Investigation on Green Synthesis, Biocompatibility, and Antibacterial Activity of Silver Nanoparticles Prepared Using Cistus incanus
Abstract
:1. Introduction
2. Experiment Methods
2.1. Preparation of Plant Extracts and Infusions
2.2. Obtaining Silver Nanoparticles
2.3. Total Phenolic Content and Antioxidant Capacity of Extracts
2.4. Size, Shape and Phase Composition of AgNPs
2.5. FT-IR Analysis
2.6. Cytotoxicity Test
2.7. Immunocompatibility Assay
2.8. Antimicrobial Activity of AgNPs
2.9. Statistical Analysis
3. Results
3.1. Extract and Infusion Antioxidant Properties
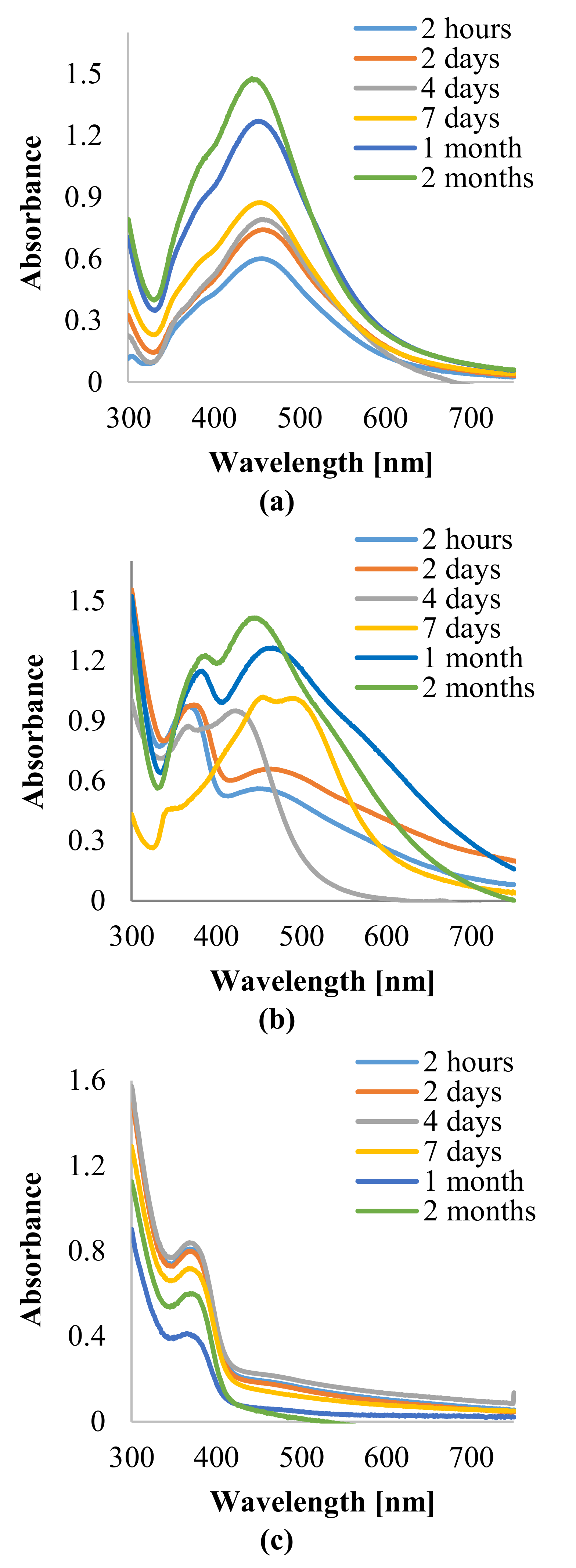
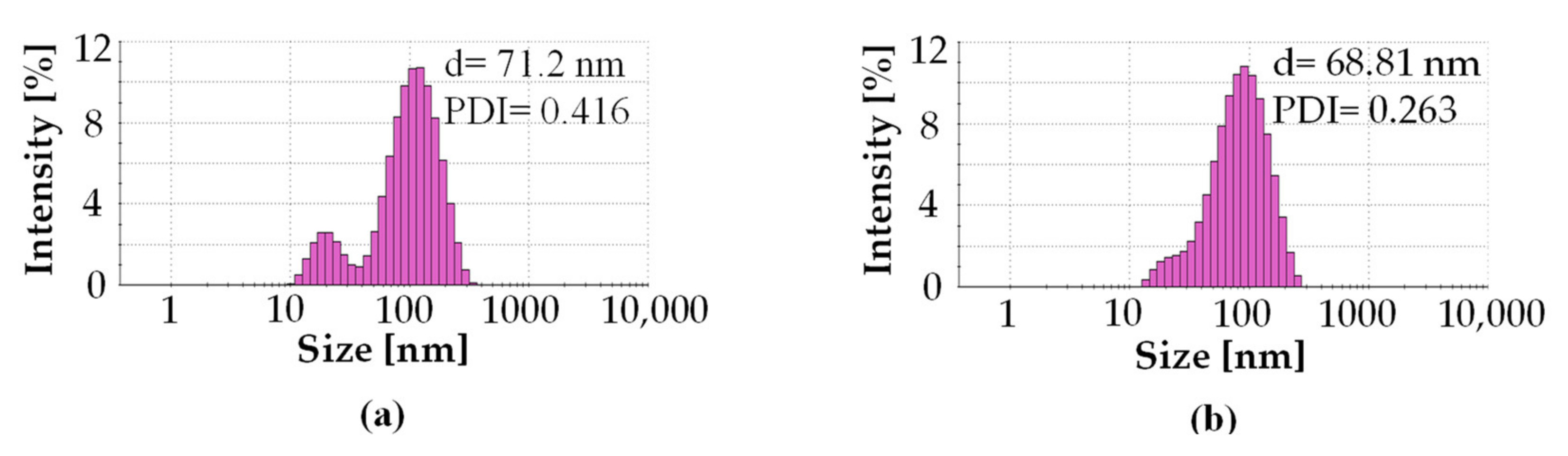
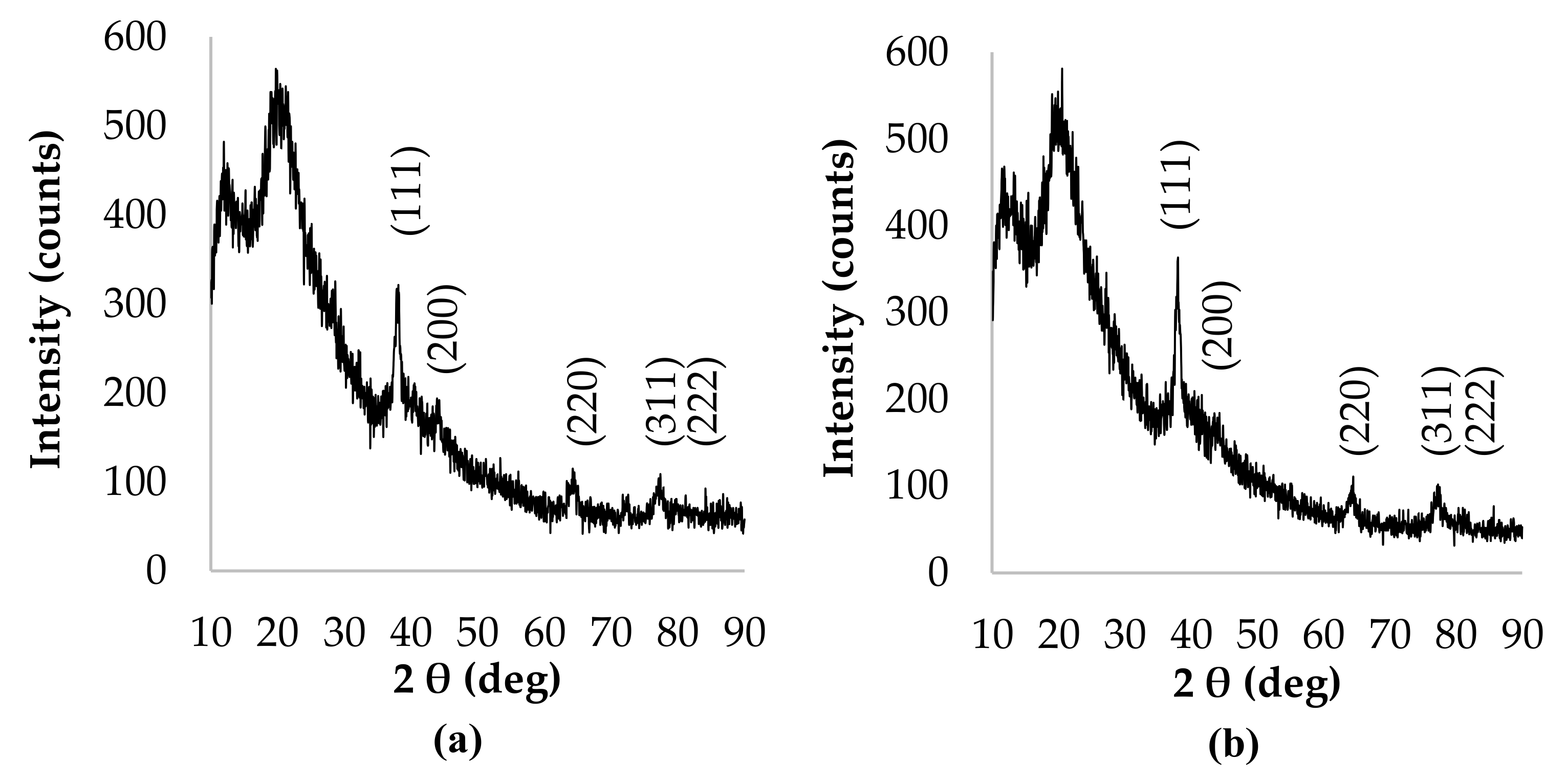
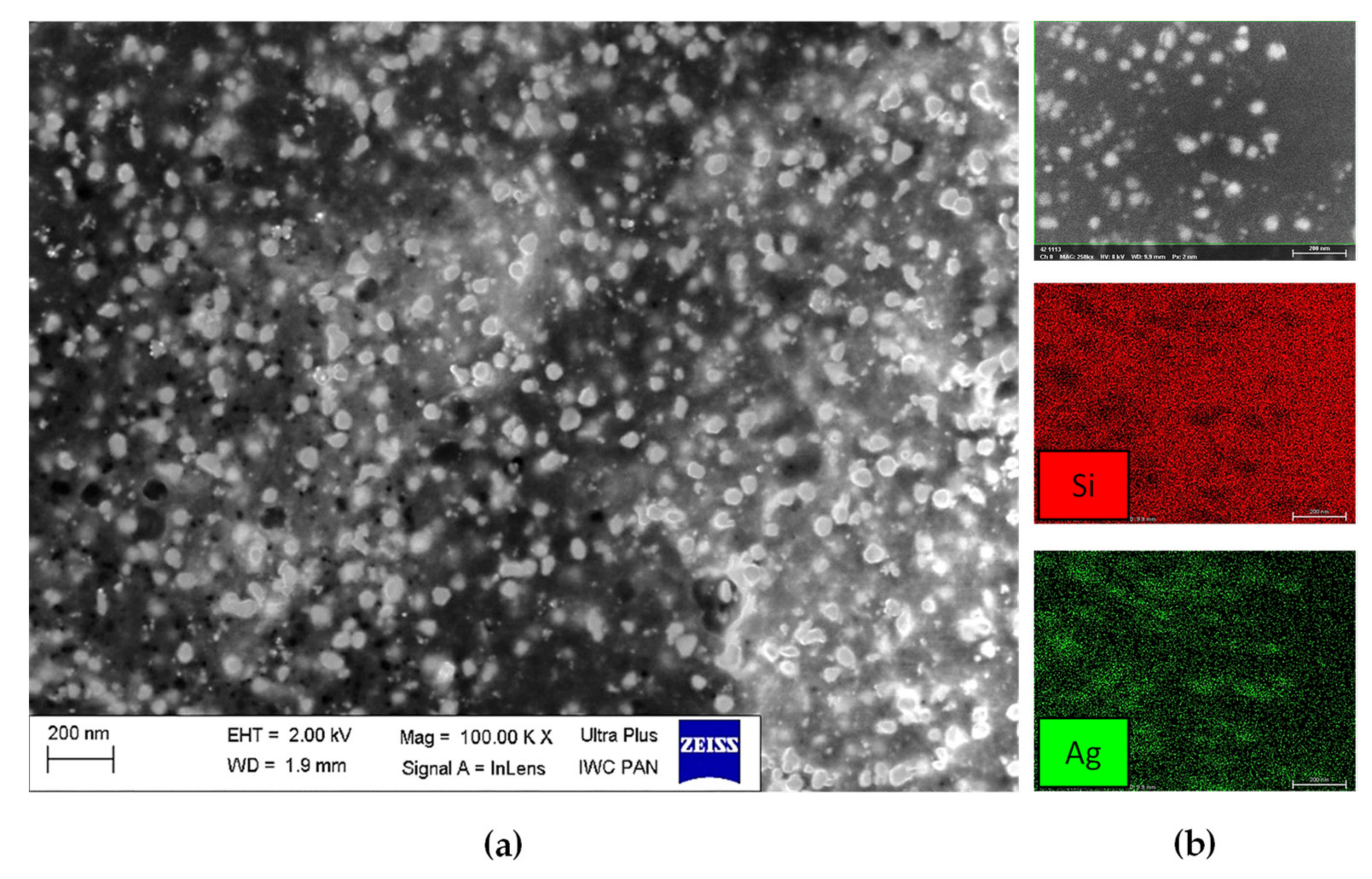
3.2. Characterization of Silver Nanoparticles
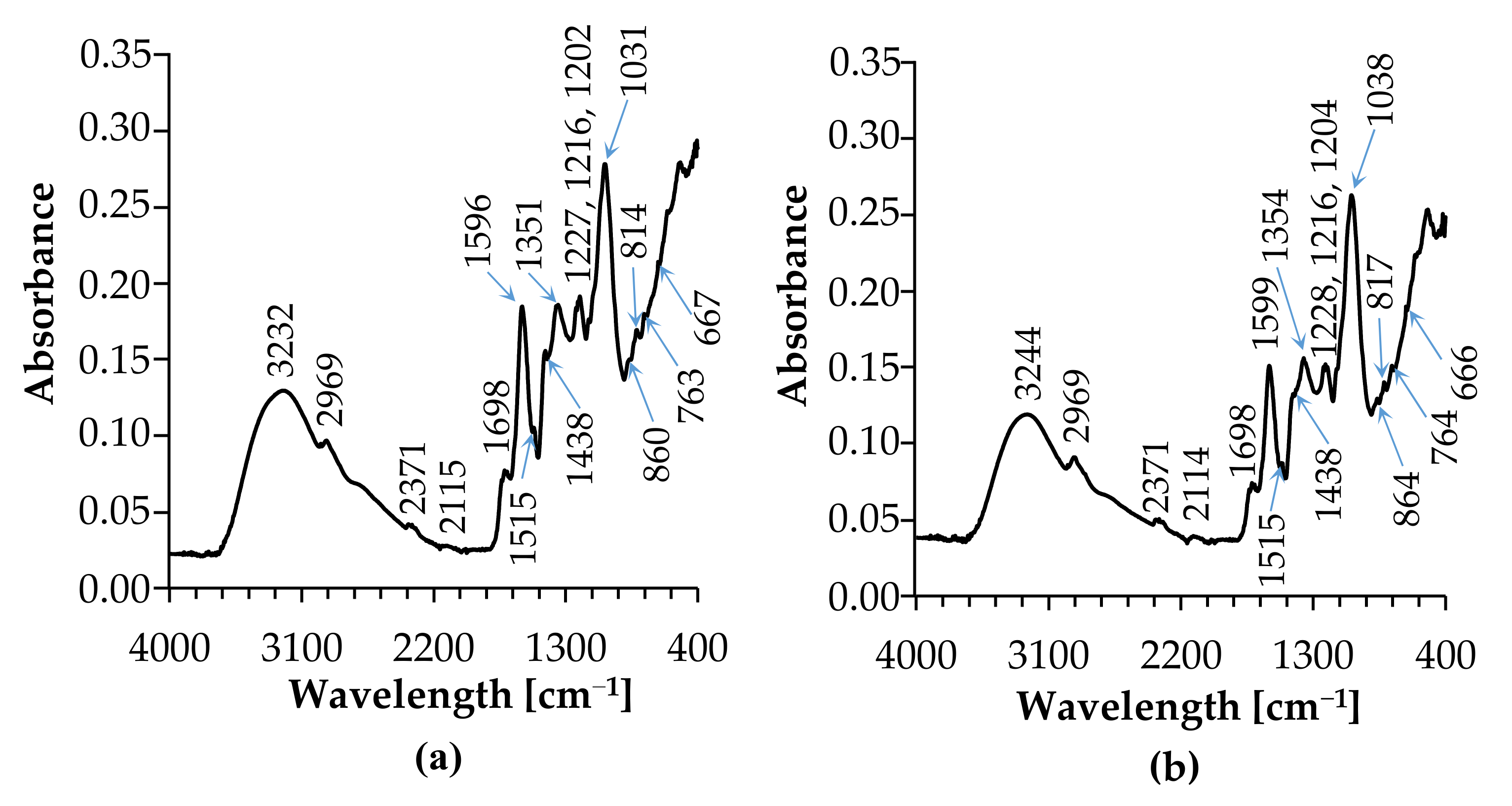
3.3. FT-IR Analysis
3.4. Direct Contact Cytotoxicity Assay
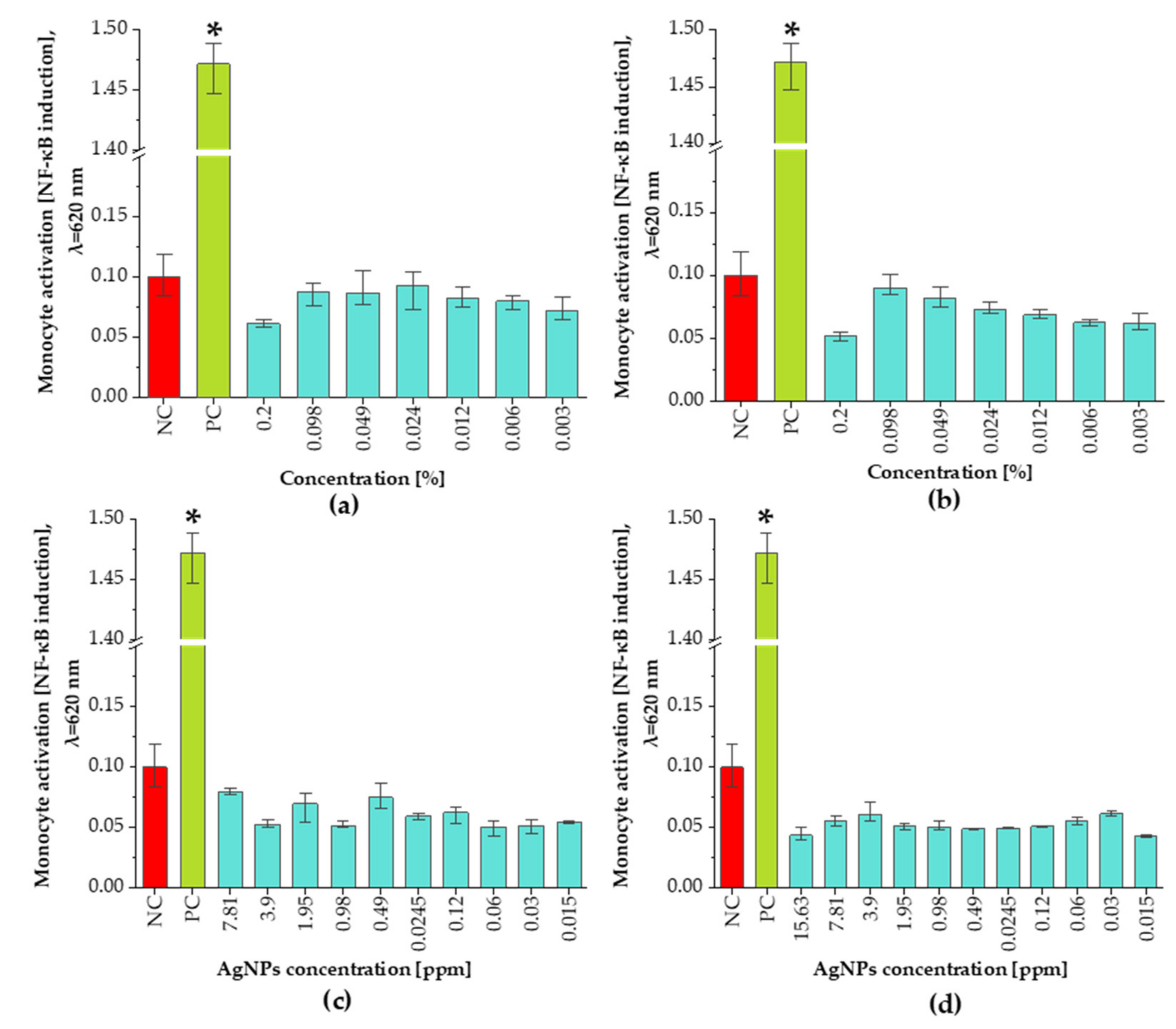
3.5. Pro-Inflammatory Assay
3.6. Antimicrobial Activity of AgNPs
4. Discussion
5. Future Perspectives
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bi, X.; Yang, L.; Wang, Z.; Zhan, Y.; Wang, S.; Zhang, C.; Li, Y.; Miao, Y.; Zha, J. Construction of a Three-Dimensional BaTiO3 Network for Enhanced Permittivity and Energy Storage of PVDF Composites. Materials 2021, 14, 3585. [Google Scholar] [CrossRef] [PubMed]
- Tetteh, E.K.; Rathilal, S. Prospects of Synthesized Magnetic TiO2-Based Membranes for Wastewater Treatment: A Review. Materials 2021, 14, 3524. [Google Scholar] [CrossRef] [PubMed]
- Astruc, D. Introduction: Nanoparticles in Catalysis. Chem. Rev. 2020, 120, 461–463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saeb, M.R.; Rabiee, N.; Mozafari, M.; Mostafavi, E. Metal-Organic Frameworks-Based Nanomaterials for Drug Delivery. Materials 2021, 14, 3652. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-pulido, G.; Medina, D.I.; Barani, M.; Rahdar, A.; Sargazi, G.; Baino, F.; Pandey, S. Nanomaterials for the Diagnosis and Treatment of Head and Neck Cancers: A Review. Materials 2021, 14, 3706. [Google Scholar] [CrossRef]
- Particles, L.; Leeuw, N.H. De A Perspective on Modelling Metallic Magnetic Nanoparticles in Biomedicine: From Monometals to Nanoalloys and Ligand-Protected Particles. Materials 2021, 14, 3611. [Google Scholar]
- Huq, M.A.; Akter, S. Bacterial mediated rapid and facile synthesis of silver nanoparticles and their antimicrobial efficacy against pathogenic microorganisms. Materials 2021, 14, 2615. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.; Bonde, S.; Golinska, P.; Trzcińska-Wencel, J.; Gade, A.; Abd-Elsalam, K.; Shende, S.; Gaikwad, S.; Ingle, A.P. Fusarium as a novel fungus for the synthesis of nanoparticles: Mechanism and applications. J. Fungi 2021, 7, 139. [Google Scholar] [CrossRef]
- Azizi, S.; Namvar, F.; Mahdavi, M.; Ahmad, M.B.; Mohamad, R. Biosynthesis of silver nanoparticles using brown marine macroalga, Sargassum muticum aqueous extract. Materials 2013, 6, 5942–5950. [Google Scholar] [CrossRef]
- Reddy, N. Properties and Applications of Nanoparticles from Plant Proteins. Materials 2021, 14, 3607. [Google Scholar] [CrossRef]
- Castillo-Henríquez, L.; Alfaro-Aguilar, K.; Ugalde-álvarez, J.; Vega-Fernández, L.; de Oca-Vásquez, G.M.; Vega-Baudrit, J.R. Green synthesis of gold and silver nanoparticles from plant extracts and their possible applications as antimicrobial agents in the agricultural area. Nanomaterials 2020, 10, 1763. [Google Scholar] [CrossRef] [PubMed]
- Alegria, E.C.B.A.; Ribeiro, A.P.C.; Mendes, M.; Ferraria, A.M.; Botelho do Rego, A.M.; Pombeiro, A.J.L. Effect of phenolic compounds on the synthesis of gold nanoparticles and its catalytic activity in the reduction of nitro compounds. Nanomaterials 2018, 8, 320. [Google Scholar] [CrossRef] [Green Version]
- Mashwani, Z.-u.-R.; Khan, M.A.; Khan, T.; Nadhman, A. Applications of plant terpenoids in the synthesis of colloidal silver nanoparticles. Adv. Colloid Interface Sci. 2016, 234, 132–141. [Google Scholar] [CrossRef] [PubMed]
- AL-Thabaiti, N.S.; Malik, M.A.; Khan, Z. Protein interactions with silver nanoparticles: Green synthesis, and biophysical approach. Int. J. Biol. Macromol. 2017, 95, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Ovais, M.; Khalil, A.T.; Ayaz, M.; Ahmad, I.; Nethi, S.K.; Mukherjee, S. Biosynthesis of metal nanoparticles via microbial enzymes: A mechanistic approach. Int. J. Mol. Sci. 2018, 19, 4100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saratale, R.G.; Saratale, G.D.; Cho, S.K.; Ghodake, G.; Kadam, A.; Kumar, S.; Mulla, S.I.; Kim, D.S.; Jeon, B.H.; Chang, J.S.; et al. Phyto-fabrication of silver nanoparticles by Acacia nilotica leaves: Investigating their antineoplastic, free radical scavenging potential and application in H2O2 sensing. J. Taiwan Inst. Chem. Eng. 2019, 99, 239–249. [Google Scholar] [CrossRef]
- Saratale, R.G.; Shin, H.S.; Kumar, G.; Benelli, G.; Ghodake, G.S.; Jiang, Y.Y.; Kim, D.S.; Saratale, G.D. Exploiting fruit byproducts for eco-friendly nanosynthesis: Citrus × clementina peel extract mediated fabrication of silver nanoparticles with high efficacy against microbial pathogens and rat glial tumor C6 cells. Environ. Sci. Pollut. Res. 2018, 25, 10250–10263. [Google Scholar] [CrossRef] [PubMed]
- Hao, D.-C. Genomics and Evolution of Medicinal Plants; Elsevier Inc.: Amsterdam, The Netherlands, 2019; ISBN 9780128142325. [Google Scholar]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J. Nat. Prod. 2012, 75, 311–335. [Google Scholar] [CrossRef] [Green Version]
- Kauroo, S.; Govinden-Soulange, J.; Ranghoo-Sanmukhiya, V.M.; Miranda, K.; Cotham, W.E.; Walla, M.D.; Nagarkatti, M.; Nagarkatti, P. Extracts of select endemic plants from the Republic of Mauritius exhibiting anti-cancer and immunomodulatory properties. Sci. Rep. 2021, 11, 4272. [Google Scholar] [CrossRef]
- Evaluation, A. In Vitro Cultures of Some Medicinal Plant Species (Cistus × incanus, Verbena officinalis, Scutellaria lateriflora, and Scutellaria baicalensis) as a Rich Potential Source of Antioxidants—Evaluation by CUPRAC and QUENCHER-CUPRAC Assays. Plants 2021, 10, 454. [Google Scholar]
- Dimcheva, V.; Karsheva, M. Cistus incanus from Strandja Mountain as a Source of Bioactive Antioxidants. Plants 2018, 7, 8. [Google Scholar] [CrossRef] [Green Version]
- Gaweł-Bęben, K.; Kukula-Koch, W.; Hoian, U.; Czop, M.; Strzępek-Gomółka, M.; Antosiewicz, B. Characterization of cistus × incanus L. and Cistus ladanifer L. extracts as potential multifunctional antioxidant ingredients for skin protecting cosmetics. Antioxidants 2020, 9, 202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Musarra-pizzo, M.; Pennisi, R.; Ben-amor, I.; Mandalari, G.; Sciortino, M.T. Antiviral Activity Exerted by Natural Products against Human Viruses. Viruses 2021, 13, 828. [Google Scholar] [CrossRef]
- Zalegh, I.; Akssira, M.; Bourhia, M.; Mellouki, F.; Rhallabi, N.; Salamatullah, A.M.; Alkaltham, M.S.; Khalil Alyahya, H.; Mhand, R.A. A review on cistus sp.: Phytochemical and antimicrobial activities. Plants 2021, 10, 1214. [Google Scholar] [CrossRef] [PubMed]
- Flemming, J.; Meyer-Probst, C.T.; Speer, K.; Kölling-Speer, I.; Hannig, C.; Hannig, M. Preventive applications of polyphenols in dentistry—A review. Int. J. Mol. Sci. 2021, 22, 4892. [Google Scholar] [CrossRef]
- Barrajõn-Catalán, E.; Fernández-Arroyo, S.; Roldán, C.; Guillén, E.; Saura, D.; Segura-Carretero, A.; Micol, V. A systematic study of the polyphenolic composition of aqueous extracts deriving from several Cistus genus species: Evolutionary relationship. Phytochem. Anal. 2011, 22, 303–312. [Google Scholar] [CrossRef]
- Klekotko, M.; Brach, K.; Olesiak-Banska, J.; Samoc, M.; Matczyszyn, K. Popcorn-shaped gold nanoparticles: Plant extract-mediated synthesis, characterization and multiphoton-excited luminescence properties. Mater. Chem. Phys. 2019, 229, 56–60. [Google Scholar] [CrossRef]
- Jing, C.; Yan, C.J.; Yuan, X.T.; Zhu, L.P. Biosynthesis of copper oxide nanoparticles and their potential synergistic effect on alloxan induced oxidative stress conditions during cardiac injury in Sprague–Dawley rats. J. Photochem. Photobiol. B Biol. 2019, 198, 111557. [Google Scholar] [CrossRef]
- Fernández, K.; Agosin, E. Quantitative analysis of red wine tannins using Fourier-transform mid-infrared spectrometry. J. Agric. Food Chem. 2007, 55, 7294–7300. [Google Scholar] [CrossRef]
- Florkiewicz, W.; Malina, D.; Pluta, K.; Rudnicka, K.; Gajewski, A.; Olejnik, E.; Tyliszczak, B.; Sobczak-Kupiec, A. Assessment of cytotoxicity and immune compatibility of phytochemicals-mediated biosynthesised silver nanoparticles using Cynara scolymus. IET Nanobiotechnol. 2019, 13, 726–735. [Google Scholar] [CrossRef]
- Vardin, H.; Tay, A.; Ozen, B.; Mauer, L. Authentication of pomegranate juice concentrate using FTIR spectroscopy and chemometrics. Food Chem. 2008, 108, 742–748. [Google Scholar] [CrossRef] [Green Version]
- Ping, L.; Pizzi, A.; Guo, Z.D.; Brosse, N. Condensed tannins from grape pomace: Characterization by FTIR and MALDI TOF and production of environment friendly wood adhesive. Ind. Crops Prod. 2012, 40, 13–20. [Google Scholar] [CrossRef]
- El-Sherbiny, I.M.; Smyth, H.D.C. Smart Magnetically Responsive Hydrogel Nanoparticles Prepared by a Novel Aerosol-Assisted Method for Biomedical and Drug Delivery Applications. J. Nanomater. 2011, 2011, 910539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carlborn, K.; Matuana, L.M. Composite materials manufactured from wood particles modified through a reactive extrusion process. Polym. Compos. 2005, 26, 534–541. [Google Scholar] [CrossRef]
- Heredia-Guerrero, J.A.; Benítez, J.J.; Domínguez, E.; Bayer, I.S.; Cingolani, R.; Athanassiou, A.; Heredia, A. Infrared and Raman spectroscopic features of plant cuticles: A review. Front. Plant Sci. 2014, 5, 305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iswantini, D.; Ramdhani, T.H.; Darusman, L.K. In vitro inhibition of celery (Apium graveolens L.) extract on the activity of xanthine oxidase and determination of its active compound. Indones. J. Chem. 2012, 12, 247–254. [Google Scholar] [CrossRef]
- Black, C.; Haughey, S.A.; Chevallier, O.P.; Galvin-King, P.; Elliott, C.T. A comprehensive strategy to detect the fraudulent adulteration of herbs: The oregano approach. Food Chem. 2016, 210, 551–557. [Google Scholar] [CrossRef] [Green Version]
- Mohamed, M.E.; Mohammed, A.M.A. Experimental and Computational Vibration Study of Amino Acids. Int. Lett. Chem. Phys. Astron. 2013, 15, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Cappelli, C.; Mennucci, B.; Monti, S. Environmental effects on the spectroscopic properties of gallic acid: A combined classical and quantum mechanical study. J. Phys. Chem. A 2005, 109, 1933–1943. [Google Scholar] [CrossRef] [PubMed]
- Coates, J. Interpretation of Infrared Spectra, A Practical Approach. In Encyclopedia of Analytical Chemistry; John Wiley & Sons Ltd.: Chichester, UK, 2006; pp. 10815–10837. [Google Scholar]
- Falcão, L.; Araújo, M.E.M. Tannins characterization in historic leathers by complementary analytical techniques ATR-FTIR, UV-Vis and chemical tests. J. Cult. Herit. 2013, 14, 499–508. [Google Scholar] [CrossRef]
- Sanches, N.B.; Pedro, R.; Diniz, M.F.; Mattos, E.d.C.; Cassu, S.N.; Dutra, R.d.C.L. Infrared spectroscopy applied to materials used as thermal insulation and coatings. J. Aerosp. Technol. Manag. 2013, 5, 421–430. [Google Scholar] [CrossRef] [Green Version]
- García, A.; Toledano, A.; Andrés, M.Á.; Labidi, J. Study of the antioxidant capacity of Miscanthus sinensis lignins. Process Biochem. 2010, 45, 935–940. [Google Scholar] [CrossRef]
- Pardeshi, S.; Dhodapkar, R.; Kumar, A. Quantum chemical density functional theory studies on the molecular structure and vibrational spectra of Gallic acid imprinted polymers. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 116, 562–573. [Google Scholar] [CrossRef]
- Pantoja-Castro, M.A.; González-Rodríguez, H. Study by infrared spectroscopy and thermogravimetric analysis of tannins and tannic acid. Rev. Latinoam. Química 2011, 39, 107–112. [Google Scholar]
- Latos-Brozio, M.; Masek, A. Effect of impregnation of biodegradable polyesters with polyphenols from cistus linnaeus and Juglans regia Linnaeus walnut green husk. Polymers 2019, 11, 669. [Google Scholar] [CrossRef] [Green Version]
- Yusnawan, E. Effects of different extraction methods on total phenolic content and antioxidant activity in soybean cultivars. IOP Conf. Ser. Earth Environ. Sci. 2018, 102, 012039. [Google Scholar] [CrossRef]
- Scherer, R.; Godoy, H.T. Efeito de métodos de extração de compostos fenólicos de Xanthium strumarium L. e suas atividades antioxidantes. Rev. Bras. Plantas Med. 2014, 16, 41–46. [Google Scholar] [CrossRef]
- Petkova, N.; Ivanova, L.; Filova, G.; Ivanov, I.; Denev, P. Antioxidants and carbohydrate content in infusions and microwave extracts from eight medicinal plants. J. Appl. Pharm. Sci. 2017, 7, 55–61. [Google Scholar]
- Chaves, J.O.; de Souza, M.C.; da Silva, L.C.; Lachos-Perez, D.; Torres-Mayanga, P.C.; Machado, A.P.d.F.; Forster-Carneiro, T.; Vázquez-Espinosa, M.; González-de-Peredo, A.V.; Barbero, G.F.; et al. Extraction of Flavonoids From Natural Sources Using Modern Techniques. Front. Chem. 2020, 8, 507887. [Google Scholar] [CrossRef] [PubMed]
- Ross, C.F.; Hoye, C.; Fernandez-Plotka, V.C. Influence of Heating on the Polyphenolic Content and Antioxidant Activity of Grape Seed Flour. J. Food Sci. 2011, 76, 884–890. [Google Scholar] [CrossRef] [PubMed]
- AKTAR, T. Cistus Criticus, Fermente Rooibos VeYeşïl Çay Infüzyonlarinin ToplamFenolïk, ToplaAntïoksïdan VeAskorbïkAsïçerïklerïnïn KarşilaştirmaliBïr Çalişmasi. DÜMF Mühendislik Derg. 2020, 11, 1197–1204. [Google Scholar]
- Dieckmann, Y.; Co, H.; Hofmann, H.; Petri-fink, A. Particle Size Distribution Measurements of Manganese-Doped ZnS Nanoparticles. Anal. Chem. 2013, 81, 3889–3895. [Google Scholar] [CrossRef] [Green Version]
- Golinska, M.S.P.; Dahm, K.R.H. Evaluation of cytotoxicity, immune compatibility and antibacterial activity of biogenic silver nanoparticles. Med. Microbiol. Immunol. 2016, 205, 603–613. [Google Scholar]
- Veeraraghavan, V.P.; Periadurai, N.D.; Karunakaran, T.; Hussain, S.; Surapaneni, K.M.; Jiao, X. Green synthesis of silver nanoparticles from aqueous extract of Scutellaria barbata and coating on the cotton fabric for antimicrobial applications and wound healing activity in fibroblast cells (L929). Saudi J. Biol. Sci. 2021, 28, 3633–3640. [Google Scholar] [CrossRef]
- Carlson, C.; Hussein, S.M.; Schrand, A.M.; Braydich-Stolle, L.K.; Hess, K.L.; Jones, R.L.; Schlager, J.J. Unique cellular interaction of silver nanoparticles: Size-dependent generation of reactive oxygen species. J. Phys. Chem. B 2008, 112, 13608–13619. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Lu, X.; Yuan, Y.; Liu, C.; Yang, B.; Hong, H.; Wang, G.; Zeng, F. Effect of size and processing method on the cytotoxicity of realgar nanoparticles in cancer cell lines. Int. J. Nanomed. 2011, 6, 1569–1577. [Google Scholar] [CrossRef] [Green Version]
- Mcshan, D.; Ray, P.C.; Yu, H. ScienceDirect Molecular toxicity mechanism of nanosilver. J. Food Drug Anal. 2014, 22, 116–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baruwati, B.; Simmons, S.O.; Varma, R.S.; Veronesi, B. “Green” Synthesized and Coated Nanosilver Alters the Membrane Permeability of Barrier (Intestinal, Brain Endothelial) Cells and Stimulates Oxidative Stress Pathways in Neurons. ACS Sustain. Chem. Eng. 2013, 1, 753–759. [Google Scholar] [CrossRef]
- Heidenau, F.; Mittelmeier, W.; Detsch, R.; Haenle, M.; Stenzel, F.; Ziegler, G.; Gollwitzer, H. A novel antibacterial titania coating: Metal ion toxicity and in vitro surface colonization. J. Mater. Sci. Mater. Med. 2005, 16, 883–888. [Google Scholar] [CrossRef]
- Liu, H.L.; Dai, S.A.; Fu, K.Y.; Hsu, S.-h. Antibacterial properties of silver nanoparticles in three different sizes and their nanocomposites with a new waterborne polyurethane. Int. J. Nanomed. 2010, 5, 1017–1028. [Google Scholar]
- Domitrovic, R. Myricitrin exhibits antioxidant, anti-inflammatory and antifibrotic activity in carbon tetrachloride-intoxicated mice Chemico-Biological Interactions Myricitrin exhibits antioxidant, anti-inflammatory and antifibrotic activity in carbon tetrachloride-intoxicated mice. Chem.-Biol. Interact. 2015, 230, 21–29. [Google Scholar] [PubMed]
- Bensaad, L.A.; Kim, K.H.; Quah, C.C.; Kim, W.R.; Shahimi, M. Anti-inflammatory potential of ellagic acid, gallic acid and punicalagin A & B isolated from Punica granatum. BMC Complement. Altern. Med. 2017, 17, 47. [Google Scholar]
- Zhu, L.; Gu, P.Q.; Shen, H. Gallic acid improved inflammation via NF-κB pathway in TNBS-induced ulcerative colitis. Int. Immunopharmacol. 2019, 67, 129–137. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Pohl, P.; Epifano, F. Phytofabrication of silver nanoparticles (Agnps) with pharmaceutical capabilities using otostegia persica (burm.) boiss. leaf extract. Nanomaterials 2021, 11, 1045. [Google Scholar] [CrossRef] [PubMed]
- Helmlinger, J.; Sengstock, C.; Groß-Heitfeld, C.; Mayer, C.; Schildhauer, T.A.; Köller, M.; Epple, M. Silver nanoparticles with different size and shape: Equal cytotoxicity, but different antibacterial effects. RSC Adv. 2016, 6, 18490–18501. [Google Scholar] [CrossRef] [Green Version]
- Dong, Y.; Zhu, H.; Shen, Y.; Zhang, W.; Zhang, L. Antibacterial activity of silver nanoparticles of different particle size against Vibrio Natriegens. PLoS ONE 2019, 14, e0222322. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Title 1 | Phenolic Content (mg GAE·g−1 d.w.) ± SD | Antioxidant Activity IC50 (mg/mL) ± SD |
|---|---|---|
| C. incanus extract | 12.65 ± 1.04 | 3.10 ± 0.14 |
| C. incanus infusion | 85.97 ± 6.54 | 10.76 ± 0.59 |
| Infusion [cm−1] | Extract [cm−1] | Peak Assignment |
|---|---|---|
| 3232 | 3244 | OH stretching, N-H stretching [30] |
| 2969 | 2969 | C-H symmetric stretching of -CH3, OH stretching [30] |
| 2930 | 2925 | C-H stretching of vibration of methyl and methoxy groups, stretching vibration of -CH3 or -CH2 groups (carboxylic acid), OH stretching [32] |
| 2371 | 2371 | -CH3 and -CH2 stretching [33] |
| 2115 | 2114 | -CH3 and -CH2 stretching [33] |
| 1698 | 1698 | C=O stretching (ketone) [34] |
| 1596 | 1599 | stretching C=C (aromatic ring) [35] |
| 1515 | 1515 | C=C (aromatic ring) [36] |
| 1438 | 1435 | C-C aromatic (conjugated with C=C), O-H bending [37] |
| 1351 | 1354 | C-H deformations, CH2-OH deformation, N-H stretching [38] |
| 1227 | 1228 | C-O (alcohol hydroxyl group), C-O-H (deformation of phenols) [39] |
| 1216 | 1216 | C-H deformations [39] |
| 1202 | 1204 | NH2 in-plane bending, aromatic C-H [40] |
| 1141 | 1140 | in—plane bending, CH3 deformation [41] |
| 1031 | 1038 | N-H symmetric bending, O-H bending [42] |
| 860 | 864 | C-O stretching benzene nucleus, C-O stretching of C-OH [43] |
| 814 | 817 | C-O-C (cyclic ethers, large rings) [43,44] |
| 763 | 764 | stretching vibration of C-O bonds, C-O-C (ester, ether, or phenols), C-N stretching (aliphatic primary amine) [44,45] |
| 667 | 666 | aromatic C-H out-of-plane bending, bending vibration of the CH‒tri-aromatic substitution group [46] |
| Sample | S. aureus | S. epidermidis | ||
|---|---|---|---|---|
| MIC [µg/mL] | MBC [µg/mL] | MIC [µg/mL] | MBC [µg/mL] | |
| Extract NPs | >256 | >256 | 32 ppm | 128 ppm |
| Infusion NPs | 2 ppm | 128 ppm | 16 ppm | 128 ppm |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Florkiewicz, W.; Pluta, K.; Malina, D.; Rudnicka, K.; Żywicka, A.; Guigou, M.D.; Tyliszczak, B.; Sobczak-Kupiec, A. Investigation on Green Synthesis, Biocompatibility, and Antibacterial Activity of Silver Nanoparticles Prepared Using Cistus incanus. Materials 2021, 14, 5028. https://doi.org/10.3390/ma14175028
Florkiewicz W, Pluta K, Malina D, Rudnicka K, Żywicka A, Guigou MD, Tyliszczak B, Sobczak-Kupiec A. Investigation on Green Synthesis, Biocompatibility, and Antibacterial Activity of Silver Nanoparticles Prepared Using Cistus incanus. Materials. 2021; 14(17):5028. https://doi.org/10.3390/ma14175028
Chicago/Turabian StyleFlorkiewicz, Wioletta, Klaudia Pluta, Dagmara Malina, Karolina Rudnicka, Anna Żywicka, Martin Duarte Guigou, Bożena Tyliszczak, and Agnieszka Sobczak-Kupiec. 2021. "Investigation on Green Synthesis, Biocompatibility, and Antibacterial Activity of Silver Nanoparticles Prepared Using Cistus incanus" Materials 14, no. 17: 5028. https://doi.org/10.3390/ma14175028
APA StyleFlorkiewicz, W., Pluta, K., Malina, D., Rudnicka, K., Żywicka, A., Guigou, M. D., Tyliszczak, B., & Sobczak-Kupiec, A. (2021). Investigation on Green Synthesis, Biocompatibility, and Antibacterial Activity of Silver Nanoparticles Prepared Using Cistus incanus. Materials, 14(17), 5028. https://doi.org/10.3390/ma14175028








