Photodegradation Process of Organic Dyes in the Presence of a Manganese-Doped Zinc Sulfide Nanowire Photocatalyst
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of ZnS and ZnS:Mn Nanowires in MW
2.3. Preparation of ZnS Nanocrystals in Conventional Heating
2.4. Characterization of Nanowires
2.5. Photocatalytic Activity Test
3. Results and Discussion
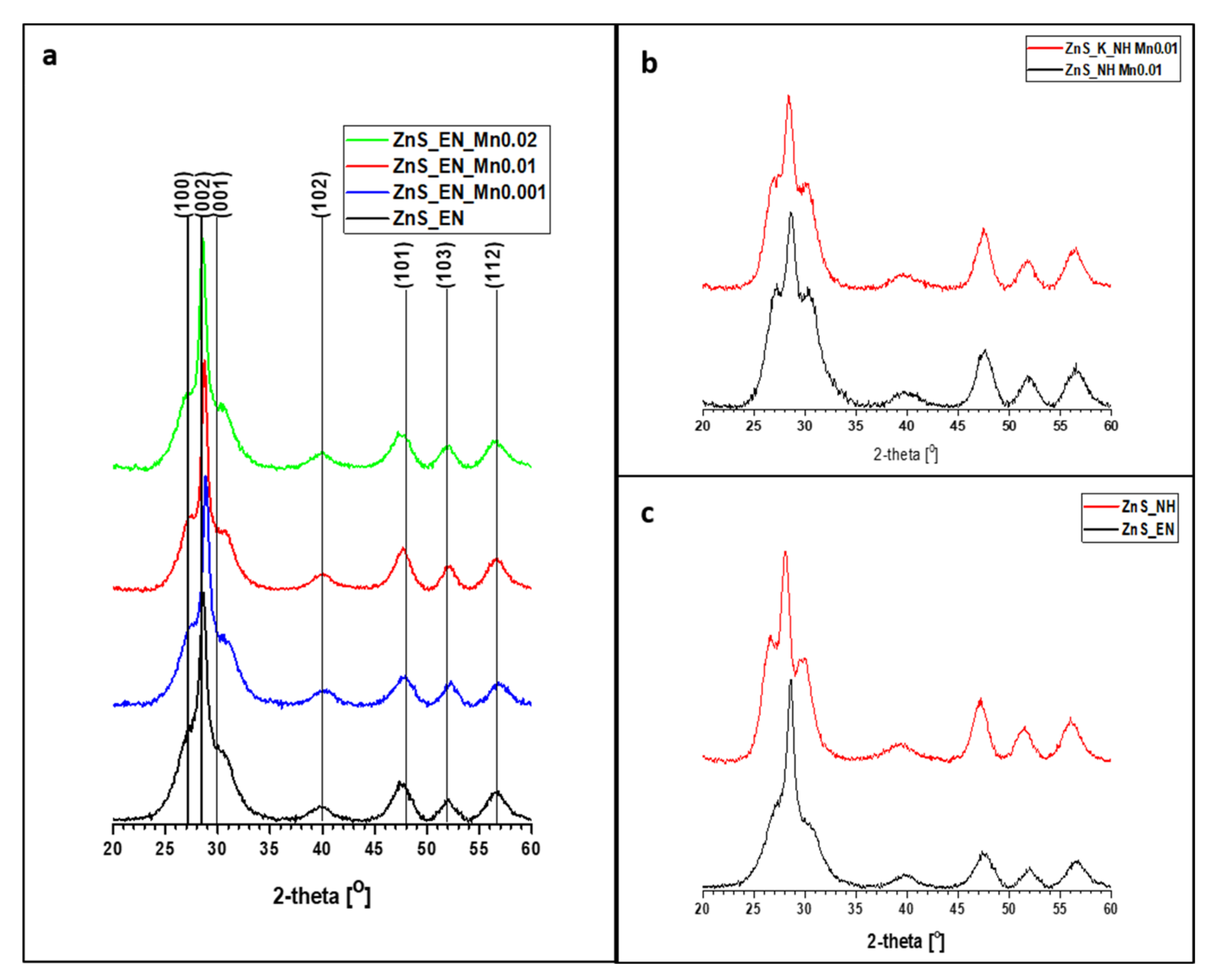
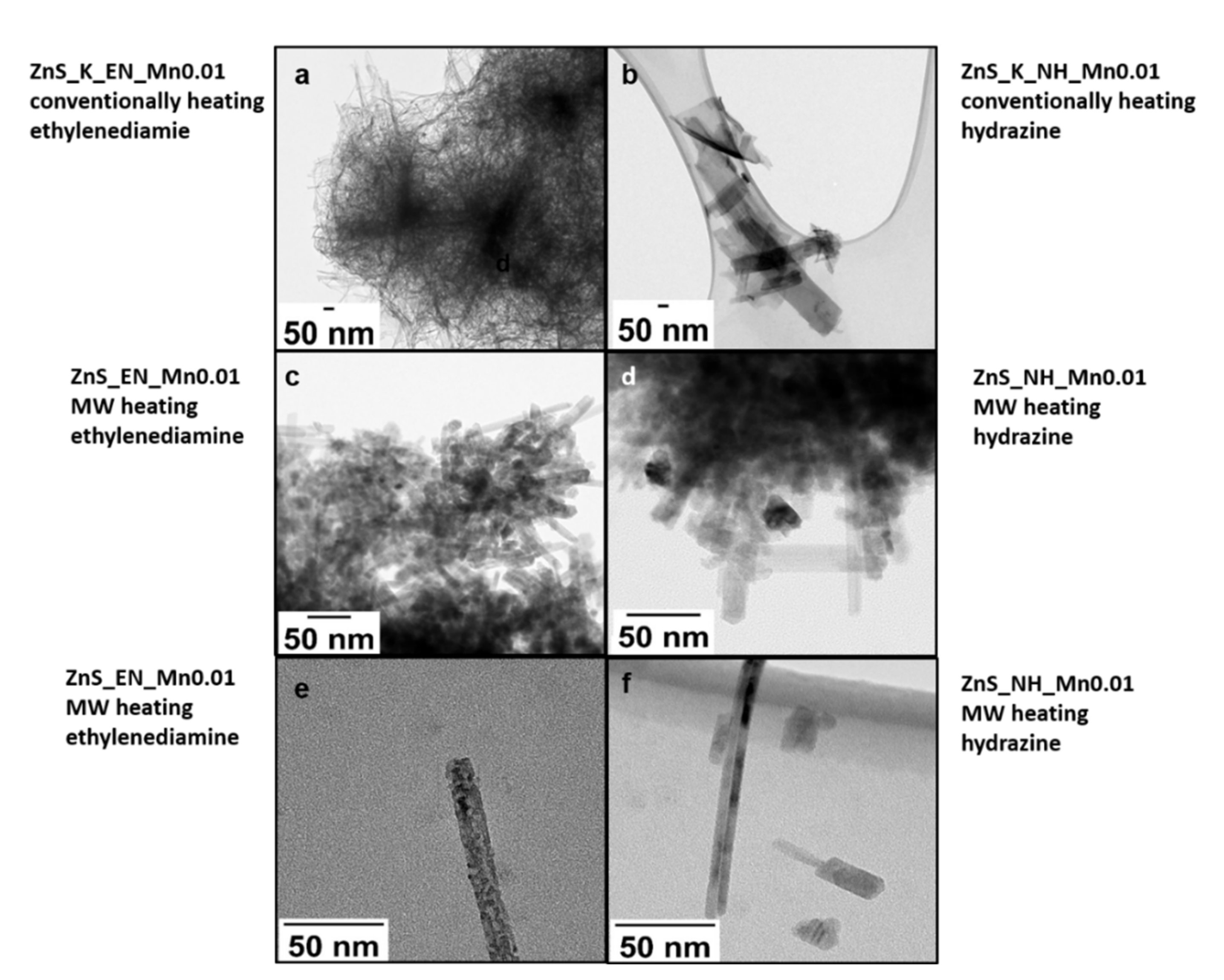
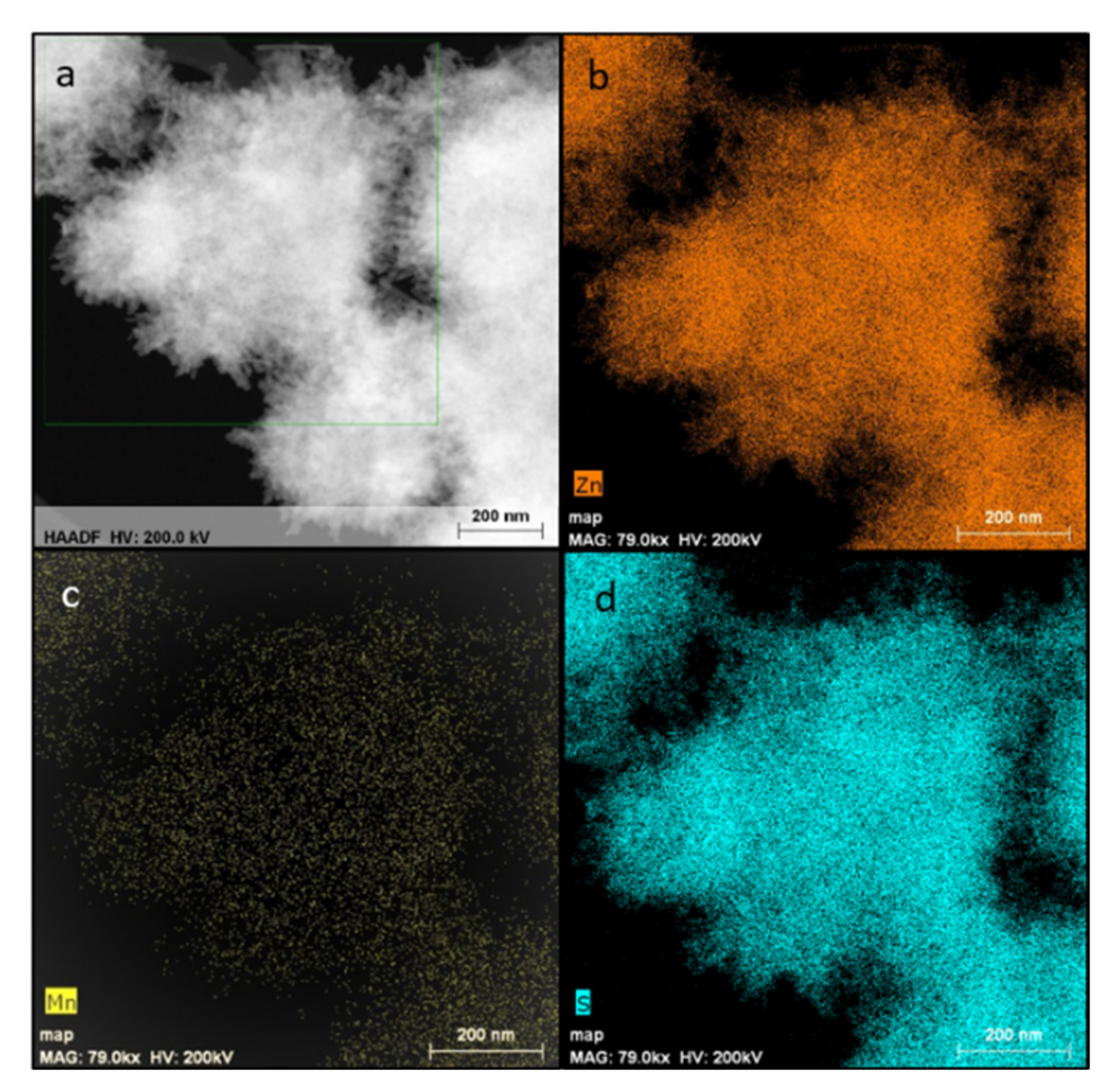
3.1. Characterization of the ZnS and ZnS:Mn Nanowires
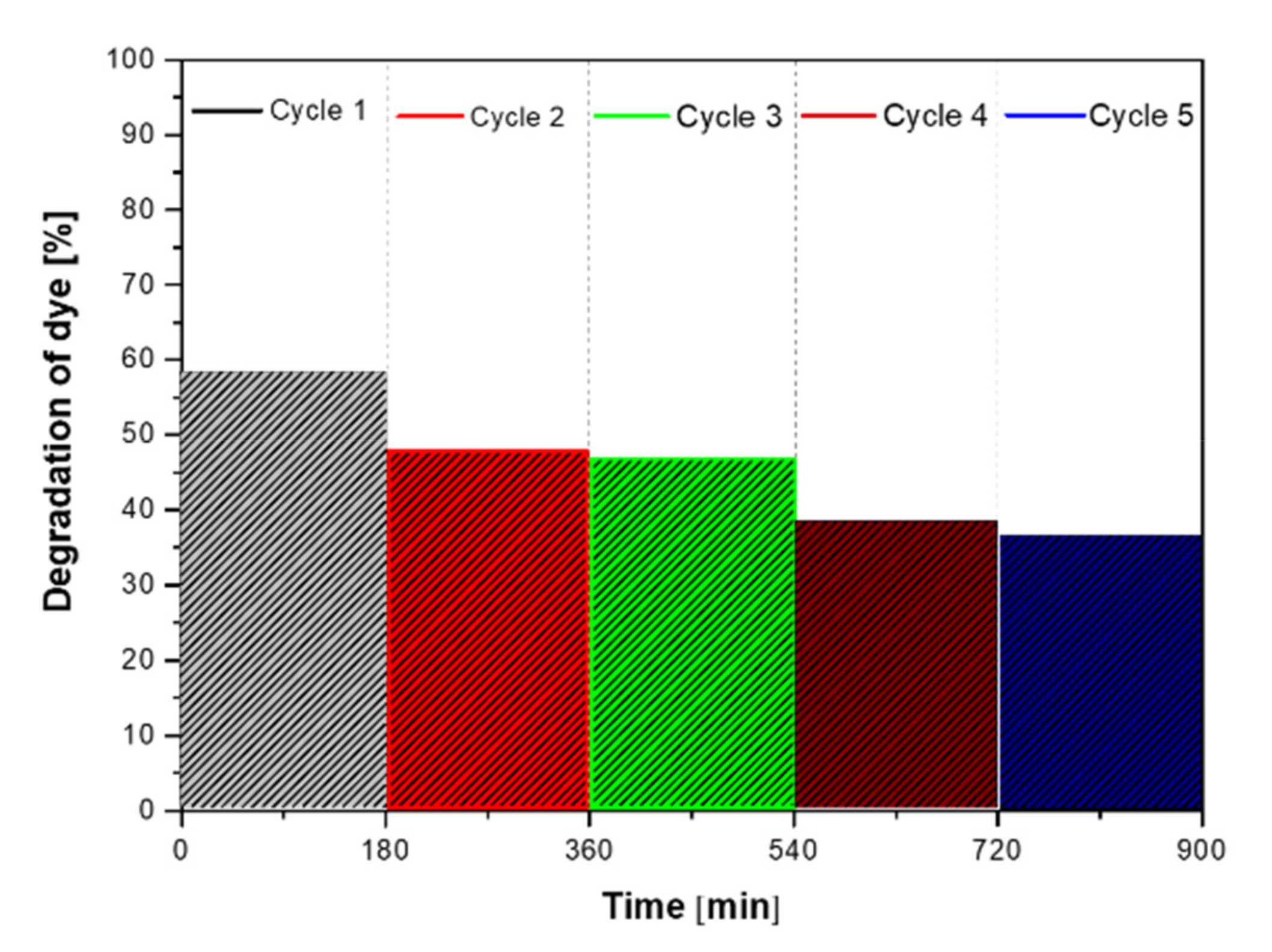
3.2. Test of Photocatalytic Activity
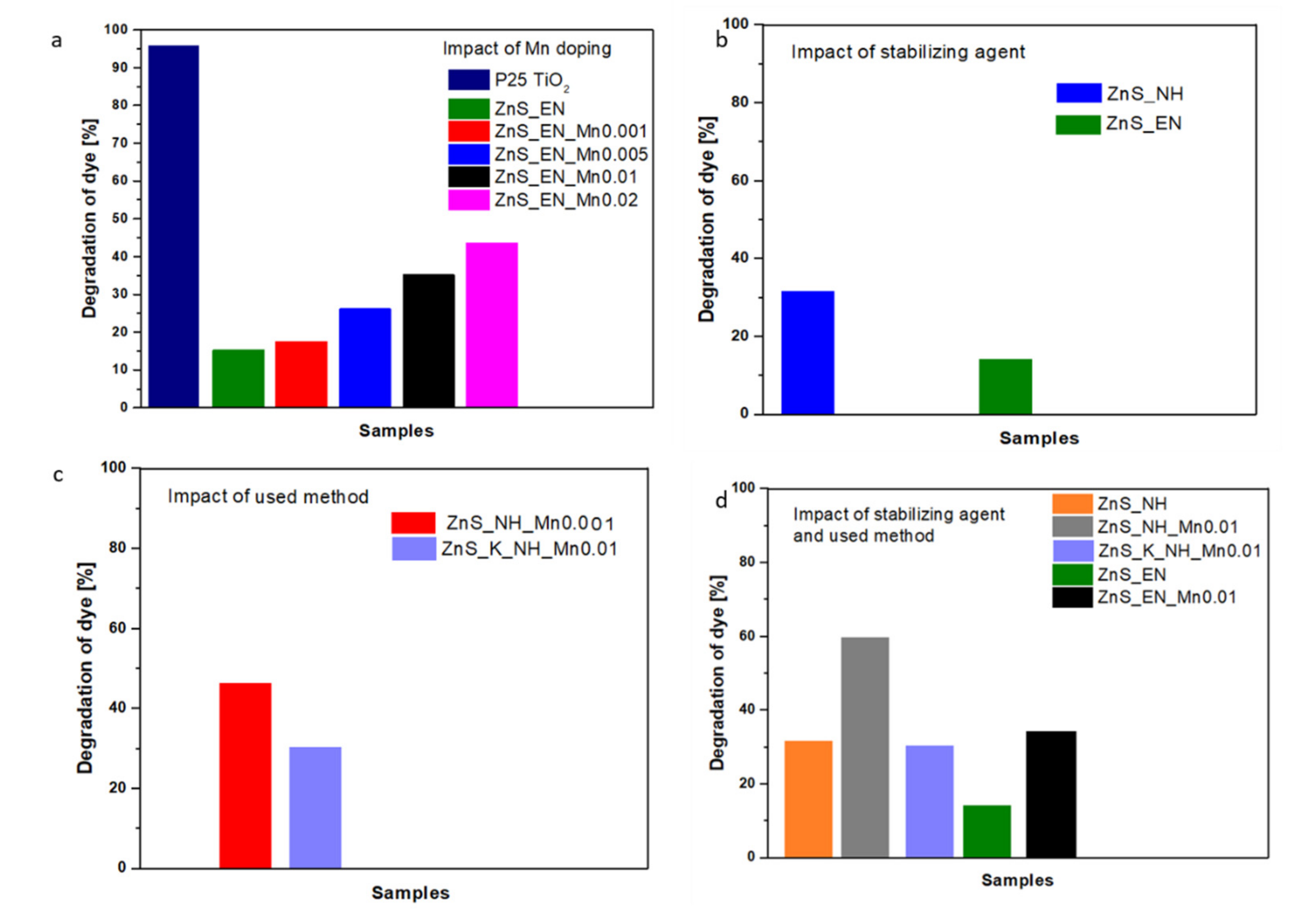
3.2.1. Effect of Mn-Doping on the Photocatalytic Activity
3.2.2. Effect of the Stabilizing Agent and the Used Synthetic Method on the Photocatalytic Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, S.-Y.; Regulacio, M.D.; Han, M.-Y. Self-assembly of colloidal one-dimensional nanocrystals. Chem. Soc. Rev. 2014, 43, 2301–2323. [Google Scholar] [CrossRef]
- Efros, A.L.; Brus, L.E. Nanocrystal Quantum Dots: From Discovery to Modern Development. ACS Nano 2021, 15, 6192–6210. [Google Scholar] [CrossRef]
- Matras-Postołek, K.; Żaba, A.; Nowak, E.; Dąbczyński, P.; Rysz, J.; Sanetra, J. Formation and characterization of one-dimensional ZnS nanowires for ZnS/P3HT hybrid polymer solar cells with improved efficiency. Appl. Surf. Sci. 2018, 451, 180–190. [Google Scholar] [CrossRef]
- Park, S.; An, S.; Mun, Y.; Lee, C. UV-activated gas sensing properties of ZnS nanorods functionalized with Pd. Curr. Appl. Phys. 2014, 14, S57–S62. [Google Scholar] [CrossRef]
- Fang, X.; Bando, Y.; Gautam, U.K.; Zhai, T.; Zeng, H.; Xu, X.; Liao, M.; Golberg, D. ZnO and ZnS Nanostructures: Ultraviolet-Light Emitters, Lasers, and Sensors. Crit. Rev. Solid State Mater. Sci. 2009, 34, 190–223. [Google Scholar] [CrossRef]
- Jafari, T.; Moharreri, E.; Amin, A.S.; Miao, R.; Song, W.; Suib, S.L. Photocatalytic Water Splitting—The Untamed Dream: A Review of Recent Advances. Molecules 2016, 21, 900. [Google Scholar] [CrossRef] [PubMed]
- Labiadh, H.; Ben Chaabane, T.; Balan, L.; Becheik, N.; Corbel, S.; Medjahdi, G.; Schneider, R. Preparation of Cu-doped ZnS QDs/TiO2 nanocomposites with high photocatalytic activity. Appl. Catal. B: Environ. 2014, 144, 29–35. [Google Scholar] [CrossRef]
- Kaur, J.; Sharma, M.; Pandey, O. Structural and optical studies of undoped and copper doped zinc sulphide nanoparticles for photocatalytic application. Superlattices Microstruct. 2015, 77, 35–53. [Google Scholar] [CrossRef]
- Chauhan, R.; Kumar, A.; Chaudhary, R.P. Photocatalytic degradation of methylene blue with Cu doped ZnS nanoparticles. J. Lumin. 2014, 145, 6–12. [Google Scholar] [CrossRef]
- Pouretedal, H.R.; Norozi, A.; Keshavarz, M.H.; Semnani, A. Nanoparticles of zinc sulfide doped with manganese, nickel and copper as nanophotocatalyst in the degradation of organic dyes. J. Hazard. Mater. 2009, 162, 674–681. [Google Scholar] [CrossRef] [PubMed]
- Jothibas, M.; Manoharan, C.; Jeyakumar, S.J.; Praveen, P.; Punithavathy, I.K.; Richard, J.P. Synthesis and enhanced photocatalytic property of Ni doped ZnS nanoparticles. Sol. Energy 2018, 159, 434–443. [Google Scholar] [CrossRef]
- Wang, C.; Ao, Y.; Wang, P.; Zhang, S.; Qian, J.; Hou, J. A simple method for large-scale preparation of ZnS nanoribbon film and its photocatalytic activity for dye degradation. Appl. Surf. Sci. 2010, 256, 4125–4128. [Google Scholar] [CrossRef]
- Sharma, M.; Jain, T.; Singh, S.; Pandey, O. Photocatalytic degradation of organic dyes under UV–Visible light using capped ZnS nanoparticles. Sol. Energy 2012, 86, 626–633. [Google Scholar] [CrossRef]
- Fang, Z.; Weng, S.; Ye, X.; Feng, W.; Zheng, Z.; Lu, M.; Lin, S.; Fu, X.; Liu, P. Defect Engineering and Phase Junction Ar-chitecture of Wide-Bandgap ZnS for Conflicting Visible Light Activity in Photocatalytic H2 Evolution. ACS Appl. Mater. Interfaces 2015, 7, 25–13915. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Hu, C.; Liu, H.; Xu, J.; Wan, B.; Wang, X. ZnS nanoparticles self-assembled from ultrafine particles and their highly photocatalytic activity. Phys. E Low-Dimens. Syst. Nanostruct. 2011, 43, 1071–1075. [Google Scholar] [CrossRef]
- Samanta, D.; Chanu, T.I.; Chatterjee, S. Citrus limetta juice as capping agent in hydrothermal synthesis of ZnS nanosphere for photocatalytic activity. Mater. Res. Bull. 2017, 88, 85–90. [Google Scholar] [CrossRef]
- Muruganandham, M.; Amutha, R.; Repo, E.; Sillanpää, M.; Kusumoto, Y.; Abdulla-Al-Mamun, M. Controlled mesoporous self-assembly of ZnS microsphere for photocatalytic degradation of Methyl Orange dye. J. Photochem. Photobiol. A Chem. 2010, 216, 2–3. [Google Scholar] [CrossRef]
- Yin, L.; Zhang, D.; Ma, J.; Kong, X.; Huang, J.; Zhang, H.; Liu, C. Facile synthesis and characterization of ZnS nano/microcrystallites with enhanced photocatalytic activity. Powder Technol. 2016, 301, 1085–1091. [Google Scholar] [CrossRef]
- Chen, F.; Cao, Y.; Jia, D. Facile synthesis of ZnS nanoparticles and their excellent photocatalytic performance. Ceram. Int. 2015, 41, 6645–6652. [Google Scholar] [CrossRef]
- Wu, X.; Li, K.; Wang, H. Facile synthesis of ZnS nanostructured spheres and their photocatalytic properties. J. Alloys Compd. 2009, 487, 537–544. [Google Scholar] [CrossRef]
- Yin, L.; Zhang, D.; Wang, D.; Kong, X.; Huang, J.; Wang, F.; Wu, Y. Size dependent photocatalytic activity of ZnS nanostructures prepared by a facile precipitation method. Mater. Sci. Eng. B 2016, 208, 15–21. [Google Scholar] [CrossRef]
- Dong, F.; Guo, Y.; Zhang, J.; Li, Y.; Yang, L.; Fang, Q.; Fang, H.; Jiang, K. Size-controllable hydrothermal synthesis of ZnS nanospheres and the application in photocatalytic degradation of organic dyes. Mater. Lett. 2013, 97, 59–63. [Google Scholar] [CrossRef]
- Kaur, M.; Nagaraja, C.M. Template-free synthesis of ZnS nanocrystals with a new sulfur source and their photocatalytic study. Mater. Lett. 2015, 154, 90–93. [Google Scholar] [CrossRef]
- Ma, Q.; Wang, Y.; Kong, J.; Jia, H. Tunable synthesis, characterization and photocatalytic properties of various ZnS nanostructures. Ceram. Int. 2016, 42, 2854–2860. [Google Scholar] [CrossRef]
- Zhu, Y.-J.; Chen, F. Microwave-Assisted Preparation of Inorganic Nanostructures in Liquid Phase. Chem. Rev. 2014, 114, 6462–6555. [Google Scholar] [CrossRef] [PubMed]
- Sakthivel, P.; Muthukumaran, S.; Ashokkumar, M. Structural, band gap and photoluminescence behavior of Mn-doped ZnS quantum dots annealed under Ar atmosphere. J. Mater. Sci. Mater. Electron. 2015, 26, 1533–1542. [Google Scholar] [CrossRef]
- Bol, A.A.; Meijerink, A. Luminescence quantum efficiency of nanocrystalline ZnS:Mn2+. 1. Surface passivation and Mn2+ concentration. J. Phys. Chem. B 2001, 105, 10197–10202. [Google Scholar] [CrossRef]
- Peng, W.; Qu, S.; Cong, G.; Zhang, X.; Wang, Z. Optical and magnetic properties of ZnS nanoparticles doped with Mn2+. J. Cryst. Growth 2005, 282, 179–185. [Google Scholar] [CrossRef]
- Erwin, S.C.; Zu, L.; Haftel, M.I.; Efros, A.L.; Kennedy, T.A.; Norris, D.J. Doping semicon-ductor nanocrystals. Nature 2005, 436, 91–94. [Google Scholar] [CrossRef]
- Matras-Postołek, K.; Sovinska, S.; Węgrzynowicz, A. Synthesis and characterization of ZnSe and ZnSe:Mn nanosheets and microflowers with high photoactive properties by microwave-assisted method. Chem. Eng. Process. Process. Intensif. 2019, 135, 204–216. [Google Scholar] [CrossRef]
- Deng, Z.-X.; Wang, C.; Sun, X.; Li, Y.-D. Structure-Directing Coordination Template Effect of Ethylenediamine in Formations of ZnS and ZnSe Nanocrystallites via Solvothermal Route. Inorg. Chem. 2002, 41, 869–873. [Google Scholar] [CrossRef]
- Zhang, J.; Li, X.; Liu, D.; Wang, S.; Yan, J.; Lu, M.; Xie, X.; Huang, L.; Huang, W. Stirring revealed new functions of ethylenediamine and hydrazine in the morphology control of copper nanowires. Nanoscale 2019, 11, 11902–11909. [Google Scholar] [CrossRef]
- Sangiorgi, N.; Aversa, L.; Tatti, R.; Verucchi, R.; Sanson, A. Spectrophotometric method for optical band gap and electronic transitions determination of semiconductor materials. Opt. Mater. 2017, 64, 18–25. [Google Scholar] [CrossRef]
- Katan, C.; Nicolas Mercier, N.; Even, J. Quantum and Dielectric Confinement Effects in Lower-Dimensional Hybrid Per-ovskite Semiconductors. Chem Rev. 2019, 13, 3140–3192. [Google Scholar] [CrossRef]
- Sangpour, P.; Hashemi, F.; Moshfegh, A.Z. Photoenhanced Degradation of Methylene Blue on Cosputtered M:TiO2 (M ) Au, Ag, Cu) Nanocomposite Systems: A Comparative Study. J. Phys. Chem. C 2010, 114, 13955–13961. [Google Scholar] [CrossRef]
- Ullah, R.; Dutta, J. Photocatalytic degradation of organic dyes with manganese-doped ZnO nanoparticles. J. Hazard. Mater. 2008, 156, 194–200. [Google Scholar] [CrossRef]
- Iqbal, M.; Thebo, A.A.; Shah, A.H.; Iqbal, A.; Thebo, K.H.; Phulpoto, S.; Mohsin, M.A. Influence of Mn-doping on the photocatalytic and solar cell efficiency of CuO nanowires. Inorg. Chem. Commun. 2017, 76, 71–76. [Google Scholar] [CrossRef]
- Ollis, D.F. Kinetics of Photocatalyzed Reactions: Five Lessons Learned. Front. Chem. 2018, 6, 378. [Google Scholar] [CrossRef] [Green Version]
- Guo, H.; Ke, Y.; Wang, D.; Lin, K.; Shen, R.; Chen, J.; Weng, W. Efficient adsorption and photocatalytic degradation of Congo red onto hydrothermally synthesized NiS nanoparticles. J. Nanoparticle Res. 2013, 15, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Hajra, P.; Shyamal, S.; Mandal, H.; Sariket, D.; Maity, A.; Kundu, S.; Bhattacharya, C. Synthesis of oxygen deficient bismuth oxide photocatalyst for improved photoelectrochemical applications. Electrochim. Acta 2019, 299, 357–365. [Google Scholar] [CrossRef]
- Ngamsinlapasathian, S.; Sreethawong, T.; Suzuki, Y.; Yoshikawa, S. Single- and double-layered mesoporous TiO2/P25 TiO2 electrode for dye-sensitized solar cell. Sol. Energy Mater. Sol. Cells 2005, 86, 269–282. [Google Scholar] [CrossRef]
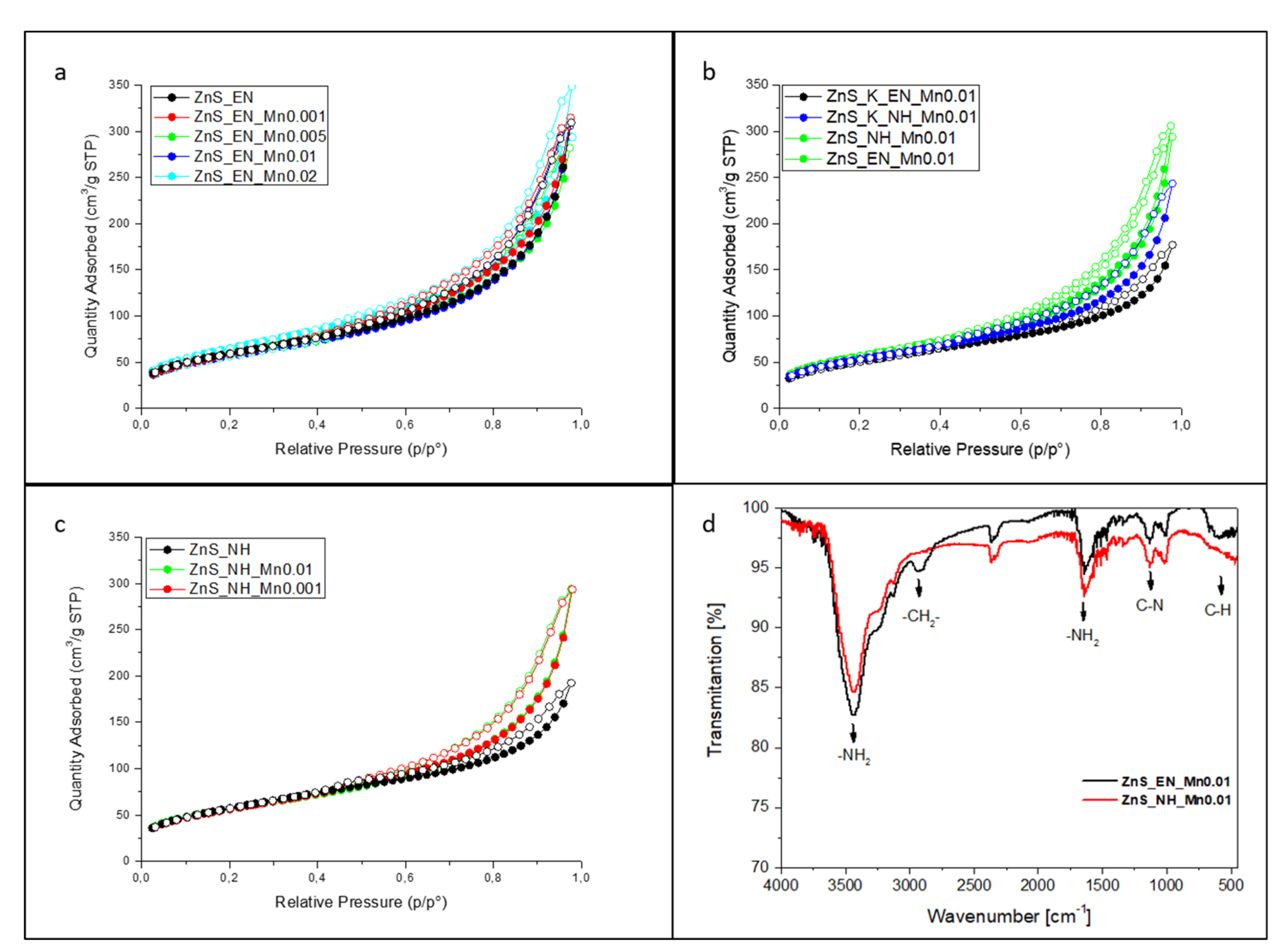
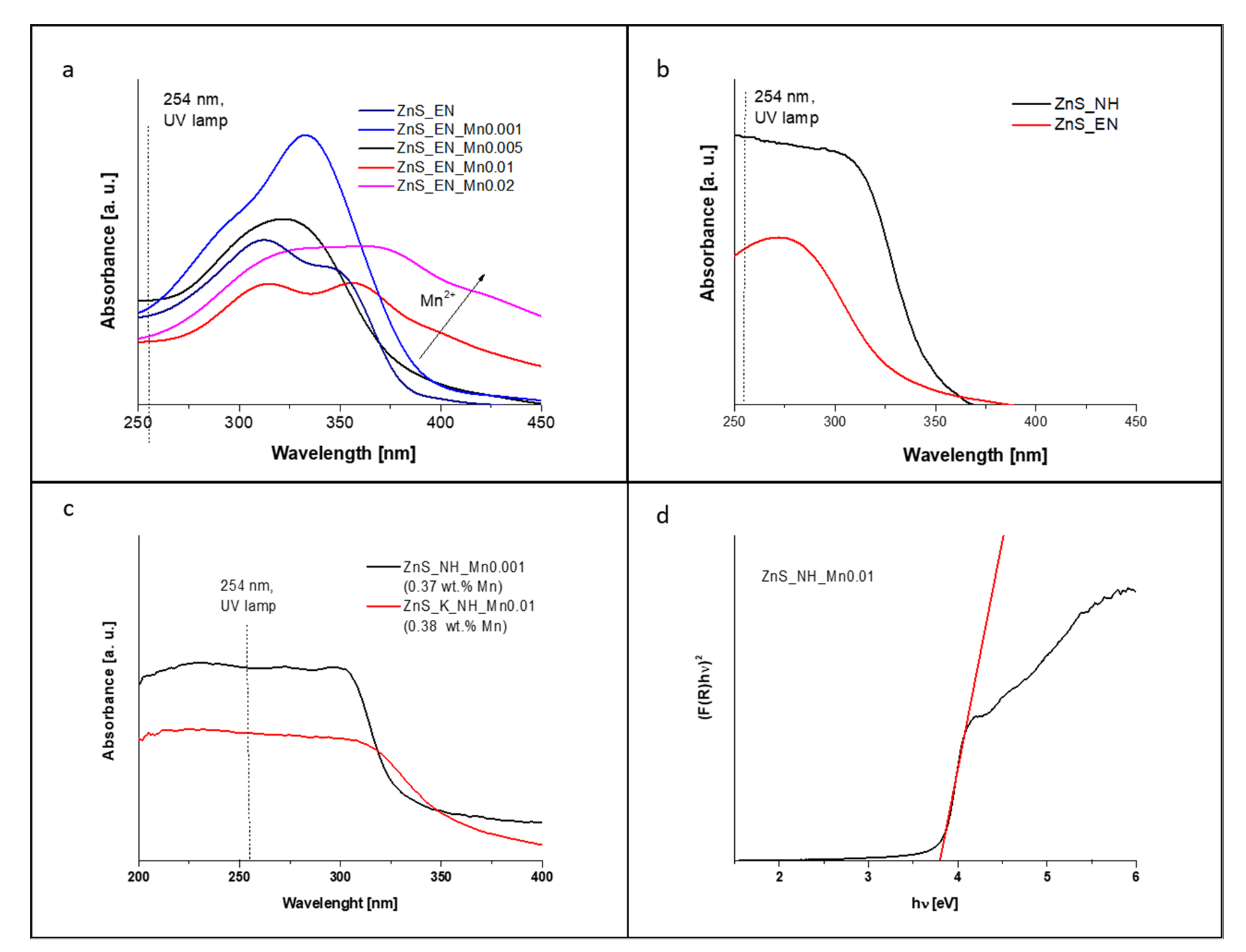
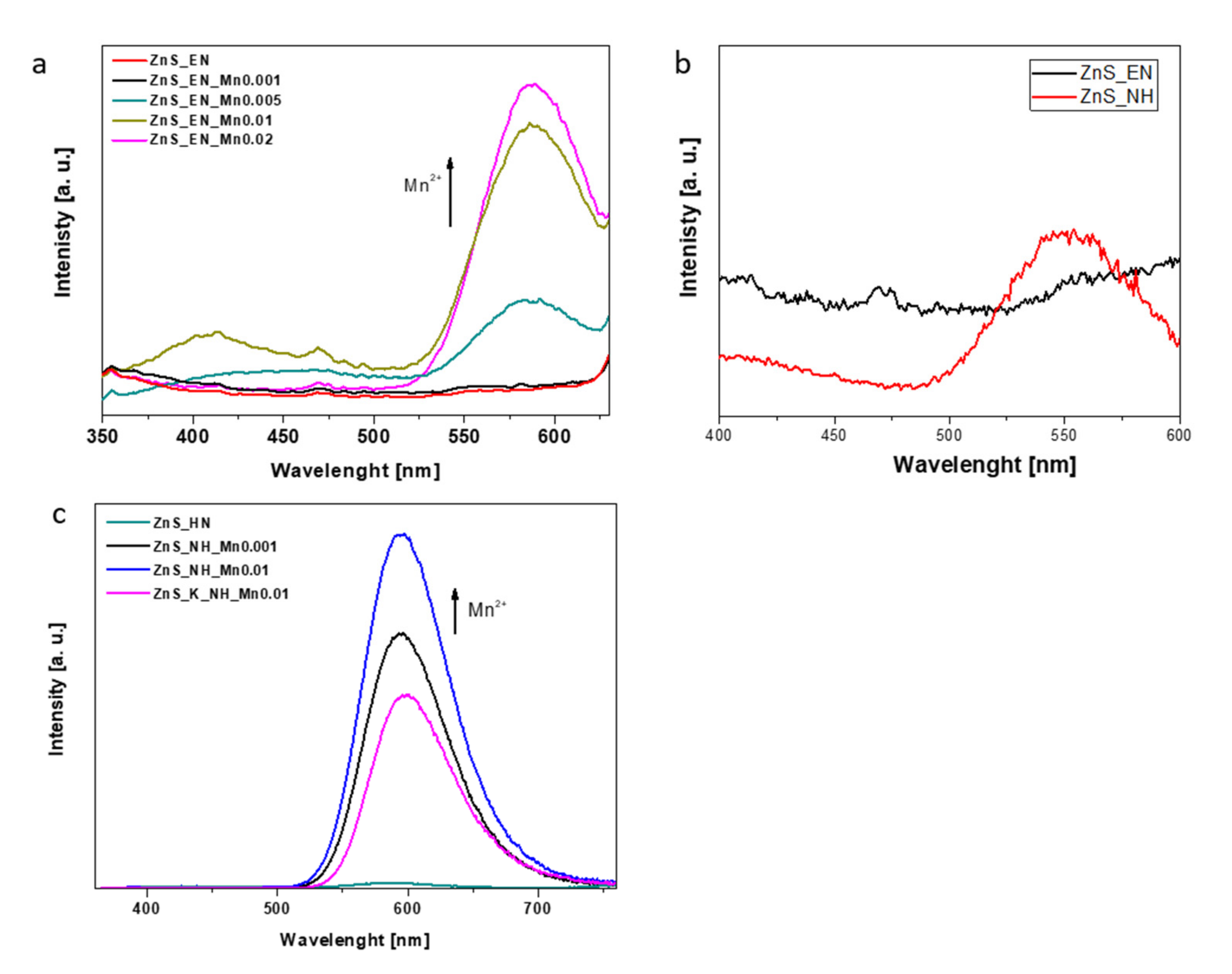
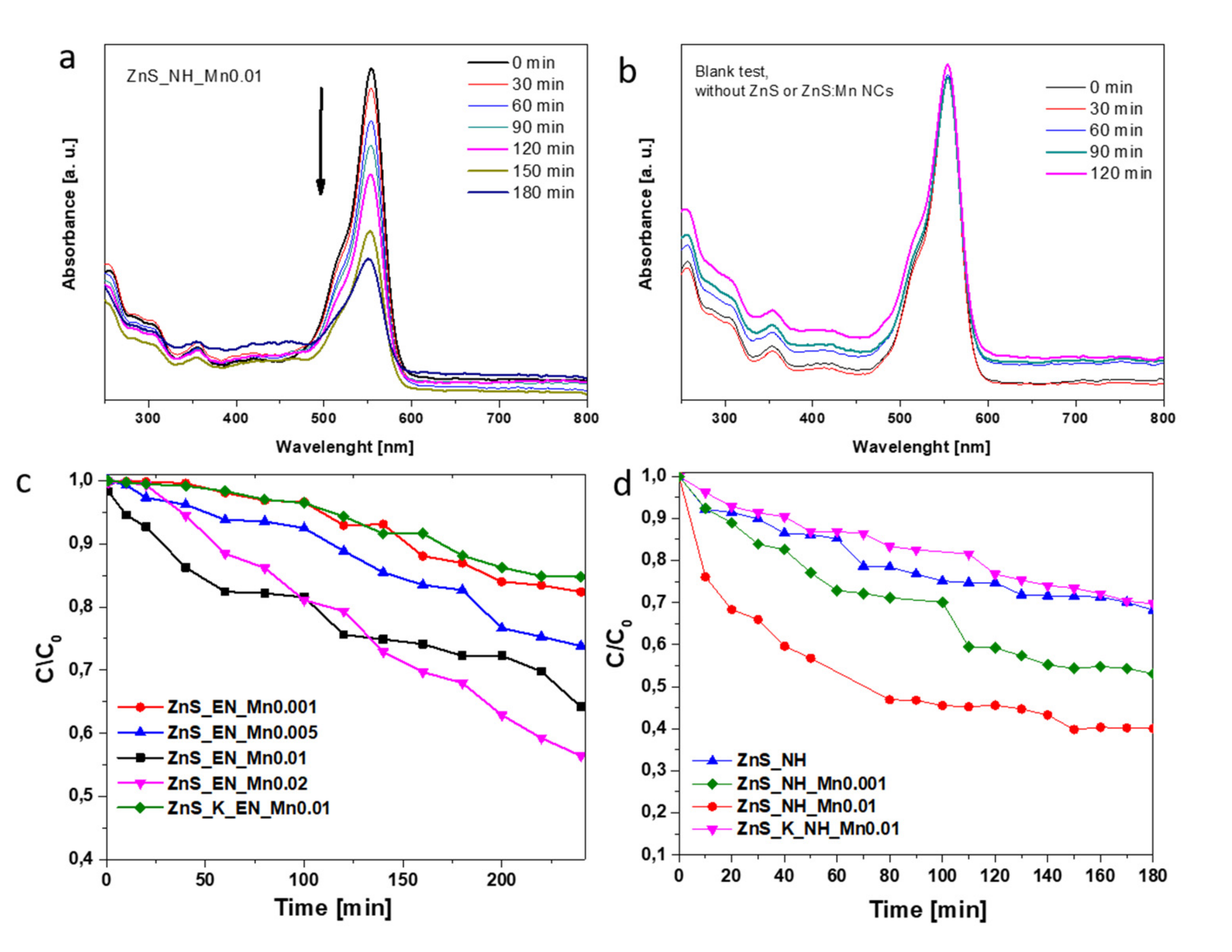
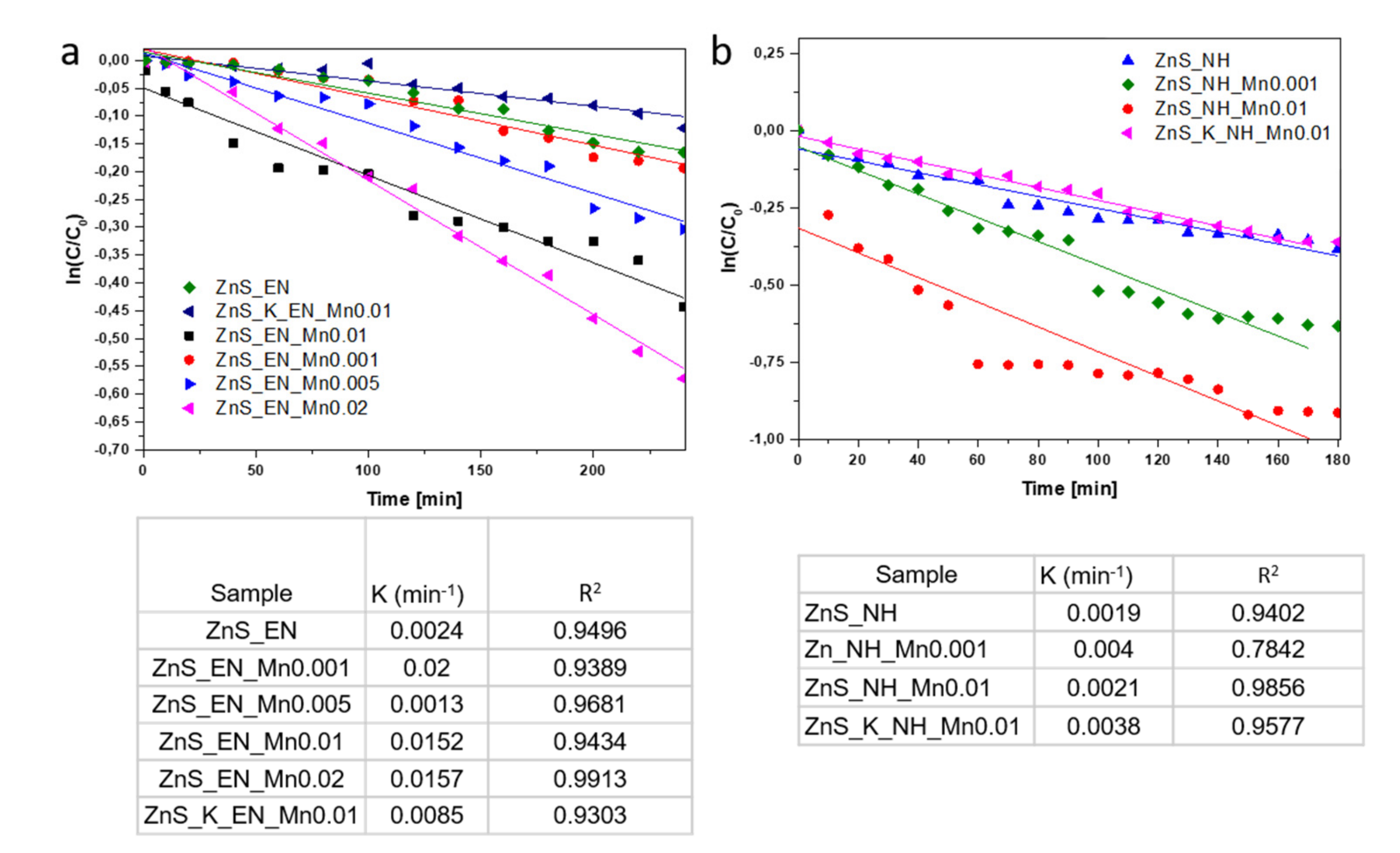

| Sample | Molar Ratio of Zn/S/Mn Precursor | Stabilizer | Bandgap [eV] (±0.02) | Surface Area [m2/g] | Average Pore Width [nm] | Mn Concentration [ppm] | Mn Concentration [wt.%] |
|---|---|---|---|---|---|---|---|
| MW, 160–170 oC, 8 bar, 20 min., stabilizer ethylenediamine (EN) | |||||||
| ZnS_EN | 1:2:0 | EN | 3.30 | 218.4 | 8.76 | – | – |
| ZnS_EN_Mn0.001 | 1:2:0.001 | EN | 2.93 | 216.24 | 9.00 | 944.97 | 0.0945 |
| ZnS_EN_Mn0.005 | 1:2:0.005 | EN | 3.31 | 226.7 | 7.72 | 1682.43 | 0.1682 |
| ZnS_EN_Mn0.01 | 1:2:0.01 | EN | 2.94 | 207.4 | 9.12 | 4705.26 | 0.4705 |
| ZnS_EN_Mn0.02 | 1:2:0.02 | EN | 2.72 | 240.1 | 8.98 | 10622.04 | 1.0622 |
| MW, 160–170 oC, 6–8 bar, 20 min., stabilizer hydrazine (N2H4) | |||||||
| ZnS_NH | 1:1:0 | N2H4 | 3.78 | 207.5 | 5.63 | – | – |
| ZnS_NH_Mn0.001 | 1:2:0.001 | N2H4 | 3.81 | 207.4 | 8.76 | 3706.19 | 0.3706 |
| ZnS_NH_Mn0.01 | 1:2:0.01 | N2H4 | 3.64 | 211.2 | 8.76 | 6847.25 | 0.6847 |
| Conventional heating, 160–170 oC, 8 bar, 20 min. | |||||||
| ZnS_K_EN_Mn0.01 | 1:2:0.01 | EN | 3.58 | 185.1 | 5.92 | 1207.21 | 0.1207 |
| ZnS_K_NH_Mn0.01 | 1:2:0.01 | N2H4 | 3.72 | 194.8 | 7.73 | 3857.24 | 0.3857 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Żaba, A.; Sovinska, S.; Kirish, T.; Węgrzynowicz, A.; Matras-Postołek, K. Photodegradation Process of Organic Dyes in the Presence of a Manganese-Doped Zinc Sulfide Nanowire Photocatalyst. Materials 2021, 14, 5840. https://doi.org/10.3390/ma14195840
Żaba A, Sovinska S, Kirish T, Węgrzynowicz A, Matras-Postołek K. Photodegradation Process of Organic Dyes in the Presence of a Manganese-Doped Zinc Sulfide Nanowire Photocatalyst. Materials. 2021; 14(19):5840. https://doi.org/10.3390/ma14195840
Chicago/Turabian StyleŻaba, Adam, Svitlana Sovinska, Tetiana Kirish, Adam Węgrzynowicz, and Katarzyna Matras-Postołek. 2021. "Photodegradation Process of Organic Dyes in the Presence of a Manganese-Doped Zinc Sulfide Nanowire Photocatalyst" Materials 14, no. 19: 5840. https://doi.org/10.3390/ma14195840
APA StyleŻaba, A., Sovinska, S., Kirish, T., Węgrzynowicz, A., & Matras-Postołek, K. (2021). Photodegradation Process of Organic Dyes in the Presence of a Manganese-Doped Zinc Sulfide Nanowire Photocatalyst. Materials, 14(19), 5840. https://doi.org/10.3390/ma14195840








