Demonstration of Near Edge X-ray Absorption Fine Structure Spectroscopy of Transition Metals Using Xe/He Double Stream Gas Puff Target Soft X-ray Source
Abstract
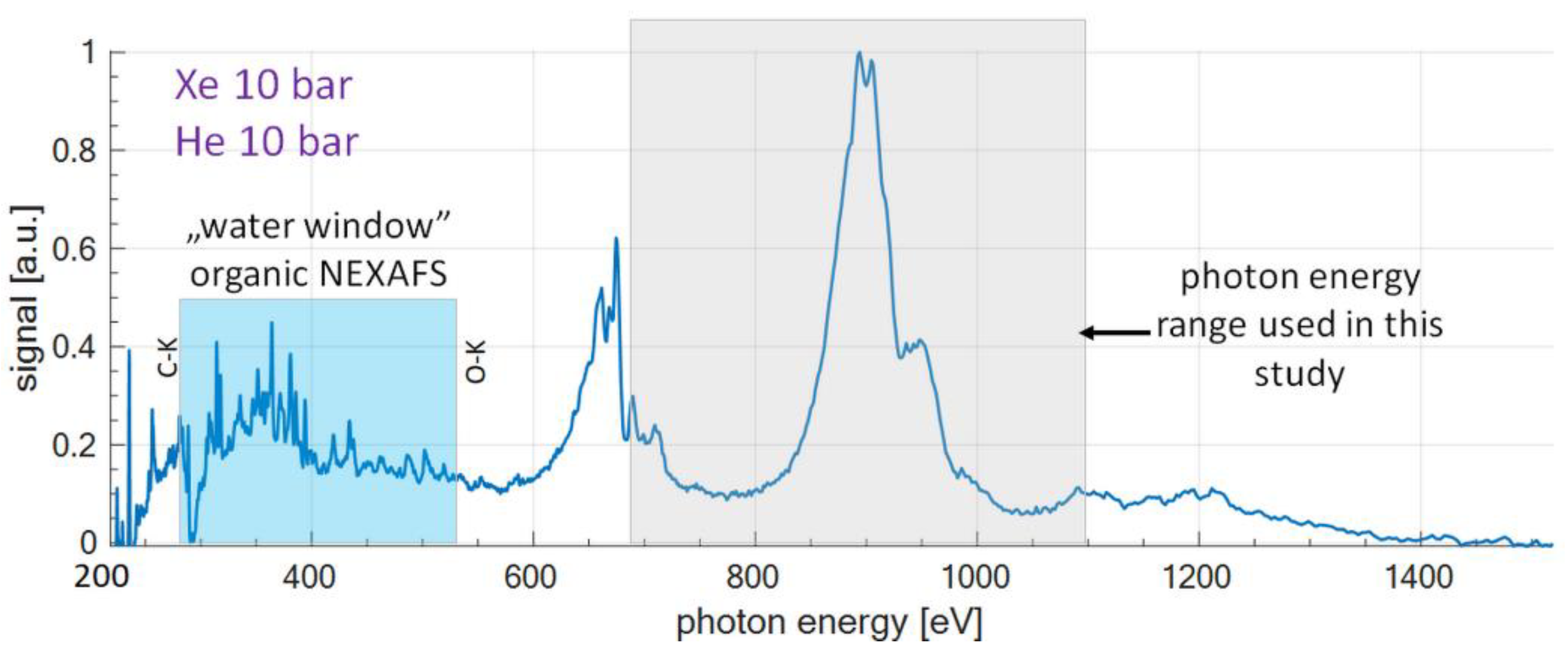
1. Introduction
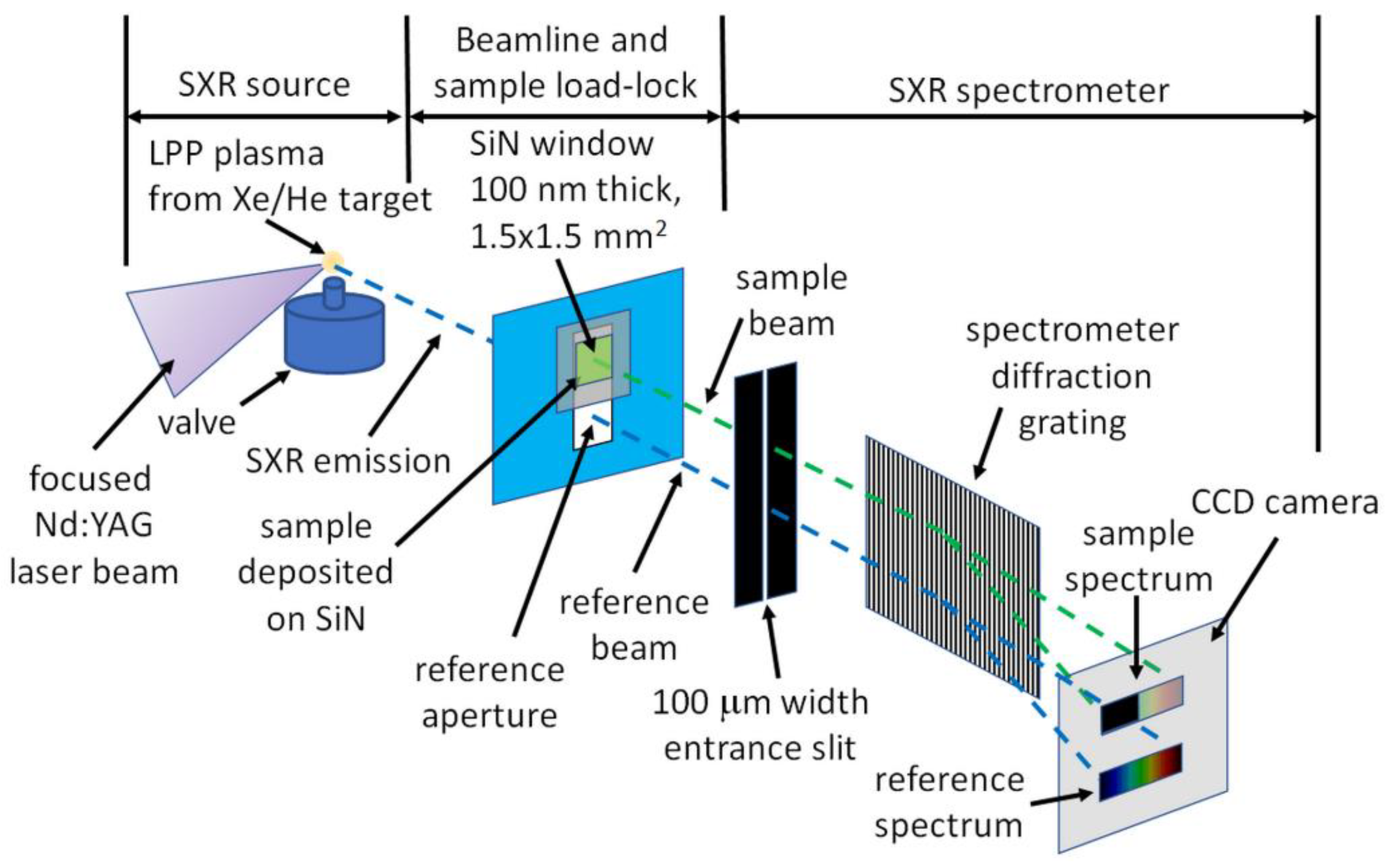
2. Experimental Setup
3. Experimental Results
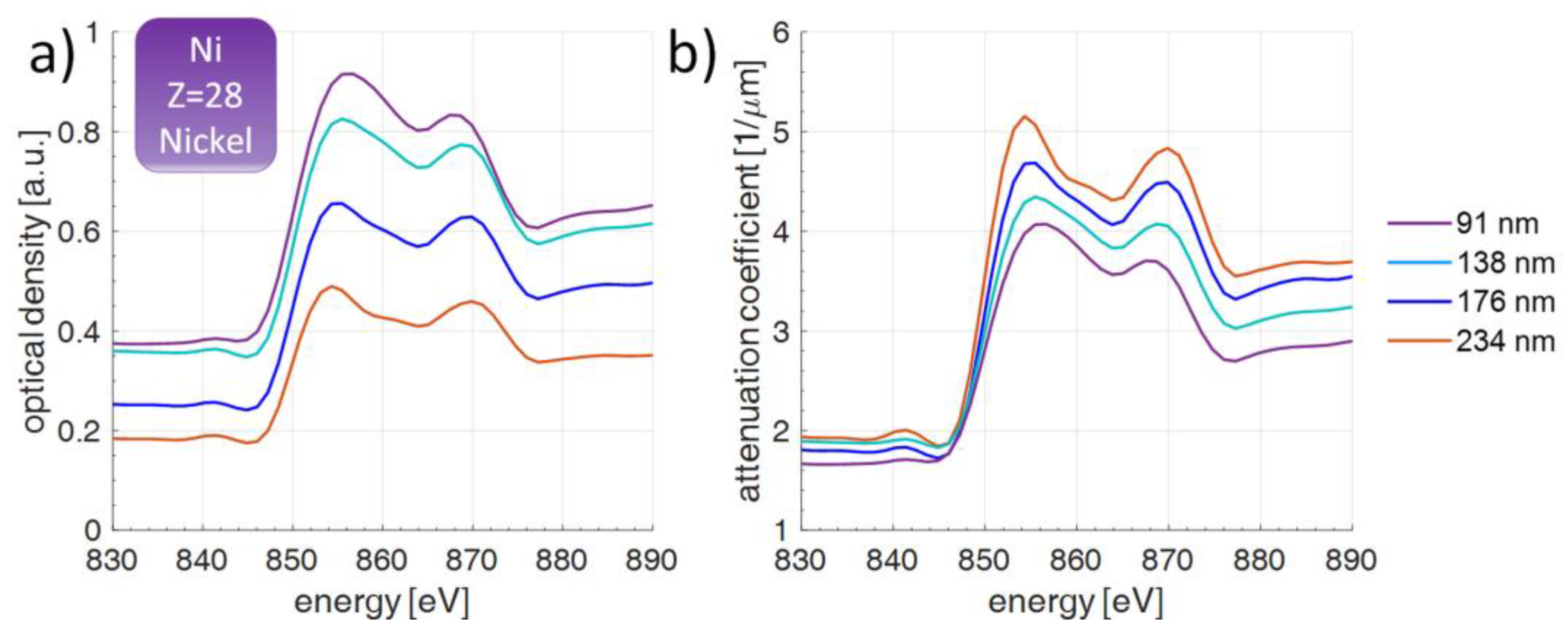
3.1. Thickness Influence on NEXAFS Measurements
3.2. NEXAFS L-Edge Spectra of Z = 26–30 Transition Metals
3.3. NEXAFS Spectrum of a Multilayer Sample
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stöhr, J. NEXAFS Spectroscopy; Springer: Berlin, Germany, 1992. [Google Scholar]
- Penner-Hahn, J.E. 2.13 X-ray Absorption Spectroscopy. In Comprehensive Coordination Chemistry II, 2nd ed.; McCleverty, J.A., Meyer, T.J., Eds.; Elsevier Science: Amsterdam, The Netherlands, 2003; Volume 2, pp. 159–186. [Google Scholar]
- Baker, M.L.; Mara, M.W.; Yan, J.J.; Hodgson, K.O.; Hedman, B.; Solomon, E.I. K- and L-edge X-ray absorption spectroscopy (XAS) and resonant inelastic X-ray scattering (RIXS) determination of differential orbital covalency (DOC) of transition metal sites. Coord. Chem. Rev. 2017, 345, 182–208. [Google Scholar] [CrossRef]
- Pattrick, R.A.D.; van der Laan, G.; Vaughan, D.J.; Henderson, C.M.B. Oxidation state and electronic configuration determination of copper in tetrahedrite group minerals by L-edge X-ray absorption spectroscopy. Phys. Chem. Miner. 1993, 20, 395–401. [Google Scholar] [CrossRef]
- Błachucki, W.; Czapla-Masztafiak, J.; Sá, J.; Szlachetko, J. A laboratory-based double X-ray spectrometer for simultaneous X-ray emission and X-ray absorption studies. J. Anal. At. Spectrom. 2019, 34, 1409–1415. [Google Scholar] [CrossRef]
- Schlesiger, C.; Anklamm, L.; Stiel, H.; Malzer, W.; Kanngießer, B. XAFS spectroscopy by an X-ray tube based spectrometer using a novel type of HOPG mosaic crystal and optimized image processing. J. Anal. At. Spectrom. 2015, 30, 1080–1085. [Google Scholar] [CrossRef]
- Jonas, A.; Stiel, H.; Glöggler, L.; Dahm, D.; Dammer, K.; Kanngießer, B.; Mantouvalou, I. Towards Poisson noise limited optical pump soft X-ray probe NEXAFS spectroscopy using a laser-produced plasma source. Opt. Express 2019, 27, 36524–36537. [Google Scholar] [CrossRef]
- Müller, M.; Schellhorn, M.; Mann, K. Laboratory-scale near-edge X-ray absorption fine structure spectroscopy with a laser-induced plasma source. J. Anal. At. Spectrom. 2019, 34, 1779–1785. [Google Scholar] [CrossRef]
- Popmintchev, D.; Galloway, B.R.; Chen, M.-C.; Dollar, F.; Mancuso, C.A.; Hankla, A.; Miaja-Avila, L.; O’Neil, G.; Shaw, J.M.; Fan, G.; et al. Near- and Extended-Edge X-Ray-Absorption Fine-Structure Spectroscopy Using Ultrafast Coherent High-Order Harmonic Supercontinua. Phys. Rev. Lett. 2018, 120, 093002. [Google Scholar] [CrossRef] [PubMed]
- Wachulak, P.; Duda, M.; Bartnik, A.; Sarzyński, A.; Wegrzynski, L.; Nowak, M.; Jancarek, A.; Fiedorowicz, H. Compact system for near edge X-ray fine structure (NEXAFS) spectroscopy using a laser-plasma light source. Opt. Express 2018, 26, 8260–8274. [Google Scholar] [CrossRef] [PubMed]
- Peth, C.; Barkusky, F.; Mann, K. Near-edge x-ray absorption fine structure measurements using a laboratory-scale XUV source. J. Phys. D Appl. Phys. 2008, 41, 105202. [Google Scholar] [CrossRef]
- Wachulak, P.W.; Bartnik, A.; Wegrzynski, L.; Kostecki, J.; Jarocki, R.; Fok, T.; Szczurek, M.; Fiedorowicz, H. Sub 1-μm resolution “water-window” microscopy using a compact, laser-plasma SXR source based on a double stream gas-puff target. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2013, 311, 42–46. [Google Scholar] [CrossRef]
- Mantouvalou, I.; Witte, K.; Martyanov, W.; Jonas, A.; Grötzsch, D.; Streeck, C.; Löchel, H.; Rudolph, I.; Erko, A.; Stiel, H.; et al. Single shot near edge x-ray absorption fine structure spectroscopy in the laboratory. Appl. Phys. Lett. 2016, 108, 201106. [Google Scholar] [CrossRef]
- Jiang, P.; Prendergast, D.; Borondics, F.; Porsgaard, S.; Giovanetti, L.; Pach, E.; Newberg, J.; Bluhm, H.; Besenbacher, F.; Salmeron, M. Experimental and theoretical investigation of the electronic structure of Cu2O and CuO thin films on Cu(110) using x-ray photoelectron and absorption spectroscopy. J. Chem. Phys. 2013, 138, 024704. [Google Scholar] [CrossRef]
- Yuste, M.; Escobar-Galindo, R.; Caretti, I.; Torres, R.; Sánchez, O. Influence of the oxygen partial pressure and post-deposition annealing on the structure and optical properties of ZnO films grown by dc magnetron sputtering at room temperature. J. Phys. D Appl. Phys. 2011, 45, 025303. [Google Scholar] [CrossRef]
- Gomes, J.; Azevedo, G.; Depeyrot, J.; Mestnik-Filho, J.; da Silva, G.; Tourinho, F.; Perzynski, R. ZnFe2O4 nanoparticles for ferrofluids: A combined XANES and XRD study. J. Magn. Magn. Mater. 2011, 323, 1203–1206. [Google Scholar] [CrossRef]
- Khan, S.; Mazher, J.; Singh, P. Ambient Zinc K-Edge Extended X-Ray Absorption Fine Structure Studies on Solid Solution Hardening of the Ternary Alloys. ISRN Spectrosc. 2013, 2013, 623409. [Google Scholar] [CrossRef]
- Ward, M.J. X-Ray Absorption Fine Structure and X-Ray Excited Optical Luminescence Studies of Gallium Nitride-Zinc Oxide Solid Solution Nanostructures. Ph.D. Thesis, The University of Western Ontario, London, ON, Canada, 2013. [Google Scholar]
- Hamilton, J.G.; Farrell, R.E.; Chen, N.; Feng, R.; Reid, J.; Peak, D. Characterizing Zinc Speciation in Soils from a Smelter-Affected Boreal Forest Ecosystem. J. Environ. Qual. 2016, 45, 684–692. [Google Scholar] [CrossRef]
- Li, D.; Bancroft, G.; Kasrai, M.; Fleet, M.; Feng, X.; Yang, B.; Tan, K. S K- and L-edge XANES and electronic structure of some copper sulfide minerals. Phys. Chem. Miner. 1994, 21, 317–324. [Google Scholar] [CrossRef]
- Ebert, H.; Stöhr, J.; Parkin, S.S.P.; Samant, M.; Nilsson, A. L-edge x-ray absorption in fcc and bcc Cu metal: Comparison of experimental and first-principles theoretical results. Phys. Rev. B 1996, 53, 16067–16073. [Google Scholar] [CrossRef] [PubMed]
- Charnock, J.; Henderson, C.; Mosselmans, J.; Pattrick, R. 3d transition metal L-edge X-ray absorption studies of the dichalcogenides of Fe, Co and Ni. Phys. Chem. Miner. 1996, 23, 403–408. [Google Scholar] [CrossRef]
- Cheng, Y.H.; Jan, J.C.; Chiou, J.W.; Pong, W.F.; Tsai, M.-H.; Hseih, H.H.; Chang, Y.K.; Dann, T.E.; Chien, F.Z.; Tseng, P.K.; et al. Electronic structure of the Fe–Cu–Nb–Si–B alloys by x-ray absorption spectroscopy. Appl. Phys. Lett. 2000, 77, 115–117. [Google Scholar] [CrossRef][Green Version]
- Bartolomé, J.; García, L.M.; Luis, F.; López-Ruiz, R.; Petroff, F.; Deranlot, C.; Wilhelm, F.; Rogalev, A.; Bencok, P.; Brookes, N.B.; et al. Magnetic polarization of noble metals by Co nanoparticles inM-capped granular multilayers (M = Cu, Ag, and Au): An x-ray magnetic circular dichroism study. Phys. Rev. B 2008, 77, 184420. [Google Scholar] [CrossRef]
- Cho, D.-Y.; Kim, J.H.; Na, K.D.; Song, J.; Hwang, C.S.; Park, B.-G.; Kim, J.-Y.; Min, C.-H.; Oh, S.-J. Spectroscopic evidence for limited carrier hopping interaction in amorphous ZnO thin film. Appl. Phys. Lett. 2009, 95, 261903. [Google Scholar] [CrossRef]
- Kuzmin, A.; Chaboy, J. EXAFS and XANES analysis of oxides at the nanoscale. IUCrJ 2014, 1, 571–589. [Google Scholar] [CrossRef]
- Wang, M.; Ren, F.; Zhou, J.; Cai, G.; Cai, L.; Hu, Y.; Wang, D.; Liu, Y.; Guo, L.; Shen, S. N Doping to ZnO Nanorods for Photoelectrochemical Water Splitting under Visible Light: Engineered Impurity Distribution and Terraced Band Structure. Sci. Rep. 2015, 5, 12925. [Google Scholar] [CrossRef]
- Wang, H.; Ralston, C.Y.; Patil, D.S.; Jones, R.M.; Gu, W.; Verhagen, M.; Adams, M.; Ge, P.; Riordan, C.; Marganian, C.A.; et al. Nickel L-Edge Soft X-ray Spectroscopy of Nickel–Iron Hydrogenases and Model CompoundsEvidence for High-Spin Nickel(II) in the Active Enzyme. J. Am. Chem. Soc. 2000, 122, 10544–10552. [Google Scholar] [CrossRef]
- Wang, H.; Patil, D.S.; Gu, W.; Jacquamet, L.; Friedrich, S.; Funk, T.; Cramer, S.P. L-edge X-ray absorption spectroscopy of some Ni enzymes: Probe of Ni electronic structure. J. Electron Spectrosc. Relat. Phenom. 2001, 114–116, 855–863. [Google Scholar] [CrossRef]
- Jahrman, E.P.; Holden, W.M.; Ditter, A.S.; Mortensen, D.R.; Seidler, G.T.; Fister, T.T.; Kozimor, S.A.; Piper, L.F.J.; Rana, J.; Hyatt, N.C.; et al. An improved laboratory-based x-ray absorption fine structure and x-ray emission spectrometer for analytical applications in materials chemistry research. Rev. Sci. Instrum. 2019, 90, 024106. [Google Scholar] [CrossRef] [PubMed]
- Mason, C.W.; Lange, F.; Saravanan, K.; Lin, F.; Nordlund, D. Beyond Divalent Copper: A Redox Couple for Sodium Ion Battery Cathode Materials. ECS Electrochem. Lett. 2015, 4, A41–A44. [Google Scholar] [CrossRef][Green Version]
- Lin, F.; Nordlund, D.; Pan, T.; Markus, I.M.; Weng, T.-C.; Xin, H.L.; Doeff, M.M. Influence of synthesis conditions on the surface passivation and electrochemical behavior of layered cathode materials. J. Mater. Chem. A 2014, 2, 19833–19840. [Google Scholar] [CrossRef]
- Benedetti, S.; Valenti, I.; di Bona, A.; Vinai, G.; Castan-Guerrero, C.; Valeri, S.; Catellani, A.; Ruini, A.; Torelli, P.; Calzolari, A. Spectroscopic identification of the chemical interplay between defects and dopants in Al-doped ZnO. Phys. Chem. Chem. Phys. 2017, 19, 29364–29371. [Google Scholar] [CrossRef]
- Sharma, A.; Varshney, M.; Park, J.; Ha, T.-K.; Chae, K.-H.; Shin, H.-J. XANES, EXAFS and photocatalytic investigations on copper oxide nanoparticles and nanocomposites. RSC Adv. 2015, 5, 21762–21771. [Google Scholar] [CrossRef]
- Henzler, K.; Heilemann, A.; Kneer, J.; Guttmann, P.; Jia, H.; Bartsch, E.; Lu, Y.; Palzer, S. Investigation of reactions between trace gases and functional CuO nanospheres and octahedrons using NEXAFS-TXM imaging. Sci. Rep. 2015, 5, 17729. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Friedrich, S.; Li, L.; Mao, Z.; Ge, P.; Balasubramanian, M.; Patil, D.S. L-edge sum rule analysis on 3d transition metal sites: From d10to d0and towards application to extremely dilute metallo-enzymes. Phys. Chem. Chem. Phys. 2018, 20, 8166–8176. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Árnadóttir, L.; Xu, Z.J.; Feng, Z. In Situ X-ray Absorption Spectroscopy Studies of Nanoscale Electrocatalysts. Nano-Micro Lett. 2019, 11, 47. [Google Scholar] [CrossRef]
- De Groot, F.M.F. Chapter 5, X-ray Absorption Near Edge Spectroscopy. In In-Situ Spectroscopy of Catalysts; Weckhuysen, B.M., Ed.; American Scientific Publishers: Valencia, CA, USA, 2004; ISBN 1-58883-026-8. [Google Scholar]
- Bunău, O.; Joly, Y. Self-consistent aspects of x-ray absorption calculations. J. Phys. Condens. Matter 2009, 21, 345501. [Google Scholar] [CrossRef] [PubMed]
- Cressey, G.; Henderson, C.M.B.; van der Laan, G. Use of L-edge X-ray absorption spectroscopy to characterize multiple valence states of 3d transition metals; a new probe for mineralogical and geochemical research. Phys. Chem. Miner. 1993, 20, 111–119. [Google Scholar] [CrossRef]
- Xu, W.; Zhang, X.-L.; Guo, Z.-Y.; Si, C.; Zhao, Y.-D.; Marcelli, A.; Chen, D.-L.; Wu, Z.-Y. CopperL-edge spectra: Multiplet vs. multiple scattering theory. J. Phys. Conf. Ser. 2013, 430, 012010. [Google Scholar] [CrossRef]
- Hocking, R.; George, S.D.; Raymond, K.N.; Hodgson, K.O.; Hedman, B.; Solomon, E.I. Fe L-Edge X-ray Absorption Spectroscopy Determination of Differential Orbital Covalency of Siderophore Model Compounds: Electronic Structure Contributions to High Stability Constants. J. Am. Chem. Soc. 2010, 132, 4006–4015. [Google Scholar] [CrossRef]
- Laskowski, R.; Blaha, P. Understanding the L2,3x-ray absorption spectra of early3dtransition elements. Phys. Rev. B 2010, 82, 205104. [Google Scholar] [CrossRef]
- Giles, L.J.; Grigoropoulos, A.; Szilagyi, R.K. Multi-edge X-ray Absorption Spectroscopy. 1. X-ray Absorption near-Edge Structure Analysis of a Biomimetic Model of FeFe-Hydrogenase. J. Phys. Chem. A 2012, 116, 12280–12298. [Google Scholar] [CrossRef]
- Wachulak, P.; Fok, T.; Węgrzyński, Ł.; Bartnik, A.; Nyga, P.; Janulewicz, K.; Fiedorowicz, H. 1-keV emission from laser-plasma source based on an Xe/He double stream gas puff target. Opt. Express 2021, 29, 20514–20525. [Google Scholar] [CrossRef]
- Wachulak, P.W.; Bartnik, A.; Fiedorowicz, H.; Feigl, T.; Jarocki, R.; Kostecki, J.; Rakowski, R.; Rudawski, P.; Sawicka, M.; Szczurek, M.; et al. A compact, quasi-monochromatic laser-plasma EUV source based on a double-stream gas-puff target at 13.8 nm wavelength. Appl. Phys. A 2010, 100, 461–469. [Google Scholar] [CrossRef]
- Nakano, N.; Kuroda, H.; Kita, T.; Harada, T. Development of a flat-field grazing-incidence XUV spectrometer and its application in picosecond XUV spectroscopy. Appl. Opt. 1984, 23, 2386–2392. [Google Scholar] [CrossRef]
- Sedlmair, J.; Gleber, S.-C.; Peth, C.; Mann, K.; Niemeyer, J.; Thieme, J. Characterization of refractory organic substances by NEXAFS using a compact X-ray source. J. Soils Sediments 2011, 12, 24–34. [Google Scholar] [CrossRef]
- Ravel, B.; Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: Data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 2005, 12, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Fogelqvist, E.; Kördel, M.; Carannante, V.; Önfelt, B.; Hertz, H.M. Laboratory cryo x-ray microscopy for 3D cell imaging. Sci. Rep. 2017, 7, 13433. [Google Scholar] [CrossRef] [PubMed]
- Wachulak, P.; Torrisi, A.; Krauze, W.; Bartnik, A.; Kostecki, J.; Maisano, M.; Sciortino, A.M.; Fiedorowicz, H. A “water window” tomography based on a laser-plasma double-stream gas-puff target soft X-ray source. Appl. Phys. A 2019, 125, 70. [Google Scholar] [CrossRef]
- Kördel, M.; Dehlinger, A.; Seim, C.; Vogt, U.; Fogelqvist, E.; Sellberg, J.A.; Stiel, H.; Hertz, H.M. Laboratory water-window x-ray microscopy. Optica 2020, 7, 658. [Google Scholar] [CrossRef]
- Jonas, A.; Dammer, K.; Stiel, H.; Kanngiesser, B.; Sánchez-De-Armas, R.; Mantouvalou, I. Transient Sub-nanosecond Soft X-ray NEXAFS Spectroscopy on Organic Thin Films. Anal. Chem. 2020, 92, 15611–15615. [Google Scholar] [CrossRef]
- National Institute of Standards and Technology (NIST). NIST Atomic Spectra Database Lines Form. Available online: https://physics.nist.gov/PhysRefData/ASD/lines_form.html (accessed on 2 January 2013).
- Hummer, A.A.; Rompel, A. X-Ray Absorption Spectroscopy. In Advances in Protein Chemistry and Structural Biology; Elsevier BV: Amsterdam, The Netherlands, 2013; Volume 93, pp. 257–305. [Google Scholar]
- Kaye&Laby, Tables of Physical & Chemical Constant. Available online: https://web.archive.org/web/20190511222636/http://www.kayelaby.npl.co.uk/atomic_and_nuclear_physics/4_2/4_2_1.html (accessed on 27 October 2021).
- X-Ray Transition Energies Database, NIST Standard Reference Database 128. Available online: https://www.nist.gov/pml/x-ray-transition-energies-database (accessed on 27 October 2021).
- Data from NIST Database for Copper. Available online: https://physics.nist.gov/cgi-bin/XrayTrans/search.pl?element=Cu&lower=600&upper=1200&units=eV (accessed on 27 October 2021).
- Bartnik, A.; Skrzeczanowski, W.; Fiedorowicz, H.; Wachulak, P.; Fok, T. Low-temperature plasmas induced in nitrogen by extreme ultraviolet (EUV) pulses. Laser Part. Beams 2018, 36, 76–83. [Google Scholar] [CrossRef]
- Kaszewska, E.A.; Sylwestrzak, M.; Marczak, J.; Skrzeczanowski, W.; Iwanicka, M.; Szmit-Naud, E.; Anglos, D.; Targowski, P. Depth-Resolved Multilayer Pigment Identification in Paintings: Combined Use of Laser-Induced Breakdown Spectroscopy (LIBS) and Optical Coherence Tomography (OCT). Appl. Spectrosc. 2013, 67, 960–972. [Google Scholar] [CrossRef] [PubMed]
- Marczak, J.; Skrzeczanowski, W.; Dajnowski, B.; Kochanowska, J.; Koss, A.; Mróz, J. Laser-induced breakdown spectroscopy and optical microscopy investigations of crowns on the Pietà from the parish church in Skrzatusz, Poland. Stud. Conserv. 2015, 60, S120–S125. [Google Scholar] [CrossRef][Green Version]
- Saber, I.; Bartnik, A.; Wachulak, P.; Skrzeczanowski, W.; Jarocki, R.; Fiedorowicz, H. Temporal variations of electron density and temperature in Kr/Ne/H2photoionized plasma induced by nanosecond pulses from extreme ultraviolet source. Phys. Plasmas 2017, 24, 063501. [Google Scholar] [CrossRef]



| Element /Sample | Z Number | Thickness [nm] | Energy Et—Theory Ee—Experiment ΔE—Difference | Absorption Edges [eV] | ||
|---|---|---|---|---|---|---|
| L1 | L2 | L3 | ||||
| Fe | 26 | 142.3 ± 2.1 | Et | 844.6 | 719.9 | 706.8 |
| Ee | 844.2 | 719.0 | 706.3 | |||
| ΔE | 0.4 | 0.9 | 0.5 | |||
| Co | 27 | 158.2 ± 3.0 | Et | 925.1 | 793.3 | 778.1 |
| Ee | - | 794.0 | 778.0 | |||
| ΔE | - | −0.7 | 0.1 | |||
| Ni | 28 | 138.0 ± 2.1 | Et | 1008.6 | 870.0 | 852.7 |
| Ee | - | 869.5 | 852.4 | |||
| ΔE | - | 0.5 | 0.3 | |||
| Cu | 29 | 163.1 ± 0.9 | Et | 1096.7 | 952.3 | 932.7 |
| Ee | 1098.0 | 949.7 | 938.2 | |||
| ΔE | −1.3 | 2.6 | −5.5 | |||
| Cu * | 29 | 163.1 ± 0.9 | Et | 1103.1 | 959.6 | 939.9 |
| Ee | 1098.0 | 949.7 | 938.2 | |||
| ΔE | 5.1 | 9.9 | 1.7 | |||
| Zn | 30 | 309.2 ± 9.0 | Et | 1196.2 | 1044.9 | 1021.8 |
| Ee | - | 1048.0 | 1021.0 | |||
| ΔE | - | −3.1 | 0.8 | |||
| EDS Composition | |||||
|---|---|---|---|---|---|
| Sample | Thickness [nm] | wg% | at% | wg% | at% |
| Si | Fe | ||||
| Fe | 142.3 ± 2.1 | 92.52 ± 0.33 | 96.09 ± 0.18 | 7.48 ± 0.33 | 3.91 ± 0.18 |
| Si | Co | ||||
| Co | 158.2 ± 3.0 | 85.83 ± 0.06 | 92.71 ± 0.03 | 14.17 ± 0.06 | 7.29 ± 0.03 |
| Si | Ni | ||||
| Ni | 90.8 ± 0.4 | 92.76 ± 0.09 | 96.40 ± 0.05 | 7.24 ± 0.09 | 3.60 ± 0.05 |
| 138.0 ± 2.1 | 90.80 ± 0.18 | 95.37 ± 0.10 | 9.20 ± 0.18 | 4.63 ± 0.10 | |
| 234.0 ± 1.4 | 81.60 ± 0.08 | 90.26 ± 0.05 | 18.40 ± 0.08 | 9.74 ± 0.05 | |
| Si | Cu | ||||
| Cu | 163.1 ± 0.9 | 88.97 ± 0.30 | 94.81 ± 0.14 | 11.03 ± 0.30 | 5.19 ± 0.14 |
| Si | Zn | ||||
| Zn | 309.2 ± 9.0 | 80.53 ± 0.31 | 90.59 ± 0.17 | 19.47 ± 0.31 | 9.41 ± 0.17 |
| Multilayer | Z Number | Thickness [nm] | Experimental Edges (Multilayer) EeM [eV] | ||
|---|---|---|---|---|---|
| L1 | L2 | L3 | |||
| Co-Ni-Fe | 26–28 | 215.7 ± 3.5 (3× ~70 nm) | Fe: 844.3 | 719.0 | 702.8 |
| Co: - | 793.5 | 777.7 | |||
| Ni: - | 869.8 | 852.6 | |||
| Constituents | Experimental edges (layer) EeL [eV] | ||||
| Fe | 26 | 142.3 ± 1.9 | 844.2 | 719.0 | 706.3 |
| Co | 27 | 158.2 ± 3.0 | - | 794.0 | 778.0 |
| Ni | 28 | 138.0 ± 2.1 | - | 869.5 | 852.4 |
| Energy difference | ΔE2 = EeM − EeL [eV] | ||||
| Fe | 26 | 142.3 ± 1.9 | 0.1 | 0 | −3.5 |
| Co | 27 | 158.2 ± 3.0 | - | −0.5 | −0.3 |
| Ni | 28 | 138.0 ± 2.1 | - | 0.3 | 0.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fok, T.; Wachulak, P.; Węgrzyński, Ł.; Bartnik, A.; Nowak, M.; Nyga, P.; Kostecki, J.; Nasiłowska, B.; Skrzeczanowski, W.; Pietruszka, R.; et al. Demonstration of Near Edge X-ray Absorption Fine Structure Spectroscopy of Transition Metals Using Xe/He Double Stream Gas Puff Target Soft X-ray Source. Materials 2021, 14, 7337. https://doi.org/10.3390/ma14237337
Fok T, Wachulak P, Węgrzyński Ł, Bartnik A, Nowak M, Nyga P, Kostecki J, Nasiłowska B, Skrzeczanowski W, Pietruszka R, et al. Demonstration of Near Edge X-ray Absorption Fine Structure Spectroscopy of Transition Metals Using Xe/He Double Stream Gas Puff Target Soft X-ray Source. Materials. 2021; 14(23):7337. https://doi.org/10.3390/ma14237337
Chicago/Turabian StyleFok, Tomasz, Przemysław Wachulak, Łukasz Węgrzyński, Andrzej Bartnik, Michał Nowak, Piotr Nyga, Jerzy Kostecki, Barbara Nasiłowska, Wojciech Skrzeczanowski, Rafał Pietruszka, and et al. 2021. "Demonstration of Near Edge X-ray Absorption Fine Structure Spectroscopy of Transition Metals Using Xe/He Double Stream Gas Puff Target Soft X-ray Source" Materials 14, no. 23: 7337. https://doi.org/10.3390/ma14237337
APA StyleFok, T., Wachulak, P., Węgrzyński, Ł., Bartnik, A., Nowak, M., Nyga, P., Kostecki, J., Nasiłowska, B., Skrzeczanowski, W., Pietruszka, R., Janulewicz, K., & Fiedorowicz, H. (2021). Demonstration of Near Edge X-ray Absorption Fine Structure Spectroscopy of Transition Metals Using Xe/He Double Stream Gas Puff Target Soft X-ray Source. Materials, 14(23), 7337. https://doi.org/10.3390/ma14237337






