UV-LED Combined with Small Bioreactor Platform (SBP) for Degradation of 17α-Ethynylestradiol (EE2) at Very Short Hydraulic Retention Time
Abstract
:1. Introduction
2. Materials and Methods
2.1. UV Light
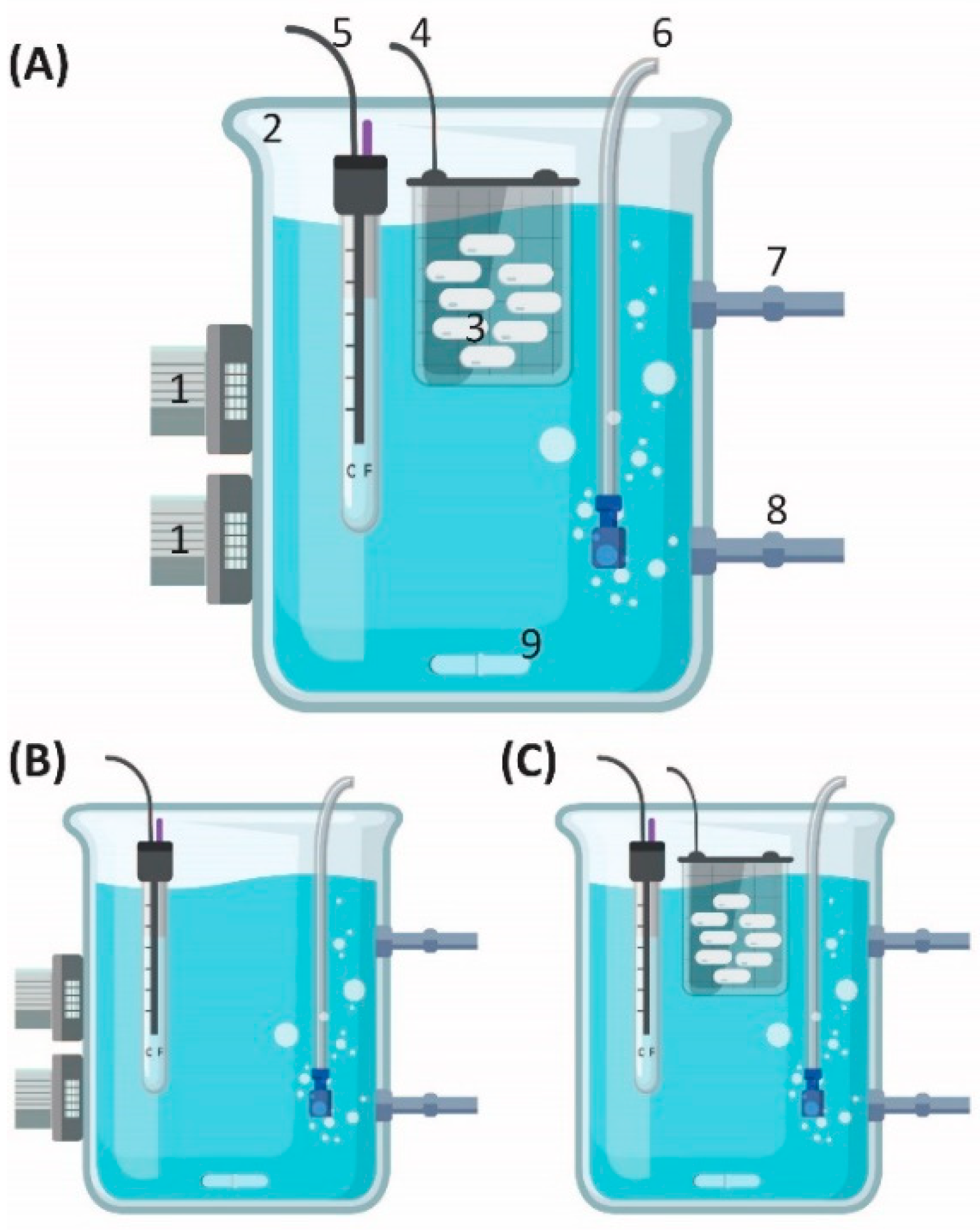
2.1.1. Batch Setup
Optimization of Wavelengths
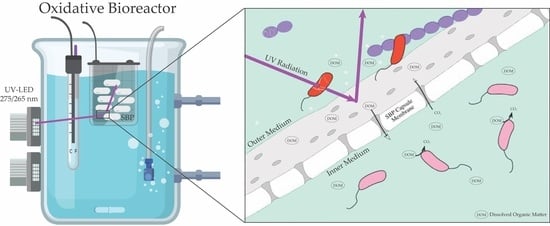
2.1.2. OBR
Total Organic Carbon (TOC) and Estrogenicity Experiments
OBR Experiments
2.2. Encapsulation of Bacterial Cultures in SBP Capsules
2.3. Experimental Stock Solution
2.4. Analytical Measurements
2.4.1. HPLC-UV
2.4.2. TOC
2.4.3. Colorimetric Estimation of H2O2 Using Strips
2.4.4. UV-LED Emission Spectra
2.4.5. Estrogenicity Assay—YES
3. Results and Discussion
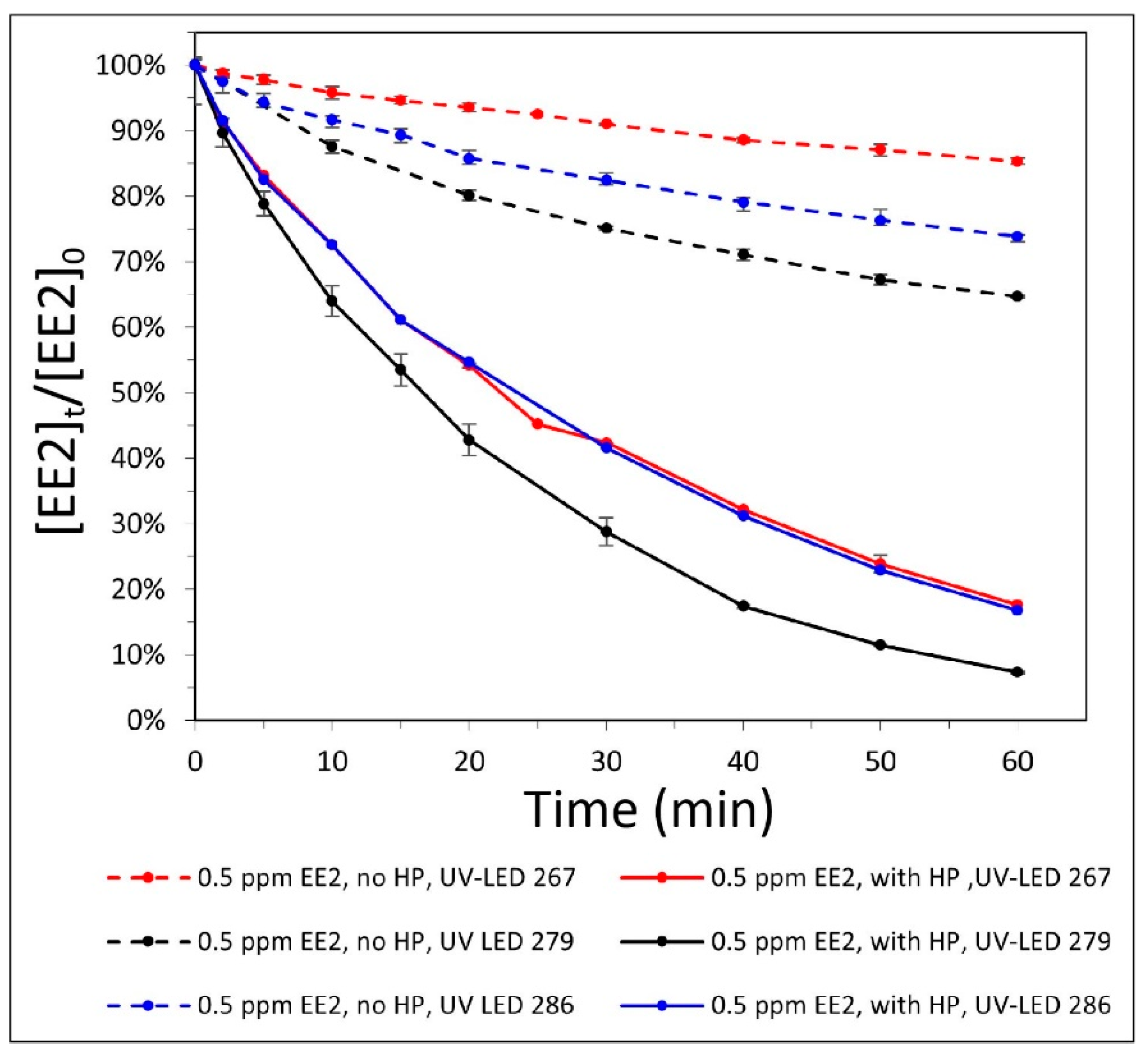
3.1. Photolysis—Wavelength Selection
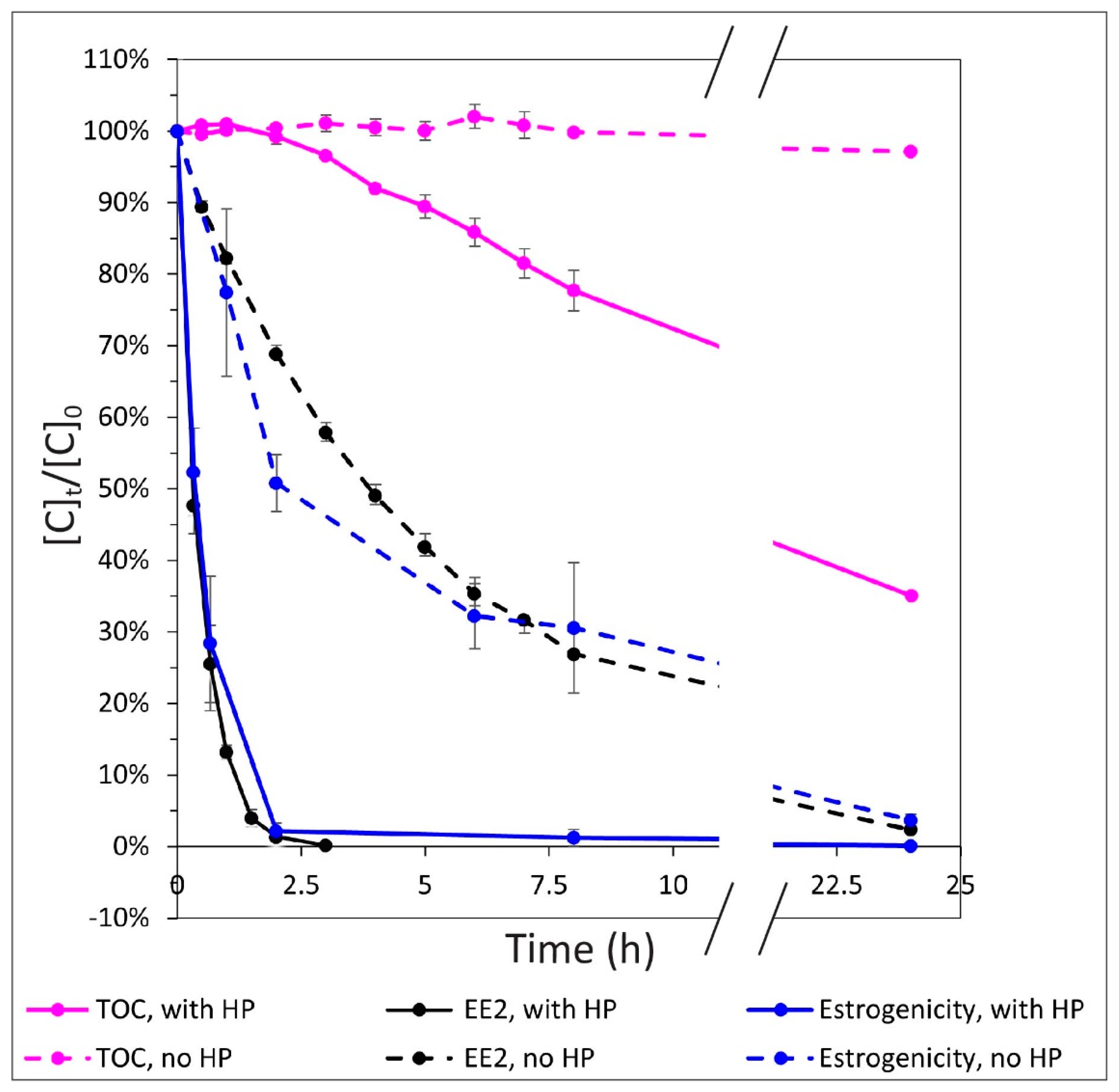
3.2. Relationship between EE2 Degradation, Estrogenicity, and Mineralization
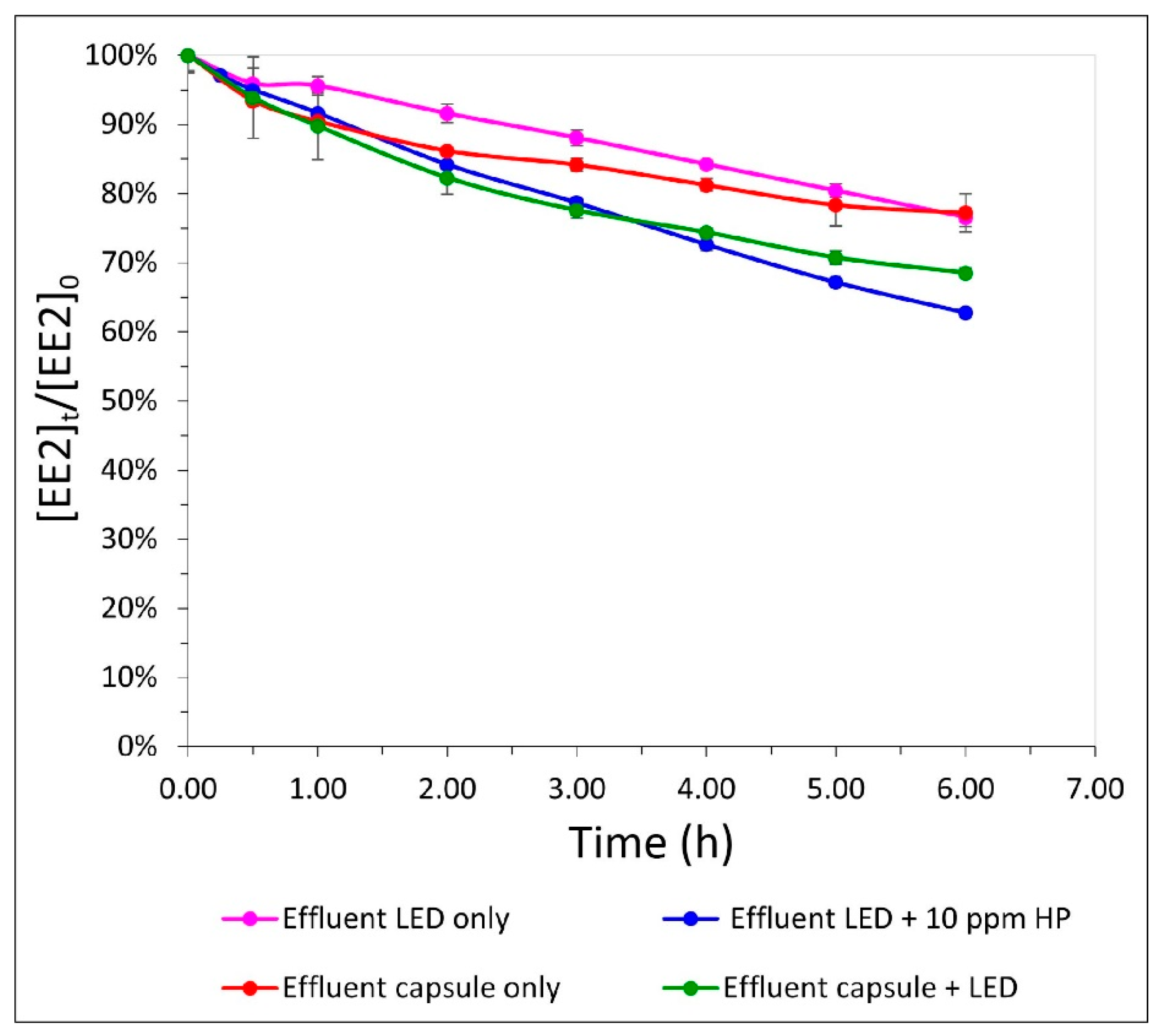
3.3. OBR as a Substitute for Physical–Chemical Treatment of Effluent
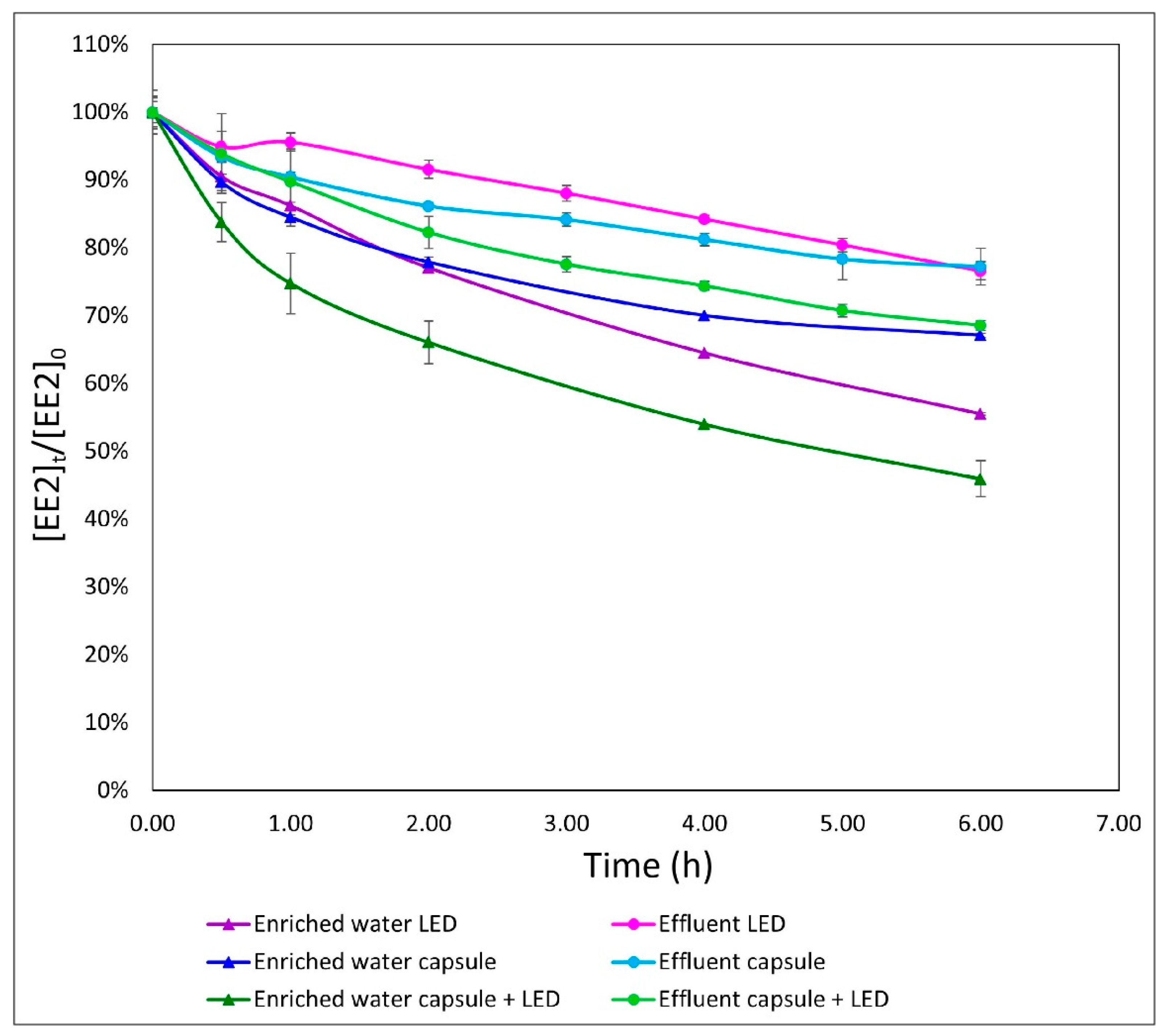
3.4. Comparison of Two Different Media in the Different Processes
4. Summary and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tang, Z.; Liu, Z.H.; Wang, H.; Dang, Z.; Liu, Y. A review of 17α-ethynylestradiol (EE2) in surface water across 32 countries: Sources, concentrations, and potential estrogenic effects. J. Environ. Manag. 2021, 292, 112804. [Google Scholar] [CrossRef] [PubMed]
- Aris, A.Z.; Shamsuddin, A.S.; Praveena, S.M. Occurrence of 17α-ethynylestradiol (EE2) in the environment and effect on exposed biota: A review. Environ. Int. 2014, 69, 104–119. [Google Scholar] [CrossRef] [PubMed]
- Menashe, O.; Raizner, Y.; Kuc, M.E.; Cohen-Yaniv, V.; Kaplan, A.; Mamane, H.; Avisar, D.; Kurzbaum, E. Biodegradation of the endocrine-disrupting chemical 17α-ethynylestradiol (EE2) by Rhodococcus zopfii and Pseudomonas putida encapsulated in small bioreactor platform (SBP) capsules. Appl. Sci. 2020, 10, 336. [Google Scholar] [CrossRef] [Green Version]
- Vaddadi, L.P.; Avisar, D.; Vadivel, V.K.; Menashe, O.; Kurzbaum, E.; Cohen-Yaniv, V.; Mamane, H. LP-UV-Nano MgO2 pretreated catalysis followed by small bioreactor platform capsules treatment for superior kinetic degradation performance of 17α-ethynylestradiol. Materials 2020, 13, 83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maniero, M.G.; Maia Bila, D.; Dezotti, M. Degradation and estrogenic activity removal of 17β-estradiol and 17α-ethinylestradiol by ozonation and O3/H2O2. Sci. Total Environ. 2008, 407, 105–115. [Google Scholar] [CrossRef]
- Kumar, V.V.; Avisar, D.; Prasanna, V.L.; Betzalel, Y.; Mamane, H. Rapid visible-light degradation of EE2 and its estrogenicity in hospital wastewater by crystalline promoted g-C3N4. J. Hazard. Mater. 2020, 398, 122880. [Google Scholar] [CrossRef]
- De Gusseme, B.; Pycke, B.; Hennebel, T.; Marcoen, A.; Vlaeminck, S.E.; Noppe, H.; Boon, N.; Verstraete, W. Biological removal of 17α-ethinylestradiol by a nitrifier enrichment culture in a membrane bioreactor. Water Res. 2009, 43, 2493–2503. [Google Scholar] [CrossRef]
- Ijpelaar, G.F.; Harmsen, D.J.H.; Beerendonk, E.F.; van Leerdam, R.C.; Metz, D.H.; Knol, A.H.; Fulmer, A.; Krijnen, S. Comparison of low pressure and medium pressure UV lamps for UV/H2O2 treatment of natural waters containing micro pollutants. Ozone Sci. Eng. 2010, 32, 329–337. [Google Scholar] [CrossRef]
- Donner, E.; Kosjek, T.; Qualmann, S.; Kusk, K.O.; Heath, E.; Revitt, D.M.; Ledin, A.; Andersen, H.R. Ecotoxicity of carbamazepine and its UV photolysis transformation products. Sci. Total Environ. 2013, 443, 870–876. [Google Scholar] [CrossRef] [Green Version]
- Gozlan, I.; Rotstein, A.; Avisar, D. Carboplatin-degradation products formed under deliberated and non-deliberated laboratory experiments: Structural elucidation. Water Air Soil Pollut. 2014, 225, 2196. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency (EPA). Ultraviolet Disinfection Guidance Manual for the Final Long Term 2 Enhanced Surface Water Treatment Rule; US EPA National Service Center for Environmental Publications: Washington, DC, USA, 2006.
- Hijnen, W.A.M.; Beerendonk, E.F.; Medema, G.J. Inactivation credit of UV radiation for viruses, bacteria and protozoan (oo)cysts in water: A review. Water Res. 2006, 40, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Zhou, H.; Cicek, N. Treatment of organic micropollutants in water and wastewater by UV-based processes: A literature review. Crit. Rev. Environ. Sci. Technol. 2014, 44, 1443–1476. [Google Scholar] [CrossRef]
- Pereira, V.J.; Linden, K.G.; Weinberg, H.S. Evaluation of UV irradiation for photolytic and oxidative degradation of pharmaceutical compounds in water. Water Res. 2007, 41, 4413–4423. [Google Scholar] [CrossRef] [PubMed]
- United Nations Environment Programme. Minamata Convention on Mercury—Text and Annexes; UN: New York, NY, USA, 2013; Available online: http://www.mercuryconvention.org/Portals/11/documents/Booklets/COP3-version/Minamata-Convention-booklet-Sep2019-EN.pdf (accessed on 1 August 2021).
- Scheer, R.; Moss, D. EarthTalk: The Dark Side of LED Lightbulbs. Sci. Am. Available online: https://www.scientificamerican.com/article/led-lightbulb-concerns/ (accessed on 18 February 2021).
- Matafonova, G.; Batoev, V. Recent advances in application of UV light-emitting diodes for degrading organic pollutants in water through advanced oxidation processes: A review. Water Res. 2018, 132, 177–189. [Google Scholar] [CrossRef]
- Sun, W.; Shatalov, M.; Deng, J.; Hu, X.; Yang, J.; Lunev, A.; Bilenko, Y.; Shur, M.; Gaska, R. Efficiency droop in 245–247 nm AlGaN light-emitting diodes with continuous wave 2 mW output power. Appl. Phys. Lett. 2010, 96, 245–248. [Google Scholar] [CrossRef]
- Fujioka, T.; Kodamatani, H.; Yoshikawa, T.; Inoue, D.; Ikehata, K. Assessment of 265-nm UV-LED for direct photolysis and advanced oxidation of N-nitrosamines and 1,4-dioxane. Environ. Technol. Innov. 2020, 20, 101147. [Google Scholar] [CrossRef]
- Zhou, S.; Li, L.; Wu, Y.; Zhu, S.; Zhu, N.; Bu, L.; Dionysiou, D.D. UV365 induced elimination of contaminants of emerging concern in the presence of residual nitrite: Roles of reactive nitrogen species. Water Res. 2020, 178, 115829. [Google Scholar] [CrossRef]
- Silveira, J.E.; Paz, W.S.; Garcia-Muñoz, P.; Zazo, J.A.; Casas, J.A. UV-LED/ilmenite/persulfate for azo dye mineralization: The role of sulfate in the catalyst deactivation. Appl. Catal. B Environ. 2017, 219, 314–321. [Google Scholar] [CrossRef]
- Silveira, J.E.; Claro, E.M.T.; Paz, W.S.; Oliveira, A.S.; Zazo, J.A.; Casas, J.A. Optimization of Disperse Blue 3 mineralization by UV-LED/FeTiO3 activated persulfate using response surface methodology. J. Taiwan Inst. Chem. Eng. 2018, 85, 66–73. [Google Scholar] [CrossRef] [Green Version]
- Beck, S.E.; Ryu, H.; Boczek, L.A.; Cashdollar, J.L.; Jeanis, K.M.; Rosenblum, J.S.; Lawal, O.R.; Linden, K.G. Evaluating UV-C LED disinfection performance and investigating potential dual-wavelength synergy. Water Res. 2017, 109, 207–216. [Google Scholar] [CrossRef]
- Kurzbaum, E.; Raizner, Y.; Kuc, M.E.; Menashe, O. Small bioreactor platform capsules provide persistent digestive biomass for continuous bioreactors operated under short hydraulic retention times. J. Water Process Eng. 2020, 37, 101516. [Google Scholar] [CrossRef]
- Kurzbaum, E.; Raizner, Y.; Kuc, M.E.; Kulikov, A.; Hakimi, B.; Kruh, L.I.; Menashe, O. Phenol biodegradation by bacterial cultures encapsulated in 3D microfiltration-membrane capsules. Environ. Technol. 2020, 41, 2875–2883. [Google Scholar] [CrossRef] [PubMed]
- Azaizeh, H.; Kurzbaum, E.; Said, O.; Jaradat, H.; Menashe, O. The potential of autochthonous microbial culture encapsulation in a confined environment for phenol biodegradation. Environ. Sci. Pollut. Res. 2015, 22, 15179–15187. [Google Scholar] [CrossRef]
- Menashe, O.; Kurzbaum, E. A novel bioaugmentation treatment approach using a confined microbial environment: A case study in a Membrane Bioreactor wastewater treatment plant. Environ. Technol. 2016, 37, 1582–1590. [Google Scholar] [CrossRef]
- Menashe, O. Microorganism Comprising Particles and Uses of Same. US Patent Appl. PCT/IL2010/000256, 2010. [Google Scholar]
- Menashe, O.; Kurzbaum, E. Small-bioreactor platform technology as a municipal wastewater additive treatment. Water Sci. Technol. 2014, 69, 504–510. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, X.; Chen, L.; Rittmann, B.E. Integrated photocatalytic-biological reactor for accelerated 2,4,6-trichlorophenol degradation and mineralization. Biodegradation 2012, 23, 189–198. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, L.; Rittmann, B.E. Integrated photocatalytic-biological reactor for accelerated phenol mineralization. Appl. Microbiol. Biotechnol. 2010, 86, 1977–1985. [Google Scholar] [CrossRef]
- Zhou, D.; Xu, Z.; Dong, S.; Huo, M.; Dong, S.; Tian, X.; Cui, B.; Xiong, H.; Li, T.; Ma, D. Intimate coupling of photocatalysis and biodegradation for degrading phenol using different light types: Visible light vs UV light. Environ. Sci. Technol. 2015, 49, 7776–7783. [Google Scholar] [CrossRef]
- Yu, M.; Wang, J.; Tang, L.; Feng, C.; Liu, H.; Zhang, H.; Peng, B.; Chen, Z.; Xie, Q. Intimate coupling of photocatalysis and biodegradation for wastewater treatment: Mechanisms, recent advances and environmental applications. Water Res. 2020, 175, 115673. [Google Scholar] [CrossRef]
- Kurzbaum, E.; Raizner, Y.; Cohen, O.; Suckeveriene, R.Y.; Kulikov, A.; Hakimi, B.; Iasur Kruh, L.; Armon, R.; Farber, Y.; Menashe, O. Encapsulated Pseudomonas putida for phenol biodegradation: Use of a structural membrane for construction of a well-organized confined particle. Water Res. 2017, 121, 37–45. [Google Scholar] [CrossRef]
- Fradkin, O. The Bacterial Filling Procedure. Available online: https://www.youtube.com/watch?v=yEMjx2FZT5Y (accessed on 16 December 2020).
- Routledge, E.J.; Sumpter, J.P. Estrogenic activity of surfactants and some of their degradation products assessed using a recombinant yeast screen. Environ. Toxicol. Chem. 1996, 15, 241–248. [Google Scholar] [CrossRef]
- Chen, P.J.; Linden, K.G.; Hinton, D.E.; Kashiwada, S.; Rosenfeldt, E.J.; Kullman, S.W. Biological assessment of bisphenol A degradation in water following direct photolysis and UV advanced oxidation. Chemosphere 2006, 65, 1094–1102. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fradkin, O.; Mamane, H.; Kaplan, A.; Menashe, O.; Kurzbaum, E.; Betzalel, Y.; Avisar, D. UV-LED Combined with Small Bioreactor Platform (SBP) for Degradation of 17α-Ethynylestradiol (EE2) at Very Short Hydraulic Retention Time. Materials 2021, 14, 5960. https://doi.org/10.3390/ma14205960
Fradkin O, Mamane H, Kaplan A, Menashe O, Kurzbaum E, Betzalel Y, Avisar D. UV-LED Combined with Small Bioreactor Platform (SBP) for Degradation of 17α-Ethynylestradiol (EE2) at Very Short Hydraulic Retention Time. Materials. 2021; 14(20):5960. https://doi.org/10.3390/ma14205960
Chicago/Turabian StyleFradkin, Oran, Hadas Mamane, Aviv Kaplan, Ofir Menashe, Eyal Kurzbaum, Yifaat Betzalel, and Dror Avisar. 2021. "UV-LED Combined with Small Bioreactor Platform (SBP) for Degradation of 17α-Ethynylestradiol (EE2) at Very Short Hydraulic Retention Time" Materials 14, no. 20: 5960. https://doi.org/10.3390/ma14205960