Study on the Behavior of Electrochemical Extraction of Cobalt from Spent Lithium Cobalt Oxide Cathode Materials
Abstract
:1. Introduction
2. Experimental Method
3. Experimental Results and Discussion
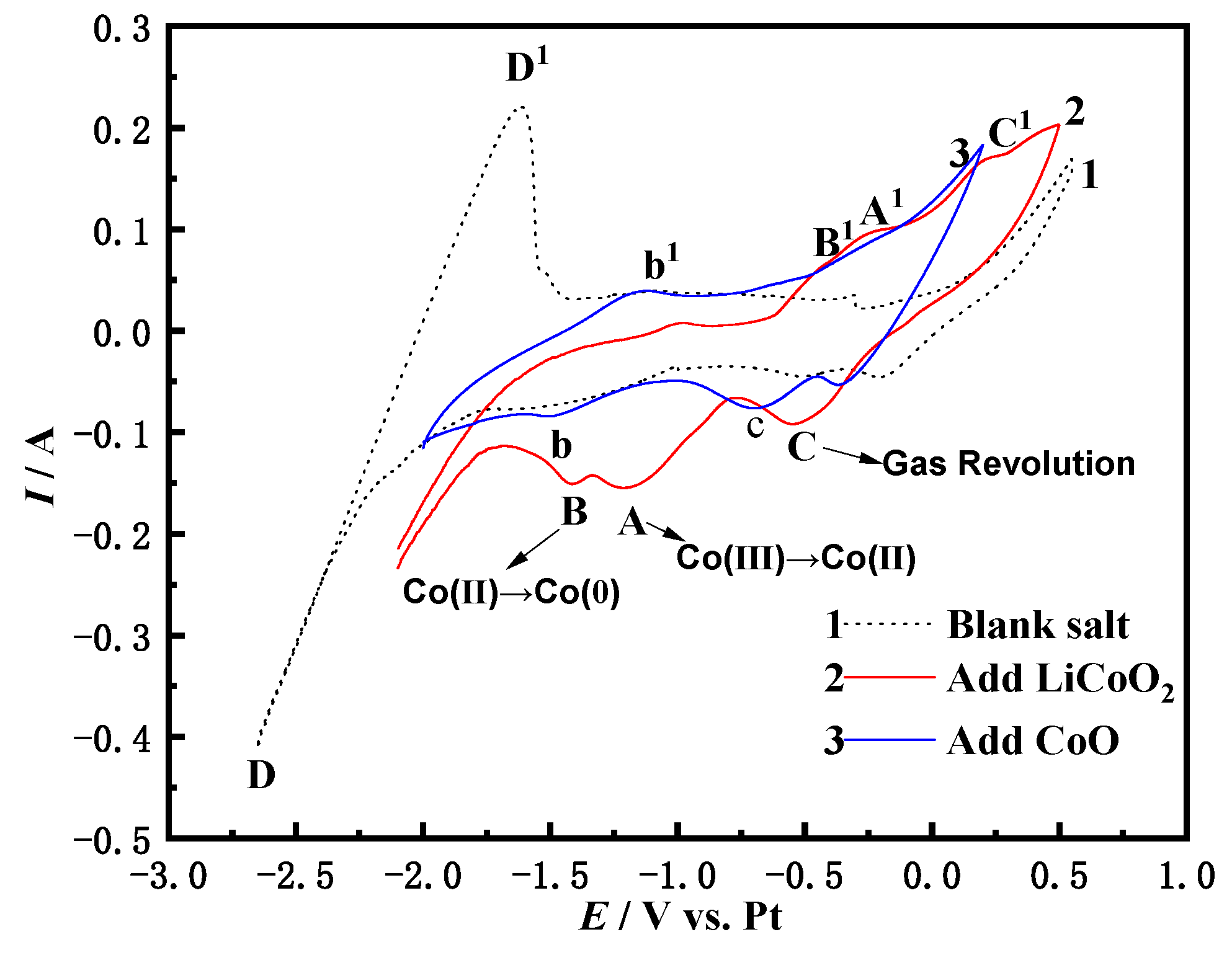
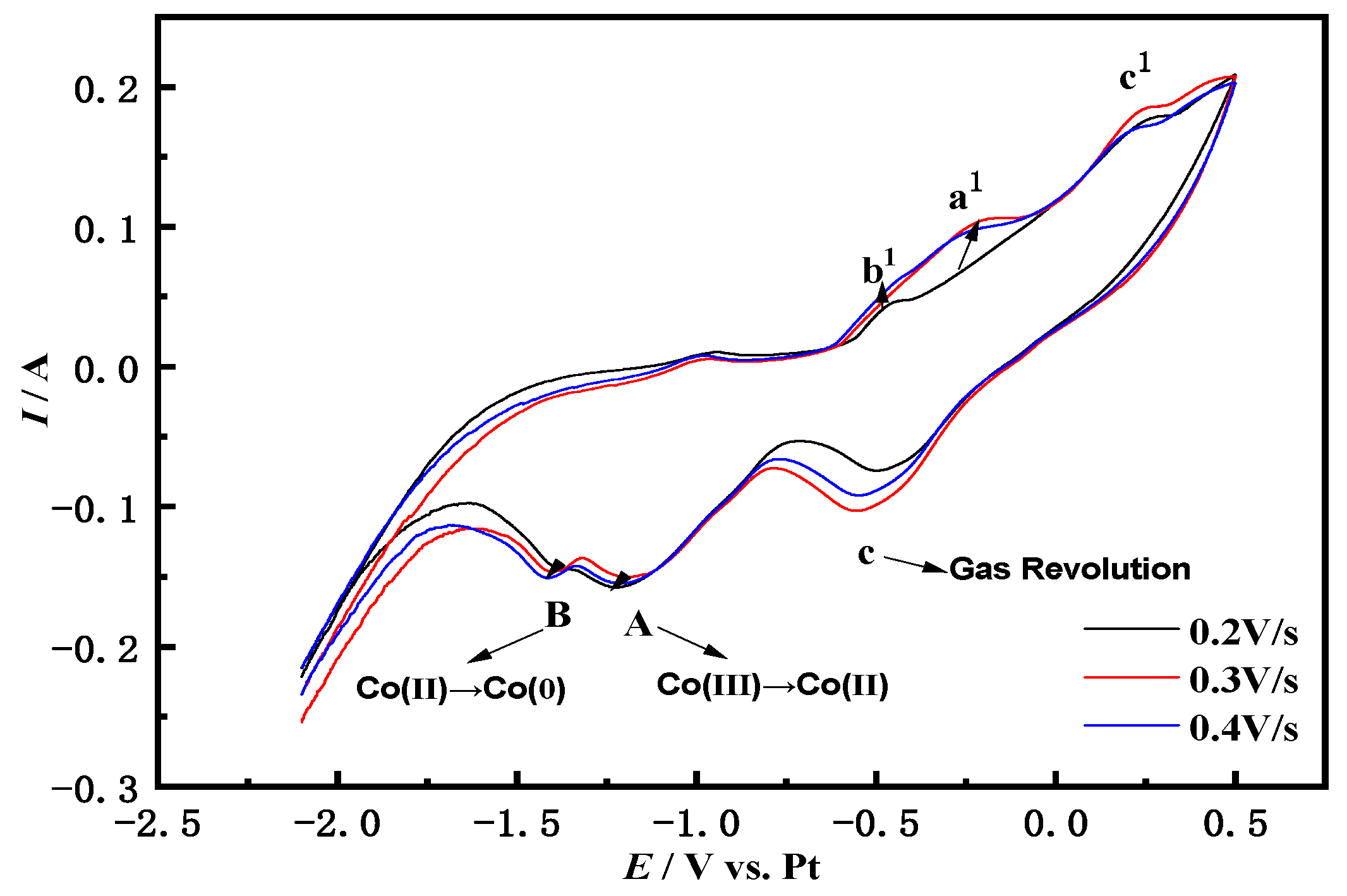
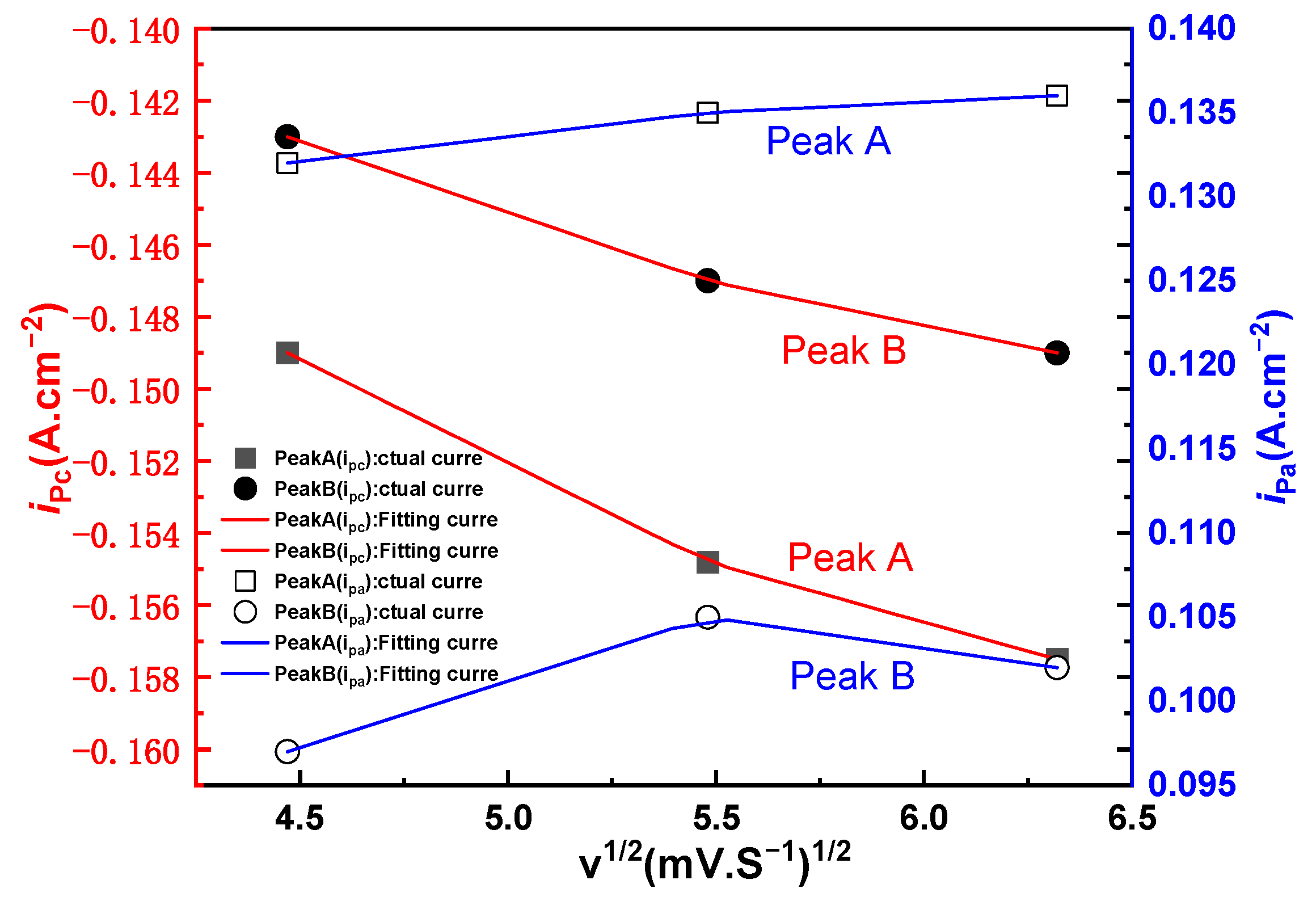
3.1. Cyclic Voltammetry
3.1.1. Cyclic Voltammetry of NaCl-CaCl2-LiCoO2
3.1.2. Cyclic Voltammetry of NaCl-CaCl2-LiCoO2 at Different Scan Rates
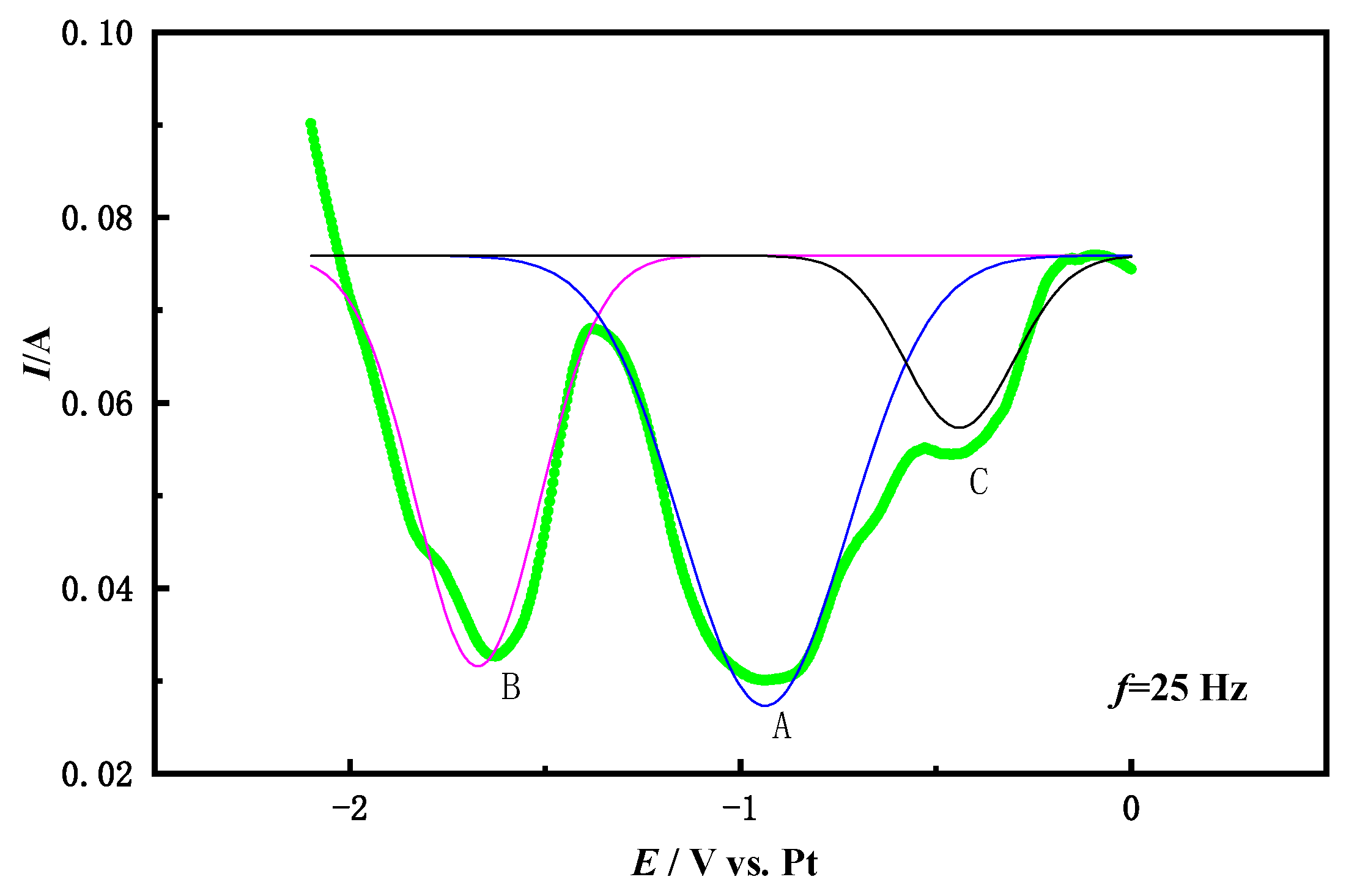
3.2. Square Wave Voltammetry
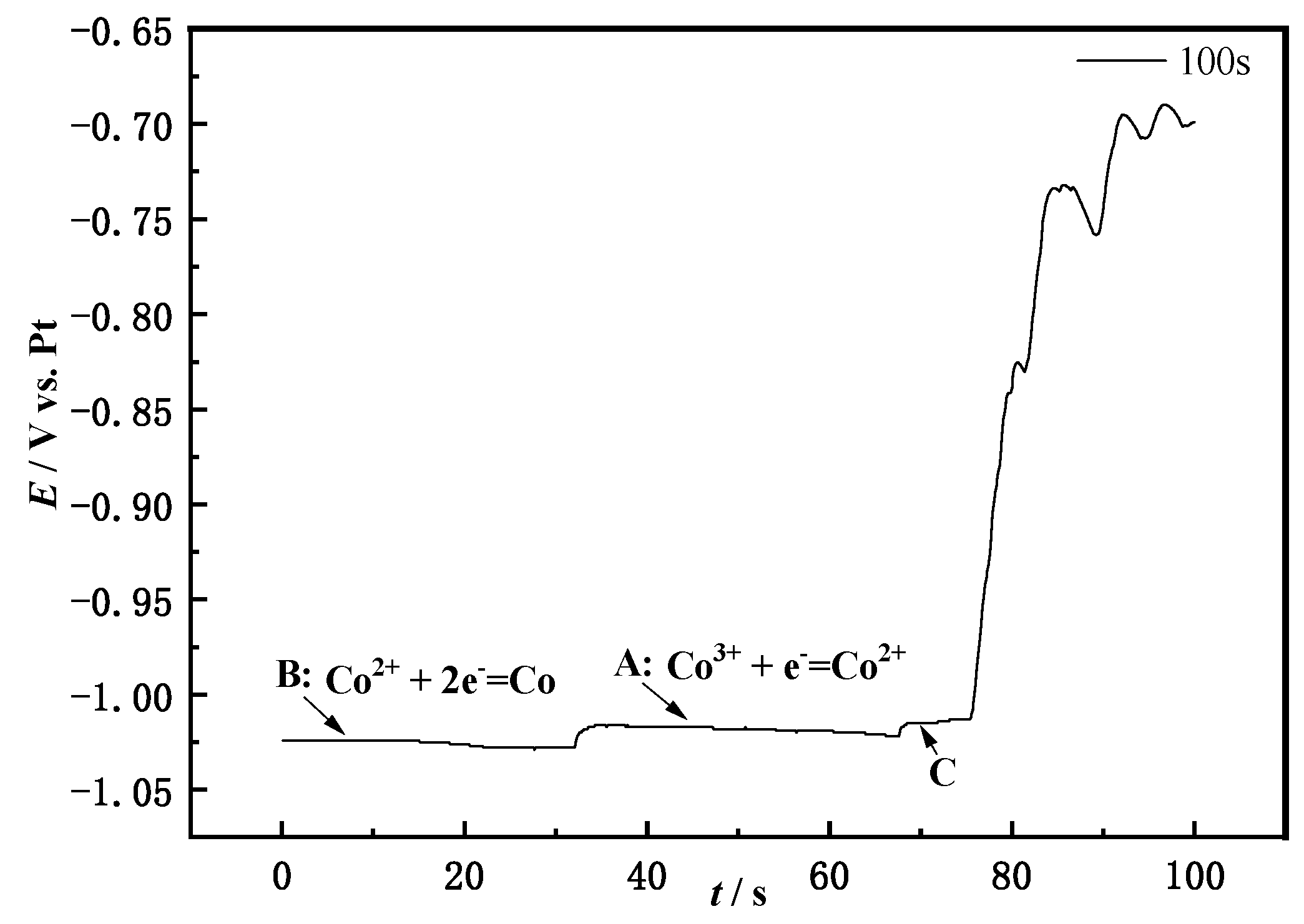
3.3. Open Circuit Chronopotential
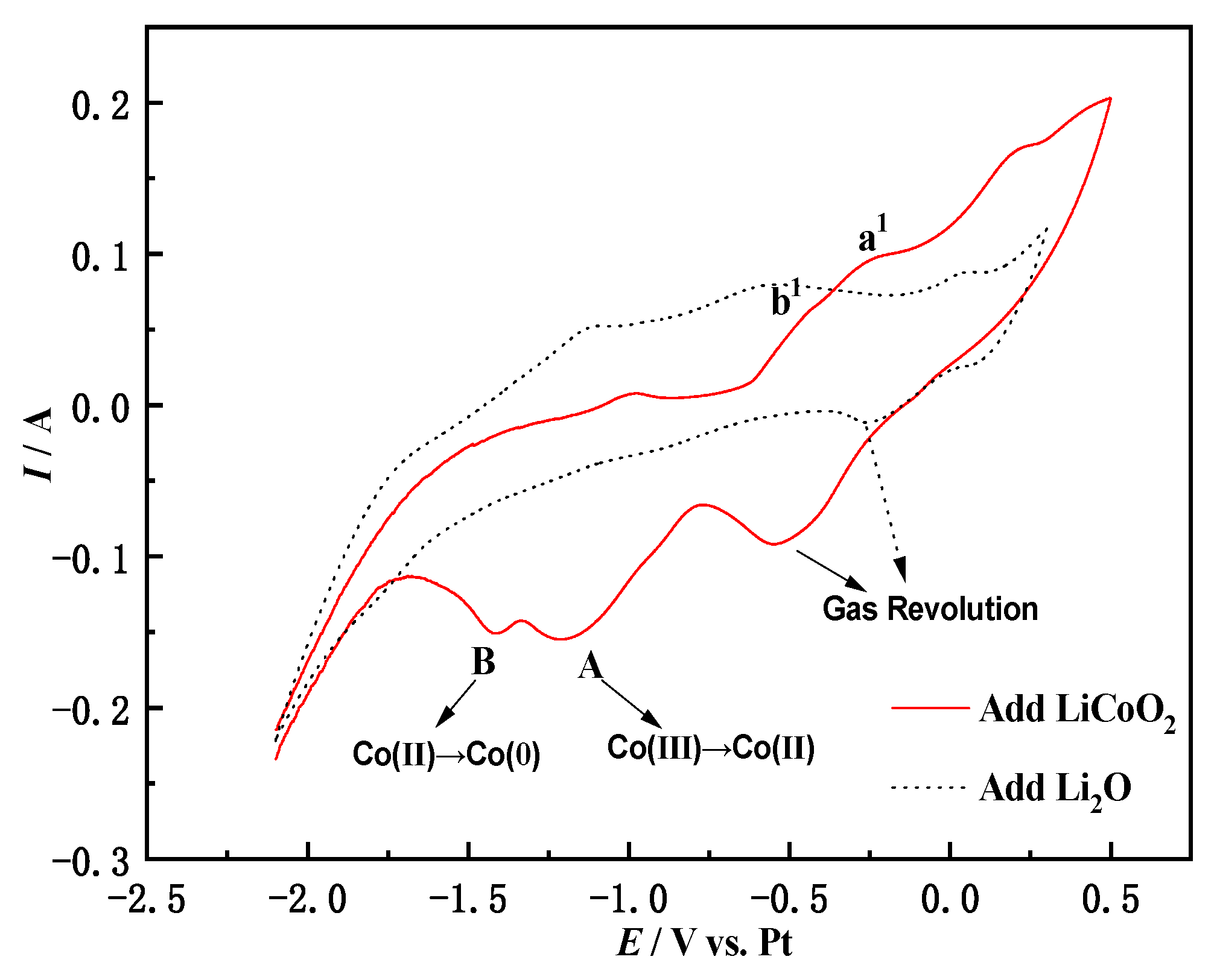
3.4. Cyclic Voltammetry of Li2O in NaCl-CaCl2
3.5. Preparation of Co by Electrolysis of LiCoO2 at Constant Cell Pressure
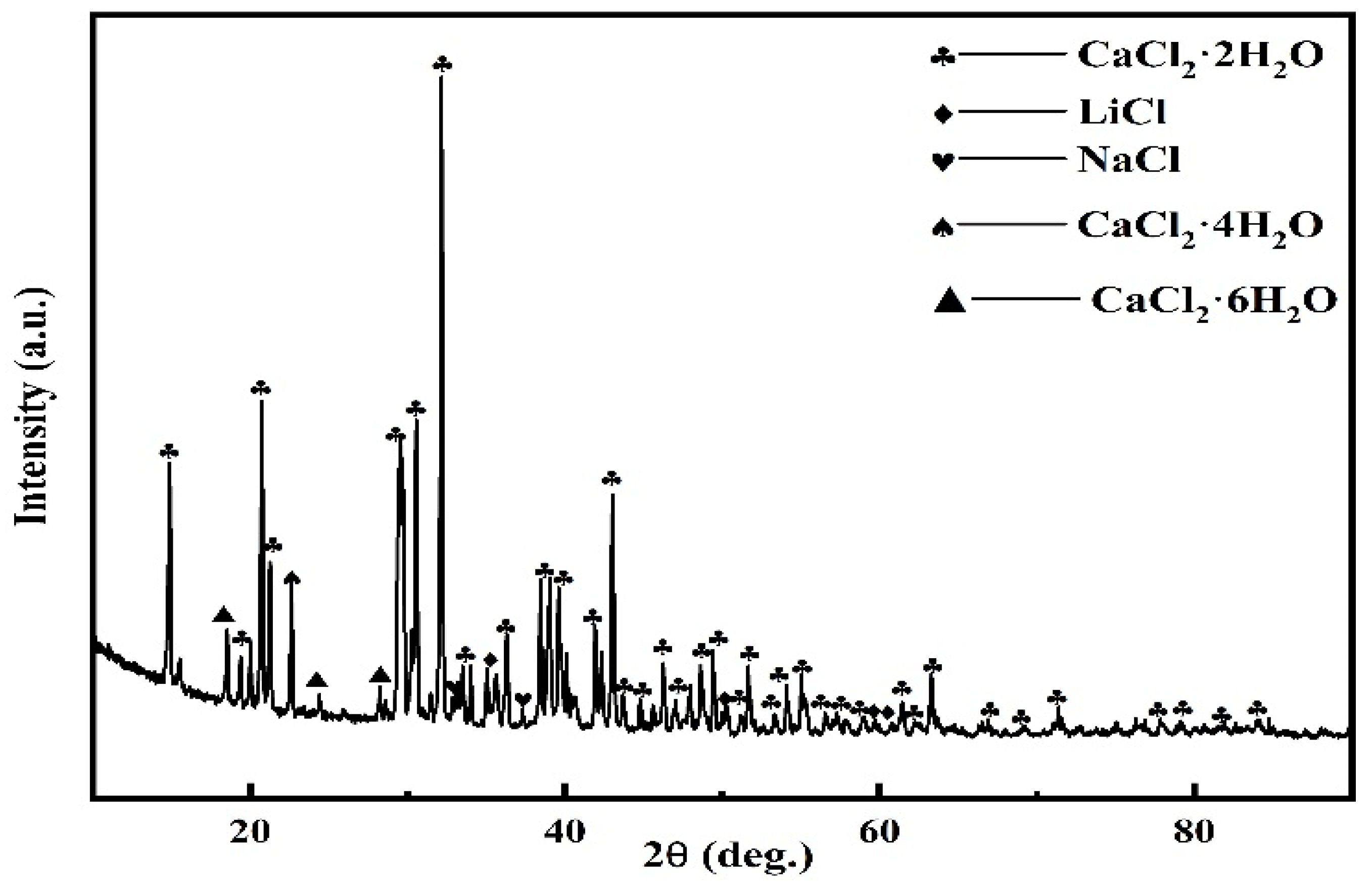
3.5.1. Immersion Test of LiCoO2 in NaCl-CaCl2 Molten Salt
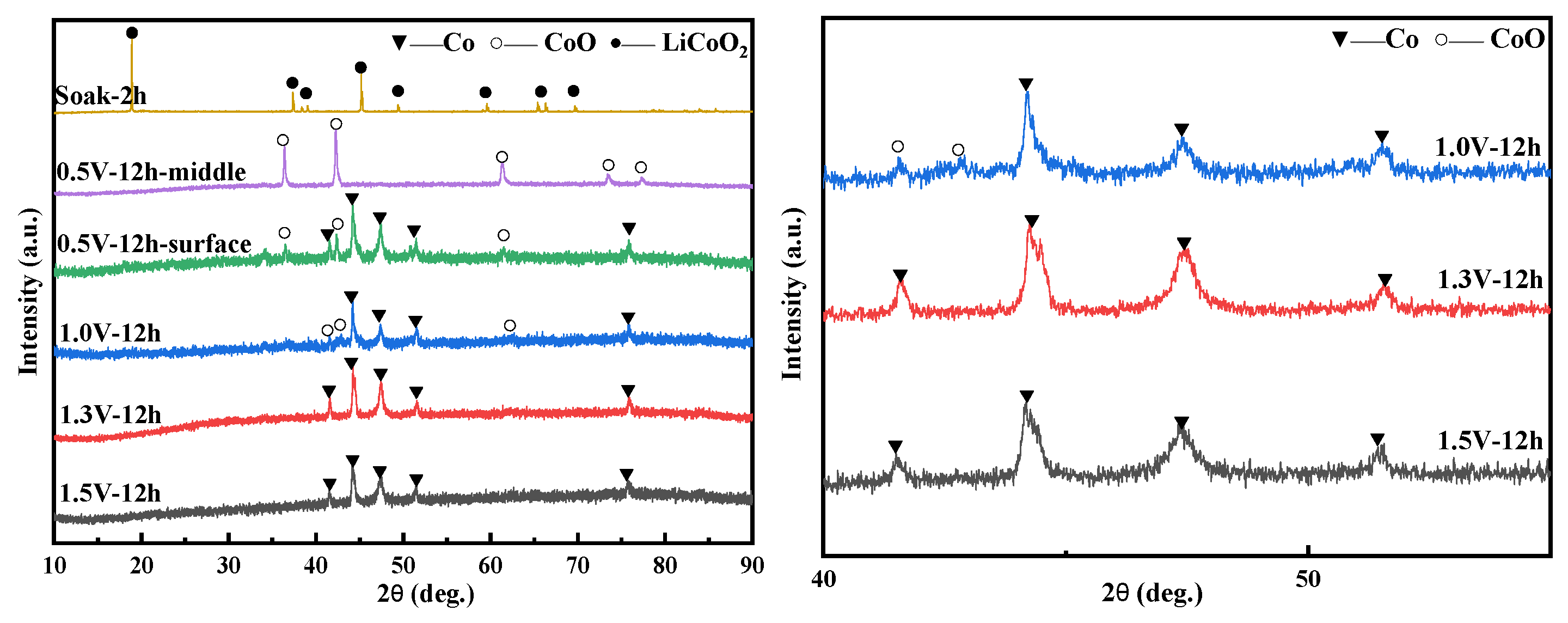
3.5.2. The Influence of Voltage Conditions on Electrolysis
4. Conclusions
- (1)
- When the potential is within −2.6 V~0.5 V, two pairs of redox peaks appear, indicating that the reduction of Co (III) is a two-step process, Co (III) → Co (II) → Co (0). In the first step, the Co (III) in the molten salt is reduced to Co (II) by an electron on the surface of the Fe electrode, and the second step is that Co (II) on the active site of the Fe electrode is reduced to Co (0) by two electrons. The reduction process of LiCoO2 is a quasi-reversible reaction controlled by diffusion. Li+ forms LiCl with Cl− in molten salt during electrochemical reaction.
- (2)
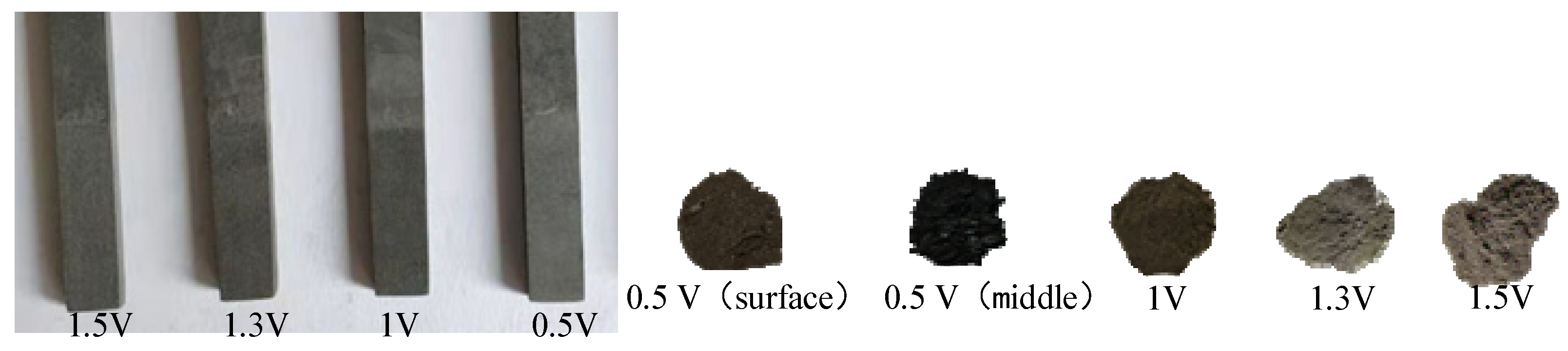
- Electrolysis experiments with different voltages of 0.5–1.5 V have confirmed the two-part reduction process of LiCoO2. The reduction reaction first proceeds on the three-phase boundary of the current collector/LiCoO2 sheet/molten salt contact, and then extends to the entire cathode surface. The inside of the LiCoO2 sheet reaction speed is slower than the surface. The inside of the LiCoO2 sheet is all CoO, which is the first reduction product, while CoO and Co are present on the outside at 0.5 V,12 h. When the voltage reaches 1.3 V, the LiCoO2 sheet is reduced to Co.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, Y.N.; Dong, Y.Y.; Han, X.; Wang, X.F.; Wang, Y.J.; Jiao, L.F.; Yuan, H.T. Application for Simply Recovered LiCoO2 Material as a High Performance Candidate for Supercapacitor in Aqueous System. ACS Sustain. Chem. Eng. 2015, 3, 2435–2442. [Google Scholar] [CrossRef]
- Takacova, Z.; Havlik, T.; Kukurugya, F.; Orac, D. Cobalt and lithium recovery from active mass of spent Li-ion batteries: Theoretical and experimental approach. J. Hydrometall. 2016, 163, 9–17. [Google Scholar] [CrossRef]
- Ma, Y.Y.; Tang, J.J.; Wanaldi, R.; Zhou, X.Y.; Wang, H.; Zhou, C.Y.; Yang, J. A promising selective recovery process of valuable metals from spent lithium-ion batteries via reduction roasting and ammonia leaching. J. Hazard. Mater. 2021, 402, 123491. [Google Scholar] [CrossRef]
- Sethurajan, M.; Gaydardzhi, E.S. Bioprocessing of spent lithium-ion batteries for critical metals recovery—A review. J. Res. Conserv. Recycl. 2020, 165, 105225. [Google Scholar] [CrossRef]
- Chen, X.P.; Guo, C.X.; Ma, H.R.; Li, J.Z.; Zhou, T.; Cao, L.; Kang, D. Organic reductants based leaching: A sustainable process for the recovery of valuable metals from spent lithium ion batteries. J. Was. Manag. 2018, 75, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Chagnes, A.; Pospiech, B. A brief review on hydrometallurgical technologies for recycling spent lithium-ion batteries. J. Chem. Technol. Biotechnol. 2013, 88, 1191–1199. [Google Scholar] [CrossRef]
- Meng, Q.; Zhang, Y.J.; Dong, P.; Liang, F. Recovery of Co and Li from spent lithium ion batteries. J. ISSN 2017, 36, 3485–3491. [Google Scholar] [CrossRef]
- Dorella, G.; Mansur, M.B. A study of the separation of cobalt from spent Li-ion battery residues. J. Power Sources 2007, 170, 210–215. [Google Scholar] [CrossRef]
- Xin, B.P.; Zhang, D.; Zhang, X.; Xia, Y.T.; Wu, F.; Chen, S.; Li, L. Bioleaching mechanism of Co and Li from spent lithium-ion battery by the mixed culture of acidophilic sulfur-oxidizing and iron-oxidizing bacteria. J. Bioresour. Technol. 2009, 100, 6163–6169. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Qiu, G.Z.; Hu, Y.H.; Sun, X.J.; Li, J.H.; Gu, G.H. Bioleaching of pyrite by A.ferrooxidans and L.ferriphilum. Trans. Nonferrous. Met. Soc. China 2008, 18, 1415–1420. [Google Scholar] [CrossRef]
- Işıldar, A.; Hullebusch, E.; Lenz, M.; Laing, G.D.; Cesaro, A.; Panda, S.; Akcil, A.; Kucuker, M.A.; Kuchta, K. Biotechnological strategies for the recovery of valuable and critical raw materials from waste electrical and electronic equipment (WEEE)—A review. J. Hazard. Mater. 2019, 362, 467–481. [Google Scholar] [CrossRef] [PubMed]
- Lv, W.G.; Wang, Z.H.; Cao, H.B.; Sun, Y.; Zhang, Y.; Sun, Z. A Critical Review and Analysis on the Recycling of Spent Lithium-Ion Batteries. ACS Sustain. Chem. Eng. 2018, 6, 1504–1521. [Google Scholar] [CrossRef]
- He, L.P.; Sun, S.; Mu, Y.Y.; Song, X.; Yu, J. Recovery of lithium, nickel, cobalt, and manganese from spent lithium-ion batteries using L-tartaric acid as a leachant. ACS Sustain. Chem. Eng. 2017, 5, 714–721. [Google Scholar] [CrossRef]
- Bai, Y.C.; Muralidharan, N.; Sun, Y.K.; Passerini, S.; Stanley, W.M.; Belharouak, L. Energy and environmental aspects in recycling lithium-ion batteries: Concept of Battery Identity Global Passport. J. Mater. 2020, 41, 304–315. [Google Scholar] [CrossRef]
- Qian, W.; Zhang, W.Y.; Dong, H.X.; Shi, H. Experimental Study on Ultrasonic Leaching of Cobalt from Waste Lithium-ion Battery with. J. Chem. Ind. 2021, 48, 48–49+84. [Google Scholar]
- Sun, L.Y.; Liu, B.R.; Wu, T.; Wang, G.G.; Huang, Q.; Su, Y.F.; Wu, F. Hydrometallurgical recycling of valuable metals from spent lithium-ion batteries by reductive leaching with stannous chloride. Int. J. Miner. Metal. Mater. 2021, 28, 997–1000. [Google Scholar]
- Ma, Y.Y.; Zhou, X.Y.; Tang, J.J.; Liu, X.J.; Gan, H.X.; Yang, J. One-step selective recovery and cyclic utilization of valuable metals from spent lithium-ion batteries via low-temperature chlorination pyrolysis. J. Resour. Conserv. Recycl. 2021, 175, 105840. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Meng, Q.; Dong, P.; Duan, J.G.; Lin, Y. Use of grape seed as reductant for leaching of cobalt from spent lithium-ion batteries. J. Ind. Eng. Chem. 2018, 66, 86–93. [Google Scholar] [CrossRef]
- Chang, W.; Man, R.L.; Yi, X.Y.; Zhang, J. Leaching LiCoO2 from spent lithium-ion batteries by electrochemical reduction. Chin. J. Nonferrous. 2014, 24, 787–792. [Google Scholar] [CrossRef]
- Zhang, B.L.; Xie, H.W.; Lu, B.H.; Chen, X.; Xing, P.F.; Qu, J.K.; Song, Q.S.; Yin, H.Y. A Green Electrochemical Process to Recover Co and Li from Spent LiCoO2 -Based Batteries in Molten Salts. ACS Sustain. Chem. Eng. 2019, 7, 13391–13399. [Google Scholar] [CrossRef]
- Rong, L.B.; He, R.; Wang, Z.Y.; Peng, J.J.; Jin, X.B.; Chen, G.Z. Investigation of electrochemical reduction of GeO2 to Ge in molten CaCl2-NaCl. J. Electrochim. Acta 2014, 147, 352–359. [Google Scholar] [CrossRef]
- Weng, W.; Wang, M.Y.; Gong, X.Z.; Wang, Z.; Wang, D.; Guo, Z.C. Direct electro-deposition of metallic chromium from K2CrO4 in the equimolar CaCl2-KCl molten salt and its reduction mechanism. Electrochim. Acta 2016, 212, 162–170. [Google Scholar] [CrossRef]
- Li, C.Y.; Han, W.; Li, M.; Wang, W.; Yang, X.G. Electrochemistry of Zr (IV) in molten LiCl-KCl-K2ZrF6 system. Int. J. Electrochem. Sci. 2018, 13, 11795–11807. [Google Scholar] [CrossRef]
- Li, M.; Xi, X.L.; Nie, Z.R.; Ma, L.W.; Liu, Q.Q. Recovery of tungsten from WC–Co hard metal scraps using molten salts electrolysis. J. Mater. Res. Technol. 2018, 8, 1440–1450. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Li, H.; Li, C.; Liang, J.; Yan, H.; Xu, Z. Study on the Behavior of Electrochemical Extraction of Cobalt from Spent Lithium Cobalt Oxide Cathode Materials. Materials 2021, 14, 6110. https://doi.org/10.3390/ma14206110
Li H, Li H, Li C, Liang J, Yan H, Xu Z. Study on the Behavior of Electrochemical Extraction of Cobalt from Spent Lithium Cobalt Oxide Cathode Materials. Materials. 2021; 14(20):6110. https://doi.org/10.3390/ma14206110
Chicago/Turabian StyleLi, Hui, Haotian Li, Chenxiao Li, Jinglong Liang, Hongyan Yan, and Zhengzhen Xu. 2021. "Study on the Behavior of Electrochemical Extraction of Cobalt from Spent Lithium Cobalt Oxide Cathode Materials" Materials 14, no. 20: 6110. https://doi.org/10.3390/ma14206110
APA StyleLi, H., Li, H., Li, C., Liang, J., Yan, H., & Xu, Z. (2021). Study on the Behavior of Electrochemical Extraction of Cobalt from Spent Lithium Cobalt Oxide Cathode Materials. Materials, 14(20), 6110. https://doi.org/10.3390/ma14206110




