Eu-Doped Zeolitic Imidazolate Framework-8 Modified Mixed-Crystal TiO2 for Efficient Removal of Basic Fuchsin from Effluent
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Adsorption and Photocatalyst
2.2.1. Preparation of Mixed-Crystal Structure TiO2(Mc-TiO2)
2.2.2. Preparation of Eu-Doped ZIF-8
2.2.3. Preparation of ZIF-8(Eu)@Mc-TiO2
2.2.4. Evaluation of Photocatalytic Activity
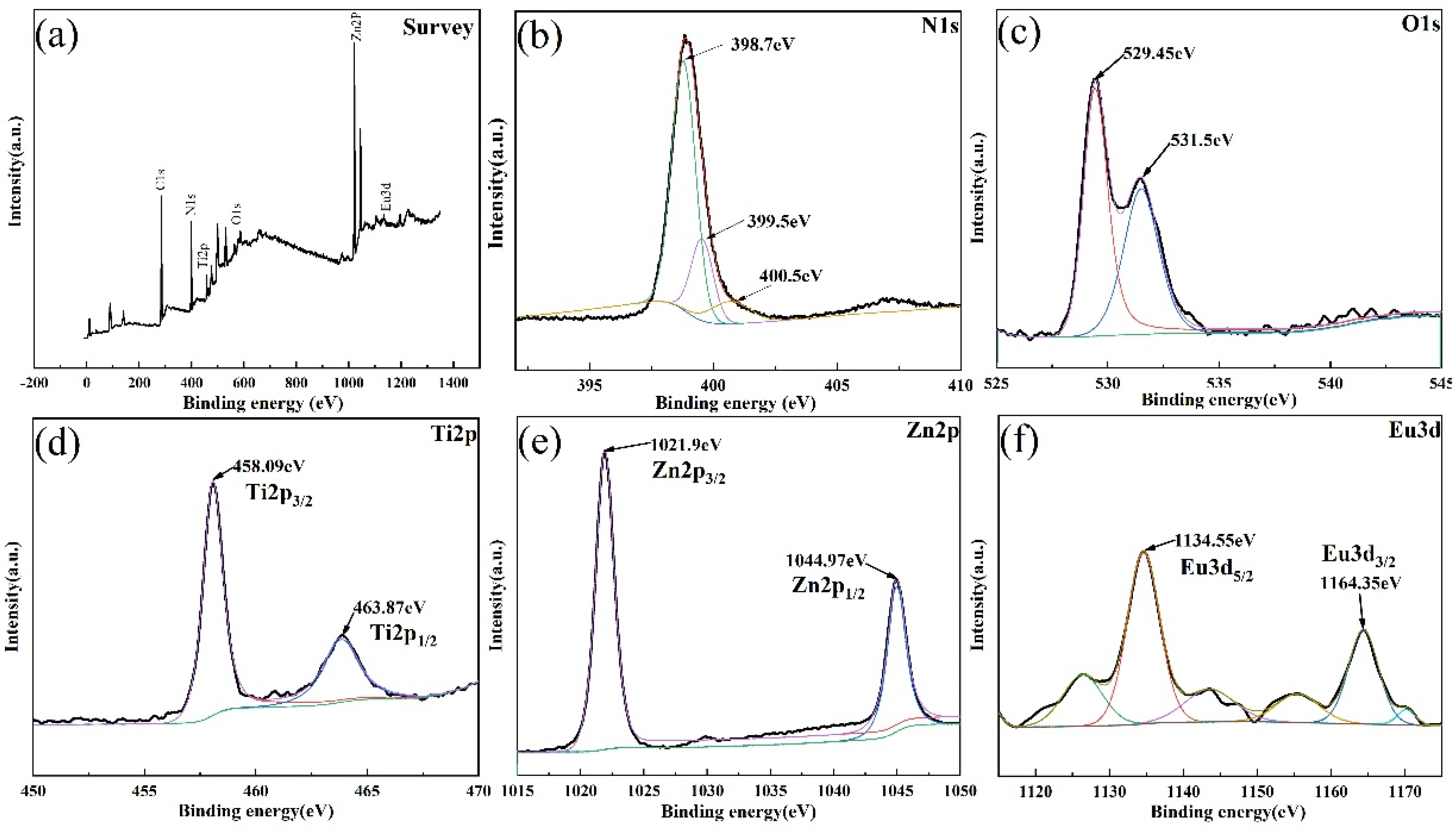
2.3. Characterization
3. Results
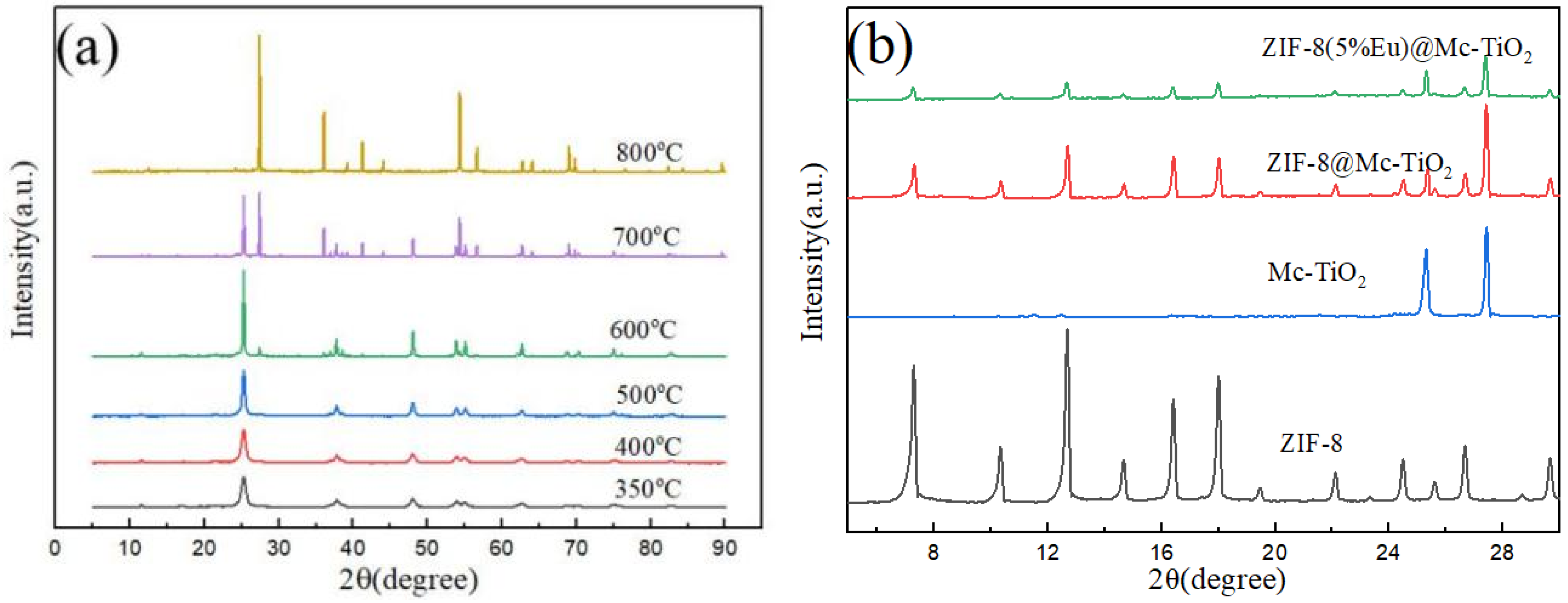
3.1. Crystal Structure Analysis of Photocatalyst
3.2. Morphology Analysis
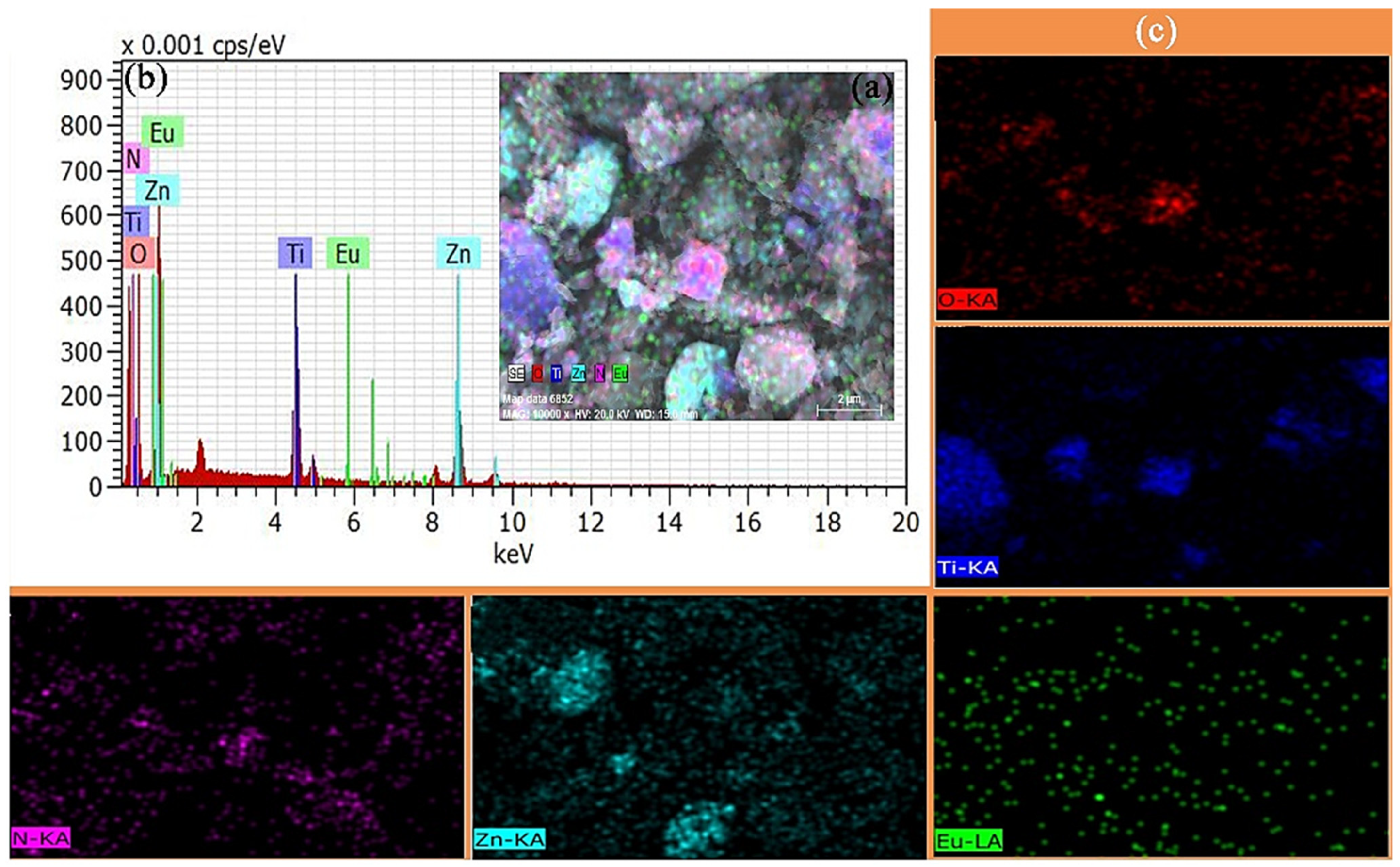
3.3. Element Analysis
3.4. Analysis of Specific Surface Area
3.5. Analysis of UV-Vis Diffuse Reflectance Spectroscopy (UV-Vis DRS)
3.6. Evaluation of Photocatalyst Performance
3.6.1. Effect of Eu Doping Amount for Photocatalytic Efficiency
3.6.2. Effect of Basic Fuchsin Concentration for Photocatalytic Efficiency
3.6.3. Effect of Dosage of ZIF-8(Eu)@Mc-TiO2 for Photocatalytic Efficiency
3.6.4. Reuse
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rai, H.S.; Bhattacharyya, M.S.; Singh, J.; Bansal, T.; Vats, P.; Banerjee, U. Removal of dyes from the effluent of textile and dyestuff manufacturing industry: A review of emerging techniques with reference to biological treatment. Crit. Rev. Environ. Sci. Technol. 2005, 35, 219–238. [Google Scholar] [CrossRef]
- Shindy, H. Fundamentals in the chemistry of cyanine dyes: A review. Dye. Pigment. 2017, 145, 505–513. [Google Scholar] [CrossRef]
- Yamjala, K.; Nainar, M.S.; Ramisetti, N.R. Methods for the analysis of azo dyes employed in food industry—A review. Food Chem. 2016, 192, 813–824. [Google Scholar] [CrossRef] [PubMed]
- Mali, P.; Mahajan, M.; Patil, D.; Kulkarni, M. Biodecolourisation of members of triphenylmethane and azo group of dyes. J. Sci. Ind. Res. 2000, 59, 221–224. [Google Scholar]
- Jain, R.; Mendiratta, S.; Kumar, L.; Srivastava, A. Green synthesis of iron nanoparticles using Artocarpus heterophyllus peel extract and their application as a heterogeneous Fenton-like catalyst for the degradation of Fuchsin Basic dye. Curr. Res. Green Sustain. Chem. 2021, 4, 100086. [Google Scholar] [CrossRef]
- Rashid, R.; Shafiq, I.; Akhter, P.; Iqbal, M.J.; Hussain, M. A state-of-the-art review on wastewater treatment techniques: The effectiveness of adsorption method. Environ. Sci. Pollut. Res. 2021, 28, 9050–9066. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.; Tareen, A.K.; Aslam, M.; Sagar, R.U.R.; Zhang, B.; Huang, W.; Mahmood, A.; Mahmood, N.; Khan, K.; Zhang, H. Recent progress, challenges, and prospects in two-dimensional photo-catalyst materials and environmental remediation. Nano-Micro Lett. 2020, 12, 1–77. [Google Scholar] [CrossRef] [PubMed]
- Busuioc-Tomoiagă, A.; Mertens, M.; Cool, P.; Bilba, N.; Vansant, E. A simple way to design highly active titania/mesoporous silica photocatalysts. In Studies in Surface Science and Catalysis; Elsevier: Amsterdam, The Netherlands, 2008; Volume 174, pp. 377–380. [Google Scholar]
- Akpan, U.G.; Hameed, B.H. Parameters affecting the photocatalytic degradation of dyes using TiO2-based photocatalysts: A review. J. Hazard. Mater. 2009, 170, 520–529. [Google Scholar] [CrossRef] [PubMed]
- Yemmireddy, V.K.; Hung, Y.-C. Effect of binder on the physical stability and bactericidal property of titanium dioxide (TiO2) nanocoatings on food contact surfaces. Food Control 2015, 57, 82–88. [Google Scholar] [CrossRef] [Green Version]
- Serga, V.; Burve, R.; Krumina, A.; Romanova, M.; Kotomin, E.A.; Popov, A.I. Extraction–Pyrolytic Method for TiO2 Polymorphs Production. Crystals 2021, 11, 431. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhao, X.; Zhang, M.; Sun, X. Charge-Transfer Process in Surface-Enhanced Raman Scattering Based on Energy Level Locations of Rare-Earth Nd3+-Doped TiO2 Nanoparticles. Nanomaterials 2021, 11, 2063. [Google Scholar] [CrossRef] [PubMed]
- Tsebriienko, T.; Popov, A.I. Effect of poly (titanium oxide) on the viscoelastic and thermophysical properties of interpenetrating polymer networks. Crystals 2021, 11, 794. [Google Scholar] [CrossRef]
- Qian, R.; Zong, H.; Schneider, J.; Zhou, G.; Zhao, T.; Li, Y.; Yang, J.; Bahnemann, D.W.; Pan, J.H. Charge carrier trapping, recombination and transfer during TiO2 photocatalysis: An overview. Catal. Today 2019, 335, 78–90. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, Z.; Wang, X. Well-defined metal–organic-framework hollow nanostructures for catalytic reactions involving gases. Adv. Mater. 2015, 27, 5365–5371. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-R.; Jang, M.-S.; Cho, H.-Y.; Kwon, H.-J.; Kim, S.; Ahn, W.-S. ZIF-8: A comparison of synthesis methods. Chem. Eng. J. 2015, 271, 276–280. [Google Scholar] [CrossRef]
- Pan, Y.; Liu, Y.; Zeng, G.; Zhao, L.; Lai, Z. Rapid synthesis of zeolitic imidazolate framework-8 (ZIF-8) nanocrystals in an aqueous system. Chem. Commun. 2011, 47, 2071–2073. [Google Scholar] [CrossRef]
- Jiang, X.; He, S.; Han, G.; Long, J.; Li, S.; Lau, C.H.; Zhang, S.; Shao, L. Aqueous one-step modulation for synthesizing monodispersed ZIF-8 nanocrystals for mixed-matrix membrane. ACS Appl. Mater. Interfaces 2021, 13, 11296–11305. [Google Scholar] [CrossRef]
- Li, X.; He, W.; Li, C.; Song, B.; Liu, S. Synergetic surface modulation of ZnO/Pt@ ZIF-8 hybrid nanorods for enhanced photocatalytic CO2 valorization. Appl. Catal. B Environ. 2021, 287, 119934. [Google Scholar] [CrossRef]
- Zhao, K.; Zhang, Z.; Feng, Y.; Lin, S.; Li, H.; Gao, X. Surface oxygen vacancy modified Bi2MoO6/MIL-88B (Fe) heterostructure with enhanced spatial charge separation at the bulk & interface. Appl. Catal. B Environ. 2020, 268, 118740. [Google Scholar]
- Serga, V.; Burve, R.; Krumina, A.; Pankratova, V.; Popov, A.I.; Pankratov, V. Study of phase composition, photocatalytic activity, and photoluminescence of TiO2 with Eu additive produced by the extraction-pyrolytic method. J. Mater. Res. Technol. 2021, 13, 2350–2360. [Google Scholar] [CrossRef]
- Reszczyńska, J.; Grzyb, T.; Sobczak, J.W.; Lisowski, W.; Gazda, M.; Ohtani, B.; Zaleska, A. Lanthanide co-doped TiO2: The effect of metal type and amount on surface properties and photocatalytic activity. Appl. Surf. Sci. 2014, 307, 333–345. [Google Scholar] [CrossRef]
- Sivasankari, J.; Sankar, S.; Selvakumar, S.; Vimaladevi, L.; Krithiga, R. Synthesis, structural and optical properties of Er doped, Li doped and Er+ Li co-doped ZnO nanocrystallites by solution-combustion method. Mater. Chem. Phys. 2014, 143, 1528–1535. [Google Scholar] [CrossRef]
- Dhiman, M.; Chudasama, B.; Kumar, V.; Tikoo, K.; Singhal, S. Augmenting the photocatalytic performance of cobalt ferrite via change in structural and optical properties with the introduction of different rare earth metal ions. Ceram. Int. 2019, 45, 3698–3709. [Google Scholar] [CrossRef]
- Patra, C.R.; Moneim, S.S.A.; Wang, E.; Dutta, S.; Patra, S.; Eshed, M.; Mukherjee, P.; Gedanken, A.; Shah, V.H.; Mukhopadhyay, D. In vivo toxicity studies of europium hydroxide nanorods in mice. Toxicol. Appl. Pharmacol. 2009, 240, 88–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, R.; Zhang, S.; Wen, T.; Gu, P.; Li, L.; Zhao, G.; Niu, F.; Huang, Q.; Tang, Z.; Wang, X. A critical review on visible-light-response CeO2-based photocatalysts with enhanced photooxidation of organic pollutants. Catal. Today 2019, 335, 20–30. [Google Scholar] [CrossRef]
- Wei, Q.; Wang, S.; Li, W.; Yuan, X.; Bai, Y. Hydrophobic ZnO-TiO2 nanocomposite with photocatalytic promoting self-cleaning surface. Int. J. Photoenergy 2015, 2015. [Google Scholar] [CrossRef] [Green Version]
- Zhu, H.Q.; Lou, C.Y.; Liu, F.R.; Jiang, G.J.; Ye, X.Y.; Yuan, R.H. Nanosized S-Doped TiO2 with Effective Visible-Light Degradation of BTEX from Wood-Based Panels. In Key Engineering Materials; Trans Tech Publications Ltd.: Stafa-Zurich, Switzerland, 2021; Volume 880, pp. 139–145. [Google Scholar]
- Hou, J.; Wang, Y.; Zhou, J.; Lu, Y.; Liu, Y.; Lv, X. Photocatalytic degradation of methylene blue using a ZnO/TiO2 heterojunction nanomesh electrode. Surf. Interfaces 2021, 22, 100889. [Google Scholar] [CrossRef]
- Chen, J.; Guo, Y.; Kang, T.; Liu, X.; Wang, X.; Zhang, X. In Situ Growth of ZIF-8 Nanocrystals on the Pore Walls of 3D Ordered Macroporous TiO2 for a One-Pot Cascade Reaction. Catalysts 2021, 11, 533. [Google Scholar] [CrossRef]
- Tuncel, D.; Ökte, A. Improved adsorption capacity and photoactivity of ZnO-ZIF-8 nanocomposites. Catal. Today 2021, 361, 191–197. [Google Scholar] [CrossRef]
- Parkash, A. Copper doped zeolitic imidazole frameworks (ZIF-8): A new generation of single-atom catalyst for oxygen reduction reaction in alkaline media. J. Electrochem. Soc. 2020, 167, 155504. [Google Scholar] [CrossRef]
- Hu, C.; Huang, Y.-C.; Chang, A.-L.; Nomura, M. Amine functionalized ZIF-8 as a visible-light-driven photocatalyst for Cr (VI) reduction. J. Colloid Interface Sci. 2019, 553, 372–381. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; An, Y.; Li, S.; Liu, Z.; Chen, Z.; Ren, Y.; Wang, S.; Zhang, X.; Wang, X. Enhanced heavy metal removal from an aqueous environment using an eco-friendly and sustainable adsorbent. Sci. Rep. 2020, 10, 16453. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Tang, S.; Gao, H.; Chen, X.; Liu, H.; Yu, C.; Yin, Z.; Zhao, X.; Pan, X.; Yang, H. Microstructure, optical, photoluminescence properties and the intrinsic mechanism of photoluminescence and photocatalysis for the BaTiO3, BaTiO3/TiO2 and BaTiO3/TiO2/CeO2 smart composites. Opt. Mater. 2021, 118, 111273. [Google Scholar] [CrossRef]
- Tvrdy, K.; Frantsuzov, P.A.; Kamat, P.V. Photoinduced electron transfer from semiconductor quantum dots to metal oxide nanoparticles. Proc. Natl. Acad. Sci. USA 2011, 108, 29–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mora-Sero, I.; Bisquert, J.; Dittrich, T.; Belaidi, A.; Susha, A.; Rogach, A. Photosensitization of TiO2 layers with CdSe quantum dots: Correlation between light absorption and photoinjection. J. Phys. Chem. C 2007, 111, 14889–14892. [Google Scholar] [CrossRef]
- Wang, X.; Yu, R.; Wang, P.; Chen, F.; Yu, H. Co-modification of F− and Fe (III) ions as a facile strategy towards effective separation of photogenerated electrons and holes. Appl. Surf. Sci. 2015, 351, 66–73. [Google Scholar] [CrossRef]
- Zhang, M.; Shang, Q.; Wan, Y.; Cheng, Q.; Liao, G.; Pan, Z. Self-template synthesis of double-shell TiO2@ ZIF-8 hollow nanospheres via sonocrystallization with enhanced photocatalytic activities in hydrogen generation. Appl. Catal. B Environ. 2019, 241, 149–158. [Google Scholar] [CrossRef]
- Xu, A.-W.; Gao, Y.; Liu, H.-Q. The preparation, characterization, and their photocatalytic activities of rare-earth-doped TiO2 nanoparticles. J. Catal. 2002, 207, 151–157. [Google Scholar] [CrossRef]
- Nosaka, Y.; Nosaka, A.Y. Kinetic processes in the presence of photogenerated charge carriers. In Photocatalysis: Fundamentals and Perspectives; Royal Society of Chemistry: London, UK, 2016; pp. 163–184. [Google Scholar]
- Xiang, Q.; Yu, J.; Wong, P.K. Quantitative characterization of hydroxyl radicals produced by various photocatalysts. J. Colloid Interface Sci. 2011, 357, 163–167. [Google Scholar] [CrossRef]
- Xiong, S.; Liu, X.; Zhu, X.; Liang, G.; Jiang, Z.; Cui, B.; Bai, J. One-step preparation of well-dispersed spindle-like Fe2O3 nanoparticles on g-C3N4 as highly efficient photocatalysts. Ecotoxicol. Environ. Saf. 2021, 208, 111519. [Google Scholar] [CrossRef]
- Zhao, W.; Guo, Y.; Faiz, Y.; Yuan, W.-T.; Sun, C.; Wang, S.-M.; Deng, Y.-H.; Zhuang, Y.; Li, Y.; Wang, X.-M. Facile in-suit synthesis of Ag/AgVO3 one-dimensional hybrid nanoribbons with enhanced performance of plasmonic visible-light photocatalysis. Appl. Catal. B Environ. 2015, 163, 288–297. [Google Scholar] [CrossRef]
- Yu, L.; Huang, Y.; Yang, Y.; Xu, Y.; Wang, G.; Yang, S. Photocatalytic degradation of organic dyes by H4SiW6Mo6O40/SiO2 sensitized by H2O2. Int. J. Photoenergy 2013, 2013, 812376. [Google Scholar] [CrossRef]
- Ning, J.; Wang, M.; Luo, X.; Hu, Q.; Hou, R.; Chen, W.; Chen, D.; Wang, J.; Liu, J. SiO2 stabilized magnetic nanoparticles as a highly effective catalyst for the degradation of basic fuchsin in industrial dye wastewaters. Molecules 2018, 23, 2573. [Google Scholar] [CrossRef] [PubMed] [Green Version]

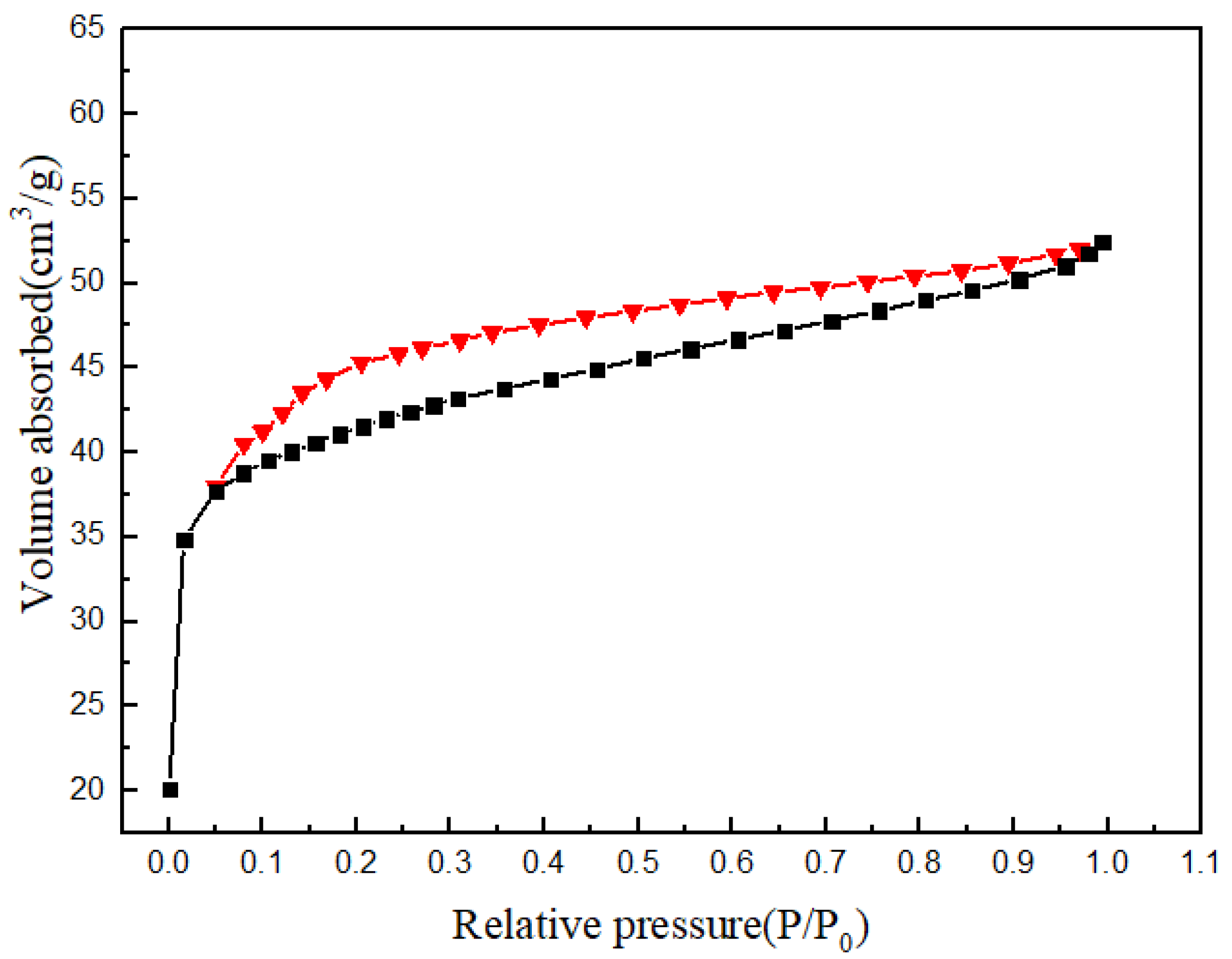


| Sample | SBET (m2/g) | VPore (cm3/g) | RAve (nm) |
|---|---|---|---|
| ZIF-8(Eu)@Mc-TiO2 | 159.23 | 0.0808 | 20,305 |
| Sample | Mc-TiO2 | ZIF-8@Mc-TiO2 | ZIF-8(0.5%Eu)@Mc-TiO2 | ZIF-8(1%Eu)@Mc-TiO2 | ZIF-8(3%Eu)@Mc-TiO2 | ZIF-8(5%Eu)@Mc-TiO2 | ZIF-8(8%Eu)@Mc-TiO2 | ZIF-8(10%Eu)@Mc-TiO2 |
|---|---|---|---|---|---|---|---|---|
| Eg(eV) | 2.97 | 2.94 | 2.92 | 2.94 | 2.92 | 2.90 | 2.85 | 2.83 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Liu, H.; Liu, Z.; An, Y.; Zhong, Y.; Hu, Z.; Li, S.; Chen, Z.; Wang, S.; Sheng, X.; et al. Eu-Doped Zeolitic Imidazolate Framework-8 Modified Mixed-Crystal TiO2 for Efficient Removal of Basic Fuchsin from Effluent. Materials 2021, 14, 7265. https://doi.org/10.3390/ma14237265
Zhang W, Liu H, Liu Z, An Y, Zhong Y, Hu Z, Li S, Chen Z, Wang S, Sheng X, et al. Eu-Doped Zeolitic Imidazolate Framework-8 Modified Mixed-Crystal TiO2 for Efficient Removal of Basic Fuchsin from Effluent. Materials. 2021; 14(23):7265. https://doi.org/10.3390/ma14237265
Chicago/Turabian StyleZhang, Wanqi, Hui Liu, Zhechen Liu, Yuhong An, Yuan Zhong, Zichu Hu, Shujing Li, Zhangjing Chen, Sunguo Wang, Xianliang Sheng, and et al. 2021. "Eu-Doped Zeolitic Imidazolate Framework-8 Modified Mixed-Crystal TiO2 for Efficient Removal of Basic Fuchsin from Effluent" Materials 14, no. 23: 7265. https://doi.org/10.3390/ma14237265
APA StyleZhang, W., Liu, H., Liu, Z., An, Y., Zhong, Y., Hu, Z., Li, S., Chen, Z., Wang, S., Sheng, X., Zhang, X., & Wang, X. (2021). Eu-Doped Zeolitic Imidazolate Framework-8 Modified Mixed-Crystal TiO2 for Efficient Removal of Basic Fuchsin from Effluent. Materials, 14(23), 7265. https://doi.org/10.3390/ma14237265







