | FO-BC-450 | Cu(II) | 219.8 | 2 | 25 | [348] | |||
| FO-BC-450 | Pb(II) | 271.9 | 2 | 25 | [348] | ||||
| Fe2O3@SiO2@SH | Hg(II) | 98 | [349] | ||||||
| γ-Fe2O3 coated Bacillus subtilis | Cd(II) | 32.6 | 4 | 30 | [350] | ||||
| Fe3O4-HBPA-ASA | Cu(II) | 136.66 | 8 | 25 | 5 | [351] | |||
| Fe3O4-HBPA-ASA | Cd(II) | 88.36 | 8 | 25 | 5 | [351] | |||
| Fe3O4-HBPA-ASA | Pb(II) | 165.46 | 8 | 25 | 5 | [351] | |||
| Crystalline iron oxide nanoparticles (IO-NPs) | Pb(II) | 9.206 | 6 | 40 | [352] | ||||
| Crystalline iron oxide nanoparticles (IO-NPs) | Ni(II) | 9.666 | 6 | 40 | [352] | ||||
| Crystalline iron oxide nanoparticles (IO-NPs) | Cu(II) | 8.355 | 6 | 40 | [352] | ||||
| Crystalline iron oxide nanoparticles (IO-NPs) | Zn(II) | 9.106 | 6 | 40 | [352] | ||||
| Pec-g-PHEAA/Fe3O4 | Cu(II) | 248.6 | [353] | ||||||
| Pec-g-PHEAA/Fe3O4 | Hg(II) | 240.2 | [353] | ||||||
| Fe3O4 fibre | Pb(II) | 16.78 | 6 | [354] | |||||
| Fe3O4 powder | Pb(II) | 15.80 | 6 | [354] | |||||
| Iron oxide (MNPs) grafted (HPG) | Cu(II) | 0.700 mg/mg | 120 | 9 | 20 | [355] | |||
| Iron oxide (MNPs) grafted (HPG) | Ni(II) | 0.451 mg/mg | 120 | 9 | 20 | [355] | |||
| Nano-CI | Pb(II) | 1900 μmol/g | 30 | 6 | [356] | ||||
| Nano-CI | Cu(II) | 2250 μmol/g | 30 | 7 | [356] | ||||
| Nano-CI | Cd(II) | 850 μmol/g | 30 | 7 | [356] | ||||
| Nano-CIC | Pb(II) | 2700 μmol/g | 30 | 4 | [356] | ||||
| Nano-CIC | Cu(II) | 4250 μmol/g | 30 | 6 | [356] | ||||
| Nano-CIC | Cd(II) | 1800 μmol/g | 30 | 6 | [356] | ||||
| Nano-CIS | Pb(II) | 2600 μmol/g | 10 | 4 | [356] | ||||
| Nano-CIS | Cu(II) | 4700 μmol/g | 10 | 6 | [356] | ||||
| Nano-CIS | Cd(II) | 1900 μmol/g | 10 | 6 | [356] |
| Pollutant | 1st Cycle | 2nd Cycle | 3rd Cycle | 4th Cycle | 5th Cycle | Ref. |
|---|---|---|---|---|---|---|
| Pb(II) | 90.12 | 88.05 | 85.65 | 81.35 | [360] | |
| Cu(II) | 93.70 | 58.66 | [196] | |||
| Pb(II) | 90 | [197] | ||||
| Cr(VI) | 55 | 88 | 90 | [361] | ||
| Pb(II) | 97.34 | 90 | [175] | |||
| Hg(II) | ≥96 | [157] | ||||
| Pb(II) | 90 | [201] | ||||
| Pb(II) | 93.5 | 89.3 | [335] | |||
| Cu(II) | 80.64 | 73.3 | [335] | |||
| As(V) | 95 | 56 | [342] | |||
| Cd(II) | 76.4 | [348] | ||||
| Cu(II) | 80.4 | [348] | ||||
| Pb(II) | 70.2 | [348] | ||||
| Hg(II) | >90 | ~75.5 | [324] | |||
| Cd(II) | 98.8 | 95.1 | 91.7 | 84.6 | 78.3 | [294] |
| Cd(II) | 99.96 | 97.25 | [302] | |||
| Cu(II) | 88.05 | 84.15 | [302] | |||
| Pb(II) | 90.79 | 87.12 | [302] | |||
| Pb(II) | 96.2 | 86.4 | [257] | |||
| Hg(II) | 95.1 | 85.9 | [257] | |||
| Cu(II) | 96.5 | 87.6 | [257] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liosis, C.; Papadopoulou, A.; Karvelas, E.; Karakasidis, T.E.; Sarris, I.E. Heavy Metal Adsorption Using Magnetic Nanoparticles for Water Purification: A Critical Review. Materials 2021, 14, 7500. https://doi.org/10.3390/ma14247500
Liosis C, Papadopoulou A, Karvelas E, Karakasidis TE, Sarris IE. Heavy Metal Adsorption Using Magnetic Nanoparticles for Water Purification: A Critical Review. Materials. 2021; 14(24):7500. https://doi.org/10.3390/ma14247500
Chicago/Turabian StyleLiosis, Christos, Athina Papadopoulou, Evangelos Karvelas, Theodoros E. Karakasidis, and Ioannis E. Sarris. 2021. "Heavy Metal Adsorption Using Magnetic Nanoparticles for Water Purification: A Critical Review" Materials 14, no. 24: 7500. https://doi.org/10.3390/ma14247500
APA StyleLiosis, C., Papadopoulou, A., Karvelas, E., Karakasidis, T. E., & Sarris, I. E. (2021). Heavy Metal Adsorption Using Magnetic Nanoparticles for Water Purification: A Critical Review. Materials, 14(24), 7500. https://doi.org/10.3390/ma14247500









