Abstract
Two new diamond-like (DL) chalcogenides, Li2MgGeSe4 and Li2MgSnSe4, have been successfully synthesized using a conventional high-temperature solid-state method. The two compounds crystallize in the non-centrosymmetric space group Pmn21 with a = 8.402 (14) Å, b = 7.181 (12) Å, c = 6.728 (11) Å, Z = 2 for Li2MgSnSe4, and a = 8.2961 (7) Å, b = 7.0069 (5) Å, c = 6.6116 (6) Å, Z = 2 for Li2MgGeSe4. The calculated results show that the second harmonic generation (SHG) coefficients of Li2MgSnSe4 (d33 = 12.19 pm/v) and Li2MgGeSe4 (d33 = −14.77 pm/v), mainly deriving from the [MSe4] (M = Ge, Sn) tetrahedral units, are close to the one in the benchmark AgGaS2 (d14 = 13.7 pm/V). The calculated band gaps for Li2MgSnSe4 and Li2MgGeSe4 are 2.42 and 2.44 eV, respectively. Moreover, the two compounds are the first series of alkali and alkaline-earth metal DL compounds in the I2-II-IV-VI4 family, enriching the structural diversity of DL compounds.
1. Introduction
The exploration of advanced functional materials, as well as the development of structural chemistry, depends on the fabrication of new compounds with a special crystal structure, which contains distinctive physical and chemical behaviors [1,2,3,4,5,6,7,8,9,10,11,12]. A diamond-like (DL) structure compound, exhibiting abundant chemical diversities and adjustable optical properties, has been proven as a valid structural framework for the design and fabrication of new infrared (IR) optical materials, especially for the mid- or far-IR nonlinear optical (NLO) materials. Over the past few decades, a large number of non-centrosymmetric DL chalcogenide compounds, such as Li4HgGe2S7 [13] and Li4MgGe2S7 [14] in the I4-II-IV2-VI7 family, and Li2CdGeS4 [15], Li2CdGeSe4 [16], Li2ZnGeSe4 [17] and Cu2ZnSnS4 [18] in the I2-II-IV-VI4 family, with outstanding optical properties, have been developed using an atomic substitution or co-substitution strategy.
In a DL compound, the cation is coordinated with four anions, and follows the Pauling’s electrostatic valency rule [19,20,21,22,23]. Hence, the optical properties including band gap and SHG response in the DL chalcogenide compounds could be effectively regulated by organizing proper tetrahedral units in the structure. On the basis of the statistical analyses, the DL chalcogenide compounds mainly consisted of univalent metal tetrahedral units, such as alkali metal tetrahedral LiQ4 (Q = S, Se) and/or IB group metal tetrahedral MIQ4 (MI = Cu, Ag; Q = S, Se), with IIB (Zn, Cd and Hg), IIIA (B, Al, Ga and In), IVA (Si, Ge and Sn) and VA (P and As) group element tetrahedral units [24,25,26,27]. Most recently, Pan and Li et al. [14] demonstrated that the alkaline-earth metal AQ4 (A = Be, Mg; Q = S, Se) tetrahedral units, which without d-d and f-f electronic transitions, can be used to regulate the optical properties of DL chalcogenide compounds. By introducing alkaline-earth metal tetrahedral unit MgS4 into the I4-II-IV2-Q7 system, the first alkali and alkaline-earth metal DL sulfide Li4MgGe2S7 with excellent IR NLO optical performances was discovered. However, owing to the experimental challenges to obtain the four-coordinated alkaline-earth metal AQ4 tetrahedral units in a crystal structure, the number of reported alkaline-earth metal containing DL compounds is very limited, and the exploration of new IR NLO materials, especially with excellent optical properties in alkali and alkaline-earth metal DL chalcogenide compounds, is just in the initial stage.
Considering the above discussions, the alkali metal tetrahedral LiS4 and alkaline-earth metal tetrahedral MgSe4 units were successfully introduced into the classical I2-II-IV-VI4 family in this work. Two new alkali and alkaline-earth metal DL selenides Li2MgMSe4 (M = Ge, Sn) were synthesized by conventional high temperature solid state reactions in sealed quartz tubes. Li2MgMSe4 (M = Ge, Sn) are isostructural compounds, crystallizing in the orthorhombic Pmn21 space group. The compounds exhibit a three dimensional channel structure, which is built by [LiSe4], [(Li/Mg)Se4] and [MSe4] (M = Ge, Sn) tetrahedral units. The theoretical investigations show that the calculated band gap for the two compounds is 2.44 eV for Li2MgGeSe4, and 2.42 eV for Li2MgSnSe4 (matched with the experimental value of 2.62 eV). The calculated SHG coefficients of the title compounds are d33 = 12.19 pm/V for Li2MgSnSe4 and d33 = −14.77 pm/V for Li2MgGeSe4, which are close to the one in AgGaS2 (d14 = 13.7 pm/V) [28]. The SHG coefficients are mainly contributed by the MSe4 (M = Ge, Sn) tetrahedral units. Meanwhile, the calculated birefringences are 0.011 for Li2MgSnSe4 and 0.012 for Li2MgGeSe4.
2. Experimental Sections
2.1. Chemical Syntheses
High purity (99.99%) raw materials (Li, Mg, Sn, Ge and Se) were obtained from Aladdin Industrial Corporation (Fengxian District, Shanghai, China) and utilized without extra purification.
Li2MgMSe4 (M = Ge, Sn) single crystals for structural determination were prepared using a melting method in sealed quartz tubes. The starting mixture samples (Li:Mg:Ge:Se = 2:1:1:4; Li:Mg:Sn:Se = 2:1:1:4) were packaged in graphite crucibles in a glove box. After that the graphite crucibles were moved into quartz tubes, and the quartz tubes were sealed by flame under a vacuum atmosphere (about 10−3 Pa). Then, the samples were heated to 880 °C in 46 h, and kept at 880 °C for 50 h, then cooled to room temperature in 48 h. Breaking the tubes, the yellow Li2MgGeSe4 and Li2MgSnSe4 single crystals were harvested in the graphite crucibles. It is worth mentioning that the two crystals show strong moisture absorptions in air.
The syntheses of Li2MgMSe4 (M = Ge, Sn) powder samples for performance characterization were tried at a higher temperature. The mixtures of Li, Mg, Ge/Sn and Se elements with an atomic stoichiometric ratio were first weighed, ground and sealed in quartz tubes. The sealed samples were slowly heated to 900 °C (in 60 h) in a muffle furnace, and kept at this temperature for 100 h, then cooled to room temperature in 100 h.
2.2. Single-Crystal X-ray Diffractions
Li2MgMSe4 (M = Ge, Sn) single crystals were manually picked out and utilized for structural determinations. The X-ray diffraction data of Li2MgMSe4 (M = Ge, Sn) single crystals were collected in a Bruker D8 Venture diffractometer that was equipped with monochromatic Mo-Kα radiation (λ = 0.71073 Å) operating at 50 kV and 40 mA. The structure refinements of the two compounds were carried out in the SHELX-97 crystallography software package. The XPREP program was used for the absorption correction (multiscan), the structures of Li2MgMSe4 (M = Sn, Ge) were checked by PLATON in case of additional symmetry elements [29,30,31]. The detailed processes can be found in previous works [9,14,32,33]. It is worth noting that the initial Li/Mg occupation from refinement was 0.53715:0.462850 for Li2MgSnSe4, and 0.48933:0.51067 for Li2MgGeSe4, which is close to 1:1. To maintain the charge balance in the whole structures, the atomic ratio of Li/Mg in both title compounds was set to 1:1. The crystal data and structural refinements of Li2MgMSe4 (M = Ge, Sn) are listed in Table 1. Meanwhile, the corresponding atomic coordinates, bond distances and angles, isotropic displacement parameters and atomic parameters are shown in Tables S1–S7. Since Li2MgGeSe4 deliquesces quickly in air, the data collection for Li2MgGeSe4 was repeated several times using different single crystals. However, the data integrity of Li2MgGeSe4 is still lower than Li2MgSnSe4.

Table 1.
Crystal data and structural refinements of Li2MgSnSe4 and Li2MgGeSe4.
2.3. Powder X-ray Diffraction (PXRD)
The Powder X-ray diffraction (PXRD) pattern of Li2MgSnSe4 was characterized using a Bruker D2 Phaser diffractometer (Bruker Corporation, Karlsruhe, Germany) under Cu-Kα radiation (λ = 1.5418 Å) with a metal holder. Meanwhile, the experimental XRD pattern of Li2MgSnSe4 (Figure S1) was recorded from 10 to 70° (2θ) with a scan step width of 0.02°. The experimental and calculated PXRD patterns of Li2MgSnSe4 are shown in Figure S1. Owing to the experimental challenge in synthesizing and characterizing the moisture-sensitive compounds, impurities such as SnSe2 and SnSe were observed in the synthesized Li2MgSnSe4 powder samples. However, based on the XRD patterns, the main phase can be determined to be Li2MgSnSe4. Meanwhile, compared with Li2MgSnSe4, Li2MgGeSe4 powder samples exhibit more serious moisture absorption. It was deliquesced too fast in air (the samples were deliquesced in 1 min at room temperature) to finish the PXRD measurement. GSAS was used to fit and refine the powder diffraction data of Li2MgSnSe4. The main phase Li2MgSnSe4 and impurity phases SnSe and SnSe2 were refined. A certain peak function was fitted with experimental intensity data, and the values of peaks and structural parameters (including background function, lattice parameters, peak parameters, atomic position, preference orientation, etc.) were constantly adjusted during the fitting process until the difference between calculated intensity and experimental intensity stabilized [34]. The multi-phase Rietveld refinement yielded tiny impurities contents such as SnSe2 and SnSe (total 9.7%) remaining from the staring materials, and a weight fraction of 90.3% of target Li2MgSnSe4 (Figure S2). The refined structural parameters are provided in Table S8. The large difference in the refinement can be attributed to the experimental challenge to obtain long time and high quality PXRD data for the moisture-sensitive Li2MgSnSe4. However, the refined results are helpful in judging the purity of the product.
2.4. UV–Vis–NIR Diffuse Reflectance Spectroscopy
The diffuse reflectance spectrum of the synthesized Li2MgSnSe4 powder samples was characterized using a DUV spectrophotometer (Shimadzu SolidSpec-3700, Shimadzu Corporation, Shanghai, China) at room temperature in air. Based on the reflection spectrum, the corresponding absorption spectrum was obtained using the Kubelka–Munk formula [35,36]. The process was completed in 5–10 min.
2.5. Raman Spectroscopy
The Raman spectrum of Li2MgSnSe4 was characterized on a single crystal in a LABRAM HR Evolution spectrometer.
The Li2MgSnSe4 single crystal was firstly placed onto a transparent glass slide. Then, a suitable objective lens was used to select the measured area on the crystal. The maximum power of the used laser beam was about 60 mW with a spot size of ~35 μm.
2.6. Theoretical Calculations
Based on the density functional theory (DFT) and CASTEP program, the plane wave pseudopotential was applied to calculate the electronic structures of Li2MgMSe4 (M = Ge, Sn) [37]. Meanwhile, the exchange-correlation effects of the compounds were analyzed by using the generalized gradient approximation (GGA) with the Perdew–Burke–Ernzerhof (PBE) function [38,39]. Under the norm conserving pseudopotentials for wave function expansion, the kinetic energy cutoff of the models was set to 450 eV. Moreover, the Brillouin zone [40] contained 2 × 2 × 2 Monkhorst-pack k-point sampling [41]. The virtual unit cells were used to process the occupancy [42,43].
3. Results and Discussion
3.1. Crystal Structure
As shown in Figure 1a,f, the two compounds are isomorphic structures. Herein, Li2MgSnSe4 is taken as an example of the structure description. Li2MgSnSe4 crystallizes in the noncentrosymmetric space group Pmn21 with a = 8.402 (14) Å, b = 7.181 (12) Å, c = 6.728 (11) Å and Z = 2. In the asymmetric unit of Li2MgSnSe4, there are two Li, one Mg, one Sn and three Se atoms that are crystallographically independent. In Li2MgSnSe4, the Li2 and Sn1 atoms are bonded to four Se atoms to build up the [LiSe4] and [SnSe4] tetrahedra with Li-Se bond lengths ranging from 2.50 Å–2.65 Å and Sn-Se bond lengths ranging from 2.505 Å–2.528 Å, respectively. The Li1 and Mg1 atoms are set to share the same sites with the atomic ratio of 1:1 in the initial refinements with the identical anisotropic displacement parameters, which can help to obtain better R values and reasonable temperature factors, similar to the situation of Cu/Mg atomic co-occupation in Cu2MgSiS4 [44], Cu2MgGeS4 [44] and Cu2MgSiSe4 [44]. Furthermore, Li/Mg atomic co-occupation is very common, which can be found in the LiMg(IO3)3 [45] and Li0.8Mg2.1B2O5F [46]. Similar to the Li2 and Sn1 atoms, the co-occupied Li1 and Mg1 atoms are bonded to four Se atoms to construct the [(Li/Mg)Se4] tetrahedra units at the Wyckoff position 4b (Table S7). Furthermore, the formed tetrahedra groups are connected with each other by sharing Se atoms to constitute the final DL structure. For both compounds, there is a similar channel-like structure with a channel diameter of about 6 Ångstrom on the ab plane, as shown in Figure 1e,j. On the basis of the detailed investigations in the Inorganic Crystal Structure Database (ICSD), the two compounds should be the first series of alkali and alkaline earth metal DL compounds in the I2-II-IV-VI4 family.
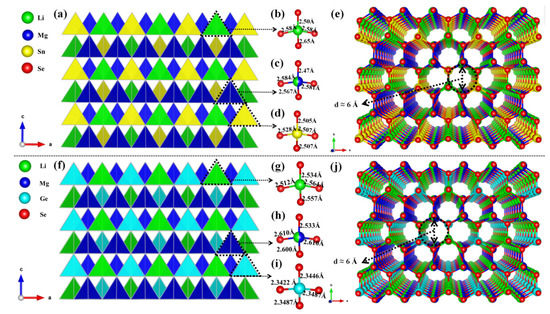
Figure 1.
The DL structure of Li2MgSnSe4 (a) and Li2MgGeSe4 (f) on the ac plane; (b–d,g–i) The structures of [LiSe4], [(Li/Mg)Se4], [SnSe4] and [GeSe4] tetrahedral units. The channel-like structures of Li2MgSnSe4 (e) and Li2MgGeSe4 (j) on ab plane.
3.2. Optical Properties
Based on the UV–Vis–NIR diffuse-reflectance spectrum, the experimental band gap of Li2MgSnSe4 was determined to be 2.62 eV (Figure 2a). To confirm chemical bonding, the Raman spectrum of Li2MgSnSe4 was characterized on a single crystal. As shown in Figure 2b, the peaks below 193 cm−1 are related to the vibrations of Li-Se and Mg-Se bonding, matched with the previous results [47,48,49]. The peak at 193 cm−1 and the overlapping peaks around 235 cm−1 could be assigned to the asymmetric and symmetric stretching vibrations of Sn-Se bonding in SnSe4 tetrahedral groups [49,50].
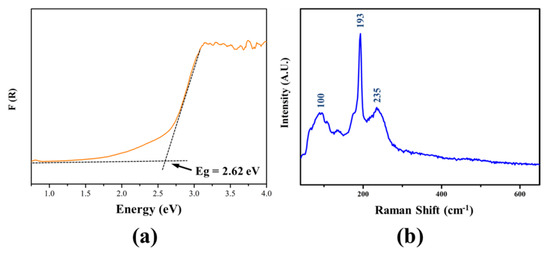
Figure 2.
(a) Experimental band gap of Li2MgSnSe4 samples; and (b) Raman spectrum of Li2MgSnSe4 single crystal.
3.3. Theoretical Calculations
To study the linear and nonlinear optical properties of Li2MgMSe4 (M = Ge, Sn), DFT calculations were implemented. Considering the Li/Mg atomic co-occupation at the Wyckoff position 4b in the structures, the virtual unit cells were built for the calculations, as shown in Table S9 and Figure S3. The calculated theoretical band gaps, SHG coefficients and birefringences of the two compounds are shown in Table 2; the calculated band gap for the two compounds is 2.44 eV for Li2MgGeSe4, and 2.42 eV for Li2MgSnSe4 (matched with the experimental value of 2.62 eV). The SHG coefficients of Li2MgSnSe4 in d33 = 12.19 pm/v and Li2MgGeSe4 in d33 = −14.77 pm/v are close to the one of AgGaS2 in d14 = 13.7 pm/v. The calculated birefringences for the two compounds are 0.011 (Li2MgSnSe4) and 0.012 (Li2MgGeSe4), respectively.

Table 2.
Calculated band gaps, SHG coefficients and birefringence of Li2MgSnSe4 and Li2MgGeSe4.
To detect the origin of the optical properties, the electronic structures, SHG densities and band-resolved NLO susceptibilities of Li2MgMSe4 (M = Ge, Sn) were further investigated. Figure 3 shows the calculated band structures, total and partial density of states and the band-resolved NLO susceptibility χ(2) of the two compounds. The band structures (Figure 3a,b) indicate that Li2MgGeSe4 is an indirect band gap compound with a band gap of 2.44 eV, while Li2MgSnSe4 is a direct band gap compound with a band gap of 2.42 eV (matched with the experimental value of 2.62 eV). Furthermore, as shown from the total and partial density of states (PDOS) curves (Figure 3c,d), the valence bands maximum (VBM), which, around the Fermi level, is mainly occupied by Se-4p (83%) orbitals with the minor contribution of Sn-5p (8%), Li/Mg-2p (5%) and Li-2s (4%) orbitals for Li2MgSnSe4 and Se-4p (83%) orbitals with the minor contribution of Ge-4p (8%), Li/Mg-2p (5%) and Li-2s (4%) orbitals for Li2MgGeSe4, respectively (the range from -4.0 to 0 eV). The conduction bands minimum (CBM) originates from Se-4p (10%), Sn-5p (9%), Li-2s (19%) and Li/Mg-2p (21%) orbitals for Li2MgSnSe4, Se-4p (11%), Ge-4p (8%), Li-2s (17%) and Li/Mg-2p (21%) orbitals for Li2MgGeSe4, respectively (the range from 2.4 to 12 eV). The results indicate that the optical band gaps of Li2MgMSe4 (M = Ge, Sn) are mainly determined by the Se-4p, Li/Mg-2p and Li-2s orbitals.
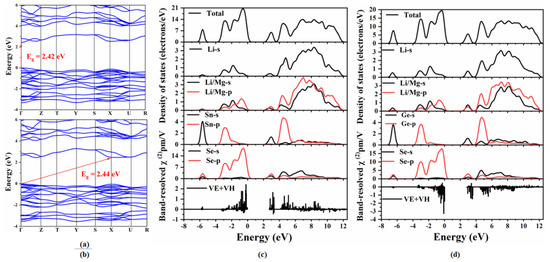
Figure 3.
Calculated band structures of (a) Li2MgSnSe4 and (b) Li2MgGeSe4; total and partial density of states and the band-resolved NLO susceptibility χ(2) of (c) Li2MgSnSe4 and (d) Li2MgGeSe4.
Figure 4 shows the calculated SHG densities for the two compounds. Combined with the band-resolved NLO susceptibility χ(2) in Figure 3c–d, the SHG responses of Li2MgMSe4 (M = Ge, Sn) can be mainly derived from the [MSe4] (M = Ge, Sn) tetrahedra units and with minor contributions from [LiSe4] and [(Li/Mg)Se4] groups.
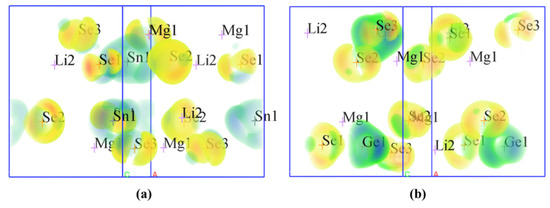
Figure 4.
SHG densities of (a) Li2MgSnSe4 and (b) Li2MgGeSe4.
4. Conclusions
In summary, the first series of DL selenides in the I2-II-IV-VI4 family, Li2MgGeSe4 and Li2MgSnSe4, have been rationally designed and synthesized. Their crystal structures were determined using single crystal X-ray diffractions, and the optical properties were studied using experimental spectra and DFT calculations. Li2MgMSe4 (M = Ge, Sn) crystallize in the non-centrosymmetric space group Pmn21 and show channel structures built by [LiSe4], [(Li/Mg)Se4] and [MSe4] (M = Ge, Sn) tetrahedra units. The two compounds exhibit large theoretical SHG coefficients in d33 (12.19 pm/v for Li2MgSnSe4, and −14.77 pm/v for Li2MgGeSe4), moderate band gaps (2.42 for Li2MgSnSe4, and 2.44 for Li2MgGeSe4) in selenides. The results demonstrated that introducing alkali metal and alkaline earth metal tetrahedral units into the I2-II-IV-VI4 family is a feasible way for the development of diamond-like IR nonlinear optical materials with good properties.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/ma14206166/s1, Table S1: Atomic coordinates and equivalent isotropic displacement parameters of Li2MgSnSe4, Table S2: Anisotropic displacement parameters (Å2 × 103) of Li2MgSnSe4, Table S3: Symmetry, selected bond lengths and angles of crystal data and structural refinements of Li2MgSnSe4, Table S4: Atomic coordinates and equivalent isotropic displacement parameters of Li2MgGeSe4, Table S5: Anisotropic displacement parameters (Å2 × 103) of Li2MgGeSe4, Table S6: Symmetry, selected bond lengths and angles of crystal data and structural refinements of Li2MgGeSe4, Table S7: Atomic parameters of Li2MgSnSe4 and Li2MgGeSe4, Table S8: The refined structural parameters of Li2MgSnSe4, Table S9: The crystallographic data of Li2MgMSe4 (M = Sn, Ge), Figure S1: Experimental and calculated PXRD patterns of Li2MgSnSe4, Figure S2: The PXRD Rietveld refinement of the obtained Li2MgSnSe4 samples, Figure S3: The atomic models of (a) Li2MgSnSe4 and (b) Li2MgGeSe4.
Author Contributions
H.G. synthesized the crystals, tested the samples, and wrote the manuscript. K.Z. and Z.Y. performed the electronic structure and optical property calculations. A.A. provided the support for the syntheses and structure determination of crystals. C.B. and J.L. provided comments on the revision of the manuscript. J.L., K.L. and S.P. designed and supervised the study. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Tianshan Cedar Program (2019XS24), the High-level Talent Project of Xinjiang Uygur Autonomous Region (2020000039), Partnership and International Cooperation Program of Shanghai Cooperation Organization (2020E01040), West Light Foundation of the Chinese Academy of Sciences (2019-YDYLTD-002), National Natural Science Foundation of China (52002398).
Data Availability Statement
The data presented in this study are available in Supplementary Material. The X-ray crystallographic coordinates for structures reported in this study have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition numbers 2106592-2106593. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/datarequest/cif (accessed on 5 October 2021).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Geng, L.; Meng, C.; Lu, H.; Luo, Z.; Lin, C.; Cheng, W. Bi2Te(IO3)O5Cl: A novel polar iodate oxychloride exhibiting a second-order nonlinear optical response. Dalton Trans. 2015, 44, 2469–2475. [Google Scholar] [CrossRef]
- Lu, X.; Chen, Z.; Shi, X.; Jing, Q.; Lee, M. Two Pyrophosphates with Large Birefringences and Second-Harmonic Responses as Ultraviolet Nonlinear Optical Materials. Angew. Chem. Int. Ed. 2020, 59, 17648–17656. [Google Scholar] [CrossRef]
- Liang, F.; Kang, L.; Lin, Z.; Wu, Y. Mid-Infrared Nonlinear Optical Materials Based on Metal Chalcogenides: Structure–Property Relationship. Cryst. Growth Des. 2017, 17, 2254–2289. [Google Scholar] [CrossRef]
- Kotb, H.; Khater, H.; Saber, O.; Ahmad, M. Sintering Temperature, Frequency, and Temperature Dependent Dielectric Properties of Na0.5Sm0.5Cu3Ti4O12 Ceramics. Materials 2021, 14, 4805. [Google Scholar] [CrossRef] [PubMed]
- Rong, L.; Xu, Z.; Sun, J.; Guo, G. New methyl formate synthesis method: Coal to methyl formate. J. Energy Chem. 2018, 27, 238–242. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.; Yang, G.; Humphrey, M.G.; Zhang, C. Recent Advances in Ultraviolet and Deep-Ultraviolet Second-Order Non-linear Optical Crystals. Coord. Chem. Rev. 2018, 375, 459–488. [Google Scholar] [CrossRef]
- Mutailipu, M.; Zhang, M.; Zhang, B.B.; Wang, L.Y.; Yang, Z.H.; Zhou, X.; Pan, S.L. SrB5O7F3 Functionalized with [B5O9F3]6− Chromophores: Accelerating the Rational Design of Deep-Ultraviolet Nonlinear Optical Materials. Angew. Chem. Int. Ed. 2018, 57, 6095–6099. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, B.; Yang, Z.; Pan, S. Cation-Tuned Synthesis of Fluorooxoborates: Towards Optimal Deep-Ultraviolet Nonlinear Optical Materials. Angew. Chem. Int. Ed. 2018, 57, 2150–2154. [Google Scholar] [CrossRef]
- Huang, Y.; Gao, L.; Yu, H.; Yang, Z.; Li, J.; Pan, S. Na6MQ4 (M = Zn, Cd; Q = S, Se): Promising New Ternary Infrared Nonlinear Optical Materials. Chem. Eur. J. 2021, 27, 6538–6544. [Google Scholar] [CrossRef]
- Xu, F.; Peng, G.; Lin, C.; Zhao, D.; Li, B.-X.; Zhang, G.; Yang, S.; Ye, N. Na3Sc2(PO4)2F3: Rational design and synthesis of an alkali rare-earth phosphate fluoride as an ultraviolet nonlinear optical crystal with an enlarged birefringence. J. Mater. Chem. C 2020, 8, 4965–4972. [Google Scholar] [CrossRef]
- Lee, H.; Ok, K.M. Na2Mg1-xZnxSiO4 (0 ≤ x ≤ 1): Noncentrosymmetric Sodium Metal Silicate Solid Solutions with Ultraviolet Nonlinear Optical Properties. Bull. Korean Chem. Soc. 2020, 41, 139–142. [Google Scholar] [CrossRef]
- Kee, J.; Ok, K.M. Hydrogen-Bond-Driven Synergistically Enhanced Hyperpolarizability: Chiral Coordination Polymers with Nonpolar Structure Exhibiting Unusually Strong Second-Harmonic Generation. Angew. Chem. Int. Ed. 2021, 60, 20656–20660. [Google Scholar] [CrossRef]
- Wu, K.; Yang, Z.; Pan, S. The first quaternary diamond-like semiconductor with 10-membered LiS4 rings exhibiting excellent nonlinear optical performances. Chem. Commun. 2017, 53, 3010–3013. [Google Scholar] [CrossRef]
- Abudurusuli, A.; Huang, J.; Wang, P.; Yang, Z.; Pan, S.; Li, J. Li4MgGe2S7: The First Alkali and Alkaline-Earth Diamond-Like Infrared Nonlinear Optical Material with Exceptional Large Band Gap. Angew. Chem. Int. Ed. 2021. [Google Scholar] [CrossRef]
- Lekse, J.W.; Moreau, M.A.; McNerny, K.L.; Yeon, J.; Halasyamani, S.; Aitken, J. Second-Harmonic Generation and Crystal Structure of the Diamond-like Semiconductors Li2CdGeS4 and Li2CdSnS4. Inorg. Chem. 2009, 48, 7516–7518. [Google Scholar] [CrossRef]
- Zhang, J.H.; Clark, D.J.; Weiland, A.; Stoyko, S.S.; Kim, Y.S.; Jang, J.I.; Aitken, J.A. Li2CdGeSe4 and Li2CdSnSe4: Biaxial Nonlinear Optical Materials with Strong Infrared Second-Order Responses and Laser-Induced Damage Thresholds Influenced by Photoluminescence. Inorg. Chem. Front. 2017, 4, 1472–1484. [Google Scholar] [CrossRef]
- Zhang, J.-H.; Clark, D.J.; Brant, J.A.; Sinagra, C.W.; Kim, Y.S.; Jang, J.I.; Aitken, J.A. Infrared nonlinear optical properties of lithium-containing diamond-like semiconductors Li2ZnGeSe4 and Li2ZnSnSe4. Dalton Trans. 2015, 44, 11212–11222. [Google Scholar] [CrossRef]
- Ritscher, A.; Hoelzel, M.; Lerch, M. The order-disorder transition in Cu2ZnSnS4-A neutron scattering investigation. J. Solid State Chem. 2016, 238, 68–73. [Google Scholar] [CrossRef] [Green Version]
- Liang, F.; Kang, L.; Lin, Z.; Wu, Y.; Chen, C. Analysis and prediction of mid-IR nonlinear optical metal sulfides with diamond-like structures. Coord. Chem. Rev. 2017, 333, 57–70. [Google Scholar] [CrossRef]
- Kang, L.; Liang, F.; Jiang, X.; Lin, Z.; Chen, C. First-Principles Design and Simulations Promote the Development of Nonlinear Optical Crystals. Accounts Chem. Res. 2019, 53, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.-M.; Xue, H.-G.; Guo, S.-P. Multinary metal chalcogenides with tetrahedral structures for second-order nonlinear optical, photocatalytic, and photovoltaic applications. Coord. Chem. Rev. 2018, 368, 115–133. [Google Scholar] [CrossRef]
- Pauling, L. The principles determining the structure of complex ionic crystals. J. Am. Chem. Soc. 1929, 51, 1010–1026. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, B.; Yu, H.; Hu, Z.; Wang, J.; Wu, Y.; Halasyamani, P.S. Designing Silicates as Deep-UV Nonlinear Optical (NLO) Materials using Edge-Sharing Tetrahedra. Angew. Chem. Int. Ed. 2020, 59, 8922–8926. [Google Scholar] [CrossRef]
- Zhang, J.-H.; Clark, D.J.; Brant, J.A.; Rosmus, K.A.; Grima, P.; Lekse, J.W.; Jang, J.I.; Aitken, J.A. α-Li2ZnGeS4: A Wide-Bandgap Diamond-like Semiconductor with Excellent Balance between Laser-Induced Damage Threshold and Second Harmonic Generation Response. Chem. Mater. 2020, 32, 8947–8955. [Google Scholar] [CrossRef]
- Brant, J.A.; Clark, D.J.; Kim, Y.S.; Jang, J.I.; Zhang, J.-H.; Aitken, J.A. Li2CdGeS4, A Diamond-Like Semiconductor with Strong Second-Order Optical Nonlinearity in the Infrared and Exceptional Laser Damage Threshold. Chem. Mater. 2014, 26, 3045–3048. [Google Scholar] [CrossRef]
- Li, G.; Chu, Y.; Zhou, Z. From AgGaS2 to Li2ZnSiS4: Realizing Impressive High Laser Damage Threshold Together with Large Second-Harmonic Generation Response. Chem. Mater. 2018, 30, 602–606. [Google Scholar] [CrossRef]
- Rosmus, K.A.; Brant, J.A.; Wisneski, S.D.; Clark, D.J.; Kim, Y.S.; Jang, J.I.; Brunetta, C.D.; Zhang, J.-H.; Srnec, M.N.; Aitken, J.A. Optical Nonlinearity in Cu2CdSnS4 and α/β-Cu2ZnSiS4: Diamond-like Semiconductors with High Laser-Damage Thresholds. Inorg. Chem. 2014, 53, 7809–7811. [Google Scholar] [CrossRef]
- Chen, J.; Chen, H.; Xu, F.; Cao, L.; Jiang, X.; Yang, S.; Sun, Y.; Zhao, X.; Lin, C.; Ye, N. Mg2In3Si2P7: A Quaternary Diamond-like Phosphide Infrared Nonlinear Optical Material Derived from ZnGeP2. J. Am. Chem. Soc. 2021, 143, 10309–10316. [Google Scholar] [CrossRef]
- SAINT, version 7.60A; Bruker Analytical X-ray Instruments Incorporated: Madison, WI, USA, 2008.
- Spek, A.L. Single-crystal structure validation with the program PLATON. J. Appl. Crystallogr. 2003, 36, 7–13. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A Found. Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef] [Green Version]
- Mei, D.J.; Cao, W.Z.; Wang, N.Z.; Jiang, X.X.; Zhao, J.; Wang, W.K.; Dang, J.H.; Zhang, S.Y.; Wu, Y.D.; Rao, P.H.; et al. Breaking Through the “3.0 eV wall” of Energy Band Gap in Mid-Infrared Nonlinear Optical Rare Earth Chalcogenides by Charge-Transfer Engineering. Mater. Horiz. 2021, 8, 2330–2334. [Google Scholar] [CrossRef]
- Jiang, J.Q.; Mei, D.J.; Gong, P.F.; Lin, Z.S.; Zhong, J.B.; Wu, Y.D. Wide Band Gap Design of New Chalcogenide Compounds: KSrPS4 and CsBaAsS4. RSC Adv. 2017, 7, 38044–38051. [Google Scholar] [CrossRef] [Green Version]
- Larson, A.C.; Dreele, R.B.V. General Structure Analysis System (GSAS); Los Alamos National Laboratory, University of California: Oakland, CA, USA, 2004; pp. 86–748. [Google Scholar]
- Kortüm, G. Reflectance Spectroscopy; Springer: Berlin, Germany, 1969. [Google Scholar]
- Tauc, J. Absorption edge and internal electric fields in amorphous semiconductors. Mater. Res. Bull. 1970, 5, 721–729. [Google Scholar] [CrossRef]
- Clark, S.; Segall, M.D.; Pickard, C.J.; Hasnip, P.J.; Probert, M.I.J.; Refson, K.; Payne, M.C. First principles methods using CASTEP. Z. Krist. 2005, 220, 567–570. [Google Scholar] [CrossRef] [Green Version]
- Ceperley, D.; Alder, B.J. Ground State of the Electron Gas by a Stochastic Method. Phys. Rev. Lett. 1980, 45, 566–569. [Google Scholar] [CrossRef] [Green Version]
- Perdew, J.P.; Zunger, A. Self-interaction correction to density-functional approximations for many-electron systems. Phys. Rev. B 1981, 23, 5048–5079. [Google Scholar] [CrossRef] [Green Version]
- Perdew, J.P.; Wang, Y. Accurate and simple analytic representation of the electron-gas correlation energy. Phys. Rev. B 1992, 45, 13244–13249. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Bellaiche, L.; Vanderbilt, D. Virtual crystal approximation revisited: Application to dielectric and piezoelectric properties of perovskites. Phys. Rev. B 2000, 61, 7877–7882. [Google Scholar] [CrossRef] [Green Version]
- Winkler, B.; Pickard, C.; Milman, V. Applicability of a quantum mechanical virtual crystal approximation’ to study Al/Si-disorder. Chem. Phys. Lett. 2002, 362, 266–270. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.W.; Zhang, M.J.; Zhao, Z.Y.; Zeng, H.Y.; Zheng, F.K.; Guo, G.C.; Huang, J.S. Synthesis, Structure, and Optical Properties of the Quaternary Diamond-Like Compounds I2-II-IV-VI4 (I = Cu; II = Mg; IV = Si, Ge; VI = S, Se). J. Solid State Chem. 2013, 204, 251–256. [Google Scholar] [CrossRef]
- Chen, J.; Hu, C.L.; Mao, F.F.; Zhang, X.H.; Yang, B.P.; Mao, J.G. LiMg(IO3)3: An Excellent SHG Material Designed by Single-Site Aliovalent Substitution. Chem. Sci. 2019, 10, 10870–10875. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Zhang, M.; Pan, S.L.; Wang, Y.; Zhang, H.; Chen, Z.H. Li0.8Mg2.1B2O5F: The First Borate Fluoride with Magnesium-Oxygen-Fluorine Octahedral Chains. Dalton Trans. 2014, 43, 2828–2834. [Google Scholar] [CrossRef] [PubMed]
- Abudurusuli, A.; Li, J.J.; Tong, T.H.; Yang, Z.H.; Pan, S.L. LiBa4Ga5Q12 (Q = S, Se): Noncentrosymmetric Metal Chalcogenides with a Cesium Chloride Topological Structure Displaying a Remarkable Laser Damage Threshold. Inorg. Chem. 2020, 59, 5674–5682. [Google Scholar] [CrossRef]
- Abudurusuli, A.; Wu, K.; Pan, S.L. Four New Quaternary Chalcogenides A2Ba7Sn4Q16 (A = Li, Na; Q = S, Se): Syntheses, Crystal Structures Determination, Nonlinear Optical Performances Investigation. New J. Chem. 2018, 42, 3350–3355. [Google Scholar] [CrossRef]
- Wu, K.; Su, X.; Yang, Z.; Pan, S. An investigation of new infrared nonlinear optical material: BaCdSnSe4, and three new related centrosymmetric compounds: Ba2SnSe4, Mg2GeSe4, and Ba2Ge2S6. Dalton Trans. 2015, 44, 19856–19864. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Pan, S.L.; Yang, Z.H. Ba2GeS4 and Mg2SnS4: Synthesis, Structures, Optical Properties and Electronic Structures. RSC Adv. 2015, 5, 33646–33652. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).