On the Structure and Properties of AlMgB14-TiB2 Composites Obtained from SHS Powders by Spark Plasma Sintering
Abstract
1. Introduction
2. Materials and Methods
2.1. The First Step
2.2. The Second Step
2.3. Characterization
3. Results and Discussion
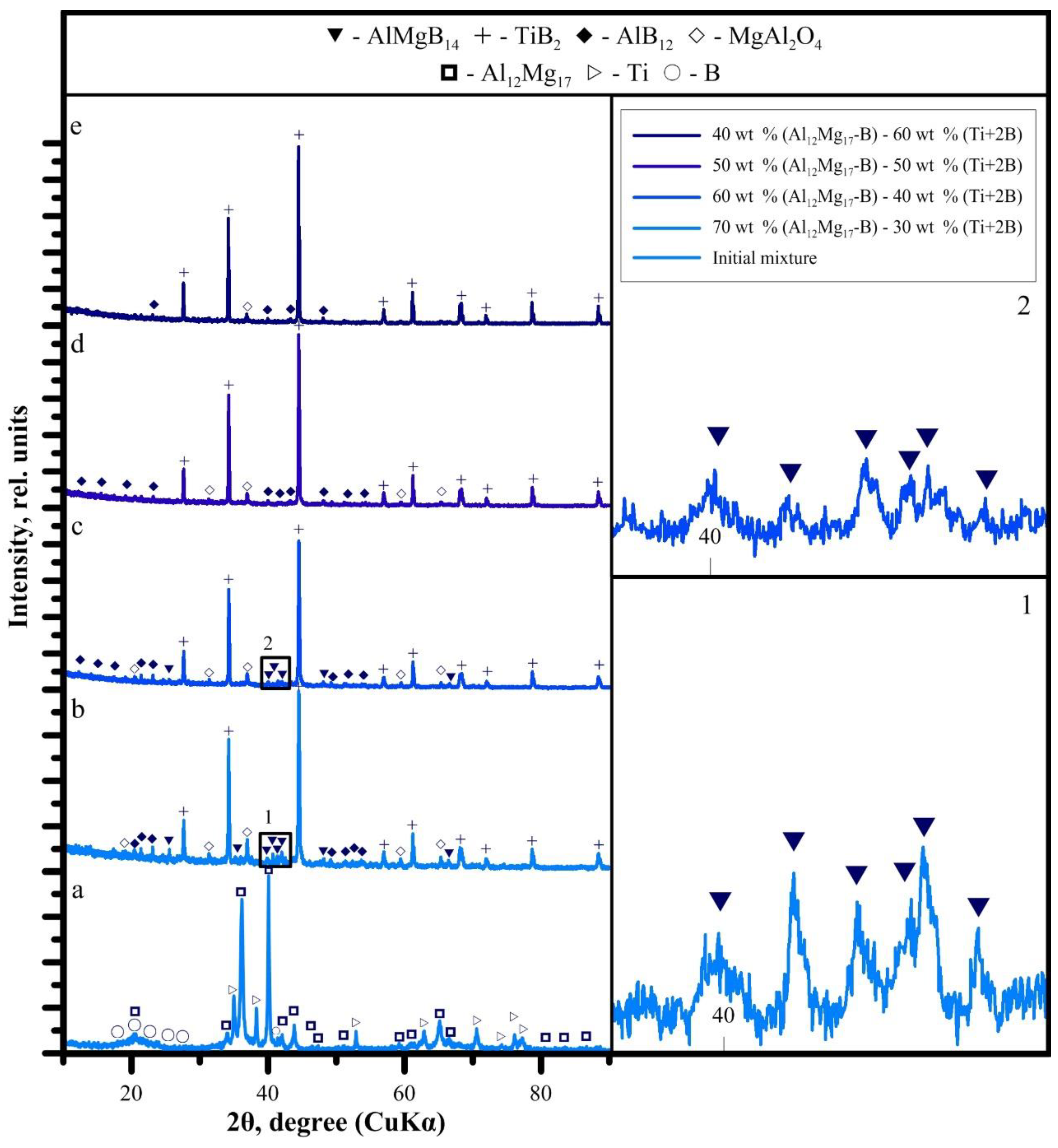
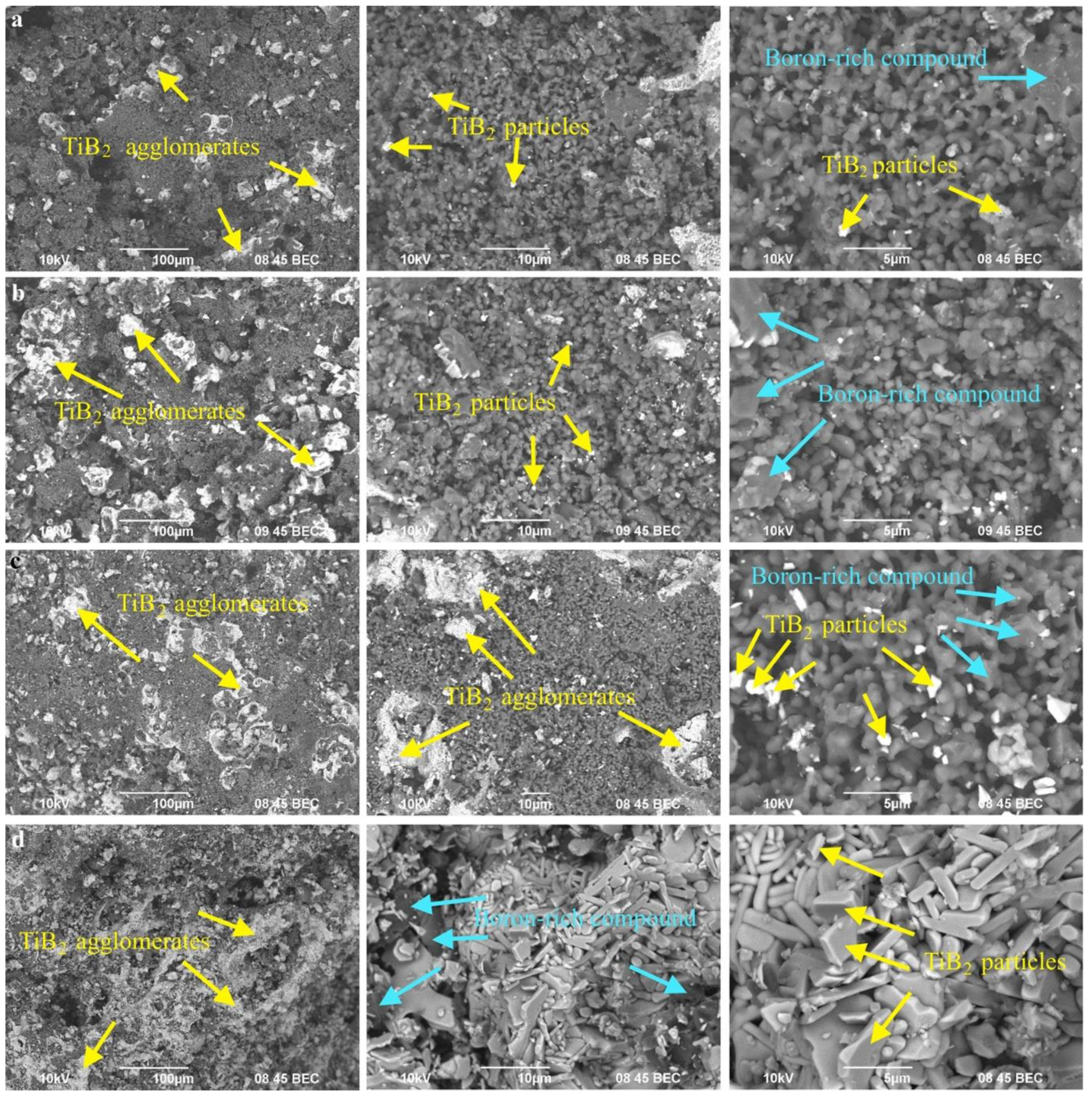
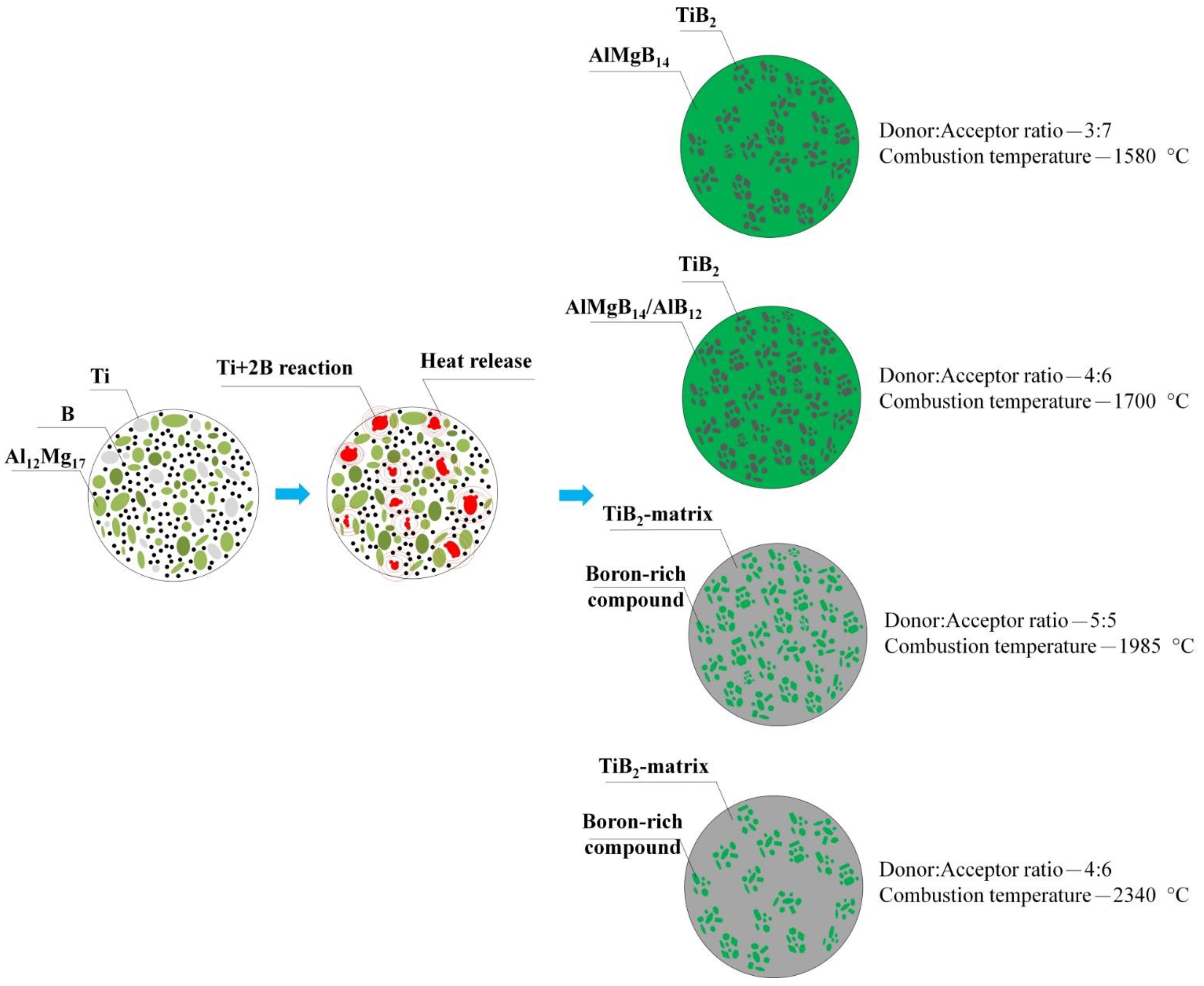
3.1. SHS Powders
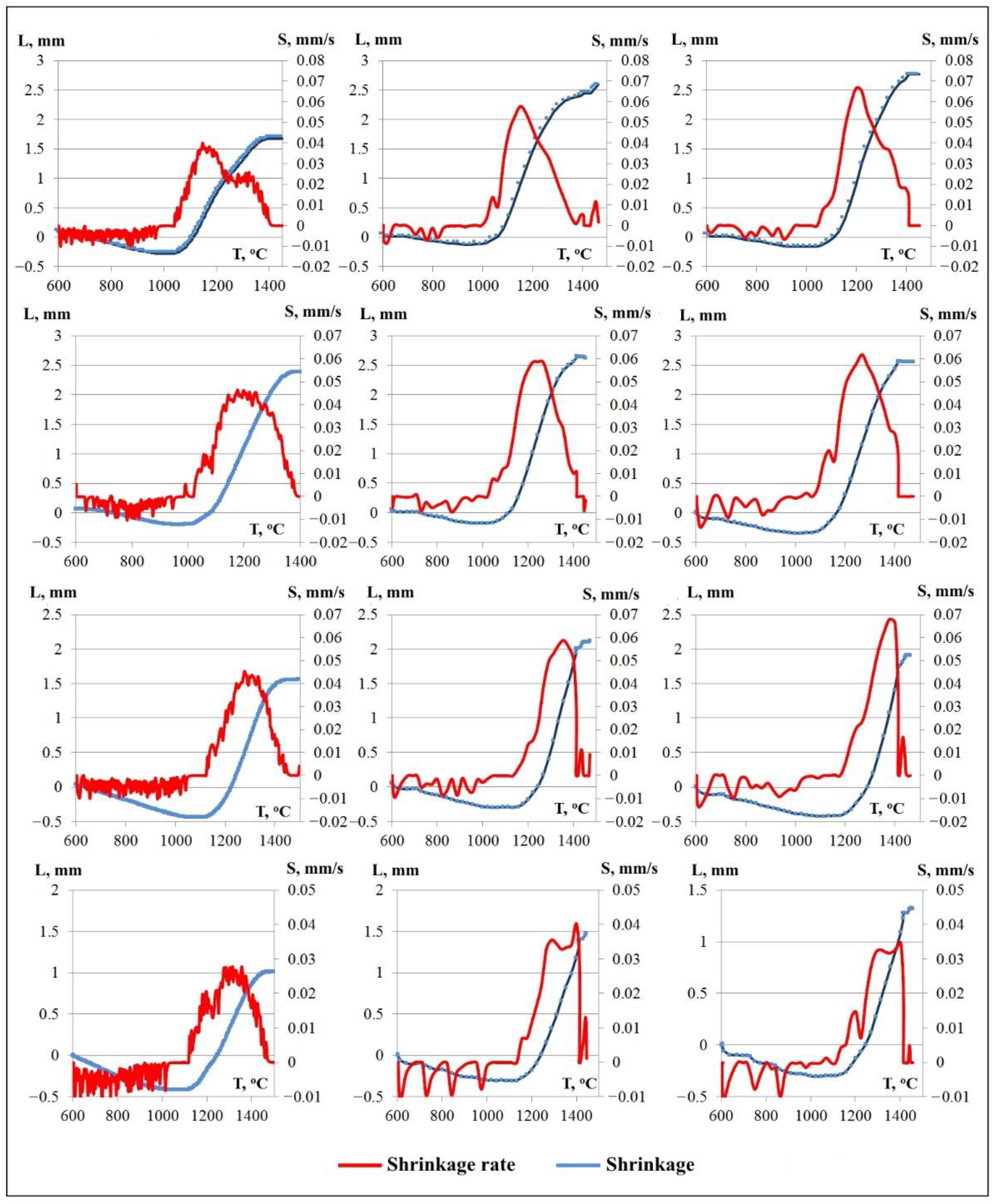
3.2. Spark Plasma Sintered Specimens
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cook, B.A.; Harringa, J.L.; Lewis, T.L.; Russel, A.M. A new class of ultra-hard materials based on AlMgB14. Scr. Mater. 2000, 42, 597–602. [Google Scholar] [CrossRef]
- Kevorkijan, V.; Škapin, S.D.; Jelen, M.; Krnel, K.; Meden, A. Cost-effective synthesis of AlMgB14–xTiB2. J. Eur. Ceram. Soc. 2007, 27, 493–497. [Google Scholar] [CrossRef]
- Xie, Z.; DeLucca, V.; Haber, R.A.; Restrepo, D.T.; Todd, J.; Blair, R.G.; Orlovskaya, N. Aluminum magnesium boride: Synthesis, sintering and microstructure. Adv. Appl. Ceram. 2017, 116, 341–347. [Google Scholar] [CrossRef]
- Roberts, D.J.; Zhao, J.; Munir, Z.A. Mechanism of reactive sintering of MgAlB14 by pulse electric current. Int. J. Refract. Hard Met. 2009, 27, 556–563. [Google Scholar] [CrossRef]
- Tian, Y.; Bastawros, A.F.; Lo, C.C.; Constant, A.P.; Russell, A.M.; Cook, B.A. Superhard self-lubricating AlMgB14 films for microelectromechanical devices. Appl. Phys. Lett. 2003, 83, 2781–2783. [Google Scholar] [CrossRef]
- Tian, Y.; Li, G.; Shinar, J.; Wang, N.L.; Cook, B.A.; Anderegg, J.W.; Snyder, J.E. Electrical transport in amorphous semiconducting AlMgB14 films. Appl. Phys. Lett. 2004, 85, 1181–1183. [Google Scholar] [CrossRef][Green Version]
- Zhukov, I.A.; Nikitin, P.Y.; Vorozhtsov, A.B.; Perevislov, S.N.; Sokolov, S.D.; Ziatdinov, M.H. The use of intermetallic AlxMgy powder to obtain AlMgB14-based materials. Mater. Today Commun. 2020, 22, 100848. [Google Scholar] [CrossRef]
- Nikitin, P.Y.; Zhukov, I.A.; Vorozhtsov, A.B. Decomposition mechanism of AlMgB14 during the spark plasma sintering. J. Mater. Res. Technol. 2020, 11, 687–692. [Google Scholar] [CrossRef]
- Lei, Y.; Meng, Q.; Zhuang, L.; Chen, S.; Hu, L.; Cheng, H. Friction and wear behavior of AlMgB14–TiB2 composite at elevated temperature. Tribol. Lett. 2014, 56, 435–442. [Google Scholar] [CrossRef]
- Cheng, J.; Ma, J.; Li, F.; Qiao, Z.; Yang, J.; Liu, W. Dry-sliding tribological properties of Cu/AlMgB14 composites. Tribol. Lett. 2014, 55, 35–44. [Google Scholar] [CrossRef]
- Lu, X.; Yao, K.; Ouyang, J.; Tian, Y. Tribological characteristics and tribo-chemical mechanisms of Al–Mg–Ti–B coatings under water–glycol lubrication. Wear 2015, 326, 68–73. [Google Scholar] [CrossRef]
- Cook, B.A.; Harringa, J.L.; Anderegg, J.; Russell, A.M.; Qu, J.; Blau, P.J.; Elmoursi, A.A. Analysis of wear mechanisms in low-friction AlMgB14–TiB2 coatings. Surf. Coat. Technol. 2010, 205, 2296–2301. [Google Scholar] [CrossRef]
- Chen, J.; Cheng, J.; Li, F.; Zhu, S.; Li, W.; Yang, J.; Liu, W. Tribological study on a novel wear-resistant AlMgB14-Si composite. Ceram. Int. 2017, 43, 12362–12371. [Google Scholar] [CrossRef]
- Sun, Y.Y.; Zhang, P.X.; Liu, G.Q.; Xiong, X.M.; Yang, F.; Jiao, G.F.; Yan, G. Effect of two-step heat treatment on the phase formation of MgAlB14. Mater. Lett. 2011, 65, 2158–2160. [Google Scholar] [CrossRef]
- Nikitin, P.Y.; Zhukov, I.A.; Matveev, A.E.; Sokolov, S.D.; Boldin, M.S.; Vorozhtsov, A.B. AlMgB14–TiB2 composite materials obtained by self-propagating high-temperature synthesis and spark plasma sintering. Ceram. Int. 2020, 46, 22733–22737. [Google Scholar] [CrossRef]
- Amosov, A.P.; Borovinskaya, I.P.; Merzhanov, A.G. Powder Technology of Self-Propagating High-Temperature Synthesis of Materials; Mashinostroenie: Moscow, Russia, 2007. (In Russian)
- Zhukov, I.A.; Promakhov, V.V.; Matveev, A.E.; Platov, V.V.; Khrustalev, A.P.; Dubkova, Y.A.; Potekaev, A.I. Principles of Structure and Phase Composition Formation in Composite Master Alloys of the Al–Ti–B/B4C Systems Used for Aluminum Alloy Modification. Russ. Phys. J. 2018, 60, 2025–2031. [Google Scholar] [CrossRef]
- Nikitin, P.Y.; Matveev, A.E.; Zhukov, I.A. Energy-effective AlMgB14 production by self-propagating high-temperature synthesis (SHS) using the chemical furnace as a source of heat energy. Ceram. Int. 2021, 47, 21698–21704. [Google Scholar] [CrossRef]
- Zhuang, L.; Lei, Y.; Chen, S.; Hu, L.; Meng, Q. Microstructure and mechanical properties of AlMgB14–TiB2 associated with metals prepared by the field-assisted diffusion bonding sintering process. Appl. Surf. Sci. 2015, 328, 125–132. [Google Scholar] [CrossRef]
- Nečina, V.; Pabst, W. Transparent MgAl2O4 spinel ceramics prepared via sinter-forging. J. Eur. Ceram. Soc. 2021, 41, 4313–4318. [Google Scholar] [CrossRef]
- Hříbalová, S.; Pabst, W. Theoretical study of the influence of carbon contamination on the transparency of spinel ceramics prepared by spark plasma sintering (SPS). J. Eur. Ceram. Soc. 2021, 41, 4337–4342. [Google Scholar] [CrossRef]





| Specimen | #30-1 | #30-2 | #30-3 | #40-1 | #40-2 | #40-3 | #50-1 | #50-2 | #50-3 | #60-1 | #60-2 | #60-3 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| donor:acceptor mass ratio | 3:7 | 3:7 | 3:7 | 4:6 | 4:6 | 4:6 | 5:5 | 5:5 | 5:5 | 6:4 | 6:4 | 6:4 |
| Specimen | Temperature, °C | Heating Rate, °C/min | Isothermal Holding, min | Pressure, MPa |
|---|---|---|---|---|
| #30-1 | 1450 | 50 | 0 | 70 |
| #30-2 | 1470 | 250 | 0 | 70 |
| #30-3 | 1470 | 250 | 5 | 70 |
| #40-1 | 1400 | 50 | 0 | 70 |
| #40-2 | 1470 | 250 | 0 | 70 |
| #40-3 | 1470 | 250 | 5 | 70 |
| #50-1 | 1500 | 50 | 0 | 70 |
| #50-2 | 1470 | 250 | 0 | 70 |
| #50-3 | 1470 | 250 | 5 | 70 |
| #60-1 | 1510 | 50 | 0 | 70 |
| #60-2 | 1470 | 250 | 0 | 70 |
| #60-3 | 1470 | 250 | 5 | 70 |
| Specimen | Density, g/cm3 | * Theoretical Density, g/cm3 | Hardness, GPa |
|---|---|---|---|
| #30-1 | 2.977 | 2.97 | 24.7 ± 2.2 |
| #30-2 | 3.089 | 24.4 ± 1.6 | |
| #30-3 | 3.087 | 32.1 ± 3.5 | |
| #40-1 | 3.251 | 3.12 | 27.7 ± 1.8 |
| #40-2 | 3.244 | 29.1 ± 1.4 | |
| #40-3 | 3.291 | 29.4 ± 1.2 | |
| #50-1 | 3.364 | 3.29 | 28.1 ± 1.6 |
| #50-2 | 3.416 | 30.1 ± 0.8 | |
| #50-3 | 3.141 | 29.7 ± 0.9 | |
| #60-1 | 3.535 | 3.48 | 28.7 ± 1.1 |
| #60-2 | 3.625 | 29.3 ± 1.3 | |
| #60-3 | 3.661 | 29.8 ± 1.3 |
| Hardness, GPa | 29.2 | 30.1 | 30.7 | 31.7 | 30.1 | 29.6 | 32.1 | 29.3 | 29.2 | 29.2 | 29.2 | 30.1 |
| D1, µm | 24.88 | 24.42 | 22.10 | 24.12 | 24.09 | 25.12 | 23.34 | 25.15 | 25.30 | 24.63 | 24.88 | 24.42 |
| D2, µm | 25.54 | 25.18 | 24.21 | 24.24 | 25.57 | 24.91 | 24.72 | 25.15 | 25.06 | 25.75 | 25.54 | 25.18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikitin, P.; Zhukov, I.; Matveev, A.; Sokolov, S.; Grigoriev, M.; Vorozhtsov, A. On the Structure and Properties of AlMgB14-TiB2 Composites Obtained from SHS Powders by Spark Plasma Sintering. Materials 2021, 14, 5521. https://doi.org/10.3390/ma14195521
Nikitin P, Zhukov I, Matveev A, Sokolov S, Grigoriev M, Vorozhtsov A. On the Structure and Properties of AlMgB14-TiB2 Composites Obtained from SHS Powders by Spark Plasma Sintering. Materials. 2021; 14(19):5521. https://doi.org/10.3390/ma14195521
Chicago/Turabian StyleNikitin, Pavel, Ilya Zhukov, Aleksey Matveev, Sergei Sokolov, Mikhail Grigoriev, and Alexander Vorozhtsov. 2021. "On the Structure and Properties of AlMgB14-TiB2 Composites Obtained from SHS Powders by Spark Plasma Sintering" Materials 14, no. 19: 5521. https://doi.org/10.3390/ma14195521
APA StyleNikitin, P., Zhukov, I., Matveev, A., Sokolov, S., Grigoriev, M., & Vorozhtsov, A. (2021). On the Structure and Properties of AlMgB14-TiB2 Composites Obtained from SHS Powders by Spark Plasma Sintering. Materials, 14(19), 5521. https://doi.org/10.3390/ma14195521






