Structural and Electrical Characterization of Pure and Al-Doped ZnO Nanorods
Abstract
:1. Introduction
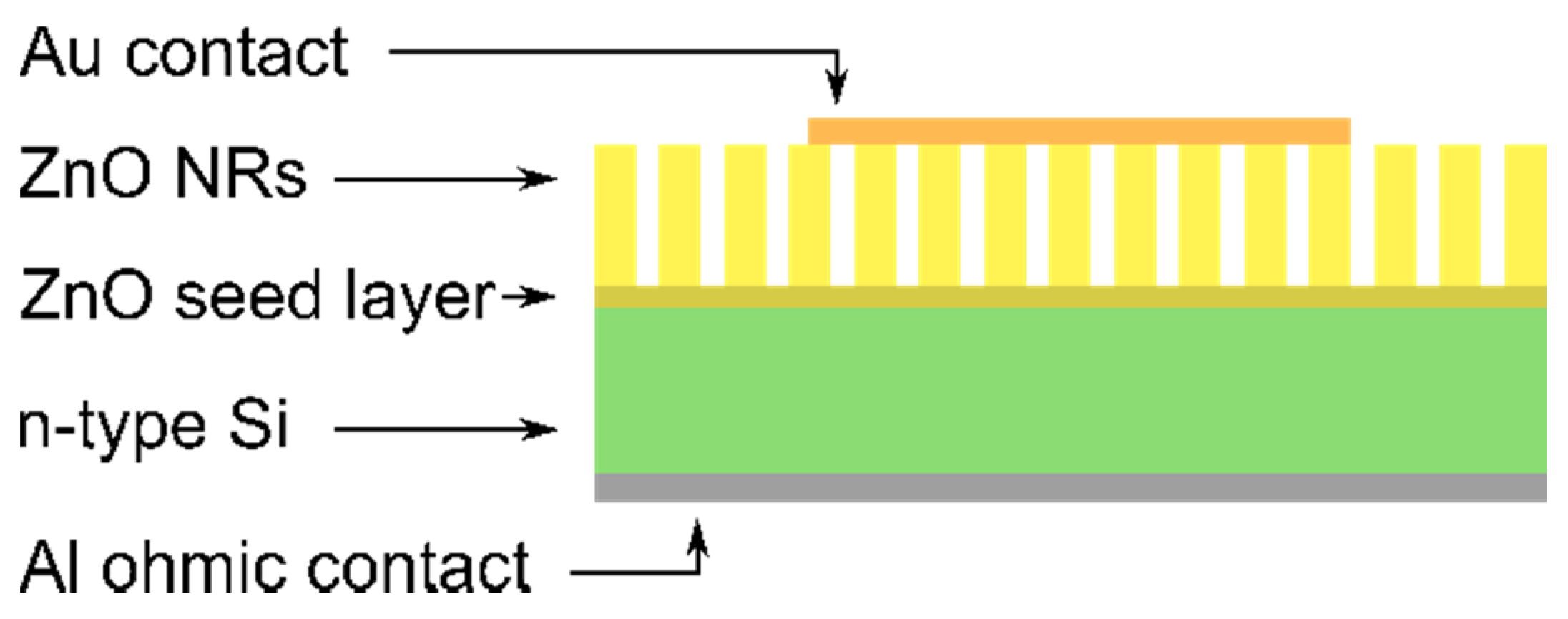
2. Materials and Methods
3. Results and Discussion
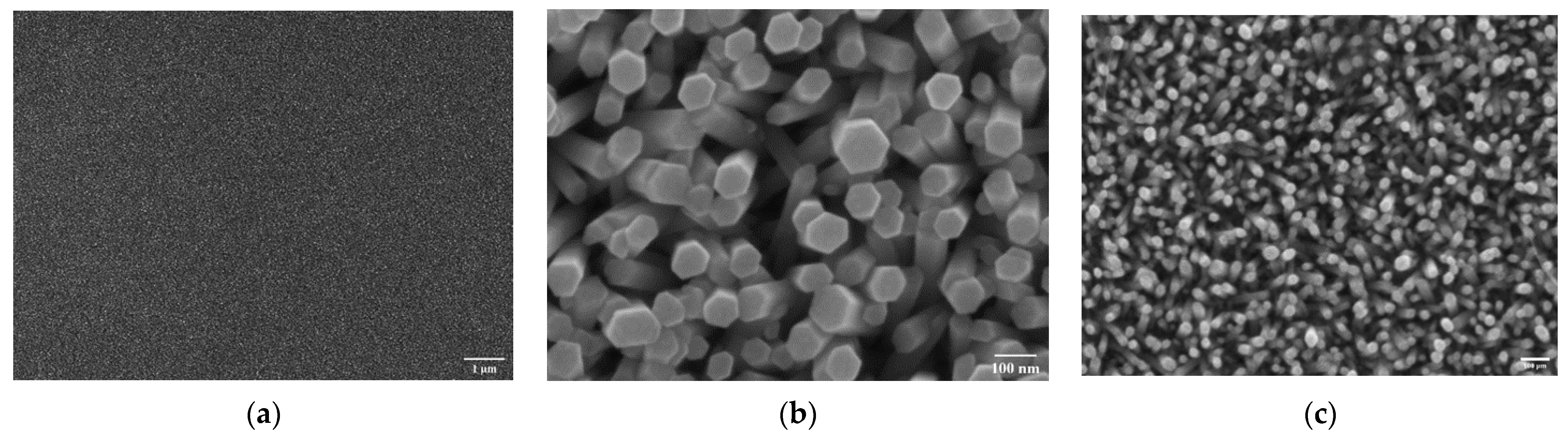
3.1. Electron Microscopy Analysis
3.2. Atomic Force Microscopy
3.3. Diffraction Analysis
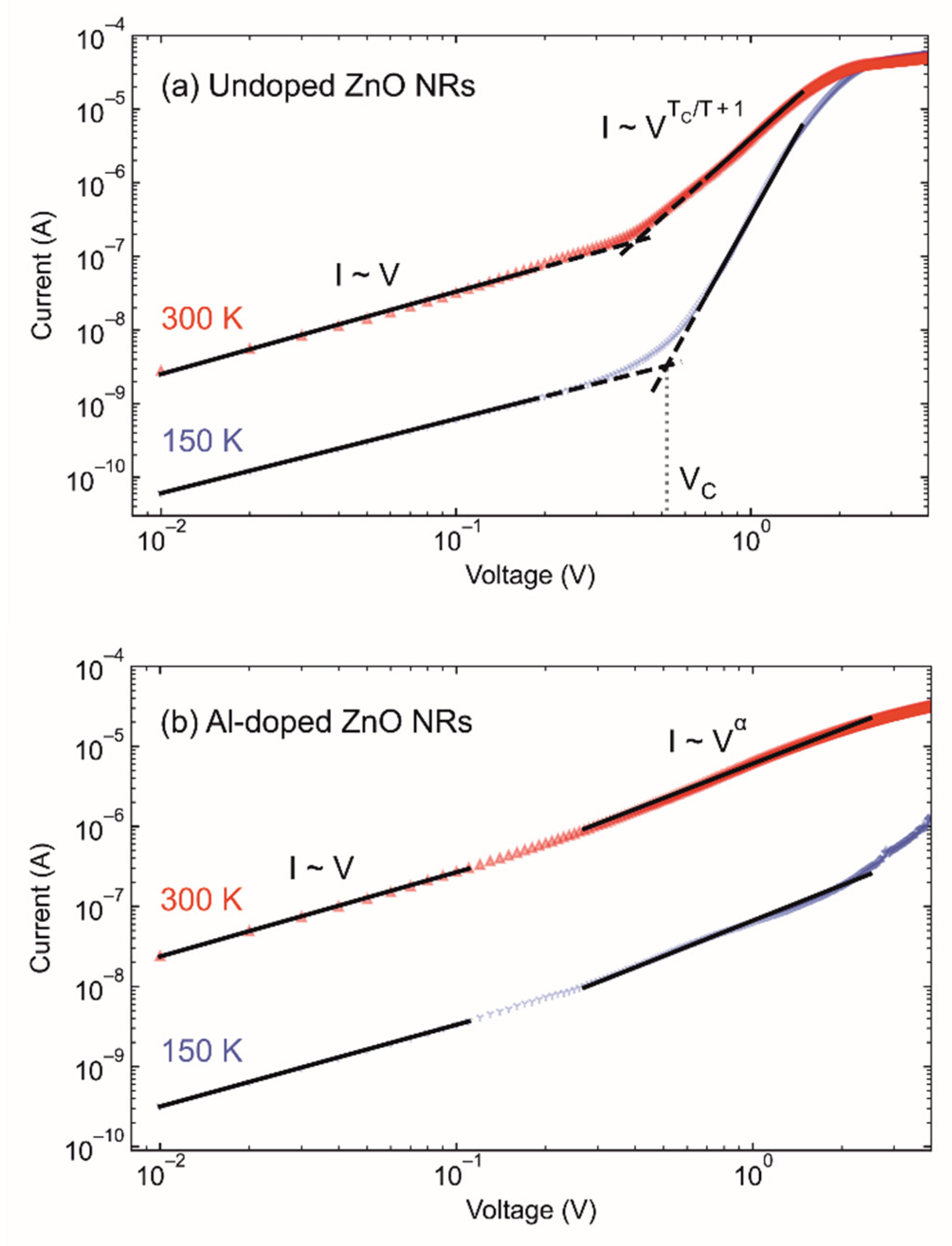
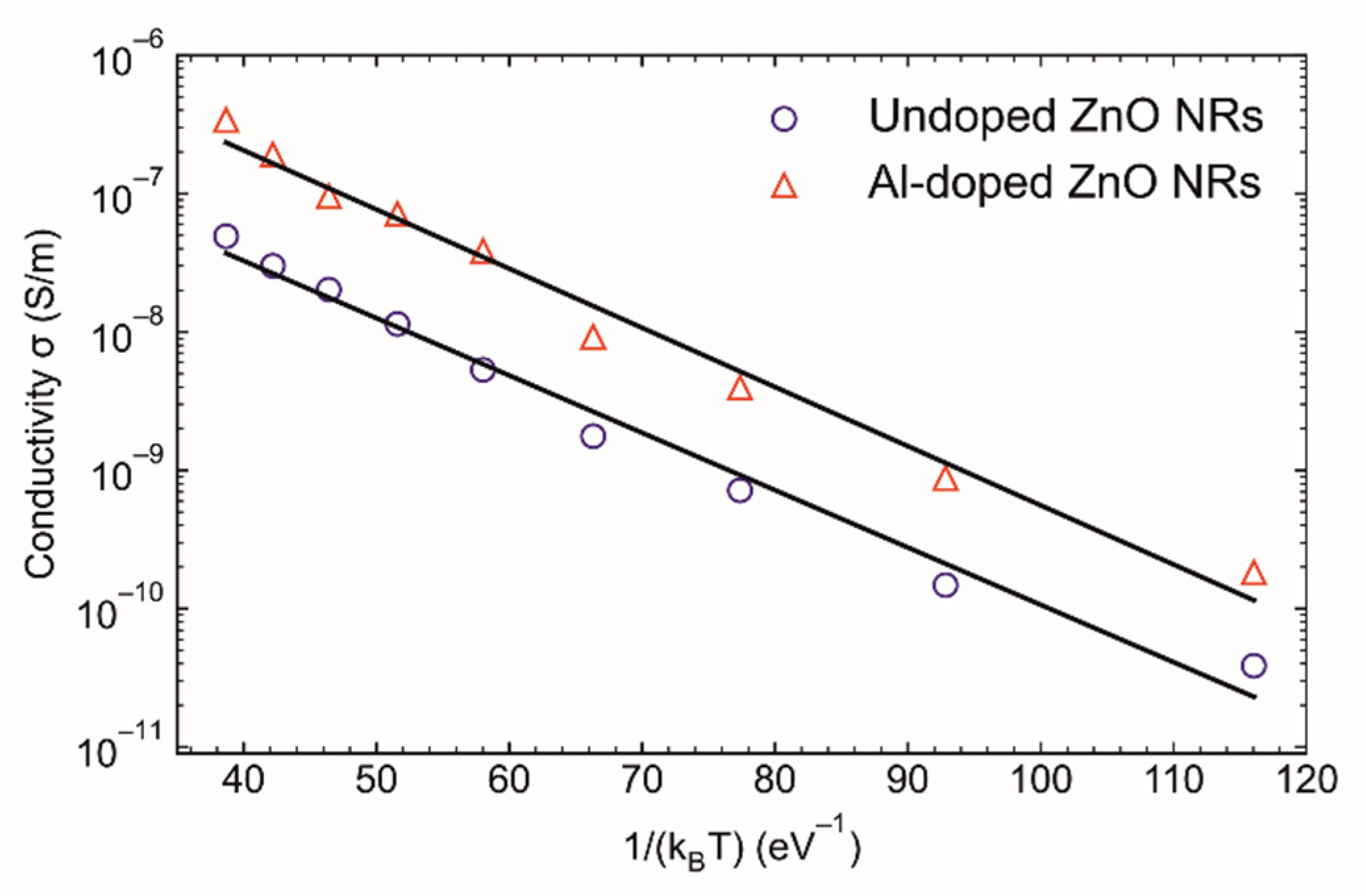
3.4. Optical and Electrical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Janotti, A.; Van De Walle, C.G. Fundamentals of zinc oxide as a semiconductor. Rep. Prog. Phys. 2009, 72, 126501. [Google Scholar] [CrossRef] [Green Version]
- Nirwal, V.S.; Rao, K. Interface state density distribution in Au/n-ZnO nanorods Schottky diodes. IOP Conf. Ser. Mater. Sci. Eng. 2012, 34, 12006. [Google Scholar] [CrossRef]
- Hofmann, D.M.; Hofstaetter, A.; Leiter, F.; Zhou, H.; Henecker, F.; Meyer, B.K.; Orlinskii, S.B.; Schmidt, J.; Baranov, P.G. Hydrogen: A Relevant Shallow Donor in Zinc Oxide. Phys. Rev. Lett. 2002, 88, 45504. [Google Scholar] [CrossRef]
- Lao, J.Y.; Wen, J.W.; Ren, Z.F. Hierarchical ZnO nanostructures. Nano Lett. 2002, 2, 1287–1291. [Google Scholar] [CrossRef]
- Cui, J. Zinc oxide nanowires. Mater. Charact. 2012, 64, 43–52. [Google Scholar] [CrossRef]
- Lim, S.K.; Hong, S.H.; Hwang, S.H.; Choi, W.M.; Kim, S.; Park, H.; Jeong, M.G. Synthesis of Al-doped ZnO Nanorods via Microemulsion Method and Their Application as a CO Gas Sensor. J. Mater. Sci. Technol. 2015, 31, 639–644. [Google Scholar] [CrossRef]
- Sung, Y.M.; Hsu, F.C.; Chen, Y.F. Improved charge transport in inverted polymer solar cells using surface engineered ZnO-nanorod array as an electron transport layer. Sol. Energy Mater. Sol. Cells 2014, 125, 239–247. [Google Scholar] [CrossRef]
- Placzek-Popko, E.; Gwozdz, K.; Gumienny, Z.; Zielony, E.; Pietruszka, R.; Witkowski, B.S.; Wachnicki, L.; Gieraltowska, S.; Godlewski, M.; Jacak, W.; et al. Si/ZnO nanorods/Ag/AZO structures as promising photovoltaic plasmonic cells. J. Appl. Phys. 2015, 117, 193101. [Google Scholar] [CrossRef]
- Park, W.I.; Kim, D.H.; Jung, S.; Yi, G. Metalorganic vapor-phase epitaxial growth of vertically well-aligned ZnO nanorods. Appl. Phys. Lett. 2002, 80, 4232–4234. [Google Scholar] [CrossRef]
- Yao, B.; Chan, Y.; Wang, N. Formation of ZnO nanostructures by a simple way of thermal evaporation. Appl. Phys. Lett. 2002, 81, 757. [Google Scholar] [CrossRef]
- Zhang, D.; Xue, Z.; Wang, Q. The mechanisms of blue emission from ZnO films deposited on glass substrate by rf magnetron sputtering. J. Phys. D Appl. Phys. 2002, 35, 2837–2840. [Google Scholar] [CrossRef]
- Choi, J.H.; Tabata, H.; Kawai, T. Initial preferred growth in zinc oxide thin films on Si and amorphous substrates by a pulsed laser deposition. J. Cryst. Growth 2001, 226, 493–500. [Google Scholar] [CrossRef]
- Paraguay, D.F.; Miki-Yoshida, M.; Morales, J.; Solis, J.; Estrada, L.W. Influence of Al, In, Cu, Fe and Sn dopants on the response of thin film ZnO gas sensor to ethanol vapour. Thin Solid Film 2000, 373, 137–140. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, Z.; Han, M.; Hao, X.; Zhu, F. A General Low-Temperature Route for Large-Scale Fabrication of Highly Oriented ZnO Nanorod/Nanotube Arrays. J. Am. Chem. Soc. 2005, 127, 2378–2379. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Mende, L.; MacManus-Driscoll, J.L. ZnO–nanostructures, defects, and devices. Mater. Today 2007, 10, 40–48. [Google Scholar] [CrossRef]
- Ashfold, M.N.R.; Doherty, R.P.; Ndifor-Angwafor, N.G.; Riley, D.J.; Sun, Y. The kinetics of the hydrothermal growth of ZnO nanostructures. Thin Solid Film 2007, 515, 8679–8683. [Google Scholar] [CrossRef]
- Mahmood, M.A.; Jan, S.; Shah, I.A.; Khan, I. Growth Parameters for Films of Hydrothermally Synthesized One-Dimensional Nanocrystals of Zinc Oxide. Int. J. Photoenergy 2016, 3153170. [Google Scholar] [CrossRef]
- Chen, R.; Zhu, P.; Deng, L.; Zhao, T.; Sun, R.; Wong, C. Effect of aluminum doping on the growth and optical and electrical properties of ZnO nanorods. ChemPlusChem 2014, 79, 743–750. [Google Scholar] [CrossRef]
- Kim, S.; Lee, S.H.; Kim, J.S.; Kim, J.S.; Kim, D.Y.; Kim, S.O.; Leem, J.Y. Effects of in doping on structural and optical properties of ZnO nanorods grown by hydrothermal method. Electron. Mater. Lett. 2013, 9, 509–512. [Google Scholar] [CrossRef]
- Khan, M.M.; Saadah, N.H.; Khan, M.E.; Harunsani, M.H.; Tan, A.L.; Cho, M.H. Potentials of Costus woodsonii leaf extract in producing narrow band gap ZnO nanoparticles. Mater. Sci. Semicond. Process. 2019, 91, 194–200. [Google Scholar] [CrossRef]
- Umar, K.; Aris, A.; Parveen, T.; Jaafar, J.; Majid, Z.A.; Reddy, A.V.B.; Talib, J. Synthesis, characterization of Mo and Mn doped ZnO and their photocatalytic activity for the decolorization of two different chromophoric dyes. Appl. Cat A Gen. 2015, 505, 507–514. [Google Scholar] [CrossRef]
- Khan, M.M.; Saadah, N.H.; Khan, M.E.; Harunsani, M.H.; Tan, A.L.; Cho, M.H. Phytogenic synthesis of band gap-narrowed ZnO nanoparticles using the bulb extract of Costus woodsonii. Bionanoscience 2019, 9, 334–344. [Google Scholar] [CrossRef]
- Jamjoum, H.A.A.; Umar, K.; Adnan, R.; Razali, M.R.; Ibrahim, M.N.M. Synthesis, characterization, and photocatalytic activities of graphene oxide/metal oxides nanocomposites: A review. Front. Chem. 2021, 9, 752276. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Umar, K.; Adnan, R.; Ibrahim, M.N.M.; Rashid, M. Graphene oxide–ZnO nanocomposite: An efficient visible light photocatalyst for degradation of rhodamine B. Appl. Nanosci. 2021, 11, 1291–1302. [Google Scholar] [CrossRef]
- Liu, X.; Pan, K.; Li, W.; Hu, D.; Liu, S.; Wang, Y. Optical and gas sensing properties of Al-doped ZnO transparent conducting films prepared by sol-gel method under different heat treatments. Ceram. Int. 2014, 40, 9931–9939. [Google Scholar] [CrossRef]
- Zhang, S.B.; Wei, S.; Zunger, A. Intrinsic n-type versus p-type doping asymmetry and the defect physics of ZnO. Phys. Rev. B 2001, 63, 75205. [Google Scholar] [CrossRef] [Green Version]
- Simpkins, B.S.; Mastro, M.A.; Eddy, C.R.; Hite, J.K.; Pehrsson, P.E. Space-charge-limited currents and trap characterization in coaxial AlGaN/GaN nanowires. J. Appl. Phys. 2011, 110, 44303. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, J.; Shi, S.; Gao, Y.; Wang, C.; Zhang, X.; Yin, S.; Li, L. Development of Solution-Processed ZnO Nanorod Arrays Based Photodetectors and the Improvement of UV Photoresponse via AZO Seed Layers. ACS Appl. Mater. Interfaces 2016, 8, 22647–22657. [Google Scholar] [CrossRef]
- Behrens, M.; Lolli, G.; Muratova, N.; Kasatkin, I.; Hävecker, M.; D’Alnoncourt, R.N.; Storcheva, O.; Köhler, K.; Muhler, M.; Schlögl, R. The effect of Al-doping on ZnO nanoparticles applied as catalyst support. Phys. Chem. Chem. Phys. 2013, 15, 1374–1381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hjiri, M.; El Mir, L.; Leonardi, S.G.; Pistone, A.; Mavilia, L.; Neri, G. Al-doped ZnO for highly sensitive CO gas sensors. Sens. Actuators B Chem. 2014, 196, 413–420. [Google Scholar] [CrossRef]
- Nečas, D.; Klapetek, P. Gwyddion: An Open-Source Software for SPM Data Analysis. Cent. Eur. J. Phys. 2012, 10, 181–188. [Google Scholar] [CrossRef]
- Khalid, N.R.; Hammad, A.; Tahir, M.B.; Iqbal, T.; Nabi, G.; Hussain, M.K. Enhanced photocatalytic activity of Al and Fe co-doped ZnO nanorods for methylene blue degradation. Ceram. Int. 2019, 45, 21430–21435. [Google Scholar] [CrossRef]
- Pelicano, C.M.; Yanagi, H. Enhanced charge transport in Al-doped ZnO nanotubes designed via simultaneous etching and Al doping of H2O-oxidized ZnO nanorods for solar cell aplications. J. Mater. Chem. C 2019, 7, 4653–4661. [Google Scholar] [CrossRef]
- Burgelman, M.; Nollet, P.; Degrave, S. Modelling polycrystalline semiconductor solar cells. Thin Solid Films 2000, 361–362, 527–532. [Google Scholar] [CrossRef]
- Nambala, F.J.; Nel, J.M.; Machatine, A.G.J.; Mwakikunga, B.W.; Njoroge, E.G.; Maabong, K.; Das, A.G.M.; Diale, M. Role of substrate and annealing temperature on the structure of ZnO and AlxZn1-xO thin films for solar cell applications. Phys. B Condens. Matter 2016, 480, 72–79. [Google Scholar] [CrossRef] [Green Version]
- Tansley, T.L.; Owen, S.J.T. Conductivity of Si-ZnO p-n and n-n heterojunctions. J. Appl. Phys. 1984, 55, 454–459. [Google Scholar] [CrossRef]
- Yildiz, A.; Cansizoglu, H.; Karabacak, T. Electrical transport in AZO nanorods. Appl. Surf. Sci. 2015, 352, 2–4. [Google Scholar] [CrossRef] [Green Version]
- Guo, Z.; Zhao, D.; Liu, Y.; Shen, D.; Zhang, J.; Li, B. Visible and ultraviolet light alternative photodetector based on ZnO nanowire/heterojunction nanowire/n-Si heterojunction. Appl. Phys. Lett. 2008, 93, 163501. [Google Scholar] [CrossRef] [Green Version]
- Lampert, M.A. Volume-controlled current injection in insulators. Rep. Prog. Phys. 1964, 27, 329. [Google Scholar] [CrossRef]
- Rose, A. Space-Charge-Limited Currents in Solids. Phys. Rev. 1955, 97, 1538–1544. [Google Scholar] [CrossRef]
- Lampert, M.A. Simplified theory of space-charge-limited currents in an insulator with traps. Phys Rev. 1956, 103, 1648–1656. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, W.; Wang, X.; Wang, Z.; Qi, J.; Zhang, Y. Size Dependence of Dielectric Constant in a Single Pencil-Like ZnO Nanowire. Nano Lett. 2012, 12, 1919–1922. [Google Scholar] [CrossRef] [PubMed]
- Hussain, I.; Soomro, M.Y.; Bano, N.; Nur, O.; Willander, M. Systematic study of interface trap and barrier inhomogeneities using I-V-T characteristics of Au/ZnO nanorods Schottky diode. J. Appl. Phys. 2013, 113, 234509. [Google Scholar] [CrossRef] [Green Version]
- Sankar Ganesh, R.; Navaneethan, M.; Mani, G.K.; Ponnusamy, S.; Tsuchiya, K.; Muthamizhchelvan, C.; Kawasaki, S.; Hayakawa, Y. Influence of Al doping on the structural, morphological, optical, and gas sensing properties of ZnO nanorods. J. Alloys Comp. 2017, 698, 555–564. [Google Scholar] [CrossRef]



| Technique | Specifications | Reference |
|---|---|---|
| Chemical vapor deposition | Accurate growth control, high T, carrier gas | [9] |
| Thermal evaporation | Simple, no catalysts, high temperature | [10] |
| RF magnetron sputtering | High sample purity, low cost, low pressure | [11] |
| Pulsed laser deposition | Gas pressure control, high T, low pressure | [12] |
| Spray pyrolysis | Does not require high-quality targets | [13] |
| Element | Mass (%) | MassNorm. (%) | Atom (%) |
|---|---|---|---|
| Oxygen | 4.85 | 4.72 | 8.11 |
| Aluminum | 0.91 | 0.88 | 0.90 |
| Silicon | 94.28 | 91.65 | 89.83 |
| Zinc | 2.83 | 2.75 | 1.16 |
| Total | 102.87 | 100.00 | 100.00 |
| Sample | σ (mS·cm−1) at 300 K | σ (mS·cm−1) at 150 K |
|---|---|---|
| Undoped ZnO NR | 3–5 × 10−5 | 5–9 × 10−7 |
| Al-doped ZnO NR | 2–4 × 10−4 | 3–5 × 10−6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panžić, I.; Capan, I.; Brodar, T.; Bafti, A.; Mandić, V. Structural and Electrical Characterization of Pure and Al-Doped ZnO Nanorods. Materials 2021, 14, 7454. https://doi.org/10.3390/ma14237454
Panžić I, Capan I, Brodar T, Bafti A, Mandić V. Structural and Electrical Characterization of Pure and Al-Doped ZnO Nanorods. Materials. 2021; 14(23):7454. https://doi.org/10.3390/ma14237454
Chicago/Turabian StylePanžić, Ivana, Ivana Capan, Tomislav Brodar, Arijeta Bafti, and Vilko Mandić. 2021. "Structural and Electrical Characterization of Pure and Al-Doped ZnO Nanorods" Materials 14, no. 23: 7454. https://doi.org/10.3390/ma14237454
APA StylePanžić, I., Capan, I., Brodar, T., Bafti, A., & Mandić, V. (2021). Structural and Electrical Characterization of Pure and Al-Doped ZnO Nanorods. Materials, 14(23), 7454. https://doi.org/10.3390/ma14237454






