Recovery of Uranium by Se-Derivatives of Amidoximes and Composites Based on Them
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Materials
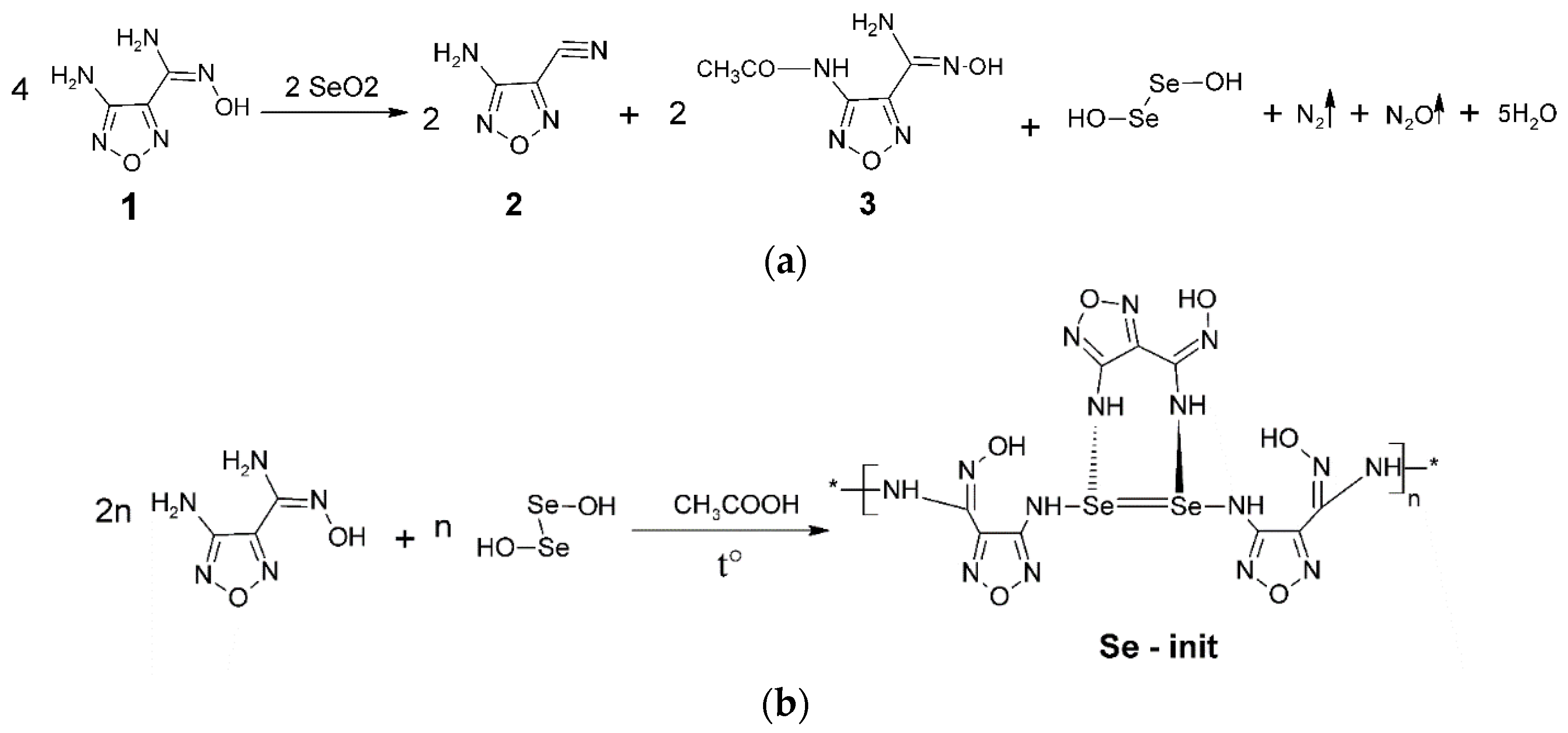
2.2. Synthesis of the Se-Derivative N’-hydroxy-1,2,5-oxadiazole-3-carboximidamide
2.3. Synthesis of Composite Sorbents
2.4. Study of Sorption Characteristics under Static Conditions
2.5. Equipment
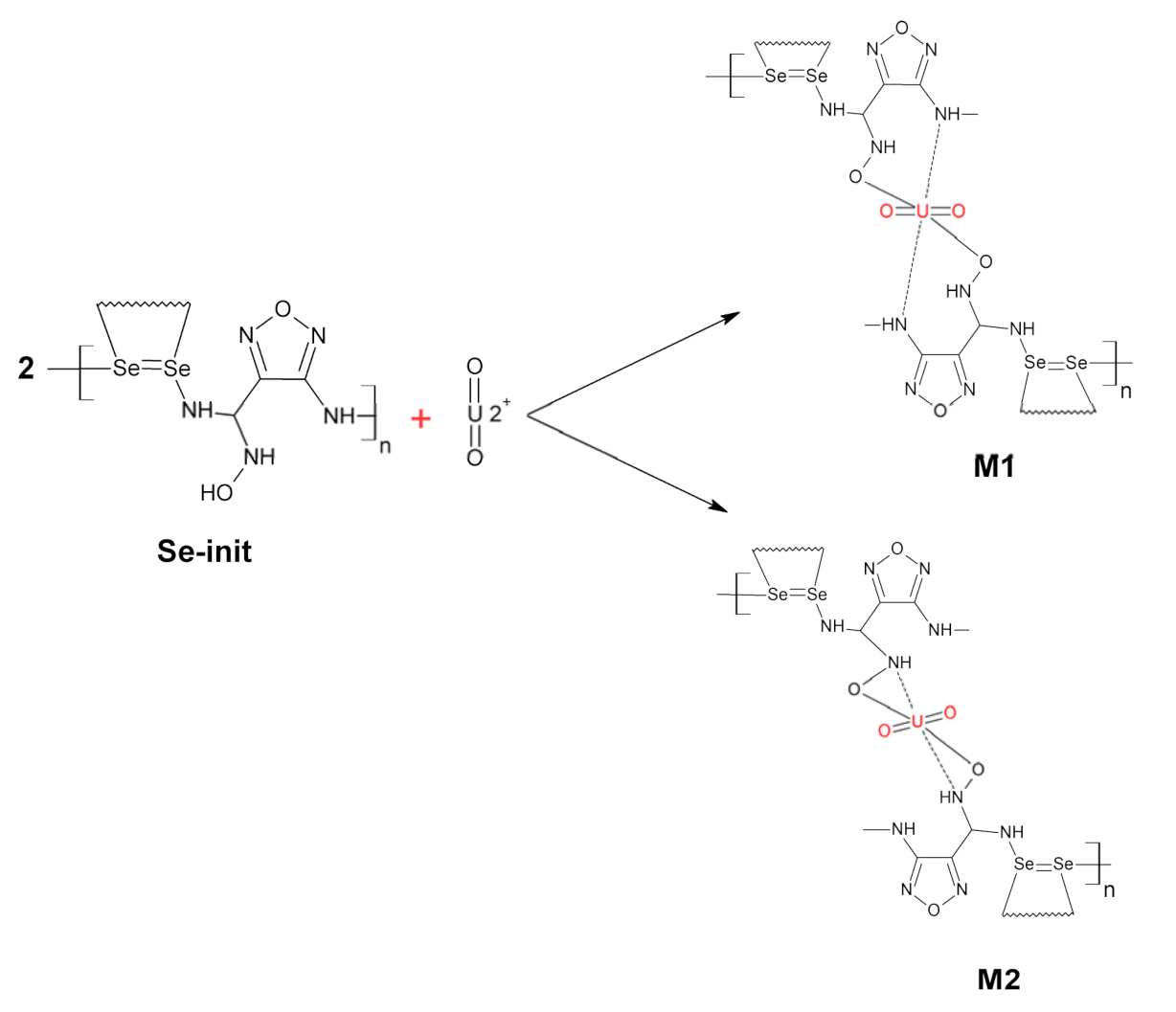
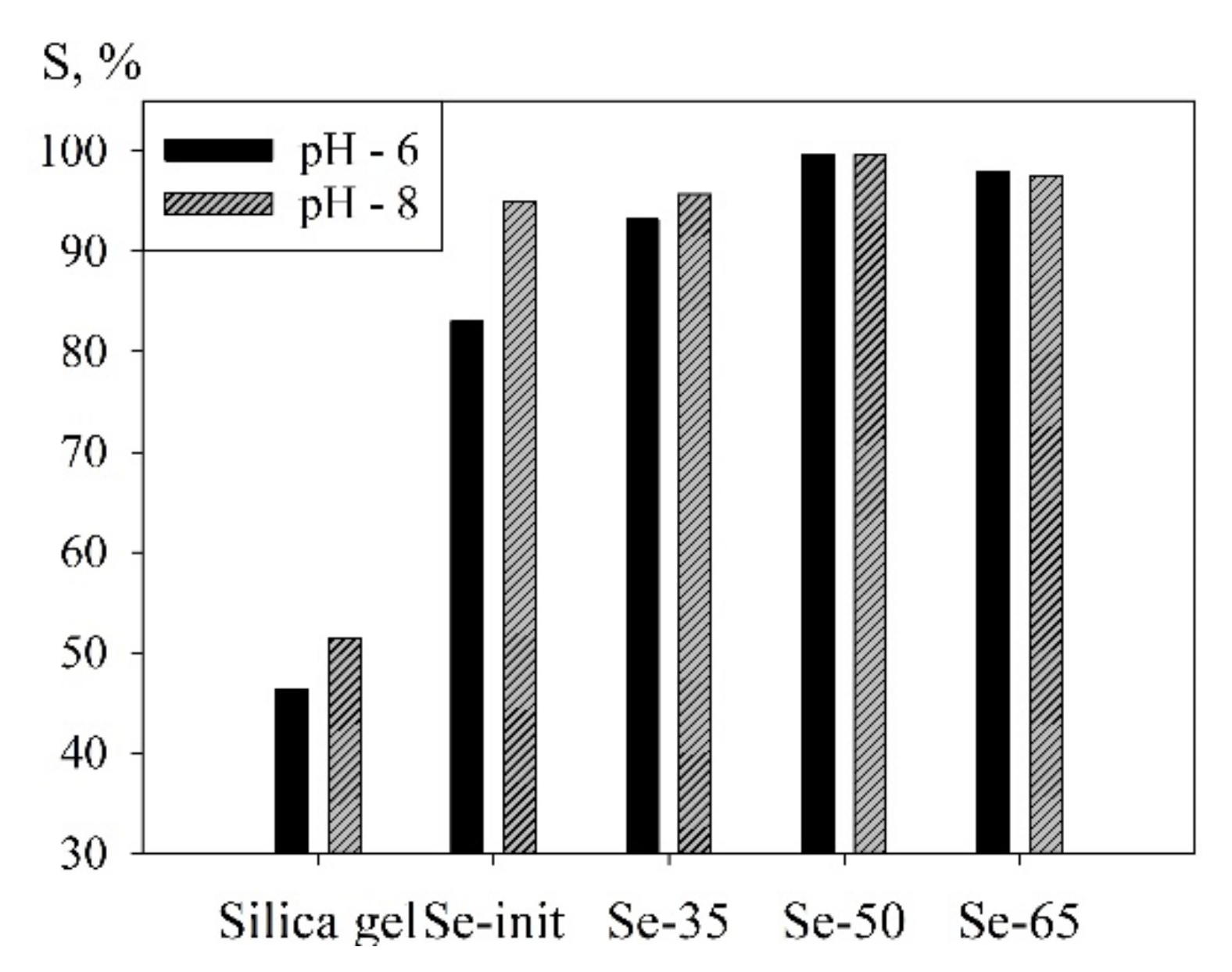
3. Results and Discussion
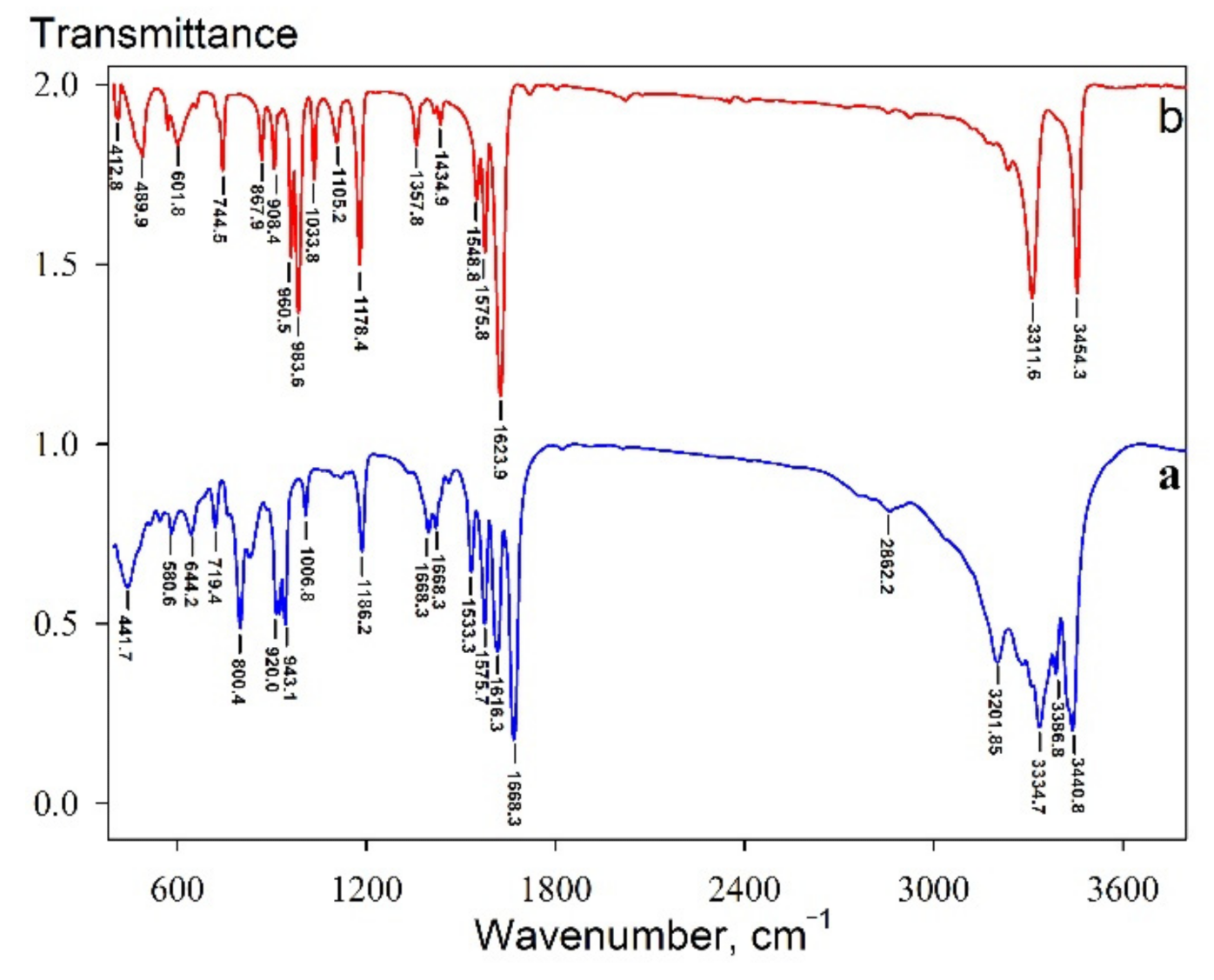
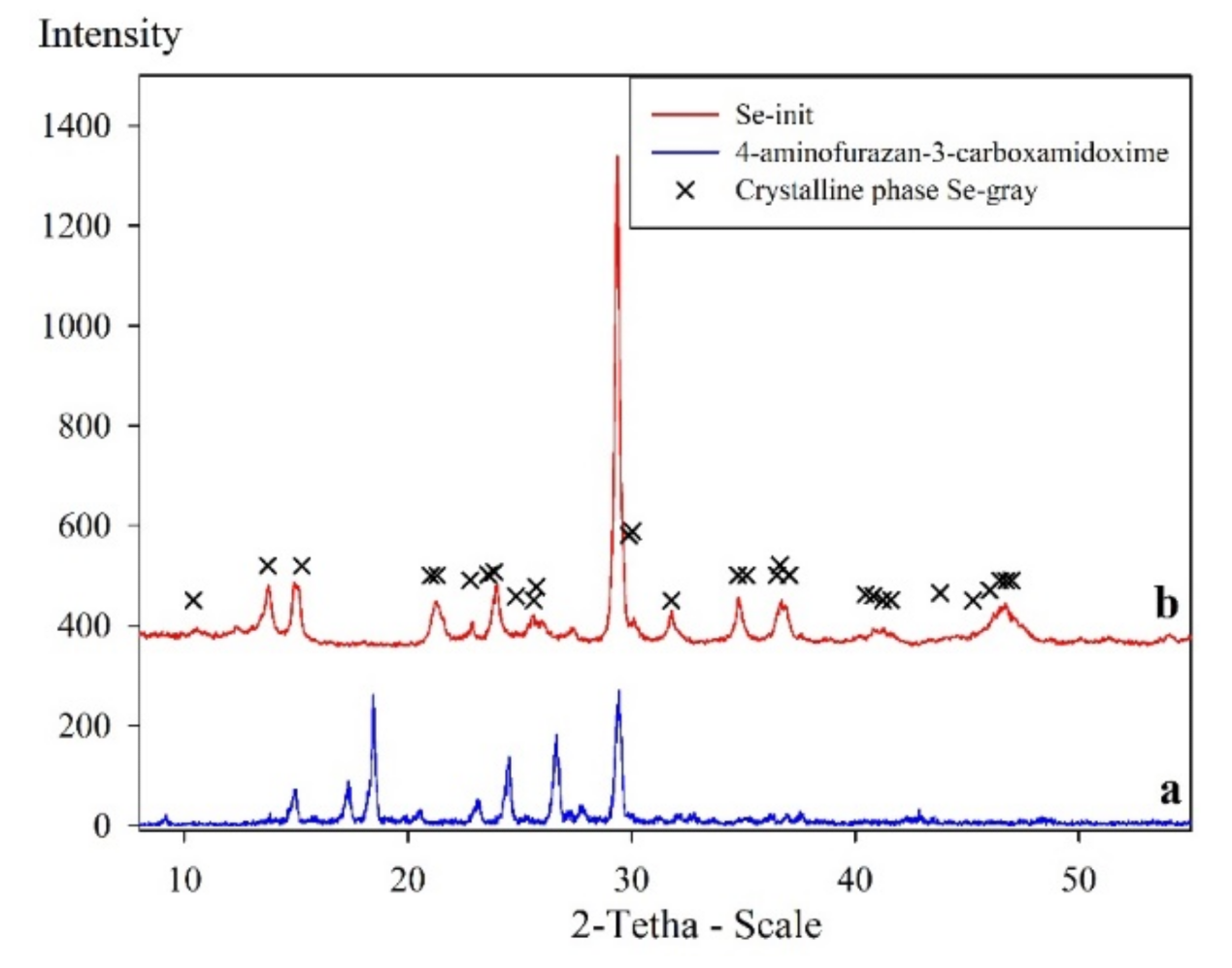
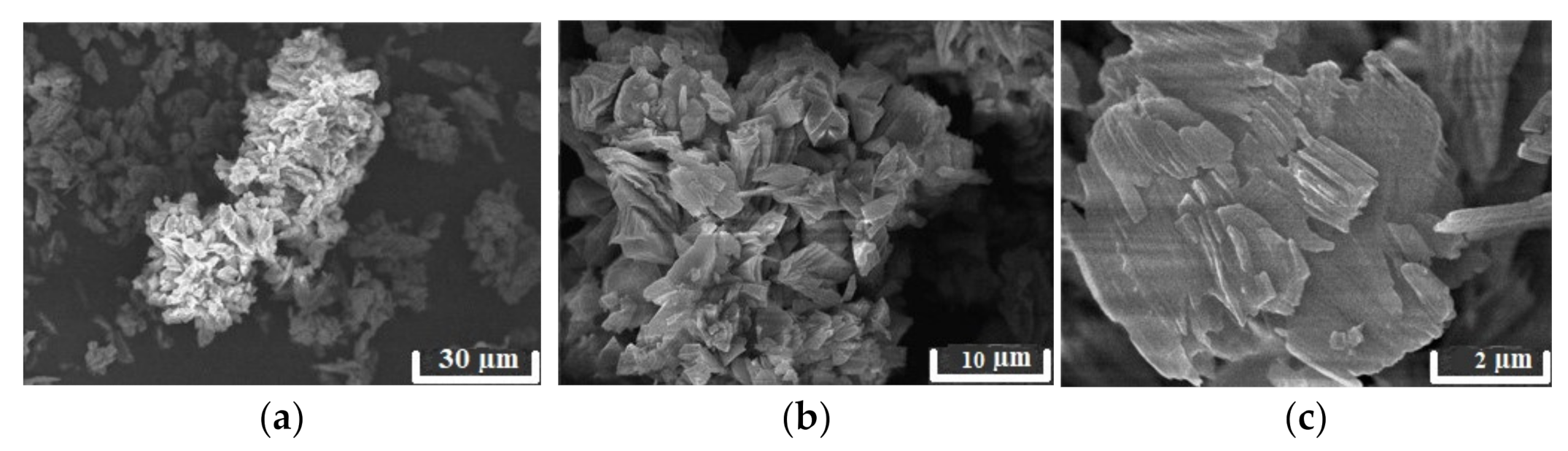
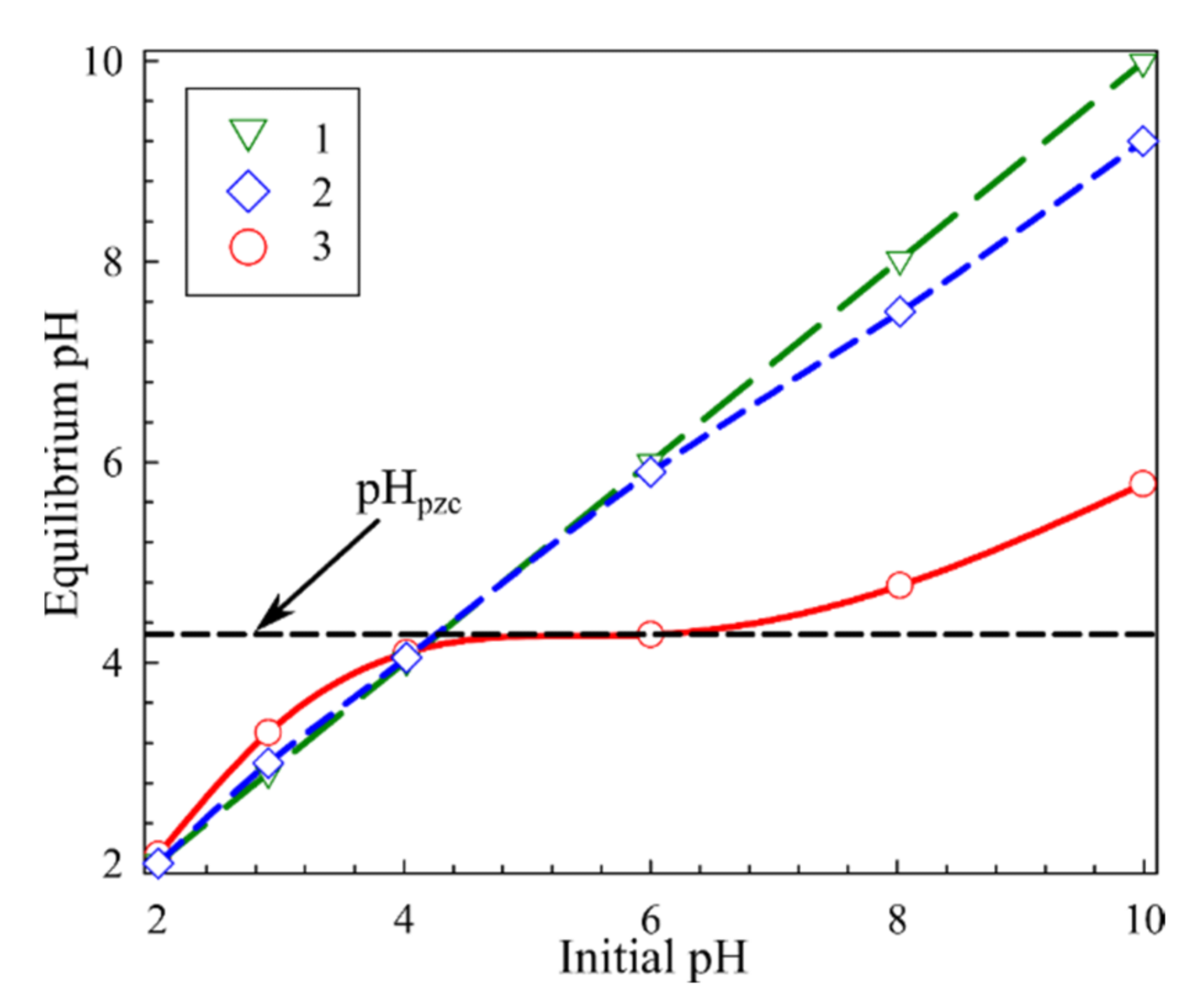
3.1. Physiochemical Properties
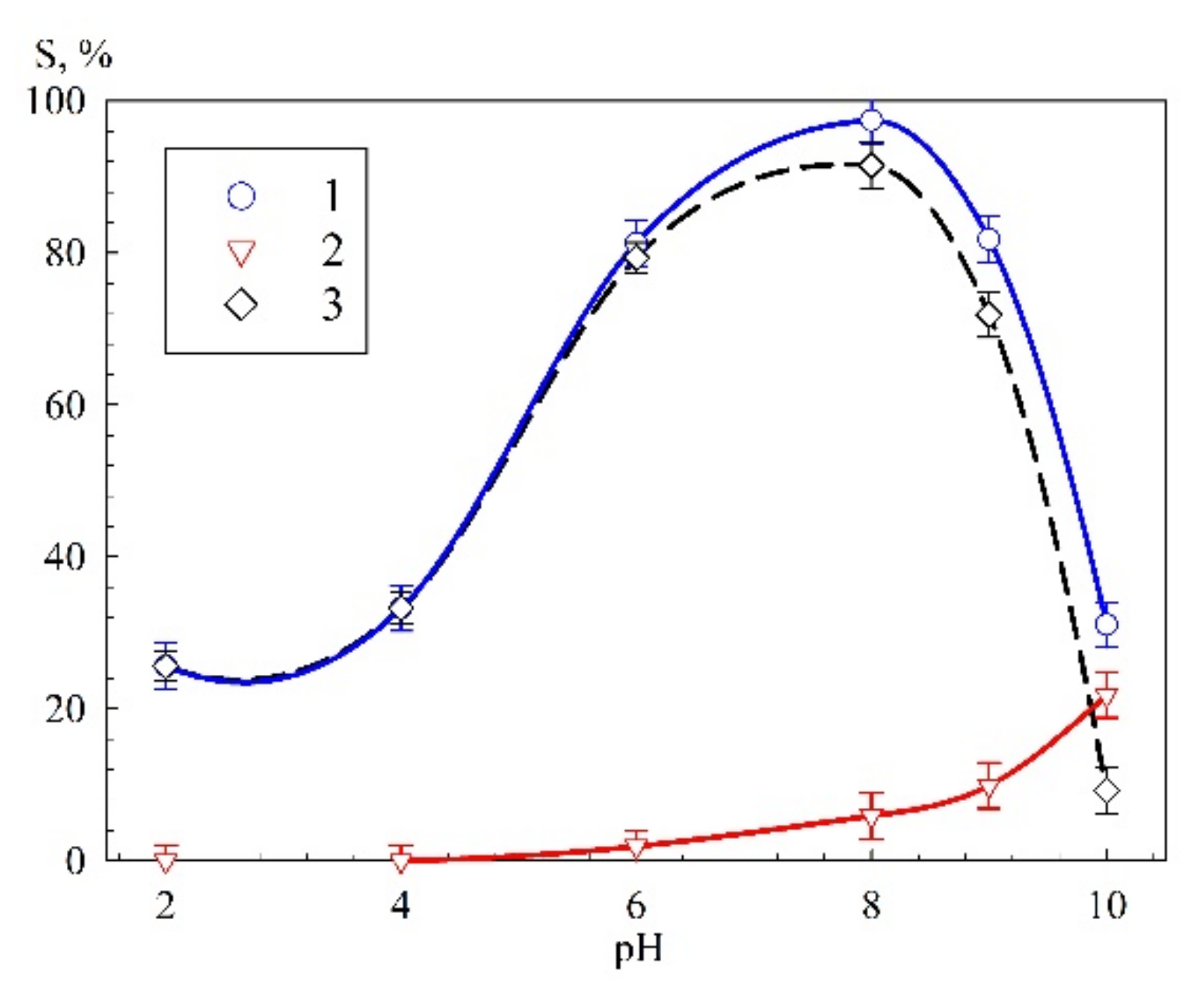
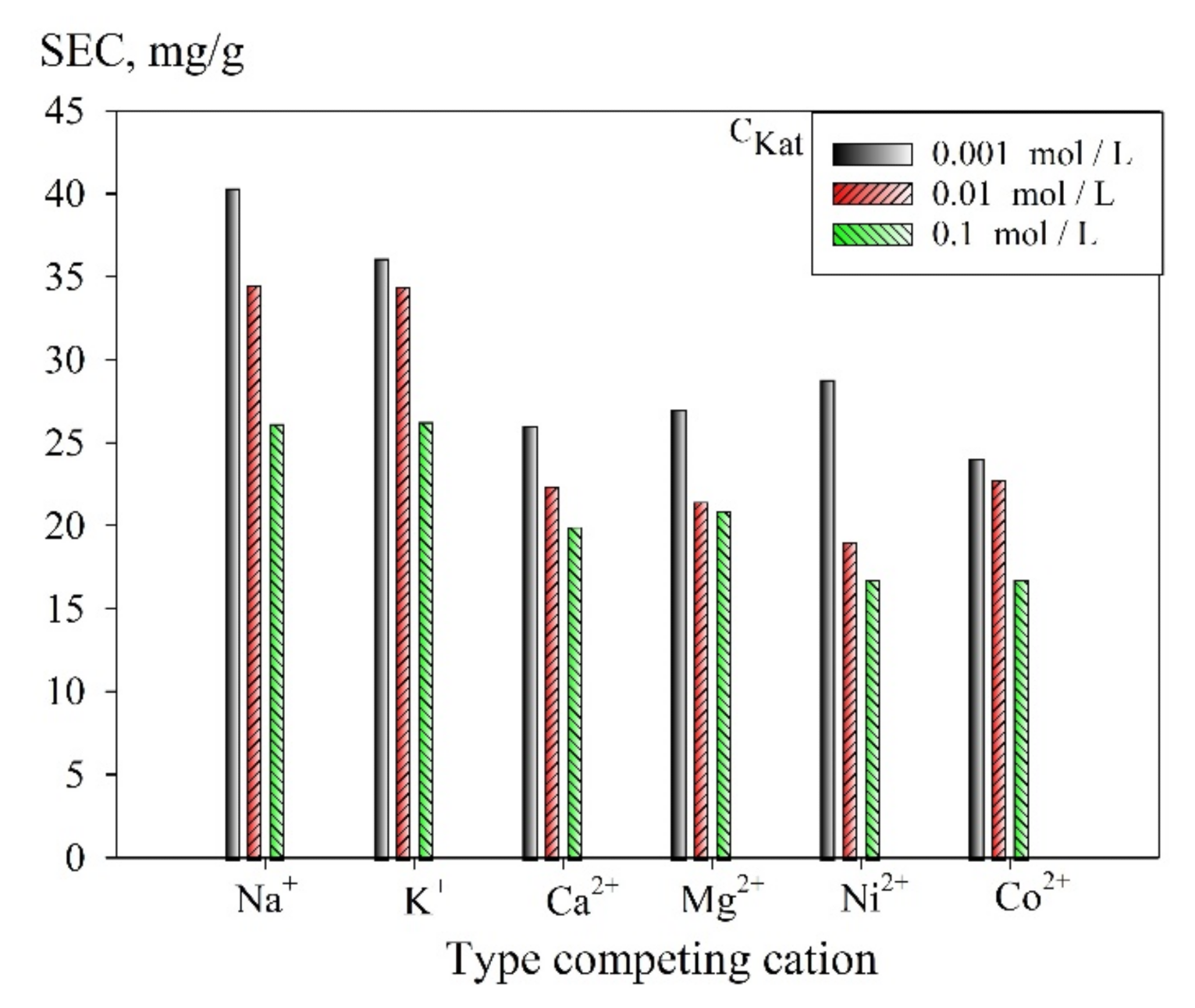
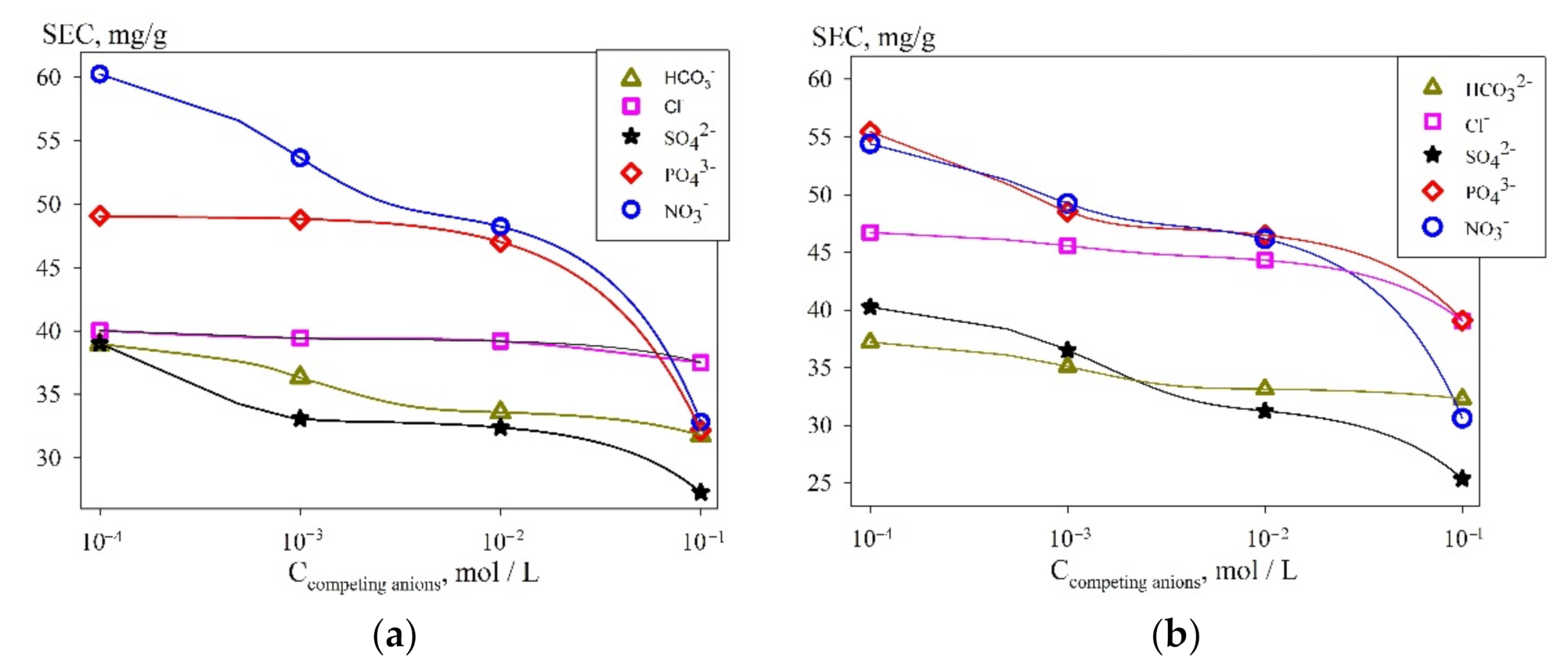
3.2. Sorption Selective Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schnug, E.; Lottermoser, B.G. Fertilizer-Derived Uranium and Its Threat to Human Health. Environ. Sci. Technol. 2013, 47, 2433–2434. [Google Scholar] [CrossRef]
- Beltrami, D.; Cote, G.; Mokhtari, H.; Courtaud, B.; Moyer, B.A.; Chagnes, A. Recovery of Uranium from Wet Phosphoric Acid by Solvent Extraction Processes. Chem. Rev. 2014, 114, 12002–12023. [Google Scholar] [CrossRef]
- Shen, J.; Schäfer, A. Removal of Fluoride and Uranium by Nanofiltration and Reverse Osmosis: A Review. Chemosphere 2014, 117, 679–691. [Google Scholar] [CrossRef]
- Chen, K.; Chen, C.; Ren, X.; Alsaedi, A.; Hayat, T. Interaction Mechanism between Different Facet TiO2 and U(VI): Experimental and Density-Functional Theory Investigation. Chem. Eng. J. 2019, 359, 944–954. [Google Scholar] [CrossRef]
- Singh, D.K.; Hareendran, K.N.; Sreenivas, T.; Kain, V.; Dey, G.K. Development of a Phosphate Precipitation Method for the Recovery of Uranium from Lean Tenor Alkaline Leach Liquor. Hydrometallurgy 2017, 171, 228–235. [Google Scholar] [CrossRef]
- Li, P.; Zhun, B.; Wang, X.; Liao, P.; Wang, G.; Wang, L.; Guo, Y.; Zhang, W. Highly Efficient Interception and Precipitation of Uranium(VI) from Aqueous Solution by Iron-Electrocoagulation Combined with Cooperative Chelation by Organic Ligands. Environ. Sci. Technol. 2017, 51, 14368–14378. [Google Scholar] [CrossRef]
- Bhalara, P.D.; Punetha, D.; Balasubramanian, K. A Review of Potential Remediation Techniques for Uranium(VI) Ion Retrieval from Contaminated Aqueous Environment. J. Environ. Chem. Eng. 2014, 2, 1621–1634. [Google Scholar] [CrossRef]
- Pauling, L. The Structure of the Chlorites. Proc. Natl. Acad. Sci. USA 1930, 16, 578–582. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, M.M.; Baeyens, B.; Dähn, R.; Scheinost, A.C.; Bradbury, M.H. U(VI) Sorption on Montmorillonite in the Absence and Presence of Carbonate: A Macroscopic and Microscopic Study. Geochim. Cosmochim. Acta 2012, 93, 262–277. [Google Scholar] [CrossRef]
- Psareva, T.S.; Zakutevsk, O.I.; Strelko, V.V. Sorption of Uranium by Titanosilicate Ion Exchanger. Rep. NAS Ukr. 2003, 130–135. (In Russian) [Google Scholar]
- Lv, Z.; Wang, H.; Chen, C.; Yang, S.; Chen, L.; Alsaedi, A.; Hayat, T. Enhanced Removal of Uranium(VI) from Aqueous Solution by a Novel Mg-MOF-74-Derived Porous MgO/Carbon Adsorbent. J. Colloid Interface Sci. 2019, 537, A1–A10. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Bao, H.; Wu, Q.; Wang, H.; Gai, T.; Shao, L.; Wang, S.; Tang, H.; Li, Y.; Wang, X. The Physical Chemistry of Uranium (VI) Immobilization on Manganese Oxides. J. Hazard. Mater. 2020, 391, 122207. [Google Scholar] [CrossRef] [PubMed]
- Papynov, E.K.; Tkachenko, I.A.; Maiorov, V.Y.; Pechnikov, V.S.; Fedorets, A.N.; Portnyagin, A.S.; Dran’kov, A.N.; Buravlev, I.Y.; Grishin, A.V.; Tananaev, I.G.; et al. Nanostructured Magnetic Sorbents for Selective Recovery of Uranium(VI) from Aqueous Solutions. Radiochemistry 2019, 61, 28–36. [Google Scholar] [CrossRef]
- Amesh, P.; Suneesh, A.S.; Selvan, B.R.; Venkatesan, K.A.; Chandra, M. Magnetic Assisted Separation of Uranium(VI) from Aqueous Phase Using Diethylenetriamine Modified High Capacity Iron Oxide Adsorbent. J. Environ. Chem. Eng. 2020, 8, 103661. [Google Scholar] [CrossRef]
- Pshinko, G.N. Impact of Humic Matter on Sorption of Radionuclides by Montmorrilonite. J. Water Chem. Technol. 2009, 31, 163–171. [Google Scholar] [CrossRef]
- Zheleznov, V.V.; Maiorov, V.Y.; Polyakova, N.V.; Silant’ev, V.E.; Sokol’nitskaya, T.A.; Sushkov, Y.V.; Voit, E.I. Sorption of U(VI) onto TiO2/ZrO2/SiO2 Mesoporous Materials from Sulfate Solutions. Radiochemistry 2018, 60, 618–624. [Google Scholar] [CrossRef]
- Müller, K.; Foerstendorf, H.; Brendler, V.; Rossberg, A.; Stolze, K.; Gröschel, A. The Surface Reactions of U(VI) on γ-Al2O3—In Situ Spectroscopic Evaluation of the Transition from Sorption Complexation to Surface Precipitation. Chem. Geol. 2013, 357, 75–84. [Google Scholar] [CrossRef]
- Latham, A.H.; Williams, M.E. Controlling Transport and Chemical Functionality of Magnetic Nanoparticles. Acc. Chem. Res. 2008, 41, 411–420. [Google Scholar] [CrossRef]
- Strelko, V.V.; Psareva, T.S.; Zakutevskij, O.I.; Kanibolotskij, V.A.; Meleshevich, S.I. Sorption of U by titanosilicate ionites. Dopov. Natsyional’noyi Akad. Nauk Ukrayini 2005, 37, 142–147. (In Russian) [Google Scholar]
- Kosyakov, V.N.; Veleshko, I.E.; Yakovlev, N.G.; Gorovoi, L.F. Preparation, Properties, and Application of Modified Mikoton Sorbents. Radiochemistry 2004, 46, 385–390. [Google Scholar] [CrossRef]
- Veleshko, I.E.; Veleshko, A.N.; Rumyantseva, E.V. Chitin and Chitosan Based Materials from Different Sources for Radionuclides Adsorption. Prikladnaya Fizika i Matematika 2015, 3, 12–18. (In Russian) [Google Scholar]
- Szlachta, M.; Neitola, R.; Peräniemi, S.; Vepsäläinen, J. Effective Separation of Uranium from Mine Process Effluents Using Chitosan as a Recyclable Natural Adsorbent. Sep. Purif. Technol. 2020, 253, 117493. [Google Scholar] [CrossRef]
- Kumar, G.P.; Kumar, P.A.; Chakraborty, S.; Ray, M. Uptake and Desorption of Copper Ion Using Functionalized Polymer Coated Silica Gel in Aqueous Environment. Sep. Purif. Technol. 2007, 57, 47–56. [Google Scholar] [CrossRef]
- Sun, S.; Wang, A. Adsorption Properties of Carboxymethyl-Chitosan and Cross-Linked Carboxymethyl-Chitosan Resin with Cu(II) as Template. Sep. Purif. Technol. 2006, 49, 197–204. [Google Scholar] [CrossRef]
- Gao, B.; Gao, Y.; Li, Y. Preparation and Chelation Adsorption Property of Composite Chelating Material Poly(Amidoxime)/SiO2 towards Heavy Metal Ions. Chem. Eng. J. 2010, 158, 542–549. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, J.; Zhao, L.; Zhang, S.; Huang, Y.; Wu, X.; Wang, X. Synthesis of Amidoxime-Functionalized Fe3O4@SiO2 Core–Shell Magnetic Microspheres for Highly Efficient Sorption of U(VI). Chem. Eng. J. 2014, 235, 275–283. [Google Scholar] [CrossRef]
- Hirotsu, T.; Katoh, S.; Sugasaka, K.; Sen, M.; Itagaki, T. Binding Properties of a Polymer Having Amidoxime Groups with Proton and Metal Ions. Sep. Sci. Technol. 1986, 21, 1101–1110. [Google Scholar] [CrossRef]
- Yu, S.; Wang, X.; Tan, X.; Wang, X. Sorption of Radionuclides from Aqueous Systems onto Graphene Oxide-Based Materials: A Review. Inorg. Chem. Front. 2015, 2, 593–612. [Google Scholar] [CrossRef]
- Das, S.; Oyola, Y.; Mayes, R.T.; Janke, C.J.; Kuo, L.-J.; Gill, G.; Wood, J.R.; Dai, S. Extracting Uranium from Seawater: Promising AF Series Adsorbents. Ind. Eng. Chem. Res. 2016, 55, 4110–4117. [Google Scholar] [CrossRef]
- Antonik, L.M.; Khabibulina, A.G. Polyoximes: Synthesis, Structure, and Properties. Polym. Sci. Ser. C 2004, 46, 37–48. (In Russian) [Google Scholar]
- Al-Harahsheh, M.; AlJarrah, M.; Mayyas, M.; Alrebaki, M. High-Stability Polyamine/Amide-Functionalized Magnetic Nanoparticles for Enhanced Extraction of Uranium from Aqueous Solutions. J. Taiwan Inst. Chem. Eng. 2018, 86, 148–157. [Google Scholar] [CrossRef]
- Li, B.; Sun, Q.; Zhang, Y.; Abney, C.W.; Aguila, B.; Lin, W.; Ma, S. Functionalized Porous Aromatic Framework for Efficient Uranium Adsorption from Aqueous Solutions. ACS Appl. Mater. Interfaces 2017, 9, 12511–12517. [Google Scholar] [CrossRef] [PubMed]
- Lindner, H.; Schneider, E. Review of Cost Estimates for Uranium Recovery from Seawater. Energy Econ. 2015, 49, 9–22. [Google Scholar] [CrossRef]
- Stepanov, A. The usage OF AMIDOXOME of 4-amonofurazan -3-carboxylic acid in the synthesis of heterocyclic compound S (review). Bull. St PbSIT(TU) 2014, 51, 32–46. [Google Scholar] [CrossRef][Green Version]
- Tokar, E.A.; Maslov, K.V.; Egorin, A.M. Selenium-Derivative of N-Hydroxyamidine Aminofurazan for Extraction of Uranium from Liquid Media Patent RU 2741909C1. Priority to RU2020128578A 2021. Available online: https://patents.google.com/patent/RU2741909C1/en?oq=Selenium-derivative+of+N-hydroxyamidine+aminofurazan+for+extraction+of+uranium+from+liquid+media+Patent+RU+2741909+C1 (accessed on 25 August 2021).
- Busev, A.I.; Tiptsova, V.G.; Ivanov, V.M. Guide to the Analytical Chemistry of Rare Elements.-Rec. and Add. M Chem. 1978, 2432. (In Russian) [Google Scholar]
- Putilina, V.S.; Galitskaia, I.V.; Iuganova, T.I. Sorbtsionnye Protsessy pri Zagriaznenii Podzemnykh vod TiAZhelymi Metallami i Radioaktivnymi Ėlementami; URAN: Analiticheskiĭ obzor; Ėkologiia; GPNTB SO RAN: Novosibirsk, Russia, 2014; ISBN 9785945602533. [Google Scholar]
- Bogolepov, A.A.; Pshinko, G.N.; Kornilovich, B.Y. The Impact of Complexing Agents on the Processes of Sorption Treatment of Waters Containing Uranium. J. Water Chem. Technol. 2007, 29, 9–14. [Google Scholar] [CrossRef]
- Uranium Geochemistry and Kd Values//Understanding Variation in Partition Coefficient, Kd. Values. In Geochemistry and Available Kd Values for Selected Inorganic Contaminants; Chapter 5; EPA 402-R-99-004B; US EPA, US DOE: Washington, DC, USA, 1999; Volume 2, pp. 5.65–5.77. Available online: http://www.epa.gov/radiation/docs/kdreport/vol2/402-r-99-004b_ch5b.pdf (accessed on 20 August 1999).
- Giles, C.H.; Smith, D.; Huitson, A. A General Treatment and Classification of the Solute Adsorption Isotherm. I. Theoretical. J. Colloid Interface Sci. 1974, 47, 755–765. [Google Scholar] [CrossRef]
- Da’na, E. Adsorption of Heavy Metals on Functionalized-Mesoporous Silica: A Review. Microporous Mesoporous Mater. 2017, 247, 145–157. [Google Scholar] [CrossRef]
- Sandoval, O.G.M.; Trujillo, G.C.D.; Orozco, A.E.L. Amorphous Silica Waste from a Geothermal Central as an Adsorption Agent of Heavy Metal Ions for the Regeneration of Industrial Pre-Treated Wastewater. Water Resour. Ind. 2018, 20, 15–22. [Google Scholar] [CrossRef]

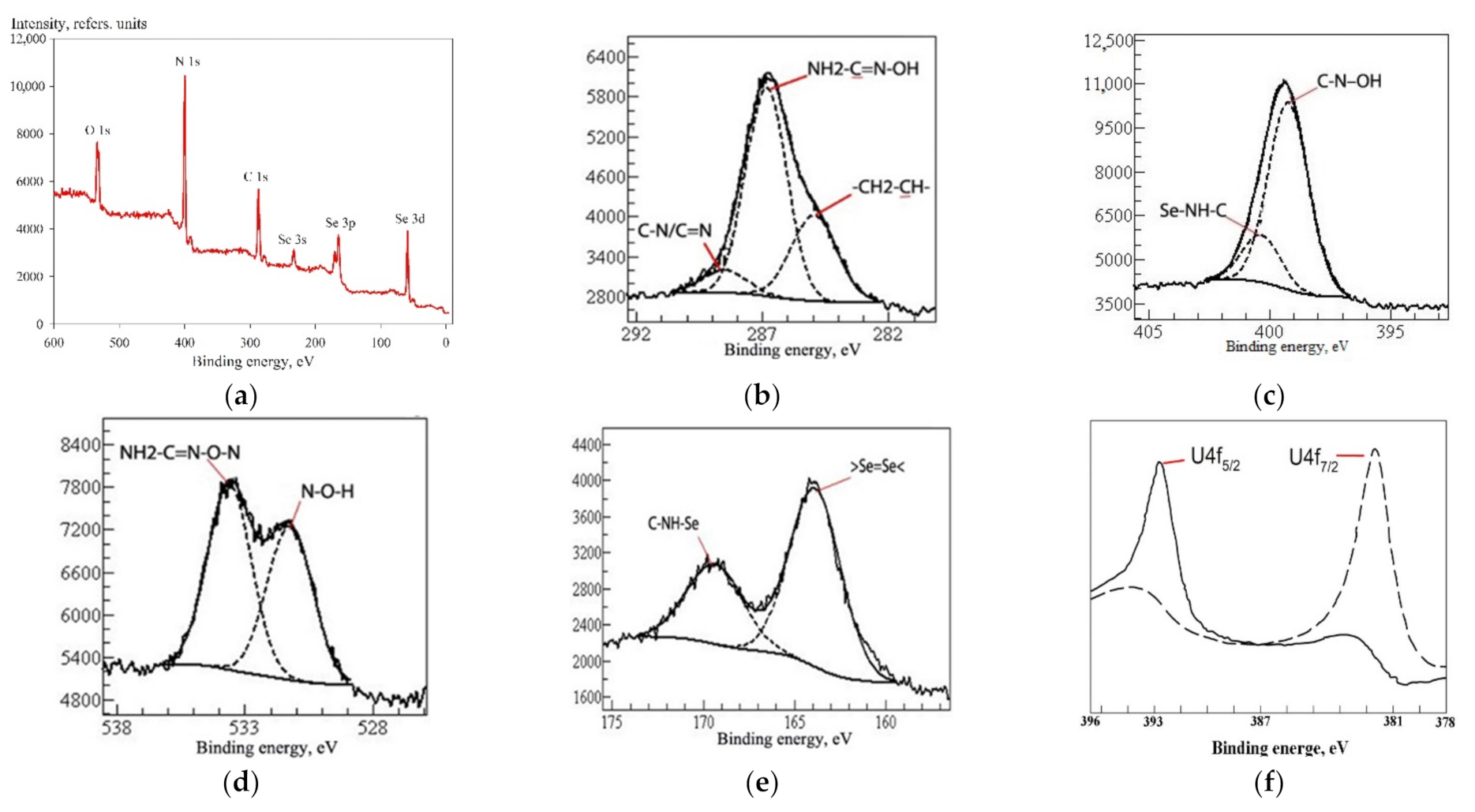

| Analysis Method | C, at.% | N, at.% | O, at.% | Se, at.% |
|---|---|---|---|---|
| Theoretical | 29.97 | 46.70 | 16.67 | 6.66 |
| EDS Analysis | 29.41 | 44.38 | 17.94 | 8.28 |
| XPS | 33.80 | 45.20 | 16.10 | 6.85 |
| REM | 31.88 | 43.97 | 17.05 | 6.50 |
| pH Initial | Kd × 10−3, cm3 g−1 | SEC, mg g−1 |
|---|---|---|
| 2 | 0.3 | 3.0 |
| 4 | 0.5 | 4.3 |
| 6 | 5.0 | 9.7 |
| 8 | 37.9 | 15.8 |
| 9 | 4.2 | 9.1 |
| 10 | 0.5 | 2.9 |
| Parameter | Si-Init | Se-35 | Se-50 | Se-65 |
|---|---|---|---|---|
| Silica gel content, wt.% | 0 | 35 | 50 | 65 |
| Pore volume, cm3 g−1 | 0.05 | 0.45 | 0.51 | 0.65 |
| Specific surface area, ml g−1 | 2 | 210 | 243 | 298 |
| Specific pore size, nm | 1.21 | 12.1 | 12.1 | 12.1 |
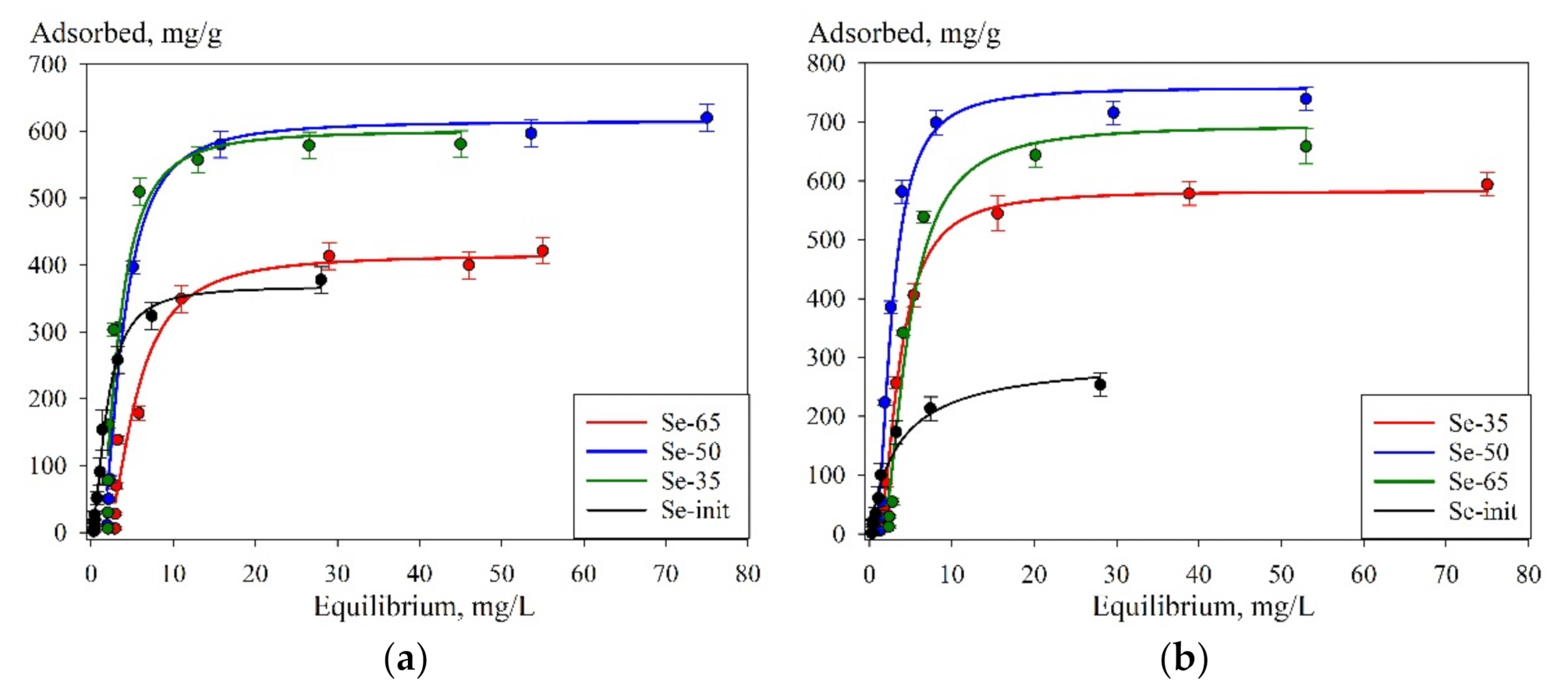
| Parameter | Se-Init | Se-35 | Se-50 | Se-65 | |
|---|---|---|---|---|---|
| pH-6 | Gmax | 370 ± 20 | 420 ± 20 | 620 ± 20 | 600 ± 30 |
| Kl | 0.24 ± 0.05 | 0.14 ± 0.07 | 0.14 ± 0.05 | 0.14 ± 0.07 | |
| R2 | 0.98 | 0.96 | 0.98 | 0.94 | |
| pH-8 | Gmax | 270 ± 10 | 580 ± 20 | 760 ± 30 | 690 ± 20 |
| Kl | 0.30 ± 0.07 | 0.19 ± 0.05 | 0.18 ± 0.07 | 0.21 ± 0.09 | |
| R2 | 0.98 | 0.99 | 0.97 | 0.98 |
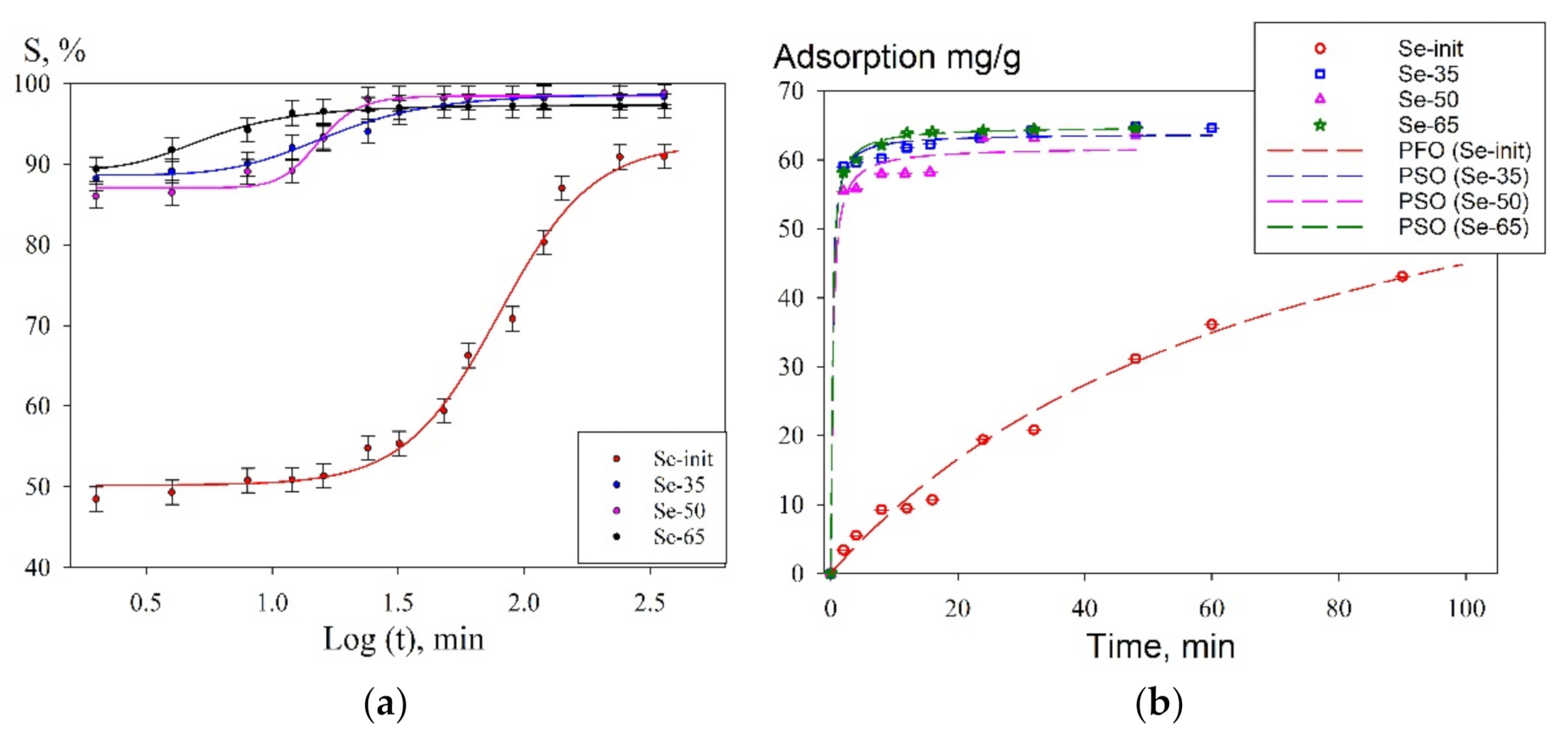
| Equation Type | R2 | A, mg g−1 | k1, min−1 | k2, mg g−1 min−1 | |
|---|---|---|---|---|---|
| Se-init | PFO | 0.999 | 54.7 ± 5.5 | 0.003 ± 0.0002 | - |
| Se-35 | 0.932 | 62.2 ± 3.7 | 0.015 ± 0.003 | - | |
| Se-50 | 0.953 | 60.1 ± 4.7 | 0.010 ± 0.002 | - | |
| Se-65 | 0.931 | 62.7 ± 3.8 | 0.012 ± 0.002 | - | |
| Se-init | PSO | 0.853 | 28.9 ± 7.7 | - | 0.80 ± 0.04 |
| Se-35 | 0.998 | 62.2 ± 1.5 | - | 9.50 ± 0.80 | |
| Se-50 | 0.999 | 62.4 ± 2.4 | - | 7.80 ± 0.60 | |
| Se-65 | 0.998 | 64.8 ± 2.5 | - | 6.60 ± 0.50 |
| Material | Se-init | Se-35 | Se-50 | Se-65 |
|---|---|---|---|---|
| Degree of destruction% | 8.1 | 5.2 | 0.8 | 0.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tokar, E.; Maslov, K.; Tananaev, I.; Egorin, A. Recovery of Uranium by Se-Derivatives of Amidoximes and Composites Based on Them. Materials 2021, 14, 5511. https://doi.org/10.3390/ma14195511
Tokar E, Maslov K, Tananaev I, Egorin A. Recovery of Uranium by Se-Derivatives of Amidoximes and Composites Based on Them. Materials. 2021; 14(19):5511. https://doi.org/10.3390/ma14195511
Chicago/Turabian StyleTokar, Eduard, Konstantin Maslov, Ivan Tananaev, and Andrei Egorin. 2021. "Recovery of Uranium by Se-Derivatives of Amidoximes and Composites Based on Them" Materials 14, no. 19: 5511. https://doi.org/10.3390/ma14195511
APA StyleTokar, E., Maslov, K., Tananaev, I., & Egorin, A. (2021). Recovery of Uranium by Se-Derivatives of Amidoximes and Composites Based on Them. Materials, 14(19), 5511. https://doi.org/10.3390/ma14195511






