Hydroxyapatite Film Coating by Er:YAG Pulsed Laser Deposition Method for the Repair of Enamel Defects
Abstract
1. Introduction
2. Materials and Methods
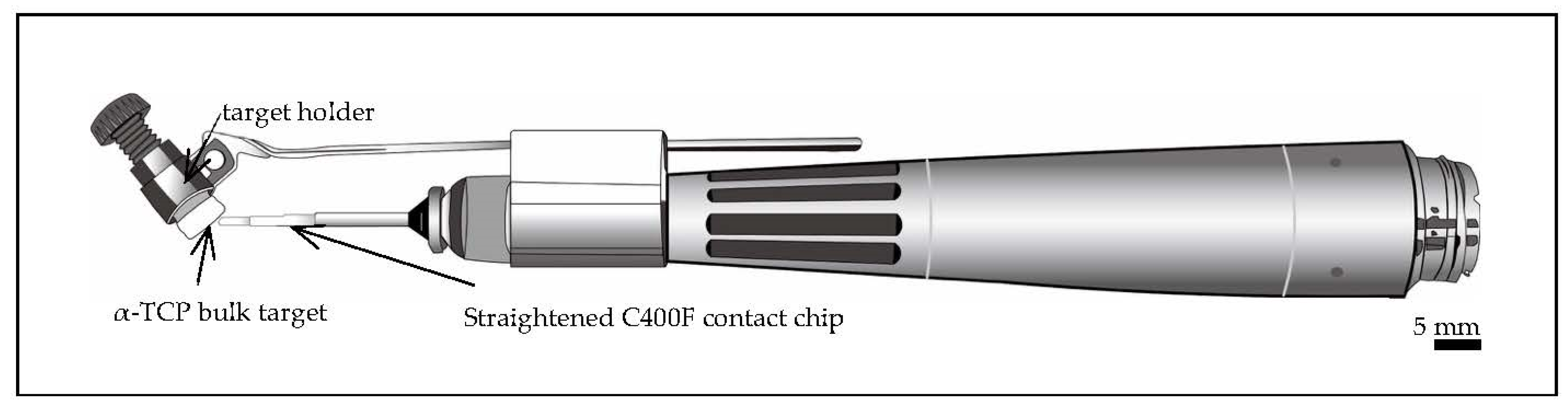
2.1. Preparation of Targets for Er:YAG-PLD
2.2. Preparation of Bovine Tooth Slabs (Specimens)
2.3. Chairside Er:YAG-PLD Device
2.4. Preparation of Artificial Saliva
2.5. Microstructural Analysis
2.6. Elemental Analysis
2.7. Toothbrush Abrasion and Its Measurements
2.8. Mechanical Property Measurements
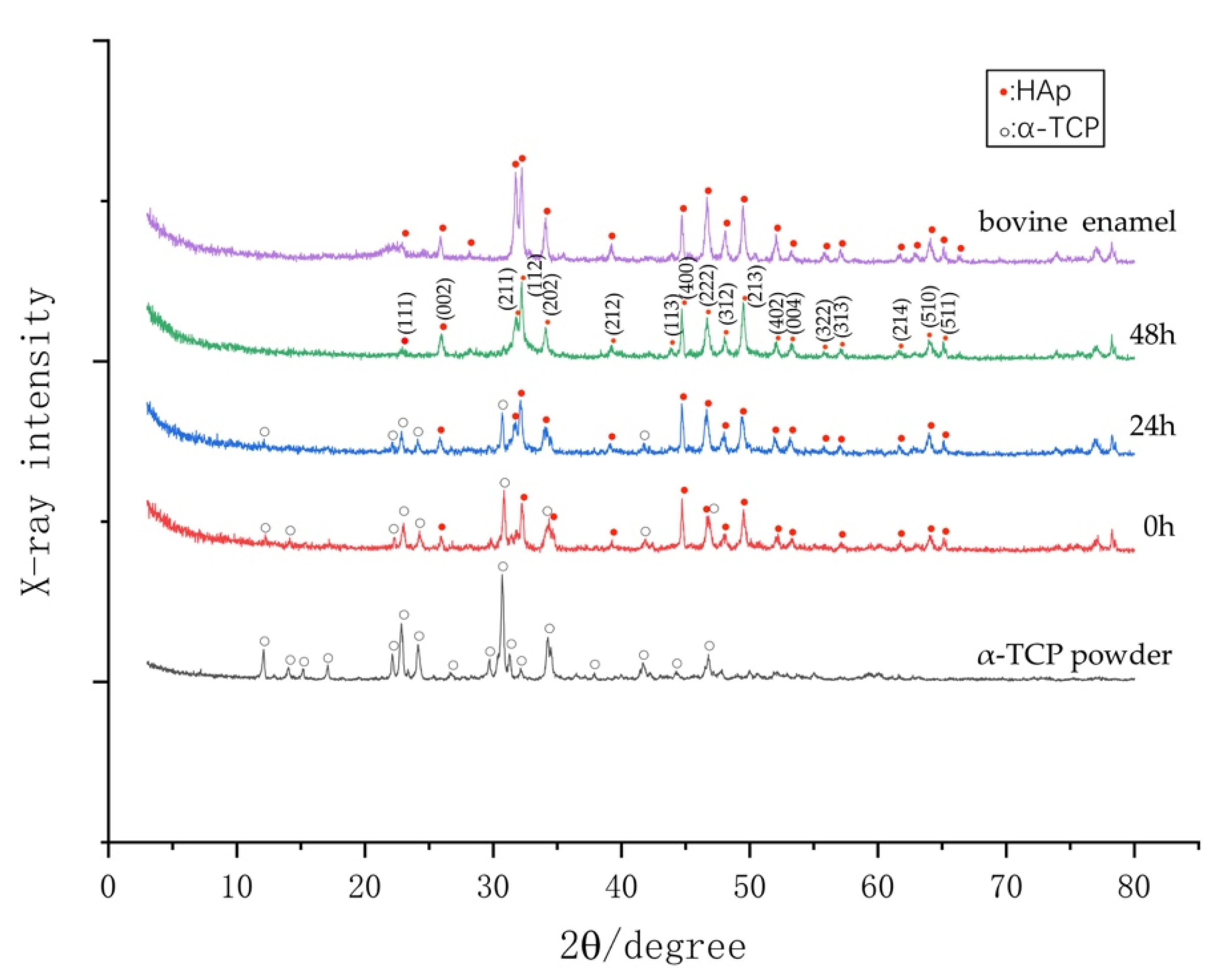
2.9. Crystal Structural Analysis
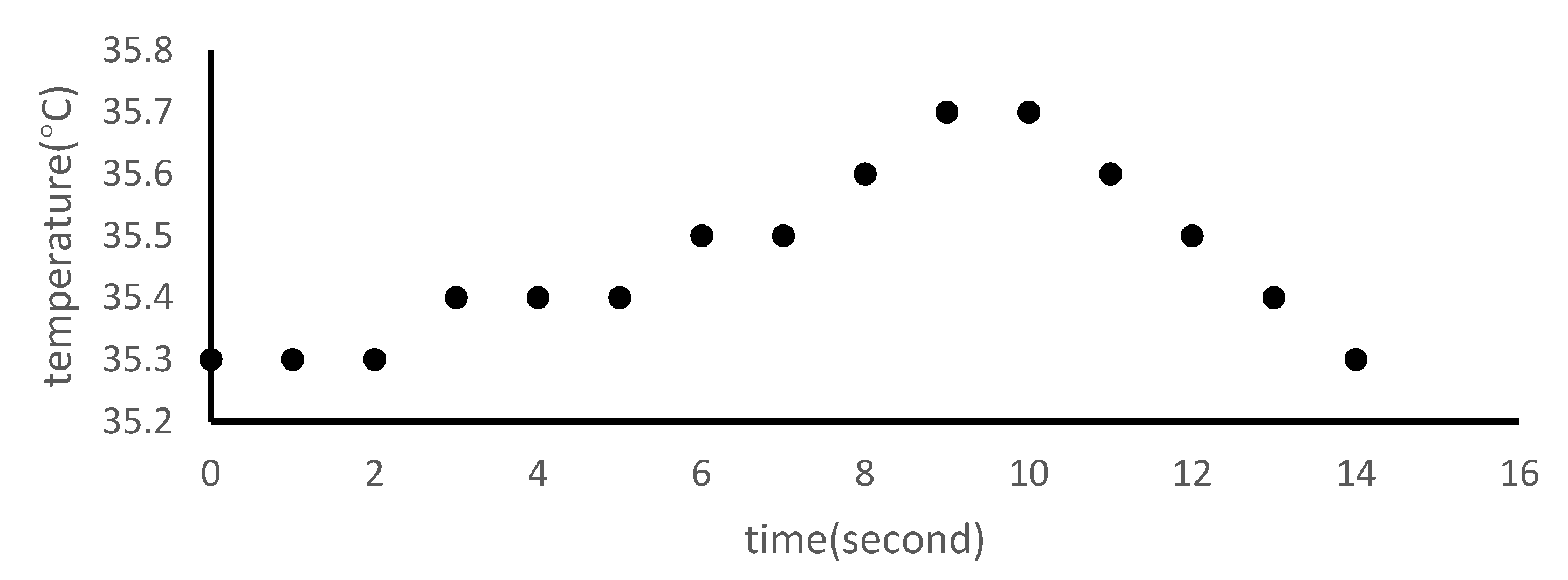
2.10. Measurement of Temperature Change during Er:YAG-PLD Procedure
2.11. Effect of HAp Film Coating on Cell Attachment
2.12. Statistical Analysis
3. Results
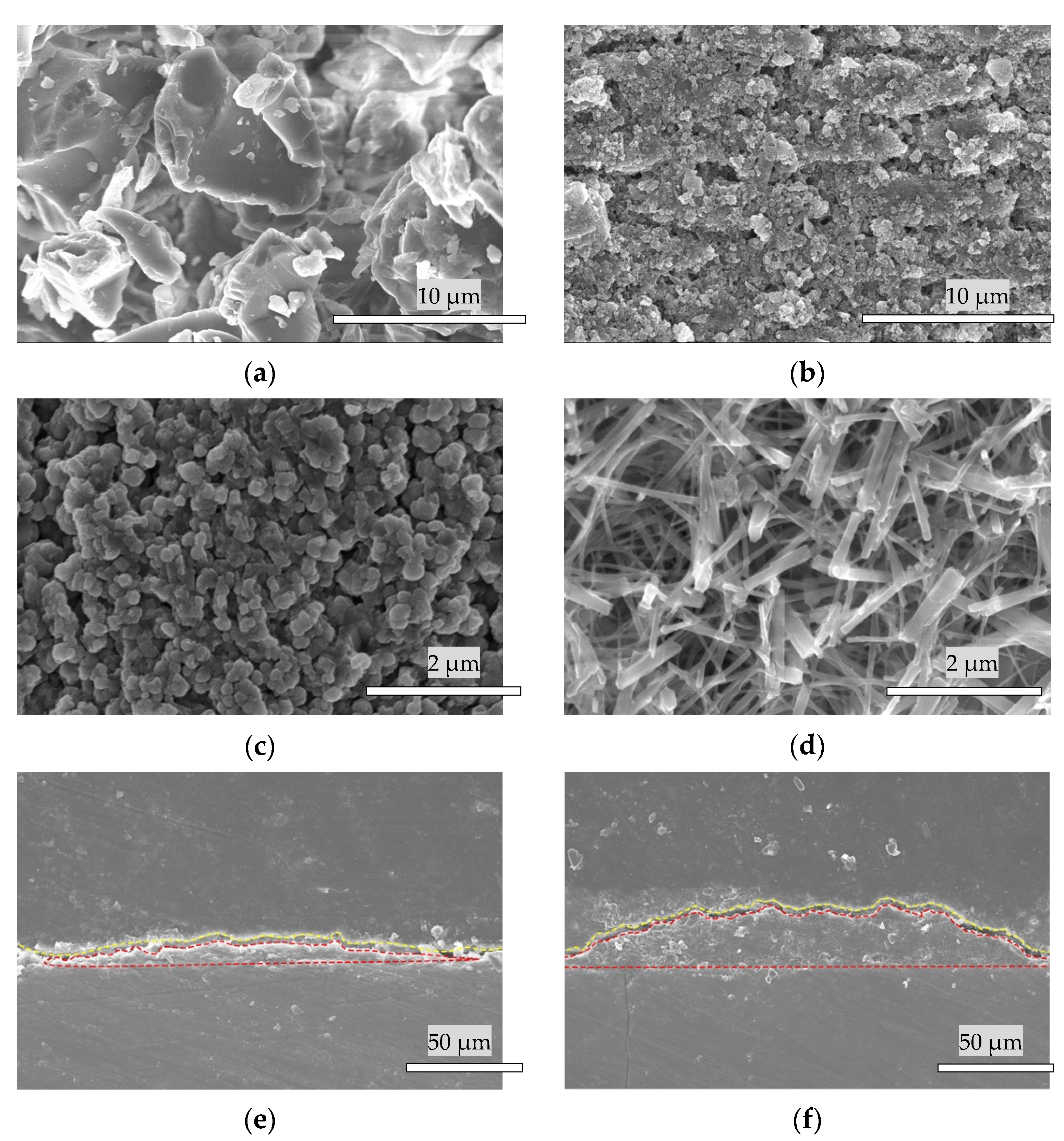
3.1. Materials Fabrication
3.2. Scanning Electron Microscope Analysis
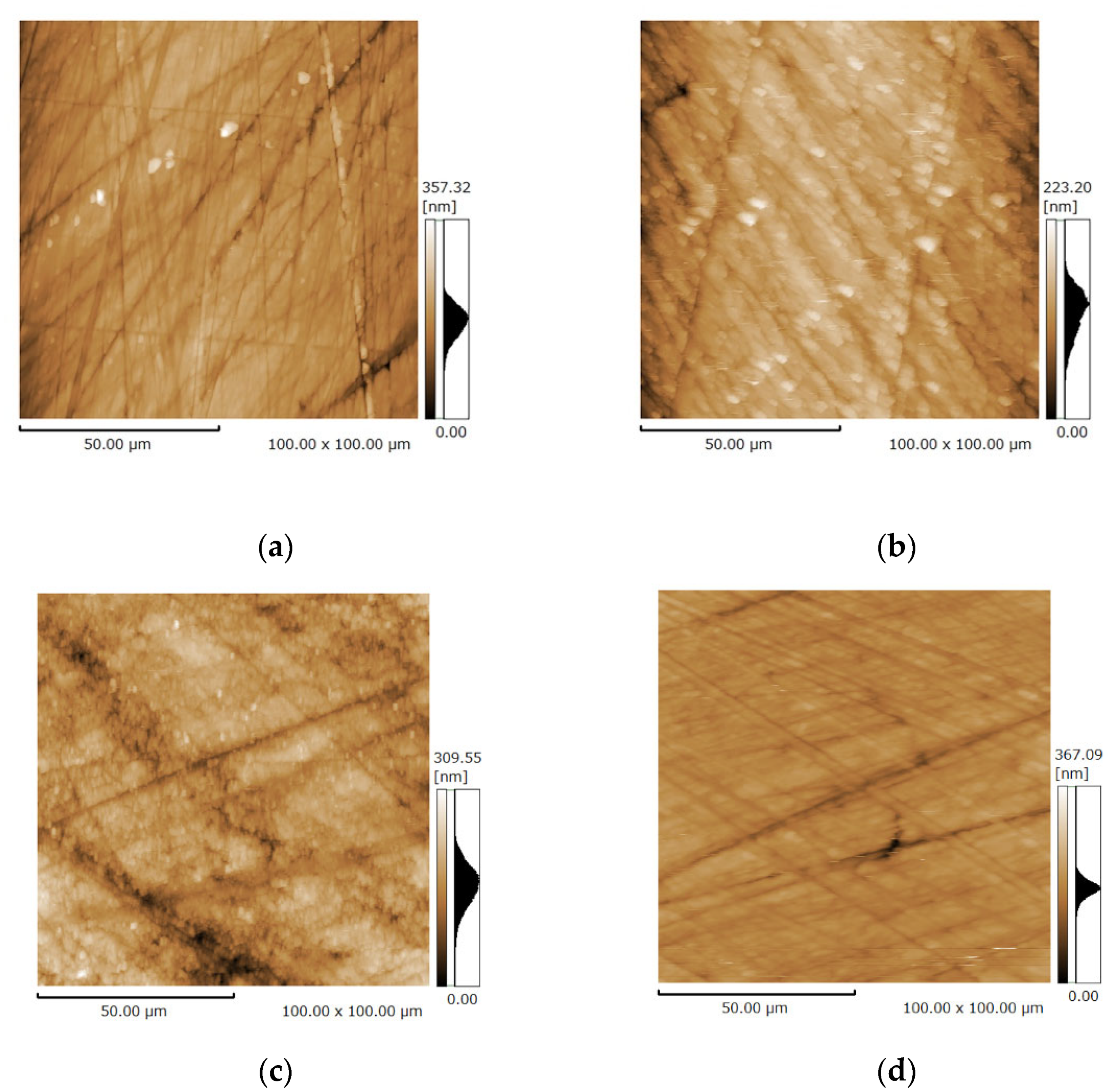
3.3. Surface Microstructure of the Bovine Enamel and HAp Film Coating Deposited Specimens before and after the Brushing Test
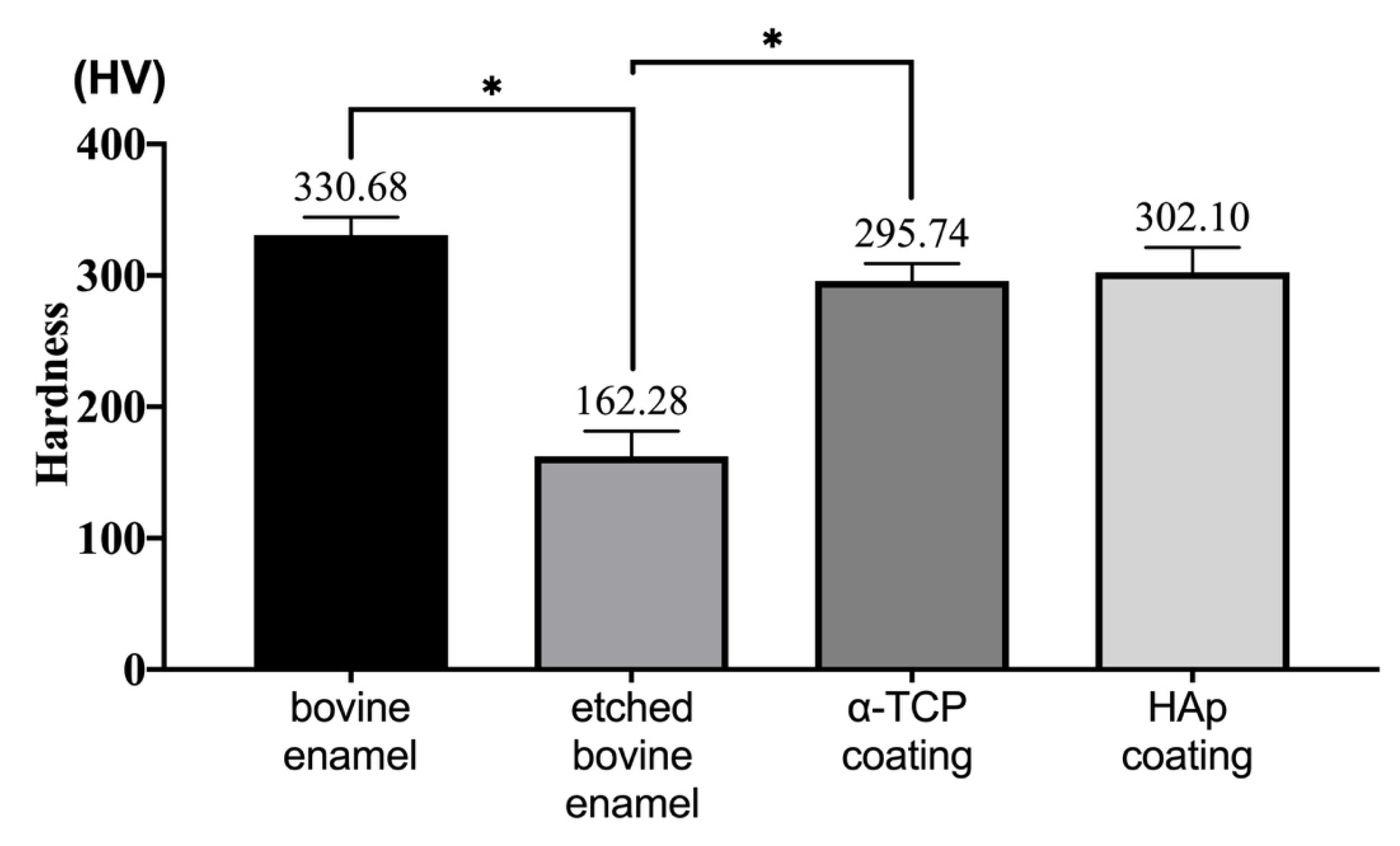
3.4. Hardness Analysis
3.5. Phase Determination by XRD
3.6. Temperature Changes Following Er:YAG-PLD
3.7. Calcium-to-Phosphate (Ca/P) Analysis of HAp Film Coating by Energy-Dispersive X-ray Spectroscopy (EDS)
3.8. Cell Attachment of HAp Film Coating
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kitahara-Céia, F.M.F.; Mucha, J.N.; dos Santos, P.A.M. Assessment of enamel damage after removal of ceramic brackets. Am. J. Orthod. Dentofacial Orthop. 2008, 134, 548–555. [Google Scholar] [CrossRef] [PubMed]
- Shao, C.; Jin, B.; Mu, Z.; Lu, H.; Zhao, Y.; Wu, Z.; Yan, L.; Zhang, Z.; Zhou, Y.; Pan, H.; et al. Repair of tooth enamel by a biomimetic mineralization frontier ensuring epitaxial growth. Sci. Adv. 2019, 5, eaaw9569. [Google Scholar] [CrossRef] [PubMed]
- Caruso, S.; Bernardi, S.; Pasini, M.; Giuca, M.; Docimo, R.; Continenza, M.; Gatto, R. The process of mineralisation in the development of human tooth. Eur. J. Paediatr. Dent. 2016, 17, 322. [Google Scholar] [PubMed]
- Milosevic, M. Polymerization Mechanics of Dental Composites—Advantages and Disadvantages. Procedia Eng. 2016, 149, 313–320. [Google Scholar] [CrossRef]
- Arcos, D.; Vallet-Regí, M. Substituted hydroxyapatite coatings of bone implants. J. Mater. Chem. B 2020, 8, 1781–1800. [Google Scholar] [CrossRef]
- Palmer, L.C.; Newcomb, C.J.; Kaltz, S.R.; Spoerke, E.D.; Stupp, S.I. Biomimetic systems for hydroxyapatite mineralization inspired by bone and enamel. Chem. Rev. 2008, 108, 4754–4783. [Google Scholar] [CrossRef]
- Akatsuka, R.; Sasaki, K.; Zahmaty, M.S.S.; Noji, M.; Anada, T.; Suzuki, O.; Kuriyagawa, T. Characteristics of hydroxyapatite film formed on human enamel with the powder jet deposition technique. J. Biomed. Mater. Res. Part B 2011, 98, 210–216. [Google Scholar] [CrossRef]
- Kato, N.; Ido, Y.; Yamamoto, E.; Hontsu, S. Development of Ultra-Thin Opaque White Hydroxyapatite Sheet for Restoration of Enamel and Aesthetic Treatments of Teeth. Key Eng. Mater. 2017, 758, 162–165. [Google Scholar] [CrossRef]
- Hontsu, S.; Nakamori, M.; Tabata, H.; Ishii, J.; Kawai, T. Pulsed laser deposition of bioceramic hydroxyapatite thin films on polymer materials. Jpn. J. Appl. Phys. 1996, 35, L1208. [Google Scholar]
- Boj, J.R.; Poirier, C.; Hernandez, M.; Espasa, E.; Espanya, A. Laser soft tissue treatments for paediatric dental patients. Eur. Arch. Paediatr. Dent. 2011, 12, 100–105. [Google Scholar] [CrossRef]
- Harashima, T.; Kinoshita, J.-I.; Kimura, Y.; Brugnera, A., Jr.; Zanin, F.; Pecora, J.D.; Matsumoto, K. Morphological comparative study on ablation of dental hard tissues at cavity preparation by Er:YAG and Er,Cr:YSGG lasers. Photomed. Laser Surg. 2005, 23, 52–55. [Google Scholar] [CrossRef]
- Ohsugi, Y.; Aoki, A.; Mizutani, K.; Katagiri, S.; Komaki, M.; Noda, M.; Takagi, T.; Kakizaki, S.; Meinzer, W.; Izumi, Y. Evaluation of bone healing following Er:YAG laser ablation in rat calvaria compared with bur drilling. J.Biophotonics 2019, 12, e201800245. [Google Scholar] [CrossRef] [PubMed]
- Chimello-Sousa, D.T.; de Souza, A.E.; Chinelatti, M.A.; Pécora, J.D.; Palma-Dibb, R.G.; Corona, S.A.M. Influence of Er:YAG laser irradiation distance on the bond strength of a restorative system to enamel. J. Dent. 2006, 34, 245–251. [Google Scholar] [CrossRef]
- Duffo, G.; Castillo, E.Q. Development of an artificial saliva solution for studying the corrosion behavior of dental alloys. Corrosion 2004, 60, 594–602. [Google Scholar] [CrossRef]
- Diaz-Arnold, A.M.; Marek, C.A. The impact of saliva on patient care: A literature review. J. Prosthet. Dent. 2002, 88, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Kubala, E.; Strzelecka, P.; Grzegocka, M.; Lietz-Kijak, D.; Gronwald, H.; Skomro, P.; Kijak, E. A review of selected studies that determine the physical and chemical properties of saliva in the field of dental treatment. BioMed Res. Int. 2018, 2018, 6572381. [Google Scholar] [CrossRef]
- McCracken, G.; Janssen, J.; Swan, M.; Steen, N.; De Jager, M.; Heasman, P. Effect of brushing force and time on plaque removal using a powered toothbrush. J. Clin. Periodontol. 2003, 30, 409–413. [Google Scholar] [CrossRef] [PubMed]
- Ganss, C.; Schlueter, N.; Preiss, S.; Klimek, J. Tooth brushing habits in uninstructed adults—Frequency, technique, duration and force. Clin. Oral Investig. 2009, 13, 203–208. [Google Scholar] [CrossRef]
- Yamamoto, E.; Kato, N.; Hontsu, S. Adhesive Evaluation by Brushing Tests for Hydroxyapatite Films Fabricated on Dentins Using a Water Mist Assisted Er:YAG Laser Deposition Method. Key Eng. Mater. 2017, 758, 97. [Google Scholar] [CrossRef]
- Souza-Gabriel, A.; Colucci, V.; Turssi, C.; Serra, M.; Corona, S. Microhardness and SEM after CO2 laser irradiation or fluoride treatment in human and bovine enamel. Microsc. Res. Tech. 2010, 73, 1030–1035. [Google Scholar] [CrossRef]
- Mangkonsu, C.; Kunio, I.; Bunhan, L.; Otman, R.; Noor, A.-F.M. The effect of microwave sintering on the microstructure and properties of calcium phosphate ceramic. Procedia Chem. 2016, 19, 498–504. [Google Scholar] [CrossRef][Green Version]
- Yamamoto, E.; Nakazawa, M.; Hirai, M.; Hashimoto, Y.; Baba, S.; Hontsu, S. Cell Adhesion Ability of α-Tricalcium Phosphate Films Formed on Titanium Substrates by an Er:YAG Laser Deposition Method: Imprecations for Management of Peri-Implant Inflammation. Key Eng. Mater. 2019, 829, 157. [Google Scholar] [CrossRef]
- Liao, W.; Okada, M.; Inami, K.; Hashimoto, Y.; Matsumoto, N. Cell survival and gene expression under compressive stress in a three-dimensional in vitro human periodontal ligament-like tissue model. Cytotechnology 2016, 68, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.-M.; Tsai, R.; Chang, E.; Yang, C.-Y.; Yang, M. The cell attachment and morphology of neonatal rat calvarial osteoblasts on the surface of Ti-6Al-4V and plasma-sprayed HA coating: Effect of surface roughness and serum contents. J. Mater. Sci. Mater. Med. 2002, 13, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Dennison, D.; Ong, J.L. Protein Adsorption and Osteoblast Precursor Cell Attachment to Hydroxyapatite of Different Crystallinities. Int. J. Oral Maxillofac. Implants 2005, 20, 187–192. [Google Scholar]
- Kaul-Ghanekar, R.; Singh, S.; Mamgain, H.; Jalota-Badhwar, A.; Paknikar, K.M.; Chattopadhyay, S. Tumor suppressor protein SMAR1 modulates the roughness of cell surface: Combined AFM and SEM study. BMC Cancer 2009, 9, 350. [Google Scholar] [CrossRef] [PubMed]
- Son, W.-S.; Park, H.J.; Lee, C.-J.; Kim, S.-N.; Song, S.U.; Park, G.; Lee, Y.-W. Supercritical drying of vascular endothelial growth factor in mesenchymal stem cells culture fluids. J. Supercrit. Fluids 2020, 157, 104710. [Google Scholar] [CrossRef]
- Mischo, J.; Faidt, T.; McMillan, R.B.; Dudek, J.; Gunaratnam, G.; Bayenat, P.; Holtsch, A.; Spengler, C.; Mueller, F.; Hähl, H. Hydroxyapatite pellets as versatile model surfaces for systematic studies on enamel. bioRxiv 2021, bioRxiv:01.11.426207. [Google Scholar]
- Kato, N.; Yamamoto, E.; Isai, A.; Nishikawa, H.; Kusunoki, M.; Yoshikawa, K. Ultrathin amorphous calcium phosphate freestanding sheet for dentin tubule sealing. Bioceram. Dev. Appl. 2013, 1, 007. [Google Scholar] [CrossRef]
- Dostalova, T.; Jelinkova, H.; Nemec, M.; Sulc, J.; Miyagi, M. Er:YAG laser ablation: 5–11 years prospective study. Proc. SPIE 2005, 5687, 63–68. [Google Scholar]
- Iaria, G. Clinical, morphological, and ultrastructural aspects with the use of Er:YAG and Er,Cr:YSGG lasers in restorative dentistry. Gen. Dent. 2008, 56, 636. [Google Scholar] [PubMed]
- Ferna, E.; Gil, F.; Ginebra, M.; Driessens, F.; Planell, J.; Best, S. Calcium phosphate bone cements for clinical applications. Part I: Solution chemistry. J. Mater. Sci. Mat. Med. 1999, 10, 169–176. [Google Scholar]
- Brown, P.W. Hydration behavior of calcium phosphates is analogous to hydration behavior of calcium silicates. Cem. Concr. Res. 1999, 29, 1167–1171. [Google Scholar] [CrossRef]
- Ginebra, M.; Driessens, F.; Planell, J. Effect of the particle size on the micro and nanostructural features of a calcium phosphate cement: A kinetic analysis. Biomaterials 2004, 25, 3453–3462. [Google Scholar] [CrossRef] [PubMed]
- Ginebra, M.P.; Fernández, E.; Driessens, F.C.; Planell, J.A. Modeling of the Hydrolysis of α-Tricalcium Phosphate. J. Am. Ceram. Soc. 1999, 82, 2808–2812. [Google Scholar] [CrossRef]
- Durucan, C.; Brown, P.W. Kinetic model for α–tricalcium phosphate hydrolysis. J. Am. Ceram. Soc. 2002, 85, 2013–2018. [Google Scholar] [CrossRef]
- Voronets, J.; Lussi, A. Thickness of softened human enamel removed by toothbrush abrasion: An in vitro study. Clin. Oral Investig. 2010, 14, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Hicks, J.; Garcia-Godoy, F.; Flaitz, C. Biological factors in dental caries enamel structure and the caries process in the dynamic process of demineralization and remineralization (part 2). J. Clin. Pediatr. Dent. 2005, 28, 119–124. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Nishikawa, H.; Kusunoki, M.; Li, P.; Hontsu, S. A Novel Membrane-Type Apatite Scaffold Engineered by Pulsed Laser Ablation. Dent. Mater. J. 2015, 34, 345–350. [Google Scholar] [CrossRef]
- Nahas, P.; Zeinoun, T.; Namour, M.; Ayach, T.; Nammour, S. Effect of Er:YAG laser energy densities on thermally affected dentin layer: Morphological study. Laser Ther. 2018, 27, 91–97. [Google Scholar] [CrossRef]
- Dostalova, T. Dentin and pulp respose to Erbium: YAG laser ablation: A preliminary evaluation of human teeth. J. Clin. Laser Med. Surg. 1997, 15, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Rizoiu, I. Pulpal thermal responses to an erbium, chromium: YSGG pulsed laser hydrokinetic system. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1998, 86, 220–223. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Ueda, M.; Kohiga, Y.; Imura, K.; Hontsu, S. Application of fluoridated hydroxyapatite thin film coatings using KrF pulsed laser deposition. Dent. Mater. J. 2018, 37, 408–413. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Cavin, R.; Ong, J.L. Protein Adsorption on Titanium Surfaces and Their Effect on Osteoblast Attachment. J. Biomed. Mater. Res. Part A 2003, 67, 344–349. [Google Scholar] [CrossRef]
- Yamamoto, E.; Kato, N.; Yoshikawa, K.; Yasuo, K.; Yamamoto, K.; Hontsu, S. Adhesion properties of an apatite film deposited on dentine using Er:YAG laser ablation method. Key Eng. Mater. 2016, 696, 69–73. [Google Scholar] [CrossRef]




| Specimen | Element | C K | O K | Na K | Mg K | P K | Cl K | Ca k | Totals |
|---|---|---|---|---|---|---|---|---|---|
| Bovine enamel | Weight % | 4.97 ± 0.43 | 47.18 ± 0.42 | 1.22 ± 0.24 | 0.26 ± 0.02 | 17.14 ± 0.15 | 0.20 ± 0.01 | 29.03 ± 0.63 | 100 |
| Atomic % | 8.79 ± 0.71 | 62.61 ± 0.27 | 1.13 ± 0.22 | 0.23 ± 0.02 | 11.75 ± 0.19 | 0.12 ± 0.01 | 15.38 ± 0.44 | 100 | |
| HAp coating | Weight % | 5.60 ± 1.49 | 41.66 ± 1.06 | 1.41 ± 0.63 | 0.28 ± 0.02 | 17.79 ± 0.32 | - | 33.25 ± 1.18 | 100 |
| Atomic % | 10.23 ± 2.58 | 57.28 ± 1.60 | 1.35 ± 0.60 | 0.26 ± 0.02 | 12.63 ± 0.41 | - | 18.25 ± 0.87 | 100 |
| Specimens | Ca/P |
|---|---|
| Bovine enamel | 1.31 ± 0.02 |
| HAp coating | 1.45 ± 0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Hontsu, S.; Komasa, S.; Yamamoto, E.; Hashimoto, Y.; Matsumoto, N. Hydroxyapatite Film Coating by Er:YAG Pulsed Laser Deposition Method for the Repair of Enamel Defects. Materials 2021, 14, 7475. https://doi.org/10.3390/ma14237475
Chen L, Hontsu S, Komasa S, Yamamoto E, Hashimoto Y, Matsumoto N. Hydroxyapatite Film Coating by Er:YAG Pulsed Laser Deposition Method for the Repair of Enamel Defects. Materials. 2021; 14(23):7475. https://doi.org/10.3390/ma14237475
Chicago/Turabian StyleChen, Liji, Shigeki Hontsu, Satoshi Komasa, Ei Yamamoto, Yoshiya Hashimoto, and Naoyuki Matsumoto. 2021. "Hydroxyapatite Film Coating by Er:YAG Pulsed Laser Deposition Method for the Repair of Enamel Defects" Materials 14, no. 23: 7475. https://doi.org/10.3390/ma14237475
APA StyleChen, L., Hontsu, S., Komasa, S., Yamamoto, E., Hashimoto, Y., & Matsumoto, N. (2021). Hydroxyapatite Film Coating by Er:YAG Pulsed Laser Deposition Method for the Repair of Enamel Defects. Materials, 14(23), 7475. https://doi.org/10.3390/ma14237475






