Microstructure Evolution and Mechanical Properties of X6CrNiMoVNb11-2 Stainless Steel after Heat Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation and Initial Properties
2.1.1. Casting Process and Chemical Composition of X6CrNiMoVNb11-2 Steel
2.1.2. The Hot-Rolling Process and the Initial As-Rolled Mechanical Properties
2.2. Heat Treatment Schedule and Properties Measurements
2.2.1. Experimental Schedule of the Heat-Treatment Process
2.2.2. Experimental Measurement of Mechanical Properties
3. Results and Discussion
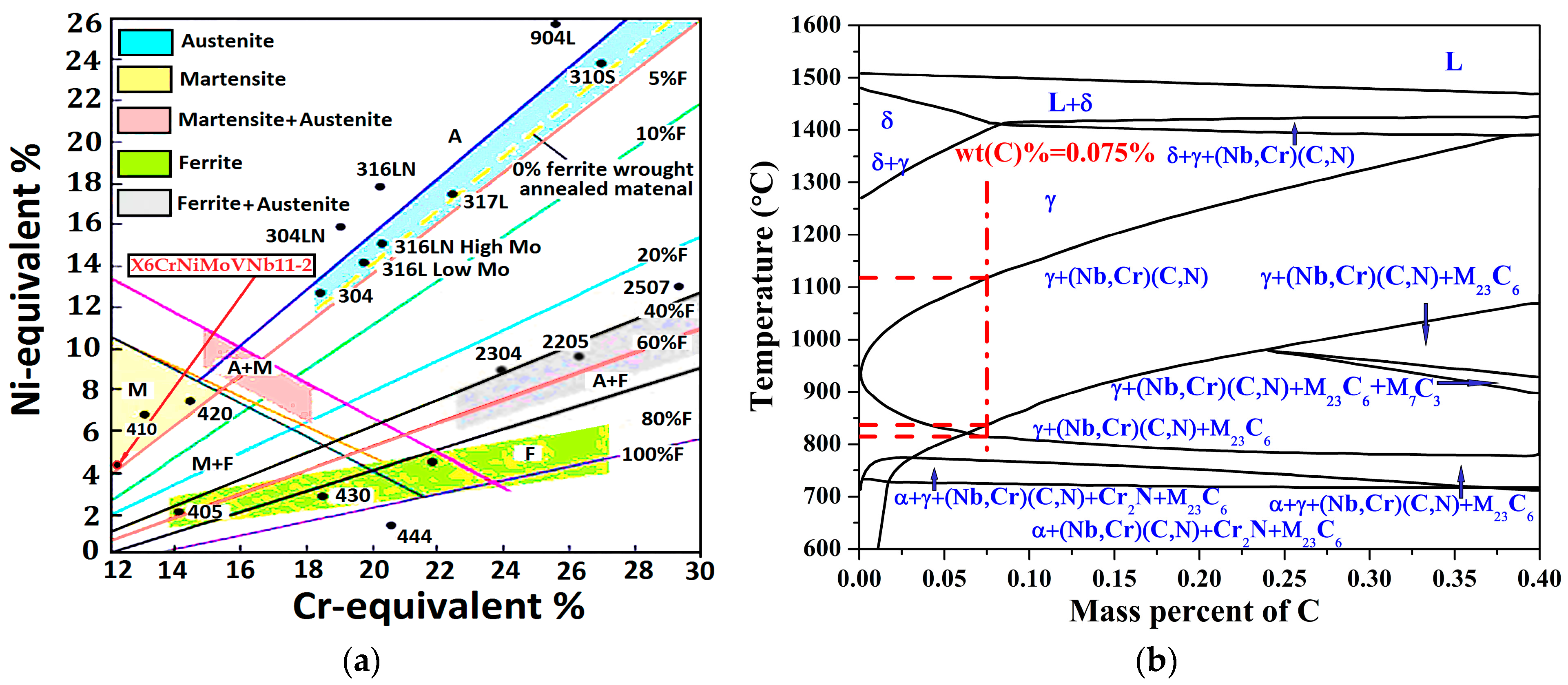
3.1. Analysis of the Alloy Phase Diagram and TTT/CCT Curve
3.1.1. Analysis of the Alloy Phase Diagram
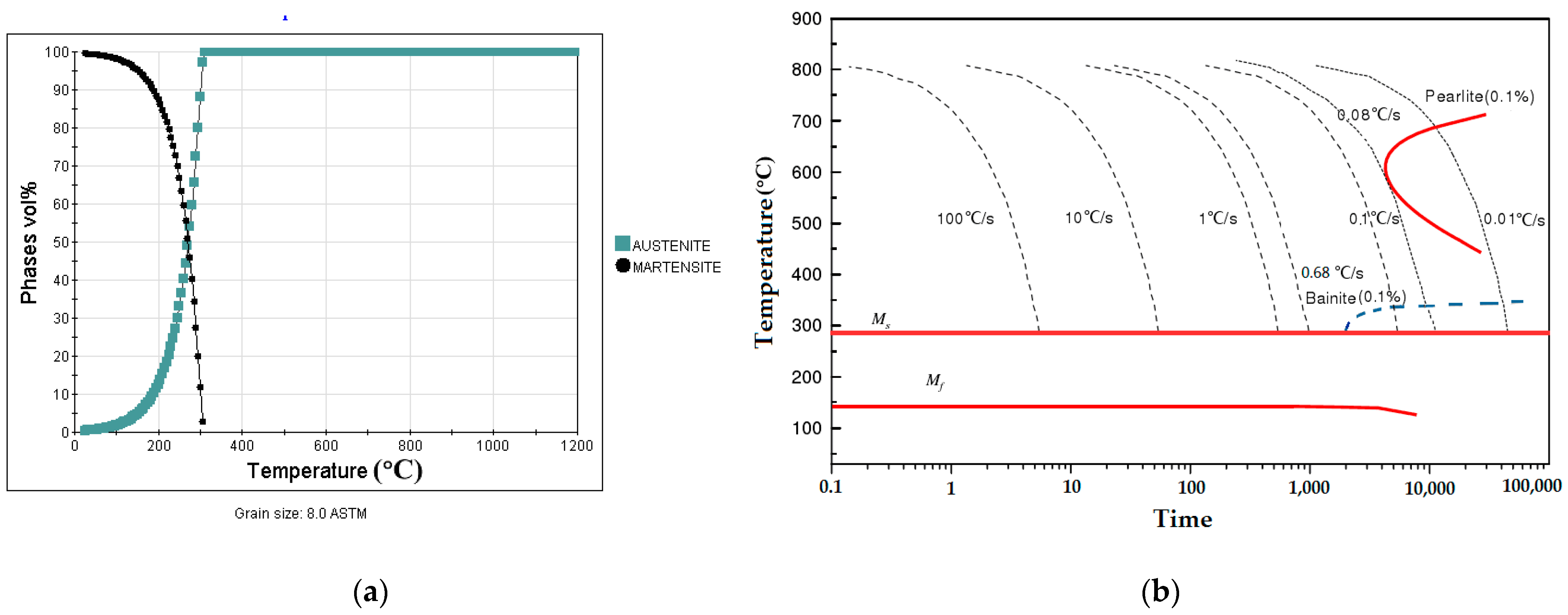
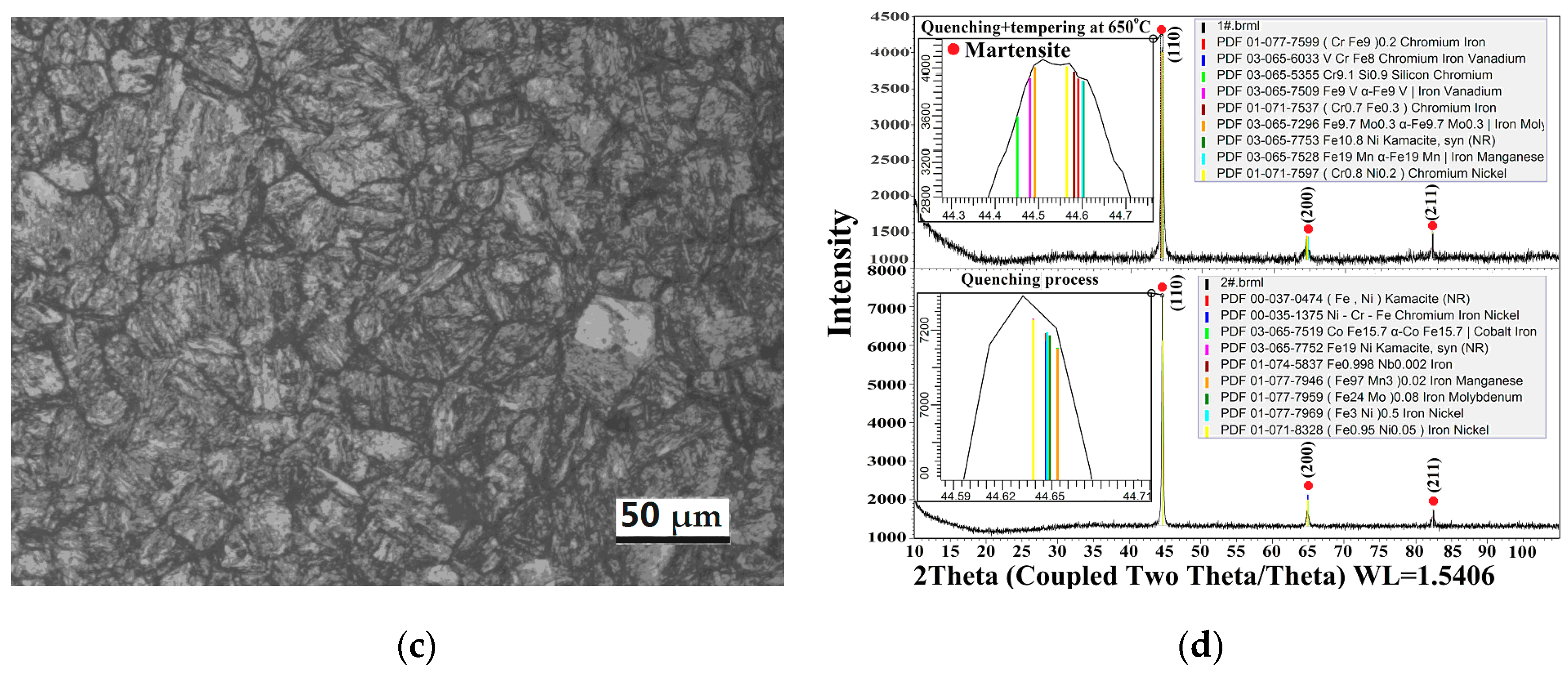
3.1.2. Analysis of the Phase Composition and CCT Curve
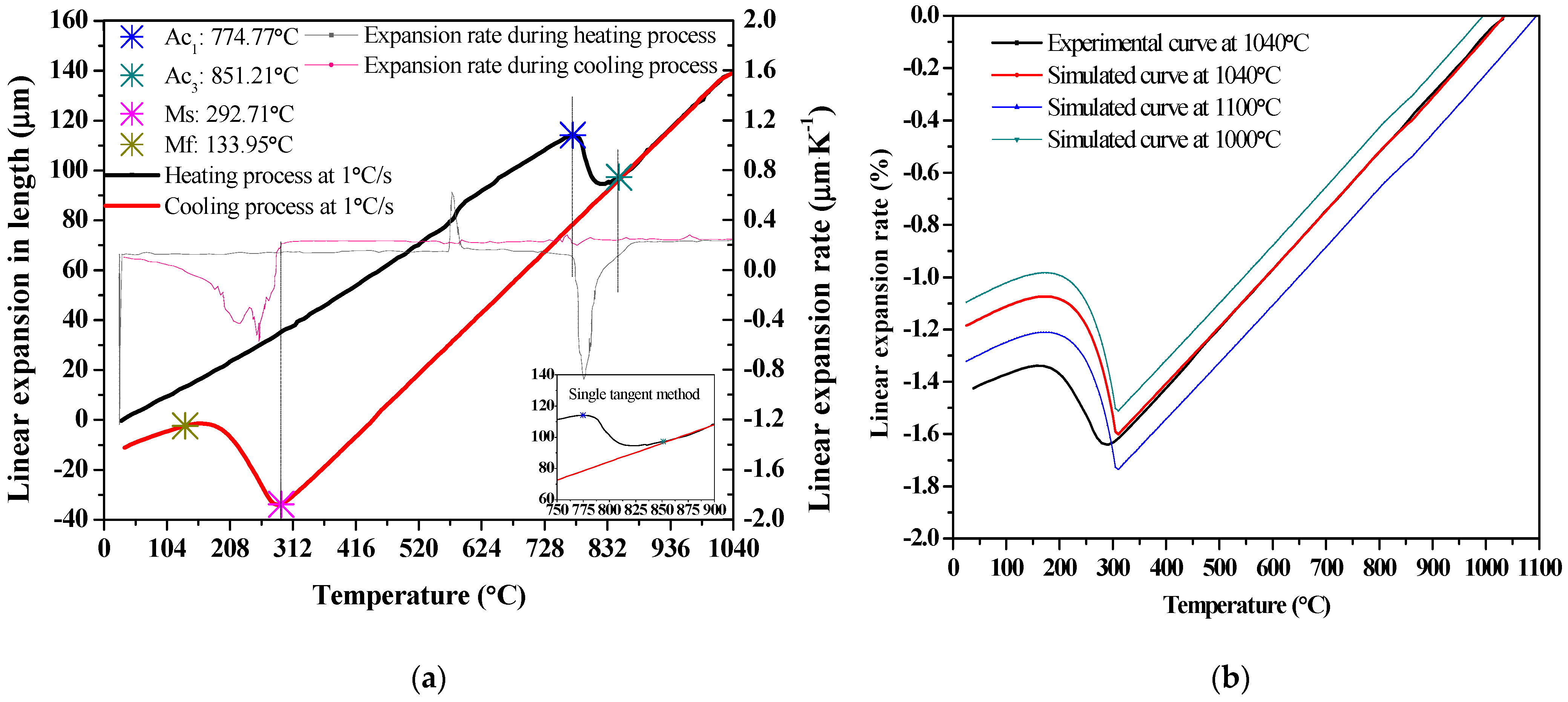
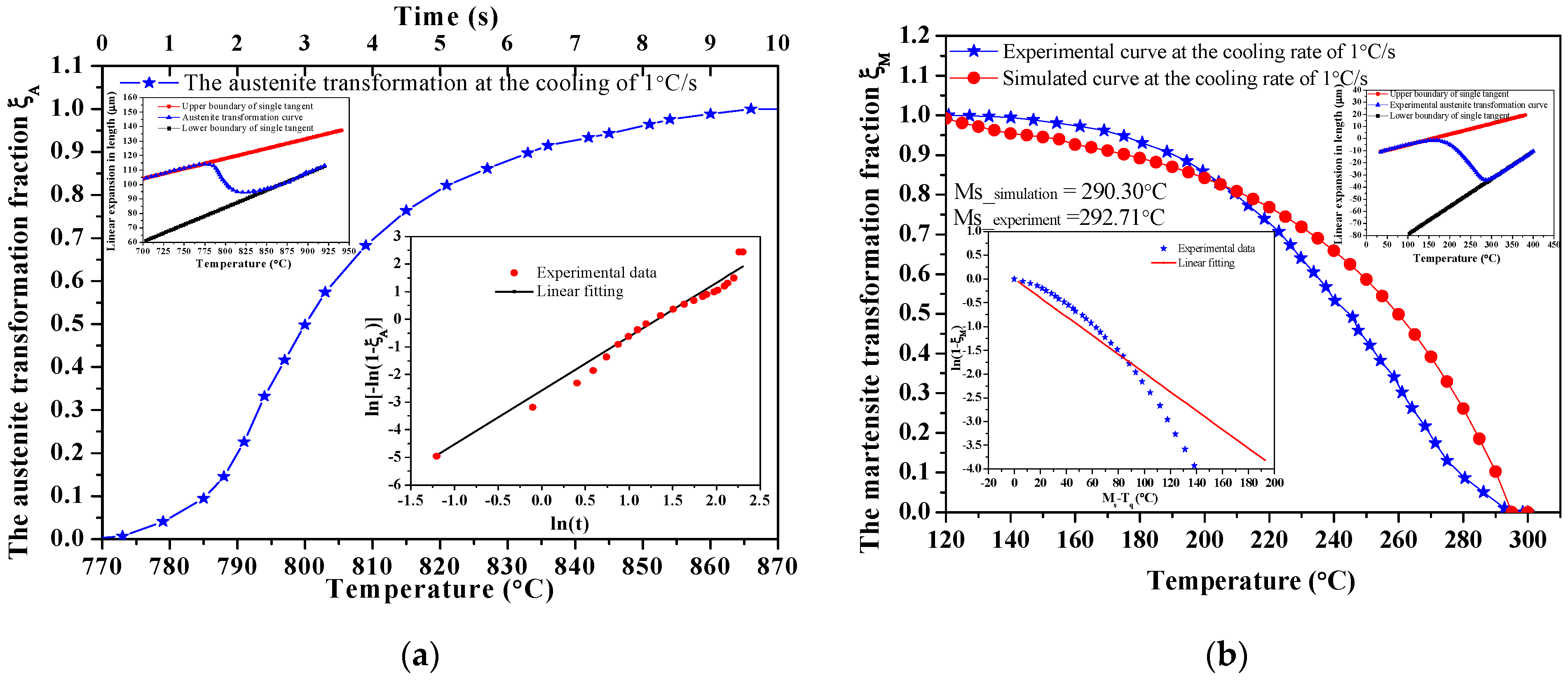
3.2. Phase Transformation Temperatures and Martensitic Kinetic Equation
3.2.1. Determination of Martensitic Phase Transition Point
3.2.2. Calculation of the Phase Transformation Kinetic Equation
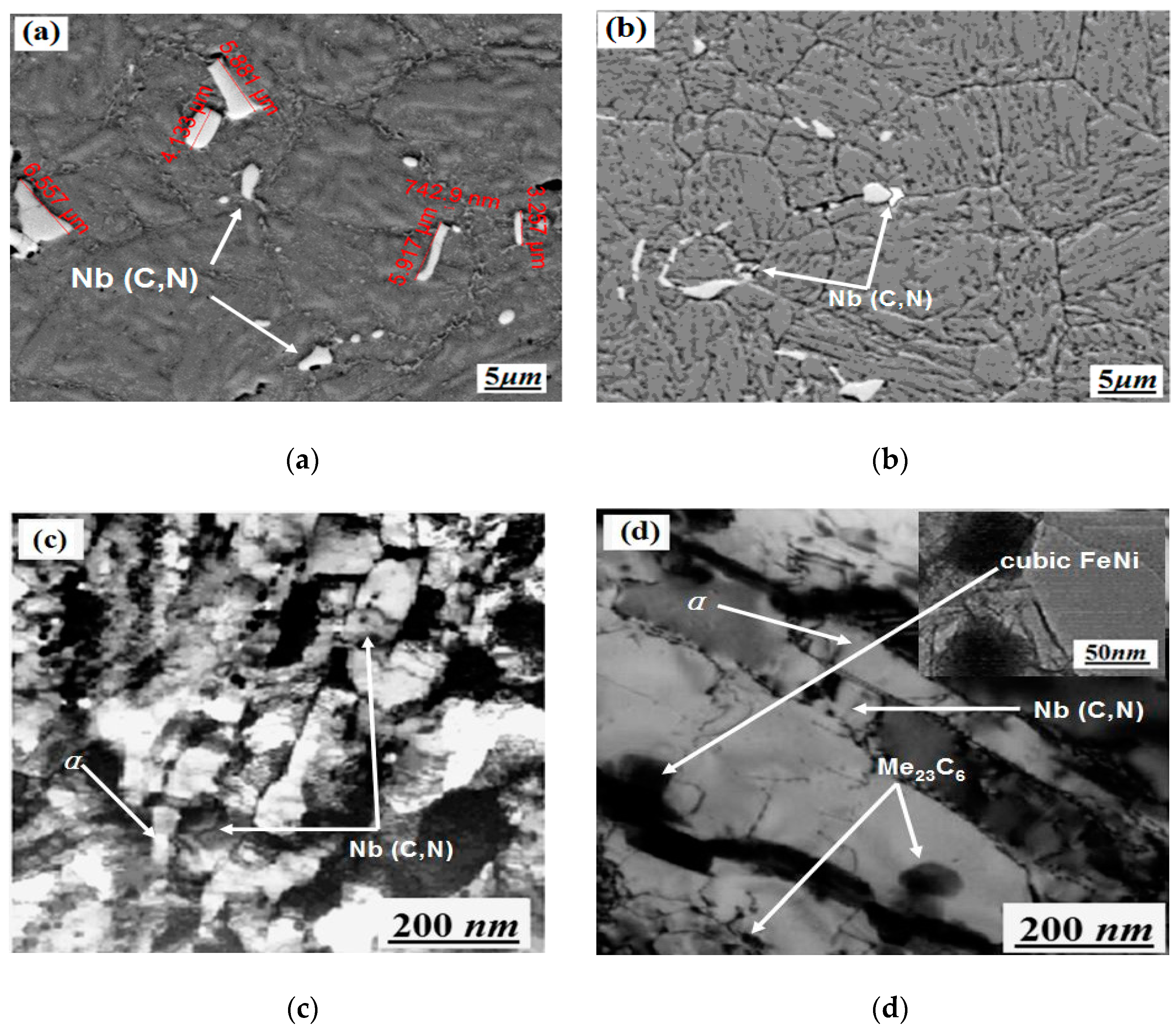
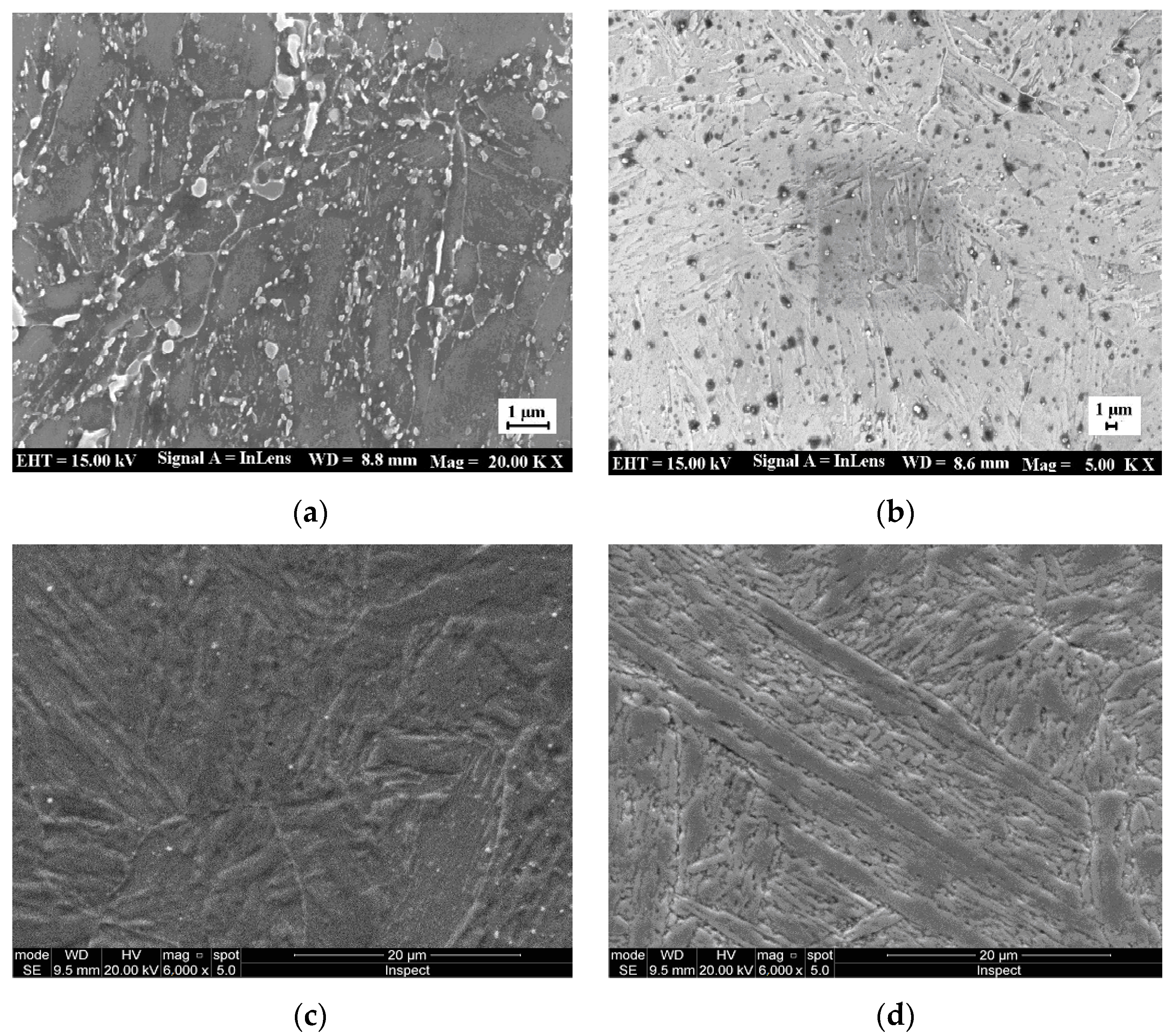
3.3. Analysis of SEM, XRD, EDS and Phase before and after Heat Treatment
3.3.1. SEM and XRD Analysis before and after Heat Treatment
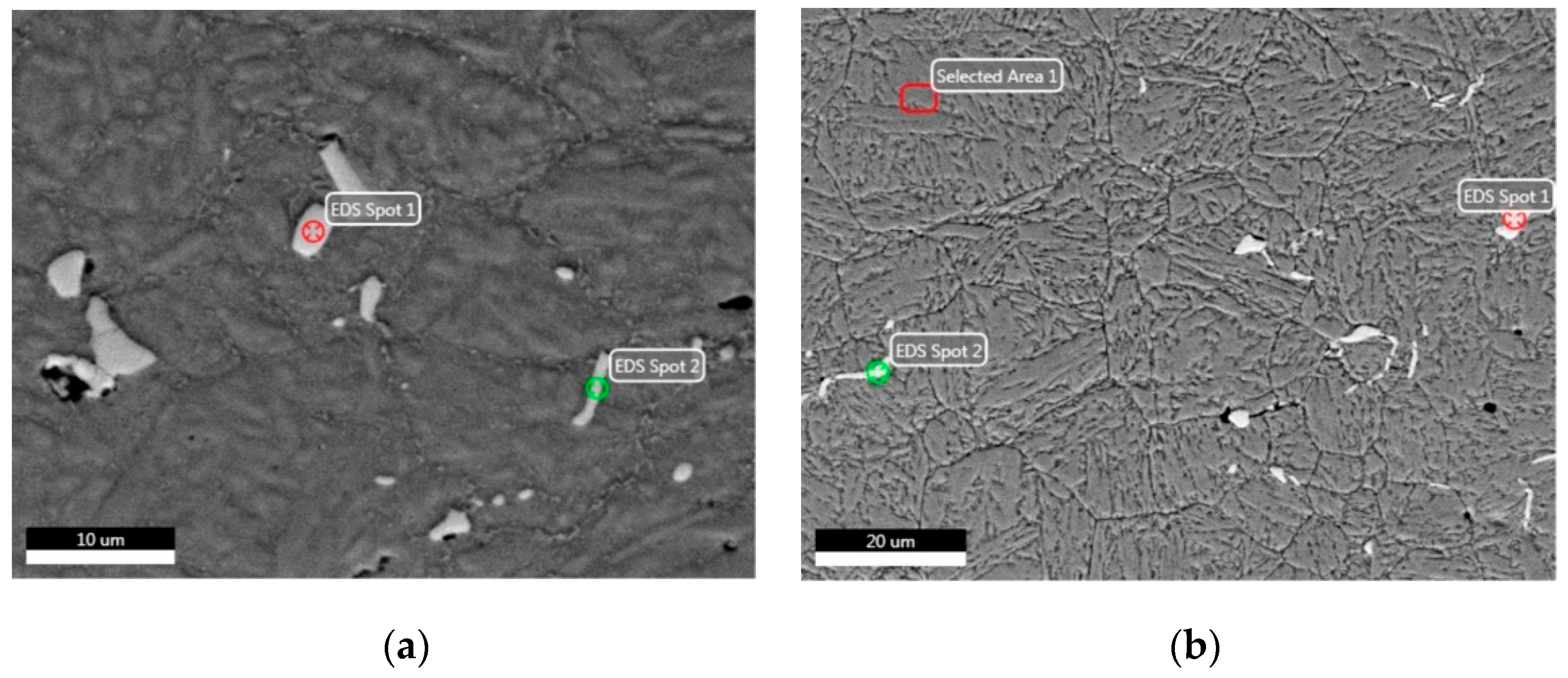
3.3.2. SEM-EDS Analysis before and after Heat Treatment
3.4. Phase Analysis before and after Heat Treatment
3.4.1. EDS Analysis before and after Heat Treatment
3.4.2. Phase Analysis before and after Heat Treatment
3.4.3. Hardness Analysis after Various Heat Treatment Processes
3.5. Experimental Mechanical Properties under Various Heat Treatments
3.5.1. Effect of the Quenching Cooling Rate on Properties
3.5.2. Effect of Tempering Cooling Rate on Properties
3.5.3. Comparison of Hardness under Various Processes
3.6. Comparison of Mechanical Properties under Various Processes
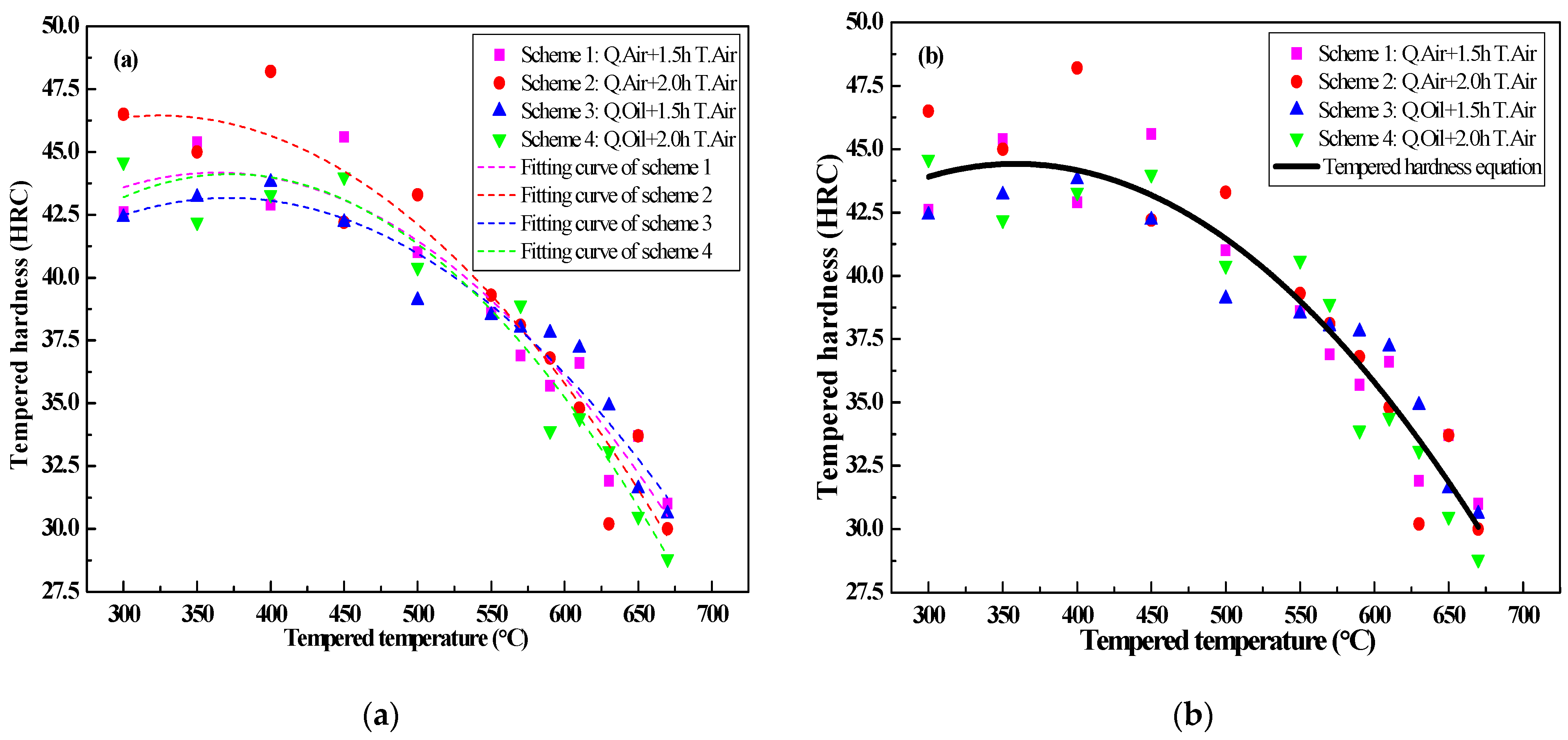
3.6.1. Determination of the Tempering Hardness Equation
3.6.2. Comparison of Tensile Properties under Various Processes
4. Conclusions
- According to the thermal expansion curve, the Ac1, Ac3, Ms and Mf points are determined to be Ac1 = 774.77 °C, Ac3 = 851.21 °C, Ms = 292.71 °C, Mf = 133.95 °C, and the tempering hardness equation under the quenching-tempering process is obtained;
- Secondary hardening occurs at the tempering temperature of 550 °C, due to the formation of M7C3 in martensite. When the tempering temperature increases up to 650 °C, the M7C3 carbides become coarser and begin to partially convert into M23C6 carbides;
- During the tempering process, the cooling rate has a greater impact on the impact toughness. The faster the cooling rate is, the higher the impact toughness is. With the continuing phase transformation from austenite into martensite that depletes the carbon element, the hardness greatly increases and the elongation slightly decreases;
- The grain size of martensite is finer and more uniform under the process of the oil quenching and tempering with air cooling, compared with that of the oil quenching process. The optimized process, namely, oil quenching at 1040 °C for 1.0 h and then tempering at 650 °C for 1.5 h with the air cooling, yields the best comprehensive properties;
- The ideal heat treatment is to quench at 1040 °C with oil cooling and then temper at 650 °C with air cooling, resulting in hardness on the surface of 313 HV, the hardness at the center is 285 HV, the elongation is 17.9%, and the impact toughness is 65.0 J/cm2;
- The cooling medium has an important effect on mechanical properties. With the decrease in the cooling rate, carbides gradually precipitate during quenching, and the subsequent temper embrittlement appears during tempering, resulting in a great decrease in impact toughness and strength. Thus, high impact toughness can be obtained through rapid cooling during the quenching and tempering processes.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jiang, S.; Hui, W.; Yuan, W.; Liu, X.; Lu, Z. Ultrastrong steel via minimal lattice misfit and high-density nanoprecipitation. Nature 2017, 544, 460–464. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Lu, J.; Chan, K.C.; Sun, L.; Zhu, L. Dual-phase nanostructuring as a route to high-strength magnesium alloys. Nature 2017, 545, 80–83. [Google Scholar] [CrossRef]
- Lo, K.H.; Shek, C.H.; Lai, J.K.L. Recent developments in stainless steels. Mater. Sci. Eng. R 2009, 65, 39–104. [Google Scholar] [CrossRef]
- Cai, X.; Hu, X.Q.; Zheng, L.G.; Li, D.Z. Hot deformation behavior and processing maps of 0.3C–15Cr–1Mo–0.5N high nitrogen martensitic stainless steel. Acta Metall. Sin.-Engl. Lett. 2020, 33, 693–704. [Google Scholar] [CrossRef]
- Kumar, B.R.; Sharma, S.; Munda, P.; Minz, R.K. Structure and microstructure evolution of a ternary Fe–Cr–Ni alloy akin to super martensitic stainless steel. Mater. Des. 2013, 50, 392–398. [Google Scholar] [CrossRef]
- Dong-Seok, L.; Yong-Deuk, L.; Joong-Hwan, J.; Chong-Sool, C. Amount of retained austenite at room temperature after reverse transformation of martensite to austenite in an Fe–13%Cr–7%Ni–3%Si martensitic stainless steel. Scr. Mater. 2001, 45, 767–772. [Google Scholar]
- Blinova, E.N.; Glezer, A.M.; Libman, M.A.; Estrin, E.I. Structure of the martensite–austenite transition zone after a local pulse heating of the martensite. Russ. Phys. J. 2014, 57, 429–435. [Google Scholar] [CrossRef]
- Wang, B.; He, Y.; Liu, Y.; Tian, Y.; You, J.; Wang, Z.; Wang, G. Mechanism of the microstructural evolution of 18Cr2Ni4WA steel during vacuum low-pressure carburizing heat treatment and its effect on case hardness. Materials 2020, 13, 2352. [Google Scholar] [CrossRef]
- Fu, J.; Zhang, Y. Mechanism of crack initiation and propagation of 316LN stainless steel during the high-temperature tensile deformation. Mater. Res. Express 2020, 7, 121684. [Google Scholar] [CrossRef]
- Wang, L.X.; Song, C.J.; Sun, F.M.; Li, L.J.; Zhai, Q.J. Microstructure and mechanical properties of 12 wt.%Cr ferritic stainless steel with Ti and Nb dual stabilization. Mater. Des. 2009, 30, 49–56. [Google Scholar] [CrossRef]
- Aghaie-Khafri, M.; Anijdan, S.H.M.; Amirkamali, M. Microstructural evolution under ausforming and aging conditions in 17-4 PH stainless steel. Mater. Res. Express 2019, 6, 106532. [Google Scholar] [CrossRef]
- Bilmes, P.D.; Solari, M.; Llorente, C.L. Characteristics and effects of austenite resulting from tempering of 13CrNiMo martensitic steel weld metals. Mater. Charact. 2011, 46, 285–296. [Google Scholar] [CrossRef]
- Kondo, K.; Ogawa, K.; Amaya, H.; Ueda, M.; Ohtani, H. Development of weldable super 13Cr martensitic stainless steel for flowline. In Proceedings of the Twelfth International Offshore and Polar Engineering Conference, Kitakyushu, Japan, 26–31 May 2002; pp. 26–31. [Google Scholar]
- Zou, D.N.; Han, Y.; Zhang, W.; Fang, X.D. Influence of tempering process on mechanical properties of 00Cr13Ni4Mo supermartensitic stainless steel. J. Iron. Steel Res. Int. 2010, 17, 50–54. [Google Scholar] [CrossRef]
- AlHazaa, A.; Haneklaus, N. Diffusion Bonding and Transient Liquid Phase (TLP) Bonding of Type 304 and 316 Austenitic Stainless Steel—A Review of Similar and Dissimilar Material Joints. Metals 2020, 10, 613. [Google Scholar] [CrossRef]
- Liu, Y.R.; Dong, Y.E.; Yong, Q.L.; Jie, S.U.; Zhao, K.Y.; Jiang, W. Effect of heat treatment on microstructure and property of Cr13 super martensitic stainless steel. J. Iron. Steel Res. Int. 2011, 18, 60–66. [Google Scholar] [CrossRef]
- Song, Y.Y.; Ping, D.H.; Yin, F.X.; Li, X.Y.; Li, Y.Y. Microstructural evolution and low temperature impact toughness of a Fe-13%Cr-4%Ni-Mo martensitic stainless steel. Mater. Sci. Eng. A 2010, 527, 614–618. [Google Scholar] [CrossRef]
- Golanski, G.; Slania, J. Effect of different heat treatments on microstructure and mechanical properties of the martensitic GX12CrMoVNbN9-1 cast steel. Arch. Metall. Mater. 2013, 58, 25–30. [Google Scholar] [CrossRef] [Green Version]
- Fu, J.; Li, F.G.; Li, Y.T. Characterization and the improved Arrhenius model of 0Cr11Ni2MoVNb steel during hot deformation process. IOP Conf. Series Mater. Sci. Eng. 2018, 382, 022027. [Google Scholar] [CrossRef] [Green Version]
- Fu, J.; Li, F.G.; Li, Y.T. Establishment of hot processing map and work hardening mechanism of 0Cr11Ni2MoVNb stainless steel during hot compression process. IOP Conf. Series Mater. Sci. Eng. 2018, 382, 022029. [Google Scholar] [CrossRef]
- Li, Q.; Wu, L.; Li, F.; Liu, T.; Su, C. Experiments study on the rolling process for heavy disk. Int. J. Adv. Manuf. Technol. 2012, 65, 1171–1175. [Google Scholar] [CrossRef]
- Topic, M.; Allen, C.; Tait, R. The effect of cold work and heat treatment on the fatigue behaviour of 3Cr12 corrosion resistant steel wire. Int. J. Fatigue 2007, 29, 49–56. [Google Scholar] [CrossRef]
- Krakhmalev, P.; Yadroitsava, I.; Fredriksson, G.; Yadroitsev, I. In situ heat treatment in selective laser melted martensitic AISI420 stainless steels. Mater. Des. 2015, 87, 380–385. [Google Scholar] [CrossRef]
- Warmelo, M.V.; Nolan, D.; Norrish, J. Mitigation of sensitization effects in unstabilised 12% Cr ferritic stainless steel welds. Mater. Sci. Eng. A 2007, 464, 157–169. [Google Scholar] [CrossRef]
- Tkalcec, I.; Mari, D. Internal friction in martensitic, ferritic and bainitic carbon steel: Cold work effects. Mater. Sci. Eng. A 2004, 370, 213–217. [Google Scholar] [CrossRef]
- Chadha, K.; Yuan, T.; Bocher, P.; Spray, J.G.; Aranas, C. Microstructure evolution, mechanical properties and deformation behavior of an additively manufactured maraging steel. Materials 2020, 13, 2380. [Google Scholar] [CrossRef]
- Scheuer, C.J.; Zanetti, F.I.; Cardoso, R.P.; Brunatto, S.F. Ultra-low-to high-temperature plasma-assisted nitriding: Revisiting and going further on the martensitic stainless steel treatment. Mater. Res. Express 2019, 6, 026529. [Google Scholar] [CrossRef]
- Saeidi, K.; Zapata, D.L.; Lofaj, F.; Kvetkova, L.; Akhtar, F. Ultra-high strength martensitic 420 stainless steel with high ductility. Addit. Manuf. 2019, 29, 100803. [Google Scholar] [CrossRef]
- Tsuchiyama, T.; Tobata, J.; Tao, T.; Nakada, N.; Takaki, S. Quenching and partitioning treatment of a low-carbon martensitic stainless steel. Mater. Sci. Eng. A 2012, 532, 585–592. [Google Scholar] [CrossRef]
- Ebrahimi, G.; Momeni, A.; Jahazi, M.; Bocher, P. Dynamic recrystallization and precipitation in 13Cr super-martensitic stainless steels. Metall. Trans. A 2014, 45, 2219–2231. [Google Scholar] [CrossRef]
- Yang, H.S.; Bhadeshia, H.K.D.H. Austenite grain size and the martensite–start temperature. Scr. Mater. 2009, 60, 493–495. [Google Scholar] [CrossRef]
- Kung, C.Y.; Rayment, J.J. An examination of the validity of existing empirical formulae for the calculation of Ms temperature. Metall. Trans. A 1982, 13, 328–331. [Google Scholar] [CrossRef] [Green Version]
- Andrews, K.W. Enlpirical formulae for calculation of some transformation temperatures. JISI 1965, 203, 721–727. [Google Scholar]
- Watt, D.F.; Coon, L.; Bibby, M.; Goldak, J.; Henwood, C. An algorithm for modelling microstructural development in weld heat-affected zones (Part A) reaction kinetics. Acta Metall. 1988, 36, 3029–3035. [Google Scholar] [CrossRef]
- Kudryavtsev, A.S.; Okhapkin, K.A.; Mikhailov, M.S.; Skutin, V.S.; Zubova, G.E.; Fedotov, B.V. Analysis of factors responsible for the accelerated creep rupture of 12% Cr martensitic steel weld joints. Phys. Met. Metallogr. 2016, 117, 602–610. [Google Scholar] [CrossRef]
- Babu, K.; Kumar, T.S.P. Comparison of austenite decomposition models during finite element simulation of water quenching and air cooling of AISI 4140 steel Metall. Mater. Trans. B 2014, 45, 1530–1544. [Google Scholar] [CrossRef]
- Hunkel, M.; Dong, J.; Epp, J.; Kaiser, D.; Dietrich, S.; Schulze, V.; Rajaei, A.; Hallstedt, B.; Broeckmann, C. Comparative study of the tempering behavior of different martensitic steels by means of in-situ diffractometry and dilatometry. Materials 2020, 13, 5058. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhong, Y.; Lv, C.; Tan, L.; Yuan, X.; Li, S. Effect of carbon partition in the reverted austenite of supermartensitic stainless steel. Mater. Res. Express 2019, 6, 086518. [Google Scholar] [CrossRef]
- Pryds, N.H.; Huang, X. The effect of cooling rate on the microstructure formed during solidification of ferritic steels. Metall. Mater. Trans. A 2000, 31, 3155–3166. [Google Scholar] [CrossRef]
- Ma, X.P.; Wang, L.J.; Liu, C.M. Subramanian, S.V. Role of Nb in low interstitial 13Cr super martensitic stainless steel. Mater. Sci. Eng. A 2011, 528, 6812–6818. [Google Scholar] [CrossRef]
- Liao, J. Nitride precipitation in weld HAZs of duplex stainless steel. ISIJ Int. 2001, 41, 460–467. [Google Scholar] [CrossRef] [Green Version]
- Stiller, K.; Danoix, F.; Bostel, A. Investigation of precipitation in a new maraging stainless steel. Appl. Surf. Sci. 1996, 94–95, 326–333. [Google Scholar] [CrossRef]
- Khodabakhshi, F.; Farshidianfar, M.H.; Gerlich, A.P.; Nosko, M.; Trembosova, V.; Khajepour, A. Microstructure, strain-rate sensitivity, work hardening, and fracture behavior of laser additive manufactured austenitic and martensitic stainless steel structures. Mater. Sci. Eng. A 2019, 756, 545–561. [Google Scholar] [CrossRef]
- Fujita, N.; Ohmura, K.; Yamamoto, A. Changes of microstructures and high temperature properties during high temperature service of Niobium added ferritic stainless steels. Mater. Sci. Eng. A 2003, 351, 272–281. [Google Scholar] [CrossRef]
- Sim, G.M.; Ahn, J.C.; Hong, S.C.; Lee, K.J.; Lee, K.S. Effect of Nb precipitate coarsening on the high temperature strength in Nb containing ferritic stainless steels. Mater. Sci. Eng. A 2005, 396, 159–165. [Google Scholar] [CrossRef]
- Silva, C.; Farias, J.P.; Miranda, H.C.; Guimarães, R.; Menezes, J.W.A.; Neto, M.A.M. Microstructural characterization of the HAZ in AISI444 ferritic stainless steel welds. Mater. Charact. 2008, 59, 528–533. [Google Scholar] [CrossRef]
- Takano, K.; Sakakibara, M.; Matsui, T.; Takaki, S. Effect of alloying elements and microstructure on corrosion resistance and hardness of martensitic stainless steel. J. Iron. Steel. Inst. Jpn. 2000, 86, 123–130. [Google Scholar] [CrossRef]
- Calliari, I.; Zanesco, M.; Dabalà, M.; Brunelli, K.; Ramous, E. Investigation of microstructure and properties of a Ni–Mo martensitic stainless steel. Mater. Des. 2008, 29, 246–250. [Google Scholar] [CrossRef]
- Kitahara, H.; Ueji, R.; Tsuji, N.; Minamino, Y. Crystallographic features of lath martensite in low-carbon steel. Acta Mater. 2006, 54, 1279–1288. [Google Scholar] [CrossRef]
- Lu, S.Y.; Yao, K.F.; Chen, Y.B.; Wang, M.H.; Ge, X.Y. Effect of quenching and partitioning on the microstructure evolution and electrochemical properties of a martensitic stainless steel. Corros. Sci. 2016, 103, 95–104. [Google Scholar] [CrossRef]
- Klotz, U.E.; Solenthaler, C.; Uggowitzer, P.J. Martensitic-austenitic 9–12% Cr steels-alloy design, microstructural stability and mechanical properties. Mater. Sci. Eng. A 2008, 476, 186–194. [Google Scholar] [CrossRef]
- Gao, P.; Liu, J.; Chen, W.; Li, F.; Pang, J.; Zhao, Z. Quasi-Situ Characterization of Retained Austenite Orientation in Quenching and Partitioning Steel via Uniaxial Tensile Tests. Materials 2020, 13, 4609. [Google Scholar] [CrossRef]
- Lv, Z.; Qian, L.; Liu, S.; Zhan, L.; Qin, S. Preparation and mechanical behavior of ultra-high strength low-carbon steel. Materials 2020, 13, 459. [Google Scholar] [CrossRef] [Green Version]
- Xue, F.M.; Li, F.G.; Cai, J.; Yuan, Z.W.; Bo, C.; Liu, T.Y. Characterization of the elasto-plastic properties of 0Cr12Mn5Ni4Mo3Al steel by microindentation. Mater. Des. 2012, 36, 81–87. [Google Scholar] [CrossRef]
- Guo, C.S. A mathematical model of the tempering procedure of hardened steel. Acta Metall. Sin. 1999, 35, 865–868. (In Chinese) [Google Scholar]
- Isfahany, A.N.; Saghafian, H.; Borhani, G. The effect of heat treatment on mechanical properties and corrosion behavior of AISI420 martensitic stainless steel. J. Alloys Compd. 2011, 509, 3931–3936. [Google Scholar] [CrossRef]
- Ma, X.; Wang, L.; Subramanian, S.V.; Liu, C. Studies on Nb microalloying of 13Cr super martensitic stainless steel. Metall. Mater. Trans. A 2012, 43, 4475. [Google Scholar] [CrossRef]
- Qin, F.C.; Li, Y.T.; Qi, H.P.; Ju, L. Microstructure-texture-mechanical properties in hot rolling of a centrifugal casting ring blank. J. Mater. Eng. Perform. 2016, 25, 1237–1248. [Google Scholar] [CrossRef]




| Tensile Properties | Hardness | |||||
|---|---|---|---|---|---|---|
| σb (MPa) | σ0.2 (MPa) | δ (%) | Ψ (%) | Ak (J/cm2) | HV | |
| As-cast | 815 | 695 | 8.3 | 45.0 | 45.0 | 238 |
| As-rolled (Initial) | 925 | 735 | 12.1 | 46.0 | 55.0 | 328 |
| Quenching Process | Tempering Process | ||||
|---|---|---|---|---|---|
| Temperature | Time | Cooling | Temperature | Time | Cooling |
| Oil cooling | |||||
| 1040 °C | 1 h | Air cooling | 300–670 °C | 1.5–2 h | Oil cooling |
| Furnace cooling | Air cooling | ||||
| PAG medium | |||||
| Spot Position | Element | Weight % | Atomic % | Net Int. | Spot Position | Element | Weight % | Atomic % | Net Int. |
|---|---|---|---|---|---|---|---|---|---|
| Spot 1 in Figure 9a | NbL | 91.46 | 86.20 | 1472.15 | Spot 1 in Figure 9b | NbL | 93.01 | 88.56 | 982.32 |
| TiK | 0.67 | 1.22 | 10.64 | V K | 1.14 | 1.97 | 10.38 | ||
| CrK | 2.01 | 3.38 | 26.53 | CrK | 1.63 | 2.77 | 14.16 | ||
| FeK | 5.87 | 9.20 | 62.60 | FeK | 4.22 | 6.69 | 29.70 | ||
| Spot 2 in Figure 9a | NbL | 69.09 | 57.02 | 1051.50 | Spot 2 in Figure 9b | NbL | 86.85 | 79.54 | 843.12 |
| TiK | 0.17 | 0.28 | 2.93 | V K | 1.02 | 1.71 | 8.87 | ||
| CrK | 4.98 | 7.35 | 69.55 | CrK | 2.34 | 3.82 | 19.21 | ||
| FeK | 25.75 | 35.35 | 273.78 | FeK | 9.79 | 14.92 | 64.28 |
| Regime of Heat Treatment | Hardness (HV) | Phase Composition |
|---|---|---|
| Quenching at 1040 °C by oil | 380.0–420.0 | α + Nb(C, N) |
| Quenching at 1040 °C by oil + tempering at 550 °C | 375.0–395.0 | α + Nb(C, N) + M7C3 + others [48] |
| Quenching at 1040 °C by oil + tempering at 650 °C | 300.0–330.0 | α + Nb(C, N) + M23C6 + others [48] |
| Outer Layer | Center Position | |||||||
|---|---|---|---|---|---|---|---|---|
| Oil. + Air. | Air. + Air. | Fur. + Air. | Con. + Air. | Oil. + Air. | Air. + Air. | Fur. + Air. | Con. + Air. | |
| Tensile strength (MPa) | 1045 | 1060 | 1050 | 1020 | 1052 | 1060 | 1050 | 1020 |
| Yield strength (MPa) | 962 | 948 | 933 | 916 | 975 | 946 | 933 | 910 |
| Impact toughness (J/cm2) | 65.0 | 58.8 | 56.8 | 55.0 | 64.0 | 58.8 | 57.9 | 54.5 |
| Outer Layer | Center Position | |||||
|---|---|---|---|---|---|---|
| Oil. + Oil. | Oil. + Air. | Oil. + Fur. | Oil. + Oil. | Oil. + Air. | Oil. + Fur. | |
| Tensile strength (MPa) | 944 | 951 | 941 | 941 | 948 | 939 |
| Yield strength (MPa) | 835 | 853 | 830 | 830 | 837 | 826 |
| Impact Toughness (J/cm2) | 95.0 | 67.0 | 57.5 | 92.0 | 65.0 | 45.0 |
| Quenching and Tempering Medium | Surface Hardness | Central Hardness | Difference between Surface and Center |
|---|---|---|---|
| Air | 308 HV | 279 HV | 29 HV |
| Oil | 313 HV | 285 HV | 27 HV |
| PAG medium | 335 HV | 297 HV | 38 HV |
| Oil (quenching) + air (tempering) | 340 HV | 318 HV | 22 HV |
| Condition | σb (MPa) | σ0.2 (MPa) | δ (%) | Ψ (%) | Ak(J/cm2) | Phase | Hardness (HV) |
|---|---|---|---|---|---|---|---|
| Quenching by oil (Q.Oil) | 952.5 | 853.5 | 12.0 | 44.5 | 65.0 | α + Nb(C, N) | 418 |
| Quenching by air (Q.Air) | 965.0 | 838.0 | 11.9 | 44.5 | 65.0 | α + Nb(C, N) | 408 |
| Q.Oil + 550 °C tempering | 951.0 | 884.0 | 15.5 | 48.0 | 70.0 | α + Nb(C, N) + M7C3 | 392 |
| Q.Air + 550 °C tempering | 993.0 | 850.0 | 14.2 | 46.0 | 64.0 | α + Nb(C, N) + M7C3 | 382 |
| Q.Oil + 650 °C tempering | 910.5 | 885.5 | 17.9 | 55.0 | 65.0 | α + Nb(C, N) + M23C6 | 313 |
| Q.Air + 650 °C tempering | 925.0 | 820.0 | 16.8 | 53.0 | 60.5 | α + Nb(C, N) + M23C6 | 308 |
| Q.Oil + 670 °C tempering | 910.0 | 810.5 | 16.5 | 52.0 | 62.0 | α + Nb(C, N) + M23C6 (coarser) | 315 |
| Q.Air + 670 °C tempering | 920.0 | 805.5 | 15.5 | 49.0 | 60.0 | α + Nb(C, N) + M23C6 (coarser) | 298 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, J.; Xia, C. Microstructure Evolution and Mechanical Properties of X6CrNiMoVNb11-2 Stainless Steel after Heat Treatment. Materials 2021, 14, 5243. https://doi.org/10.3390/ma14185243
Fu J, Xia C. Microstructure Evolution and Mechanical Properties of X6CrNiMoVNb11-2 Stainless Steel after Heat Treatment. Materials. 2021; 14(18):5243. https://doi.org/10.3390/ma14185243
Chicago/Turabian StyleFu, Jia, and Chaoqi Xia. 2021. "Microstructure Evolution and Mechanical Properties of X6CrNiMoVNb11-2 Stainless Steel after Heat Treatment" Materials 14, no. 18: 5243. https://doi.org/10.3390/ma14185243
APA StyleFu, J., & Xia, C. (2021). Microstructure Evolution and Mechanical Properties of X6CrNiMoVNb11-2 Stainless Steel after Heat Treatment. Materials, 14(18), 5243. https://doi.org/10.3390/ma14185243







