Nanomaterials Application in Endodontics
Abstract
1. Introduction
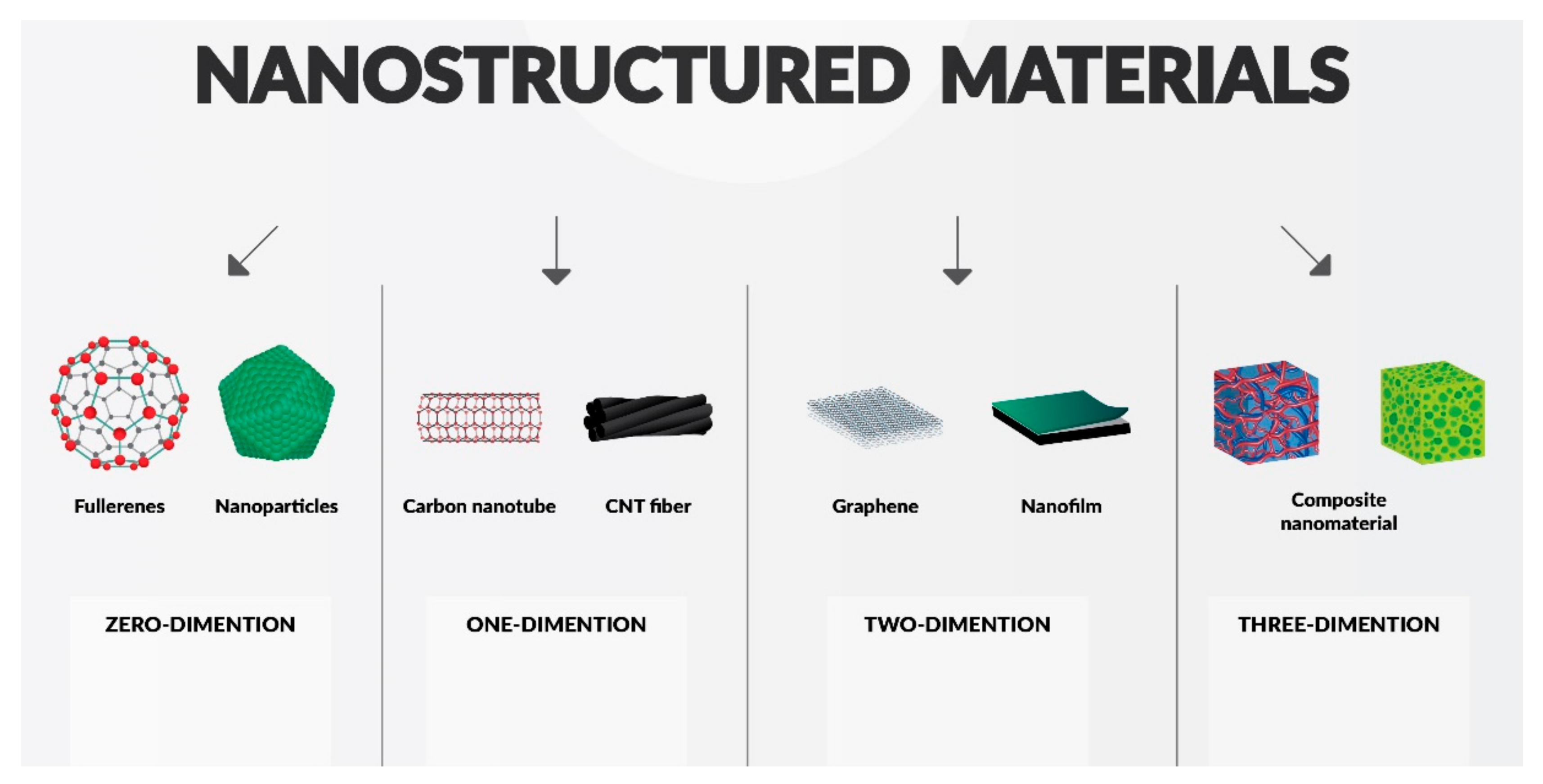
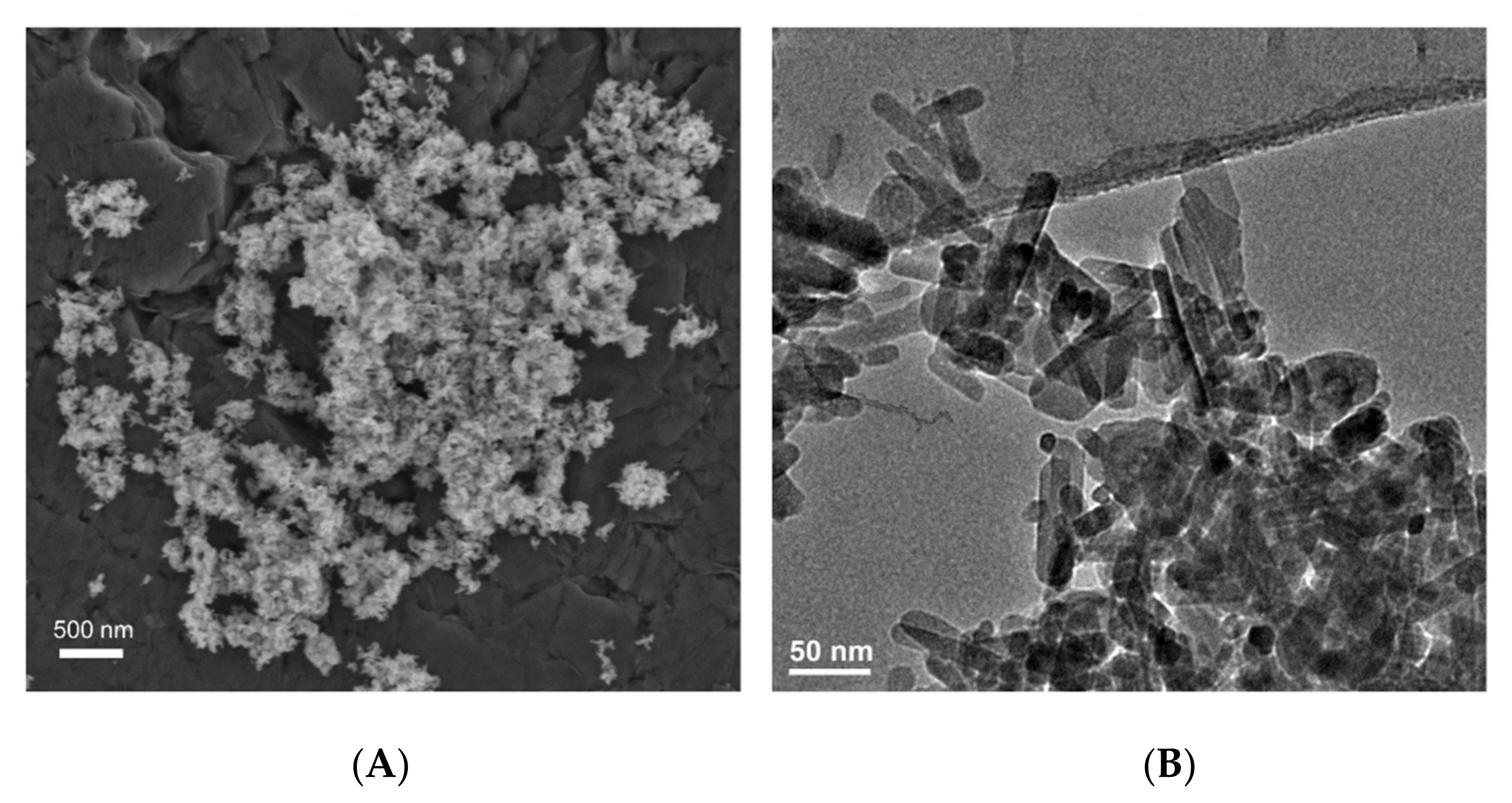
2. Classification of Nanomaterials, Materials Modification
2.1. Quantum Influence on Nanomaterials
2.1.1. Quantum Confinement Effects
2.1.2. Surface Effects
2.2. Chitosan Nanoparticles
2.3. Hydroxyapatite (HAp)
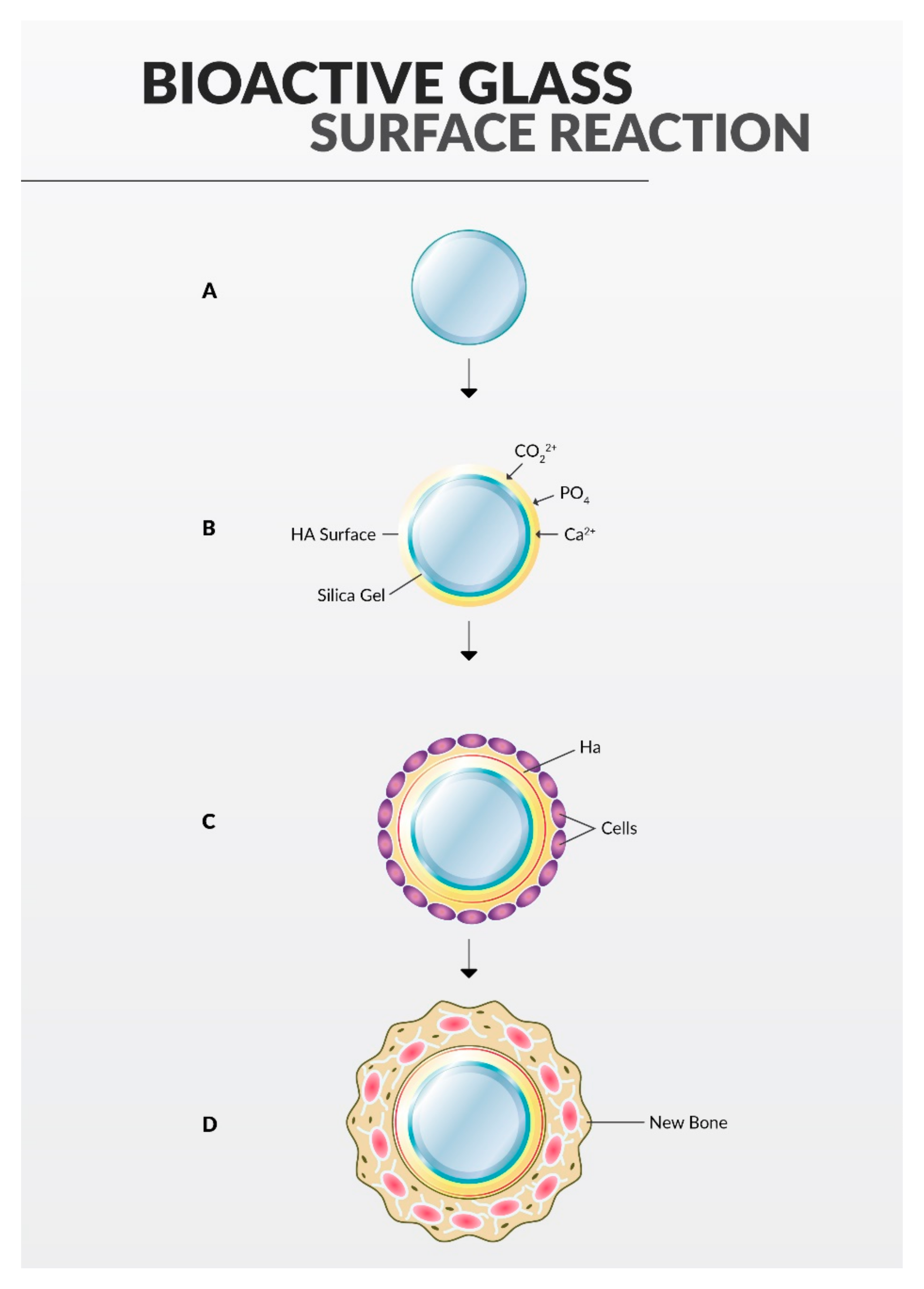
2.4. Bioactive Glass
2.5. Zirconia (Zr)
2.6. Nanosilver
2.7. Zinc Oxide (ZnO)
2.8. Exosomes
2.9. Graphene
2.10. Nanopolymers
3. Clinical Applications
3.1. Sealers
Chitosan as a Sealer
3.2. Obturating Materials
3.3. Nano-Size Related Drug Delivery Applications in Endodontics
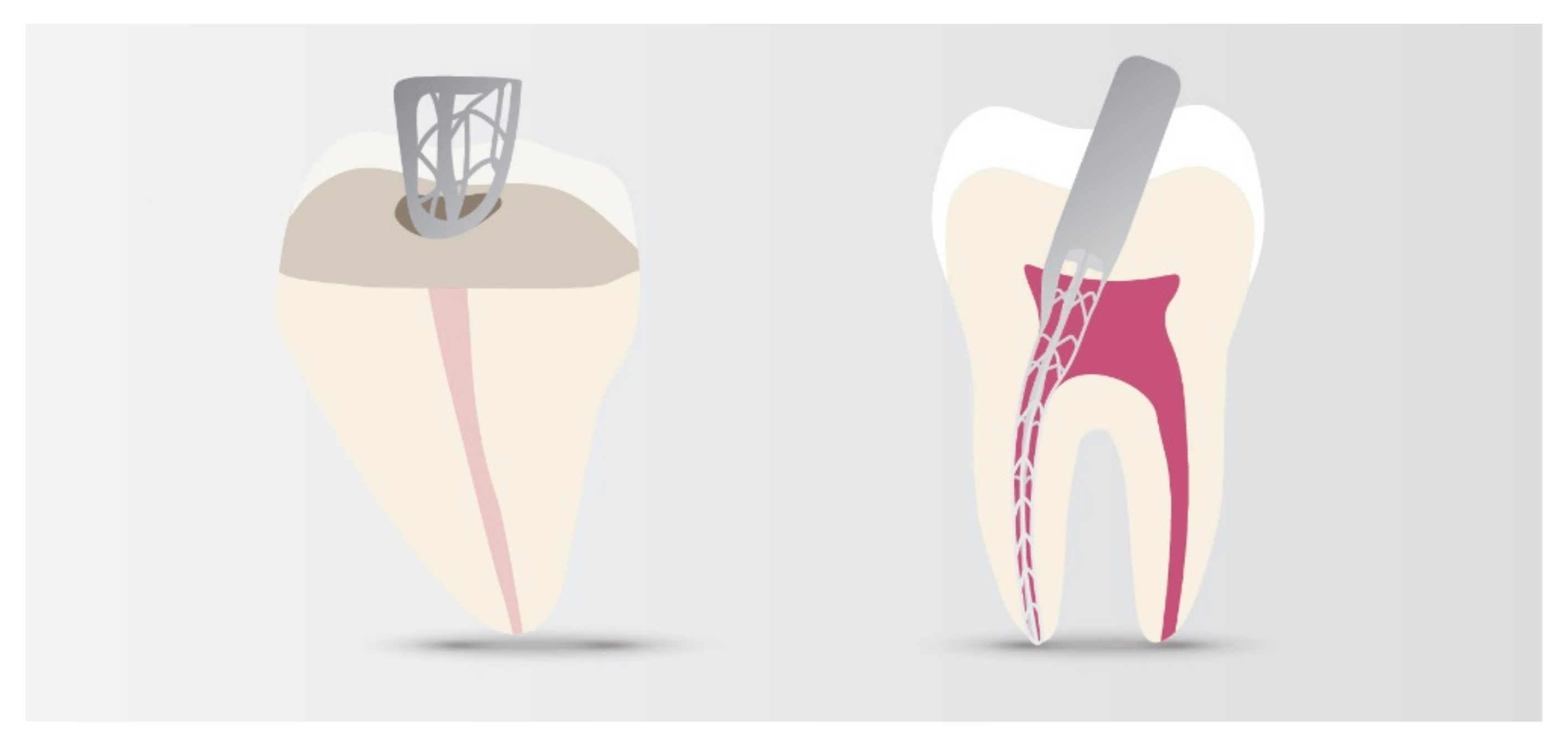
3.4. Root Repair Materials
Polymer Nanocomposites (PCN)
3.5. Nanoparticle-Based Disinfection in Endodontics
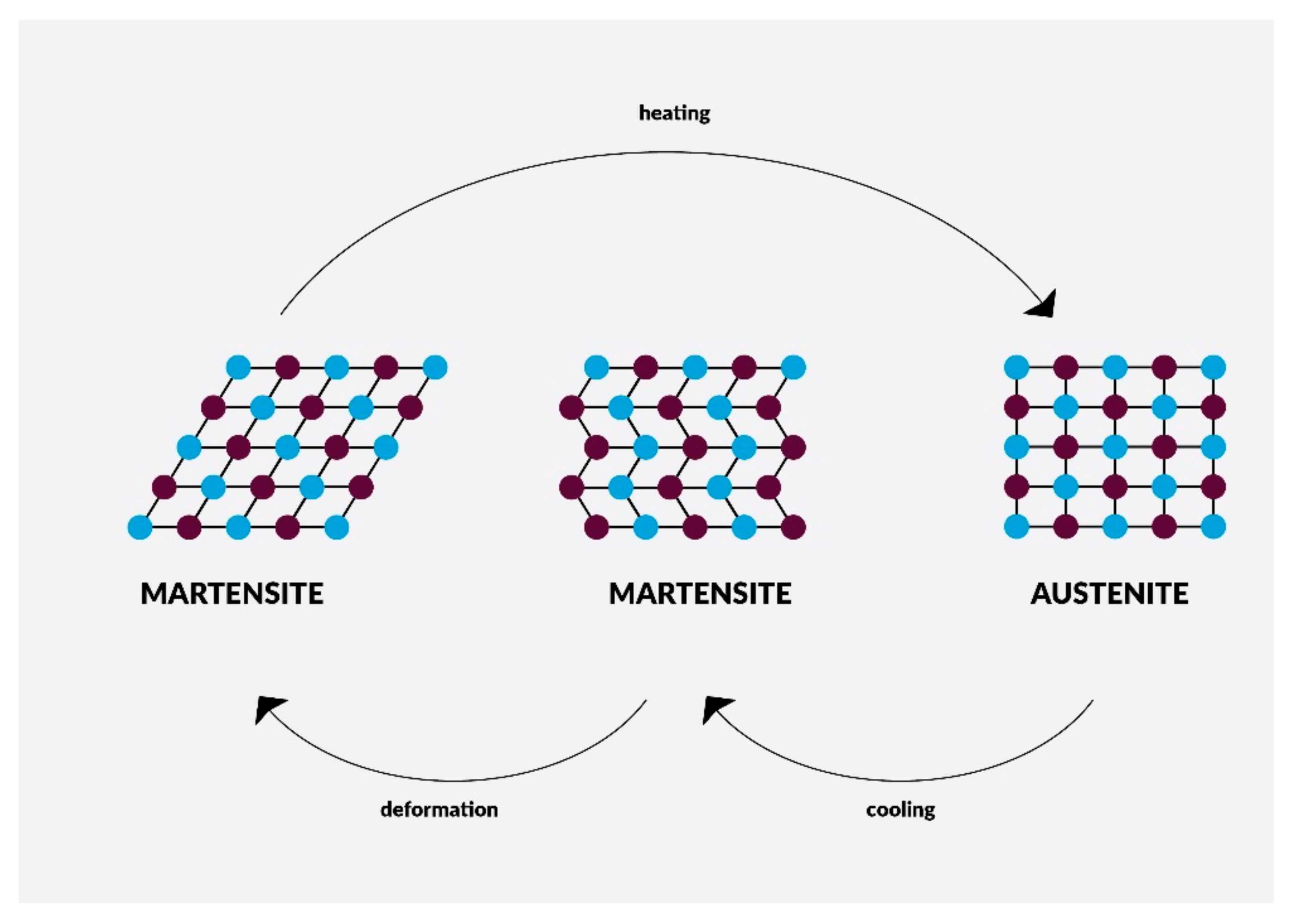
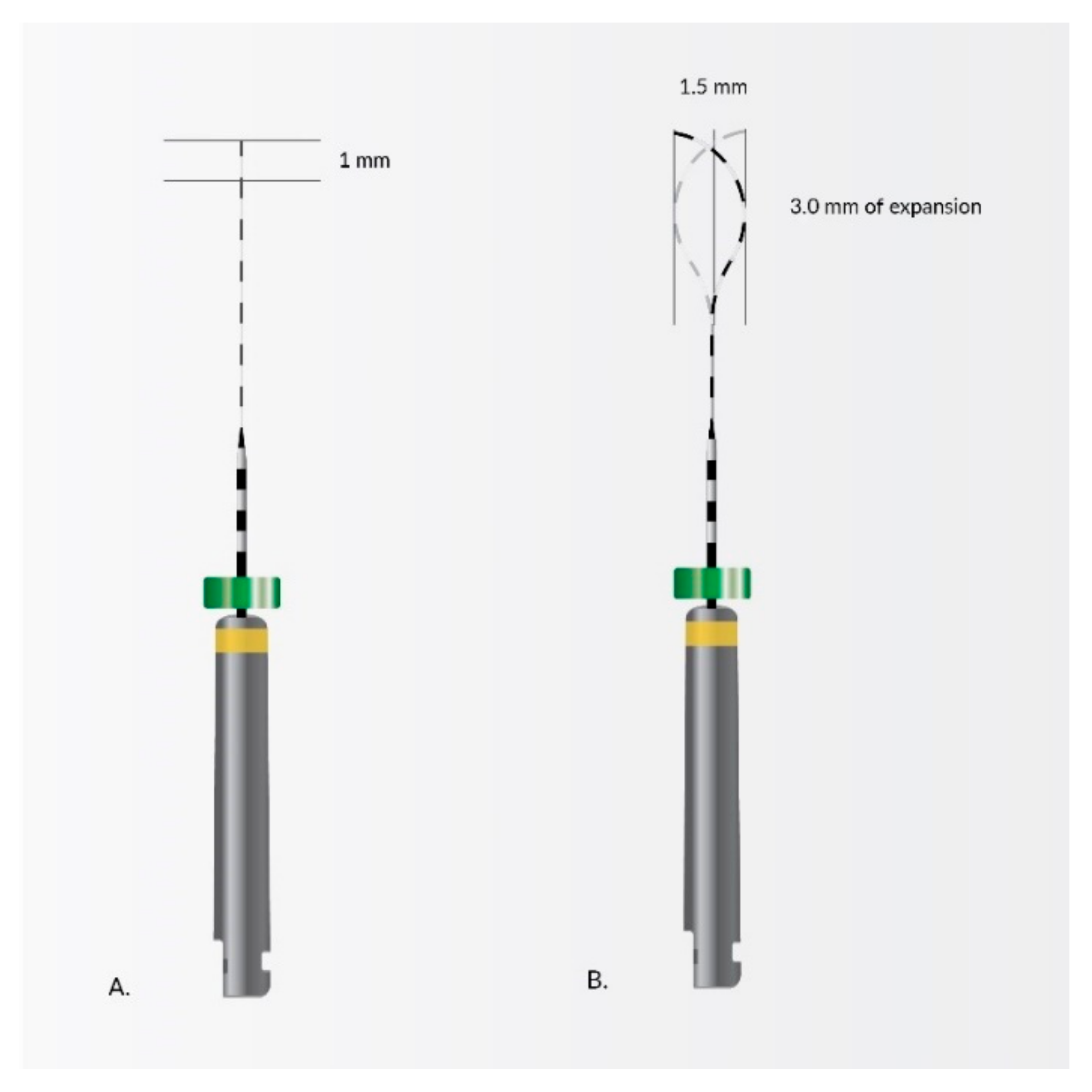
4. Nanomaterials in Endodontic Instruments and Their Effects
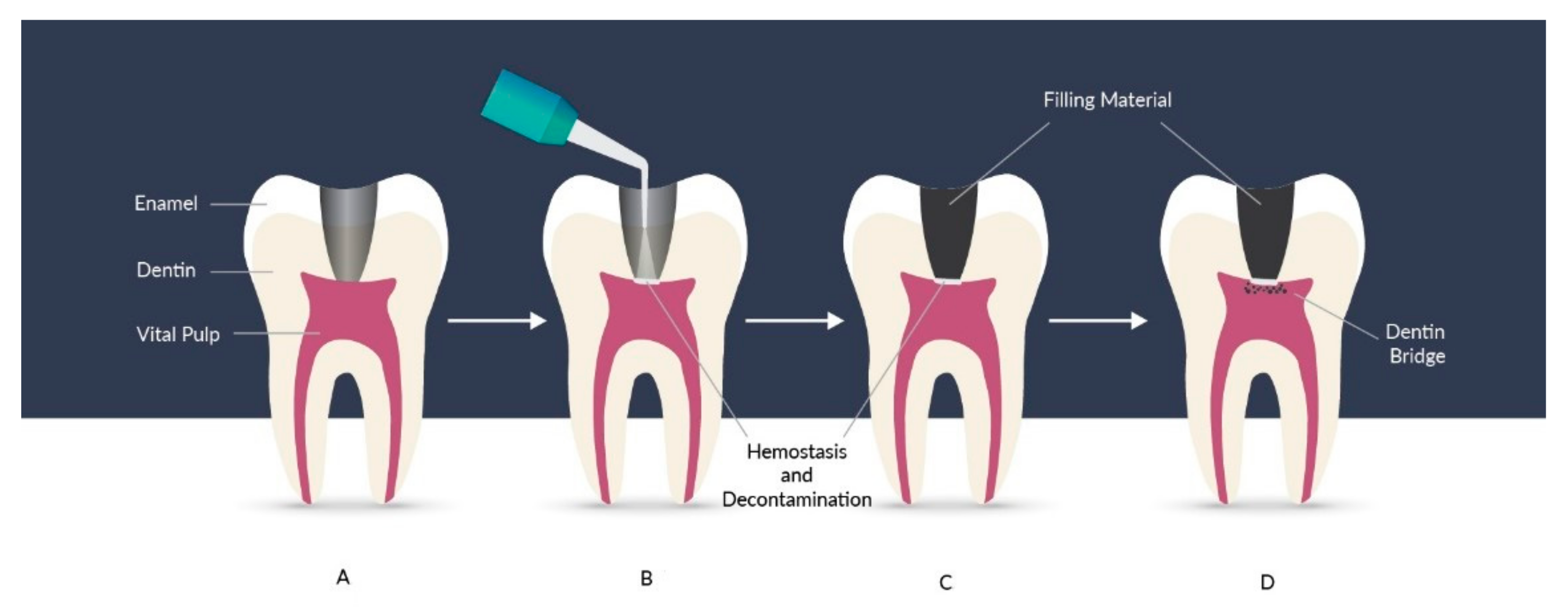
5. Nanoapplications for Repair and Pulp Regeneration
6. Discussion
Challenges and Advantages of Endodontic Nanotechnology
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jhajharia, K.; Mehta, L.; Parolia, A.; Shetty, K. Biofilm in endodontics: A review. J. Int. Soc. Prev. Community Dent. 2015, 5, 1. [Google Scholar] [CrossRef] [PubMed]
- Arabska-Przedpełska, B. Współczesna endodoncja w praktyce. 2011. Available online: https://netaro.pl/wspolczesna-endodoncja-w-praktyce-nowa-2297.html (accessed on 20 April 2021).
- Lubojanski, A.; Dobrzynski, M.; Nowak, N.; Rewak-Soroczynska, J.; Sztyler, K.; Zakrzewski, W.; Dobrzynski, W.; Szymonowicz, M.; Rybak, Z.; Wiglusz, K.; et al. Application of selected nanomaterials and ozone in modern clinical dentistry. Nanomaterials 2021, 11, 259. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L. Nanoparticles in medicine: Therapeutic applications and developments. Clin. Pharmacol. Ther. 2008, 83, 761–769. [Google Scholar] [CrossRef] [PubMed]
- Virlan, M.J.R.; Miricescu, D.; Radulescu, R.; Sabliov, C.M.; Totan, A.; Calenic, B.; Greabu, M. Organic nanomaterials and their applications in the treatment of oral diseases. Molecules 2016, 21, 207. [Google Scholar] [CrossRef]
- Zakrzewski, W.; Dobrzynski, M.; Dobrzynski, W.; Zawadzka-Knefel, A.; Janecki, M.; Kurek, K.; Lubojanski, A.; Szymonowicz, M.; Rybak, Z.; Wiglusz, R.J. Nanomaterials Application in Orthodontics. Nanomaterials 2021, 11, 337. [Google Scholar] [CrossRef]
- Song, W.; Ge, S. Application of antimicrobial nanoparticles in dentistry. Molecules 2019, 24, 1033. [Google Scholar] [CrossRef]
- Venugopal, J.; Prabhakaran, M.; Low, S.; Choon, A.; Zhang, Y.; Deepika, G.; Ramakrishna, S. Nanotechnology for Nanomedicine and Delivery of Drugs. Curr. Pharm. Des. 2008, 14, 2184–2200. [Google Scholar] [CrossRef]
- Peng, Q.; Liu, J.; Zhang, T.; Zhang, T.X.; Zhang, C.-L.; Mu, H. Digestive Enzyme Corona Formed in the Gastrointestinal Tract and Its Impact on Epithelial Cell Uptake of Nanoparticles. Biomacromolecules 2019, 20, 1789–1797. [Google Scholar] [CrossRef]
- Lo Giudice, M.C.; Herda, L.M.; Polo, E.; Dawson, K.A. In situ characterization of nanoparticle biomolecular interactions in complex biological media by flow cytometry. Nat. Commun. 2016, 7, 13475. [Google Scholar] [CrossRef]
- Vidal, C.L.; Ferreira, I.; Ferreira, P.S.; Valente, M.L.C.; Teixeira, A.B.V.; Reis, A.C. Incorporation of hybrid nanomaterial in dental porcelains: Antimicrobial, chemical, and mechanical properties. Antibiotics 2021, 10, 98. [Google Scholar] [CrossRef]
- Ferreira, I.; Vidal, C.L.; Botelho, A.L.; Ferreira, P.S.; da Costa Valente, M.L.; Schiavon, M.A.; Alves, O.L.; Dos Reis, A.C. Effect of nanomaterial incorporation on the mechanical and microbiological properties of dental porcelain. J. Prosthet. Dent. 2020, 123, 529.e1–529.e5. [Google Scholar] [CrossRef]
- Shrestha, A.; Hamblin, M.R.; Kishen, A. Photoactivated rose bengal functionalized chitosan nanoparticles produce antibacterial/biofilm activity and stabilize dentin-collagen. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 491–501. [Google Scholar] [CrossRef]
- Shrestha, A. Antibiofilm efficacy of photosensitizer-functionalized bioactive nanoparticles on multispecies biofilm. J. Endod. 2014, 40, 1604–1610. [Google Scholar] [CrossRef]
- The Effectiveness of Nanomaterials in the Management of Dentine Hypersensitivity—A Review. Available online: https://www.researchgate.net/publication/328496221_The_Effectiveness_of_Nanomaterials_in_the_Management_of_Dentine_Hypersensitivity-A_Review (accessed on 30 May 2021).
- Chieruzzi, M.; Pagano, S.; Moretti, S.; Pinna, R.; Milia, E.; Torre, L.; Eramo, S. Nanomaterials for tissue engineering in dentistry. Nanomaterials 2016, 6, 134. [Google Scholar] [CrossRef]
- Veerapandian, M.; Yun, K. Functionalization of biomolecules on nanoparticles: Specialized for antibacterial applications. Appl. Microbiol. Biotechnol. 2011, 90, 1655–1667. [Google Scholar] [CrossRef]
- Roduner, E. Size matters: Why nanomaterials are different. Chem. Soc. Rev. 2006, 35, 583–592. [Google Scholar] [CrossRef]
- Neikov, O. Quantum Confinement Effect—An Overview. Available online: https://www.sciencedirect.com/topics/engineering/quantum-confinement-effect (accessed on 21 April 2021).
- Cassidy, J.; Zamkov, M. Nanoshell quantum dots: Quantum confinement beyond the exciton Bohr radius. J. Chem. Phys. 2020, 152, 110902. [Google Scholar] [CrossRef]
- Barmparis, G.D.; Kopidakis, G.; Remediakis, I.N. Shape-dependent single-electron levels for Au nanoparticles. Materials 2016, 9, 301. [Google Scholar] [CrossRef]
- Mody, V.V.; Singh, A.; Wesley, B. Basics of magnetic nanoparticles for their application in the field of magnetic fluid hyperthermia. Eur. J. Nanomed. 2013, 5, 11–21. [Google Scholar] [CrossRef]
- Gubin, S. Magnetic Nanoparticles; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2005. [Google Scholar]
- The Promise and Peril of Nanotechnology. Available online: https://phys.org/news/2014-03-peril-nanotechnology.html (accessed on 5 May 2021).
- Ghosh, C.K. Quantum effect on properties of nanomaterials. In Introduction to Nano; Springer: Berlin/Heidelberg, Germany, 2015; pp. 73–111. [Google Scholar]
- Using Quantum Effects in Nanomaterials for Unique Identification. Available online: https://spie.org/news/6250-using-quantum-effects-in-nanomaterials-for-unique-identification?SSO=1 (accessed on 6 June 2021).
- Quantum Size Effect—An Overview. ScienceDirect Topics. Available online: https://www.sciencedirect.com/topics/chemistry/quantum-size-effect (accessed on 6 June 2021).
- Kanaparthy, R.; Kanaparthy, A. The changing face of dentistry: Nanotechnology. Int. J. Nanomed. 2011, 6, 2799–2804. [Google Scholar] [CrossRef]
- Yu, X.; Zhan, Z. The effects of the size of nanocrystalline materials on their thermodynamic and mechanical properties. Nanoscale Res. Lett. 2014, 9, 1–6. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Meyers, M.A.; Mishra, A.; Benson, D.J. Mechanical properties of nanocrystalline materials. Prog. Mater. Sci. 2006, 51, 427–556. [Google Scholar] [CrossRef]
- Giebultowicz, T. Breathing life into an old model. Nature 2000, 408, 299–301. [Google Scholar] [CrossRef]
- Qiu, L.; Zhu, N.; Feng, Y.; Michaelides, E.E.; Żyła, G.; Jing, D.; Zhang, X.; Norris, P.M.; Markides, C.N.; Mahian, O. A review of recent advances in thermophysical properties at the nanoscale: From solid state to colloids. Phys. Rep. 2020, 843, 1–81. [Google Scholar] [CrossRef]
- Callaway, J. Model for lattice thermal conductivity at low temperatures. Phys. Rev. 1959, 113, 1046–1051. [Google Scholar] [CrossRef]
- Wang, Z.; Alaniz, J.E.; Jang, W.; Garay, J.E.; Dames, C. Thermal conductivity of nanocrystalline silicon: Importance of grain size and frequency-dependent mean free paths. Nano Lett. 2011, 11, 2206–2213. [Google Scholar] [CrossRef] [PubMed]
- Rowe, D.M. (Ed.) Thermoelectrics Handbook: Macro to Nano, 1st ed.; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Slack, G.A.; Galginaitis, S. Thermal conductivity and phonon scattering by magnetic impurities in CdTe. Phys. Rev. 1964, 133, A253. [Google Scholar] [CrossRef]
- Zhang, L.; Huang, H. Young’s moduli of ZnO nanoplates: Ab initio determinations. Appl. Phys. Lett. 2006, 89, 183111. [Google Scholar] [CrossRef]
- Nanda, K.K. Size-dependent density of nanoparticles and nanostructured materials. Phys. Lett. A 2012, 376, 3301–3302. [Google Scholar] [CrossRef]
- Safaei, A. Shape, structural, and energetic effects on the cohesive energy and melting point of nanocrystals. J. Phys. Chem. C 2010, 114, 13482–13496. [Google Scholar] [CrossRef]
- Jiang, Q.; Li, J.C.; Chi, B.Q. Size-dependent cohesive energy of nanocrystals. Chem. Phys. Lett. 2002, 366, 551–554. [Google Scholar] [CrossRef]
- Sun, C.Q.; Wang, Y.; Tay, B.K.; Li, S.; Huang, H.; Zhang, Y.B. Correlation between the melting point of a nanosolid and the cohesive energy of a surface atom. J. Phys. Chem. B 2002, 106, 10701–10705. [Google Scholar] [CrossRef]
- Guisbiers, G.; Buchaillot, L. Modeling the melting enthalpy of nanomaterials. J. Phys. Chem. C 2009, 113, 3566–3568. [Google Scholar] [CrossRef]
- Guisbiers, G. Review on the analytical models describing melting at the nanoscale. J. Nanosci. Lett. 2012, 2, 8. [Google Scholar]
- Gelb, L.D.; Gubbins, K.E.; Radhakrishnan, R.; Sliwinska-Bartkowiak, M. Phase separation in confined systems. Rep. Prog. Phys. 2000, 63, 727. [Google Scholar] [CrossRef]
- Berry, R.S. Phases and Phase Changes of Small Systems. In Theory of Atomic and Molecular Clusters; Springer: Berlin/Heidelberg, Germany, 1999; pp. 1–26. [Google Scholar]
- Agnihotri, S.A.; Mallikarjuna, N.N.; Aminabhavi, T.M. Recent advances on chitosan-based micro- and nanoparticles in drug delivery. J. Control. Release 2004, 100, 5–28. [Google Scholar] [CrossRef]
- Del Carpio-Perochena, A.; Kishen, A.; Shrestha, A.; Bramante, C.M. Antibacterial Properties Associated with Chitosan Nanoparticle Treatment on Root Dentin and 2 Types of Endodontic Sealers. J. Endod. 2015, 41, 1353–1358. [Google Scholar] [CrossRef]
- Wang, N.; Ji, Y.; Zhu, Y.; Wu, X.; Mei, L.; Zhang, H.; Deng, J.; Wang, S. Antibacterial effect of chitosan and its derivative on Enterococcus faecalis associated with endodontic infection. Exp. Ther. Med. 2020, 19, 3805. [Google Scholar] [CrossRef] [PubMed]
- Ballal, N.V.; Kundabala, M.; Bhat, K.S.; Acharya, S.; Ballal, M.; Kumar, R. Susceptibility of Candida albicans and Enterococcus faecalis to Chitosan, Chlorhexidine gluconate and their combination in vitro. Aust. Endod. J. 2009, 35, 29–33. [Google Scholar] [CrossRef]
- Kumar, M.N.V.R.; Muzzarelli, R.A.A.; Muzzarelli, C.; Sashiwa, H.; Domb, A.J. Chitosan chemistry and pharmaceutical perspectives. Chem. Rev. 2004, 104, 6017–6084. [Google Scholar] [CrossRef]
- Shi, Z.; Neoh, K.G.; Kang, E.T.; Wang, W. Antibacterial and mechanical properties of bone cement impregnated with chitosan nanoparticles. Biomaterials 2006, 27, 2440–2449. [Google Scholar] [CrossRef] [PubMed]
- Shariatinia, Z. Pharmaceutical applications of chitosan. Adv. Colloid Interface Sci. 2019, 263, 131–194. [Google Scholar] [CrossRef] [PubMed]
- Sahariah, P.; Gaware, V.S.; Lieder, R.; Jónsdóttir, S.; Hjálmarsdóttir, M.Á.; Sigurjonsson, O.E.; Másson, M. The effect of substituent, degree of acetylation and positioning of the cationic charge on the antibacterial activity of quaternary chitosan derivatives. Mar. Drugs 2014, 12, 4635–4658. [Google Scholar] [CrossRef] [PubMed]
- Savitha, A.; SriRekha, A.; Vijay, R.; Ashwija Champa, C.; Jaykumar, T. An in vivo comparative evaluation of antimicrobial efficacy of chitosan, chlorhexidine gluconate gel and their combination as an intracanal medicament against Enterococcus faecalis in failed endodontic cases using real time polymerase chain reaction (qPCR). Saudi Dent. J. 2019, 31, 360–366. [Google Scholar] [CrossRef]
- Loyola-Rodríguez, J.P.; Torres-Méndez, F.; Espinosa-Cristobal, L.F.; García-Cortes, J.O.; Loyola-Leyva, A.; González, F.J.; Soto-Barreras, U.; Nieto-Aguilar, R.; Nieto-Aguilar, R. Antimicrobial activity of endodontic sealers and medications containing chitosan and silver nanoparticles against Enterococcus faecalis. J. Appl. Biomater. Funct. Mater. 2019, 17, 2280800019851771. [Google Scholar] [CrossRef]
- Sebdani, M.M.; Fathi, M.H. Novel hydroxyapatite-forsterite-bioglass nanocomposite coatings with improved mechanical properties. J. Alloys Compd. 2011, 509, 2273–2276. [Google Scholar] [CrossRef]
- Sung, Y.M.; Lee, J.C.; Yang, J.W. Crystallization and sintering characteristics of chemically precipitated hydroxyapatite nanopowder. J. Cryst. Growth 2004, 262, 467–472. [Google Scholar] [CrossRef]
- Fathi, M.H.; Hanifi, A. Evaluation and characterization of nanostructure hydroxyapatite powder prepared by simple sol-gel method. Mater. Lett. 2007, 61, 3978–3983. [Google Scholar] [CrossRef]
- Zakrzewski, W.; Dobrzynski, M.; Nowicka, J.; Pajaczkowska, M.; Szymonowicz, M.; Targonska, S.; Sobierajska, P.; Wiglusz, K.; Dobrzynski, W.; Lubojanski, A.; et al. The Influence of Ozonated Olive Oil-Loaded and Copper-Doped Nanohydroxyapatites on Planktonic Forms of Microorganisms. Nanomaterials 2020, 10, 1997. [Google Scholar] [CrossRef]
- Al-Hazmi, F.; Alnowaiser, F.; Alghamdi, A.A.; Aly, M.M.; Al-Tuwirqi, R.M.; El-Tantawy, F. A new large—Scale synthesis of magnesium oxide nanowires: Structural and antibacterial properties. Superlattices Microstruct. 2012, 52, 200–209. [Google Scholar] [CrossRef]
- Szymonowicz, M.; Korczynski, M.; Dobrzynski, M.; Zawisza, K.; Mikulewicz, M.; Karuga-Kuzniewska, E.; Zywickab, B.; Rybak, Z.; Wiglusz, R.J. Cytotoxicity Evaluation of High-Temperature Annealed Nanohydroxyapatite in Contact with Fibroblast Cells. Materials 2017, 10, 590. [Google Scholar] [CrossRef]
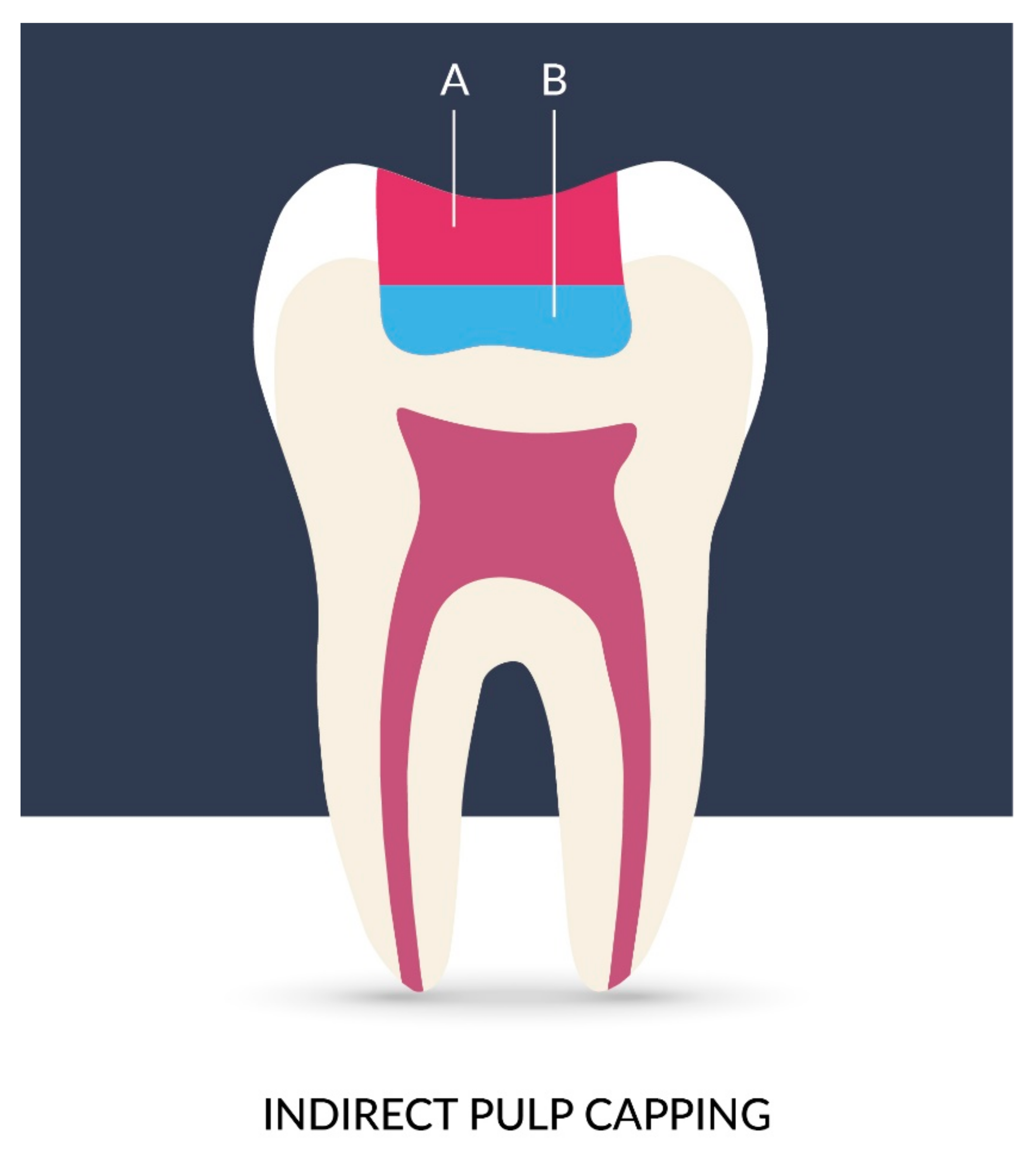
- Zaen El-Din, A.M.; Hamama, H.H.; Abo El-Elaa, M.A.; Grawish, M.E.; Mahmoud, S.H.; Neelakantan, P. The effect of four materials on direct pulp capping: An animal study. Aust. Endod. J. 2020, 46, 249–256. [Google Scholar] [CrossRef]
- Pathak, D.S.D. Advances in Pulp Capping Materials: A Review. IOSR J. Dent. Med. Sci. 2017, 16, 31–37. [Google Scholar] [CrossRef]
- Wiatrak, B.; Sobierajska, P.; Szandruk-Bender, M.; Jawien, P.; Janeczek, M.; Dobrzynski, M.; Pistor, P.; Szelag, A.; Wiglusz, R.J. Nanohydroxyapatite as a biomaterial for peripheral nerve regeneration after mechanical damage—In vitro study. Int. J. Mol. Sci. 2021, 22, 4454. [Google Scholar] [CrossRef]
- Al-Hamdan, R.S.; Almutairi, B.; Kattan, H.F.; Alsuwailem, N.A.; Farooq, I.; Vohra, F.; Abduljabbar, T. Influence of hydroxyapatite nanospheres in dentin adhesive on the dentin bond integrity and degree of conversion: A scanning electron microscopy (SEM), raman, fourier transform-infrared (FTIR), and microtensile study. Polymers 2020, 12, 2948. [Google Scholar] [CrossRef]
- Liu, Y.; Tjäderhane, L.; Breschi, L.; Mazzoni, A.; Li, N.; Mao, J.; Pashley, D.H.; Tay, F.R. Limitations in bonding to dentin and experimental strategies to prevent bond degradation. J. Dent. Res. 2011, 90, 953–968. [Google Scholar] [CrossRef]
- Herman, K.; Wujczyk, M.; Dobrzynski, M.; Diakowska, D.; Wiglusz, K.; Wiglusz, R.J. In Vitro Assessment of Long-Term Fluoride Ion Release from Nanofluorapatite. Materials 2021, 14, 3747. [Google Scholar] [CrossRef]
- Allaker, R.P.; Ren, G. Potential impact of nanotechnology on the control of infectious diseases. Trans. R. Soc. Trop. Med. Hyg. 2008, 102, 1–2. [Google Scholar] [CrossRef]
- Liu, H.; Peng, H.; Wu, Y.; Zhang, C.; Cai, Y.; Xu, G.; Li, Q.; Chen, X.; Ji, J.; Zhang, Y.; et al. The promotion of bone regeneration by nanofibrous hydroxyapatite/chitosan scaffolds by effects on integrin-BMP/Smad signaling pathway in BMSCs. Biomaterials 2013, 34, 4404–4417. [Google Scholar] [CrossRef]
- Gaharwar, A.K.; Dammu, S.A.; Canter, J.M.; Wu, C.J.; Schmidt, G. Highly extensible, tough, and elastomeric nanocomposite hydrogels from poly(ethylene glycol) and hydroxyapatite nanoparticles. Biomacromolecules 2011, 12, 1641–1650. [Google Scholar] [CrossRef]
- Cao, W.; Hench, L.L. Bioactive materials. Ceram. Int. 1996, 22, 493–507. [Google Scholar] [CrossRef]
- Zakrzewski, W.; Dobrzynski, M.; Rybak, Z.; Szymonowicz, M.; Wiglusz, R.J. Selected Nanomaterials’ Application Enhanced with the Use of Stem Cells in Acceleration of Alveolar Bone Regeneration during Augmentation Process. Nanomaterials 2020, 10, 1216. [Google Scholar] [CrossRef] [PubMed]
- Piotrowski, G.; Hench, L.L.; Allen, W.C.; Miller, G.J. Mechanical studies of the bone bioglass interfacial bond. J. Biomed. Mater. Res. 1975, 9, 47–61. [Google Scholar] [CrossRef] [PubMed]
- Profeta, A.C.; Prucher, G.M. Bioactive-glass in Endodontic Therapy and Associated Microsurgery. Open Dent. J. 2017, 11, 164–170. [Google Scholar] [CrossRef]
- Profeta, A.C. Dentine bonding agents comprising calcium-silicates to support proactive dental care: Origins, development and future. Dent. Mater. J. 2014, 33, 443–452. [Google Scholar] [CrossRef]
- Mohn, D.; Zehnder, M.; Imfeld, T.; Stark, W.J. Radio-opaque nanosized bioactive glass for potential root canal application: Evaluation of radiopacity, bioactivity and alkaline capacity. Int. Endod. J. 2010, 43, 210–217. [Google Scholar] [CrossRef]
- Mohn, D.; Bruhin, C.; Luechinger, N.A.; Stark, W.J.; Imfeld, T.; Zehnder, M. Composites made of flame-sprayed bioactive glass 45S5 and polymers: Bioactivity and immediate sealing properties. Int. Endod. J. 2010, 43, 1037–1046. [Google Scholar] [CrossRef]
- Stoor, P.; Soderling, E.; Salonen, J.I. Antibacterial effects of a bioactive glass paste on oral microorganisms. Acta Odontol. Scand. 1998, 56, 161–165. [Google Scholar] [CrossRef]
- Deville, S.; Gremillard, L.; Chevalier, J.; Fantozzi, G. A critical comparison of methods for the determination of the aging sensitivity in biomedical grade yttria-stabilized zirconia. J. Biomed. Mater. Res. Part B Appl. Biomater. 2005, 72, 239–245. [Google Scholar] [CrossRef]
- Chevalier, J. What future for zirconia as a biomaterial? Biomaterials 2006, 27, 535–543. [Google Scholar] [CrossRef]
- Kumar, G.; Shivrayan, A. Comparative study of mechanical properties of direct core build-up materials. Contemp. Clin. Dent. 2015, 6, 16–20. [Google Scholar] [CrossRef]
- Asakawa, Y.; Takahashi, H.; Iwasaki, N.; Kobayashi, M. Effect of ultraviolet light irradiation period on bond strengths between fber-reinforced composite post and core build-up composite resin. Dent. Mater. J. 2014, 33, 133–140. [Google Scholar] [CrossRef]
- Hambire, U.; Tripathi, V. Influence of Zirconia Nanoclusters on the Compressive Strength of Bis-Gma and Tegdma Based Dental Composites. ARPN J. Eng. Appl. Sci. 2012, 7, 9. [Google Scholar]
- Prabhu, S.; Poulose, E.K. Silver nanoparticles: Mechanism of antimicrobial action, synthesis, medical applications, and toxicity effects. Int. Nano Lett. 2012, 2, 1–10. [Google Scholar] [CrossRef]
- Chladek, G.; Barszczewska-Rybarek, I.; Lukaszczyk, J. Developing the procedure of modifying the denture soft liner by silver nanoparticles. Acta Bioeng. Biomech. 2012, 14, 23–29. [Google Scholar]
- Chávez-Andrade, G.M.; Tanomaru-Filho, M.; Basso Bernardi, M.I.; de Toledo Leonardo, R.; Faria, G.; Guerreiro-Tanomaru, J.M. Antimicrobial and biofilm anti-adhesion activities of silver nanoparticles and farnesol against endodontic microorganisms for possible application in root canal treatment. Arch. Oral Biol. 2019, 107. [Google Scholar] [CrossRef]
- Martinez-Andrade, J.M.; Avalos-Borja, M.; Vilchis-Nestor, A.R.; Sanchez-Vargas, L.O.; Castro-Longoria, E. Dual function of EDTA with silver nanoparticles for root canal treatment–A novel modification. PLoS ONE 2018, 13. [Google Scholar] [CrossRef]
- Senges, C.; Wrbas, K.T.; Altenburger, M.; Follo, M.; Spitzmüller, B.; Wittmer, A.; Hellwig, E.; Al-Ahmad, A. Bacterial and candida albicans adhesion on different root canal filling materials and sealers. J. Endod. 2011, 37, 1247–1252. [Google Scholar] [CrossRef]
- George, S.; Basrani, B.; Kishen, A. Possibilities of gutta-percha-centered infection in endodontically treated teeth: An in vitro study. J. Endod. 2010, 36, 1241–1244. [Google Scholar] [CrossRef]
- Siqueira, J.F.; Favieri, A.; Gahyva, S.M.M.; Moraes, S.R.; Lima, K.C.; Lopes, H.P. Antimicrobial activity and flow rate of newer and established root canal sealers. J. Endod. 2000, 26, 274–277. [Google Scholar] [CrossRef]
- Ørstavik, D. Antibacterial properties of root canal sealers, cements and pastes. Int. Endod. J. 1981, 14, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Kishen, A.; Shi, Z.; Shrestha, A.; Neoh, K.G. An Investigation on the Antibacterial and Antibiofilm Efficacy of Cationic Nanoparticulates for Root Canal Disinfection. J. Endod. 2008, 34, 1515–1520. [Google Scholar] [CrossRef] [PubMed]
- Sawai, J. Quantitative evaluation of antibacterial activities of metallic oxide powders (ZnO, MgO and CaO) by conductimetric assay. J. Microbiol. Methods 2003, 54, 177–182. [Google Scholar] [CrossRef]
- Reddy, K.M.; Feris, K.; Bell, J.; Wingett, D.G.; Hanley, C.; Punnoose, A. Selective toxicity of zinc oxide nanoparticles to prokaryotic and eukaryotic systems. Appl. Phys. Lett. 2007, 90, 213902. [Google Scholar] [CrossRef]
- Lai, R.C.; Arslan, F.; Lee, M.M.; Sze, N.S.K.; Choo, A.; Chen, T.S.; Salto-Tellez, M.; Timmers, L.; Lee, C.N.; El Oakley, R.M.; et al. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010, 4, 214–222. [Google Scholar] [CrossRef]
- Mendt, M.; Rezvani, K.; Shpall, E. Mesenchymal stem cell-derived exosomes for clinical use. Bone Marrow Transplant. 2019, 542, 789–792. [Google Scholar] [CrossRef]
- Yu, S.; Chen, H.; Gao, B. Potential Therapeutic Effects of Exosomes in Regenerative Endodontics. Arch. Oral Biol. 2020, 120, 104946. [Google Scholar] [CrossRef]
- Jing, H.; He, X.; Zheng, J. Exosomes and regenerative medicine: State of the art and perspectives. Transl. Res. 2018, 196, 1–16. [Google Scholar] [CrossRef]
- Shah, N.; Logani, A.; Bhaskar, U.; Aggarwal, V. Efficacy of revascularization to induce apexification/apexogensis in infected, nonvital, immature teeth: A pilot clinical study. J. Endod. 2008, 34, 919–925. [Google Scholar] [CrossRef]
- Zhou, H.; Li, X.; Yin, Y.; He, X.-T.; An, Y.; Tian, B.-M.; Hong, Y.-L.; Wu, L.-A.; Chen, F.-M. The proangiogenic effects of extracellular vesicles secreted by dental pulp stem cells derived from periodontally compromised teeth. Stem Cell Res. Ther. 2020, 11, 1–18. [Google Scholar] [CrossRef]
- Gandolfi, M.G.; Gardin, C.; Zamparini, F.; Ferroni, L.; Esposti, M.D.; Parchi, G.; Ercan, B.; Manzoli, L.; Fava, F.; Fabbri, P.; et al. Mineral-Doped Poly(L-lactide) Acid Scaffolds Enriched with Exosomes Improve Osteogenic Commitment of Human Adipose-Derived Mesenchymal Stem Cells. Nanomaterials 2020, 10, 432. [Google Scholar] [CrossRef]
- Ge, Z.; Yang, L.; Xiao, F.; Wu, Y.; Yu, T.; Chen, J.; Lin, J.; Zhang, Y. Graphene Family Nanomaterials: Properties and Potential Applications in Dentistry. Int. J. Biomater. 2018, 2018. [Google Scholar] [CrossRef]
- Paulus, G.L.C.; Nelson, J.T.; Lee, K.Y.; Wang, Q.H.; Reuel, N.F.; Grassbaugh, B.R.; Kruss, S.; Landry, M.P.; Kang, J.W.; Vander Ende, E.; et al. A graphene-based physiometer array for the analysis of single biological cells. Sci. Rep. 2014, 4, 1–11. [Google Scholar] [CrossRef]
- Chatterjee, N.; Eom, H.J.; Choi, J. A systems toxicology approach to the surface functionality control of graphene-cell interactions. Biomaterials 2014, 35, 1109–1127. [Google Scholar] [CrossRef]
- Pranno, N.; La Monaca, G.; Polimeni, A.; Sarto, M.S.; Uccelletti, D.; Bruni, E.; Cristalli, M.P.; Cavallini, D.; Vozza, I. Antibacterial Activity against Staphylococcus Aureus of Titanium Surfaces Coated with Graphene Nanoplatelets to Prevent Peri-Implant Diseases. An In-Vitro Pilot Study. Int. J. Environ. Res. Public Health 2020, 17, 1568. [Google Scholar] [CrossRef]
- Wu, X.; Ding, S.J.; Lin, K.; Su, J. A review on the biocompatibility and potential applications of graphene in inducing cell differentiation and tissue regeneration. J. Mater. Chem. B 2017, 5, 3084–3102. [Google Scholar] [CrossRef]
- Moradi, F.; Haghgoo, R. Evaluation of antimicrobial efficacy of nanosilver solution, sodium hypochlorite and normal saline in root canal irrigation of primary teeth. Contemp. Clin. Dent. 2018, 9, S227–S232. [Google Scholar] [CrossRef]
- Dubey, N.; Rajan, S.S.; Bello, Y.D.; Min, K.S.; Rosa, V. Graphene Nanosheets to Improve Physico-Mechanical Properties of Bioactive Calcium Silicate Cements. Materials 2017, 10, 606. [Google Scholar] [CrossRef]
- Beyth, N.; Houri-Haddad, Y.; Baraness-Hadar, L.; Yudovin-Farber, I.; Domb, A.J.; Weiss, E.I. Surface antimicrobial activity and biocompatibility of incorporated polyethylenimine nanoparticles. Biomaterials 2008, 29, 4157–4163. [Google Scholar] [CrossRef]
- Abramovitz, I.; Wisblech, D.; Zaltsman, N.; Weiss, E.I.; Beyth, N. Intratubular Antibacterial Effect of Polyethyleneimine Nanoparticles: An Ex Vivo Study in Human Teeth. J. Nanomater. 2015, 2015. [Google Scholar] [CrossRef]
- Toledano, M.; Osorio, E.; Aguilera, F.S.; Muñoz-Soto, E.; Toledano-Osorio, M.; López-López, M.T. Polymeric nanoparticles for endodontic therapy. J. Mech. Behav. Biomed. Mater. 2020, 103. [Google Scholar] [CrossRef] [PubMed]
- Toledano-Osorio, M.; Osorio, E.; Aguilera, F.S.; Luis Medina-Castillo, A.; Toledano, M.; Osorio, R. Improved reactive nanoparticles to treat dentin hypersensitivity. Acta Biomater. 2018, 72, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Batool, F.; Strub, M.; Petit, C.; Bugueno, I.M.; Bornert, F.; Clauss, F.; Huck, O.; Kuchler-Bopp, S.; Benkirane-Jessel, N. Periodontal Tissues, Maxillary Jaw Bone, and Tooth Regeneration Approaches: From Animal Models Analyses to Clinical Applications. Nanomaterials 2018, 8, 337. [Google Scholar] [CrossRef] [PubMed]
- Md Said, H.; Bakar, W.; Farea, M.; Husein, A. The effect of different sealer placement techniques on sealing Ability: An in vitro study. J. Conserv. Dent. 2012, 15, 257–260. [Google Scholar] [CrossRef]
- Siqueira, J.F.; Guimarães-Pinto, T.; Rôças, I.N. Effects of Chemomechanical Preparation with 2.5% Sodium Hypochlorite and Intracanal Medication with Calcium Hydroxide on Cultivable Bacteria in Infected Root Canals. J. Endod. 2007, 33, 800–805. [Google Scholar] [CrossRef]
- AlShwaimi, E.; Bogari, D.; Ajaj, R.; Al-Shahrani, S.; Almas, K.; Majeed, A. In Vitro Antimicrobial Effectiveness of Root Canal Sealers against Enterococcus faecalis: A Systematic Review. J. Endod. 2016, 42, 1588–1597. [Google Scholar] [CrossRef]
- Kontakiotis, E.G.; Wu, M.-K.; Wesselink, P.R. Effect of sealer thickness on long-term sealing ability: A 2-year follow-up study. Int. Endod. J. 2003, 30, 307–312. [Google Scholar] [CrossRef]
- Schäfer, E.; Bering, N.; Bürklein, S. Selected physicochemical properties of AH Plus, EndoREZ and RealSeal SE root canal sealers. Odontology 2015, 103, 61–65. [Google Scholar] [CrossRef]
- Lodiene, G.; Morisbak, E.; Bruzell, E.; Ørstavik, D. Toxicity evaluation of root canal sealers in vitro. Int. Endod. J. 2008, 41, 72–77. [Google Scholar] [CrossRef]
- Barros, J.; Silva, M.G.; Rodrigues, M.A.; Alves, F.R.F.; Lopes, M.A.; Pina-Vaz, I.; Siqueira, J.F. Antibacterial, physicochemical and mechanical properties of endodontic sealers containing quaternary ammonium polyethylenimine nanoparticles. Int. Endod. J. 2014, 47, 725–734. [Google Scholar] [CrossRef]
- Abramovitz, I.; Beyth, N.; Weinberg, G.; Borenstein, A.; Polak, D.; Kesler-Shvero, D.; Houri-Haddad, Y. In vitro biocompatibility of endodontic sealers incorporating antibacterial nanoparticles. J. Nanomater. 2012, 2012. [Google Scholar] [CrossRef]
- Shrestha, A.; Kishen, A. Antibacterial Nanoparticles in Endodontics: A Review. J. Endod. 2016, 42, 1417–1426. [Google Scholar] [CrossRef]
- Gong, S.Q.; Huang, Z.; Shi, W.; Ma, B.; Tay, F.R.; Zhou, B. In vitro evaluation of antibacterial effect of AH plus incorporated with quaternary ammonium epoxy silicate against enterococcus faecalis. J. Endod. 2014, 40, 1611–1615. [Google Scholar] [CrossRef]
- Makvandi, P.; Gu, J.T.; Zare, E.N.; Ashtari, B.; Moeini, A.; Tay, F.R.; Niu, L.N. Polymeric and inorganic nanoscopical antimicrobial fillers in dentistry. Acta Biomater. 2020, 101, 69–101. [Google Scholar] [CrossRef]
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial properties of chitosan and mode of action: A state of the art review. Int. J. Food Microbiol. 2010, 144, 51–63. [Google Scholar] [CrossRef]
- Wong, J.; Zou, T.; Lee, A.H.C.; Zhang, C. The potential translational applications of nanoparticles in endodontics. Int. J. Nanomed. 2021, 16, 2087–2106. [Google Scholar] [CrossRef]
- Love, R.M. Enterococcus faecalis—A mechanism for its role in endodontic failure. Int. Endod. J. 2001, 34, 399–405. [Google Scholar] [CrossRef]
- Nair, N.; James, B.; Devadathan, A.; Johny, M.K.; Mathew, J.; Jacob, J. Comparative evaluation of antibiofilm efficacy of chitosan nanoparticle- and zinc oxide nanoparticle-incorporated calcium hydroxide-based sealer: An in vitro study. Contemp. Clin. Dent. 2018, 9, 434–439. [Google Scholar] [CrossRef]
- del Carpio-Perochena, A.; Kishen, A.; Felitti, R.; Bhagirath, A.Y.; Medapati, M.R.; Lai, C.; Cunha, R.S. Antibacterial Properties of Chitosan Nanoparticles and Propolis Associated with Calcium Hydroxide against Single- and Multispecies Biofilms: An In Vitro and In Situ Study. J. Endod. 2017, 43, 1332–1336. [Google Scholar] [CrossRef]
- Al-Haddad, A.; Aziz, Z.A.C.A. Bioceramic-Based Root Canal Sealers: A Review. Int. J. Biomater. 2016, 2016. [Google Scholar] [CrossRef]
- Cherng, A.M.; Chow, L.C.; Takagi, S. In vitro evaluation of a calcium phosphate cement root canal filler/sealer. J. Endod. 2001, 27, 613–615. [Google Scholar] [CrossRef]
- Tomson, R.M.E.; Polycarpou, N.; Tomson, P.L. Contemporary obturation of the root canal system. Br. Dent. J. 2014, 216, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Whitworth, J. Methods of filling root canals: Principles and practices. Endod. Top. 2005, 12, 2–24. [Google Scholar] [CrossRef]
- Vishwanath, V.; Rao, H. Gutta-percha in—A comprehensive review of material science. J. Conserv. Dent. 2019, 22, 216. [Google Scholar] [CrossRef]
- Moorer, W.R.; Genet, J.M. Evidence for antibacterial activity of endodontic gutta-percha cones. Oral Surg. Oral Med. Oral Pathol. 1982, 53, 503–507. [Google Scholar] [CrossRef]
- Hamann, C.; Rodgers, P.A.; Alenius, H.; Halsey, J.F.; Sullivan, K. Cross-reactivity between gutta-percha and natural rubber latex: Assumptions vs. reality. J. Am. Dent. Assoc. 2002, 133, 1357–1367. [Google Scholar] [CrossRef]
- Lee, D.K.; Kim, S.V.; Limansubroto, A.N.; Yen, A.; Soundia, A.; Wang, C.Y.; Shi, W.; Hong, C.; Tetradis, S.; Kim, Y.; et al. Nanodiamond-Gutta Percha Composite Biomaterials for Root Canal Therapy. ACS Nano 2015, 9, 11490–11501. [Google Scholar] [CrossRef]
- Melker, K.B.; Vertucci, F.J.; Rojas, M.F.; Progulske-Fox, A.; Bélanger, M. Antimicrobial efficacy of medicated root canal filling materials. J. Endod. 2006, 32, 148–151. [Google Scholar] [CrossRef]
- Corrêa, J.M.; Mori, M.; Sanches, H.L.; Da Cruz, A.D.; Poiate, E.; Poiate, I.A.V.P. Silver nanoparticles in dental biomaterials. Int. J. Biomater. 2015, 2015. [Google Scholar] [CrossRef]
- Shantiaee, Y.; Dianat, O.; Mohammadkhani, H.; Akbarzadeh Baghban, A. Cytotoxicity Comparison of Nanosilver Coated Gutta-Percha with Guttaflow and Normal Gutta-Percha on L929 Fibroblast with Mtt Assay. J. Dent. Sch. Shahid Beheshti Univ. Med. Sci. 2011, 29, 63–69. [Google Scholar]
- Shantiaee, Y.; Maziar, F.; Dianat, O.; Mahjour, F. Comparing microleakage in root canals obturated with nanosilver coated gutta-percha to standard gutta-percha by two different methods. Iran Endod. J. 2011, 6, 140–145. [Google Scholar] [PubMed]
- Barnard, A.S. Self-assembly in nanodiamond agglutinates. J. Mater. Chem. 2008, 18, 4038–4041. [Google Scholar] [CrossRef]
- Pattanaik, S.; Jena, A.; Shashirekha, G. In vitro comparative evaluation of antifungal efficacy of three endodontic sealers with and without incorporation of chitosan nanoparticles against Candida albicans. J. Conserv. Dent. 2019, 22, 564–567. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Xu, K.; Wang, H.; Jeremy Tan, P.K.; Fan, W.; Venkatraman, S.S.; Li, L.; Yang, Y.Y. Self-assembled cationic peptide nanoparticles as an efficient antimicrobial agent. Nat. Nanotechnol. 2009, 4, 457–463. [Google Scholar] [CrossRef]
- Kaur, A.; Shah, N.; Logani, A.; Mishra, N. Biotoxicity of commonly used root canal sealers: A meta-analysis. J. Conserv. Dent. 2015, 18, 83–88. [Google Scholar] [CrossRef]
- Versiani, M.A.; Abi Rached-Junior, F.J.; Kishen, A.; Pécora, J.D.; Silva-Sousa, Y.T.; de Sousa-Neto, M.D. Zinc Oxide Nanoparticles Enhance Physicochemical Characteristics of Grossman Sealer. J. Endod. 2016, 42, 1804–1810. [Google Scholar] [CrossRef]
- Javidi, M.; Zarei, M.; Omidi, S.; Ghorbani, A.; Gharechahi, M.; Shayani Rad, M. Cytotoxicity of a new nano zinc-oxide eugenol sealer on murine fibroblasts. Iran. Endod. J. 2015, 10, 231–235. [Google Scholar] [CrossRef]
- Omidi, S.; Javidi, M.; Zarei, M.; Mushakhian, S.; Jafarian, A. Subcutaneous connective tissue reaction to a new nano zinc-oxide eugenol sealer in rat model. Iran. Endod. J. 2017, 12, 79–84. [Google Scholar] [CrossRef]
- Almadi, K.H.; Ahmed, M.A.; Ghazal, T.; Jouhar, R.; Alkahtany, M.F.; Abduljabbar, T.; Vohra, F. Antimicrobial Efficacy of Propolis in Comparison to Chlorhexidine against Enterococcus faecalis: A Systematic Review and Meta-Analysis. Appl. Sci. 2021, 11, 3469. [Google Scholar] [CrossRef]
- Seung, J.; Weir, M.D.; Melo, M.A.S.; Romberg, E.; Nosrat, A.; Xu, H.H.K.; Tordik, P.A. A Modified Resin Sealer: Physical and Antibacterial Properties. J. Endod. 2018, 44, 1553–1557. [Google Scholar] [CrossRef]
- Franci, G.; Falanga, A.; Galdiero, S.; Palomba, L.; Rai, M.; Morelli, G.; Galdiero, M. Silver nanoparticles as potential antibacterial agents. Molecules 2015, 20, 8856–8874. [Google Scholar] [CrossRef]
- Teixeira, A.B.V.; Vidal, C.L.; de Castro, D.T.; de Oliveira-Santos, C.; Schiavon, M.A.; Dos Reis, A.C. Incorporating antimicrobial nanomaterial and its effect on the antimicrobial activity, flow and radiopacity of endodontic sealers. Eur. Endod. J. 2017, 2. [Google Scholar] [CrossRef]
- Baras, B.H.; Sun, J.; Melo, M.A.S.; Tay, F.R.; Oates, T.W.; Zhang, K.; Weir, M.D.; Xu, H.H.K. Novel root canal sealer with dimethylaminohexadecyl methacrylate, nano-silver and nano-calcium phosphate to kill bacteria inside root dentin and increase dentin hardness. Dent. Mater. 2019, 35, 1479–1489. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, D.; Sun, D.; Gu, J. Current status of in vivo bioanalysis of nano drug delivery systems. J. Pharm. Anal. 2020, 10, 221–232. [Google Scholar] [CrossRef]
- Renugalakshmi, A.; Sekar Vinothkumar, T.; Kandaswamy, D. Nanodrug Delivery Systems in Dentistry: A Review on Current Status and Future Perspectives. Curr. Drug Deliv. 2011, 8, 586–594. [Google Scholar] [CrossRef]
- Sousa, M.G.C.; Maximiano, M.R.; Costa, R.A.; Rezende, T.M.B.; Franco, O.L. Nanofibers as drug-delivery systems for infection control in dentistry. Expert Opin. Drug Deliv. 2020, 17, 919–930. [Google Scholar] [CrossRef]
- Ribeiro, J.S.; Münchow, E.A.; Ferreira Bordini, E.A.; de Oliveira da Rosa, W.L.; Bottino, M.C. Antimicrobial Therapeutics in Regenerative Endodontics: A Scoping Review. J. Endod. 2020, 46, S115–S127. [Google Scholar] [CrossRef]
- Bottino, M.C.; Pankajakshan, D.; Nör, J.E. Advanced Scaffolds for Dental Pulp and Periodontal Regeneration. Dent. Clin. N. Am. 2017, 61, 689–711. [Google Scholar] [CrossRef]
- Senthil Kumar, R.; Ravikumar, N.; Kavitha, S.; Mahalaxmi, S.; Jayasree, R.; Sampath Kumar, T.S.; Haneesh, M. Nanochitosan modified glass ionomer cement with enhanced mechanical properties and fluoride release. Int. J. Biol. Macromol. 2017, 104, 1860–1865. [Google Scholar] [CrossRef]
- Garg, U.; Chauhan, S.; Nagaich, U.; Jain, N. Current advances in chitosan nanoparticles based drug delivery and targeting. Adv. Pharm. Bull. 2019, 9, 195–204. [Google Scholar] [CrossRef]
- Chengzhu, L.; Yuchao, L.; Sie Chin, T. Graphene Nanomaterials: Synthesis, Biocompatibility, and Cytotoxicity. Int. J. Mol. Sci. 2018, 19, 3564. [Google Scholar] [CrossRef]
- Guazzo, R.; Gardin, C.; Bellin, G.; Sbricoli, L.; Ferroni, L.; Ludovichetti, F.S.; Piattelli, A.; Antoniac, I.; Bressan, E.; Zavan, B. Graphene-Based Nanomaterials for Tissue Engineering in the Dental Field. Nanomaterials 2018, 8, 349. [Google Scholar] [CrossRef]
- Liu, J.; Dong, J.; Zhang, T.; Peng, Q. Graphene-based nanomaterials and their potentials in advanced drug delivery and cancer therapy. J. Control. Release 2018, 286, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.; Yu, H.; Yang, X.; Yang, X.; Wang, Y.; Liu, Q.; Jin, L.; Yang, Y. Application of Nanomaterials in Stem Cell Regenerative Medicine of Orthopedic Surgery. J. Nanomater. 2017, 2017. [Google Scholar] [CrossRef]
- Damas, B.A.; Wheater, M.A.; Bringas, J.S.; Hoen, M.M. Cytotoxicity comparison of mineral trioxide aggregates and endosequence bioceramic root repair materials. J. Endod. 2011, 37, 372–375. [Google Scholar] [CrossRef] [PubMed]
- Brzęcka, D.M.; Staniowski, T. Nowe materiały bioceramiczne do naprawy korzenia—Przegląd piśmiennictwa. Dent. Med. Probl. 2016, 53, 551–558. [Google Scholar] [CrossRef]
- Alenazy, M.S.; Mosadomi, H.A.; Al-Nazhan, S.; Rayyan, M.R. Clinical considerations of nanobiomaterials in endodontics: A systematic review. Saudi Endod. J. 2018, 8, 163–169. [Google Scholar] [CrossRef]
- Krishnan, P.S.G.; Joshi, M.; Bhargava, P.; Valiyaveettil, S.; He, C. Effect of heterocyclic based organoclays on the properties of polyimide—Clay nanocomposites. J. Nanosci. Nanotechnol. 2005, 5, 1148–1157. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Sato, Y.; Haniu, H.; Nomura, H.; Kobayashi, S.; Takanashi, S.; Okamoto, M.; Takizawa, T.; Aoki, K.; Usui, Y.; et al. A three-dimensional block structure consisting exclusively of carbon nanotubes serving as bone regeneration scaffold and as bone defect filler. PLoS ONE 2017, 12, e0172601. [Google Scholar] [CrossRef]
- Pandey, J.K.; Pratheep Kumar, A.; Misra, M.; Mohanty, A.K.; Drzal, L.T.; Singh, R.P. Recent advances in biodegradable nanocomposites. J. Nanosci. Nanotechnol. 2005, 5, 497–526. [Google Scholar] [CrossRef]
- Li, X.G.; Zhang, R.R.; Huang, M.R. Synthesis of electroconducting narrowly distributed nanoparticles and nanocomposite films of orthanilic acid/aniline copolymers. J. Comb. Chem. 2006, 8, 174–183. [Google Scholar] [CrossRef]
- Shaikh, S.; Birdi, A.; Qutubuddin, S.; Lakatosh, E.; Baskaran, H. Controlled release in transdermal pressure sensitive adhesives using organosilicate nanocomposites. Ann. Biomed. Eng. 2007, 35, 2130–2137. [Google Scholar] [CrossRef]
- Modareszadeh, M.R.; Chogle, S.A.; Mickel, A.K.; Jin, G.; Kowsar, H.; Salamat, N.; Shaikh, S.; Qutbudin, S. Cytotoxicity of set polymer nanocomposite resin root-end filling materials. Int. Endod. J. 2011, 44, 154–161. [Google Scholar] [CrossRef]
- Chogle, S.M.A.; Duhaime, C.F.; Mickel, A.K.; Shaikh, S.; Reese, R.; Bogle, J.H.; Chan, D.K.; Potluri, S.; Qutubuddin, S. Preliminary evaluation of a novel polymer nanocomposite as a root-end filling material. Int. Endod. J. 2011, 44, 1055–1060. [Google Scholar] [CrossRef]
- Subramani, K.; Ahmed, W. (Eds.) Nanobiomaterials in Clinical Dentistry, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Johnston, H.J.; Hutchison, G.; Christensen, F.M.; Peters, S.; Hankin, S.; Stone, V. A review of the in vivo and in vitro toxicity of silver and gold particulates: Particle attributes and biological mechanisms responsible for the observed toxicity. Crit. Rev. Toxicol. 2010, 40, 328–346. [Google Scholar] [CrossRef] [PubMed]
- Pagonis, T.C.; Chen, J.; Fontana, C.R.; Devalapally, H.; Ruggiero, K.; Song, X.; Foschi, F.; Dunham, J.; Skobe, Z.; Yamazaki, H.; et al. Nanoparticle-based Endodontic Antimicrobial Photodynamic Therapy. J. Endod. 2010, 36, 322–328. [Google Scholar] [CrossRef]
- De Almeida, J.; Cechella, B.; Bernardi, A.; De Lima Pimenta, A.; Felippe, W. Effectiveness of nanoparticles solutions and conventional endodontic irrigants against Enterococcus faecalis biofilm. Indian J. Dent. Res. 2018, 29, 347–351. [Google Scholar] [CrossRef]
- Jowkar, Z.; Hamidi, S.A.; Shafiei, F.; Ghahramani, Y. The effect of silver, zinc oxide, and titanium dioxide nanoparticles used as final irrigation solutions on the fracture resistance of root-filled teeth. Clin. Cosmet. Investig. Dent. 2020, 12, 141–148. [Google Scholar] [CrossRef]
- Balakrishnan1, S.; Amin2, S. Licensed Under Creative Commons Attribution CC BY Nanoparticles in Endodontic Irrigation-A Review. Int. J. Sci. Res. 2018. [Google Scholar] [CrossRef]
- Shaw, J.A.; Churchill, C.B.; Iadicola, M.A. Tips and Tricks for Characterizing Shape Memory Alloy Wire: Part 1-Differential Scanning Calorimetry and Basic Phenomena. Exp. Tech. 2008, 32, 55–62. [Google Scholar] [CrossRef]
- Metzger, Z. The self- A djusting file (SAF) system: An evidence-based update. J. Conserv. Dent. 2014, 17, 401–419. [Google Scholar] [CrossRef] [PubMed]
- Adini, A.R.; Feldman, Y.; Cohen, S.R.; Rapoport, L.; Moshkovich, A.; Redlich, M.; Moshonov, J.; Shay, B.; Tenne, R. Alleviating fatigue and failure of NiTi endodontic files by a coating containing inorganic fullerene-like WS2 nanoparticles. J. Mater. Res. 2011, 26, 1234–1242. [Google Scholar] [CrossRef]
- Ahn, S.; Ha, J.H.; Kwak, S.W.; Kim, H.C. Advancement of mechanical properties of nickel-titanium rotary endodontic instruments by spring machining on the file shaft. Materials 2020, 13, 5246. [Google Scholar] [CrossRef] [PubMed]
- Fioretti, F.; Mendoza-Palomares, C.; Avoaka-Boni, M.C.; Ramaroson, J.; Bahi, S.; Richert, L.; Granier, F.; Benkirane-Jessel, N.; Haikel, Y. Nano-odontology: Nanostructured assemblies for endodontic regeneration. J. Biomed. Nanotechnol. 2011, 7, 471–475. [Google Scholar] [CrossRef]
- Kim, S. Microcirculation of the dental pulp in health and disease. J. Endod. 1985, 11, 465–471. [Google Scholar] [CrossRef]
- Oishi, Y.; Manabe, I. Macrophages in inflammation, repair and regeneration. Int. Immunol. 2018, 30, 511–528. [Google Scholar] [CrossRef]
- Schultz, P. Polyelectrolyte multilayers functionalized by a synthetic analogue of an anti-inflammatory peptide, alpha-MSH, for coating a tracheal prosthesis. Biomaterials 2005, 26, 2621–2630. [Google Scholar] [CrossRef]
- Smith, I.O.; Liu, X.H.; Smith, L.A.; Ma, P.X. Nanostructured polymer scaffolds for tissue engineering and regenerative medicine. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2009, 1, 226–236. [Google Scholar] [CrossRef]
- Yang, X.; Yang, F.; Walboomers, X.F.; Bian, Z.; Fan, M.; Jansen, J.A. The performance of dental pulp stem cells on nanofibrous PCL/gelatin/nHA scaffolds. J. Biomed. Mater. Res. Part A 2010, 93, 247–257. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X.; Jin, X.; Ma, H.; Hu, J.; Ni, L.; Ma, P.X. The odontogenic differentiation of human dental pulp stem cells on nanofibrous poly(l-lactic acid) scaffolds in vitro and in vivo. Acta Biomater. 2010, 6, 3856–3863. [Google Scholar] [CrossRef]
- Gupte, M.J.; Ma, P.X. Nanofibrous scaffolds for dental and craniofacial applications. J. Dent. Res. 2012, 91, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Bonilla-Represa, V.; Abalos-Labruzzi, C.; Herrera-Martinez, M.; Guerrero-Pérez, M.O. Nanomaterials in dentistry: State of the art and future challenges. Nanomaterials 2020, 10, 1770. [Google Scholar] [CrossRef] [PubMed]
- Subramani, K.; Ahmed, W.; Hartsfield, J.K. Nanobiomaterials in Clinical Dentistry; Elsevier Inc.: Amsterdam, The Netherlands, 2012; ISBN 9781455731275. [Google Scholar]
- Chogle, S.; Kinaia, B.M.; Goodis, H.E. Scope of nanotechnology in endodontics. In Nanobiomaterials in Clinical Dentistry; Elsevier: Amsterdam, The Netherlands, 2019; pp. 517–539. ISBN 9780128158869. [Google Scholar]
- Raura, N.; Garg, A.; Arora, A.; Roma, M. Nanoparticle technology and its implications in endodontics: A review. Biomater. Res. 2020, 24, 1–8. [Google Scholar] [CrossRef]
- Desouky, A.A.; Negm, M.M.; Ali, M.M. Sealability of Different Root Canal Nanosealers: Nano Calcium Hydroxide and Nano Bioactive Glass. Open Dent. J. 2019, 13, 308–315. [Google Scholar] [CrossRef]
- Eskandarinezhad, M.; Shahvaghar-Asl, N.; Sharghi, R.; Shirazi, S.; Shakouie, S.; Milani, A.S.; Balaei, E. Sealing efficacy of mineral trioxide aggregate with and without nanosilver for root end filling: An in-vitro bacterial leakage study. J. Clin. Exp. Dent. 2017, 9, e27–e33. [Google Scholar] [CrossRef][Green Version]
- Javidi, M.; Zarei, M.; Naghavi, N.; Mortazavi, M.; Nejat, A. Zinc oxide nano-particles as sealer in endodontics and its sealing ability. Contemp. Clin. Dent. 2014, 5, 20–24. [Google Scholar] [CrossRef]
- Teixeira, A.B.V.; de Castro, D.T.; Schiavon, M.A.; dos Reis, A.C. Cytotoxicity and release ions of endodontic sealers incorporated with a silver and vanadium base nanomaterial. Odontology 2020, 108, 661–668. [Google Scholar] [CrossRef]
- Enggardipta, R.A.; Untara, R.T.E.; Santosa, P.; Kartikaningtyas, A.T.; Widyastuti, A.; Ratih, D.N. Apical sealing ability of chitosan nanoparticles in epoxy-resin-based endodontic sealer. Maj. Kedokt. Gigi Indones. 2020, 5, 69. [Google Scholar] [CrossRef]
- del Carpio-Perochena, A.; Bramante, C.M.; Duarte, M.A.H.; de Moura, M.R.; Aouada, F.A.; Kishen, A. Chelating and antibacterial properties of chitosan nanoparticles on dentin. Restor. Dent. Endod. 2015, 40, 195. [Google Scholar] [CrossRef]
- Ng, X.W.; Mundargi, R.C.; Venkatraman, S.S. Nanomedicine: Size-related drug delivery applications, including periodontics and endodontics. In Nanotechnology in Endodontics: Current and Potential Clinical Applications; Springer International Publishing: Basel, Switzerland, 2015; pp. 71–96. ISBN 9783319135755. [Google Scholar]
- Panácek, A.; Smékalová, M.; Kilianová, M.; Prucek, R.; Bogdanová, K.; Věcěrová, R.; Kolár, M.; Havrdová, M.; Płaza, G.A.; Chojniak, J.; et al. Strong and nonspecific synergistic antibacterial efficiency of antibiotics combined with silver nanoparticles at very low concentrations showing no cytotoxic effect. Molecules 2016, 21, 26. [Google Scholar] [CrossRef]
- Shrestha, A.; Kishen, A. Antibacterial efficacy of photosensitizer functionalized biopolymeric nanoparticles in the presence of tissue inhibitors in root canal. J. Endod. 2014, 40, 566–570. [Google Scholar] [CrossRef] [PubMed]
- Baras, B.H.; Melo, M.A.S.; Thumbigere-Math, V.; Tay, F.R.; Fouad, A.F.; Oates, T.W.; Weir, M.D.; Cheng, L.; Xu, H.H.K. Novel Bioactive and Therapeutic Root Canal Sealers with Antibacterial and Remineralization Properties. Materials 2020, 13, 1096. [Google Scholar] [CrossRef]
- Ioannidis, K.; Niazi, S.; Mylonas, P.; Mannocci, F.; Deb, S. The synthesis of nano silver-graphene oxide system and its efficacy against endodontic biofilms using a novel tooth model. Dent. Mater. 2019, 35, 1614–1629. [Google Scholar] [CrossRef]
- Anil, K. (Ed.) Nanotechnology in Endodontics—Current and Potential Clinical Applications; Springer: Berlin, Germany, 2015. [Google Scholar]
- Bellamy, C.; Shrestha, S.; Torneck, C.; Kishen, A. Effects of a Bioactive Scaffold Containing a Sustained Transforming Growth Factor-β1–releasing Nanoparticle System on the Migration and Differentiation of Stem Cells from the Apical Papilla. J. Endod. 2016, 42, 1385–1392. [Google Scholar] [CrossRef]
- Patel, E.; Pradeep, P.; Kumar, P.; Choonara, Y.E.; Pillay, V. Oroactive dental biomaterials and their use in endodontic therapy. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 108, 201–212. [Google Scholar] [CrossRef]
- Shrestha, A.; Zhilong, S.; Gee, N.K.; Kishen, A. Nanoparticulates for antibiofilm treatment and effect of aging on its antibacterial activity. J. Endod. 2010, 36, 1030–1035. [Google Scholar] [CrossRef]
- Barros, J.; Costa-Rodrigues, J.; Lopes, M.A.; Pina-Vaz, I.; Fernandes, M.H. Response of human osteoblastic and osteoclastic cells to AH plus and pulp canal sealer containing quaternary ammonium polyethylenimine nanoparticles. J. Endod. 2014, 40, 1149–1155. [Google Scholar] [CrossRef]
- Baras, B.H.; Wang, S.; Melo, M.A.S.; Tay, F.; Fouad, A.F.; Arola, D.D.; Weir, M.D.; Xu, H.H.K. Novel bioactive root canal sealer with antibiofilm and remineralization properties. J. Dent. 2019, 83, 67–76. [Google Scholar] [CrossRef]
- Hashmi, A.; Zhang, X.; Kishen, A. Impact of Dentin Substrate Modification with Chitosan-Hydroxyapatite Precursor Nanocomplexes on Sealer Penetration and Tensile Strength. J. Endod. 2019, 45, 935–942. [Google Scholar] [CrossRef]
- Akbarianrad, N.; Mohammadian, F.; Nazari, A.A.; Nobar, R. Applications of nanotechnology in endodontic: A Review. Nanomed. J. 2018, 5, 121–126. [Google Scholar] [CrossRef]
- Gopee, N.V.; Roberts, D.W.; Webb, P.; Cozart, C.R.; Siitonen, P.H.; Warbritton, A.R.; Yu, W.V.; Colvin, V.L.; Walker, N.J.; Howard, P.C. Migration of intradermally injected quantum dots to sentinel organs in mice. Toxicol. Sci. 2007, 98, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Fröhlich, E. Cellular Targets and Mechanisms in the Cytotoxic Action of Non-biodegradable Engineered Nanoparticles. Curr. Drug Metab. 2013, 14, 976–988. [Google Scholar] [CrossRef] [PubMed]
- Jeevanandam, J.; Barhoum, A.; Chan, Y.S.; Dufresne, A.; Danquah, M.K. Review on nanoparticles and nanostructured materials: History, sources, toxicity and regulations. Beilstein J. Nanotechnol. 2018, 9, 1050–1074. [Google Scholar] [CrossRef]
| Nanomaterial | Application | Method of Use | Details | Reference |
|---|---|---|---|---|
| Ca(OH)2 | Sealing | Sealing Application of a sealer on a guttapercha point followed by canal obturation | Study focused on reduction of microleakage from the apical to coronal third by exchanging traditional ZnoE sealer with Ca(OH)2 | [197] |
| nAg | Root restoration | Application into root cavities | Material has been mixed with MTA and placed in the cavity | [198] |
| Nano zinc oxide | Sealing | Canal obturation with a sealer | Zinc oxide nanoparticles mixed in a zinc-oxide eugenol cement | [199] |
| nAgVO3 | Sealing | Canal obturation with a sealer enriched with a nanomaterial | AH Plus endodontic, Sealer 26, Endomethasone N sealers were incorporated with AgVO3 | [200] |
| Nanodiamond | Sealing, Antimicrobial action | Canal obturation with a nanodiamond guttapercha | Nanodiamond-amoxicillin conjugates are conjugated and incorporated into a gutta-percha point | [137] |
| Chitosan nanoparticles | Sealing | Nanomaterial added to the sealer | Chitosan nanoparticles added to the epoxy resin sealer elevate the apical sealing ability of root canal obturation | [201] |
| Chitosan nanoparticles | antimicrobial | Nanoparticles as a part of irrigating solution | Several irrigants were compared, including one with chitosan nanoparticles, according to the study, it can be a useful alternative to EDTA irrigants | [202] |
| Nano-sized Liposomes | Drug delivery | Nano-sized biomaterial used for application of drugs | 80–100 nm sized liposomes with incorporated drugs specifically used for resistant bacterial biofilm that causes persistent endodontic and periodontic disease | [203] |
| nAg | antimicrobial | Nanoparticles used as an antimicrobial material | Different concentrations of nAg particles were tested for their antimicrobial effect on E. coli, S. aureus and P. aeruginosa | [204] |
| Chitosan nanoparticles | Photodynamic therapy | Nanoparticles have been a photosensitizer increased its efficacy | Application of chitosan nanoparticles into a photosensitizer increased activity of the material. Their higher surface area resulted in increased binding of the photosensitizer to the particles. | [205] |
| Quaternary Ammonium Polyethyleneimine nanoparticles (QPEI) | Antimicrobial resins | Nanoparticles were mixed with dental resins | QPEI have been chemically incorporated into several polymeric resin matrices of dental materials, which resulted in a long-lasting antimicrobial effect. | [206] |
| Nanohydroxyapatite | Regeneration therapy | Dental pulp stem cells have been seeded on the nanocomposite scaffolds | Nanohydroxyapatite scaffolds elevated in vivo and in vitro odontogenic differentiation of the Dental pulp stem cells | [190] |
| Nanofibrous poly (L-lactic acid) scaffolds | Regeneration therapy | Dental pulp stem cells have been seeded on the nanomaterial scaffold | Nanofibrous poly (L-lactic acid) scaffold increased the odontogenic differentiation of dental pulp stem cells | [191] |
| Exosomes | Regeneration therapy | Exosomes derived from dental pulp stem cells were tested for their influence for endothelial cells regeneration | Exosomes derived from dental pulp stem cells highly enhanced the proliferation, angiogenesis and migration of endothelial cells in vitro and accelerated in vivo cutaneous wound healing | [100] |
| Graphene oxide | Antimicrobial | Silver nanoparticles were synthesized on graphene oxide particles | On an ex vivo model of an infected tooth, silver-graphene oxide material has successfully achieved and enhanced antimicrobial activity | [207] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zakrzewski, W.; Dobrzyński, M.; Zawadzka-Knefel, A.; Lubojański, A.; Dobrzyński, W.; Janecki, M.; Kurek, K.; Szymonowicz, M.; Wiglusz, R.J.; Rybak, Z. Nanomaterials Application in Endodontics. Materials 2021, 14, 5296. https://doi.org/10.3390/ma14185296
Zakrzewski W, Dobrzyński M, Zawadzka-Knefel A, Lubojański A, Dobrzyński W, Janecki M, Kurek K, Szymonowicz M, Wiglusz RJ, Rybak Z. Nanomaterials Application in Endodontics. Materials. 2021; 14(18):5296. https://doi.org/10.3390/ma14185296
Chicago/Turabian StyleZakrzewski, Wojciech, Maciej Dobrzyński, Anna Zawadzka-Knefel, Adam Lubojański, Wojciech Dobrzyński, Mateusz Janecki, Karolina Kurek, Maria Szymonowicz, Rafał Jakub Wiglusz, and Zbigniew Rybak. 2021. "Nanomaterials Application in Endodontics" Materials 14, no. 18: 5296. https://doi.org/10.3390/ma14185296
APA StyleZakrzewski, W., Dobrzyński, M., Zawadzka-Knefel, A., Lubojański, A., Dobrzyński, W., Janecki, M., Kurek, K., Szymonowicz, M., Wiglusz, R. J., & Rybak, Z. (2021). Nanomaterials Application in Endodontics. Materials, 14(18), 5296. https://doi.org/10.3390/ma14185296










