Performance and Nanostructure Simulation of Phosphogypsum Modified by Sodium Carbonate and Alum
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Sample Preparation
2.3. Test Methods
2.4. Molecular Dynamics Simulation Methods
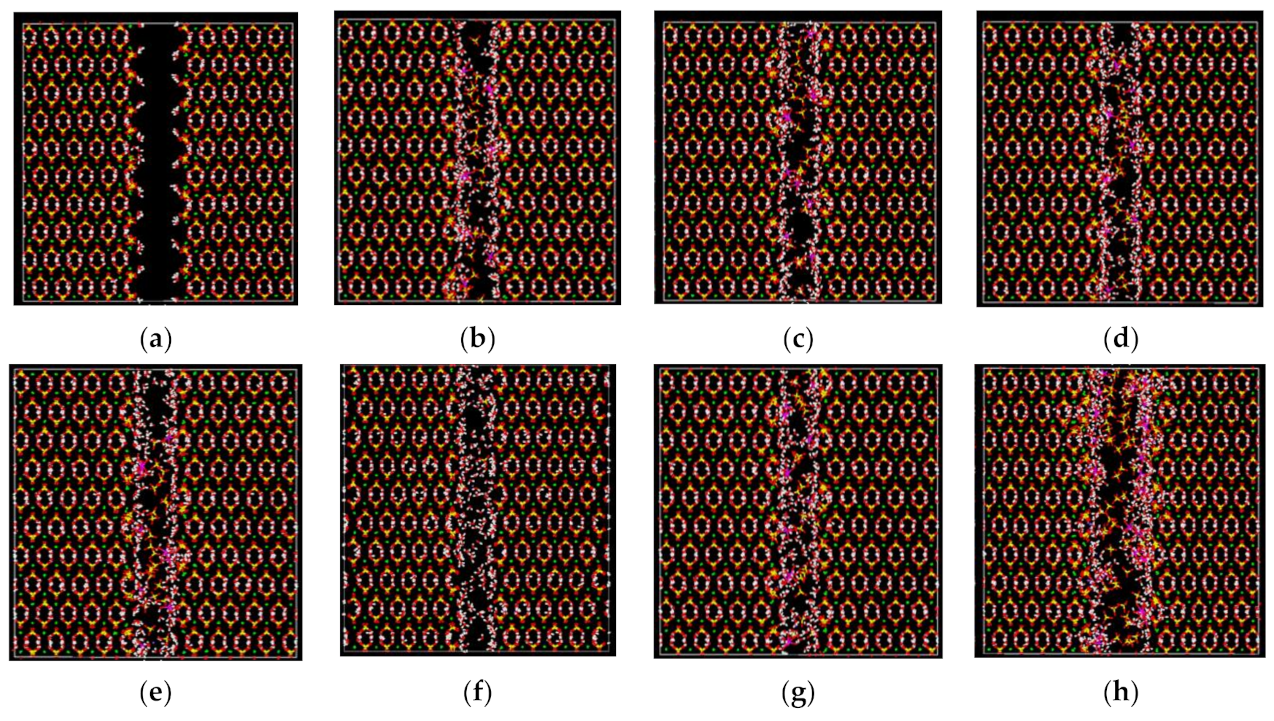
2.4.1. Simulation of Pore Formation Mechanisms
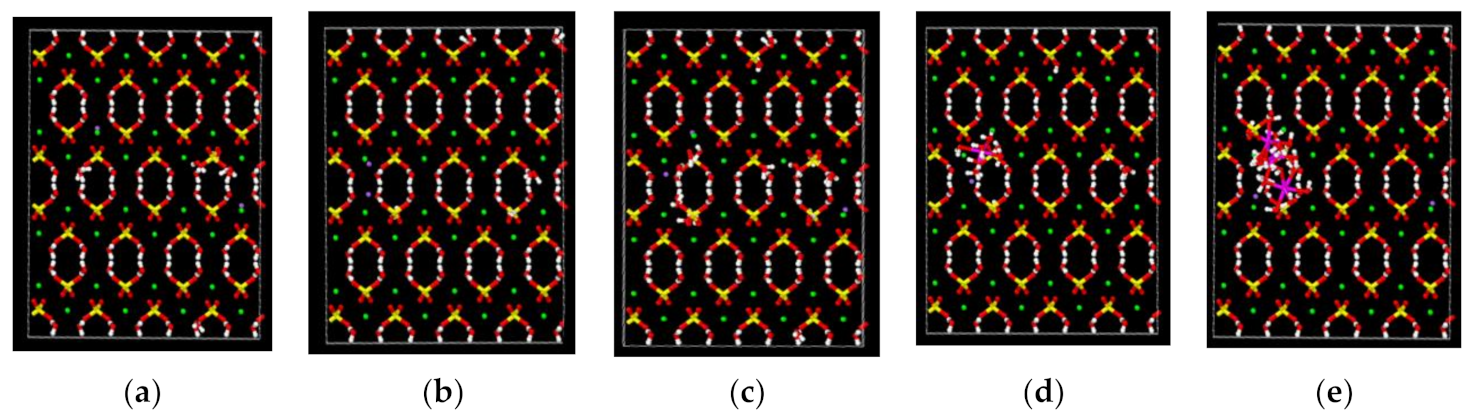
2.4.2. Effects of Pores on Mechanical Properties
3. Results and Discussion
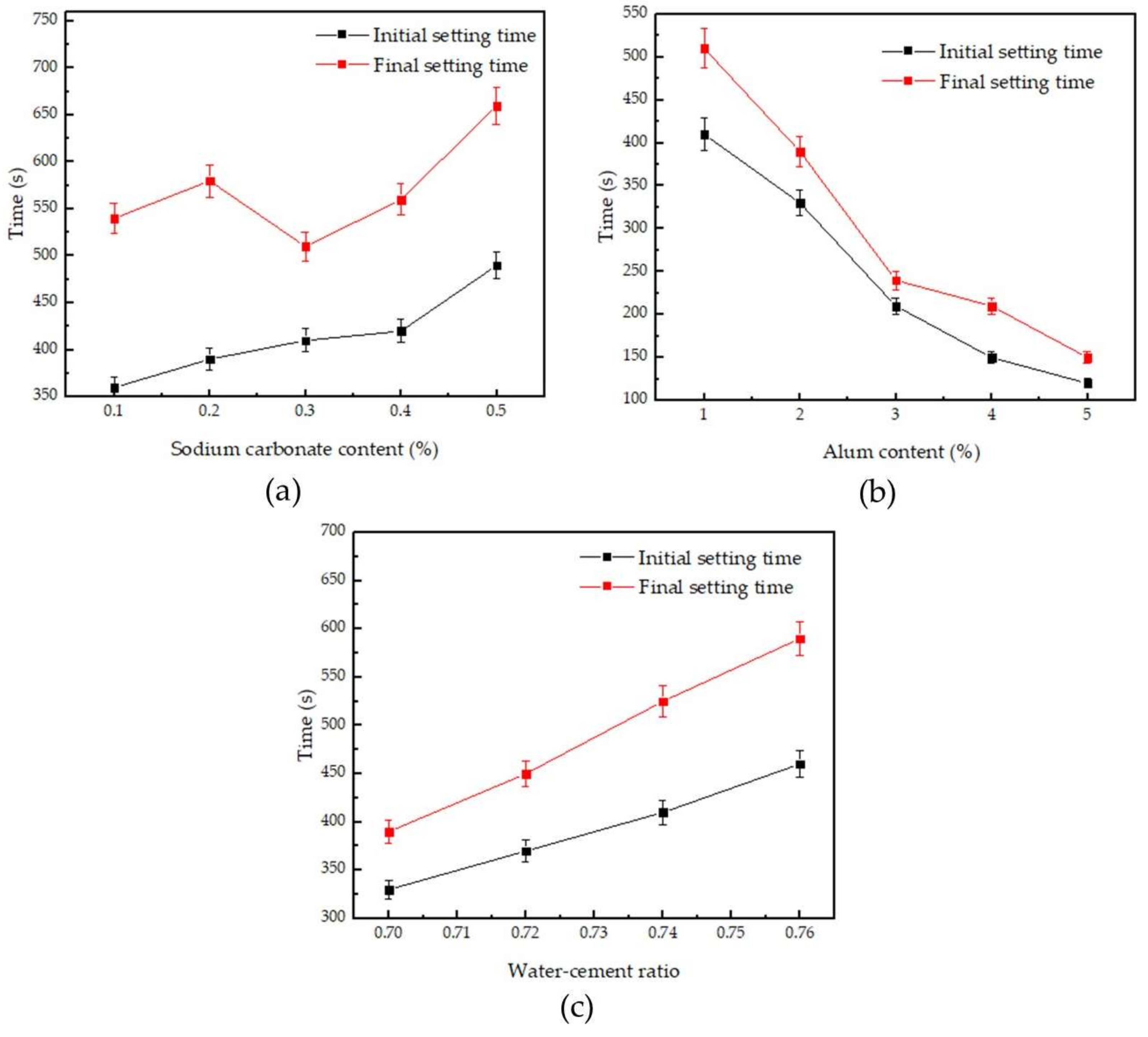
3.1. Setting Time
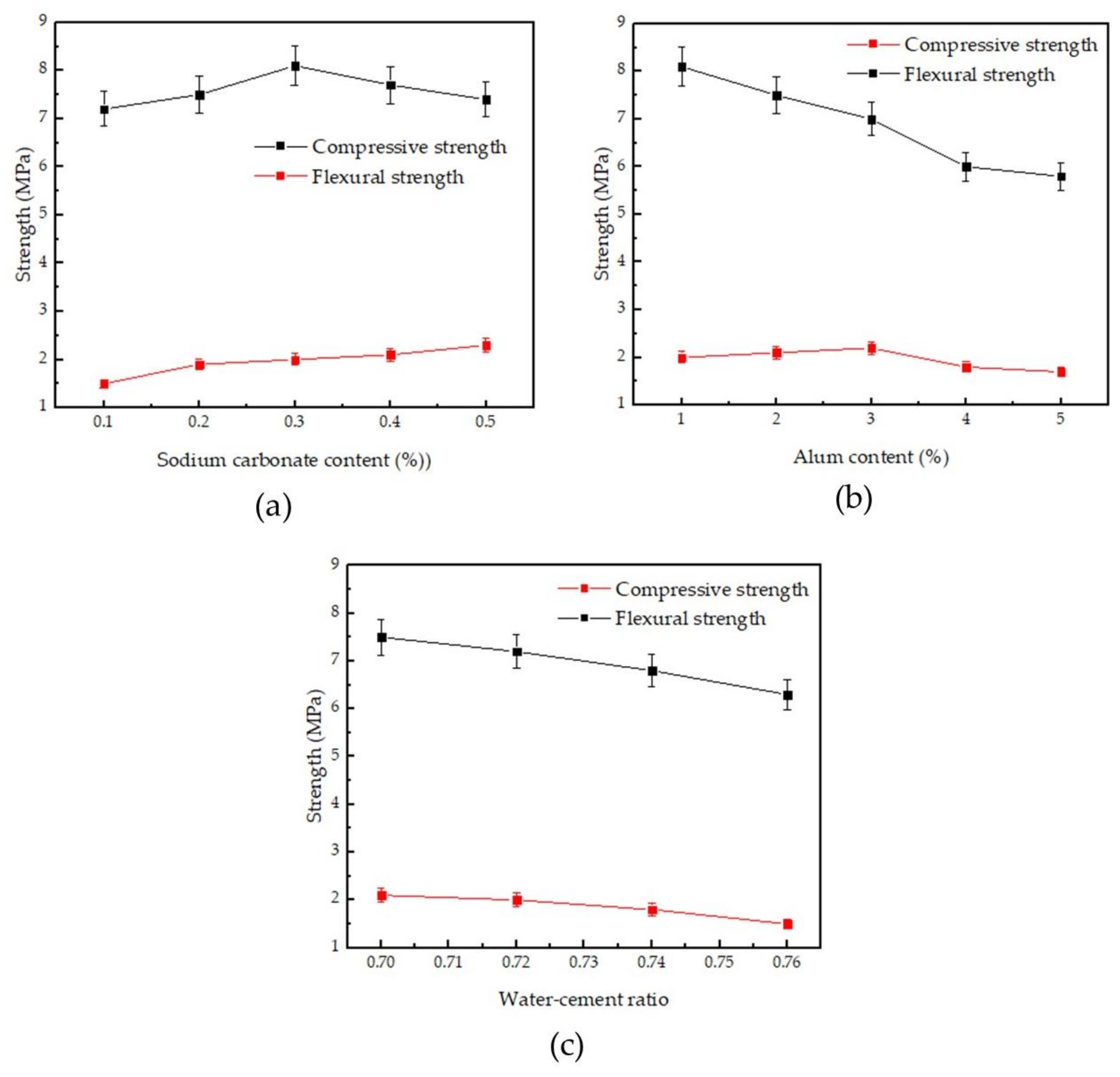
3.2. Mechanical Properties
3.3. Absolute Dry Density
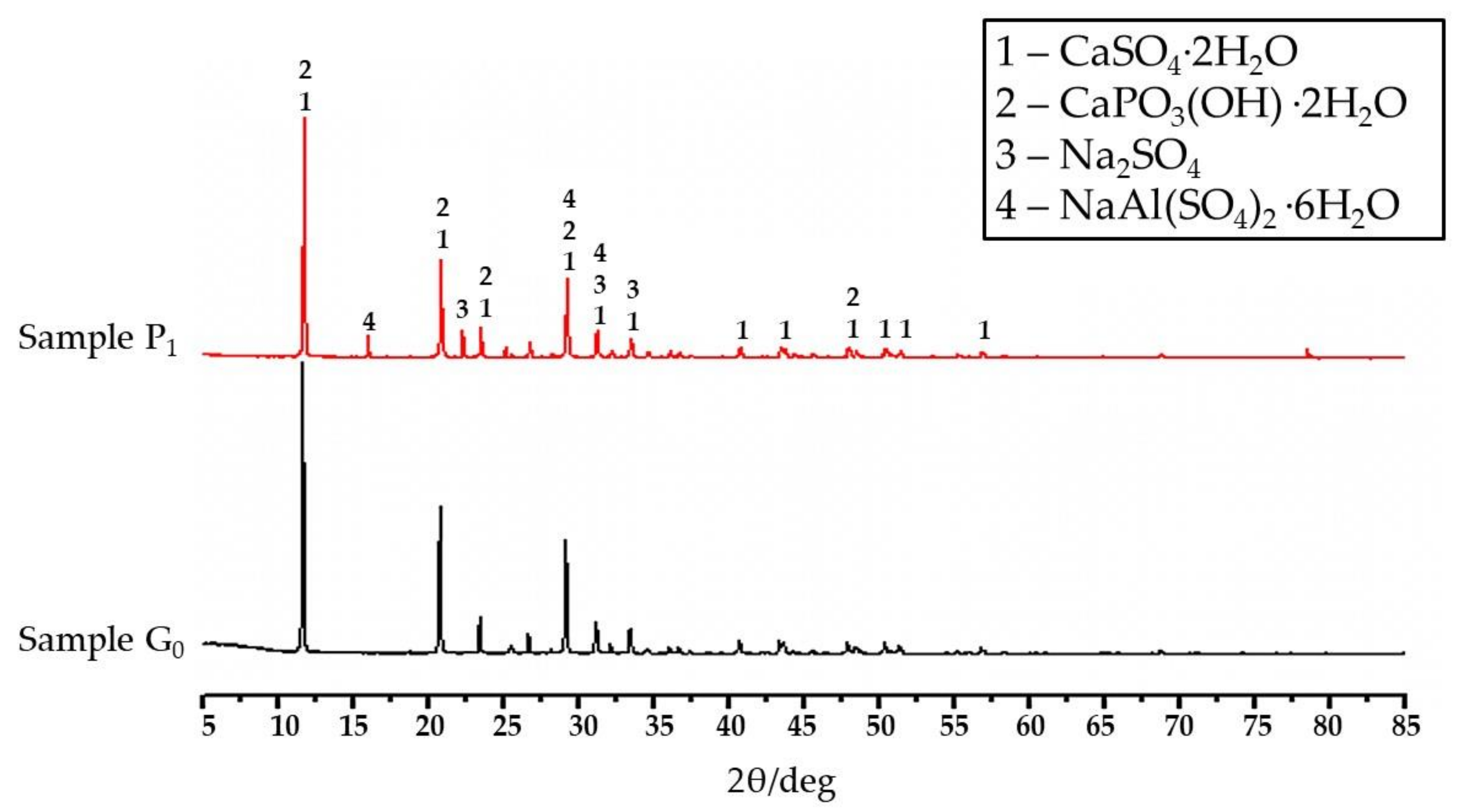
3.4. XRD Analysis
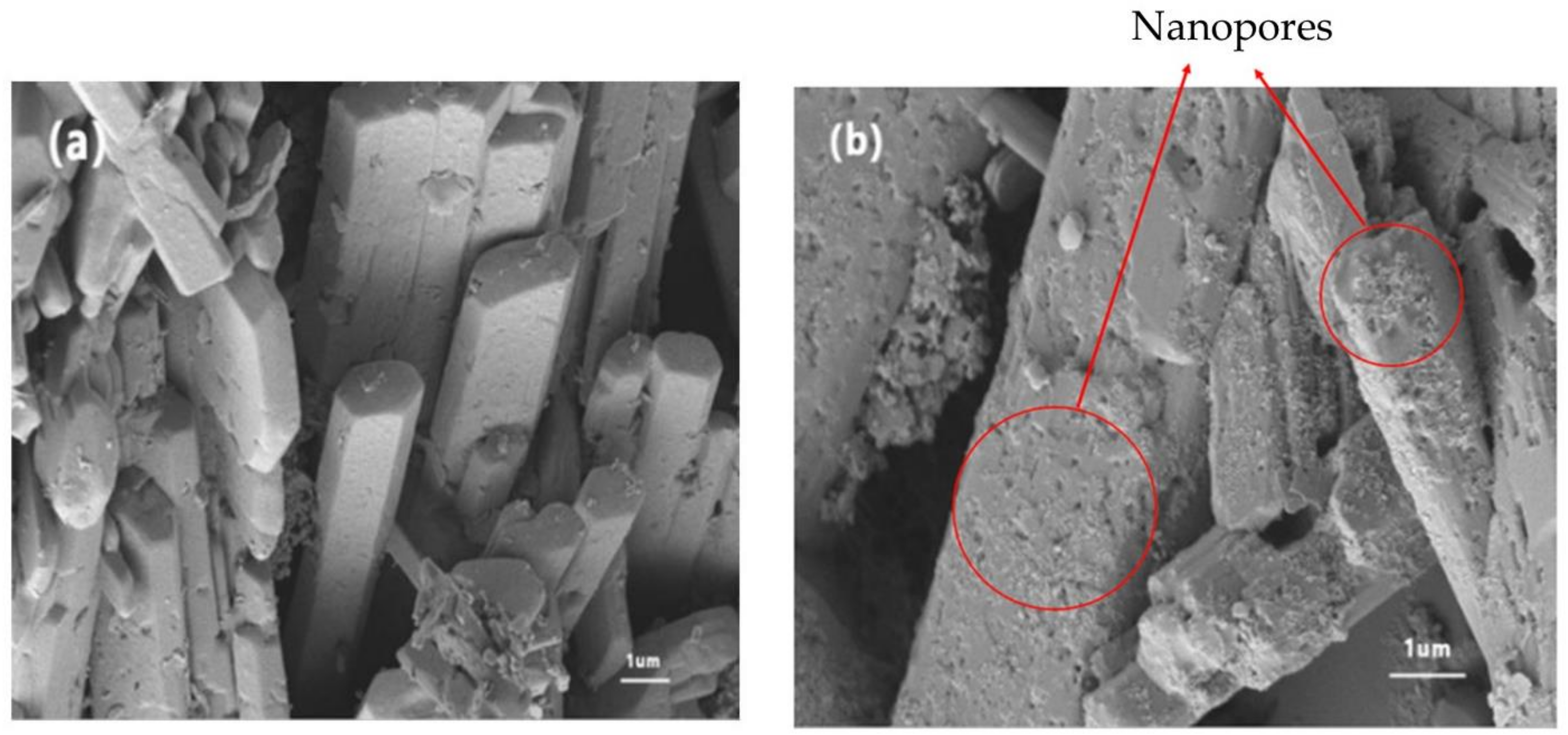
3.5. SEM Morphology
3.6. Molecular Dynamics Simulation Analysis
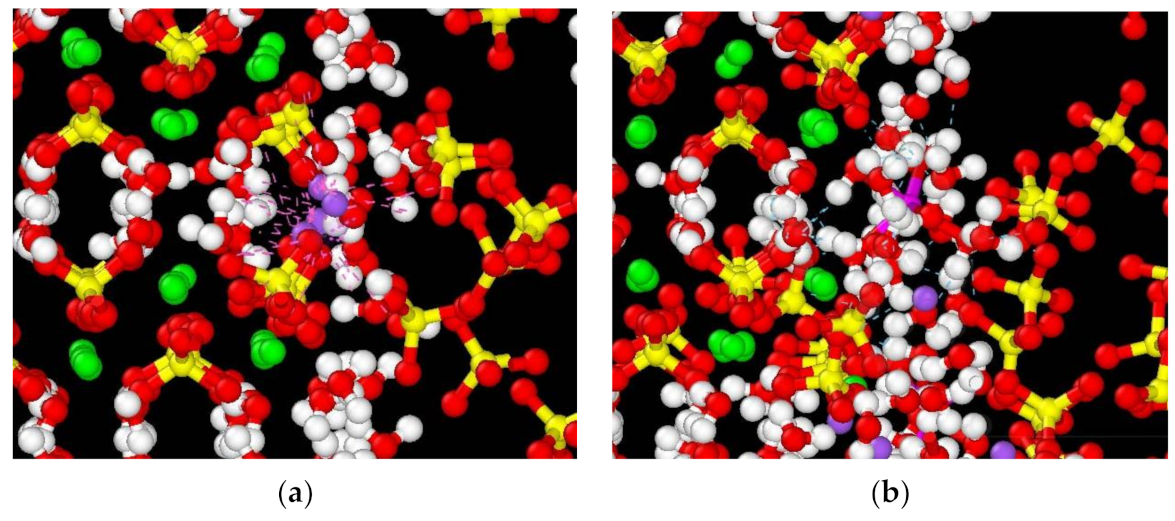
3.6.1. Pore Formation Mechanisms
3.6.2. Effects of Pore Diameter on Mechanical Properties
4. Conclusions
- Considering the mechanical strength and absolute dry density of phosphogypsum, the best content of sodium carbonate is 0.3% and the best content of alum is 2%. At this time, the initial setting time is 330 s, the final setting time is 390 s, the flexural strength of the phosphogypsum test block is 2.1 MPa, the compressive strength is 7.5 MPa, and the absolute dry density is 990 kg/m3.
- When phosphogypsum is mixed with sodium carbonate and alum, new hydration products are generated, and the CaSO4·2H2O crystal grains contain a large number of nanopores. Molecular dynamics simulations show that the hydration products are responsible for the surface deformation and the generation of surface nanopores. This is mainly caused by the Van der Waals forces between the Na ions and sulfate groups and by hydrogen bonding of the eroded CaSO4·2H2O water molecules and NaAl(SO4)2·6H2O. The nanopore structure in the CaSO4·2H2O crystal yields enhanced mechanical properties by providing structural freedom and molecular rearrangement.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rashad, A.M. Phosphogypsum as a construction material. J. Clean. Prod. 2017, 166, 732–743. [Google Scholar] [CrossRef]
- Neto, J.; Bersch, J.D.; Silva, T.; Rodríguez, E.D.; Kirchheim, A.P. Influence of phosphogypsum purification with lime on the properties of cementitious matrices with and without plasticizer. Constr. Build. Mater. 2021, 299, 123935. [Google Scholar] [CrossRef]
- Wu, K.; Han, H.; Xu, L.; Gao, Y.; Yang, Z.; Jiang, Z.; De Schutter, G. The improvement of freezing–thawing resistance of concrete by cellulose/polyvinyl alcohol hydrogel. Constr. Build. Mater. 2021, 291, 123274. [Google Scholar] [CrossRef]
- Attallah, M.F.; Metwally, S.S.; Moussa, S.I.; Soliman, M.A. Environmental impact assessment of phosphate fertilizers and phosphogypsum waste: Elemental and radiological effects. Microchem. J. 2019, 146, 789–797. [Google Scholar] [CrossRef]
- Liu, S.; Fang, P.; Ren, J.; Li, S. Application of lime neutralised phosphogypsum in supersulfated cement. J. Clean. Prod. 2020, 272, 122660. [Google Scholar] [CrossRef]
- Rosales, J.; Pérez, S.M.; Cabrera, M.; Gázquez, M.J.; Bolivar, J.P.; de Brito, J.; Agrela, F. Treated phosphogypsum as an alternative set regulator and mineral addition in cement production. J. Clean. Prod. 2020, 244, 118752. [Google Scholar] [CrossRef]
- Liu, S.; Wang, L.; Yu, B. Effect of modified phosphogypsum on the hydration properties of the phosphogypsum-based supersulfated cement. Constr. Build. Mater. 2019, 214, 9–16. [Google Scholar] [CrossRef]
- Cánovas, C.R.; Chapron, S.; Arrachart, G.; Pellet-Rostaing, S. Leaching of rare earth elements (REEs) and impurities from phosphogypsum: A preliminary insight for further recovery of critical raw materials. J. Clean. Prod. 2019, 219, 225–235. [Google Scholar] [CrossRef]
- Campos, M.P.; Costa, L.J.P.; Nisti, M.B.; Mazzilli, B.P. Phosphogypsum recycling in the building materials industry: Assessment of the radon exhalation rate. J. Environ. Radioact. 2017, 172, 232–236. [Google Scholar] [CrossRef]
- Ding, W.; Chen, Q.; Sun, H.; Peng, T. Modified mineral carbonation of phosphogypsum for CO2 sequestration. J. CO2 Util. 2019, 34, 507–515. [Google Scholar] [CrossRef]
- Morales, B.R.D.S.C.; García-Martínez, A.; Pineda, P.; García-Tenório, R. Valorization of phosphogypsum in cement-based materials: Limits and potential in eco-efficient construction. J. Build. Eng. 2021, 44, 102506. [Google Scholar] [CrossRef]
- Tao, T.I.A.N.; Zhang, C.L.; Feng, Z.H.U.; Yuan, S.X.; Ying, G.U.O.; Xue, S.G. Effect of phosphogypsum on saline-alkalinity and aggregate stability of bauxite residue. Trans. Nonferrous Met. Soc. China 2021, 31, 1484–1495. [Google Scholar]
- Syczewski, M.D.; Borkowski, A.; Gąsiński, A.; Raczko, J.; Mordak, K.; Grądziel, I.; Siuda, R. Phosphogypsum and clay mineral/phosphogypsum ceramic composites as useful adsorbents for uranium uptake. Appl. Geochem. 2020, 123, 104793. [Google Scholar] [CrossRef]
- Mashifana, T.P. Chemical treatment of phosphogypsum and its potential application for building and construction. Procedia Manuf. 2019, 35, 641–648. [Google Scholar] [CrossRef]
- Gu, K.; Chen, B.; Pan, Y. Utilization of untreated-phosphogypsum as filling and binding material in preparing grouting materials. Constr. Build. Mater. 2020, 265, 120749. [Google Scholar] [CrossRef]
- Chen, Q.; Ding, W.; Sun, H.; Peng, T.; Ma, G. Indirect mineral carbonation of phosphogypsum for CO2 sequestration. Energy 2020, 206, 118148. [Google Scholar] [CrossRef]
- Vaičiukynienė, D.; Nizevičienė, D.; Kielė, A.; Janavičius, E.; Pupeikis, D. Effect of phosphogypsum on the stability upon firing treatment of alkali-activated slag. Constr. Build. Mater. 2018, 184, 485–491. [Google Scholar] [CrossRef]
- Alla, M.; El Hafiany, M.L.; Gharibi, E.K.; Ghalit, M. Thermodynamic study of the desulfurization process of phosphogypsum. Mater. Today Proc. 2019, 13, 556–561. [Google Scholar] [CrossRef]
- Wang, T.; Wu, K.; Wu, M. Development of green binder systems based on flue gas desulfurization gypsum and fly ash incorporating slag or steel slag powders. Constr. Build. Mater. 2020, 265, 120275. [Google Scholar] [CrossRef]
- Xu, L.; Wu, K.; Li, N.; Zhou, X.; Wang, P. Utilization of flue gas desulfurization gypsum for producing calcium sulfoaluminate cement. J. Clean. Prod. 2017, 161, 803–811. [Google Scholar] [CrossRef]
- Wu, Q.; Zhu, Z.; Li, S.; Wang, S.; Chen, B. Effect of polyacrylic ester emulsion on mechanical properties of macro-defect free desulphurization gypsum plaster. Constr. Build. Mater. 2017, 153, 656–662. [Google Scholar] [CrossRef]
- Song, B.; Shi, C.; Hu, X.; Ouyang, K.; Ding, Y.; Ke, G. Effect of early CO2 curing on the chloride transport and binding behaviors of fly ash-blended Portland cement. Constr. Build. Mater. 2021, 288, 123113. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, S.; Xuan, D.; Guan, X.; Zhang, H. Improving the mechanical properties of sulfoaluminate cement-based grouting material by incorporating limestone powder for a double fluid system. Materials 2020, 13, 4854. [Google Scholar] [CrossRef]
- Liu, J.; Shi, C.; Farzadnia, N.; Ma, X. Effects of pretreated fine lightweight aggregate on shrinkage and pore structure of ultra-high strength concrete. Constr. Build. Mater. 2019, 204, 276–287. [Google Scholar] [CrossRef]
- Nizevičienė, D.; Vaičiukynienė, D.; Michalik, B.; Bonczyk, M.; Vaitkevičius, V.; Jusas, V. The treatment of phosphogypsum with zeolite to use it in binding material. Constr. Build. Mater. 2018, 180, 134–142. [Google Scholar] [CrossRef]
- Liu, S.; Ouyang, J.; Ren, J. Mechanism of calcination modification of phosphogypsum and its effect on the hydration properties of phosphogypsum-based supersulfated cement. Constr. Build. Mater. 2020, 243, 118226. [Google Scholar] [CrossRef]
- Contreras, M.; Teixeira, S.R.; Santos, G.T.A.; Gázquez, M.J.; Romero, M.; Bolívar, J.P. Influence of the addition of phosphogypsum on some properties of ceramic tiles. Constr. Build. Mater. 2018, 175, 588–600. [Google Scholar] [CrossRef] [Green Version]
- Mohammed, F.; Biswas, W.K.; Yao, H.; Tadé, M. Sustainability assessment of symbiotic processes for the reuse of phosphogypsum. J. Clean. Prod. 2018, 188, 497–507. [Google Scholar] [CrossRef] [Green Version]
- Pérez-López, R.; Carrero, S.; Cruz-Hernández, P.; Asta, M.P.; Macías, F.; Cánovas, C.R.; Nieto, J.M. Sulfate reduction processes in salt marshes affected by phosphogypsum: Geochemical influences on contaminant mobility. J. Hazard. Mater. 2018, 350, 154–161. [Google Scholar] [CrossRef]
- Lachehab, A.; Mertah, O.; Kherbeche, A.; Hassoune, H. Utilization of phosphogypsum in CO2 mineral sequestration by producing potassium sulphate and calcium carbonate. Mater. Sci. Energy Technol. 2020, 3, 611–625. [Google Scholar] [CrossRef]
- Pinto, S.R.; da Luz, C.A.; Munhoz, G.S.; Medeiros-Junior, R.A. Resistance of phosphogypsum-based supersulfated cement to carbonation and chloride ingress. Constr. Build. Mater. 2020, 263, 120640. [Google Scholar] [CrossRef]
- Islam, G.S.; Chowdhury, F.H.; Raihan, M.T.; Amit, S.K.S.; Islam, M.R. Effect of phosphogypsum on the properties of Portland cement. Procedia Eng. 2017, 171, 744–751. [Google Scholar] [CrossRef]
- Liu, X. New widely used material—Foam plaster. J. Achiev. Sci. Technol. 1995, 4, 32–33. [Google Scholar]
- Vimmrova, A.; Nazmunnahar, M.; Cerny, R. Lightweight gypsum-based materials prepared with aluminum powder as foaming agent. J. Cem. Wapno Beton 2016, 19, 299–307. [Google Scholar]
- Zhu, Z.; Wang, Z.; Zhou, Y.; Wei, Y.; She, A. Synthesis and structure of calcium silicate hydrate (C-S-H) modified by hydroxyl-terminated polydimethylsiloxane (pdms). Constr. Build. Mater. 2020, 267, 120731. [Google Scholar] [CrossRef]
- Plimpton, S.; Crozier, P.; Thompson, A. Lammps-large-scale atomic/molecular massively parallel simulator. Sandia Natl. Lab. 2007, 18, 43. [Google Scholar]
- Sarkar, P.K.; Mitra, N. Gypsum under tensile loading: A molecular dynamics study. Constr. Build. Mater. 2019, 201, 1–10. [Google Scholar] [CrossRef]
- Sun, H. COMPASS: An ab initio force-field optimized for condensed-phase applications overview with details on alkane and benzene compound. J. Phys. Chem. B 1998, 102, 7338–7364. [Google Scholar] [CrossRef]
- Chang, X.; Xue, Q.; Li, X.; Zhang, J.; Zhu, L.; He, D.; Zheng, H.; Lu, S.; Liu, Z. Inherent wettability of different rock surfaces at nanoscale: A theoretical study. Appl. Surf. Sci. 2018, 434, 73–81. [Google Scholar] [CrossRef]
- Khalkhali, M.; Ma, X.; Zhang, H.; Liu, Q. Bulk and surface properties of gypsum: A comparison between classical force fields and dispersion-corrected DFT calculations. Comput. Mater. Sci. 2019, 164, 8–16. [Google Scholar] [CrossRef]
- Su, M.; Bai, Y.; Han, J.; Chen, J.; Sun, H. Adhesion of gypsum crystals to polymer membranes: Mechanisms and prediction. J. Membr. Sci. 2018, 566, 104–111. [Google Scholar] [CrossRef]
- Hou, D.; Zhao, T.; Ma, H.; Li, Z. Reactive molecular simulation on water confined in the nanopores of the calcium silicate hydrate gel: Structure, reactivity, and mechanical properties. J. Phys. Chem. C 2015, 119, 1346–1358. [Google Scholar] [CrossRef]
- Rogge, S.M.; Goeminne, R.; Demuynck, R.; Gutiérrez-Sevillano, J.J.; Vandenbrande, S.; Vanduyfhuys, L.; Waroquier, M.; Verstraelen, T.; Van Speybroeck, V. Modeling Gas Adsorption in Flexible Metal–Organic Frameworks via Hybrid Monte Carlo/Molecular Dynamics Schemes. Adv. Theory Simul. 2019, 2, 1800177. [Google Scholar] [CrossRef]
- Widom, M.; Huhn, W.P.; Maiti, S.; Steurer, W. Hybrid Monte Carlo/molecular dynamics simulation of a refractory metal high entropy alloy. Metall. Mater. Trans. A 2014, 45, 196–200. [Google Scholar] [CrossRef] [Green Version]
- Hou, D.; Ma, H.; Li, Z.; Jin, Z. Molecular simulation of “hydrolytic weakening”: A case study on silica. Acta Mater. 2014, 80, 264–277. [Google Scholar] [CrossRef]
- Zhang, N.; Carrez, P.; Shahsavari, R. Screw-dislocation-induced strengthening–toughening mechanisms in complex layered materials: The case study of tobermorite. ACS Appl. Mater. Interfaces 2017, 9, 1496–1506. [Google Scholar] [CrossRef] [Green Version]
- Tao, L.; Shahsavari, R. Diffusive, displacive deformations and local phase transformation govern the mechanics of layered crystals: The case study of tobermorite. Sci. Rep. 2017, 7, 5907. [Google Scholar] [CrossRef] [Green Version]
- Zhang, N.; Shahsavari, R. Balancing strength and toughness of calcium-silicate-hydrate via random nanovoids and particle inclusions: Atomistic modeling and statistical analysis. J. Mech. Phys. Solids 2016, 96, 204–222. [Google Scholar] [CrossRef] [Green Version]
- Singh, N.B.; Middendorf, B. Calcium sulphate hemihydrate hydration leading to gypsum crystallization. Prog. Cryst. Growth Charact. Mater. 2007, 53, 57–77. [Google Scholar] [CrossRef]
- Zheng, G.; Xia, J.; Han, Y. The influence of retarders on the properties of phosphogypsum-based building gypsum. J. Non Met. Miner. 2019, 42, 79–81. [Google Scholar]
- Plachy, T.; Tesarek, P.; Padevet, P.; Polak, M. Determination of Young’s modulus of gypsum blocks using two different experimental methods. In Recent Advances in Applied and Theoretical Mechanics, Proceedings of 5th WSEAS International Conference on Applied and Theoretical Mechanics, Puerto De La Cruz, Spain, 14–16 December 2009; Bulucea, C., Ed.; WSEAS Press: Athens, Greece, 2009. [Google Scholar]
- Mao, X.; Song, X.; Lu, G.; Xu, Y.; Sun, Y.; Yu, J. Effect of additives on the morphology of calcium sulfate hemihydrate: Experimental and molecular dynamics simulation studies. Chem. Eng. J. 2015, 278, 320–327. [Google Scholar] [CrossRef]




| No. | Sodium Carbonate Content (%) | Alum Content (%) | Water–Cement Ratio |
|---|---|---|---|
| G0 | 0 | 0 | 0.70 |
| G1 | 0 | 0 | 0.88 |
| F1 | 0.1 | 1 | 0.70 |
| F2 | 0.2 | 1 | 0.70 |
| F3 | 0.3 | 1 | 0.70 |
| F4 | 0.4 | 1 | 0.70 |
| F5 | 0.5 | 1 | 0.70 |
| P1 | 0.3 | 2 | 0.70 |
| P2 | 0.3 | 3 | 0.70 |
| P3 | 0.3 | 4 | 0.70 |
| P4 | 0.3 | 5 | 0.70 |
| W1 | 0.3 | 2 | 0.72 |
| W2 | 0.3 | 2 | 0.74 |
| W3 | 0.3 | 2 | 0.76 |
| No. | Initial Setting Time (s) | Final Setting Time (s) | Flexural Strength (MPa) | Compressive Strength (MPa) | Absolute Dry Density (kg/m3) |
|---|---|---|---|---|---|
| G0 | 330 | 598 | 2.4 | 8.6 | 1289 |
| G1 | 530 | 740 | 1.7 | 6.8 | 993 |
| F1 | 360 | 540 | 1.5 | 7.2 | 1050 |
| F2 | 390 | 580 | 1.9 | 7.5 | 1050 |
| F3 | 410 | 510 | 2.0 | 8.1 | 1030 |
| F4 | 420 | 560 | 2.1 | 7.7 | 1040 |
| F5 | 490 | 660 | 2.3 | 7.4 | 1050 |
| P1 | 330 | 390 | 2.1 | 7.5 | 990 |
| P2 | 210 | 240 | 2.2 | 7.0 | 1000 |
| P3 | 150 | 210 | 1.8 | 6.0 | 980 |
| P4 | 120 | 150 | 1.7 | 5.8 | 1000 |
| W1 | 370 | 450 | 2.0 | 7.2 | 980 |
| W2 | 410 | 525 | 1.8 | 6.8 | 972 |
| W3 | 460 | 590 | 1.5 | 6.3 | 965 |
| No. | Na2SO4 Content (%) | NaAl(SO4)2·6H2O Content (%) | Total Pore Volume (Å3) |
|---|---|---|---|
| G0 | 0 | 0 | 97,853.75 |
| F1 | 0 | 1 | 105,481.02 |
| F2 | 0.1 | 1 | 105,747.92 |
| F3 | 0.2 | 1 | 105,676.17 |
| F4 | 0.3 | 1 | 105,965.05 |
| P1 | 0.3 | 0 | 103,818.17 |
| P2 | 0.3 | 2 | 106,379.60 |
| P3 | 0.3 | 3 | 111,369.97 |
| Pore Size | 0 Å | 2 Å | 3 Å | 4 Å | 5 Å | 6 Å |
| Young’s Modulus (GPa) | 0.73 | 2.5079 | 0.8288 | 1.1416 | 1.1567 | 1.7919 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, D.; Wang, J.; Hou, G.; Wang, L.; Wu, Q.; Lu, B. Performance and Nanostructure Simulation of Phosphogypsum Modified by Sodium Carbonate and Alum. Materials 2021, 14, 5830. https://doi.org/10.3390/ma14195830
Zhong D, Wang J, Hou G, Wang L, Wu Q, Lu B. Performance and Nanostructure Simulation of Phosphogypsum Modified by Sodium Carbonate and Alum. Materials. 2021; 14(19):5830. https://doi.org/10.3390/ma14195830
Chicago/Turabian StyleZhong, Dongqing, Jingchen Wang, Guihua Hou, Luming Wang, Qian Wu, and Bao Lu. 2021. "Performance and Nanostructure Simulation of Phosphogypsum Modified by Sodium Carbonate and Alum" Materials 14, no. 19: 5830. https://doi.org/10.3390/ma14195830
APA StyleZhong, D., Wang, J., Hou, G., Wang, L., Wu, Q., & Lu, B. (2021). Performance and Nanostructure Simulation of Phosphogypsum Modified by Sodium Carbonate and Alum. Materials, 14(19), 5830. https://doi.org/10.3390/ma14195830







