Machine Learning-Based Strength Prediction for Refractory High-Entropy Alloys of the Al-Cr-Nb-Ti-V-Zr System
Abstract
:1. Introduction
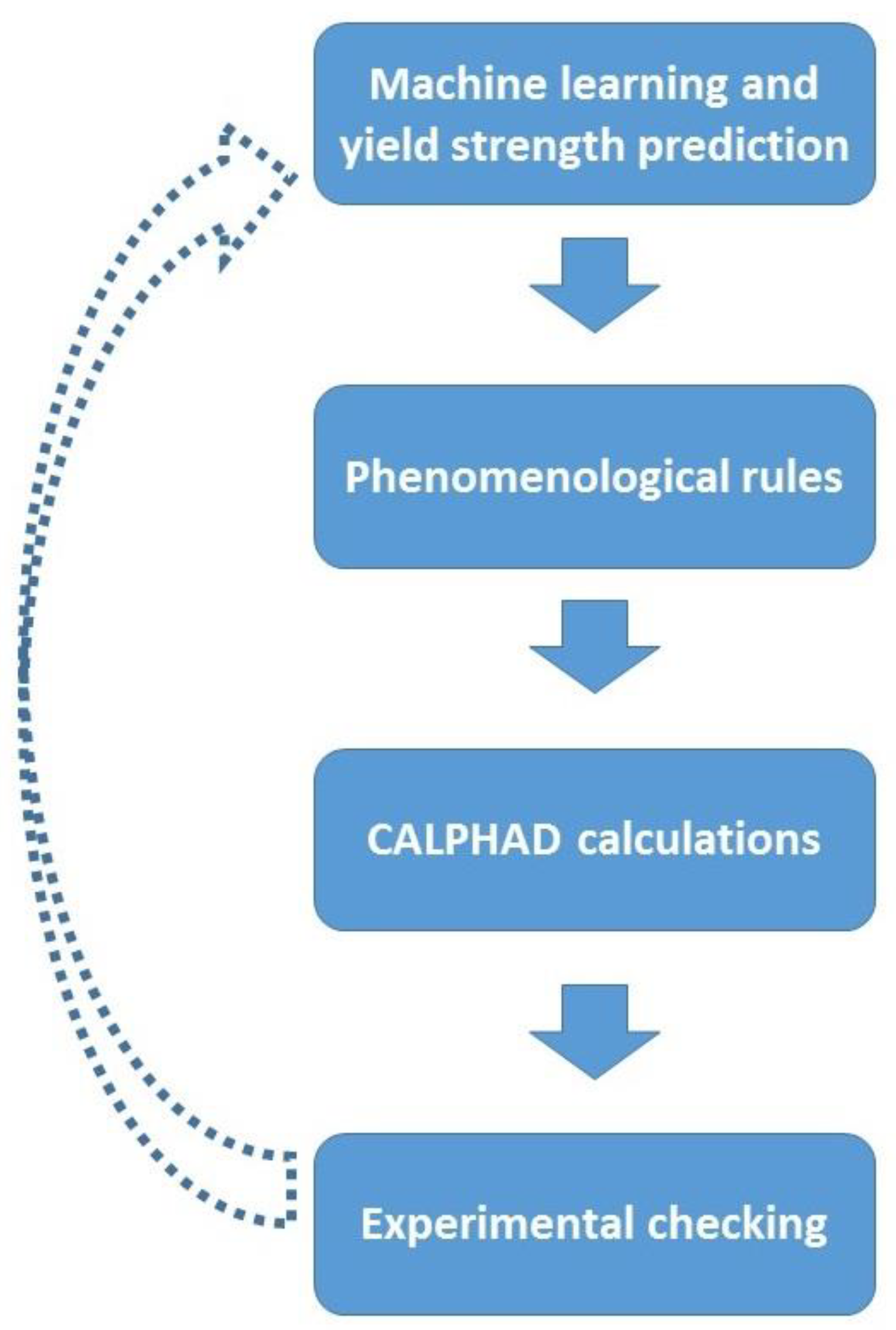
2. Materials and Methods
2.1. Computational Predictions
2.1.1. Machine Learning
2.1.2. Phenomenological Rules
2.1.3. CALPHAD Calculations
2.2. Experiment
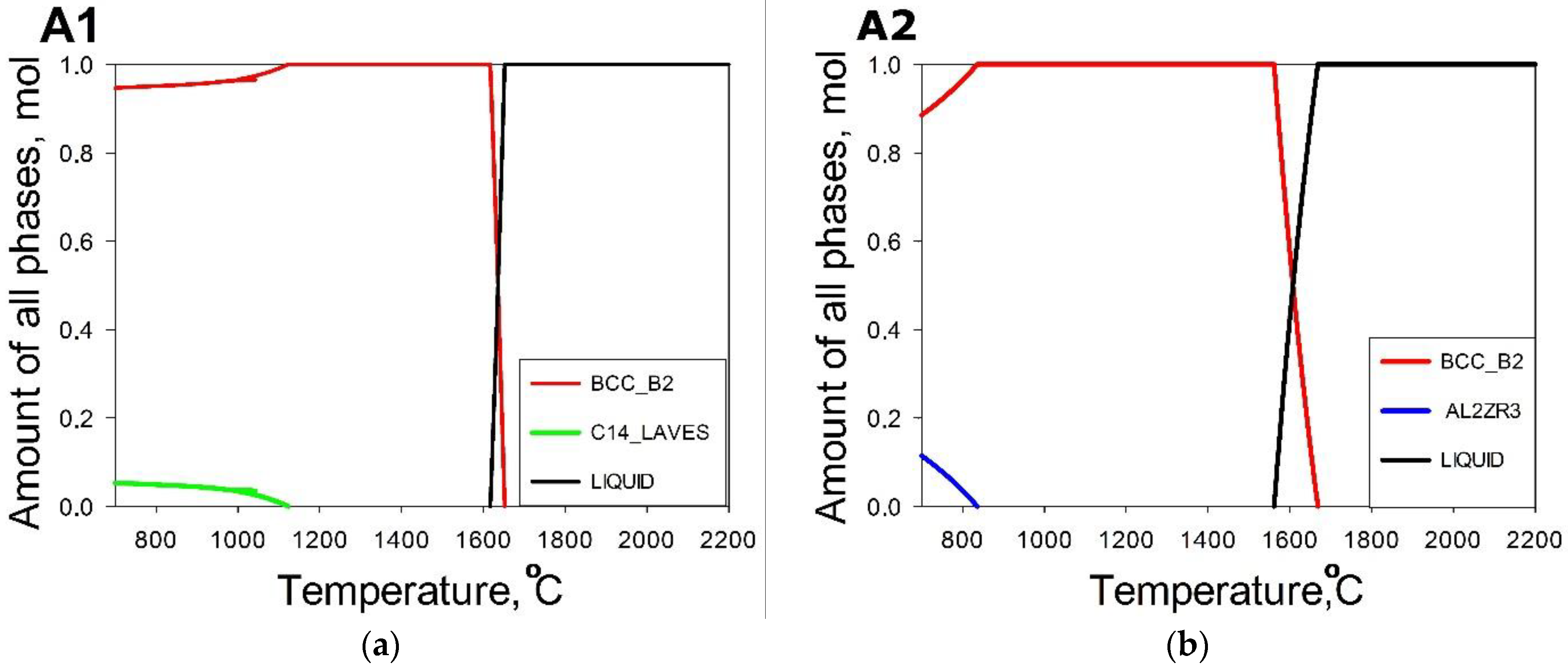
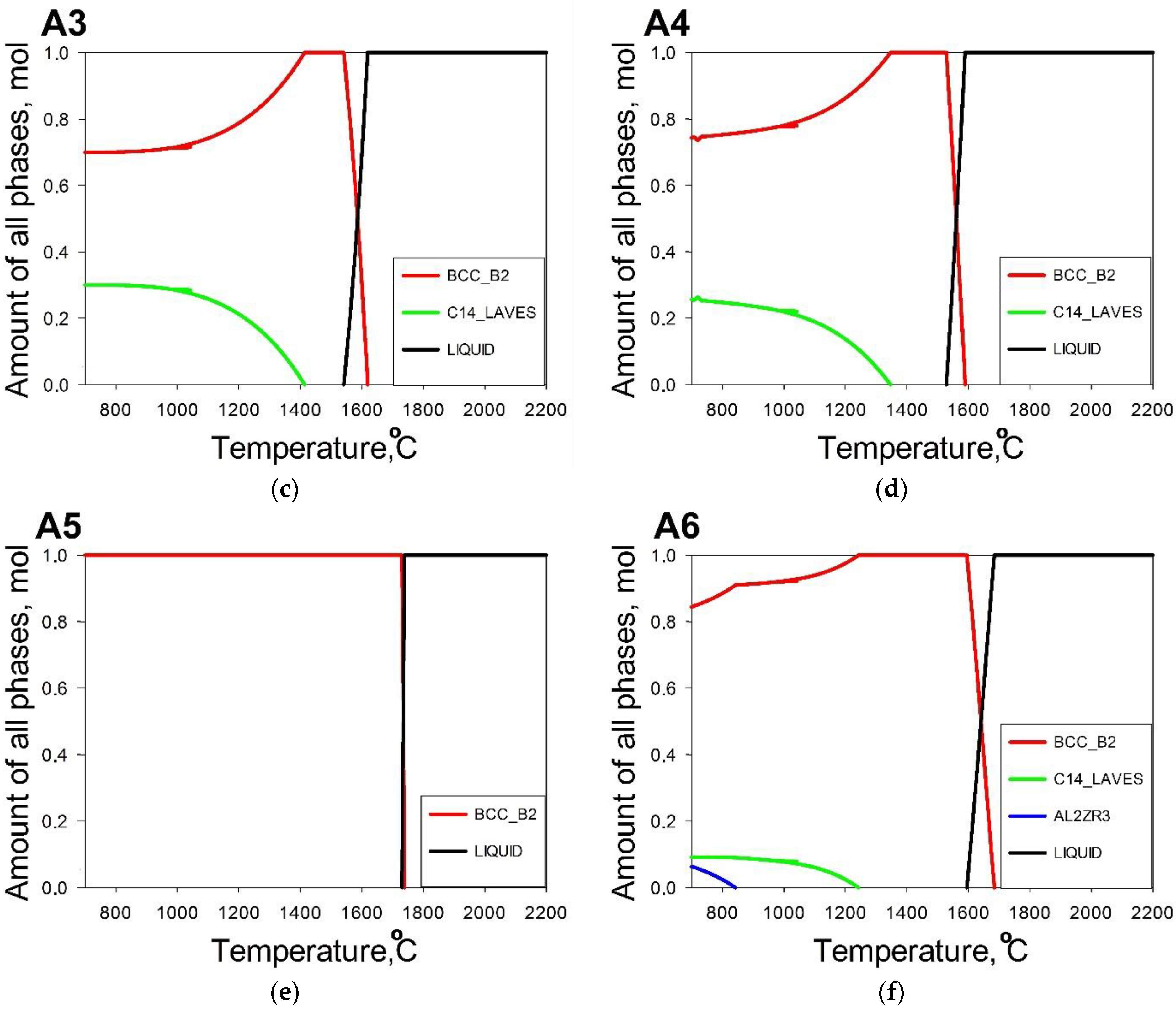
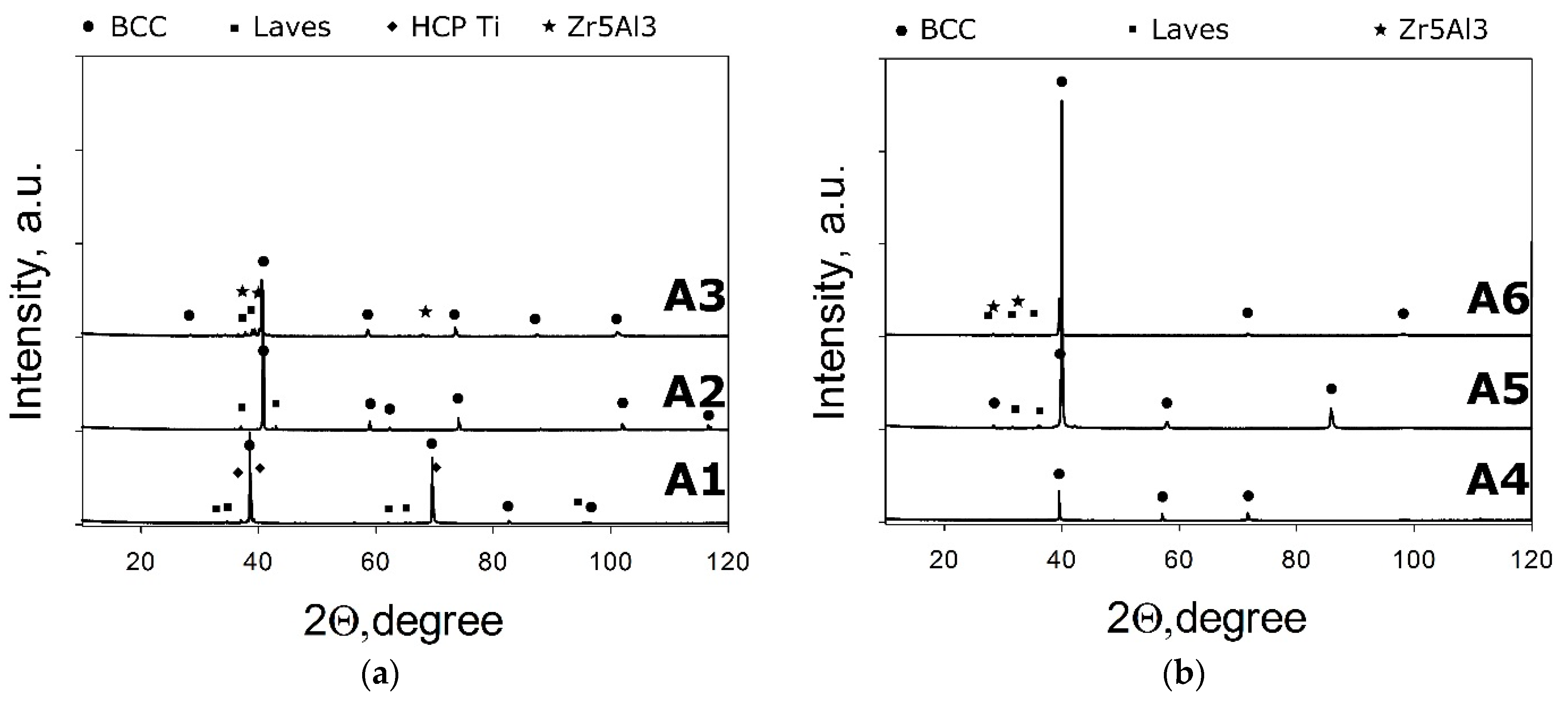
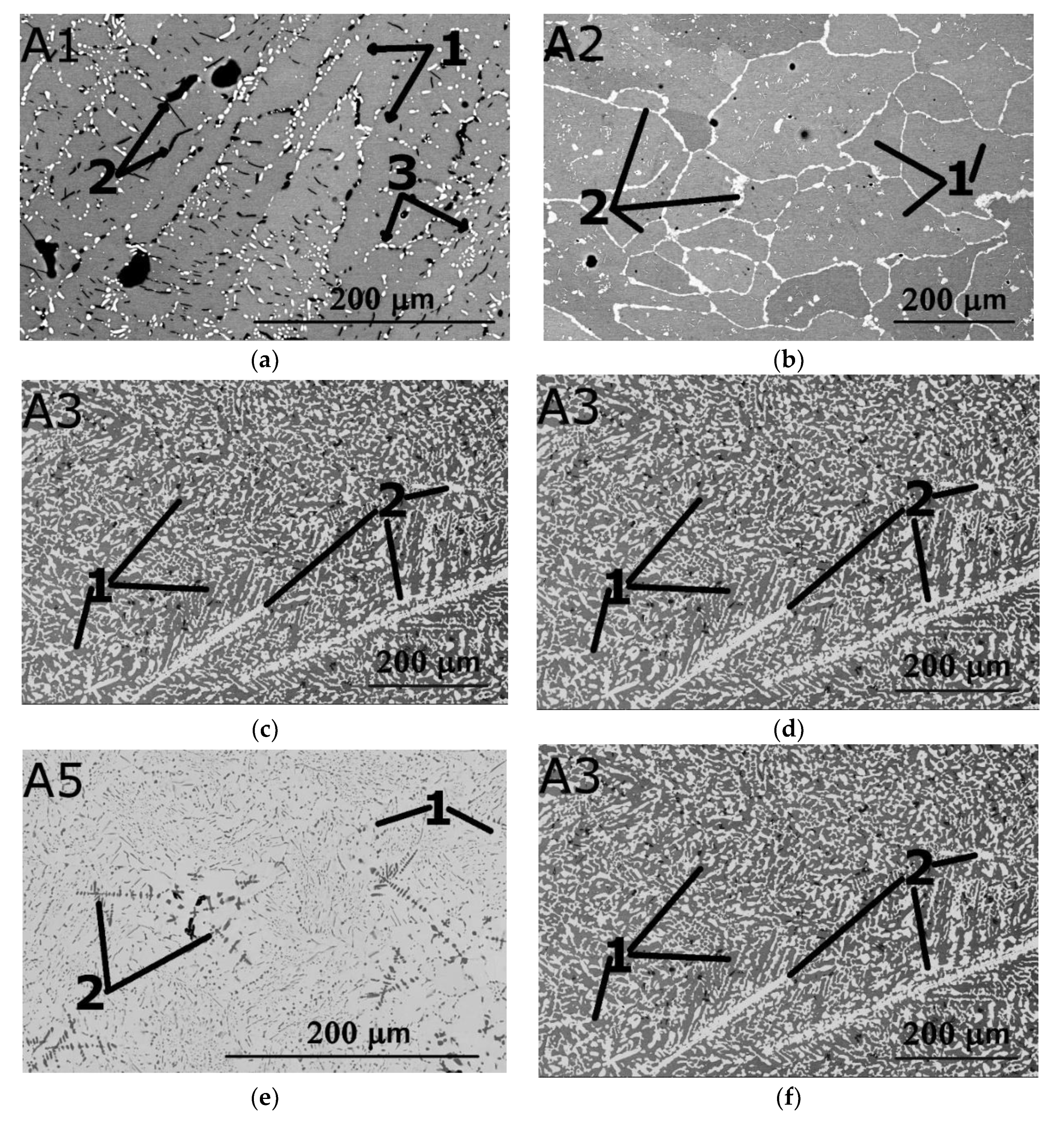
3. Results
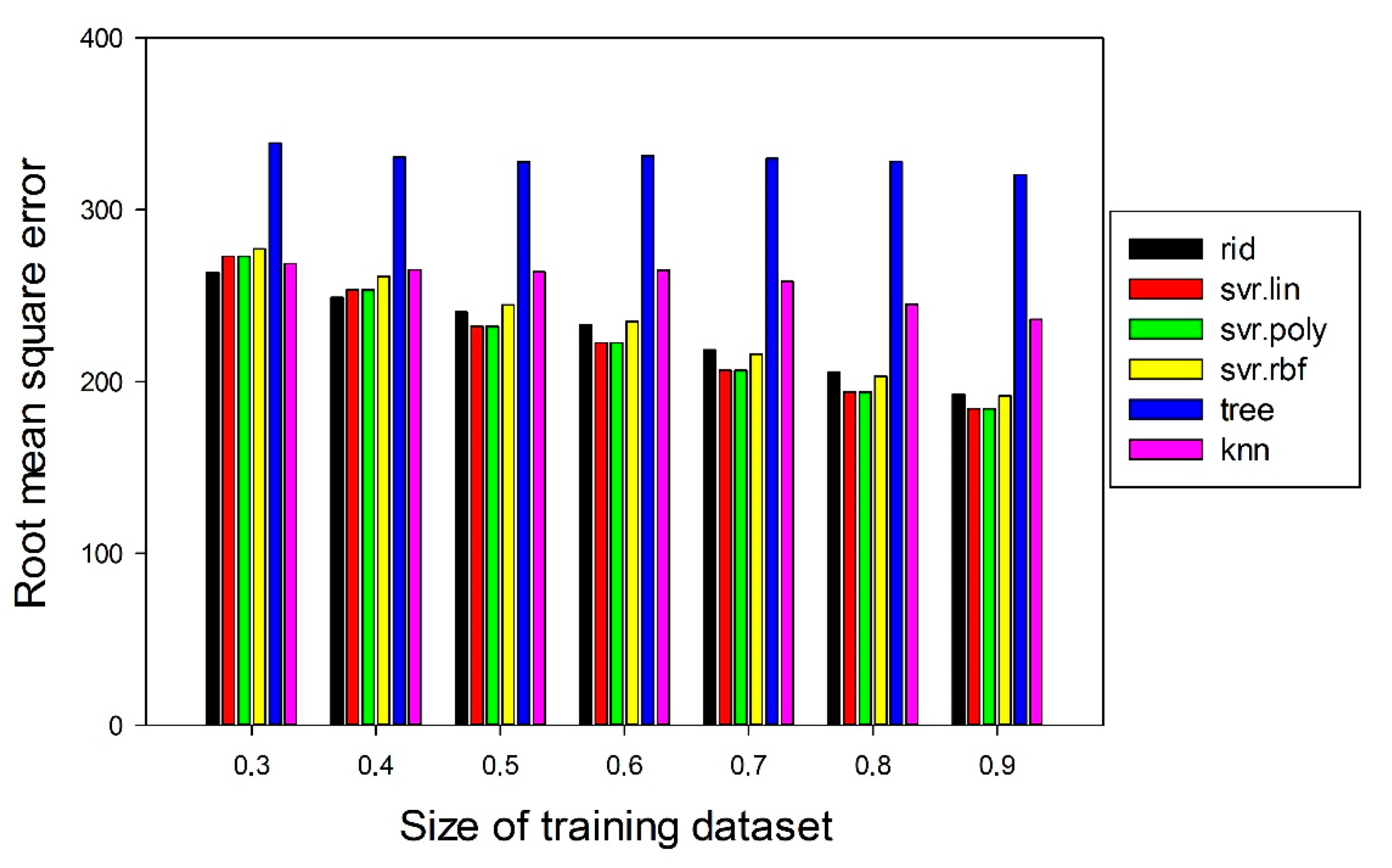
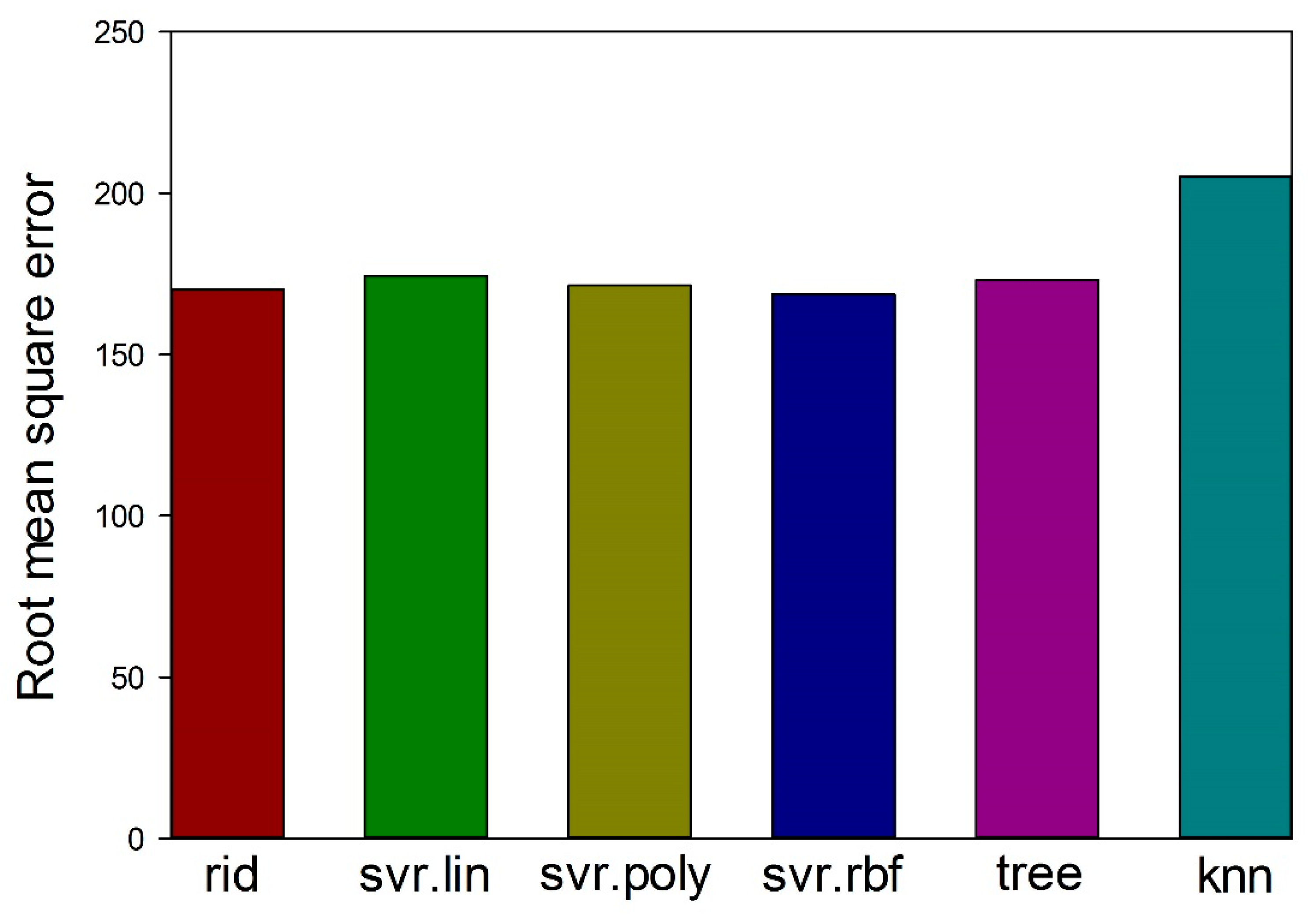
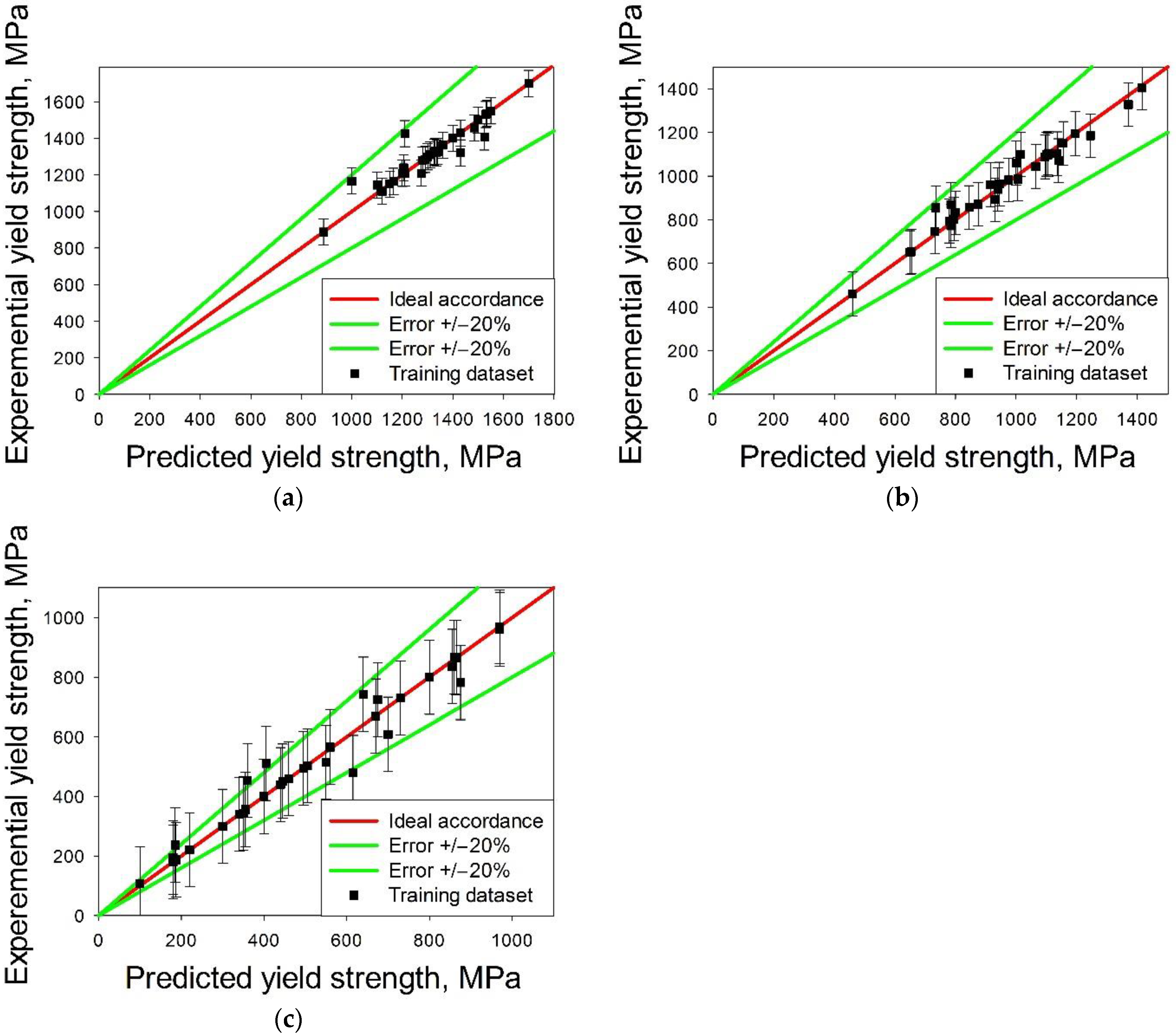
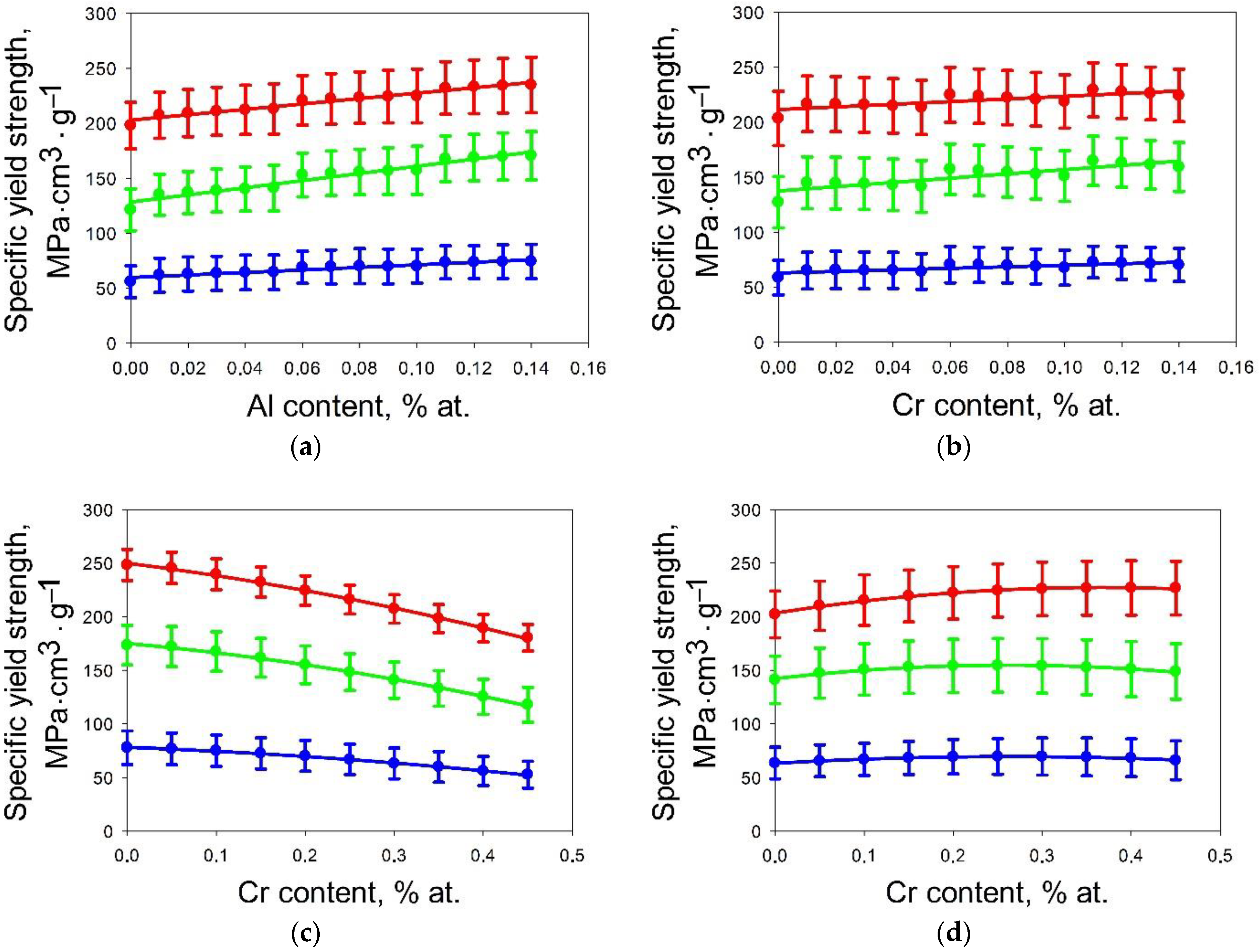
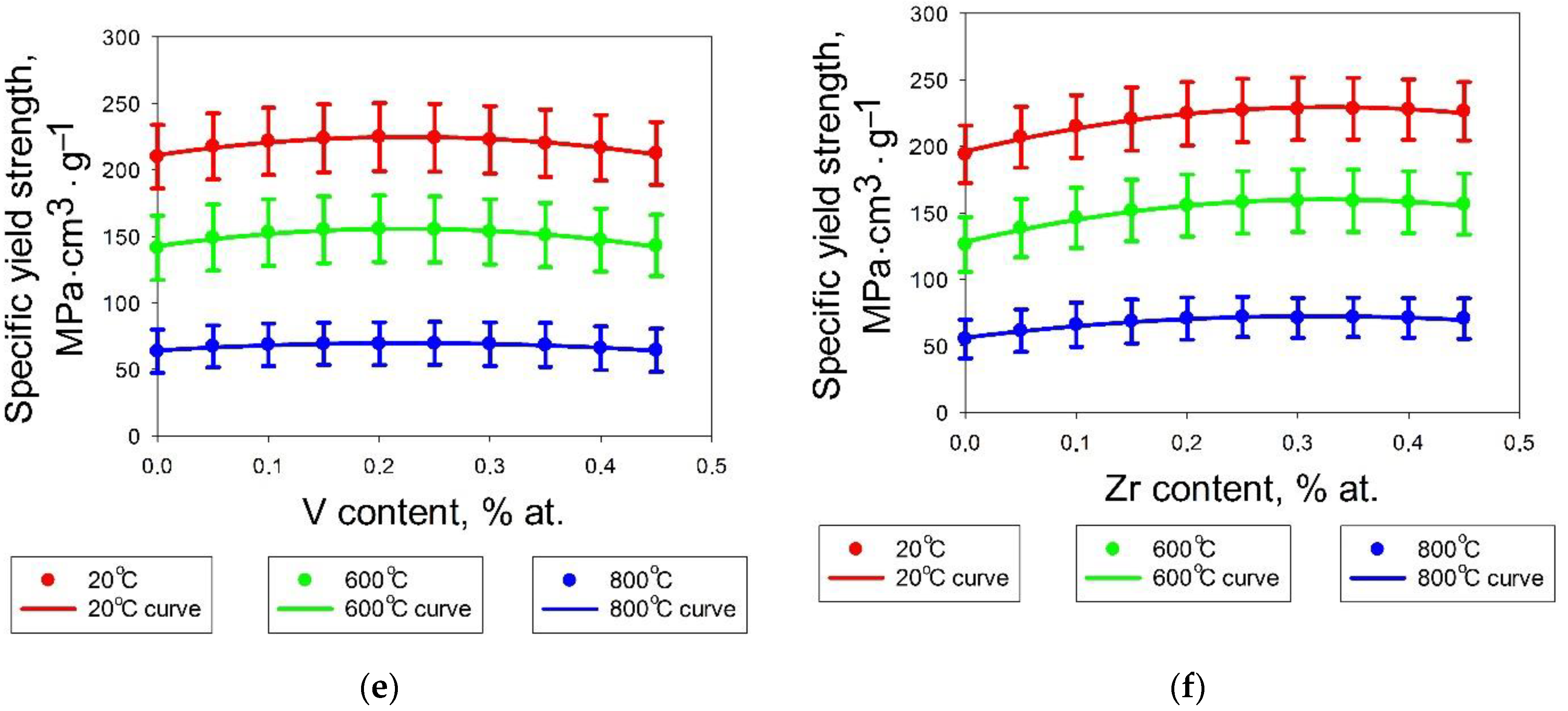
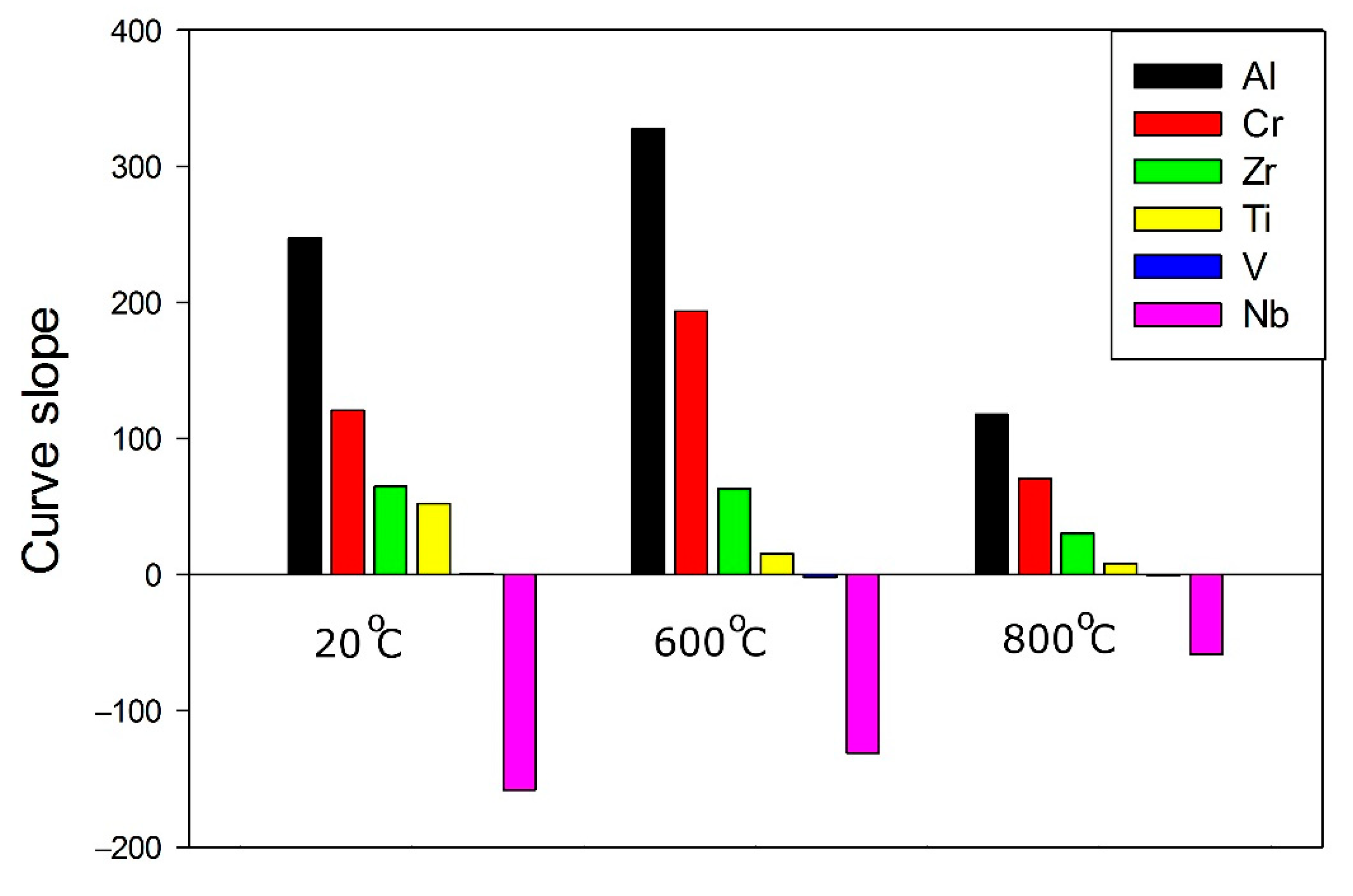
3.1. Machine Learning Prediction of Composition-Properties Relationships in Alloys of the Al-Cr-Nb-Ti-V-Zr System
3.2. Comparison between the Predicted and Actual Structure of Al-Cr-Nb-Ti-V-Zr Alloys
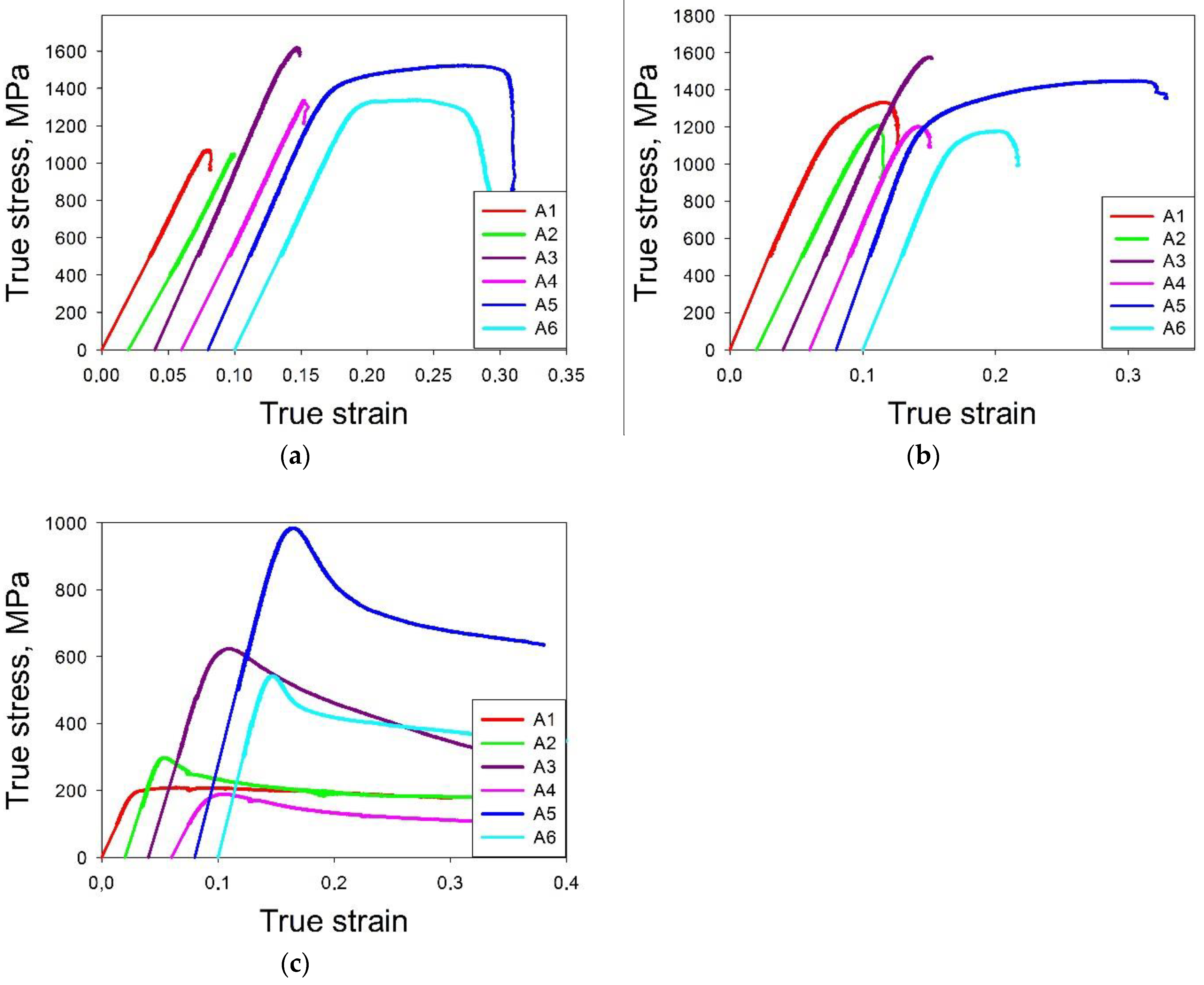
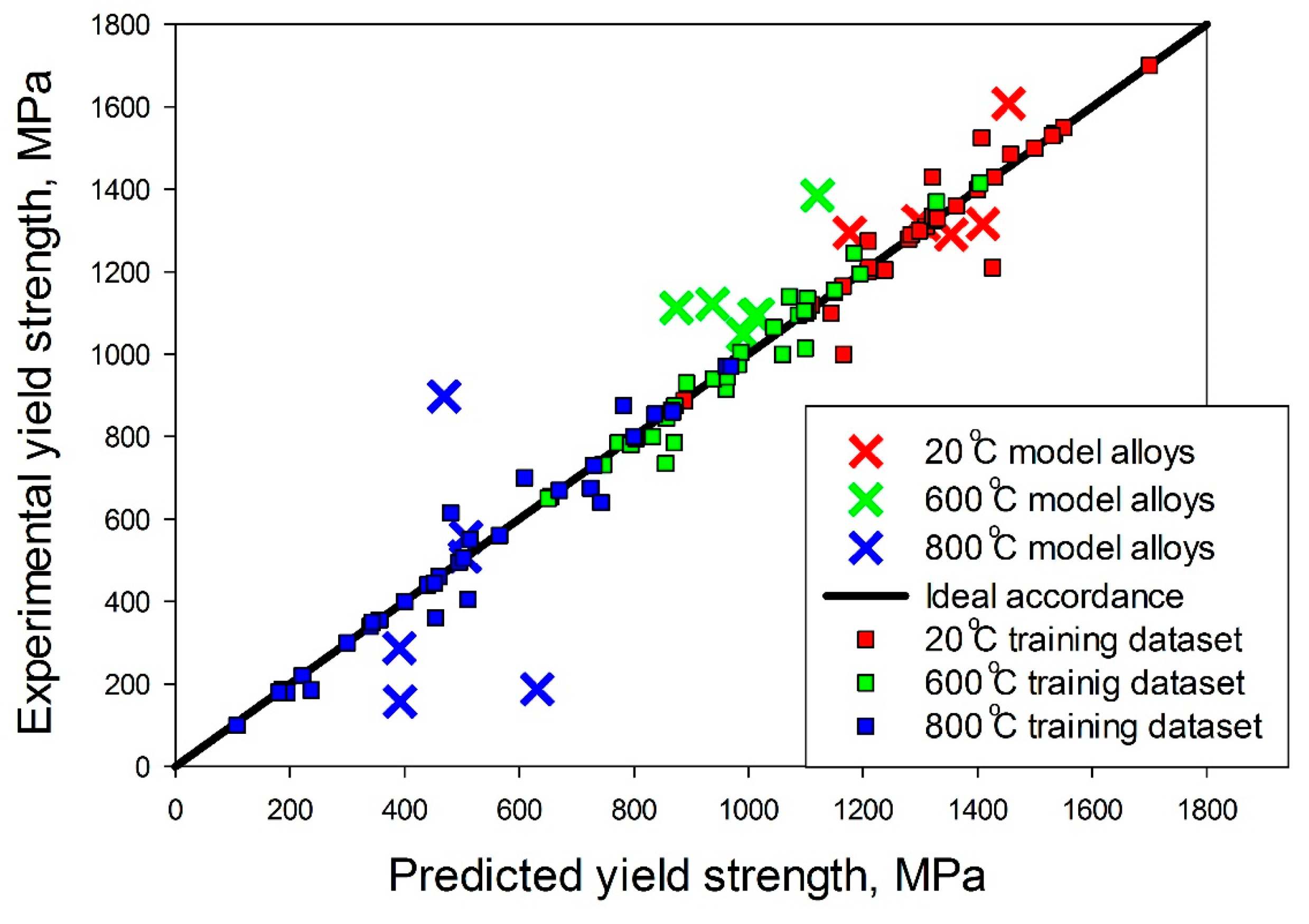
3.3. Comparison of Predicted and Measured Mechanical Properties of the Al-Cr-Nb-Ti -V-Zr Alloys
4. Conclusions
- The use of a combination of CALPHAD and phenomenological rules does not result in an accurate prediction of the phase composition of the alloys; only one of them had a desirable single-phase structure. However, in four model alloys the second phase(s) did not exceed 10%, thereby suggesting the good potential of this approach for the selection of alloys with a desirable phase composition.
- The surrogate model based on a support-vector machine algorithm for the prediction of the yield strength showed good accuracy at 20 °C and 600 °C (the error of prediction was less than 20% for all alloys except one). However, at 800 °C, the error of prediction was worse than 20% for only two model alloys. Relatively low prediction accuracy at 800 °C can be associated with the proximity of this temperature to the transition point between the athermal plateau and the strong temperature dependence in bcc alloys, causing, in turn, a severe spread in the yield strength of the training dataset alloys.
- For the Al-Cr-Nb-Ti-V-Zr system, the content of aluminum, chromium and zirconium have the greatest influence on the specific yield strength. The effect of vanadium and titanium is lower; an addition of niobium has a negative effect on specific yield strength.
- One of the predicted alloys (A5: Al13Cr12Nb20Ti20V35) possesses an excellent combination of strength (1295 MPa at 20 °C, 1113 MPa at 600 °C and 898 MPa at 800 °C) and ductility (16.8% at 20 °C, 5.5% at 600 °C and >50% at 800 °C) in the interval 20–800 °C.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yeh, J.W.; Chen, S.K.; Lin, S.J.; Gan, J.Y.; Chin, T.S.; Shun, T.T.; Tsau, C.H.; Chang, S.Y. Nanostructured High-Entropy Alloys with Multiple Principal Elements: Novel Alloy Design Concepts and Outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Cantor, B.; Chang, I.T.H.; Knight, P.; Vincent, A.J.B. Microstructural Development in Equiatomic Multicomponent Alloys. Mater. Sci. Eng. A 2004, 375–377, 213–218. [Google Scholar] [CrossRef]
- Gao, M.C.; Liaw, P.K.; Yeh, J.W.; Zhang, Y. High-Entropy Alloys: Fundamentals and Applications; Springer: Berlin/Heidelberg, Germany, 2016; ISBN 9783319270135. [Google Scholar]
- Miracle, D.B.; Senkov, O.N. A Critical Review of High Entropy Alloys and Related Concepts. Acta Mater. 2017, 122, 448–511. [Google Scholar] [CrossRef] [Green Version]
- Tsai, M.-H.; Yeh, J.-W. High-Entropy Alloys: A Critical Review. Mater. Res. Lett. 2014, 2, 107–123. [Google Scholar] [CrossRef]
- Steurer, W. Single-Phase High-Entropy Alloys—A Critical Update. Mater. Charact. 2020, 162, 110179. [Google Scholar] [CrossRef]
- Chuang, M.H.; Tsai, M.H.; Wang, W.R.; Lin, S.J.; Yeh, J.W. Microstructure and Wear Behavior of AlxCo1.5CrFeNi1.5Tiy High-Entropy Alloys. Acta Mater. 2011, 59, 6308–6317. [Google Scholar] [CrossRef]
- Gorr, B.; Azim, M.; Christ, H.J.; Mueller, T.; Schliephake, D.; Heilmaier, M. Phase Equilibria, Microstructure, and High Temperature Oxidation Resistance of Novel Refractory High-Entropy Alloys. J. Alloys Compd. 2015, 624, 270–278. [Google Scholar] [CrossRef]
- Shi, Y.; Yang, B.; Liaw, P.K. Corrosion-Resistant High-Entropy Alloys: A Review. Metals 2017, 7, 43. [Google Scholar] [CrossRef] [Green Version]
- Senkov, O.N.; Wilks, G.B.; Miracle, D.B.; Chuang, C.P.; Liaw, P.K. Refractory High-Entropy Alloys. Intermetallics 2010, 18, 1758–1765. [Google Scholar] [CrossRef]
- Senkov, O.N.; Wilks, G.B.; Scott, J.M.; Miracle, D.B. Mechanical Properties of Nb25Mo25Ta25W25 and V20Nb20Mo20Ta20W20 Refractory High Entropy Alloys. Intermetallics 2011, 19, 698–706. [Google Scholar] [CrossRef]
- Senkov, O.N.; Senkova, S.V.; Woodward, C.; Miracle, D.B. Low-Density, Refractory Multi-Principal Element Alloys of the Cr-Nb-Ti-V-Zr System: Microstructure and Phase Analysis. Acta Mater. 2013, 61, 1545–1557. [Google Scholar] [CrossRef]
- Senkov, O.N.; Senkova, S.V.; Miracle, D.B.; Woodward, C. Mechanical Properties of Low-Density, Refractory Multi-Principal Element Alloys of the Cr-Nb-Ti-V-Zr System. Mater. Sci. Eng. A 2013, 565, 51–62. [Google Scholar] [CrossRef]
- Couzinié, J.P.; Senkov, O.N.; Miracle, D.B.; Dirras, G. Comprehensive Data Compilation on the Mechanical Properties of Refractory High-Entropy Alloys. Data Br. 2018, 21, 1622–1641. [Google Scholar] [CrossRef]
- Senkov, O.N.; Miracle, D.B.; Chaput, K.J.; Couzinie, J.P. Development and Exploration of Refractory High Entropy Alloys—A Review. J. Mater. Res. 2018, 33, 3092–3128. [Google Scholar] [CrossRef] [Green Version]
- Guo, S. Phase Selection Rules for Cast High Entropy Alloys: An Overview. Mater. Sci. Technol. 2015, 31, 1223–1230. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, Y. Prediction of High-Entropy Stabilized Solid-Solution in Multi-Component Alloys. Mater. Chem. Phys. 2012, 132, 233–238. [Google Scholar] [CrossRef]
- Guo, S.; Hu, Q.; Ng, C.; Liu, C.T. More than Entropy in High-Entropy Alloys: Forming Solid Solutions or Amorphous Phase. Intermetallics 2013, 41, 96–103. [Google Scholar] [CrossRef]
- Ye, Y.F.; Wang, Q.; Lu, J.; Liu, C.T.; Yang, Y. Design of High Entropy Alloys: A Single-Parameter Thermodynamic Rule. Scr. Mater. 2015, 104, 53–55. [Google Scholar] [CrossRef]
- Singh, A.K.; Kumar, N.; Dwivedi, A.; Subramaniam, A. A Geometrical Parameter for the Formation of Disordered Solid Solutions in Multi-Component Alloys. Intermetallics 2014, 53, 112–119. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, Y.; Yang, Y.; Wang, J.; Liu, C.T. Atomic-Size Effect and Solid Solubility of Multicomponent Alloys. Scr. Mater. 2015, 94, 28–31. [Google Scholar] [CrossRef]
- Troparevsky, M.C.; Morris, J.R.; Kent, P.R.C.; Lupini, A.R.; Stocks, G.M. Criteria for Predicting the Formation of Single-Phase High-Entropy Alloys. Phys. Rev. X 2015, 5, 011041. [Google Scholar] [CrossRef] [Green Version]
- Senkov, O.N.; Miracle, D.B. A New Thermodynamic Parameter to Predict Formation of Solid Solution or Intermetallic Phases in High Entropy Alloys. J. Alloys Compd. 2016, 658, 603–607. [Google Scholar] [CrossRef] [Green Version]
- King, D.J.M.; Middleburgh, S.C.; McGregor, A.G.; Cortie, M.B. Predicting the Formation and Stability of Single Phase High-Entropy Alloys. Acta Mater. 2016, 104, 172–179. [Google Scholar] [CrossRef]
- Li, J.H.; Tsai, M.H. Theories for Predicting Simple Solid Solution High-Entropy Alloys: Classification, Accuracy, and Important Factors Impacting Accuracy. Scr. Mater. 2020, 188, 80–87. [Google Scholar] [CrossRef]
- Senkov, O.N.; Miller, J.D.; Miracle, D.B.; Woodward, C. Accelerated Exploration of Multi-Principal Element Alloys for Structural Applications. Calphad Comput. Coupling Phase Diagr. Thermochem. 2015, 50, 32–48. [Google Scholar] [CrossRef]
- Senkov, O.N.; Miller, J.D.; Miracle, D.B.; Woodward, C. Accelerated Exploration of Multi-Principal Element Alloys with Solid Solution Phases. Nat. Commun. 2015, 6, 6529. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, F.; Chen, S.; Cao, W. Computational Thermodynamics Aided High-Entropy Alloy Design. JOM 2012, 64, 839–845. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, C.; Chen, S.L.; Zhu, J.; Cao, W.S.; Kattner, U.R. An Understanding of High Entropy Alloys from Phase Diagram Calculations. Calphad Comput. Coupling Phase Diagr. Thermochem. 2014, 45, 1–10. [Google Scholar] [CrossRef]
- Gao, M.C.; Carney, C.S.; Doğan, N.; Jablonksi, P.D.; Hawk, J.A.; Alman, D.E. Design of Refractory High-Entropy Alloys. JOM 2015, 67, 2653–2669. [Google Scholar] [CrossRef]
- Huang, W.; Martin, P.; Zhuang, H.L. Machine-Learning Phase Prediction of High-Entropy Alloys. Acta Mater. 2019, 169, 225–236. [Google Scholar] [CrossRef]
- Zhang, Y.; Wen, C.; Wang, C.; Antonov, S.; Xue, D.; Bai, Y.; Su, Y. Phase Prediction in High Entropy Alloys with a Rational Selection of Materials Descriptors and Machine Learning Models. Acta Mater. 2020, 185, 528–539. [Google Scholar] [CrossRef]
- Islam, N.; Huang, W.; Zhuang, H.L. Machine Learning for Phase Selection in Multi-Principal Element Alloys. Comput. Mater. Sci. 2018, 150, 230–235. [Google Scholar] [CrossRef]
- Lederer, Y.; Toher, C.; Vecchio, K.S.; Curtarolo, S. The Search for High Entropy Alloys: A High-Throughput Ab-Initio Approach. Acta Mater. 2018, 159, 364–383. [Google Scholar] [CrossRef] [Green Version]
- Jiang, C.; Uberuaga, B.P. Efficient Ab Initio Modeling of Random Multicomponent Alloys. Phys. Rev. Lett. 2016, 116, 105501. [Google Scholar] [CrossRef] [Green Version]
- Tian, F.; Varga, L.K.; Chen, N.; Delczeg, L.; Vitos, L. Ab Initio Investigation of High-Entropy Alloys of 3d Elements. Phys. Rev. B Condens. Matter Mater. Phys. 2013, 87, 075144. [Google Scholar] [CrossRef]
- Widom, M.; Huhn, W.P.; Maiti, S.; Steurer, W. Hybrid Monte Carlo/Molecular Dynamics Simulation of a Refractory Metal High Entropy Alloy. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2014, 45, 196–200. [Google Scholar] [CrossRef] [Green Version]
- Gorsse, S.; Senkov, O.N. About the Reliability of CALPHAD Predictions in Multicomponent Systems. Entropy 2018, 20, 899. [Google Scholar] [CrossRef] [Green Version]
- Toda-Caraballo, I.; Rivera-Díaz-Del-Castillo, P.E.J. Modelling Solid Solution Hardening in High Entropy Alloys. Acta Mater. 2015, 85, 14–23. [Google Scholar] [CrossRef]
- Toda-Caraballo, I. A General Formulation for Solid Solution Hardening Effect in Multicomponent Alloys. Scr. Mater. 2017, 127, 113–117. [Google Scholar] [CrossRef]
- Wang, Z.; Fang, Q.; Li, J.; Liu, B.; Liu, Y. Effect of Lattice Distortion on Solid Solution Strengthening of BCC High-Entropy Alloys. J. Mater. Sci. Technol. 2018, 34, 349–354. [Google Scholar] [CrossRef]
- Coury, F.G.; Kaufman, M.; Clarke, A.J. Solid-Solution Strengthening in Refractory High Entropy Alloys. Acta Mater. 2019, 175, 66–81. [Google Scholar] [CrossRef]
- Fleischer, R.L. Substitutional Solution Hardening. Acta Metall. 1963, 11, 203–209. [Google Scholar] [CrossRef]
- Labusch, R. Statistical Theories of Solid Solution Hardening. Acta Metall. 1972, 20, 917–927. [Google Scholar] [CrossRef]
- Gypen, L.A.; Deruyttere, A. Multi-Component Solid Solution Hardening—Part 1 Proposed Model. J. Mater. Sci. 1977, 12, 1028–1033. [Google Scholar] [CrossRef]
- Ramprasad, R.; Batra, R.; Pilania, G.; Mannodi-Kanakkithodi, A.; Kim, C. Machine Learning in Materials Informatics: Recent Applications and Prospects. NPJ Comput. Mater. 2017, 3, 54. [Google Scholar] [CrossRef]
- Wen, C.; Zhang, Y.; Wang, C.; Xue, D.; Bai, Y.; Antonov, S.; Dai, L.; Lookman, T.; Su, Y. Machine Learning Assisted Design of High Entropy Alloys with Desired Property. Acta Mater. 2019, 170, 109–117. [Google Scholar] [CrossRef] [Green Version]
- Chang, Y.J.; Jui, C.Y.; Lee, W.J.; Yeh, A.C. Prediction of the Composition and Hardness of High-Entropy Alloys by Machine Learning. JOM 2019, 71, 3433–3442. [Google Scholar] [CrossRef]
- Li, J.; Xie, B.; Fang, Q.; Liu, B.; Liu, Y.; Liaw, P.K. High-Throughput Simulation Combined Machine Learning Search for Optimum Elemental Composition in Medium Entropy Alloy. J. Mater. Sci. Technol. 2021, 68, 70–75. [Google Scholar] [CrossRef]
- Bhandari, U.; Rafi, M.R.; Zhang, C.; Yang, S. Yield Strength Prediction of High-Entropy Alloys Using Machine Learning. Mater. Today Commun. 2021, 26, 101871. [Google Scholar] [CrossRef]
- Stepanov, N.D.; Shaysultanov, D.G.; Salishchev, G.A.; Tikhonovsky, M.A. Structure and Mechanical Properties of a Light-Weight AlNbTiV High Entropy Alloy. Mater. Lett. 2015, 142, 153–155. [Google Scholar] [CrossRef]
- Stepanov, N.D.; Yu Yurchenko, N.; Shaysultanov, D.G.; Salishchev, G.A.; Tikhonovsky, M.A. Effect of Al on Structure and Mechanical Properties of AlxNbTiVZr (x = 0, 0.5, 1, 1.5) High Entropy Alloys. Mater. Sci. Technol. 2015, 31, 1184–1193. [Google Scholar] [CrossRef]
- Klimenko, D.N.; Yurchenko, N.Y.; Stepanov, N.D.; Zherebtsov, S.V. Prediction of Strength Characteristics of High-Entropy Alloys Al-Cr-Nb-Ti-V-Zr Systems. Mater. Today Proc. 2021, 38, 1535–1540. [Google Scholar] [CrossRef]
- Yurchenko, N.; Panina, E.; Zherebtsov, S.; Stepanov, N. Design and Characterization of Eutectic Refractory High Entropy Alloys. Materialia 2021, 16, 101057. [Google Scholar] [CrossRef]
- Stepanov, N.D.; Yurchenko, N.Y.; Skibin, D.V.; Tikhonovsky, M.A.; Salishchev, G.A. Structure and Mechanical Properties of the AlCrxNbTiV (x = 0, 0.5, 1, 1.5) High Entropy Alloys. J. Alloys Compd. 2015, 652, 266–280. [Google Scholar] [CrossRef]
- Yurchenko, N.Y.; Stepanov, N.D.; Zherebtsov, S.V.; Tikhonovsky, M.A.; Salishchev, G.A. Structure and Mechanical Properties of B2 Ordered Refractory AlNbTiVZrx (x = 0–1.5) High-Entropy Alloys. Mater. Sci. Eng. A 2017, 704, 82–90. [Google Scholar] [CrossRef] [Green Version]
- Yurchenko, N.Y.; Stepanov, N.D.; Gridneva, A.O.; Mishunin, M.V.; Salishchev, G.A.; Zherebtsov, S.V. Effect of Cr and Zr on Phase Stability of Refractory Al-Cr-Nb-Ti-V-Zr High-Entropy Alloys. J. Alloys Compd. 2018, 757, 403–414. [Google Scholar] [CrossRef]
- Yurchenko, N.Y.; Stepanov, N.D.; Shaysultanov, D.G.; Tikhonovsky, M.A.; Salishchev, G.A. Effect of Al Content on Structure and Mechanical Properties of the AlxCrNbTiVZr (x = 0; 0.25; 0.5; 1) High-Entropy Alloys. Mater. Charact. 2016, 121, 125–134. [Google Scholar] [CrossRef]
- Yurchenko, N.; Stepanov, N.; Salishchev, G. Laves-Phase Formation Criterion for High-Entropy Alloys. Mater. Sci. Technol. 2017, 33, 17–22. [Google Scholar] [CrossRef]
- Stepanov, N.D.; Yurchenko, N.Y.; Gridneva, A.O.; Zherebtsov, S.V.; Ivanisenko, Y.V.; Salishchev, G.A. Structure and Hardness of B2 Ordered Refractory AlNbTiVZr0.5 High Entropy Alloy after High-Pressure Torsion. Mater. Sci. Eng. A 2018, 716, 308–315. [Google Scholar] [CrossRef] [Green Version]
- Yurchenko, N.; Panina, E.; Zherebtsov, S.; Salishchev, G.; Stepanov, N. Oxidation Behavior of Refractory AlNbTiVZr0.25 High-Entropy Alloy. Materials 2018, 11, 2526. [Google Scholar] [CrossRef] [Green Version]
- Stepanov, N.D.; Yurchenko, N.Y.; Panina, E.S.; Tikhonovsky, M.A.; Zherebtsov, S.V. Precipitation-Strengthened Refractory Al0.5CrNbTi2V0.5 High Entropy Alloy. Mater. Lett. 2017, 188, 162–164. [Google Scholar] [CrossRef]
- Varvenne, C.; Leyson, G.P.M.; Ghazisaeidi, M.; Curtin, W.A. Solute Strengthening in Random Alloys. Acta Mater. 2017, 124, 660–683. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.; Singh, P.; Johnson, D.D.; Liaw, P.K.; Balasubramanian, G. Atomistic Clustering-Ordering and High-Strain Deformation of an Al0.1CrCoFeNi High-Entropy Alloy. Sci. Rep. 2016, 6, 31028. [Google Scholar] [CrossRef] [Green Version]
- Varvenne, C.; Luque, A.; Curtin, W.A. Theory of Strengthening in Fcc High Entropy Alloys. Acta Mater. 2016, 118, 164–176. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Kauffmann, A.; Laube, S.; Choi, I.C.; Schwaiger, R.; Huang, Y.; Lichtenberg, K.; Müller, F.; Gorr, B.; Christ, H.J.; et al. Contribution of Lattice Distortion to Solid Solution Strengthening in a Series of Refractory High Entropy Alloys. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2018, 49, 772–781. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.Y.; Shang, S.L.; Wang, Y.; Han, F.; Darling, K.A.; Wu, Y.; Xie, X.; Senkov, O.N.; Li, J.; Hui, X.D.; et al. Atomic and Electronic Basis for the Serrations of Refractory High-Entropy Alloys. NPJ Comput. Mater. 2017, 3, 23. [Google Scholar] [CrossRef] [Green Version]
- Kelleher, J.; Namee, B.; D’Arcy, A. Fundamentals of Machine Learning for Predective Data Analytics; MIT Press: Cambridge, MA, USA, 2015; ISBN 9780262029445. [Google Scholar]
- Gao, M.C.; Zhang, C.; Gao, P.; Zhang, F.; Ouyang, L.Z.; Widom, M.; Hawk, J.A. Thermodynamics of Concentrated Solid Solution Alloys. Curr. Opin. Solid State Mater. Sci. 2017, 21, 238–251. [Google Scholar] [CrossRef]
- Gorsse, S.; Tancret, F. Current and Emerging Practices of CALPHAD toward the Development of High Entropy Alloys and Complex Concentrated Alloys. J. Mater. Res. 2018, 33, 2899–2923. [Google Scholar] [CrossRef]
- Roy, A.; Balasubramanian, G. Predictive Descriptors in Machine Learning and Data-Enabled Explorations of High-Entropy Alloys. Comput. Mater. Sci. 2021, 193, 110381. [Google Scholar] [CrossRef]
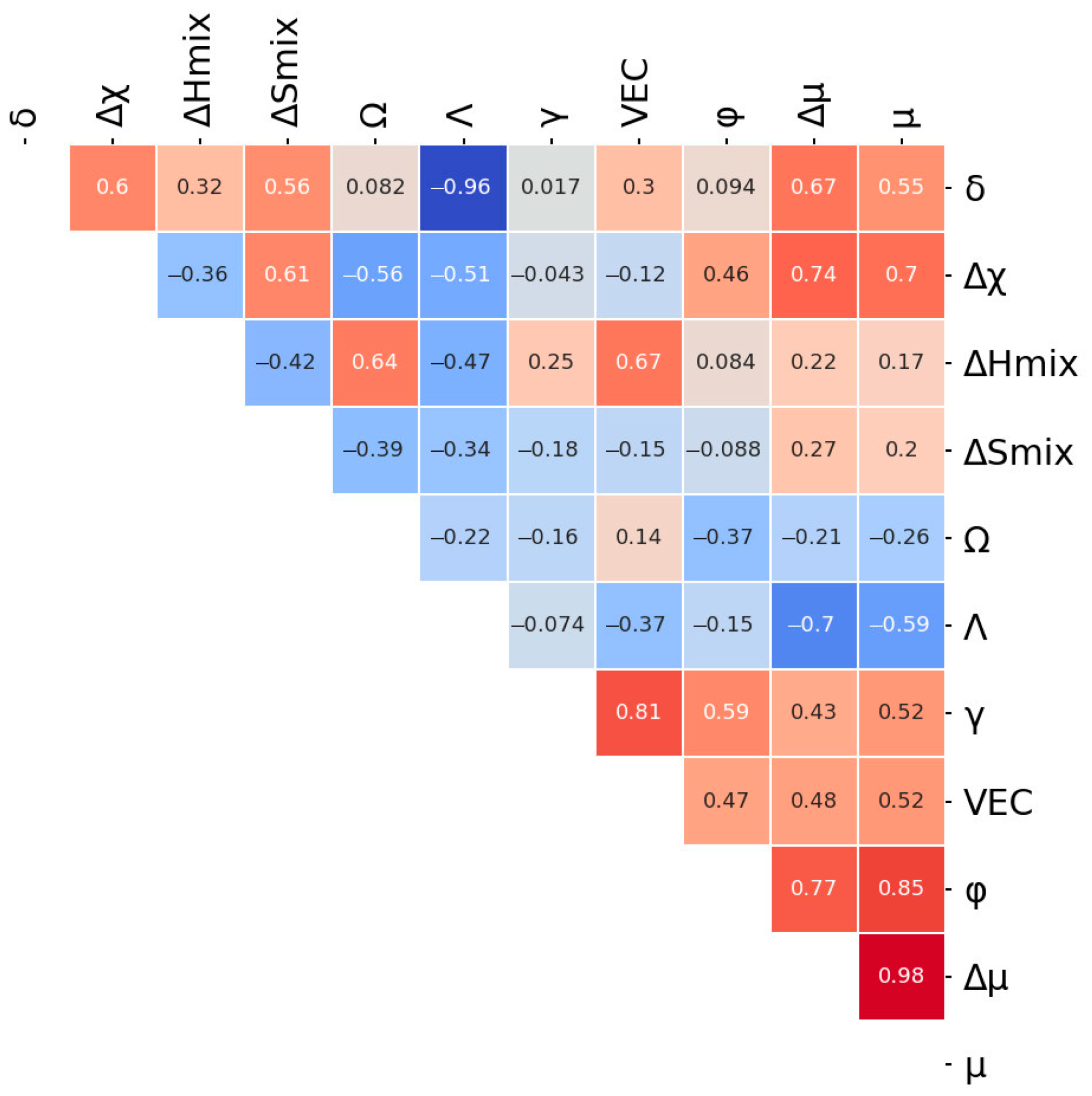
| Feature | Equation for Feature Calculation |
|---|---|
| The difference in atomic radii between elements () | , |
| Valence electron concentration | |
| Enthalpy of mixing () | |
| Difference in electronegativity between elements | |
| Configurational entropy | |
| Work function | |
| Shear modulus | |
| Difference in shear modulus | |
| parameter | |
| parameter | |
| parameter |
| Alloy | Content, at.% | |||||
|---|---|---|---|---|---|---|
| Al | Cr | Nb | Ti | V | Zr | |
| A1 | 14 | 1 | 10 | 45 | 25 | 5 |
| A2 | 10 | - | 20 | 35 | 15 | 20 |
| A3 | 14 | 11 | 5 | 35 | 25 | 10 |
| A4 | 13 | 7 | 5 | 45 | 15 | 15 |
| A5 | 13 | 12 | 20 | 20 | 35 | - |
| A6 | 13 | 2 | 20 | 30 | 25 | 10 |
| Alloys | Al | Cr | Nb | Ti | V | Zr | |
|---|---|---|---|---|---|---|---|
| A1 | Nominal composition | 14 | 1 | 10 | 45 | 25 | 5 |
| Actual chemical composition | 13 | 0.1 | 10.1 | 43.8 | 27.8 | 5.2 | |
| 1 (matrix) | 15.1 | 0.1 | 10.7 | 43.1 | 27.4 | 3.6 | |
| 2 (dark particles) | 2.2 | 0 | 3.9 | 78.6 | 4.2 | 11.1 | |
| 3 (light particles) | 16.9 | 0.4 | 11.2 | 39.1 | 25.2 | 7.2 | |
| A2 | Nominal composition | 10 | - | 20 | 35 | 15 | 20 |
| Actual chemical composition | 9.6 | 0 | 20.4 | 35.5 | 13.3 | 21.2 | |
| 1 (matrix) | 10 | 0 | 21.3 | 35.6 | 12.8 | 20.3 | |
| 2 (light phase) | 11 | 0 | 20.2 | 33 | 12.9 | 22.9 | |
| A3 | Nominal composition | 14 | 11 | 5 | 35 | 25 | 10 |
| Actual chemical composition | 15.5 | 11.3 | 6.2 | 36.2 | 20.3 | 10.5 | |
| 1 (grey) | 13.8 | 11.1 | 7.2 | 39.2 | 24.3 | 4.4 | |
| 2 (light) | 15.5 | 10.8 | 4.8 | 32.7 | 15 | 21.2 | |
| A4 | Nominal composition | 13 | 7 | 5 | 45 | 15 | 15 |
| Actual chemical composition | 11.3 | 6.8 | 7.3 | 49.6 | 14.5 | 10.5 | |
| 1 (matrix) | 12.6 | 6.7 | 7.3 | 49 | 14.3 | 10.1 | |
| A5 | Nominal composition | 13 | 12 | 20 | 20 | 35 | - |
| Actual chemical composition | 14.1 | 13.5 | 23.3 | 22.5 | 25.2 | 1.4 | |
| 1 (matrix) | 15 | 15.3 | 26.1 | 14.8 | 28.3 | 0.5 | |
| 2 (dark particles) | 3.1 | 3.3 | 7 | 78.3 | 7.5 | 0.8 | |
| A6 | Nominal composition | 13 | 2 | 20 | 30 | 25 | 10 |
| Actual chemical composition | 12 | 1.2 | 22.2 | 30.9 | 19.5 | 14.2 | |
| 1 (matrix) | 13.1 | 1.6 | 23.5 | 31.6 | 22.3 | 7.9 | |
| 2 (light phase) | 14.2 | 1.9 | 20.5 | 29.1 | 20.1 | 14.2 |
| Alloy | Microhardness, HV | Yield Strength, MPa | ||||||
|---|---|---|---|---|---|---|---|---|
| 20 °C | 600 °C | 800 °C | ||||||
| Measured | Estimated Using Microhardness | Predicted | Measured | Predicted | Measured | Predicted | ||
| A1 | 553 | 1070 * | 1316 | 1409 | 1093 | 1011 | 187 | 631 |
| A2 | 556 | 1049 * | 1323 | 1297 | 1122 | 937 | 287 | 390 |
| A3 | 650 | 1608 | 1454 | 1385 | 1120 | 556 | 506 | |
| A4 | 552 | 1337 | 1306 | 1096 | 1016 | 157 | 392 | |
| A5 | 540 | 1295 | 1177 | 1113 | 874 | 898 | 468 | |
| A6 | 489 | 1290 | 1353 | 1048 | 991 | 509 | 504 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klimenko, D.; Stepanov, N.; Li, J.; Fang, Q.; Zherebtsov, S. Machine Learning-Based Strength Prediction for Refractory High-Entropy Alloys of the Al-Cr-Nb-Ti-V-Zr System. Materials 2021, 14, 7213. https://doi.org/10.3390/ma14237213
Klimenko D, Stepanov N, Li J, Fang Q, Zherebtsov S. Machine Learning-Based Strength Prediction for Refractory High-Entropy Alloys of the Al-Cr-Nb-Ti-V-Zr System. Materials. 2021; 14(23):7213. https://doi.org/10.3390/ma14237213
Chicago/Turabian StyleKlimenko, Denis, Nikita Stepanov, Jia Li, Qihong Fang, and Sergey Zherebtsov. 2021. "Machine Learning-Based Strength Prediction for Refractory High-Entropy Alloys of the Al-Cr-Nb-Ti-V-Zr System" Materials 14, no. 23: 7213. https://doi.org/10.3390/ma14237213
APA StyleKlimenko, D., Stepanov, N., Li, J., Fang, Q., & Zherebtsov, S. (2021). Machine Learning-Based Strength Prediction for Refractory High-Entropy Alloys of the Al-Cr-Nb-Ti-V-Zr System. Materials, 14(23), 7213. https://doi.org/10.3390/ma14237213








