GTR/NBR/Silica Composites Performance Properties as a Function of Curing System: Sulfur versus Peroxides
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Measurements
3. Results and Discussion
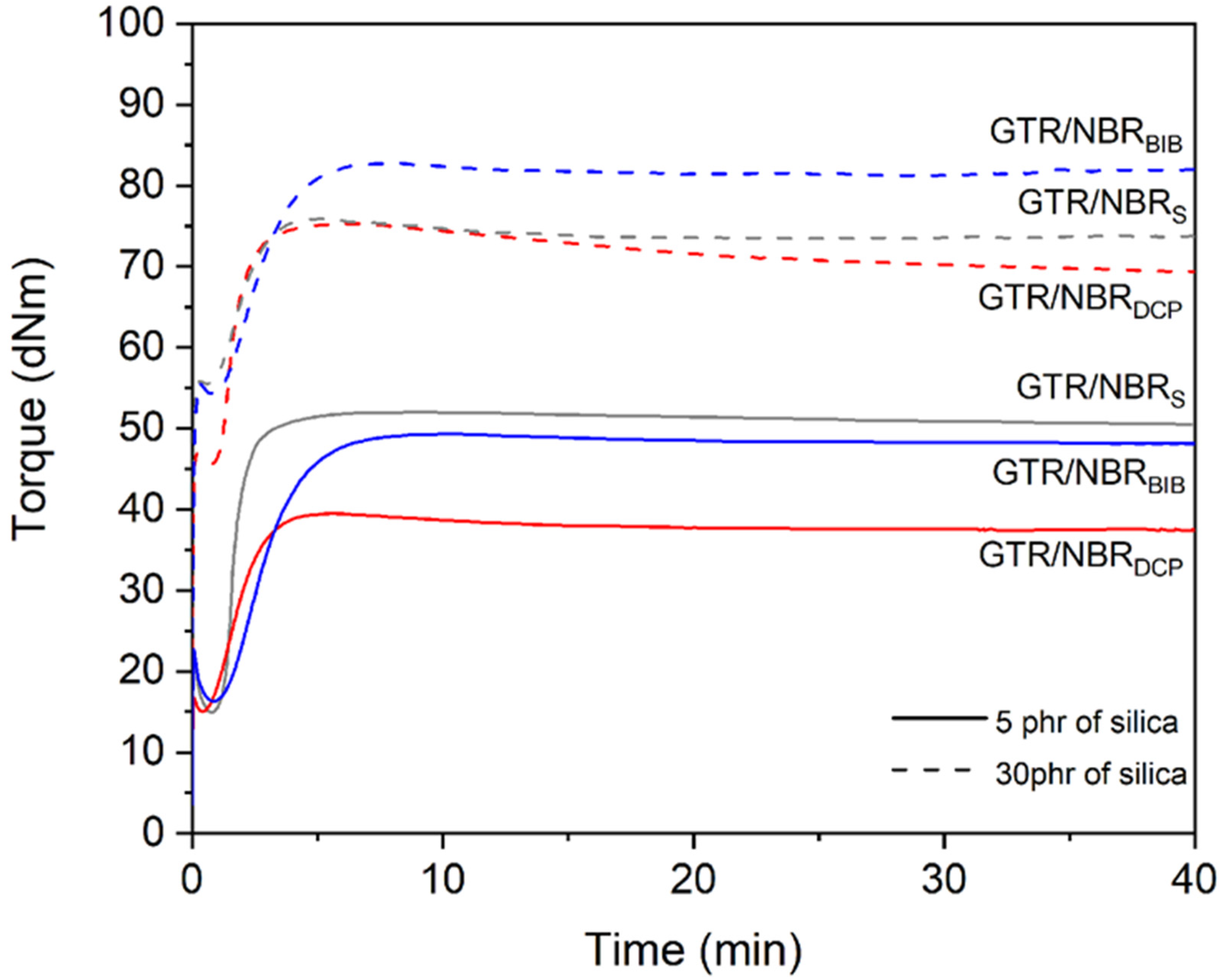
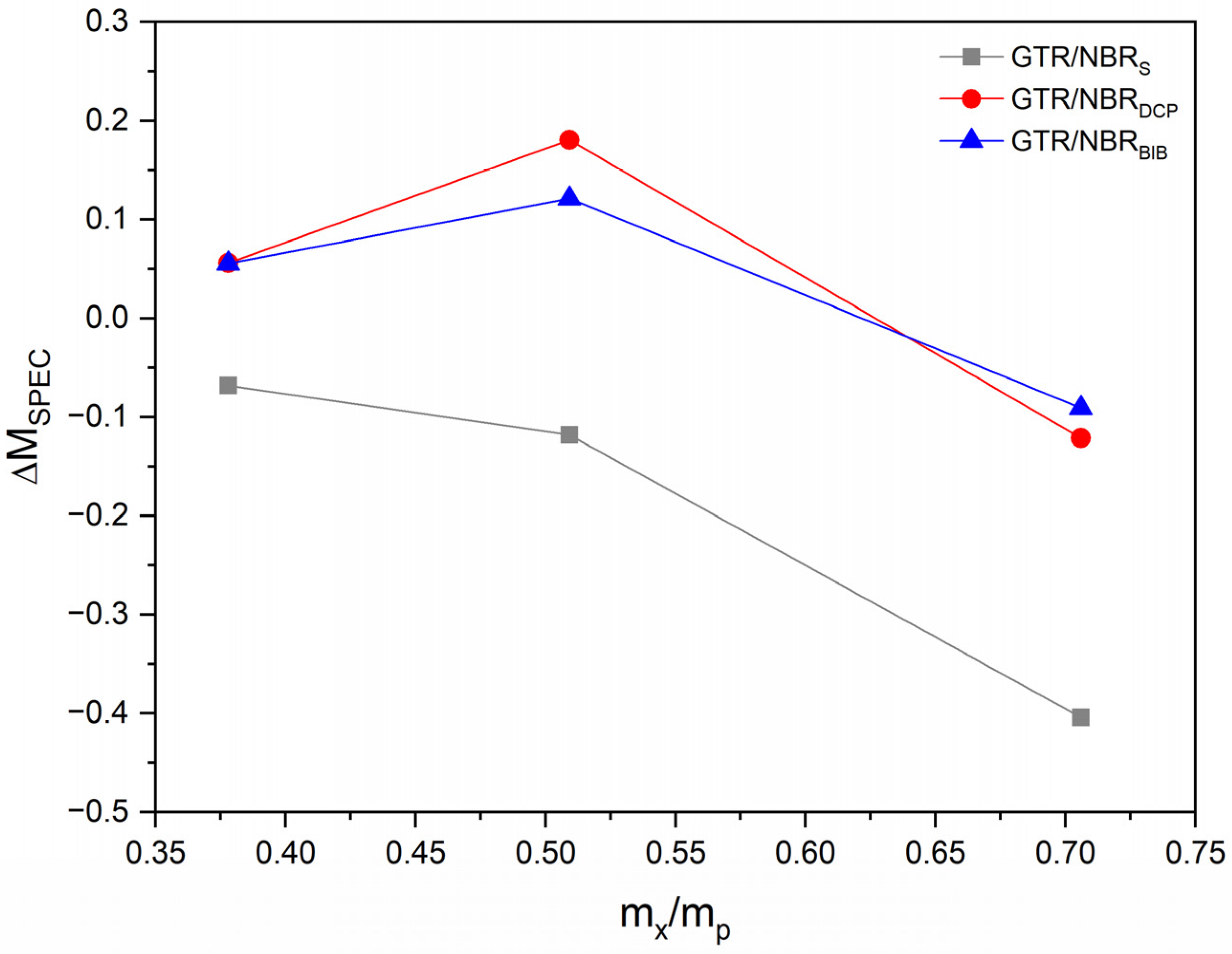
3.1. Curing Characteristics
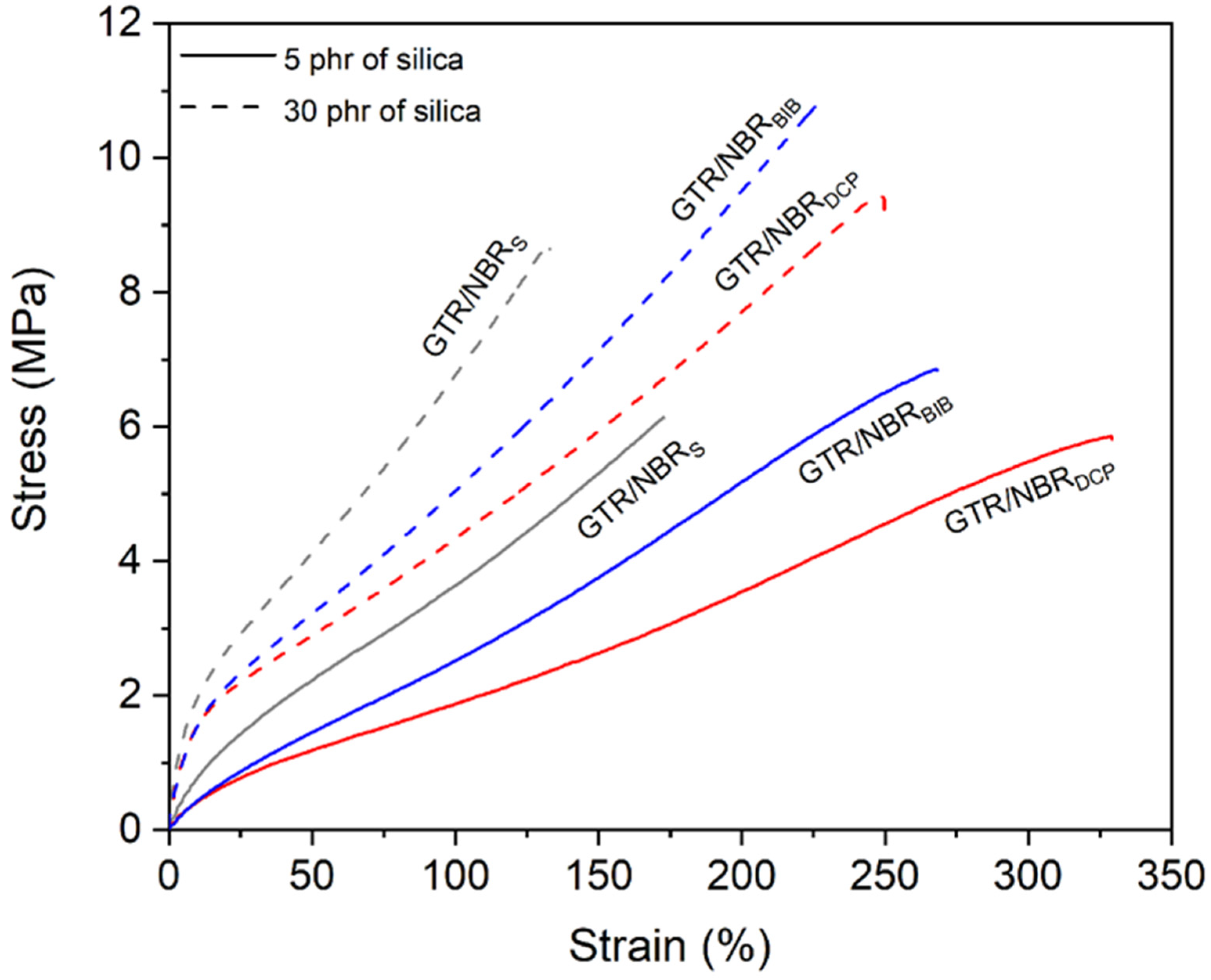
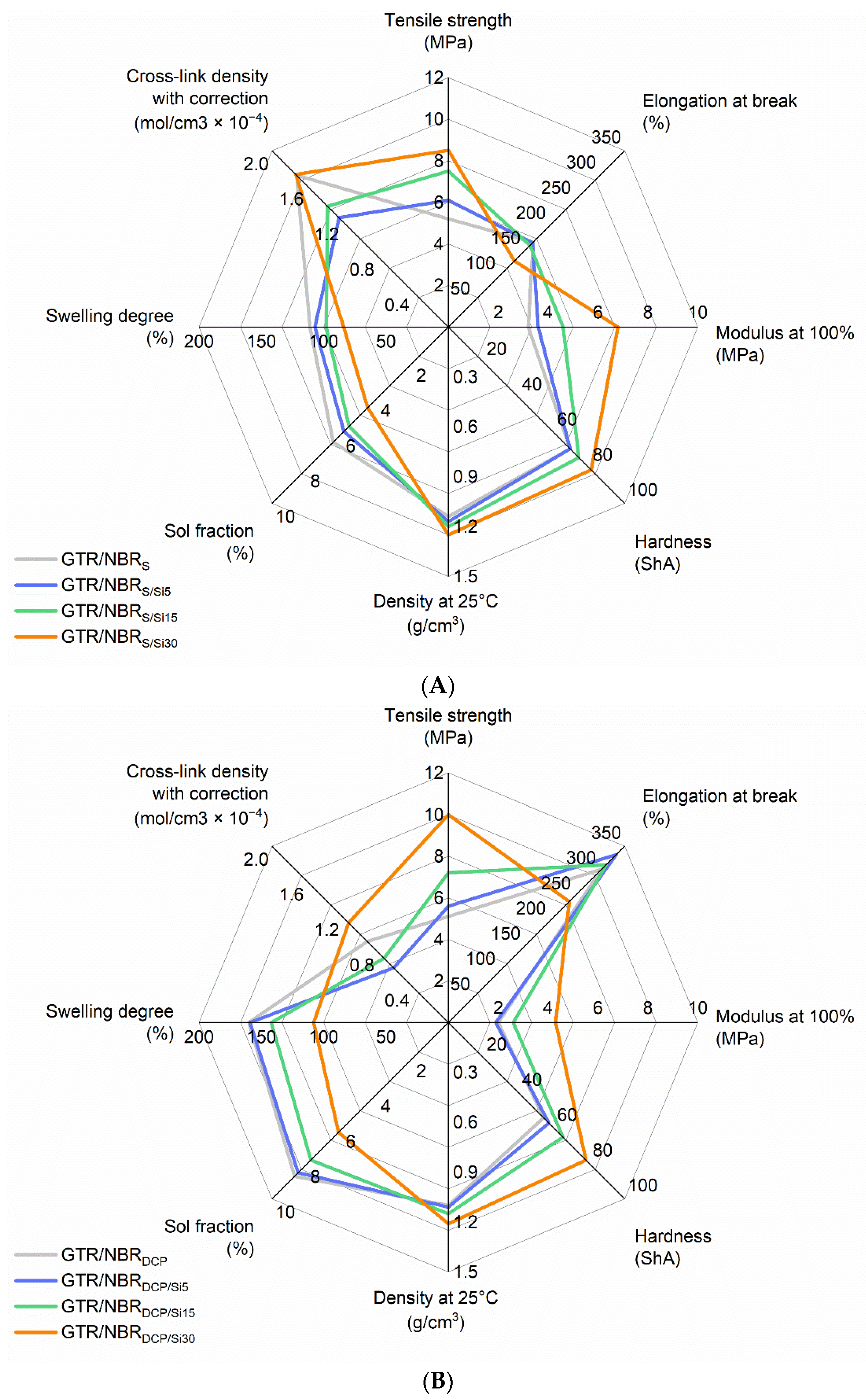
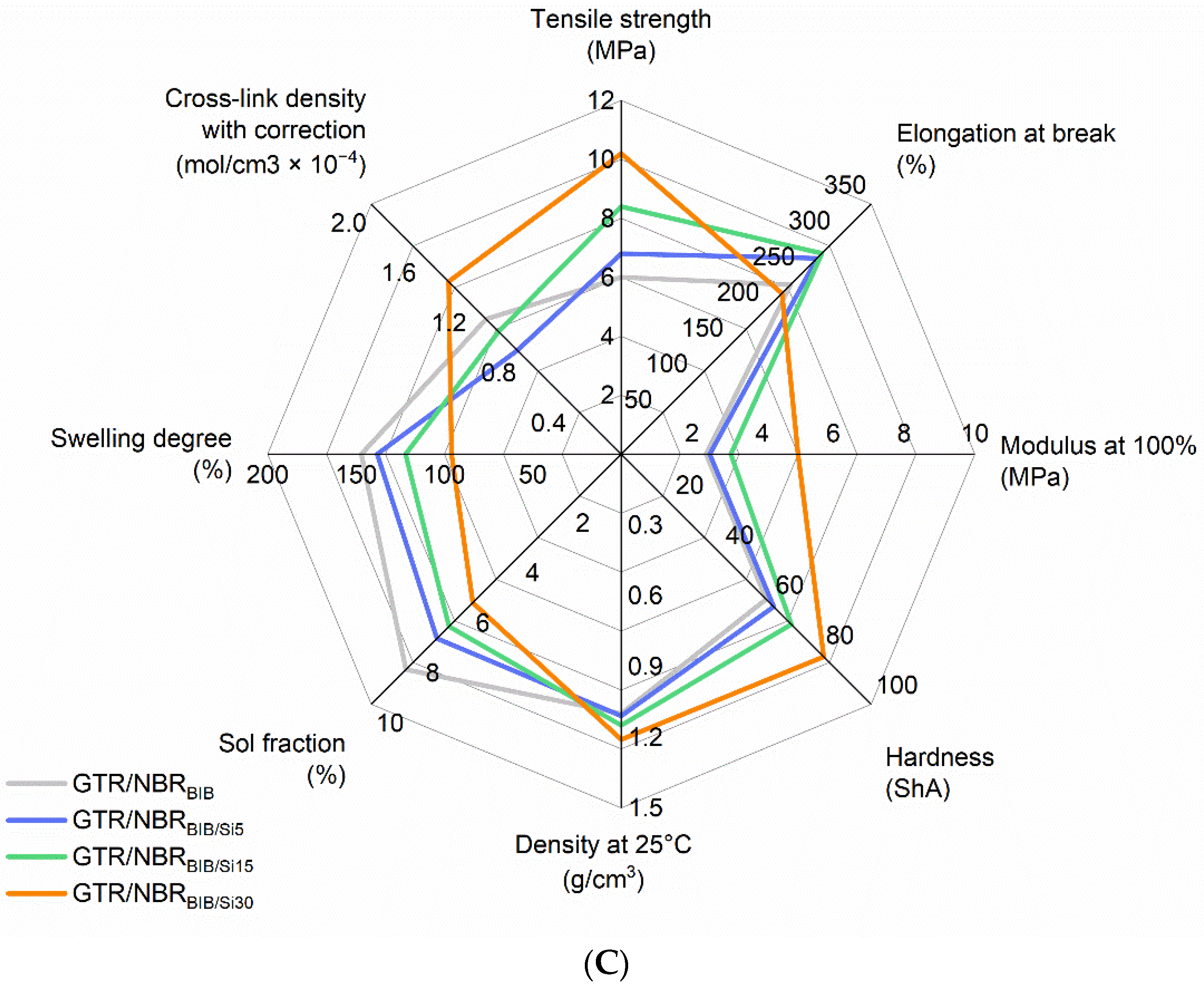
3.2. Physico-Mechanical Properties of GTR/NBR/SiO2
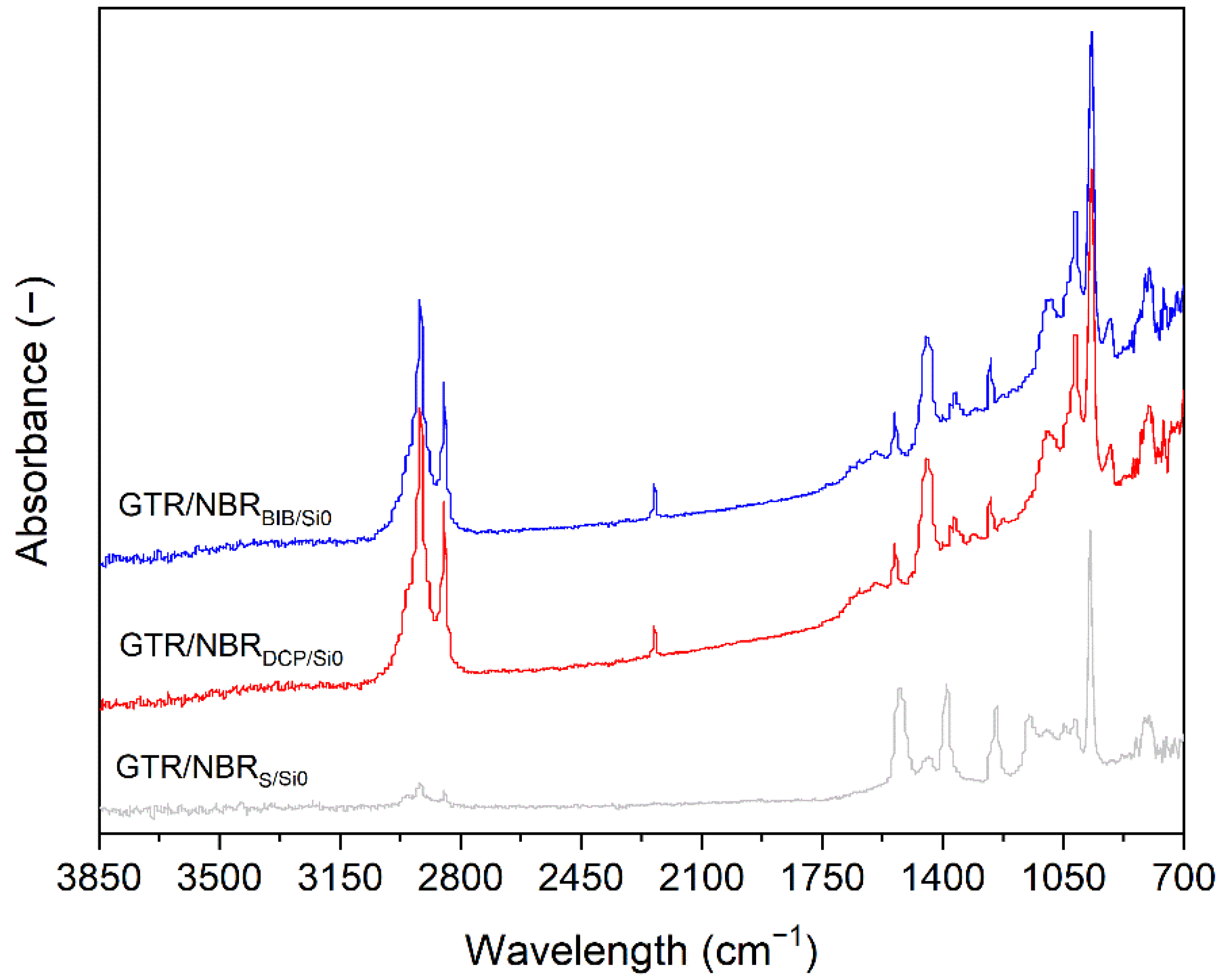
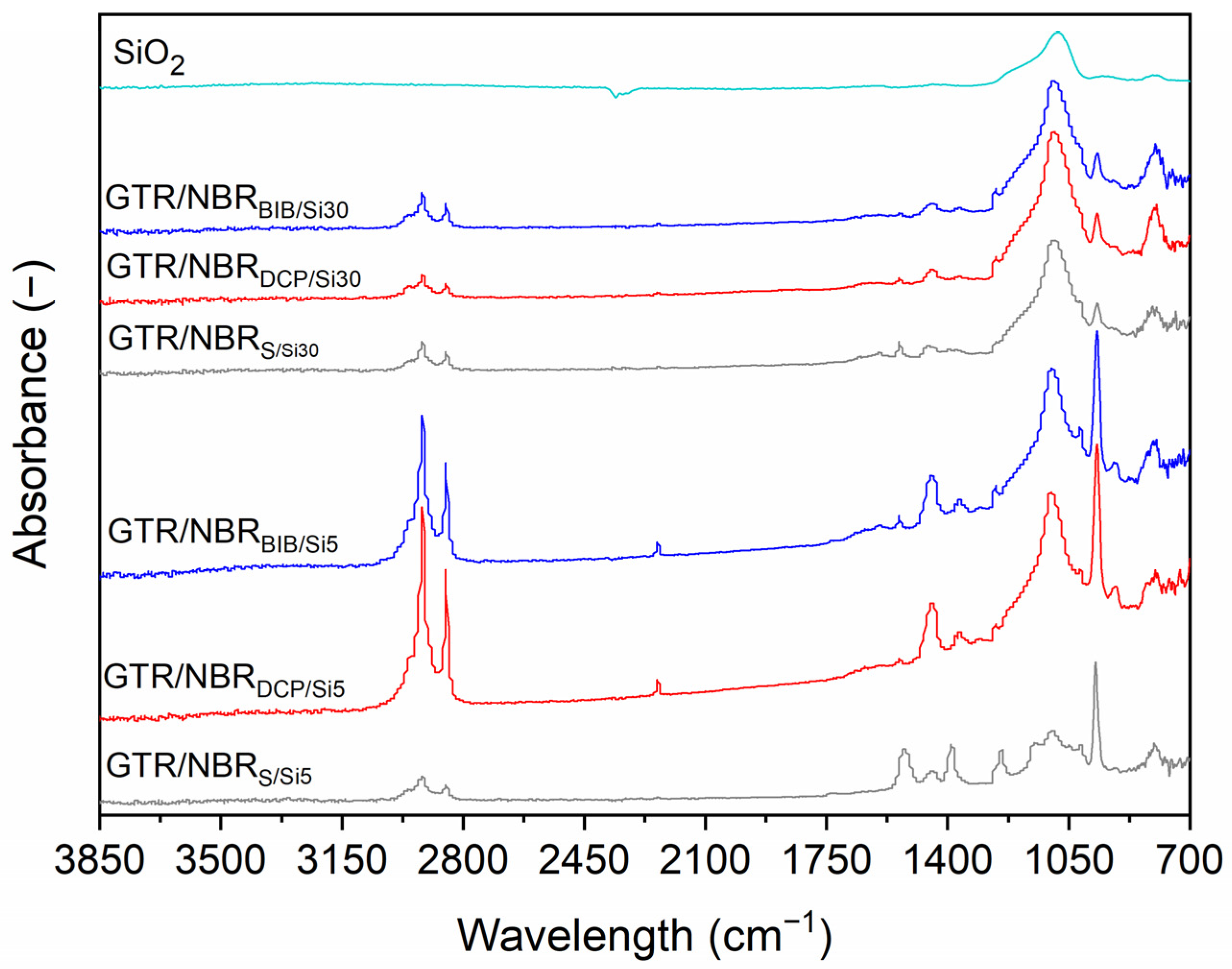
3.3. FTIR Analysis
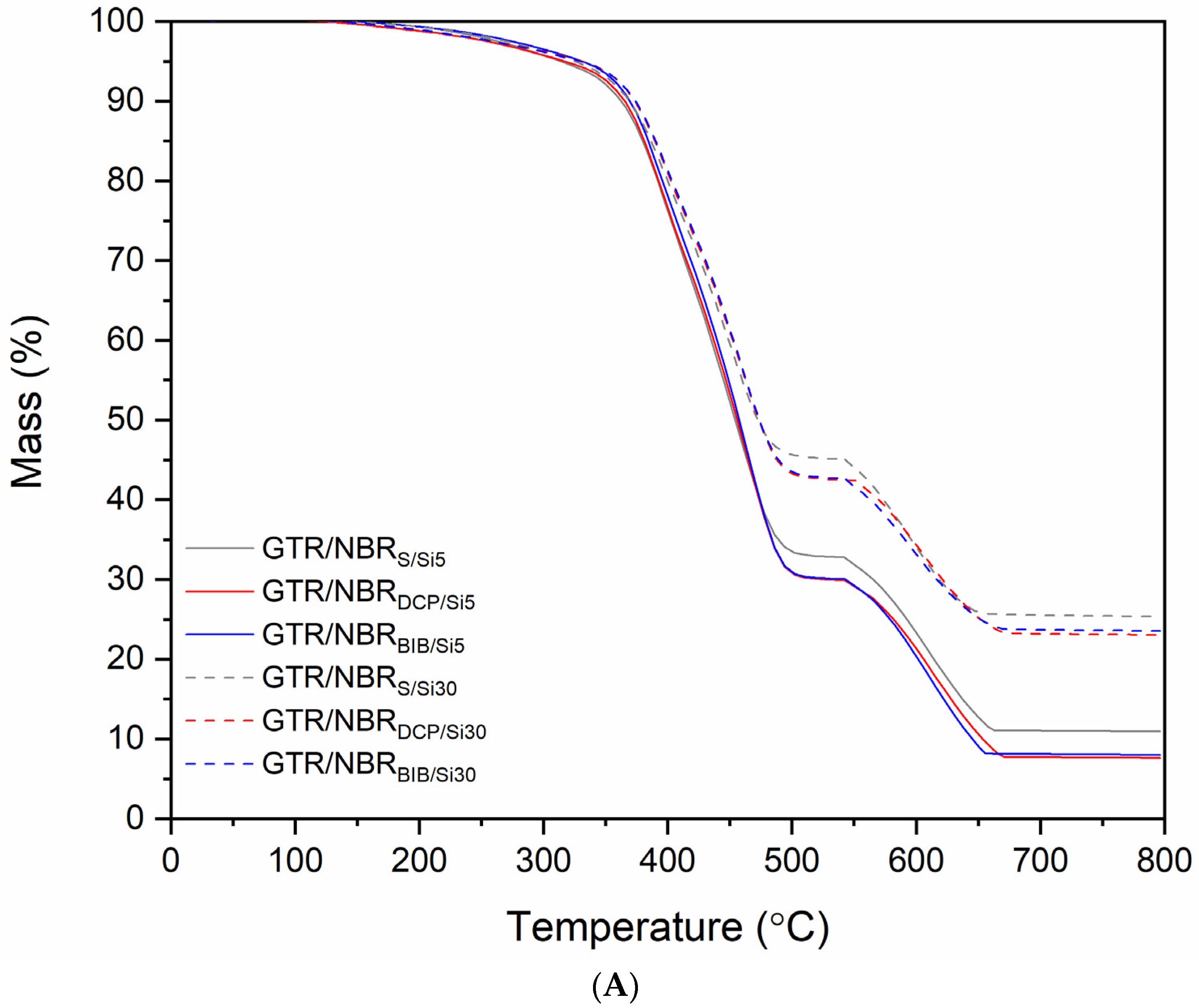
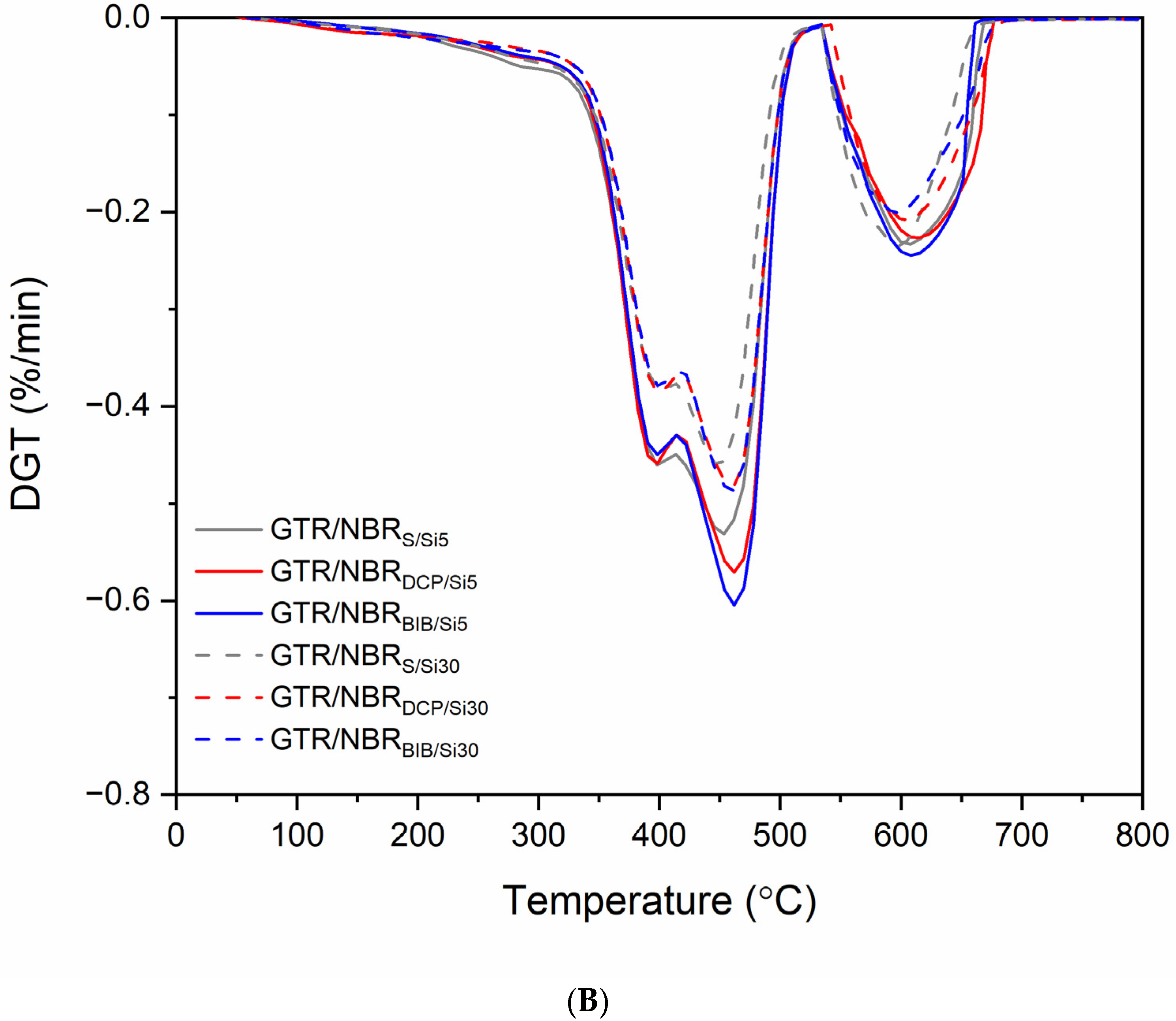
3.4. Thermogravimetric Analysis
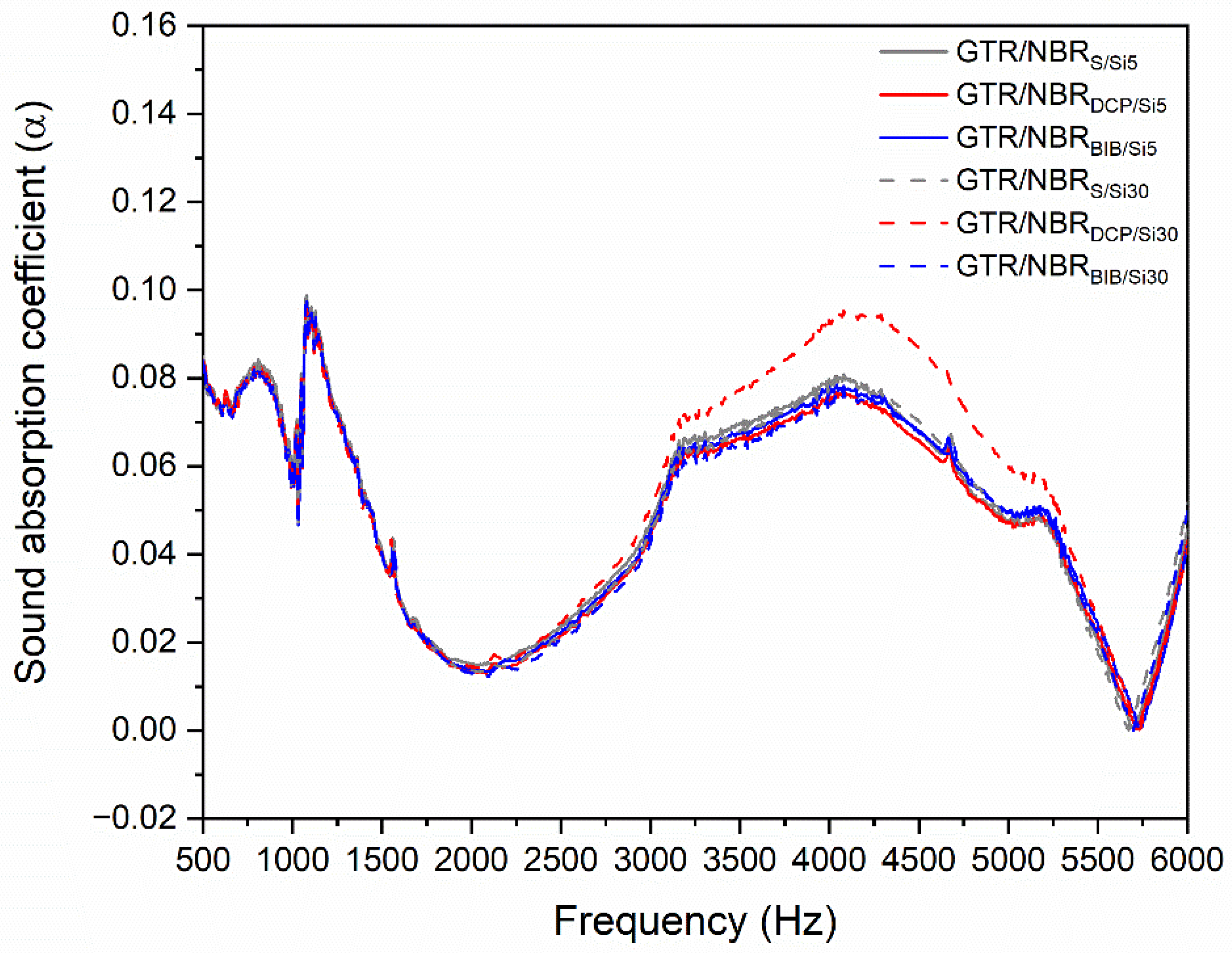
3.5. Acoustic Properties
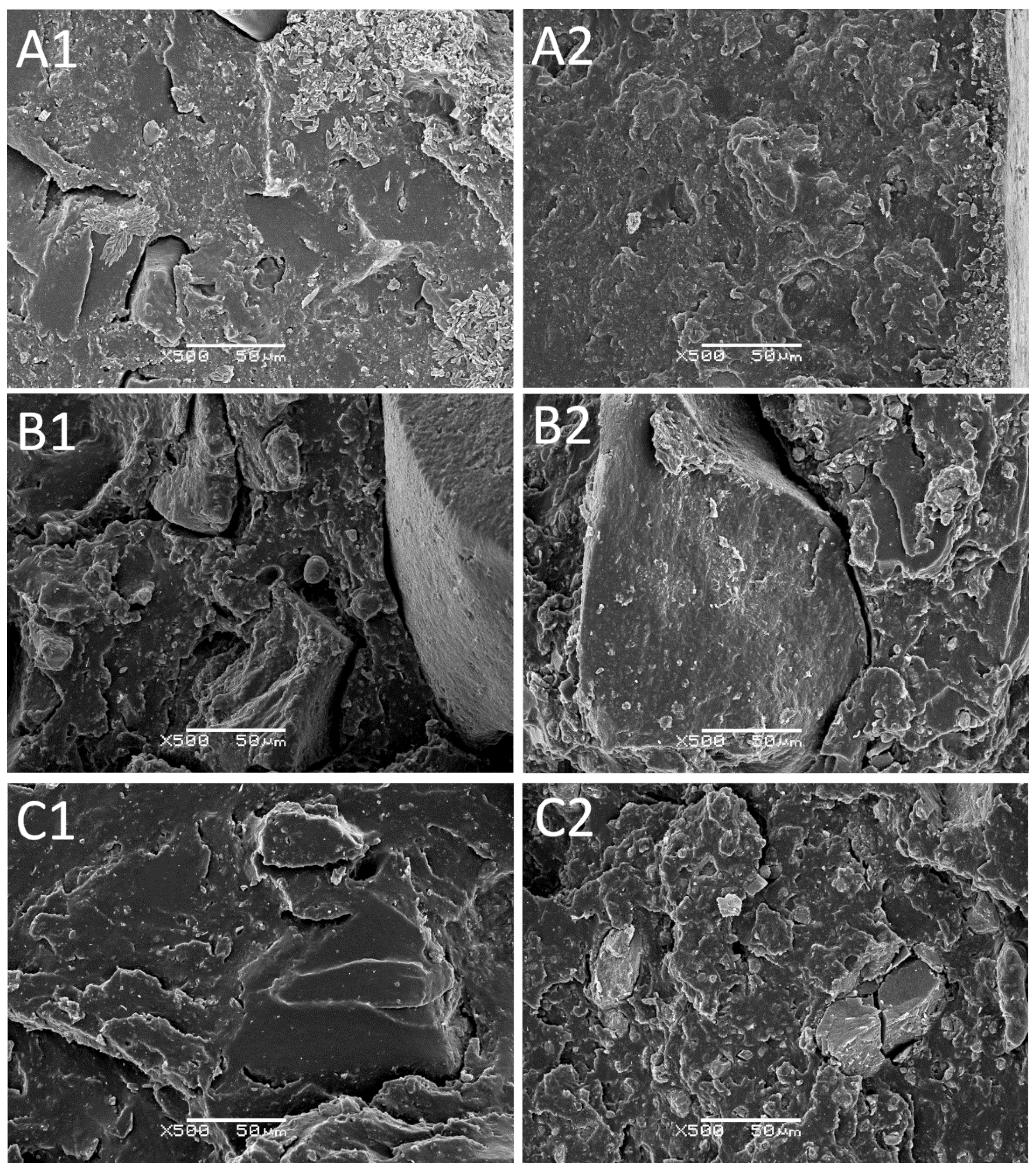
3.6. SEM
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kole, P.J.; Löhr, A.J.; Van Belleghem, F.G.A.J.; Ragas, A.M.J. Wear and tear of tyres: A stealthy source of microplastics in the environment. Int. J. Environ. Res. Public Health 2017, 14, 1265. [Google Scholar] [CrossRef]
- Asaro, L.; Gratton, M.; Seghar, S.; Aït Hocine, N. Recycling of rubber wastes by devulcanization. Resour. Conserv. Recycl. 2018, 133, 250–262. [Google Scholar] [CrossRef]
- Machin, E.B.; Pedroso, D.T.; de Carvalho, J.A. Energetic valorization of waste tires. Renew. Sustain. Energy Rev. 2017, 68, 306–315. [Google Scholar] [CrossRef] [Green Version]
- Adhikari, B.; De, D.; Maiti, S. Reclamation and recycling of waste rubber. Prog. Polym. Sci. 2000, 25, 909–948. [Google Scholar] [CrossRef]
- Formela, K.; Hejna, A.; Zedler, L.; Colom, X.; Cañavate, J. Microwave treatment in waste rubber recycling—Recent advances and limitations. Express Polym. Lett. 2019, 13, 565–588. [Google Scholar] [CrossRef]
- Colom, X.; Marín-Genescà, M.; Mujal, R.; Formela, K.; Cañavate, J. Structural and physico-mechanical properties of natural rubber/GTR composites devulcanized by microwaves: Influence of GTR source and irradiation time. J. Compos. Mater. 2018, 52, 3099–3108. [Google Scholar] [CrossRef]
- Navarro, F.J.; Partal, P.; Martínez-Boza, F.J.; Gallegos, C. Novel recycled polyethylene/ground tire rubber/bitumen blends for use in roofing applications: Thermo-mechanical properties. Polym. Test. 2010, 29, 588–595. [Google Scholar] [CrossRef]
- Rungrodnimitchai, S.; Kotatha, D. Chemically modified ground tire rubber as fluoride ions adsorbents. Chem. Eng. J. 2015, 282, 161–169. [Google Scholar] [CrossRef]
- Orrit-Prat, J.; Mujal-Rosas, R.; Rahhali, A.; Marin-Genesca, M.; Colom-Fajula, X.; Belana-Punseti, J. Dielectric and mechanical characterization of PVC composites with ground tire rubber. J. Compos. Mater. 2010, 45, 1233–1243. [Google Scholar] [CrossRef]
- Colom, X.; Cañavate, J.; Carrillo, F.; Lis, M. Acoustic and mechanical properties of recycled polyvinyl chloride/ground tyre rubber composites. J. Compos. Mater. 2013, 48, 1061–1069. [Google Scholar] [CrossRef]
- Mostafa, A.; Abouel-Kasem, A.; Bayoumi, M.R.; El-Sebaie, M.G. The influence of CB loading on thermal aging resistance of SBR and NBR rubber compounds under different aging temperature. Mater. Des. 2009, 30, 791–795. [Google Scholar] [CrossRef]
- Baeta, D.A.; Zattera, J.A.; Oliveira, M.G.; Oliveira, P.J. The use of styrene-butadiene rubber waste as a potential filler in nitrile rubber: Order of addition and size of waste particle. Braz. J. Chem. Eng. 2009, 29, 23–31. [Google Scholar] [CrossRef]
- Moon, S.C.; Choi, J.K.; Jo, B.W. Flame retardancy and foaming properties of the NBR/ground tire rubber foams containing expandable graphite. Polymer 2004, 28, 412–425. [Google Scholar]
- Choi, Y.S.; Choi, S.K.; Moon, S.C.; Jo, B.W. Halogen-free flame retarding NBR/GTR foams. J. Ind. Eng. Chem. 2008, 14, 387–395. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, X.; Liang, M.; Lu, C. Improvement of the properties of ground tire rubber (GTR)-filled nitrile rubber vulcanizates through plasma surface modification of GTR powder. J. Appl. Polym. Sci. 2009, 114, 1118–1125. [Google Scholar] [CrossRef]
- Cañavate, J.; Colom, X.; Saeb, M.R.; Przybysz, M.; Zedler, L.; Formela, K. Influence of microwave treatment conditions of GTR on physico-mechanical and structural properties of NBR/NR/GTR composites. Afinidad 2019, 76, 171–179. [Google Scholar]
- Zedler, Ł.; Przybysz, M.; Klein, M.; Saeb, M.R.; Formela, K. Processing, physico-mechanical and thermal properties of reclaimed GTR and NBR/reclaimed GTR blends as function of various additives. Polym. Degrad. Stab. 2017, 143, 186–195. [Google Scholar] [CrossRef]
- Maciejewska, M.; Siwek, M. The influence of curing systems on the cure characteristics and physical properties of styrene–butadiene elastomer. Materials 2020, 13, 5329. [Google Scholar] [CrossRef]
- Kruželák, J.; Kvasničáková, A.; Dosoudil, R.; Hudec, I.; Vilčáková, J. Combined sulfur and peroxide curing systems applied in cross-linking of rubber magnets. Polym. Polym. Compos. 2020. [Google Scholar] [CrossRef]
- Masłowski, M.; Miedzianowska, J.; Strzelec, K. Natural rubber composites filled with crop residues as an alternative to vulcanizates with common fillers. Polymers 2019, 11, 972. [Google Scholar] [CrossRef] [Green Version]
- Zedler, Ł.; Colom, X.; Saeb, M.R.; Formela, K. Preparation and characterization of natural rubber composites highly filled with brewers' spent grain/ground tire rubber hybrid reinforcement. Compos. Part B Eng. 2018, 145, 182–188. [Google Scholar] [CrossRef]
- Pérez, L.D.; Sierra, L.; López, B.L. Effect of the filler characteristics on the miscibility of styrene-butadiene rubber and nitrile-butadiene rubber blends. Polym. Eng. Sci. 2008, 48, 1986–1993. [Google Scholar] [CrossRef]
- Dierkes, W.K.; Guo, R.; Mathew, T.; Tiwari, M.; Datta, R.; Talma, A.; Noordermeer, J.W.M.; van Ooij, W.J. A key to enhancement of compatibility and dispersion in elastomer blends. Kautsch. Gummi Kunstst. 2011, 64, 28–35. [Google Scholar]
- Hejna, A.; Klein, M.; Saeb, M.R.; Formela, K. Towards understanding the role of peroxide initiators on compatibilization efficiency of thermoplastic elastomers highly filled with reclaimed GTR. Polym. Test. 2019, 73, 143–151. [Google Scholar] [CrossRef]
- Zhao, X.; Cornish, K.; Vodovotz, Y. Synergistic mechanisms underlie the peroxide and coagent improvement of natural-rubber-toughened poly(3-hydroxybutyrate-co-3-hydroxyvalerate) mechanical performance. Polymers 2019, 11, 565. [Google Scholar] [CrossRef] [Green Version]
- Menon, A.R.R.; Pillai, C.K.S.; Nando, G.B. Vulcanization of natural rubber modified with cashew nut shell liquid and its phosphorylated derivative—A comparative study. Polymer 1998, 39, 4033–4036. [Google Scholar] [CrossRef]
- Khang, T.H.; Ariff, Z.M. Vulcanization kinetics study of natural rubber compounds having different formulation variables. J. Therm. Anal. Calorim. 2012, 109, 1545–1553. [Google Scholar] [CrossRef]
- Flory, P.J.; Rehner, J. Statistical mechanics of cross-linked polymer networks I. rubberlike elasticity. J. Chem. Phys. 1943, 11, 512–520. [Google Scholar] [CrossRef]
- Kraus, G.J. Swelling of filler-reinforced vulcanizates. J. Appl. Polym. Sci. 1963, 7, 861–871. [Google Scholar] [CrossRef]
- Mangili, I.; Oliveri, M.; Anzano, M.; Collina, E.; Pitea, D.; Lasagni, M. Full factorial experimental design to study the devulcanization of ground tire rubber in supercritical carbon dioxide. J. Supercrit. Fluids 2014, 92, 249–256. [Google Scholar] [CrossRef]
- Kramárová, Z.; Alexy, P.; Chodák, I.; Špirk, E.; Hudec, I.; Koŝíková, B.; Gregorová, A.; Šúri, P.; Feranc, J.; Bugaj, P.; et al. Biopolymers as fillers for rubber blends. Polym. Adv. Technol. 2007, 18, 135–140. [Google Scholar] [CrossRef]
- Ansarifar, M.A.; Chugh, J.P.; Haghighat, S. Effects of silica on the cure properties of some compounds of styrene-butadiene rubber. Iran. Polym. J. 2000, 9, 81–87. [Google Scholar]
- Suntako, R. The rubber damper reinforced by modified silica fume (mSF) as an alternative reinforcing filler in rubber industry. J. Polym. Res. 2017, 24, 131. [Google Scholar] [CrossRef]
- Maciejewska, M.; Sowińska, A. Influence of fillers and ionic liquids on the crosslinking and performance of natural rubber biocomposites. Polymers 2021, 13, 1656. [Google Scholar] [CrossRef] [PubMed]
- Kazemi, H.; Mighri, F.; Park, K.W.; Fard, F.S.; Rodrigue, D. Vulcanization kinetics and properties of natural rubber hybrid composites based on maple/silica/carbon black. Elastomery 2019, 4, 227–240. [Google Scholar]
- Kruželák, J.; Sýkora, R.; Hudec, I. Peroxide vulcanization of natural rubber. Part I: Effect of temperature and peroxide concentration. J. Polym. Eng. 2014, 34, 617–624. [Google Scholar] [CrossRef]
- Przybysz, M.; Marć, M.; Klein, M.; Saeb, M.R.; Formela, K. Structural, mechanical and thermal behavior assessments of PCL/PHB blends reactively compatibilized with organic peroxides. Polym. Test. 2018, 67, 513–521. [Google Scholar] [CrossRef]
- Saiwari, S.; Dierkes, W.K.; Noordermeer, J.W.M. Comparative investigation of the devulcanization parameters of tire rubbers. Rubber Chem. Technol. 2014, 87, 31–42. [Google Scholar] [CrossRef] [Green Version]
- Seghar, S.; Asaro, L.; Rolland-Monnet, M.; Hocine, N.A. Thermo-mechanical devulcanization and recycling of rubber industry waste. Resour. Conserv. Recyl. 2019, 144, 180–186. [Google Scholar] [CrossRef]
- Hejna, A.; Korol, J.; Przybysz-Romatowska, M.; Zedler, Ł.; Chmielnicki, B.; Formela, K. Waste tire rubber as low-cost and environmentally-friendly modifier in thermoset polymers—A review. Waste Manag. 2020, 108, 106–118. [Google Scholar] [CrossRef]
- Arastoopour, H.; Schocke, D.A.; Bernstein, B.; Bilgili, E. Process for Recycling of Rubber Materials. U.S. Patent US5904885, 18 May 1999. [Google Scholar]
- Quadrini, F.; Santo, L.; Musacchi, E. A sustainable molding process for new rubber products from tire recycling. Prog. Rubber Plast. Recycl. Technol. 2019, 35, 41–55. [Google Scholar] [CrossRef]
- Sabzekar, M.; Chenar, M.P.; Mortazavi, S.M.; Kariminejad, M.; Asadi, S.; Zohuri, G. Influence of process variables on chemical devulcanization of sulfur-cured natural rubber. Polym. Degrad. Stab. 2015, 118, 88–95. [Google Scholar] [CrossRef]
- Colom, X.; Cañavate, J.; Formela, K.; Shadman, A.; Saeb, M.R. Assessment of the devulcanization process of EPDM waste from roofing systems by combined thermomechanical/microwave procedures. Polym. Degrad. Stab. 2021, 183, 109450. [Google Scholar] [CrossRef]
- Gibala, D.; Hamed, G.R. Cure and mechanical behavior of rubber compounds containing ground vulcanizates. Part I: Cure behavior. Rubber Chem. Technol. 1994, 67, 636–648. [Google Scholar] [CrossRef]
- Sun, Y.; Yan, X.; Liang, H.; Böhm, G.; Jia, L. Rubber recycling: Mending the interface between ground rubber particles and virgin rubber. ACS Appl. Mater. Interfaces 2020, 12, 47957–47965. [Google Scholar] [CrossRef]
- Kendall, C.E. Relation of peroxide decomposition to rubber degradation. Rubber Chem. Technol. 1951, 24, 857–864. [Google Scholar] [CrossRef]
- Kruželák, J.; Sýkora, R.; Hudec, I. Vulcanization of rubber compounds with peroxide curing systems. Rubber Chem. Technol. 2017, 90, 60–88. [Google Scholar] [CrossRef]
- Formela, K.; Haponiuk, J.T. Curing characteristics, mechanical properties and morphology of butyl rubber filled with ground tire rubber (GTR). Iran. Polym. J. 2014, 23, 185–194. [Google Scholar] [CrossRef] [Green Version]
- Song, P.; Wan, C.; Xie, Y.; Formela, K.; Wang, S. Vegetable derived-oil facilitating carbon black migration from waste tire rubbers and its reinforcement effect. Waste Manag. 2018, 78, 238–248. [Google Scholar] [CrossRef]
- Stevenson, W.T.K.; Garton, A. Infrared spectroscopy of carbon-filled polymers. J. Mater. Sci. Lett. 1987, 6, 580–582. [Google Scholar] [CrossRef]
- Zedler, Ł.; Colom, X.; Cañavate, J.; Saeb, M.R.; Haponiuk, J.T.; Formela, K. Investigating the impact of curing system on structure-property relationship of natural rubber modified with brewery by-product and ground tire rubber. Polymers 2020, 12, 545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, H.; Yang, J.; Liu, Z. Thermogravimetric analysis of two Chinese used tires. Thermochim. Acta 1999, 333, 173–175. [Google Scholar] [CrossRef]
- Ubaidillah, H.Y.I.; Kristiani, R.; Muqowi, E.; Mazlan, S.A.; Ubaidillah, H.Y.I.; Kristiani, R.; Muqowi, E.; Mazlan, S.A. Perfect sound insulation property of reclaimed waste tire rubber. AIPC 2016, 1717, 050012. [Google Scholar] [CrossRef]
- Pfretzschner, J.; Rodriguez, R.M. Acoustic properties of rubber crumbs. Polym. Test. 1999, 18, 81–92. [Google Scholar] [CrossRef]
- Zhang, H. Heat-insulating materials and sound-absorbing materials. Build. Mater. Civ. Eng. 2011, 304–423. [Google Scholar] [CrossRef]
- De, D.; Das, A.; De, D.; Panda, P.K.; Dey, B.; Roy, B.C. Reinforcing effect of silica on the properties of styrene butadiene rubber–reclaim rubber blend system. J. Appl. Polym. Sci. 2006, 99, 957–968. [Google Scholar] [CrossRef]
| Name | Abbreviation | Chemical Structure | Active Oxygen (%) * | The Half-Life Temperature (°C) * |
|---|---|---|---|---|
| di-(2-tert-butyl-peroxyisopropyl)-benzene | BIB |  | 8.98 | 169 |
| dicumyl peroxide | DCP | 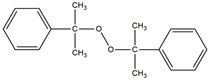 | 5.80 | 162 |
| Components (phr) | Sample Code | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GTR/ NBR S | GTR/ NBR S/Si5 | GTR/ NBR S/Si15 | GTR/ NBR S/Si30 | GTR/ NBR DCP | GTR/ NBR DCP/Si5 | GTR/ NBR DCP/Si15 | GTR/ NBR DCP/Si30 | GTR/ NBR BIB | GTR/ NBR BIB/Si5 | GTR/ NBR BIB/Si15 | GTR/ NBR BIB/Si30 | |
| GTR * | 70 | 70 | 70 | 70 | 70 | 70 | 70 | 70 | 70 | 70 | 70 | 70 |
| NBR | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 |
| Silica | - | 5 | 15 | 30 | - | 5 | 15 | 30 | - | 5 | 15 | 30 |
| Zinc Oxide | 5 | 5 | 5 | 5 | - | - | - | - | - | - | - | - |
| Stearic Acid | 3 | 3 | 3 | 3 | - | - | - | - | - | - | - | - |
| Sulfur | 2 | 2 | 2 | 2 | - | - | - | - | - | - | - | - |
| TMTD | 1 | 1 | 1 | 1 | - | - | - | - | - | - | - | - |
| DCP | - | - | - | - | 2 | 2 | 2 | 2 | - | - | - | - |
| BIB | - | - | - | - | - | - | - | - | 2 | 2 | 2 | 2 |
| Sample Code | Curing System | Curing Temp (°C) | Silica Content (phr) | Curing Parameters | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mmin. (dNm) | Mmax. (dNm) | ΔM (dNm) | ΔMSPEC (-) | t2 (min) | t90 (min) | CRI (min−1) | R300 (%) | ||||
| GTR/NBRS | Sulfur based | 160 | 0 | 10.5 | 49.8 | 39.3 | - | 1.2 | 2.9 | 57.1 | 0.3 |
| GTR/NBRS/Si5 | 5 | 14.9 | 52.0 | 37.1 | −0.07 | 1.2 | 2.7 | 68.0 | 0.6 | ||
| GTR/NBRS/Si15 | 15 | 24.0 | 59.1 | 35.1 | −0.12 | 1.0 | 2.4 | 75.2 | 1.2 | ||
| GTR/NBRS/Si30 | 30 | 45.6 | 75.3 | 29.7 | −0.40 | 1.1 | 2.7 | 62.1 | 1.8 | ||
| GTR/NBRDCP | DCP | 180 | 0 | 14.9 | 38.1 | 23.2 | - | 1.1 | 3.4 | 43.1 | 2.6 |
| GTR/NBRDCP/Si5 | 5 | 15.1 | 39.5 | 24.4 | 0.06 | 0.9 | 3.2 | 43.1 | 3.9 | ||
| GTR/NBRDCP/Si15 | 15 | 27.3 | 54.6 | 27.3 | 0.18 | 1.0 | 3.6 | 39.2 | 2.3 | ||
| GTR/NBRDCP/Si30 | 30 | 55.6 | 75.9 | 20.3 | −0.12 | 1.2 | 3.3 | 47.4 | 1.8 | ||
| GTR/NBRBIB | BIB | 180 | 0 | 13.8 | 45.0 | 31.2 | - | 1.3 | 4.8 | 28.8 | 1.0 |
| GTR/NBRBIB/Si5 | 5 | 16.3 | 49.3 | 33.0 | 0.06 | 1.5 | 5.1 | 27.5 | 1.0 | ||
| GTR/NBRBIB/Si15 | 15 | 25.4 | 60.5 | 35.0 | 0.12 | 1.1 | 4.5 | 29.6 | 1.1 | ||
| GTR/NBRBIB/Si30 | 30 | 54.3 | 82.8 | 28.5 | −0.09 | 1.4 | 4.6 | 31.3 | 1.1 | ||
| Sample code | Curing System | Curing Temp (°C) | Silica Content (phr) | Physico-Mechanical Parameters | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Tensile Strength (MPa) | Elongation at Break (%) | Modulus at 100% (MPa) | Hardness (ShA) | Density at 25 °C (g/cm3) | Swelling Degree (%) | Cross-Link Density with Correction (mol/cm3 × 10−4) | Sol Fraction (%) | ||||
| GTR/NBRS | Sulfur based | 160 | 0 | 5.2 ± 0.7 | 168 ± 17 | 3.2 ± 0.9 | 69 ± 1 | 1.14 ± 0.01 | 111 ± 1 | 1.72 ± 0.01 | 6.5 ± 0.1 |
| GTR/NBRS/Si5 | 5 | 6.1 ± 0.6 | 167 ± 19 | 3.6 ± 0.8 | 69 ± 1 | 1.17 ± 0.01 | 107 ± 1 | 1.24 ± 0.01 | 5.9 ± 0.1 | ||
| GTR/NBRS/Si15 | 15 | 7.5 ± 0.9 | 162 ± 20 | 4.6 ± 0.6 | 74 ± 2 | 1.20 ± 0.01 | 98 ± 1 | 1.37 ± 0.01 | 5.6 ± 0.1 | ||
| GTR/NBRS/Si30 | 30 | 8.5 ± 1.5 | 131 ± 21 | 6.8 ± 0.9 | 81 ± 2 | 1.25 ± 0.01 | 84 ± 1 | 1.73 ± 0.05 | 4.6 ± 0.2 | ||
| GTR/NBRDCP | DCP | 180 | 0 | 5.1 ± 0.3 | 304 ± 12 | 2.0 ± 0.8 | 54 ± 2 | 1.10 ± 0.01 | 160 ± 1 | 0.92 ± 0.02 | 8.7 ± 0.1 |
| GTR/NBRDCP/Si5 | 5 | 5.6 ± 0.3 | 333 ± 23 | 1.9 ± 0.3 | 57 ± 1 | 1.11 ± 0.01 | 159 ± 4 | 0.62 ± 0.03 | 8.5 ± 0.3 | ||
| GTR/NBRDCP/Si15 | 15 | 7.2 ± 0.6 | 313 ± 7 | 2.6 ± 0.7 | 65 ± 1 | 1.15 ± 0.01 | 142 ± 4 | 0.73 ± 0.04 | 7.8 ± 0.2 | ||
| GTR/NBRDCP/Si30 | 30 | 10.0 ± 1.0 | 240 ± 23 | 4.3 ± 0.8 | 78 ± 1 | 1.21 ± 0.01 | 108 ± 3 | 1.13 ± 0.07 | 6.2 ± 0.3 | ||
| GTR/NBRBIB | BIB | 180 | 0 | 6.0 ± 0.4 | 237 ± 23 | 2.4 ± 0.3 | 58 ± 1 | 1.10 ± 0.01 | 147 ± 3 | 1.08 ± 0.05 | 8.6 ± 0.3 |
| GTR/NBRBIB/Si5 | 5 | 6.8 ± 0.5 | 274 ± 12 | 2.5 ± 0.5 | 61 ± 1 | 1.11 ± 0.01 | 138 ± 1 | 0.83 ± 0.01 | 7.4 ± 0.2 | ||
| GTR/NBRBIB/Si15 | 15 | 8.4 ± 0.5 | 281 ± 5 | 3.1 ± 0.8 | 68 ± 1 | 1.15 ± 0.01 | 122 ± 1 | 0.98 ± 0.01 | 6.9 ± 0.1 | ||
| GTR/NBRBIB/Si30 | 30 | 10.2 ± 0.6 | 225 ± 25 | 5.0 ± 0.6 | 81 ± 1 | 1.21 ± 0.01 | 96 ± 3 | 1.38 ± 0.09 | 5.9 ± 0.3 | ||
| Sample | Mass Loss (%) | Char Residue at 750 °C (%) | |||
|---|---|---|---|---|---|
| T−2% | T−5% | T−10% | T−50% | ||
| Temperature (°C) | |||||
| GTR/NBRS/Si5 | 253.2 | 317.5 | 366.2 | 453.6 | 11.0 |
| GTR/NBRDCP/Si5 | 237.2 | 317.5 | 366.3 | 453.8 | 7.7 |
| GTR/NBRBIB/Si5 | 237.6 | 333.7 | 366.4 | 457.4 | 8.0 |
| GTR/NBRS/Si30 | 253.6 | 325.9 | 366.4 | 470.2 | 25.4 |
| GTR/NBRDCP/Si30 | 237.0 | 325.5 | 374.4 | 469.6 | 23.1 |
| GTR/NBRBIB/Si30 | 237.4 | 325.8 | 374.6 | 473.9 | 23.6 |
| Frequency (Hz) | Sample Code | |||||
|---|---|---|---|---|---|---|
| GTR/NBR S/Si5 | GTR/NBR DCP/Si5 | GTR/NBR BIB/Si5 | GTR/NBR S/Si30 | GTR/NBR DCP/Si30 | GTR/NBR BIB/Si30 | |
| Sound Absorption Coefficient (α) | ||||||
| 500 | 0.08405 | 0.08376 | 0.08147 | 0.08266 | 0.08330 | 0.08282 |
| 1000 | 0.06136 | 0.05938 | 0.06085 | 0.06012 | 0.05929 | 0.05840 |
| 2000 | 0.01491 | 0.01344 | 0.01402 | 0.01360 | 0.01439 | 0.01292 |
| 4000 | 0.07988 | 0.07610 | 0.07809 | 0.07910 | 0.09323 | 0.07543 |
| Average | 0.06005 | 0.05817 | 0.05861 | 0.05887 | 0.06255 | 0.05739 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zedler, Ł.; Colom, X.; Cañavate, J.; Formela, K. GTR/NBR/Silica Composites Performance Properties as a Function of Curing System: Sulfur versus Peroxides. Materials 2021, 14, 5345. https://doi.org/10.3390/ma14185345
Zedler Ł, Colom X, Cañavate J, Formela K. GTR/NBR/Silica Composites Performance Properties as a Function of Curing System: Sulfur versus Peroxides. Materials. 2021; 14(18):5345. https://doi.org/10.3390/ma14185345
Chicago/Turabian StyleZedler, Łukasz, Xavier Colom, Javier Cañavate, and Krzysztof Formela. 2021. "GTR/NBR/Silica Composites Performance Properties as a Function of Curing System: Sulfur versus Peroxides" Materials 14, no. 18: 5345. https://doi.org/10.3390/ma14185345







