Pathways to Green Perspectives: Production and Characterization of Polylactide (PLA) Nanocomposites Filled with Superparamagnetic Magnetite Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Treatment of Magnetite and Production of Nanocomposites by Melt-Compounding
2.3. Characterization Methods
2.3.1. Rheological Measurements
2.3.2. Thermogravimetric Analysis (TGA)
2.3.3. Differential Scanning Calorimetry (DSC)
2.3.4. Scanning Electron Microscopy (SEM)
2.3.5. Transmission Electron Microscopy (TEM)
2.3.6. Characterization of Magnetic Properties
3. Results and Discussion
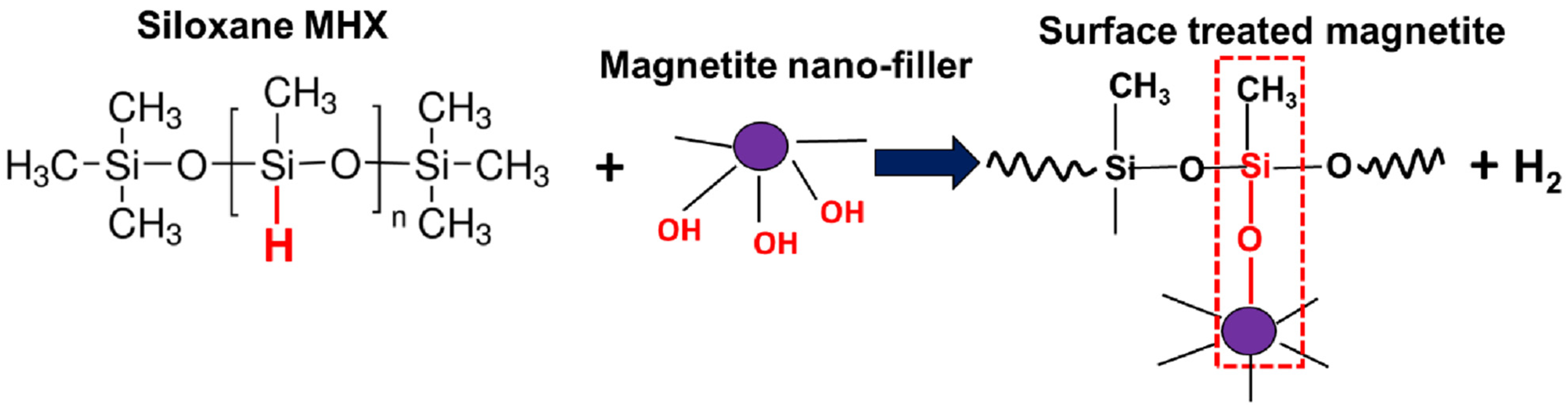
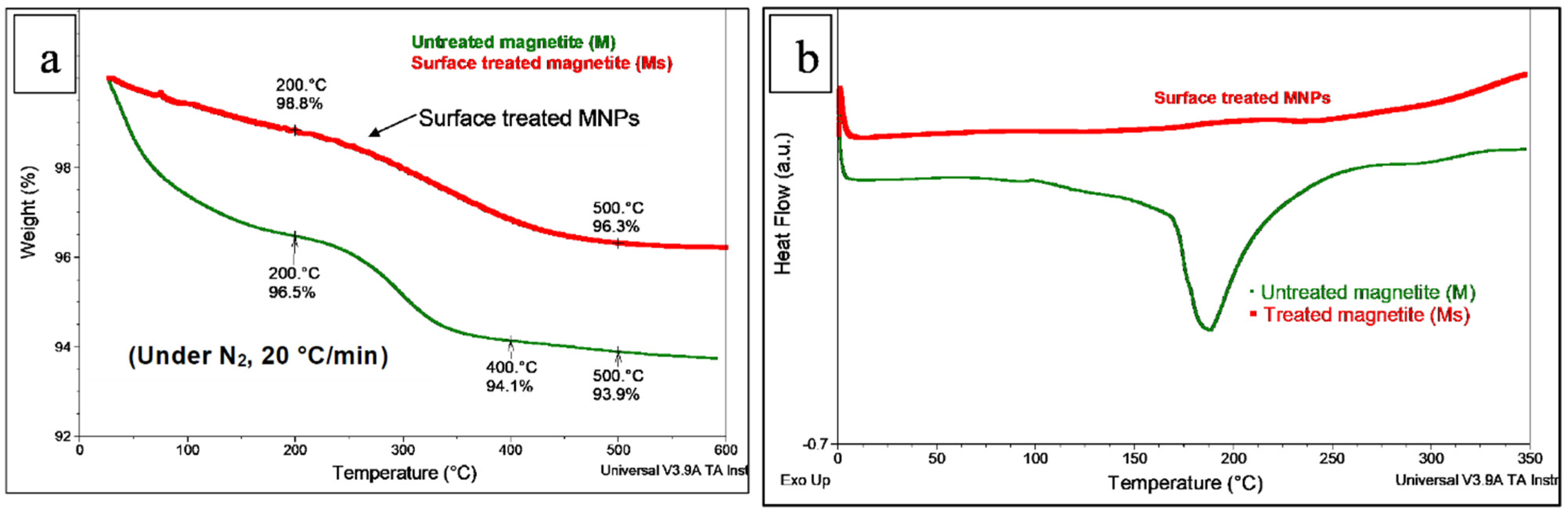
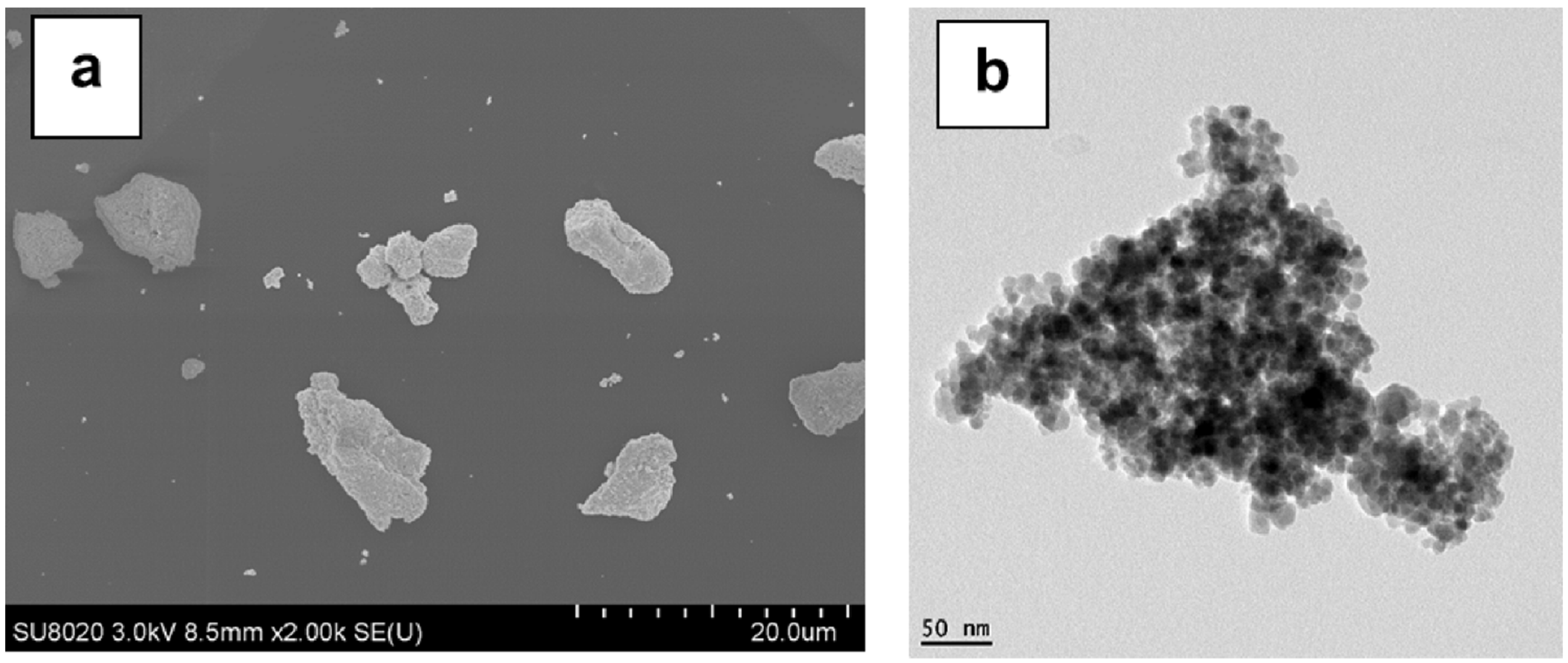
3.1. Preliminary Considerations Regarding the Surface Treatment of Magnetite NPs with MHX
3.2. Characterization of PLA-Magnetite Nanocomposites
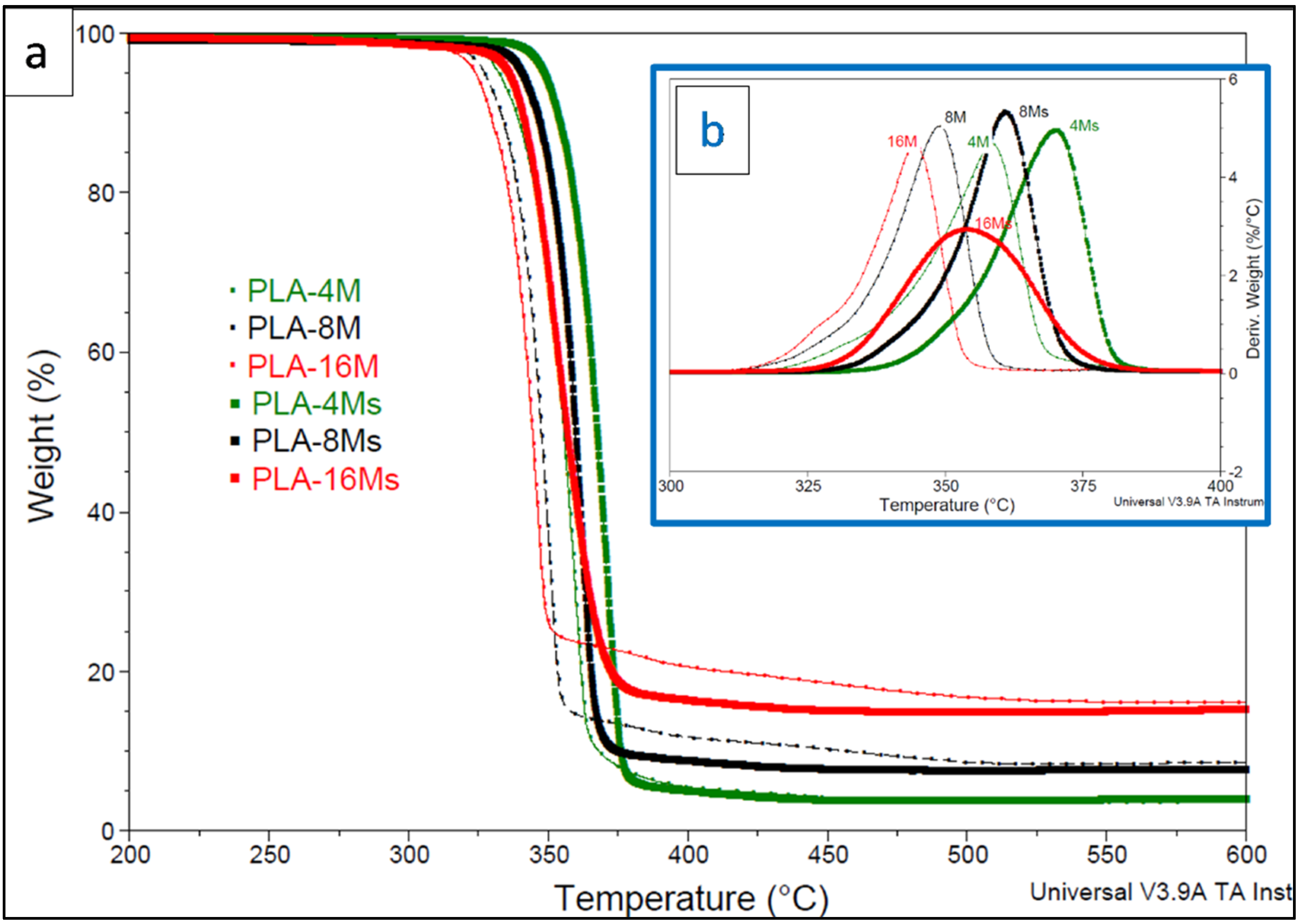
3.2.1. Effects of Magnetite Addition and MNPs Surface Treatment on PLA Thermal Properties
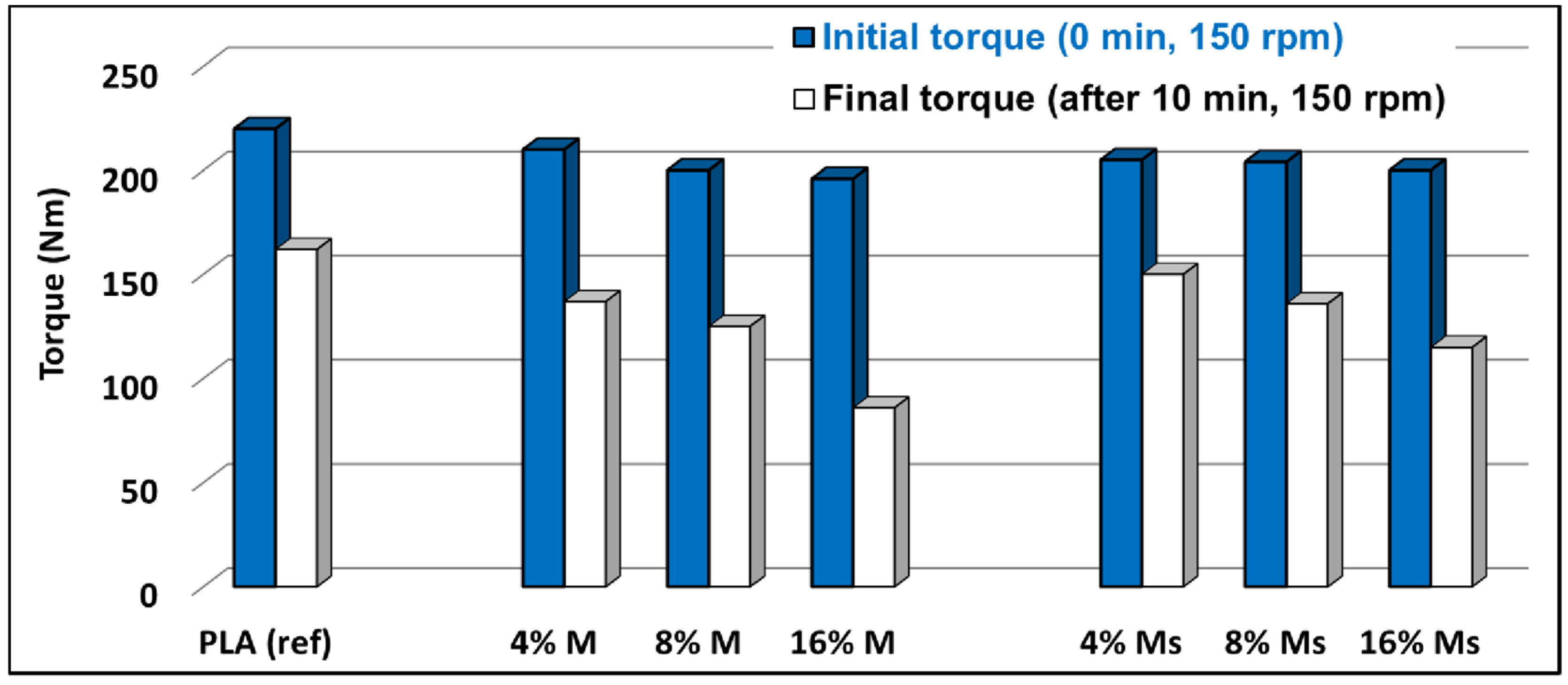
3.2.2. Rheological Characterizations
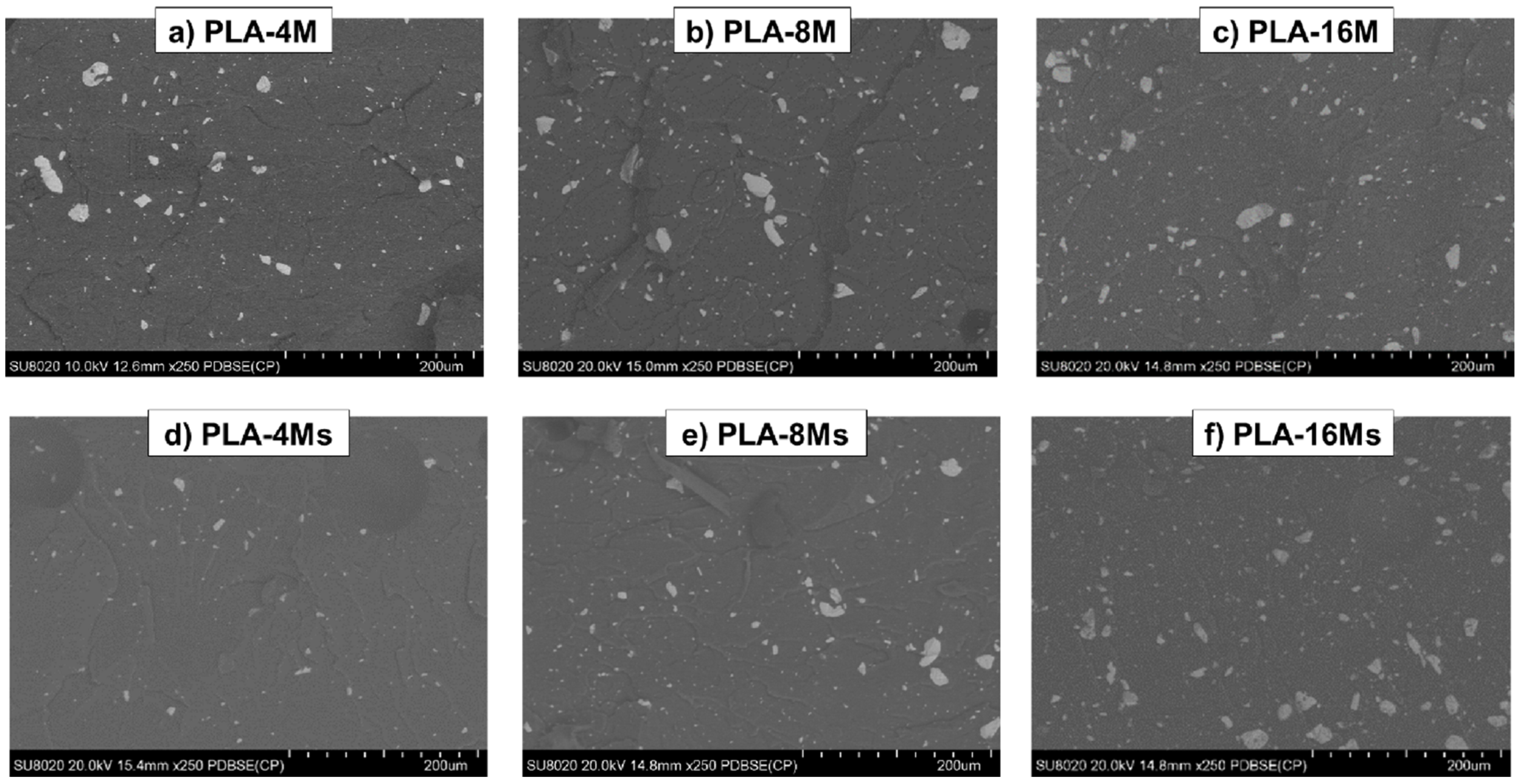
3.2.3. Morphology of PLA−Magnetite Nanocomposites
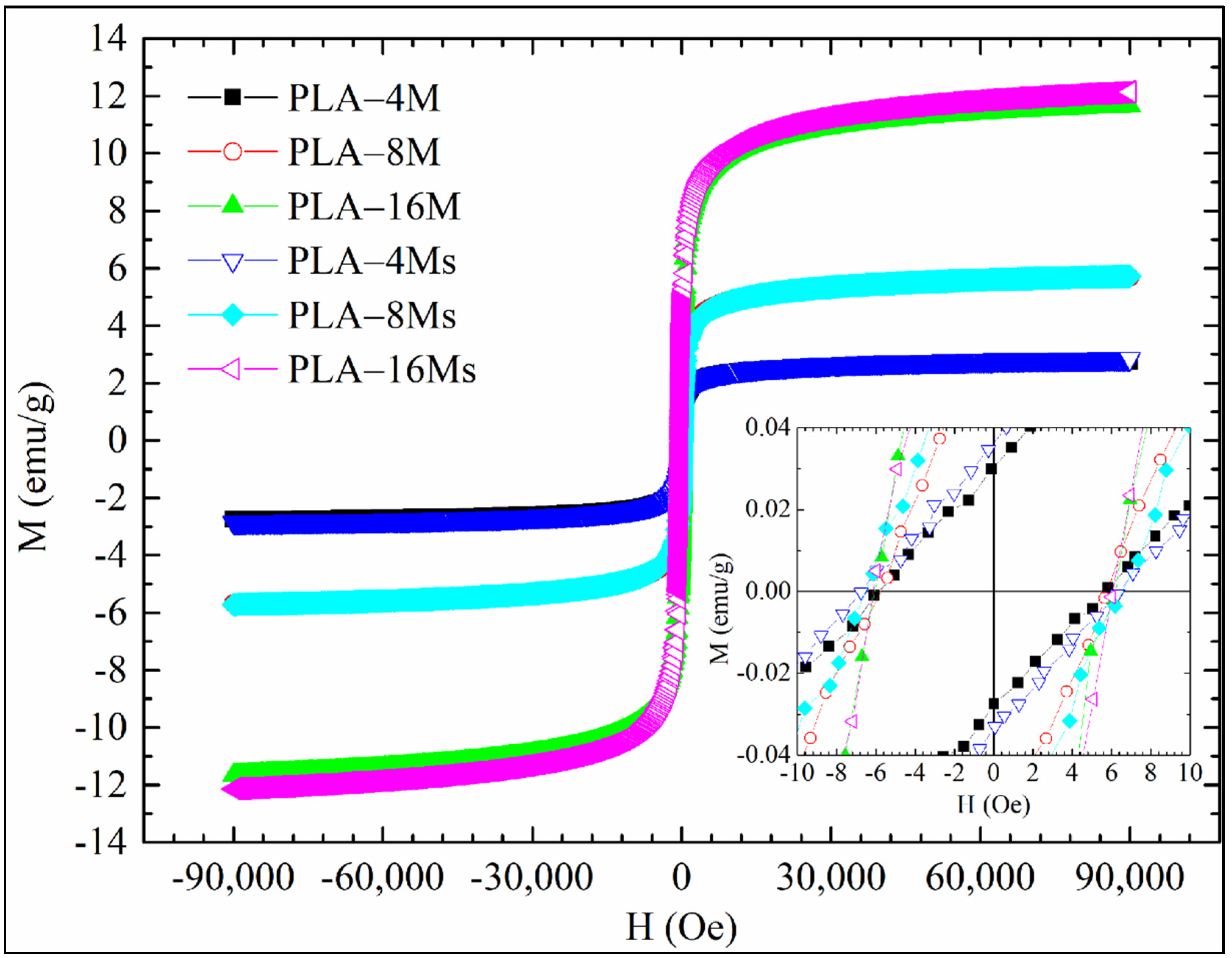
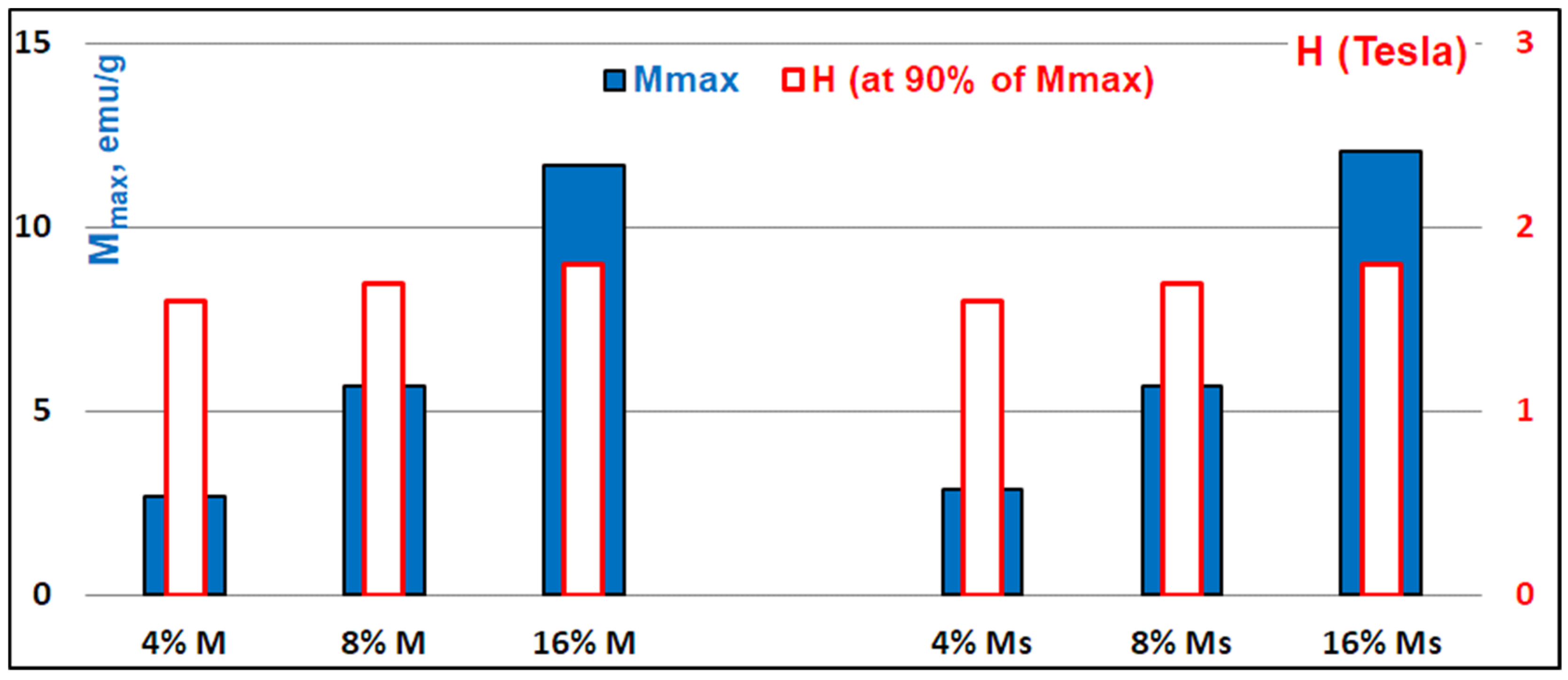
3.2.4. Magnetic Features of PLA–Magnetite Nanocomposites
3.2.5. PLA–Magnetite Nanocomposites with Superparamagnetic Properties: Current Prospects
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Raquez, J.-M.; Habibi, Y.; Murariu, M.; Dubois, P. Polylactide (PLA)-based nanocomposites. Prog. Polym. Sci. 2013, 38, 1504–1542. [Google Scholar] [CrossRef]
- Babu, R.P.; O’Connor, K.; Seeram, R. Current progress on bio-based polymers and their future trends. Prog. Biomater. 2013, 2, 8. [Google Scholar] [CrossRef]
- Rezvani Ghomi, E.; Khosravi, F.; Saedi Ardahaei, A.; Dai, Y.; Neisiany, R.E.; Foroughi, F.; Wu, M.; Das, O.; Ramakrishna, S. The life cycle assessment for polylactic acid (PLA) to make it a low-carbon material. Polymers 2021, 13, 1854. [Google Scholar] [CrossRef] [PubMed]
- Sreekumar, K.; Bindhu, B.; Veluraja, K. Perspectives of polylactic acid from structure to applications. Polym. Renew. Resour. 2021, 12, 60–74. [Google Scholar] [CrossRef]
- Banerjee, R.; Ray, S.S. An overview of the recent advances in polylactide-based sustainable nanocomposites. Polym. Eng. Sci. 2021, 61, 617–649. [Google Scholar] [CrossRef]
- Murariu, M.; Dubois, P. PLA composites: From production to properties. Adv. Drug Deliv. Rev. 2016, 107, 17–46. [Google Scholar] [CrossRef] [PubMed]
- Auras, R.; Harte, B.; Selke, S. An overview of polylactides as packaging materials. Macromol. Biosci. 2004, 4, 835–864. [Google Scholar] [CrossRef] [PubMed]
- Widiastuti, I. Polylactide nanocomposites for packaging materials: A review. AIP Conf. Proc. 2016, 1710, 030020. [Google Scholar] [CrossRef]
- Gupta, B.; Revagade, N.; Hilborn, J. Poly(lactic acid) fiber: An overview. Prog. Polym. Sci. 2007, 32, 455–482. [Google Scholar] [CrossRef]
- Nagarajan, V.; Mohanty, A.K.; Misra, M. Perspective on polylactic acid (PLA) based sustainable materials for durable applications: Focus on toughness and heat resistance. ACS Sustain. Chem. Eng. 2016, 4, 2899–2916. [Google Scholar] [CrossRef]
- Murariu, M.; Laoutid, F.; Dubois, P.; Fontaine, G.; Bourbigot, S.; Devaux, E.; Campagne, C.; Ferreira, M.; Solarski, S. Chapter 21—Pathways to biodegradable flame retardant polymer (nano)composites. In Polymer Green Flame Retardants; Elsevier: Amsterdam, The Netherlands, 2014; pp. 709–773. [Google Scholar]
- Saini, P.; Arora, M.; Kumar, M.N.V.R. Poly(lactic acid) blends in biomedical applications. Adv. Drug Deliv. Rev. 2016, 107, 47–59. [Google Scholar] [CrossRef]
- Lasprilla, A.J.R.; Martinez, G.A.R.; Lunelli, B.H.; Jardini, A.L.; Filho, R.M. Poly-lactic acid synthesis for application in biomedical devices—A review. Biotechnol. Adv. 2012, 30, 321–328. [Google Scholar] [CrossRef]
- Teixeira, S.; Eblagon, K.M.; Miranda, F.; Pereira, M.F.R.; Figueiredo, J.L. Towards controlled degradation of poly(lactic) acid in technical applications. C J. Carbon Res. 2021, 7, 42. [Google Scholar] [CrossRef]
- Ncube, L.K.; Ude, A.U.; Ogunmuyiwa, E.N.; Zulkifli, R.; Beas, I.N. Environmental impact of food packaging materials: A review of contemporary development from conventional plastics to polylactic acid based materials. Materials 2020, 13, 4994. [Google Scholar] [CrossRef] [PubMed]
- Sinha Ray, S.; Maiti, P.; Okamoto, M.; Yamada, K.; Ueda, K. New polylactide/layered silicate nanocomposites. 1. Preparation, characterization, and properties. Macromolecules 2002, 35, 3104–3110. [Google Scholar] [CrossRef]
- Okamoto, M. Polylactide/clay nano-biocomposites. In Environmental Silicate Nano-Biocomposites; Avérous, L., Pollet, E., Eds.; Springer: London, UK, 2012; pp. 77–118. [Google Scholar]
- Murariu, M.; Dechief, A.L.; Bonnaud, L.; Paint, Y.; Gallos, A.; Fontaine, G.; Bourbigot, S.; Dubois, P. The production and properties of polylactide composites filled with expanded graphite. Polym. Degrad. Stab. 2010, 95, 889–900. [Google Scholar] [CrossRef]
- Kim, I.-H.; Jeong, Y.G. Polylactide/exfoliated graphite nanocomposites with enhanced thermal stability, mechanical modulus, and electrical conductivity. J. Polym. Sci. Part B Polym. Phys. 2010, 48, 850–858. [Google Scholar] [CrossRef]
- Wang, Y.; Lin, C.-S. Preparation and characterization of maleated polylactide-functionalized graphite oxide nanocomposites. J. Polym. Res. 2013, 21, 334. [Google Scholar] [CrossRef]
- Pillai, S.K.; Ramontja, J.; Ray, S.S. Amine functionalization of carbon nanotubes for the preparation of CNT based polylactide composites—A comparative study. In Nanostructured Materials and Nanotechnology V; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011; pp. 43–51. [Google Scholar]
- Wu, D.; Lv, Q.; Feng, S.; Chen, J.; Chen, Y.; Qiu, Y.; Yao, X. Polylactide composite foams containing carbon nanotubes and carbon black: Synergistic effect of filler on electrical conductivity. Carbon 2015, 95, 380–387. [Google Scholar] [CrossRef]
- Brzeziński, M.; Biela, T. Polylactide nanocomposites with functionalized carbon nanotubes and their stereocomplexes: A focused review. Mater. Lett. 2014, 121, 244–250. [Google Scholar] [CrossRef]
- Shameli, K.; Ahmad, M.B.; Wan Yunus, W.M.Z.; Ibrahim, N.A.; Jokar, M.; Darroudi, M. Synthesis and characterization of silver/polylactide nanocomposites. World Acad. Sci. Eng. Technol. 2010, 64, 28–32. [Google Scholar]
- Kim, E.S.; Kim, S.H.; Lee, C.H. Electrospinning of polylactide fibers containing silver nanoparticles. Macromol. Res. 2010, 18, 215–221. [Google Scholar] [CrossRef]
- Chu, Z.; Zhao, T.; Li, L.; Fan, J.; Qin, Y. Characterization of antimicrobial poly (lactic acid)/nano-composite films with silver and zinc oxide nanoparticles. Materials 2017, 10, 659. [Google Scholar] [CrossRef] [PubMed]
- Marra, A.; Silvestre, C.; Duraccio, D.; Cimmino, S. Polylactic acid/zinc oxide biocomposite films for food packaging application. Int. J. Biol. Macromol. 2016, 88, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Murariu, M.; Benali, S.; Paint, Y.; Dechief, A.-L.; Murariu, O.; Raquez, J.-M.; Dubois, P. Adding value in production of multifunctional polylactide (PLA)–ZnO nanocomposite films through alternative manufacturing methods. Molecules 2021, 26, 2043. [Google Scholar] [CrossRef]
- Zhu, A.; Diao, H.; Rong, Q.; Cai, A. Preparation and properties of polylactide–silica nanocomposites. J. Appl. Polym. Sci. 2010, 116, 2866–2873. [Google Scholar] [CrossRef]
- Huang, J.-W.; Chang Hung, Y.; Wen, Y.-L.; Kang, C.-C.; Yeh, M.-Y. Polylactide/nano and microscale silica composite films. I. Preparation and characterization. J. Appl. Polym. Sci. 2009, 112, 1688–1694. [Google Scholar] [CrossRef]
- Odent, J.; Raquez, J.-M.; Samuel, C.; Barrau, S.; Enotiadis, A.; Dubois, P.; Giannelis, E.P. Shape-memory behavior of polylactide/silica ionic hybrids. Macromolecules 2017, 50, 2896–2905. [Google Scholar] [CrossRef]
- Nan, A.; Turcu, R.; Liebscher, J. Magnetite-polylactic acid core–shell nanoparticles by ring-opening polymerization under microwave irradiation. J. Polym. Sci. Part A Polym. Chem. 2012, 50, 1485–1490. [Google Scholar] [CrossRef]
- Chen, A.-Z.; Kang, Y.-Q.; Pu, X.-M.; Yin, G.-F.; Li, Y.; Hu, J.-Y. Development of Fe3O4-poly(l-lactide) magnetic microparticles in supercritical CO2. J. Colloid Interface Sci. 2009, 330, 317–322. [Google Scholar] [CrossRef]
- Zhang, X.; Xue, L.; Wang, J.; Liu, Q.; Liu, J.; Gao, Z.; Yang, W. Effects of surface modification on the properties of magnetic nanoparticles/PLA composite drug carriers and in vitro controlled release study. Colloids Surf. A Physicochem. Eng. Asp. 2013, 431, 80–86. [Google Scholar] [CrossRef]
- Shan, D.; Shi, Y.; Duan, S.; Wei, Y.; Cai, Q.; Yang, X. Electrospun magnetic poly(l-lactide) (PLLA) nanofibers by incorporating PLLA-stabilized Fe3O4 nanoparticles. Mater. Sci. Eng. C 2013, 33, 3498–3505. [Google Scholar] [CrossRef]
- Yang, G.; Zhang, B.; Wang, J.; Wang, M.; Xie, S.; Li, X. Synthesis and characterization of poly(lactic acid)-modified superparamagnetic iron oxide nanoparticles. J. Sol-Gel Sci. Technol. 2016, 77, 335–341. [Google Scholar] [CrossRef]
- Murariu, M.; Doumbia, A.; Bonnaud, L.; Dechief, A.L.; Paint, Y.; Ferreira, M.; Campagne, C.; Devaux, E.; Dubois, P. High-performance polylactide/ZnO nanocomposites designed for films and fibers with special end-use properties. Biomacromolecules 2011, 12, 1762–1771. [Google Scholar] [CrossRef]
- Natarajan, S.; Harini, K.; Gajula, G.P.; Sarmento, B.; Neves-Petersen, M.T.; Thiagarajan, V. Multifunctional magnetic iron oxide nanoparticles: Diverse synthetic approaches, surface modifications, cytotoxicity towards biomedical and industrial applications. BMC Mater. 2019, 1, 2. [Google Scholar] [CrossRef]
- Kalia, S.; Kango, S.; Kumar, A.; Haldorai, Y.; Kumari, B.; Kumar, R. Magnetic polymer nanocomposites for environmental and biomedical applications. Colloid Polym. Sci. 2014, 292, 2025–2052. [Google Scholar] [CrossRef]
- Martinez-Boubeta, C.; Simeonidis, K. Chapter 20—Magnetic Nanoparticles for Water purification. In Nanoscale Materials in Water Purification; Thomas, S., Pasquini, D., Leu, S.-Y., Gopakumar, D.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 521–552. [Google Scholar]
- Frey, N.A.; Peng, S.; Cheng, K.; Sun, S. Magnetic nanoparticles: Synthesis, functionalization, and applications in bioimaging and magnetic energy storage. Chem. Soc. Rev. 2009, 38, 2532–2542. [Google Scholar] [CrossRef]
- Wu, W.; Wu, Z.; Yu, T.; Jiang, C.; Kim, W.-S. Recent progress on magnetic iron oxide nanoparticles: Synthesis, surface functional strategies and biomedical applications. Sci. Technol. Adv. Mater. 2015, 16, 023501. [Google Scholar] [CrossRef]
- Wu, W.; He, Q.; Jiang, C. Magnetic iron oxide nanoparticles: Synthesis and surface functionalization strategies. Nanoscale Res. Lett. 2008, 3, 397. [Google Scholar] [CrossRef]
- Demirer, G.S.; Okur, A.C.; Kizilel, S. Synthesis and design of biologically inspired biocompatible iron oxide nanoparticles for biomedical applications. J. Mater. Chem. B 2015, 3, 7831–7849. [Google Scholar] [CrossRef]
- Oh, J.K.; Park, J.M. Iron oxide-based superparamagnetic polymeric nanomaterials: Design, preparation, and biomedical application. Prog. Polym. Sci. 2011, 36, 168–189. [Google Scholar] [CrossRef]
- Kandasamy, G.; Maity, D. Recent advances in superparamagnetic iron oxide nanoparticles (SPIONs) for in vitro and in vivo cancer nanotheranostics. Int. J. Pharm. 2015, 496, 191–218. [Google Scholar] [CrossRef]
- Li, Q.; Kartikowati, C.W.; Horie, S.; Ogi, T.; Iwaki, T.; Okuyama, K. Correlation between particle size/domain structure and magnetic properties of highly crystalline Fe3O4 nanoparticles. Sci. Rep. 2017, 7, 9894. [Google Scholar] [CrossRef]
- Lu, Q.; Choi, K.; Nam, J.-D.; Choi, H.J. Magnetic polymer composite particles: Design and magnetorheology. Polymers 2021, 13, 512. [Google Scholar] [CrossRef]
- Ali, A.; Zafar, H.; Zia, M.; Ul Haq, I.; Phull, A.R.; Ali, J.S.; Hussain, A. Synthesis, characterization, applications, and challenges of iron oxide nanoparticles. Nanotechnol. Sci. Appl. 2016, 9, 49–67. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, J.; Bertinetti, L.; Widdrat, M.; Hirt, A.M.; Faivre, D. Formation of magnetite nanoparticles at low temperature: From superparamagnetic to stable single domain particles. PLoS ONE 2013, 8, e57070. [Google Scholar] [CrossRef]
- Wahajuddin, S.A. Superparamagnetic iron oxide nanoparticles: Magnetic nanoplatforms as drug carriers. Int. J. Nanomed. 2012, 7, 3445–3471. [Google Scholar] [CrossRef]
- Baharvand, H. Encapsulation of ferromagnetic iron oxide particles by polyester resin. e-Polymers 2008, 8. [Google Scholar] [CrossRef][Green Version]
- Yamaura, M.; Camilo, R.L.; Sampaio, L.C.; Macêdo, M.A.; Nakamura, M.; Toma, H.E. Preparation and characterization of (3-aminopropyl)triethoxysilane-coated magnetite nanoparticles. J. Magn. Magn. Mater. 2004, 279, 210–217. [Google Scholar] [CrossRef]
- Bini, R.A.; Marques, R.F.C.; Santos, F.J.; Chaker, J.A.; Jafelicci, M. Synthesis and functionalization of magnetite nanoparticles with different amino-functional alkoxysilanes. J. Magn. Magn. Mater. 2012, 324, 534–539. [Google Scholar] [CrossRef]
- Aisida, S.O.; Akpa, P.A.; Ahmad, I.; Zhao, T.-K.; Maaza, M.; Ezema, F.I. Bio-inspired encapsulation and functionalization of iron oxide nanoparticles for biomedical applications. Eur. Polym. J. 2020, 122, 109371. [Google Scholar] [CrossRef]
- Akbarzadeh, A.; Samiei, M.; Davaran, S. Magnetic Fe3O4: Preparation, physical properties, and applications in biomedicine. Nanoscale Res. Lett. 2012, 7, 144. [Google Scholar] [CrossRef]
- Frounchi, M.; Shamshiri, S. Magnetic nanoparticles-loaded PLA/PEG microspheres as drug carriers. J. Biomed. Mater. Res. Part A 2015, 103, 1893–1898. [Google Scholar] [CrossRef]
- Wilson, K.S.; Goff, J.D.; Riffle, J.S.; Harris, L.A.; St Pierre, T.G. Polydimethylsiloxane-magnetite nanoparticle complexes and dispersions in polysiloxane carrier fluids. Polym. Adv. Technol. 2005, 16, 200–211. [Google Scholar] [CrossRef]
- de Rezende Bonesio, M.; Pissetti, F.L. Magnetite particles covered by amino-functionalized poly(dimethylsiloxane) network for copper(ii) adsorption from aqueous solution. J. Sol-Gel Sci. Technol. 2020, 94, 154–164. [Google Scholar] [CrossRef]
- Pandey, G.; Singh, S.; Hitkari, G. Synthesis and characterization of polyvinyl pyrrolidone (PVP)-coated Fe3O4 nanoparticles by chemical co-precipitation method and removal of congo red dye by adsorption process. Int. Nano Lett. 2018, 8, 111–121. [Google Scholar] [CrossRef]
- Yu, B.; Wang, M.; Sun, H.; Zhu, F.; Han, J.; Bhat, G. Preparation and properties of poly (lactic acid)/magnetic Fe3O4 composites and nonwovens. RSC Adv. 2017, 7, 41929–41935. [Google Scholar] [CrossRef]
- Cam, D.; Marucci, M. Influence of residual monomers and metals on poly (l-lactide) thermal stability. Polymer 1997, 38, 1879–1884. [Google Scholar] [CrossRef]
- Anonymous. Xiameter™ MHX-1107 Fluid 20cst. Available online: https://www.Dow.Com/en-us/pdp.Xiameter-mhx-1107-fluid-30-cst.04088544h.Html (accessed on 23 July 2021).
- Galluzzi, A.; Murariu, M.; Raquez, J.-M.; Polichetti, M.; Dubois, P. Effect of magnetite nanoparticles content on the magnetic properties of polylactide and polystyrene composites. Chem. Eng. Trans. 2021, 84, 1–6. [Google Scholar] [CrossRef]
- Lin, H.; Hu, Q.; Liao, T.; Zhang, X.; Yang, W.; Cai, S. Highly hydrophobic cotton fabrics modified by poly(methylhydrogen)siloxane and fluorinated olefin: Characterization and applications. Polymers 2020, 12, 833. [Google Scholar] [CrossRef]
- Chen, J.; Ma, Y.; Lin, H.; Zheng, Q.; Zhang, X.; Yang, W.; Li, R. Fabrication of hydrophobic ZnO/PMHS coatings on bamboo surfaces: The synergistic effect of ZnO and PMHS on anti-mildew properties. Coatings 2019, 9, 15. [Google Scholar] [CrossRef]
- Roy Choudhury, A.K. Principles of Textile Finishing; Elsevier: Amsterdam, The Netherlands, 2017; pp. 123–171. [Google Scholar]
- Khatavkar, S.N.; Sartale, S.D. α-Fe2O3 thin films by liquid phase deposition: Low-cost option for supercapacitor. J. Solid State Electrochem. 2017, 21, 2555–2566. [Google Scholar] [CrossRef]
- Taghizadeh, S.-M.; Berenjian, A.; Zare, M.; Ebrahiminezhad, A. New perspectives on iron-based macrostructure. Processes 2020, 8, 1128. [Google Scholar] [CrossRef]
- Kim, D.K.; Lee, J.W. Synthesis of non-hydrate iron oleate for eco-friendly production of monodispersed iron oxide nanoparticles. J. Korean Ceram. Soc. 2018, 55, 625–634. [Google Scholar] [CrossRef]
- Gao, C.; Wang, Y.; Hu, D.; Pan, Z.; Xiang, L. Tribological properties of magnetite nanoparticles with various morphologies as lubricating additives. J. Nanopart. Res. 2013, 15, 1502. [Google Scholar] [CrossRef]
- Saeidlou, S.; Huneault, M.A.; Li, H.; Park, C.B. Poly(lactic acid) crystallization. Prog. Polym. Sci. 2012, 37, 1657–1677. [Google Scholar] [CrossRef]
- Namanga, J.; Foba, J.; Ndinteh, D.T.; Yufanyi, D.M.; Krause, R.W.M. Synthesis and magnetic properties of a superparamagnetic nanocomposite “pectin-magnetite nanocomposite”. J. Nanomater. 2013, 2013, 137275. [Google Scholar] [CrossRef]
- Di Palma, L.; Bavasso, I.; Sarasini, F.; Tirillò, J.; Puglia, D.; Dominici, F.; Torre, L.; Galluzzi, A.; Polichetti, M.; Ramazanov, M.A.; et al. Effect of nano-magnetite particle content on mechanical, thermal and magnetic properties of polypropylene composites. Polym. Compos. 2018, 39, E1742–E1750. [Google Scholar] [CrossRef]
- Jiang, H.; Han, X.; Li, Z.; Chen, X.; Hou, Y.; Gai, L.; Li, D.; Lu, X.; Fu, T. Superparamagnetic core–shell structured microspheres carrying carboxyl groups as adsorbents for purification of genomic DNA. Colloids Surf. A Physicochem. Eng. Asp. 2012, 401, 74–80. [Google Scholar] [CrossRef]
- Pankhurst, Q.A.; Connolly, J.; Jones, S.K.; Dobson, J. Applications of magnetic nanoparticles in biomedicine. J. Phys. D: Appl. Phys. 2003, 36, R167–R181. [Google Scholar] [CrossRef]
- Tavassoli, H.; Alhosseini, S.N.; Tay, A.; Chan, P.P.Y.; Weng Oh, S.K.; Warkiani, M.E. Large-scale production of stem cells utilizing microcarriers: A biomaterials engineering perspective from academic research to commercialized products. Biomaterials 2018, 181, 333–346. [Google Scholar] [CrossRef] [PubMed]








| Sample | Code | PLA | Nanofiller, wt.% | |
|---|---|---|---|---|
| wt.% | Untreated | Treated * | ||
| PLA−4% magnetite | PLA-4M | 96 | 4 | - |
| PLA−8% magnetite | PLA-8M | 92 | 8 | - |
| PLA−16% magnetite | PLA-16M | 84 | 16 | - |
| PLA−4% treated magnetite * | PLA-4Ms | 96 | - | 4 |
| PLA−8% treated magnetite | PLA-8Ms | 92 | - | 8 |
| PLA−16% treated magnetite | PLA-16Ms | 84 | - | 16 |
| Sample | T5% Temperature at 5% Weight Loss, °C | TD Temperature at Max. Rate of Degradation, °C | Residual Product at 600 °C, % |
|---|---|---|---|
| Neat PLA | 354 | 387 | 0.3 |
| PLA-4M | 332 | 358 | 3.8 |
| PLA-4Ms | 349 | 370 | 4.1 |
| PLA-8M | 327 | 349 | 8.6 |
| PLA-8Ms | 341 | 361 | 7.7 |
| PLA-16M | 324 | 344 | 16.1 |
| PLA-16Ms | 336 | 354 | 15.2 |
| Sample | Tg (°C) | Tcc1 (°C) | ΔHcc1 (J g−1) | Tcc2 (°C) | ΔHcc2 (J g−1) | Tm (°C) | ΔHm (J g−1) | χc, % |
|---|---|---|---|---|---|---|---|---|
| PLA | 61 | 109 | 24.8 | - | - | 169 | 36.7 | 12.8 |
| PLA-4M | 60 | 106 | 27.3 | - | - | 168 | 39.5 | 13.1 |
| PLA-8M | 59 | 99 | 23.4 | 155 | 1.6 | 168 | 38.3 | 14.3 |
| PLA-16M | 58 | - | - | - | - | 167 | 37.3 | 40.1 |
| PLA-4Ms | 59 | 101 | 26.9 | 151 | 0.5 | 167 | 41.5 | 15.2 |
| PLA-8Ms | 60 | 102 | 27.7 | 151 | 1.1 | 168 | 40.9 | 13.0 |
| PLA-16Ms | 57 | 96 | 17.4 | 152 | 1.8 | 166 | 42.4 | 24.9 |
| Sample | MFI (g/10 min) |
|---|---|
| PLA (AR) | 3.1 |
| PLA (processed) | 5.8 |
| PLA-4M | 9.2 |
| PLA-8M | 10.8 |
| PLA-16M | 14.9 |
| PLA-4Ms | 7.8 |
| PLA-8Ms | 8.7 |
| PLA-16Ms | 10.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murariu, M.; Galluzzi, A.; Paint, Y.; Murariu, O.; Raquez, J.-M.; Polichetti, M.; Dubois, P. Pathways to Green Perspectives: Production and Characterization of Polylactide (PLA) Nanocomposites Filled with Superparamagnetic Magnetite Nanoparticles. Materials 2021, 14, 5154. https://doi.org/10.3390/ma14185154
Murariu M, Galluzzi A, Paint Y, Murariu O, Raquez J-M, Polichetti M, Dubois P. Pathways to Green Perspectives: Production and Characterization of Polylactide (PLA) Nanocomposites Filled with Superparamagnetic Magnetite Nanoparticles. Materials. 2021; 14(18):5154. https://doi.org/10.3390/ma14185154
Chicago/Turabian StyleMurariu, Marius, Armando Galluzzi, Yoann Paint, Oltea Murariu, Jean-Marie Raquez, Massimiliano Polichetti, and Philippe Dubois. 2021. "Pathways to Green Perspectives: Production and Characterization of Polylactide (PLA) Nanocomposites Filled with Superparamagnetic Magnetite Nanoparticles" Materials 14, no. 18: 5154. https://doi.org/10.3390/ma14185154
APA StyleMurariu, M., Galluzzi, A., Paint, Y., Murariu, O., Raquez, J.-M., Polichetti, M., & Dubois, P. (2021). Pathways to Green Perspectives: Production and Characterization of Polylactide (PLA) Nanocomposites Filled with Superparamagnetic Magnetite Nanoparticles. Materials, 14(18), 5154. https://doi.org/10.3390/ma14185154











