Refining of Precious Metal Bearing Materials from Secondary Sources-Methanesulfonic Acid Leaching of Raw Silver Granules as a Promising Approach towards a Green Way of Silver Refining
Abstract
:1. Introduction
2. Methanesulfonic Acid in Leaching and Metal Recovery—A Literature Review
3. Calculation of the Leaching Yield
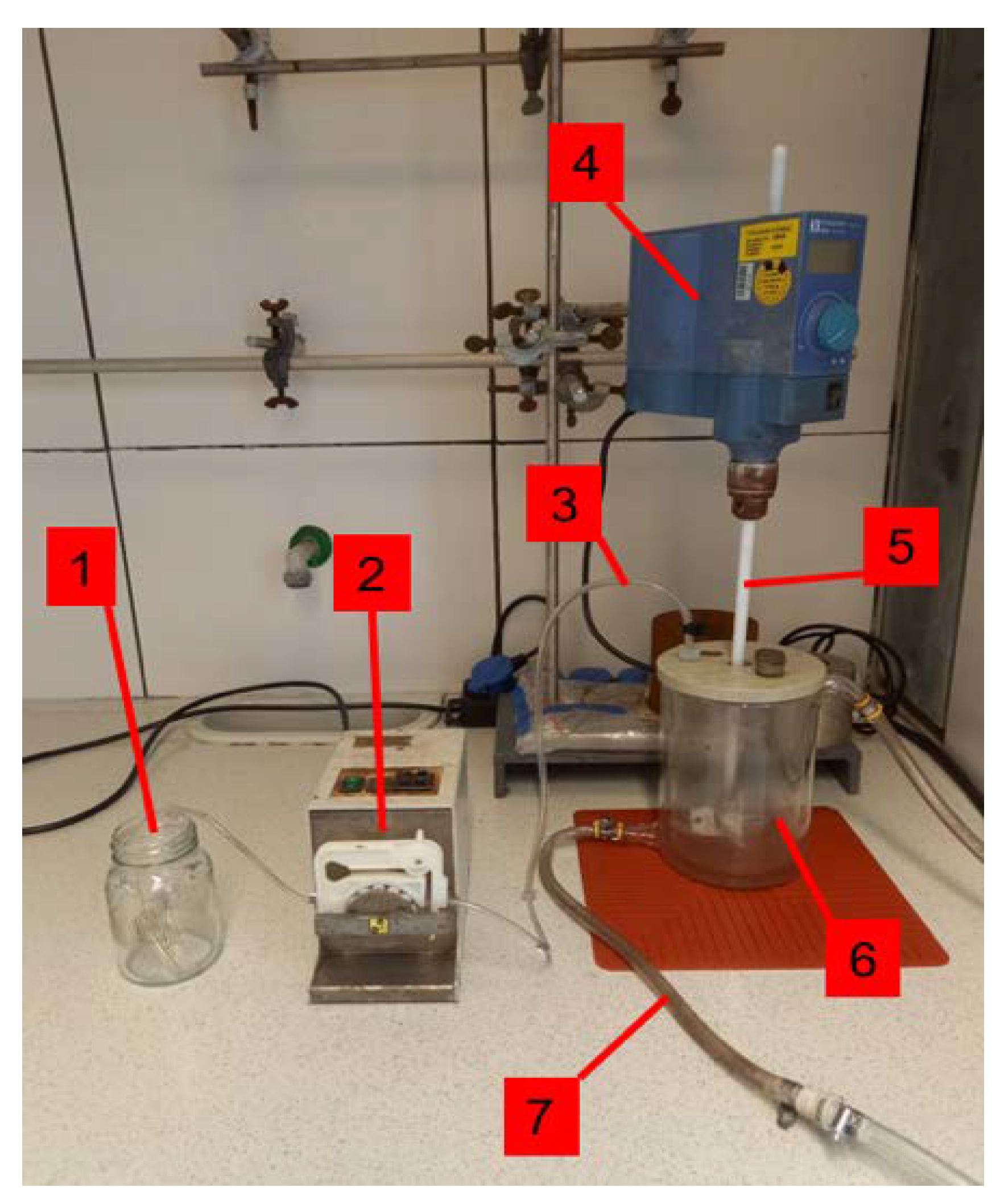
4. Materials and Method
5. Results and Discussion
5.1. The Effect of the Solid Concentration
5.2. The Effect of the H2O2 Dosage
5.3. Temperature Development during Leaching at Constant Temperatures
5.4. Leaching Results for Leaching at Different Temperatures
6. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- U.S. Geological Survey; National Minerals Information Center. Silver Statistics and Information; Annual Publications: Reston, VA, USA, 2021.
- Langner, B.; Lossin, A. Lexikon der Metalle; Inf. material of Aurubis AG Company: Hamburg, Germany, 2012. [Google Scholar]
- Pawlek, F. Metallhüttenkunde; De Gruyter: Berlin, Germany; New York, NY, USA, 1983; ISBN 3-11-007458-3. [Google Scholar]
- Dressler, A.; Keil, M.; Müller, T.; Stelter, M. The Possible Use of Methanesulfonic Acid as a New Electrolyte for Silver Electrorefining, World of Metallurgy. Erzmetall 2017, 70, 265–270. [Google Scholar]
- Gernon, M.D.; Wu, M.; Buszta, T.; Janney, P. Environmental benefits of methanesulfonic acid: Comparative properties and advantages. Green Chem. 1999, 1, 127–140. [Google Scholar] [CrossRef]
- Jaskula, M.; Kammel, R. Untersuchung zur Verbesserung des Platinmetallausbringens bei der industriellen Silberraffnation. Metall 1997, 51, 393–400. [Google Scholar]
- BASF. Lutropur®—The Friendly Acid; Inf. Sheet of BASF Company: Ludwigshafen, Germany, 2019. [Google Scholar]
- Feng, Q.; Wen, S.; Zhao, W.; Lv, C.; Bai, X. Leaching of copper from malachite with methane-sulfonic acid. Solvent Extr. Res. Dev. Jpn. 2019, 22, 159–168. [Google Scholar] [CrossRef] [Green Version]
- Ahn, J.; Wu, J.; Lee, J. Investigation on chalcopyrite leaching with methanesulfonic acid (MSA) and hydrogen peroxide. Hydrometallurgy 2019, 187, 54–62. [Google Scholar] [CrossRef]
- Palden, T.; Onghena, B.; Regadío, M.; Binnemans, K. Methanesulfonic acid: A sustainable acidic solvent for recovering metals from the jarosite residue of the zinc industry. Green Chem. 2019, 21, 5394–5404. [Google Scholar] [CrossRef]
- Wu, Z.; Dreisinger, D.; Urch, H.; Fassbender, S. The kinetics of leaching galena concentrates with ferric methanesulfonate solution. Hydrometallurgy 2014, 142, 121–130. [Google Scholar] [CrossRef]
- Wu, Z.; Dreisinger, D.; Urch, H.; Fassbender, S. Fundamental study of lead recovery from cerussite concentrate with methanesulfonic acid (MSA). Hydrometallurgy 2014, 142, 23–35. [Google Scholar] [CrossRef]
- Zhang, Q.; Wen, S.; Feng, Q.; Nie, W.; Wu, D. Dissolution kinetics of hemimorphite in methane sulfonic acid. Physiochem. Probl. Miner. Process. 2019, 55, 1–9. [Google Scholar] [CrossRef]
- Jin, B.; Dreisinger, D. A green process for production of pure lead from methanesulfonic acid medium. Sep. Purif. Technol. 2016, 170, 199–207. [Google Scholar] [CrossRef] [Green Version]
- Hollemann, A.F.; Wiberg, E. Lehrbuch der Anorganischen Chemie, 102nd ed.; Walter de Gruyter: Berlin, Germany, 2007; ISBN 978-3-11-017770-1. [Google Scholar]
- Yang, E.H.; Lee, J.K.; Lee, J.S.; Ahn, Y.S.; Kang, G.H.; Cho, C.H. Environmentally friendly recovery of Ag from end-of-life c-Si solar cell using organic acid and its electrochemical purification. Hydrometallurgy 2016, 167, 129–133. [Google Scholar] [CrossRef]
- Schosseler, J.; Trentmann, A.; Friedrich, B. Silver recovery from oxygen depolarized cathodes in methane sulfonic acid. In Proceedings of the EMC 2019, Düsseldorf, Germany, 23–26 June 2019; pp. 261–270. [Google Scholar]






| Ag | Au | Pd | Pt | Cu | Pb | ∑ | |
|---|---|---|---|---|---|---|---|
| wt.-% | 93.86 | 3.51 | 0.93 | 0.18 | 1.41 | 0.02 | 99.91 |
| H2O2/Ag Stoich. Ratio (-) | mresidue/mraw silver (%) | Leaching Yield Ag (%) | Leaching Yield Pd (%) |
|---|---|---|---|
| 3:1 | 8.43 | 95 | 11 |
| 2.5:1 | 9.93 | 92 | 11 |
| 1:1 | 18.90 | 83 | 9 |
| 3:1 (*) | 42.84 | 59 | 6 |
| Experiment | Temperature (°C) | mresidue/mraw silver (%) |
|---|---|---|
| 1 | 11 (t: 2.5 h) | 58.88 |
| 2 | 25 | 45.06 |
| 3 | 25 | 45.50 |
| 4 | T ≠ const. | 16.36 |
| 5 | 45 | 12.36 |
| 6 | 65 | 9.42 |
| 7 | 65 (t: 2.5 h) | 8.90 |
| 8 | 80 (t: 2.5 h) | 9.02 |
| 9 | 80 | 9.28 |
| Ag (Balance) | Au | Pd | Pt | Cu | Pb | ∑ | |
|---|---|---|---|---|---|---|---|
| wt.-% | 45.28 | 42.68 | 9.688 | 2.186 | 0.155 | 0.007 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hopf, J.; Weigelt, A.; Bombach, H.; Stelter, M.; Charitos, A. Refining of Precious Metal Bearing Materials from Secondary Sources-Methanesulfonic Acid Leaching of Raw Silver Granules as a Promising Approach towards a Green Way of Silver Refining. Materials 2021, 14, 6095. https://doi.org/10.3390/ma14206095
Hopf J, Weigelt A, Bombach H, Stelter M, Charitos A. Refining of Precious Metal Bearing Materials from Secondary Sources-Methanesulfonic Acid Leaching of Raw Silver Granules as a Promising Approach towards a Green Way of Silver Refining. Materials. 2021; 14(20):6095. https://doi.org/10.3390/ma14206095
Chicago/Turabian StyleHopf, Johannes, Aaron Weigelt, Hartmut Bombach, Michael Stelter, and Alexandros Charitos. 2021. "Refining of Precious Metal Bearing Materials from Secondary Sources-Methanesulfonic Acid Leaching of Raw Silver Granules as a Promising Approach towards a Green Way of Silver Refining" Materials 14, no. 20: 6095. https://doi.org/10.3390/ma14206095
APA StyleHopf, J., Weigelt, A., Bombach, H., Stelter, M., & Charitos, A. (2021). Refining of Precious Metal Bearing Materials from Secondary Sources-Methanesulfonic Acid Leaching of Raw Silver Granules as a Promising Approach towards a Green Way of Silver Refining. Materials, 14(20), 6095. https://doi.org/10.3390/ma14206095






