A Pilot Study of Seamless Regeneration of Bone and Cartilage in Knee Joint Regeneration Using Honeycomb TCP
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Animals and Ethics
2.2. Preparation of Honeycomb TCP Scaffolds
2.3. Implantation and Histological Examination
2.4. Cartilage Tissue Formation Evaluation by Area Measurement
2.5. Statistical Analysis
3. Results
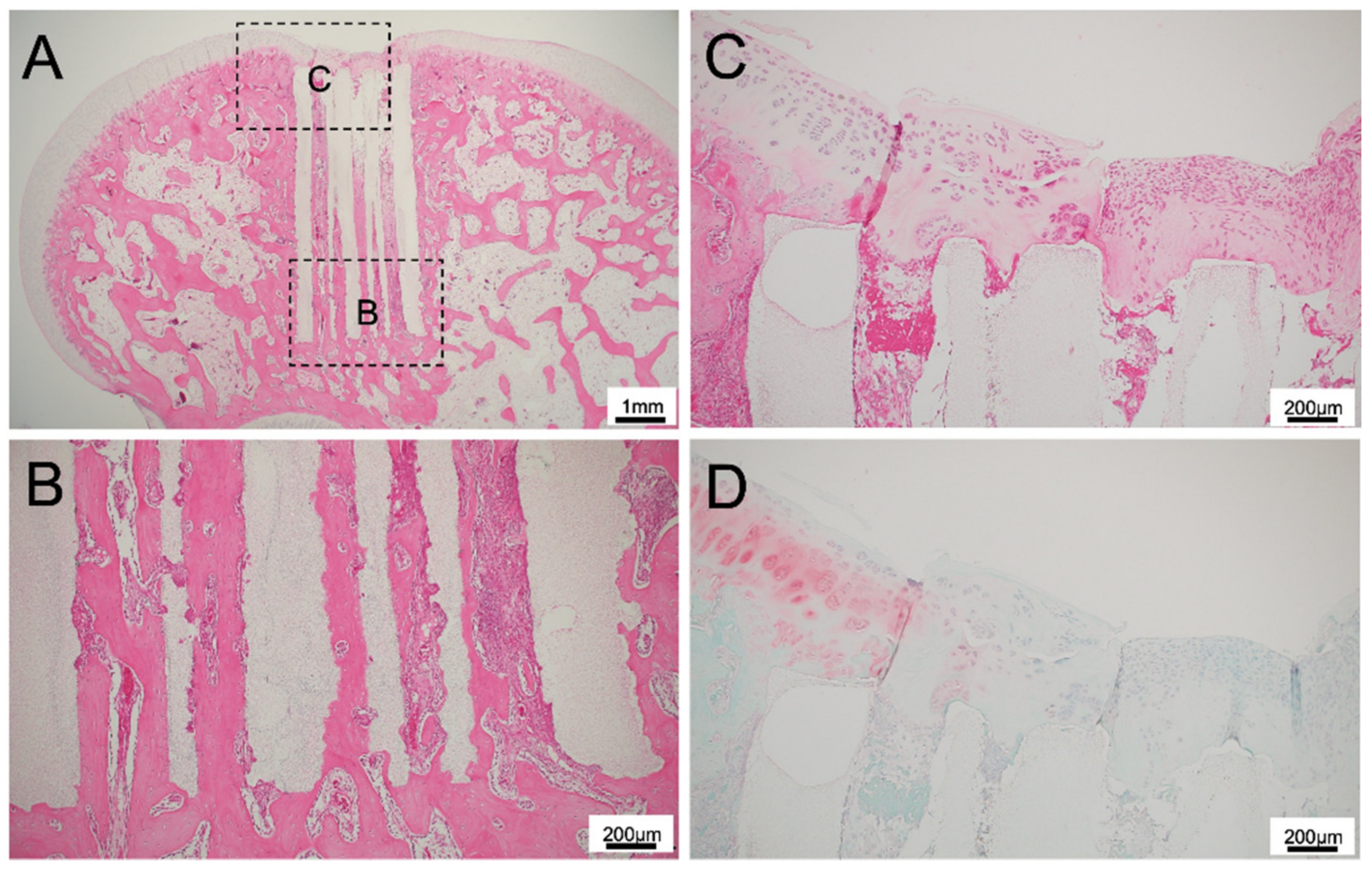
3.1. Histological Findings for 75TCP
3.2. Histological Findings for 300TCP
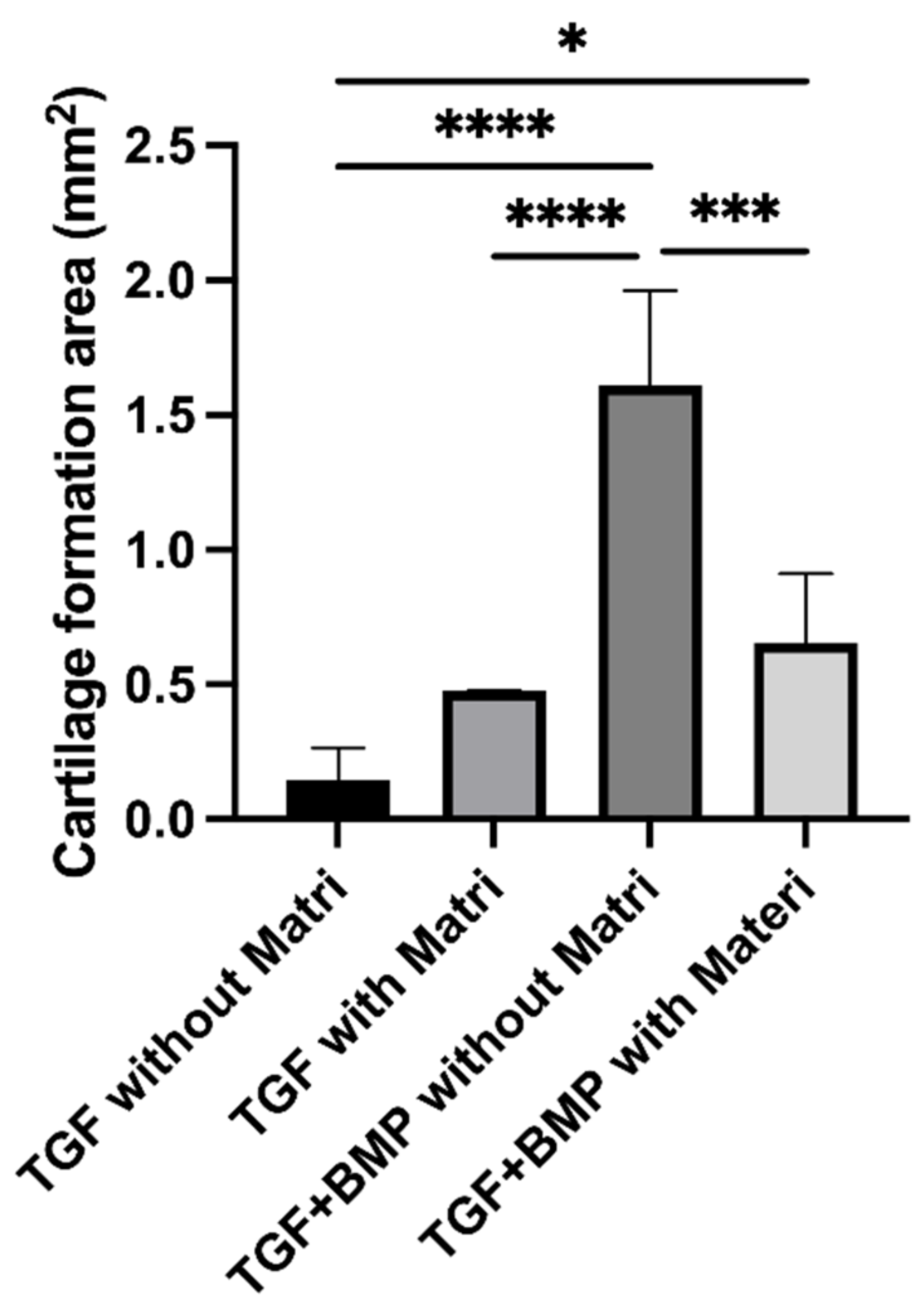
3.3. Quantitative Examination of Cartilage Tissue Formation in 300TCP
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Kaltreider, S.A.; Newman, S.A. Prevention and Management of Complications Associated with the Hydroxyapatite Implant. Ophthalmic Plast. Reconstr. Surg. 1996, 12, 18–31. [Google Scholar] [CrossRef]
- William, R.W.; Frank, V.; Dean, M.; Jason, A.; Andy, L.; Rema, O.; Yan, Y.; Hiroyuki, I.; Warwick, B. Beta-TCP bone graft substitutes in a bilateral rabbit tibial defect model. Biomaterials 2008, 29, 266–271. [Google Scholar]
- Teruo, A.; Shoji, O.; Katsuko, S.F.; Akito, T.; Takashi, U. Development of bioactive porous α-TCP/HAp beads for bone tissue engineering. J. Biomed. Mater. Res. Part A 2013, 101, 3295–3300. [Google Scholar]
- Ashish, D.; Manohar, L.B.; Prasad, V.D.; Bhumika, S.; Kiran, K.G.; Kaustubh, T.; Hiroshi, M.; Yoshihiko, S.; Hatsuhiko, M.; Mohammad, K.A. A Randomized Controlled Clinical Study of Autologous Platelet Rich Fibrin (PRF) in Combination with HA and Beta-TCP or HA and Beta-TCP Alone for Treatment of Furcation Defects. J. Hard Tissue Biol. 2019, 28, 185–190. [Google Scholar]
- Cenhao, W.; Genlin, W.; Qi, Y.; Jeffrey, C.W.; Huilin, Y.; Jun, Z. Silk Fibroin Regulates Osteoconduction of Hydroxyapatite in Rat Spine Fusion Model. J. Hard Tissue Biol. 2019, 28, 341–348. [Google Scholar]
- Kikuchi, M.; Itoh, S.; Ichinose, S.; Shinomiya, K.; Tanaka, J. Self-organization mechanism in a bone-like hydroxyapatite/collagen nanocomposite synthesized in vitro and its biological reaction in vivo. Biomaterials 2001, 22, 1705–1711. [Google Scholar] [CrossRef]
- Tsuchiya, A.; Sotome, S.; Asou, Y.; Kikuchi, M.; Koyama, Y.; Ogawa, T.; Tanaka, J.; Shinomiya, K. Effects of pore size and implant volume of porous hydroxyapatite/collagen (HAp/Col) on bone formation in a rabbit bone defect model. J. Med. Dent. Sci. 2008, 55, 91–99. [Google Scholar] [PubMed]
- Sotome, S.; Ae, K.; Okawa, A.; Ishizuki, M.; Morioka, H.; Matsumoto, S.; Nakamura, T.; Abe, S.; Beppu, Y.; Shinomiya, K. Efficacy and safety of porous hydroxyapatite/type 1 collagen composite implantation for bone regeneration: A randomized controlled study. J. Orthop. Sci. 2016, 21, 373–380. [Google Scholar] [CrossRef] [Green Version]
- Brittberg, M.; Lindahl, A.; Nilsson, A.; Ohlsson, C.; Isaksson, O.; Peterson, L. Treatment of Deep Cartilage Defects in the Knee with Autologous Chondrocyte Transplantation. N. Engl. J. Med. 1994, 331, 889–895. [Google Scholar] [CrossRef]
- Sandro, G.; Roberto, B.; Marco, C.; Alberto, R.; Annarita, C.; Carola, C.; Francesca, V. Cartilage repair evolution in post-traumatic osteochondral lesions of the talus: From open field autologous chondrocyte to bone-marrow-derived cells transplantation. Injury 2010, 41, 1196–1203. [Google Scholar]
- Caldwell, K.; Wang, J. Cell-based articular cartilage repair: The link between development and regeneration. Osteoarthr. Cartil. 2015, 23, 351–362. [Google Scholar] [CrossRef] [Green Version]
- Corvol, M.T.; Tahiri, K.; Montembault, A.; Daumard, A.; Savouret, J.F.; Rannou, F. Cell therapy in cartilage repair: Cellular and molecular bases. J. Soc. Biol. 2008, 202, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Tsumaki, N.; Okada, M.; Yamashita, A. iPS cell technologies and cartilage regeneration. Bone 2015, 70, 48–54. [Google Scholar] [CrossRef]
- Tsumaki, N. Cartilage/chondrocyte research and osteoarthritis. Regeneration of articular cartilage damage using iPS cell-derived cartilage. Clin. Calcium 2018, 28, 803–808. [Google Scholar] [PubMed]
- Taguchi, T.; Ikoma, T.; Tanaka, J. An improved method to prepare hyaluronic acid and type II collagen composite matrices. J. Biomed. Mater. Res. 2002, 61, 330–336. [Google Scholar] [CrossRef]
- Bassett, C.A.L.; Herrmann, I. Influence of Oxygen Concentration and Mechanical Factors on Differentiation of Connective Tissues in vitro. Nat. Cell Biol. 1961, 190, 460–461. [Google Scholar] [CrossRef] [PubMed]
- Schoon, J.; Ort, M.J.; Huesker, K.; Geißler, S.; Rakow, A. Diagnosis of Metal Hypersensitivity in Total Knee Arthroplasty: A Case Report. Front. Immunol. 2019, 10, 2758. [Google Scholar] [CrossRef]
- Dormer, N.H.; Singh, M.; Zhao, L.; Mohan, N.; Berkland, C.J.; Detamore, M.S. Osteochondral interface regeneration of the rabbit knee with macroscopic gradients of bioactive signals. J. Biomed. Mater. Res. Part A 2012, 100A, 162–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bailey, B.M.; Nail, L.N.; Grunlan, M.A. Continuous gradient scaffolds for rapid screening of cell–material interactions and interfacial tissue regeneration. Acta Biomater. 2013, 9, 8254–8261. [Google Scholar] [CrossRef] [Green Version]
- Nakagiri, R.; Watanabe, S.; Takabatake, K.; Tsujigiwa, H.; Watanabe, T.; Matsumoto, H.; Kimata, Y. Long-Term Effect of Honeycomb β-Tricalcium Phosphate on Zygomatic Bone Regeneration in Rats. Materials 2020, 13, 5374. [Google Scholar] [CrossRef]
- Watanabe, T.; Takabatake, K.; Tsujigiwa, H.; Watanabe, S.; Nakagiri, R.; Nakano, K.; Nagatsuka, H.; Kimata, Y. Effect of Honeycomb β-TCP Geometrical Structure on Bone Tissue Regeneration in Skull Defect. Materials 2020, 13, 4761. [Google Scholar] [CrossRef]
- Watanabe, S.; Takabatake, K.; Tsujigiwa, H.; Watanabe, T.; Tokuyama, E.; Ito, S.; Nagatsuka, H.; Kimata, Y. Efficacy of Honeycomb TCP-induced Microenvironment on Bone Tissue Regeneration in Craniofacial Area. Int. J. Med. Sci. 2016, 13, 466–476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takabatake, K.; Yamachika, E.; Tsujigiwa, H.; Takeda, Y.; Kimura, M.; Takagi, S.; Nagatsuka, H.; Iida, S. Effect of geometry and microstructure of honeycomb TCP scaffolds on bone regeneration. J. Biomed. Mater. Res. Part A 2014, 102, 2952–2960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuda, H.; Takabatake, K.; Tsujigiwa, H.; Watanabe, S.; Ito, S.; Kawai, H.; Hamada, M.; Yoshida, S.; Nakano, K.; Nagatsuka, H. Effects of the Geometrical Structure of a Honeycomb TCP on Relationship between Bone / Cartilage Formation and Angiogenesis. Int. J. Med. Sci. 2018, 15, 1582–1590. [Google Scholar] [CrossRef] [Green Version]
- Khademhosseini, A.; Vacanti, J.P.; Langer, R. Progress in Tissue Engineering. Sci. Am. 2009, 300, 64–71. [Google Scholar] [CrossRef]
- Dormer, N.H.; Berkland, C.J.; Detamore, M.S. Emerging Techniques in Stratified Designs and Continuous Gradients for Tissue Engineering of Interfaces. Ann. Biomed. Eng. 2010, 38, 2121–2141. [Google Scholar] [CrossRef]
- To, K.; Zhang, B.; Romain, K.; Mak, C.; Khan, W. Synovium-Derived Mesenchymal Stem Cell Transplantation in Cartilage Regeneration: A PRISMA Review of in vivo Studies. Front. Bioeng. Biotechnol. 2019, 7, 314. [Google Scholar] [CrossRef]
- Koga, H.; Shimaya, M.; Muneta, T.; Nimura, A.; Morito, T.; Hayashi, M.; Suzuki, S.; Ju, Y.-J.; Mochizuki, T.; Sekiya, I. Local adherent technique for transplanting mesenchymal stem cells as a potential treatment of cartilage defect. Arthritis Res. Ther. 2008, 10, R84. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, T.; Sekiya, I.; Muneta, T.; Hatsushika, D.; Horie, M.; Tsuji, K.; Kawarasaki, T.; Watanabe, A.; Hishikawa, S.; Fujimoto, Y.; et al. Arthroscopic, histological and MRI analyses of cartilage repair after a minimally invasive method of transplantation of allogeneic synovial mesenchymal stromal cells into cartilage defects in pigs. Cytotherapy 2012, 14, 327–338. [Google Scholar] [CrossRef] [Green Version]
- Zhu, S.; Qiu, H.; Bennett, S.; Kuek, V.; Rosen, V.; Xu, H.; Xu, J. Chondromodulin-1 in health, osteoarthritis, cancer, and heart disease. Cell. Mol. Life Sci. 2019, 76, 4493–4502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, W.; Friis, T.E.; Long, X.; Xiao, Y. Expression of chondromodulin-1 in the temporomandibular joint condylar cartilage and disc. J. Oral Pathol. Med. 2009, 39, 356–360. [Google Scholar] [CrossRef] [Green Version]
- Park, J.S.; Shim, M.-S.; Shim, S.H.; Na Yang, H.; Jeon, S.Y.; Woo, D.G.; Lee, D.R.; Yoon, T.K.; Park, K.-H. Chondrogenic potential of stem cells derived from amniotic fluid, adipose tissue, or bone marrow encapsulated in fibrin gels containing TGF-β3. Biomaterials 2011, 32, 8139–8149. [Google Scholar] [CrossRef] [PubMed]
- Santo, V.E.; Gomes, M.E.; Mano, J.F.; Reis, R.L. Controlled Release Strategies for Bone, Cartilage, and Osteochondral Engineering—Part II: Challenges on the Evolution from Single to Multiple Bioactive Factor Delivery. Tissue Eng. Part B Rev. 2013, 19, 327–352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delgado, R.R.A.; Solis, R.; Sanchez, E.; Hernandez, A.; Roman, J.S.; Evora, C. Cartilage repair by local delivery of transforming growth factor-β1 or bone morphogenetic protein-2 from a novel, segmented polyurethane/polylactic-co-glycolic bilayered scaffold. J. Biomed. Mater. Res. A 2014, 102, 1110–1120. [Google Scholar]
- Hakki, S.S.; Bozkurt, B.; Hakki, E.E.; Kayis, S.A.; Turac, G.; Yilmaz, I.; Karaoz, E. Bone morphogenetic protein-2, -6, and -7 differently regulate osteogenic differentiation of human periodontal ligament stem cells. J. Biomed. Mater. Res. Part B Appl. Biomater. 2014, 102, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Tominaga, H.; Maeda, S.; Miyoshi, H.; Miyazono, K.; Komiya, S.; Imamura, T. Expression of osterix inhibits bone morphogenetic protein-induced chondrogenic differentiation of mesenchymal progenitor cells. J. Bone Miner. Metab. 2008, 27, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, I.; Maeda, S.; Imamura, K.; Setoguchi, T.; Yokouchi, M.; Ishidou, Y.; Komiya, S. SnoN Suppresses Maturation of Chondrocytes by Mediating Signal Cross-talk between Transforming Growth Factor-β and Bone Morphogenetic Protein Pathways. J. Biol. Chem. 2012, 287, 29101–29113. [Google Scholar] [CrossRef] [Green Version]
- Kakoi, H.; Maeda, S.; Shinohara, N.; Matsuyama, K.; Imamura, K.; Kawamura, I.; Nagano, S.; Setoguchi, T.; Yokouchi, M.; Ishidou, Y.; et al. Bone Morphogenic Protein (BMP) Signaling Up-regulates Neutral Sphingomyelinase 2 to Suppress Chondrocyte Maturation via the Akt Protein Signaling Pathway as a Negative Feedback Mechanism. J. Biol. Chem. 2014, 289, 8135–8150. [Google Scholar] [CrossRef] [Green Version]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takabatake, K.; Tsujigiwa, H.; Yoshida, A.; Furumatsu, T.; Kawai, H.; Oo, M.W.; Nakano, K.; Nagatsuka, H. A Pilot Study of Seamless Regeneration of Bone and Cartilage in Knee Joint Regeneration Using Honeycomb TCP. Materials 2021, 14, 7225. https://doi.org/10.3390/ma14237225
Takabatake K, Tsujigiwa H, Yoshida A, Furumatsu T, Kawai H, Oo MW, Nakano K, Nagatsuka H. A Pilot Study of Seamless Regeneration of Bone and Cartilage in Knee Joint Regeneration Using Honeycomb TCP. Materials. 2021; 14(23):7225. https://doi.org/10.3390/ma14237225
Chicago/Turabian StyleTakabatake, Kiyofumi, Hidetsugu Tsujigiwa, Aki Yoshida, Takayuki Furumatsu, Hotaka Kawai, May Wathone Oo, Keisuke Nakano, and Hitoshi Nagatsuka. 2021. "A Pilot Study of Seamless Regeneration of Bone and Cartilage in Knee Joint Regeneration Using Honeycomb TCP" Materials 14, no. 23: 7225. https://doi.org/10.3390/ma14237225
APA StyleTakabatake, K., Tsujigiwa, H., Yoshida, A., Furumatsu, T., Kawai, H., Oo, M. W., Nakano, K., & Nagatsuka, H. (2021). A Pilot Study of Seamless Regeneration of Bone and Cartilage in Knee Joint Regeneration Using Honeycomb TCP. Materials, 14(23), 7225. https://doi.org/10.3390/ma14237225





