Hemodynamics Challenges for the Navigation of Medical Microbots for the Treatment of CVDs
Abstract
1. Introduction
2. Applications of Microbots in the Human Circulatory System
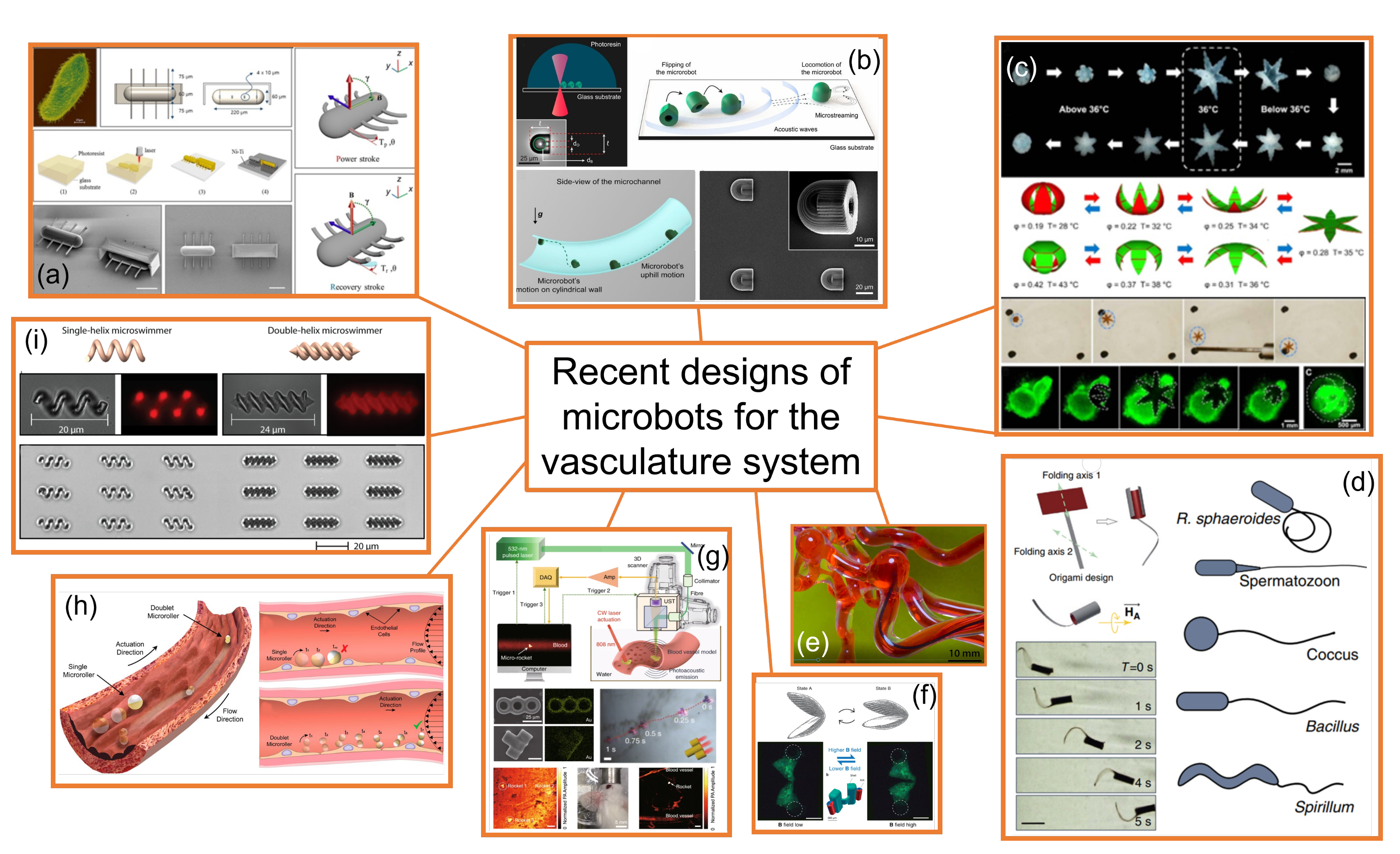
3. Microbots Designed for the Circulatory System
- (a)
- Ciliary microbots. Kim et al. [35] developed and fabricated a ciliary microbot, allowing the cilia to beat back and forth using an electromagnetic coil system, providing the possibility of moving at a speed of about 340 micrometers per second, with a much greater range of maneuvrability than previous microbots operating under magnetic attraction drive, as the controller can shift the angle of the microbot from zero to 120 degrees. Thus, ciliary microbot locomotion represents a high-efficiency swimming method in viscous fluid with non-reciprocal actuation; however, the 220 micrometer-long robot would limit the range of vessels. This kind of microbot may be useful for drug delivery purposes.
- (b)
- Soft attractor wall microbot. Aghakhani et al. [36,37] developed a 3D bullet-shaped microbot containing a spherical air bubble in its internal cavity where the bubble resonates using acoustic waves. They combined the acoustic powering with magnetic steering with the purpose of effective microbot actuation and navigation in confined and hard-to-reach body locations. This design would allow targeted drug delivery. Swimming near the vessel walls is an efficient type of propulsion as high-speed blood flow is avoided; however, lossy material, such as body tissues, would require generating sufficient acoustic radiation force, and surface microtopography of blood vessels, in the size scale of the microbots, would represent a major hurdle for robust locomotion of surface-rolling microbots against the blood flow.
- (c)
- Self-folding microbot. Breger et al. [38] proposed a self-folding functional microbot gripper with embedded iron oxide (Fe2O3) nanoparticles in the porous hydrogel layer, allowing the microgrippers to be responsive and remotely guided using magnetic fields. They illustrated the operation and functionality of these polymeric microgrippers for soft robotic and surgical applications, such as capturing and performing an excision of cells, such as fibroblast clumps. They could also be used for drug delivery purposes. Small oscillations in the temperature of blood would modify the shape of the microbot and, therefore, may alter its hemodynamics without control.
- (d)
- Sperm-shaped microbot. Another microbot inspired by natural structures consists of a head connected to a flexible tail, similar to a sperm cell [39,40]. This kind of microbot uses weak oscillating magnetic fields for steering and propulsion. Organisms that move around in a biological system use non-reciprocating devices, such as flagella or cilia, and this has been naturally proven as an efficient way of swimming in non-Newtonian fluids. It could be used for drug delivery and clearing out clogged arteries. Being soft, flexible, and motorless is positive for its application in the cardiovascular system.
- (e)
- Snake-shaped microbot [41]. Kim et al [41], introduced another class of magnetically actuated soft robots. They are based on ferromagnetic soft materials and magnetic polarities were programmed in the body of the robot, which confers the advantage of enabling navigation through complex, narrow, fragile, and tortuous environments and moving around the structure of veins in the brain. These devices may also be used for light transportation, enabling the possibility of performing a photoablation treatment of clots. Thus, they could be used for the diagnosis and treatment of blood clots and aneurysms, as well as performing other small-scale operations in the brain. Despite a hydrogel slime cover minimizing friction during travel, the increasing contact with the solid surface of the microbot may damage deteriorated vessels walls.
- (f)
- Scallop-shaped microbot. This design was proposed by Qiu et al. [42] and is able to paddle through non-Newtonian fluids such as blood and plasma, but also in other biofluids. It is striking that this design uses a reciprocal method of movement to propel the microscallops, as this generally does not work in such fluids. This device would be useful for drug delivery purposes. Its simple design allows for 3D printing fabrication; however, its 800-micron size would limit the size of the vessels through which it could swim.
- (g)
- Microrocket robot. This design was recently proposed by Li et al. [43] and solved two of the major challenges in small mobile robots in the biomedical field, that is, driving at high speed through a viscous media and observing the position of a single microbot inside the bloodstream. A moving speed of 2.8 mm/s under near-infrared light actuation in a static Newtonian analogue solution was reported; however, its performance through real blood may be different.
- (h)
- Surface microrollers. Alapan et al. [44] reported cell-sized multifunctional surface microrollers with 3.0- and 7.8-micrometer diameters for targeted drug delivery into specific cells and controlled navigation. The spherical microrollers consist of magnetically responsive Janus microparticles that can be functionalized with specific targeting antibodies against cancer cells and or light-cleavable cancer drug molecules. Swimming near the vessel walls is an efficient type of propulsion as high-speed blood flow is avoided, thus the microrollers can be propelled against physiologically relevant blood flow in endothelialized microchannels and can even move upstream on inclined three-dimensional surfaces in physiologically relevant blood flow. Surface microtopography of blood vessels, in the size scale of the microbots, is a major hurdle for robust locomotion of surface-rolling microbots against the blood flow.
- (i)
- Helical microbots. Helical microbots are another commonly studied design, aiming at targeted drug delivery, cell sorting, and cell characterization. They used helical propulsion to break time-reversal symmetry and provide locomotion at a low Reynolds number in the bloodstream via the action of a uniform or non-uniform rotating magnetic field. Helical microbots could be used for rubbing against blood clots [39]. Khalil et al. [39] experimentally investigated the influence of lysis and rubbing on the removal of clots at a low Reynolds number inside catheter segments, which was performed by helical robots. They also developed a model to predict an optimal rubbing frequency of the helical robot to reach a maximum removal rate, showing better mechanical grinding of blood clots than chemical lysis. Nevertheless, the influence of the tip geometry on the removal rate on the 3D network of blood clots will need to be tested. The size and lack of flexibility may damage the walls of the vessels and limit the size of the vessels in applications.
4. Human Circulatory Challenges
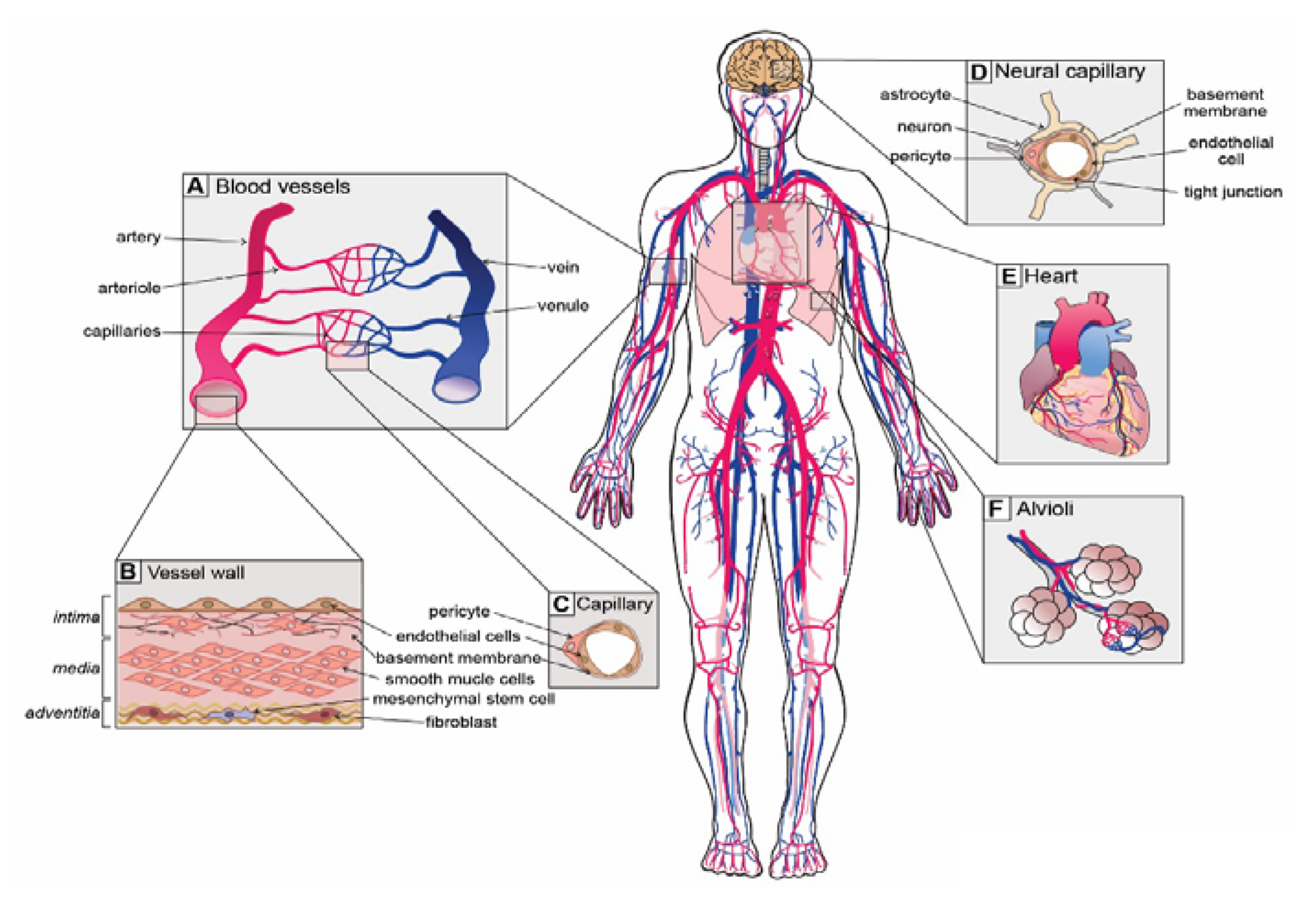
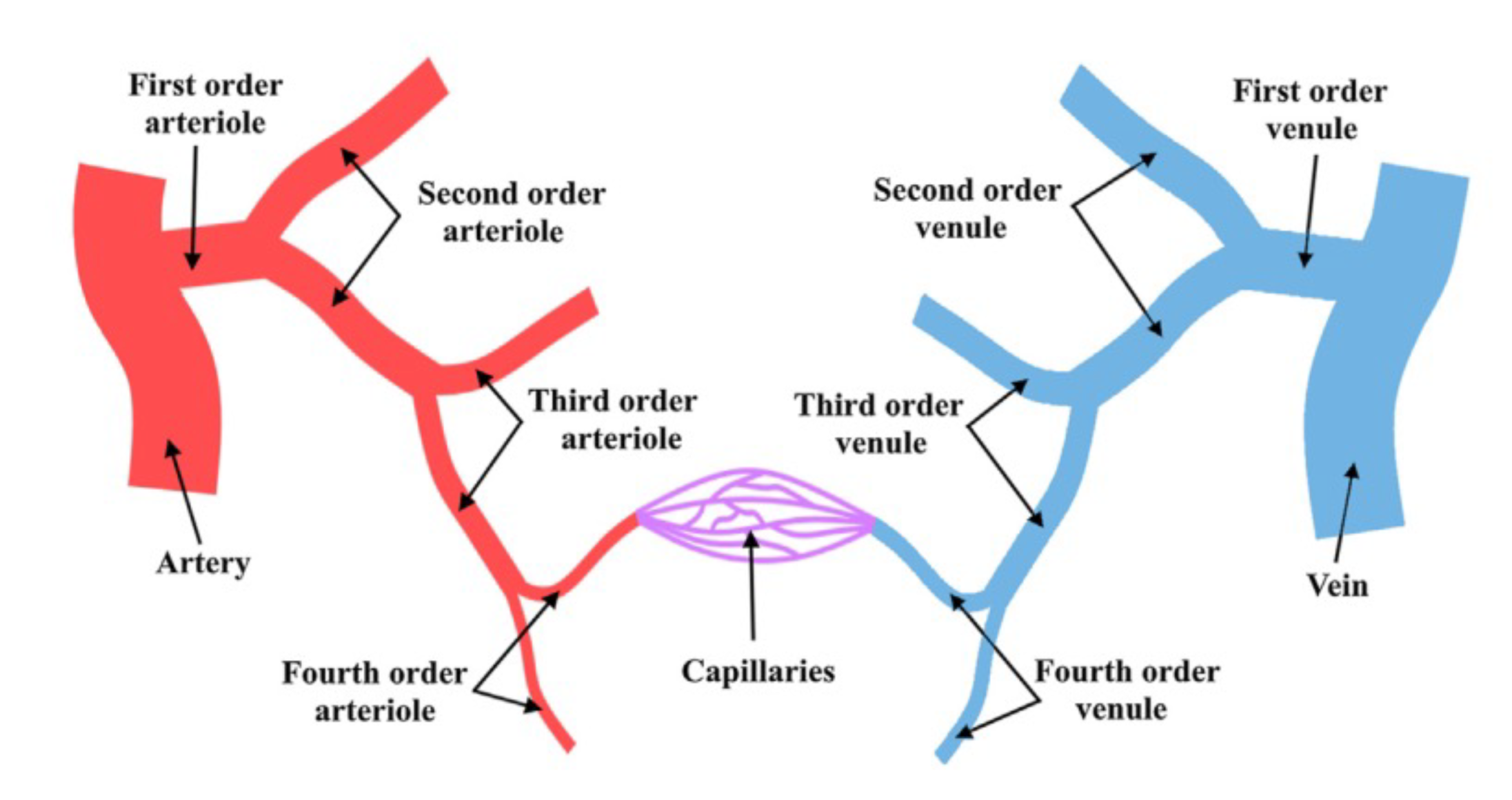
4.1. Blood Vessel Network
4.1.1. Type and Function of Blood Vessels
- Elastic arteries (i.e., aorta and pulmonary arteries), which are characterized by a thin wall and a larger diameter (normally their diameter is larger than 10 mm) and this type of artery receives blood directly from the heart, so their walls contain high elastin concentrations, allowing them to stretch and support high blood pressure.
- Muscular arteries or distributing arteries (i.e., radial and femoral arteries), as the name indicates, distribute the blood to all the organs and tissues. This type of artery is composed of a high concentration of smooth muscles. The range diameter is 0.1 to 10 mm.
- Arterioles are the smallest type of artery. They work as resistors causing a drop in blood flow pressure and their average vessel diameter is 30 m. The arteriole wall typically has one or two layers of smooth muscle.
4.1.2. Vascular Bifurcations
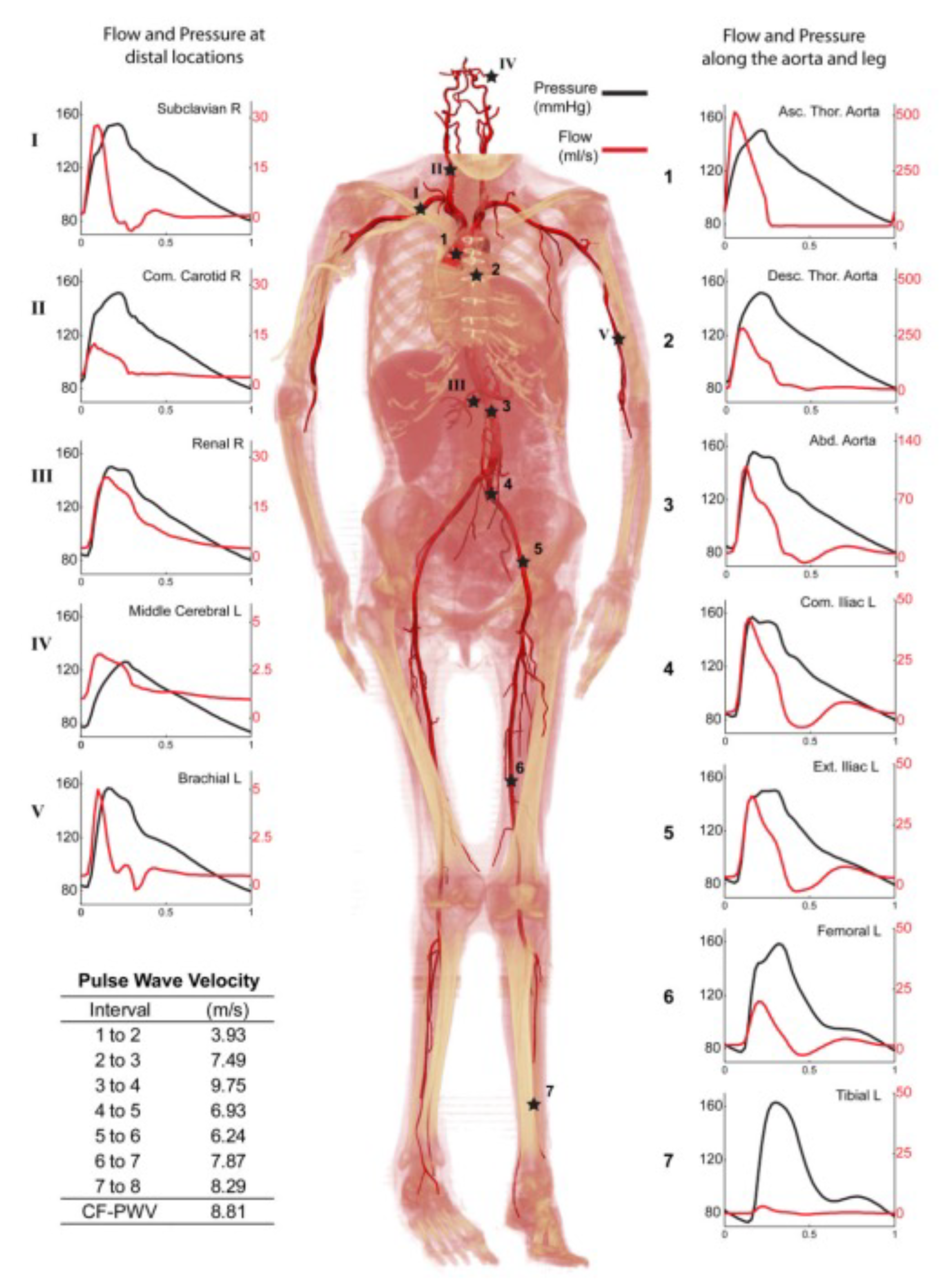
4.2. Cardiac Cycle
4.3. Characteristic Dimensionless Numbers
- The Deborah number () is the ratio of the time it takes the material to adjust to the applied stresses or deformation, i.e., the relaxation time and the characteristic time scale of the deformation process, i.e., the time of observation (T).
- The Weissenberg number (Wi) is a dimensionless group representing the ratio between elastic forces and viscous forces [65].
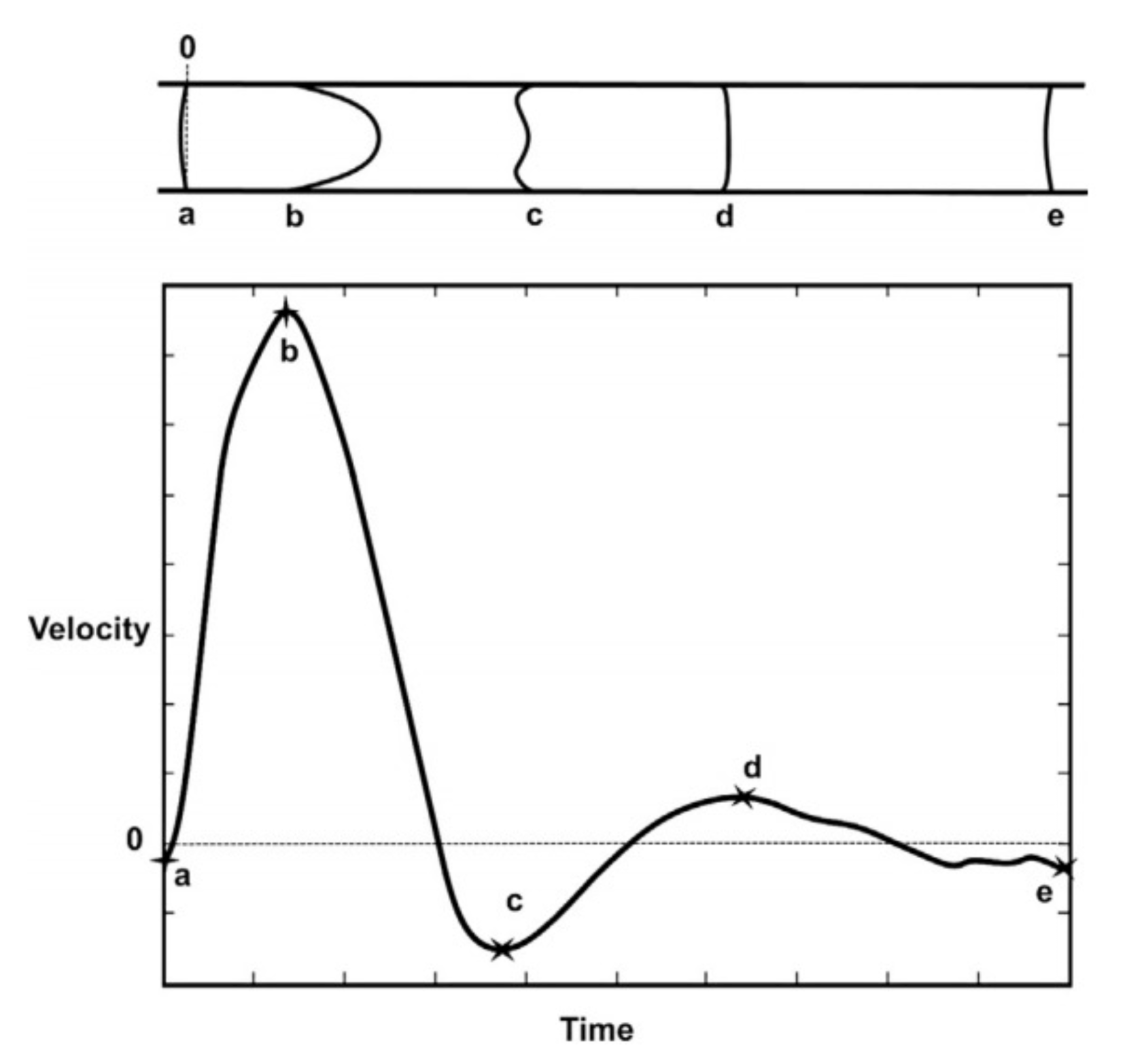
5. Pulsatile Flow
6. Final Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- World Health Organization. World Health Statistics 2020: Monitoring Health for the SDGs, Sustainable Development Goals; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- WHO Regional Office for Europe. European Health Report 2018: More than Numbers—Evidence for All; WHO Regional Office for Europe: Geneva, Switzerland, 2018. [Google Scholar]
- Tarride, J.E.; Lim, M.; DesMeules, M.; Luo, W.; Burke, N.; O’Reilly, D.; Bowen, J.; Goeree, R. A review of the cost of cardiovascular disease. Can. J. Cardiol. 2009, 25, 195–202. [Google Scholar] [CrossRef]
- Physical Intelligence. Department at Max Planck Institute for Intelligent Systems in Stuttgart, Germany. 2021. Available online: https://pi.is.mpg.de/ (accessed on 23 November 2021).
- Khalil, I.S.; Klingner, A.; Misra, S. Chapter 1—Introduction: Soft microrobots. In Mathematical Modeling of Swimming Soft Microrobots; Khalil, I.S., Klingner, A., Misra, S., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 1–15. [Google Scholar] [CrossRef]
- Martínez-Aranda, S.; Galindo-Rosales, F.J.; Campo-Deaño, L. Assessing the Dynamic Performance of Microbots in Complex Fluid FlowsComplex flow dynamics around 3D microbot prototypes. Soft Matter 2016, 12, 2334–2347. [Google Scholar] [CrossRef] [PubMed]
- Campo-Deaño, L. Assessing the Dynamic Performance of Microbots in Complex Fluid Flows. Appl. Sci. 2016, 6, 410. [Google Scholar] [CrossRef]
- Chaichana, T.; Sun, Z.; Jewkes, J. Computation of hemodynamics in the left coronary artery with variable angulations. J. Biomech. 2011, 44, 1869–1878. [Google Scholar] [CrossRef] [PubMed]
- Doutel, E.; Pinto, S.; Campos, J.; Miranda, J. μPIV Analysis and Numerical Simulation of the Flow in Mili-scale Channels Developed for Studies in Hemodynamics. APCBEE Procedia 2013, 7, 132–137. [Google Scholar] [CrossRef][Green Version]
- Doutel, E.; Pinto, S.I.; Campos, J.B.; Miranda, J.M. Link between deviations from Murray’s Law and occurrence of low wall shear stress regions in the left coronary artery. J. Theor. Biol. 2016, 402, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Temel, F.Z.; Yesilyurt, S. Simulation-based analysis of micro-robots swimming at the center and near the wall of circular mini-channels. Microfluid. Nanofluidics 2013, 14, 287–298. [Google Scholar] [CrossRef]
- Acemoglu, A.; Yesilyurt, S. Effects of geometric parameters on swimming of micro organisms with single helical flagellum in circular channels. Biophys. J. 2014, 106, 1537–1547. [Google Scholar] [CrossRef] [PubMed]
- Caldag, H.; Yesilyurt, S. Dynamics of Artificial Helical Microswimmers Under Confinement. In Proceedings of the ASME 2018 16th International Conference on Nanochannels, Microchannels, and Minichannels, Dubrovnik, Croatia, 10–13 June 2018; Volume V001T13A001. [Google Scholar]
- Gasser, T.C.; Ogden, R.W.; Holzapfel, G.A. Hyperelastic modelling of arterial layers with distributed collagen fibre orientations. J. R. Soc. Interface 2006, 3, 15–35. [Google Scholar] [CrossRef] [PubMed]
- Malvè, M.; Pérez del Palomar, A.; Chandra, S.; López-Villalobos, J.L.; Mena, A.; Fino, E.A.; Ginel, A.; Doblaré, M. FSI Analysis of a healthy and a stenotic human trachea under impedance-based boundary conditions. J. Biomech. Eng. 2011, 133, 021001. [Google Scholar] [CrossRef]
- Valencia, A.; Morales, H.; Rivera, R.; Bravo, E.; Galvez, M. Blood flow dynamics in patient-specific cerebral aneurysm models: The relationship between wall shear stress and aneurysm area index. Med. Eng. Phys. 2008, 30, 329–340. [Google Scholar] [CrossRef]
- Pinho, N.; Castro, C.F.; António, C.C.; Bettencourt, N.; Sousa, L.C.; Pinto, S. Unsteady flow and mass transfer in models of stenotic arteries considering fluid-structure interaction.Correlation between geometric parameters of the left coronary artery and hemodynamic descriptors of atherosclerosis: FSI and statistical study. Med Biol. Eng. Comput. 2019, 57, 715–729. [Google Scholar] [CrossRef]
- Drexler, K. Nanosystems: Molecular Machinery, Manufacturing, and Computation; John Wiley & Sons, Incorporated: Hoboken, NJ, USA, 1992. [Google Scholar]
- Freitas, R.A.J. Nanotechnology, nanomedicine and nanosurgery. Int. J. Surg. 2005, 3, 243–246. [Google Scholar] [CrossRef] [PubMed]
- Nelson, B.J.; Kaliakatsos, I.K.; Abbott, J.J. Microrobots for minimally invasive medicine. Annu. Rev. Biomed. Eng. 2010, 12, 55–85. [Google Scholar] [CrossRef] [PubMed]
- Montrief, T.; Koyfman, A.; Long, B. Coronary artery bypass graft surgery complications: A review for emergency clinicians. Am. J. Emerg. Med. 2018, 36, 2289–2297. [Google Scholar] [CrossRef] [PubMed]
- Goldschmidt-Clermont, P.; Kandzari, D.; Khouri, S.; Ferrari, M. Nanotechnology Needs for Cardiovascular Sciences. Biomed. Microdev. 2001, 3, 83–88. [Google Scholar] [CrossRef]
- Miloro, P.; Sinibaldi, E.; Menciassi, A.; Dario, P. Removing vascular obstructions: A challenge, yet an opportunity for interventional microdevices. Biomed. Microdev. 2012, 14, 511–532. [Google Scholar] [CrossRef] [PubMed]
- Peyer, K.E.; Zhang, L.; Nelson, B.J. Bio-inspired magnetic swimming microrobots for biomedical applications. Nanoscale 2013, 5, 1259–1272. [Google Scholar] [CrossRef]
- Singh, A.; Ansari, M.; Mahajan, M.; Srivastava, S.; Kashyap, S.; Dwivedi, P.; Pandit, V.; Katha, U. Sperm Cell Driven Microrobots—Emerging Opportunities and Challenges for Biologically Inspired Robotic Design. Micromachines 2011, 11, 448. [Google Scholar] [CrossRef] [PubMed]
- Mitragotri, S. Healing sound: The use of ultrasound in drug delivery and other therapeutic applications. Nat. Rev. Drug Discov. 2005, 4, 255–260. [Google Scholar] [CrossRef]
- Martel, S. Bacterial microsystems and microrobots. Biomed. Microdev. 2012, 14, 1033–1045. [Google Scholar] [CrossRef] [PubMed]
- Martel, S. Microrobotics in the vascular network: Present status and next challenges. J. Micro-Bio Robot. 2013, 8, 41–52. [Google Scholar] [CrossRef]
- Dogangil, G.; Ergeneman, O.; Abbott, J.J.; Pane, S.; Hall, H.; Muntwyler, S.; Nelson, B.J. Toward targeted retinal drug delivery with wireless magnetic microrobots. In Proceedings of the 2008 IEEE/RSJ International Conference on Intelligent Robots and Systems, Nice, France, 22–26 September 2008; pp. 1921–1926. [Google Scholar] [CrossRef]
- Perez, A.; Zinner, M.; Ashley, S.; Brooks, D.; Whang, E. What is the value of telerobotic technology in gastrointestinal surgery? Surg. Endosc. Other Interv. Tech. 2003, 17, 811–813. [Google Scholar] [CrossRef] [PubMed]
- Shah, B.C.; Buettner, S.L.; Lehman, A.C.; Farritor, S.M.; Oleynikov, D. Miniature In Vivo Robotics and Novel Robotic Surgical Platforms. Urol. Clin. N. Am. 2009, 36, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Mair, L.O.; Adam, G.; Chowdhury, S.; Davis, A.; Arifin, D.R.; Vassoler, F.M.; Engelhard, H.H.; Li, J.; Tang, X.; Weinberg, I.N.; et al. Soft Capsule Magnetic Millirobots for Region-Specific Drug Delivery in the Central Nervous System. Front. Robot. AI 2021, 8, 226. [Google Scholar] [CrossRef] [PubMed]
- Kafash Hoshiar, A.; Jeon, S.; Kim, K.; Lee, S.; Kim, J.Y.; Choi, H. Steering Algorithm for a Flexible Microrobot to Enhance Guidewire Control in a Coronary Angioplasty Application. Micromachines 2018, 9, 617. [Google Scholar] [CrossRef]
- Jeon, S.; Hoshiar, A.K.; Kim, K.; Lee, S.; Kim, E.; Lee, S.; Kim, J.Y.; Nelson, B.J.; Cha, H.J.; Yi, B.J.; et al. A Magnetically Controlled Soft Microrobot Steering a Guidewire in a Three-Dimensional Phantom Vascular Network. Soft Robot. 2019, 6, 54–68. [Google Scholar] [CrossRef]
- Kim, S.; Lee, S.; Lee, J.; Nelson, B.J.; Zhang, L.; Choi, H. Fabrication and Manipulation of Ciliary Microrobots with Non-reciprocal Magnetic Actuation. Sci. Rep. 2016, 6, 30713. [Google Scholar] [CrossRef] [PubMed]
- Aghakhani, A.; Yasa, O.; Wrede, P.; Sitti, M. Acoustically powered surface-slipping mobile microrobots. Proc. Natl. Acad. Sci. USA 2020, 117, 3469–3477. [Google Scholar] [CrossRef] [PubMed]
- Aghakhani, A.; Cetin, H.; Erkoc, P.; Tombak, G.I.; Sitti, M. Flexural wave-based soft attractor walls for trapping microparticles and cells. Lab A Chip 2021, 21, 582–596. [Google Scholar] [CrossRef]
- Breger, J.C.; Yoon, C.; Xiao, R.; Kwag, H.R.; Wang, M.O.; Fisher, J.P.; Nguyen, T.D.; Gracias, D.H. Self-Folding Thermo-Magnetically Responsive Soft Microgrippers. ACS Appl. Mater. Interfaces 2015, 7, 3398–3405. [Google Scholar] [CrossRef]
- Khalil, I.S.M.; Dijkslag, H.C.; Abelmann, L.; Misra, S. MagnetoSperm: A microrobot that navigates using weak magnetic fields. Appl. Phys. Lett. 2014, 104, 223701. [Google Scholar] [CrossRef]
- Huang, H.W.; Sakar, M.S.; Petruska, A.J.; Pané, S.; Nelson, B.J. Soft micromachines with programmable motility and morphology. Nat. Commun. 2016, 7, 12263. [Google Scholar] [CrossRef]
- Kim, Y.; Parada, G.A.; Liu, S.; Zhao, X. Ferromagnetic soft continuum robots. Sci. Robot. 2019, 4, eaax7329. [Google Scholar] [CrossRef] [PubMed]
- Qiu, T.; Lee, T.C.; Mark, A.G.; Morozov, K.I.; Münster, R.; Mierka, O.; Turek, S.; Leshansky, A.M.; Fischer, P. Swimming by reciprocal motion at low Reynolds number. Nat. Commun. 2014, 5, 5119. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Liu, C.; Yang, Y.; Wang, L.; Shen, Y. Micro-rocket robot with all-optic actuating and tracking in blood. Light. Sci. Appl. 2020, 9, 84. [Google Scholar] [CrossRef]
- Alapan, Y.; Bozuyuk, U.; Erkoc, P.; Karacakol, A.C.; Sitti, M. Multifunctional surface microrollers for targeted cargo delivery in physiological blood flow. Sci. Robot. 2020, 5, eaba5726. [Google Scholar] [CrossRef] [PubMed]
- Bozuyuk, U.; Alapan, Y.; Aghakhani, A.; Yunusa, M.; Sitti, M. Shape anisotropy-governed locomotion of surface microrollers on vessel-like microtopographies against physiological flows. Proc. Natl. Acad. Sci. USA 2021, 118, e2022090118. [Google Scholar] [CrossRef] [PubMed]
- Ceylan, H.; Dogan, N.O.; Yasa, I.C.; Musaoglu, M.N.; Kulali, Z.U.; Sitti, M. 3D printed personalized magnetic micromachines from patient blood-derived biomaterials. Sci. Adv. 2021, 7, eabh0273. [Google Scholar] [CrossRef] [PubMed]
- Sitti, M.; Ceylan, H.; Hu, W.; Giltinan, J.; Turan, M.; Yim, S.; Diller, E. Biomedical Applications of Untethered Mobile Milli/Microrobots. Proc. IEEE 2015, 103, 205–224. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Cho, Y.; Jeon, A.H.; Hogenauer, B.; Kensey, K.R. A new method for blood viscosity measurement. J. Non-Newton. Fluid Mech. 2000, 94, 47–56. [Google Scholar] [CrossRef]
- Barrett, K.E.; Barman, S.M.; Boitano, S.; Brooks, H.L. The Heart as a Pump; McGraw-Hill Education: New York, NY, USA, 2018. [Google Scholar]
- Bondareva, O.; Sheikh, B.N. Vascular Homeostasis and Inflammation in Health and Disease—Lessons from Single Cell Technologies. Int. J. Mol. Sci. 2020, 21, 4688. [Google Scholar] [CrossRef] [PubMed]
- Krams, R.; Back, M. ESC Textbook of Vascular Biology (The European Society of Cardiology Series); Oxford University Press: Oxford, UK, 2017. [Google Scholar]
- Campo-Deaño, L.; Oliveira, M.S.N.; Pinho, F.T. A Review of Computational Hemodynamics in Middle Cerebral Aneurysms and Rheological Models for Blood Flow. Appl. Mech. Rev. 2015, 67, 030801. [Google Scholar] [CrossRef]
- Doutel, E. Hemodynamics in the Left Coronary Artery—Numerical and In Vitro Approaches. 2016. Available online: https://repositorio-aberto.up.pt/handle/10216/85899 (accessed on 20 October 2021).
- Ballesteros, L.E.; Ramirez, L.M. Morphological expression of the left coronary artery: A direct anatomical study. Folia Morphol. 2008, 67, 135–142. [Google Scholar]
- Doutel, E.; Carneiro, J.; Campos, J.; Miranda, J. Experimental and numerical methodology to analyze flows in a coronary bifurcation. Eur. J. Mech. B/Fluids 2018, 67, 341–356. [Google Scholar] [CrossRef]
- Harvey, W. Exercitatio Anatomica de Motu Cordis et Sanguinis in Animalibus/by William Harvey; Leake, C.D., Translator; C.C. Thomas: Springfield, IL, USA, 1941. [Google Scholar]
- Ostadfar, A. Chapter 2—Macrocirculation System. In Biofluid Mechanics; Ostadfar, A., Ed.; Academic Press: Cambridge, MA, USA, 2016; pp. 61–86. [Google Scholar] [CrossRef]
- Xiao, N.; Humphrey, J.D.; Figueroa, C.A. Multi-scale computational model of three-dimensional hemodynamics within a deformable full-body arterial network. J. Comput. Phys. 2013, 244, 22–40. [Google Scholar] [CrossRef]
- Safar, M.E.; Lacolley, P. Disturbance of macro- and microcirculation: Relations with pulse pressure and cardiac organ damage. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H1–H7. [Google Scholar] [CrossRef] [PubMed]
- Ostadfar, A. Chapter 3—Microcirculation System. In Biofluid Mechanics; Academic Press: Cambridge, MA, USA, 2016; pp. 87–109. [Google Scholar] [CrossRef]
- Buckingham, E. On Physically Similar Systems; Illustrations of the Use of Dimensional Equations. Phys. Rev. 1914, 4, 345–376. [Google Scholar] [CrossRef]
- Houghton, E.; Carpenter, P.; Collicott, S.H.; Valentine, D.T. Chapter 1—Basic Concepts and Definitions. In Aerodynamics for Engineering Students, 7th ed.; Houghton, E., Carpenter, P., Collicott, S.H., Valentine, D.T., Eds.; Butterworth-Heinemann: Oxford, UK; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1–86. [Google Scholar] [CrossRef]
- Ceylan, H.; Yasa, I.C.; Kilic, U.; Hu, W.; Sitti, M. Translational prospects of untethered medical microrobots. Prog. Biomed. Eng. 2019, 1, 012002. [Google Scholar] [CrossRef]
- Galindo-Rosales, F.J.; Campo-Deaño, L.; Sousa, P.C.; Ribeiro, V.M.; Oliveira, M.S.; Alves, M.A.; Pinho, F.T. Viscoelastic instabilities in micro-scale flows. Exp. Therm. Fluid Sci. 2014, 59, 128–139. [Google Scholar] [CrossRef]
- Poole, R. The Deborah and Weissenberg numbers. Br. Soc. Rheol. Rheol. Bull. 2012, 53, 32–39. [Google Scholar]
- Vosse, V.D.F.N.; van Dongen, M.E.H. Cardiovascular Fluid Mechanics: Lecture notes; Technische Universiteit Eindhoven: Eindhoven, The Netherlands, 1998. [Google Scholar]
- Stalder, A.F.; Frydrychowicz, A.; Russe, M.F.; Korvink, J.G.; Hennig, J.; Li, K.; Markl, M. Assessment of flow instabilities in the healthy aorta using flow-sensitive MRI. J. Magn. Reson. Imaging JMRI 2011, 33, 839–846. [Google Scholar] [CrossRef]
- Mandal, S.M.; Layek, G.C. Analysis of Pulsatile Blood Flow in a Model of Arterial Aneurysm with Non-Uniform Blood Viscosity. Chin. J. Phys. 2014, 52, 1702–1717. [Google Scholar]
- Khabakhpasheva, E.; Popov, V.; Kekalov, A.; Mikhailova, E. Pulsating flow of viscoelastic fluids in tubes. J. Non Newton. Fluid Mech. 1989, 33, 289–304. [Google Scholar] [CrossRef]
- Amaratunga, M.; Rabenjafimanantsoa, H.A.; Time, R.W. Comparison of oscillatory flow conditions in Newtonian and non-Newtonian fluids using PIV and high-speed image analysis. Flow Meas. Instrum. 2019, 70, 101628. [Google Scholar] [CrossRef]
- Vazquez-Vergara, P.; Torres-Herrera, U.; Caballero-Robledo, G.A.; Olguin, L.F.; Corvera Poiré, E. Experimental Resonances in Viscoelastic Microfluidics. Front. Phys. 2021, 9, 262. [Google Scholar] [CrossRef]
- Galindo-Rosales, F.J. Complex Fluids and Rheometry in Microfluidics. In Complex Fluid Flows in Microfluidics; Galindo-Rosales, F.J., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–23. [Google Scholar] [CrossRef]
- Rath, S.; Mahapatra, B. Low Reynolds Number Pulsatile Flow of a Viscoelastic Fluid Through a Channel: Effects of Fluid Rheology and Pulsation Parameters. J. Fluids Eng. 2021, 144. [Google Scholar] [CrossRef]
- Javadzadegan, J.; Esmaeili, M.; Majidi, S.; Fakhimghanbarzadeh, B. Pulsatile flow of viscous and viscoelastic fluids in constricted tubes. J. Mech. Sci. Technol. 2009, 23, 2456–2467. [Google Scholar] [CrossRef]
- Sajid, M.; Zaman, A.; Ali, N.; Siddiqui, A.M. Pulsatile Flow of Blood in a Vessel Using an Oldroyd-B fluid. Int. J. Nonlinear Sci. Numer. Simul. 2015, 16, 197–206. [Google Scholar] [CrossRef]
- Prandtl, L. On fluid motions with very small friction. In Proceedings of the 3rd International Mathematical Congress, Heidelberg, Germany, 8–13 August 1904; pp. 484–491. [Google Scholar]
- Mikheev, N.; Molochnikov, V.; Mikheev, A.; Dushina, O. Hydrodynamics and heat transfer of pulsating flow around a cylinder. Int. J. Heat Mass Transf. 2017, 109, 254–265. [Google Scholar] [CrossRef]
- Belharet, K.; Folio, D.; Ferreira, A. Control of a magnetic microrobot navigating in microfluidic arterial bifurcations through pulsatile and viscous flow. In Proceedings of the 2012 IEEE/RSJ International Conference on Intelligent Robots and Systems, Vilamoura-Algarve, Portugal, 7–12 October 2012; pp. 2559–2564. [Google Scholar] [CrossRef]
- Sadelli, L.; Fruchard, M.; Ferreira, A. Adaptive control of microrobot in pulsatile flow. In Proceedings of the 2014 IEEE Conference on Control Applications (CCA), Juan Les Antibes, France, 8–10 October 2014; pp. 1982–1987. [Google Scholar] [CrossRef]
- Sadelli, L.; Fruchard, M.; Ferreira, A. Observer-based controller for microrobot in pulsatile blood flow. In Proceedings of the 53rd IEEE Conference on Decision and Control, Los Angeles, CA, USA, 15–17 December 2014; pp. 6993–6998. [Google Scholar] [CrossRef]
- Sadelli, L.; Fruchard, M.; Ferreira, A. 2D Observer-Based Control of a Vascular Microrobot. IEEE Trans. Autom. Control 2017, 62, 2194–2206. [Google Scholar] [CrossRef]
- Plötner, P.; Harada, K.; Sugita, N.; Mitsuishi, M. Theoretical analysis of magnetically propelled microrobots in the cardiovascular system. In Proceedings of the 2014 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Chicago, IL, USA, 26–30 August 2014; pp. 870–873. [Google Scholar] [CrossRef]

| Procedure | Physical Mechanism | Microbot Design |
|---|---|---|
| Chemical | Chemical lysis | Ciliary microbot |
| Soft attractor wall microbot | ||
| Surface microroller | ||
| Ciliary microbot | ||
| Chemomechanical | Chemical lysis + maceration | Self-folding microbot |
| Helical microbot | ||
| Mechanical | Maceration | Sperm-shaped microbot |
| Snake-shaped microbot | ||
| Helical microbot | ||
| Milling | Self-folding microbot | |
| Scallop-shaped microbot | ||
| Helical microbot | ||
| Dragging | Microrocket robot | |
| Laser | Photoablation | Snake-shaped microbot |
| Blood Vessels | Inner Diameter | Blood Velocity | Reynolds Number |
|---|---|---|---|
| [mm] | [mm/s] | [-] | |
| Aorta | 25 | 245–630 | 3000 |
| Vena cava | 30 | 50–300 | 3000 |
| Arteries | 2–6 | 100–500 | 110–850 |
| Main artery branches | 2–4 | - | - |
| Terminal artery branches | 1–2 | - | - |
| Veins | 5 | 3–50 | 150 |
| Arterioles | 0.03 | 1–100 | 0.7 |
| Venules | 0.02 | <3 | 0.01 |
| Capillaries | 0.008 | <1 | 0.002 |
| Blood Vessels | Inner Diameter | |
|---|---|---|
| Aorta | 10 | 10 |
| Large arteries | 4 | 4 |
| Small arteries | 1 | 1 |
| Arterioles | 0.1 | 0.1 |
| Capillaries | 0.01 | 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doutel, E.; Galindo-Rosales, F.J.; Campo-Deaño, L. Hemodynamics Challenges for the Navigation of Medical Microbots for the Treatment of CVDs. Materials 2021, 14, 7402. https://doi.org/10.3390/ma14237402
Doutel E, Galindo-Rosales FJ, Campo-Deaño L. Hemodynamics Challenges for the Navigation of Medical Microbots for the Treatment of CVDs. Materials. 2021; 14(23):7402. https://doi.org/10.3390/ma14237402
Chicago/Turabian StyleDoutel, Erica, Francisco J. Galindo-Rosales, and Laura Campo-Deaño. 2021. "Hemodynamics Challenges for the Navigation of Medical Microbots for the Treatment of CVDs" Materials 14, no. 23: 7402. https://doi.org/10.3390/ma14237402
APA StyleDoutel, E., Galindo-Rosales, F. J., & Campo-Deaño, L. (2021). Hemodynamics Challenges for the Navigation of Medical Microbots for the Treatment of CVDs. Materials, 14(23), 7402. https://doi.org/10.3390/ma14237402







