Butyl-Methyl-Pyridinium Tetrafluoroborate Confined in Mesoporous Silica Xerogels: Thermal Behaviour and Matrix-Template Interaction
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Characterization Methods
3. Results
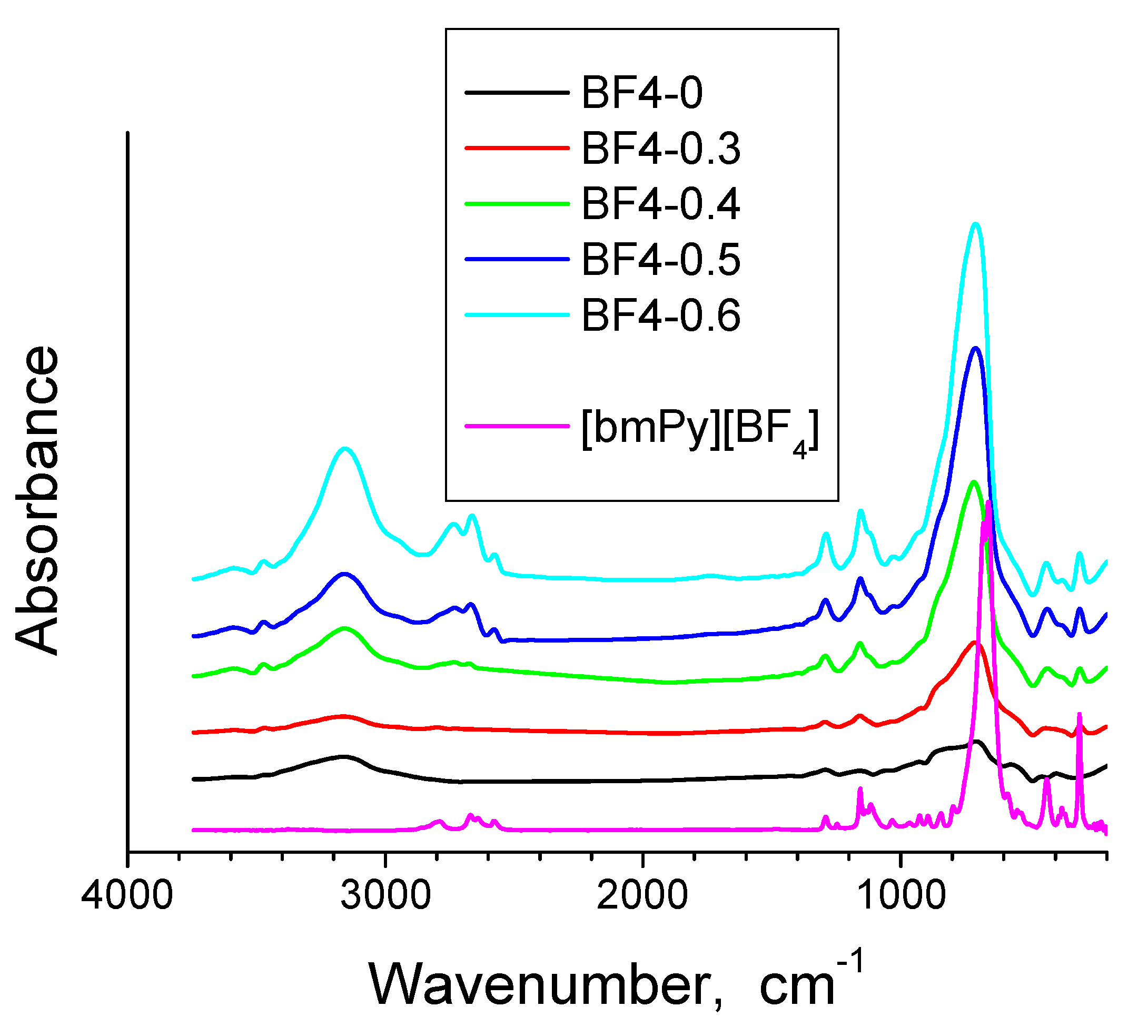
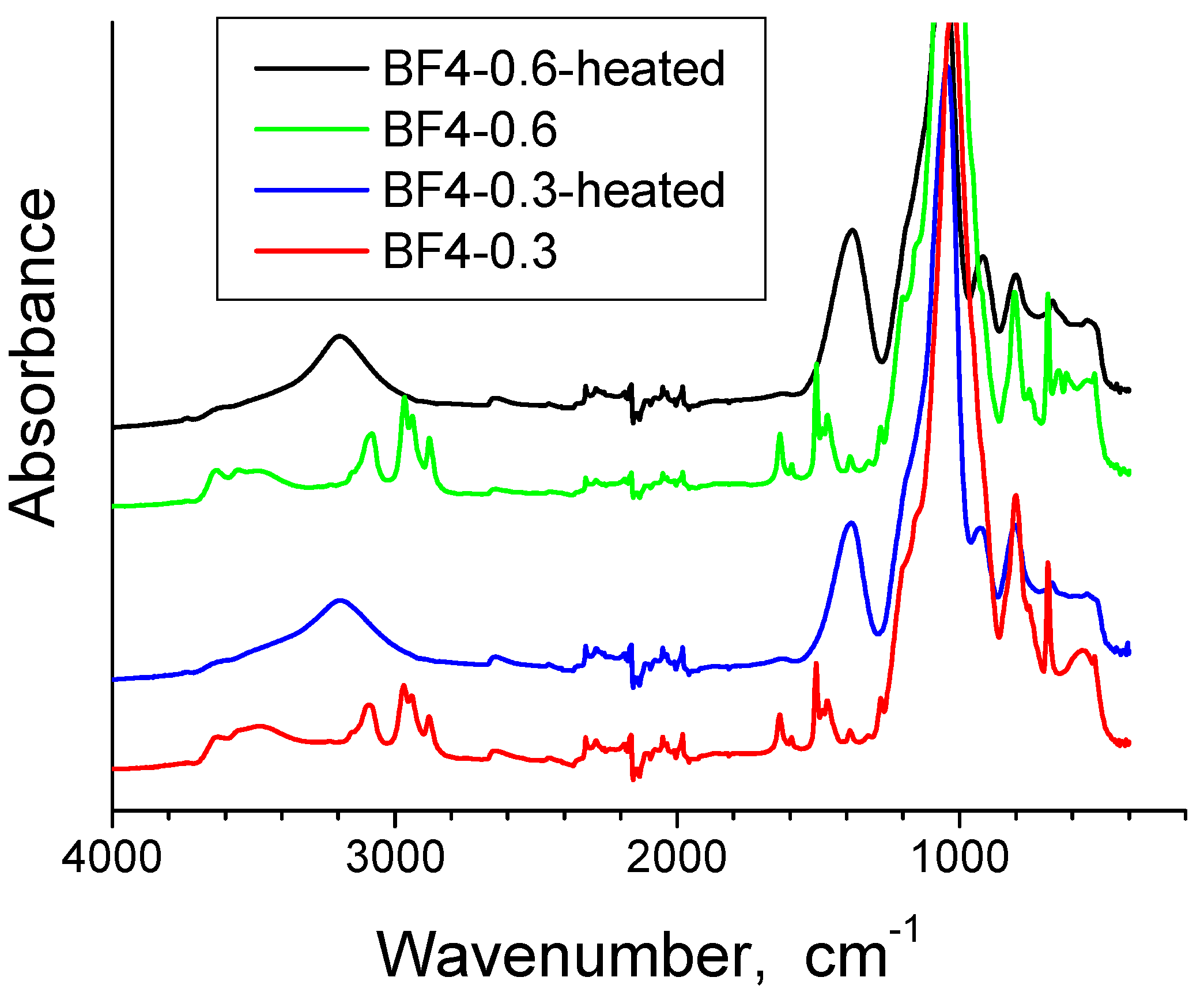
3.1. FT-IR Spectroscopy
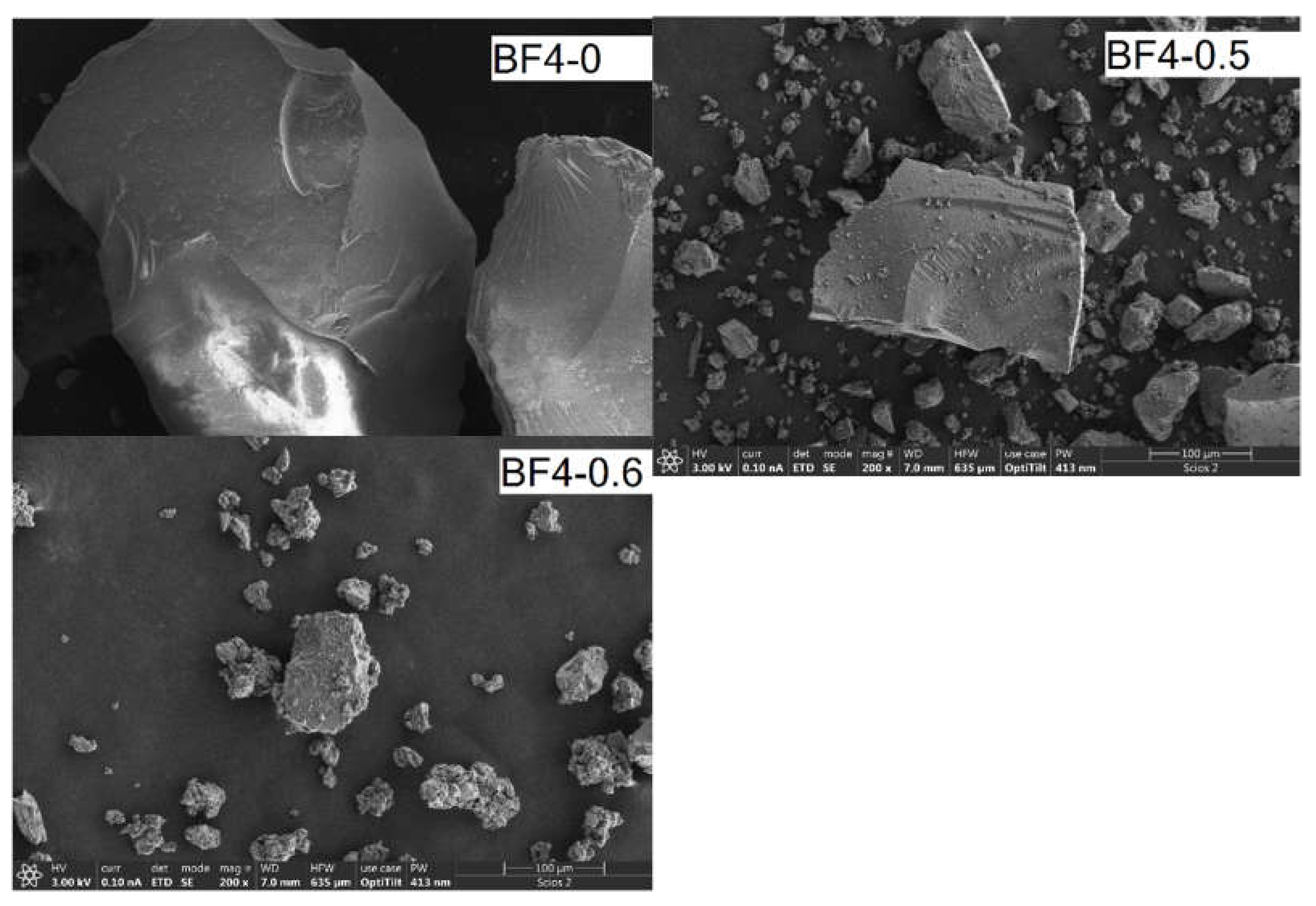
3.2. Electron Microscopy and EDS Analysis
3.3. Nitrogen Porosimetry
3.4. Thermal Analysis
3.5. Mass Spectroscopic Analysis of the Evolved Gases
3.6. SANS Analysis of the Particle and Agglomerate Morphology
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, Y. Recent advances in ionic liquids for synthesis of inorganic nanomaterials. Curr. Nanosci. 2005, 1, 35–42. [Google Scholar] [CrossRef]
- Karout, A.; Pierre, A.C. Silica xerogels and aerogels synthesized with ionic liquids. J. Non-Cryst. Solids 2007, 353, 2900–2909. [Google Scholar] [CrossRef]
- Putz, A.M.; Len, A.; Ianăşi, C.; Savii, C.; Almásy, L. Ultrasonic preparation of mesoporous silica using pyridinium ionic liquid. Korean J. Chem. Eng. 2016, 33, 749–754. [Google Scholar] [CrossRef]
- Almásy, L.; Putz, A.-M.; Len, A.; Plestil, J.; Savii, C. Small-angle scattering investigation of silica xerogels and sonogels prepared with ionic liquid pyridinium tetrafluoroborate. Process. Appl. Ceram. 2017, 11, 229–233. [Google Scholar] [CrossRef]
- Viau, L.; Néouze, M.-A.; Biolley, C.; Volland, S.; Brevet, D.; Gaveau, P.; Dieudonné, P.; Galarneanu, A.; Vioux, A. Ionic liquid mediated sol-gel synthesis in the presence of water or formic acid: Which synthesis for which material? Chem. Mater. 2012, 24, 3128–3134. [Google Scholar] [CrossRef]
- Zhou, Y.; Schattka, J.H.; Antonietti, M. Room-temperature ionic liquids as template to monolithic mesoporous silica with worm-like pores via a sol-gel nanocasting technique. Nano Lett. 2004, 4, 477–481. [Google Scholar] [CrossRef]
- Wu, C.M.; Lin, S.Y.; Chen, H.L. Structure of a monolithic silica aerogel prepared from a short-chain ionic liquid. Micropor. Mesopor. Mater. 2012, 156, 189–195. [Google Scholar] [CrossRef]
- Wu, C.M.; Lin, S.Y.; Kao, K.Y.; Chen, H.L. Self-organization of a hydrophilic short-chain ionic liquid confined within a hydrophobic nanopore. J. Phys. Chem. C 2014, 118, 17764–17772. [Google Scholar] [CrossRef]
- Zhou, Y.; Antonietti, M. Preparation of highly ordered monolithic super-microporous lamellar silica with a room-temperature ionic liquid as template via the nanocasting technique. Adv. Mater. 2003, 15, 1452–1455. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, S. Preparation of ordered mesoporous silica materials templated by ionic liquids in alkaline condition. J. Porous Mater. 2019, 26, 1–6. [Google Scholar] [CrossRef]
- Göbel, R.; Hesemann, P.; Weber, J.; Möller, E.; Friedrich, A.; Beuermann, S.; Taubert, A. Surprisingly high, bulk liquid-like mobility of silica-confined ionic liquids. Phys. Chem. Chem. Phys. 2009, 11, 3653–3662. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.P.; Singh, R.K.; Chandra, S. Thermal stability of ionic liquid in confined geometry. J. Phys. D Appl. Phys. 2010, 43, 092001. [Google Scholar] [CrossRef]
- Göbel, R.; Friedrich, A.; Taubert, A. Tuning the phase behavior of ionic liquids in organically functionalized silica ionogels. Dalton Trans. 2010, 39, 603–611. [Google Scholar] [CrossRef]
- Reinert, L.; Batouche, K.; Lévêque, J.-M.; Muller, F.; Bény, J.-M.; Kebabi, B.; Duclaux, L. Adsorption of imidazolium and pyridinium ionic liquids onto montmorillonite: Characterisation and thermodynamic calculations. Chem. Eng. J. 2012, 209, 13–19. [Google Scholar] [CrossRef]
- Askalany, A.; Olkis, C.; Bramanti, E.; Lapshin, D.; Calabrese, L.; Proverbio, E.; Freni, A.; Santori, G. Silica-supported ionic liquids for heat-powered sorption desalination. ACS Appl. Mater. Interfaces 2019, 11, 36497–36505. [Google Scholar] [CrossRef]
- Trif, L.; Franguelli, F.P.; Lendvay, G.; Majzik, E.; Béres, K.; Bereczki, L.; Szilágyi, I.M.; Pawar, R.P.; Kótai, L. Thermal analysis of solvatomorphic decakis (dimethylammonium) dihydrogendodecatungstate hydrates. J. Therm. Anal. Calorim. 2021, 144, 81–90. [Google Scholar] [CrossRef]
- Zaharescu, M.; Predoana, L.; Pandele-Cusu, J. Thermal Analysis on Gels, Glasses, and Powders. In Handbook of Sol-Gel Science and Technology; Klein, L., Aparicio, M., Pandele-Cusu, J., Eds.; Springer: Cham, Sitzerland, 2018; pp. 1833–1867. [Google Scholar] [CrossRef]
- Cao, Y.; Mu, T. Comprehensive investigation on the thermal stability of 66 ionic liquids by thermogravimetric analysis. Ind. Eng. Chem. Res. 2014, 53, 8651–8664. [Google Scholar] [CrossRef]
- Hao, Y.; Peng, J.; Hu, S.; Li, J.; Zhai, M. Thermal decomposition of allyl-imidazolium-based ionic liquid studied by TGA–MS analysis and DFT calculations. Thermochim. Acta 2010, 501, 78–83. [Google Scholar] [CrossRef]
- Clough, M.T.; Geyer, K.; Hunt, P.A.; Mertes, J.; Welton, T. Thermal decomposition ofcarboxylate ionic liquids: Trends and mechanisms. Phys. Chem. Chem. Phys. 2013, 15, 20480–20495. [Google Scholar] [CrossRef]
- Dou, Q.; Liu, L.; Yang, B.; Lang, J.; Yan, X. Silica-grafted ionic liquids for revealing the respective charging behaviors of cations and anions in supercapacitors. Nat. Comm. 2017, 8, 2188. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Park, S.-W.; Park, D.-W. Silica grafted imidazolium-based ionic liquids: Efficient heterogeneous catalysts for chemical fixation of CO2 to a cyclic carbonate. Energy Environ. Sci. 2009, 2, 1286–1292. [Google Scholar] [CrossRef]
- Siewniak, A.; Forajter, A.; Szymańska, K. Mesoporous silica-supported ionic liquids as catalysts for styrene carbonate synthesis from CO2. Catalysts 2020, 10, 1363. [Google Scholar] [CrossRef]
- Shi, T.; Livi, S.; Duchet, J.; Gérard, J.-F. Ionic liquids-containing silica microcapsules: A potential tunable platform for shaping-up epoxy-based composite materials? Nanomaterials 2020, 10, 881. [Google Scholar] [CrossRef]
- Mohamedali, M.; Ibrahim, H.; Henni, A. Imidazolium based ionic liquids confined into mesoporous silica MCM-41 and SBA-15 for carbon dioxide capture. Micropor. Mesopor. Mater. 2020, 294, 109916. [Google Scholar] [CrossRef]
- Shi, F.; Zhang, Q.; Li, D.; Deng, Y. Silica-gel-confined ionic liquids: A new attempt for the development of supported nanoliquid catalysis. Chem. Eur. J. 2005, 11, 5279–5288. [Google Scholar] [CrossRef]
- Qian, C.; Yao, C.; Yang, L.; Yang, B.; Liu, S.; Liu, Z. Preparation and application of silica films supported imidazolium-based ionic liquid as efficient and recyclable catalysts for benzoin condensations. Catal. Lett. 2020, 150, 1389–1396. [Google Scholar] [CrossRef]
- Vioux, A.; Viau, L.; Volland, S.; Le Bideou, J. Use of ionic liquids in sol-gel; ionogels and applications. Comptes Rendus Chim. 2010, 13, 242–255. [Google Scholar] [CrossRef]
- Le Bideau, J.; Viau, L.; Vioux, A. Ionogels, ionic liquid based hybrid materials. Chem. Soc. Rev. 2011, 20, 907–925. [Google Scholar] [CrossRef]
- Hesemann, P.; Viau, L.; Vioux, A. Silica Ionogels and Ionosilicas. In The Sol-Gel Handbook; Levy, D., Zayat, M., Eds.; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2015. [Google Scholar] [CrossRef]
- Andrzejewska, E.; Marcinkowska, A.; Zgrzeba, A. Ionogels—Materials containing immobilized ionic liquids. Polimery 2017, 62, 344–352. [Google Scholar] [CrossRef]
- Guo, L.Y.; Shi, J.; He, J.H.; Huang, J.Y.; Huang, P.C. Synthesis and characterization of supported on silica based ionic liquids. Appl. Mech. Mater. 2015, 727, 34–37. [Google Scholar] [CrossRef]
- Chen, X.; Put, B.; Sagara, A.; Gandrud, K.; Murata, M.; Steele, J.A.; Yabe, H.; Hantschel, T.; Roeffaers, M.; Tomiyama, M.; et al. Silica gel solid nanocomposite electrolytes with interfacial conductivity promotion exceeding the bulk Li-ion conductivity of the ionic liquid electrolyte filler. Sci. Adv. 2020, 6, eaav3400. [Google Scholar] [CrossRef] [PubMed]
- Almásy, L. New measurement control software on the Yellow Submarine SANS instrument at the Budapest Neutron Centre. J. Surf. Investig. X-ray Synchrotron Neutron Tech. 2021, 15, 527–531. [Google Scholar] [CrossRef]
- Al-Oweini, R.; El-Rassy, H. Synthesis and characterization by FTIR spectroscopy of silica aerogels prepared using several Si(OR)4 and R”Si(OR’)3 precursors. J. Mol. Struct. 2009, 919, 140–145. [Google Scholar] [CrossRef]
- Innocenzi, P. Infrared spectroscopy of sol–gel derived silica-based films: A spectra-microstructure overview. J. Non-Cryst. Solids 2003, 316, 309–319. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts, 3rd ed.; Socrates, G., Ed.; J. Wiley and Sons: Chichester, UK, 2004; ISBN 978-0-470-09307-8. [Google Scholar]
- Lenza, R.F.S.; Vasconcelos, W.L. Preparation of silica by sol-gel method using formamide. Mater. Res. 2001, 4, 189–194. [Google Scholar] [CrossRef]
- Icopini, G.A.; Brantley, S.L.; Heaney, P.J. Kinetics of silica oligomerization and nanocolloid formation as a function of pH and ionic strength at 25 °C. Geochim. Cosmochim. Acta 2005, 69, 293–303. [Google Scholar] [CrossRef]
- Gopal, N.O.; Narasimhulu, K.V.; Rao, J.L. EPR, Optical, infrared and Raman spectral studies of actinolite mineral. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2004, 60, 2441–2448. [Google Scholar] [CrossRef] [PubMed]
- Gunzler, H.; Gremlich, H.U. IR Spectroscopy: An Introduction; Wiley-VCH: Weinheim, Germany, 2004. [Google Scholar]
- Stuart, B.H. Infrared Spectroscopy: Fundamentals and Applications, Book Series: Analytical Techniques in the Sciences; Ando, D.J., Ed.; John Wiley and Sons, Ltd.: Chichester, UK, 2005. [Google Scholar]
- Shi, F.; Deng, Y. Abnormal FT-IR and FTRaman spectra of ionic liquids confined in nano-porous silica gel. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2005, 62, 239–244. [Google Scholar] [CrossRef]
- Warring, S.L.; Beattie, D.A.; McQuillan, A.J. Surficial siloxane-to-silanol interconversion during room-temperature hydration/dehydration of amorphous silica films observed by ATR-IR and TIR-Raman spectroscopy. Langmuir 2016, 32, 1568–1576. [Google Scholar] [CrossRef]
- Jitianu, A.; Crisan, M.; Meghea, A.; Rau, I.; Zaharescu, M. Influence of the silica based matrix on the formation of iron oxide nanoparticles in the Fe2O3–SiO2 system, obtained by sol–gel method. J. Mater. Chem. 2002, 12, 1401–1407. [Google Scholar] [CrossRef]
- Jin, Y.; Wang, P.; Yin, D.; Liu, J.; Qin, L.; Yu, N.; Xie, G.; Li, B. Gold nanoparticles prepared by sonochemical method in thiol-functionalized ionic liquid. Colloids Surf. A Physicochem. Eng. Asp. 2007, 302, 366–370. [Google Scholar] [CrossRef]
- Damlin, P.; Kvarnström, C.; Ivaska, A. Electrochemical synthesis and in situ spectroelectrochemical characterization of poly(3,4-ethylenedioxythiophene) (PEDOT) in room temperature ionic liquids. J. Electroanal. Chem. 2004, 570, 113–122. [Google Scholar] [CrossRef]
- Gubanova, N.N.; Baranchikov, A.Y.; Kopitsa, G.P.; Almásy, L.; Angelov, B.; Yapryntsev, A.D.; Rosta, L.; Ivanov, V.K. Combined SANS and SAXS study of the action of ultrasound on the structure of amorphous zirconia gels. Ultrason. Sonochem. 2015, 24, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Tomchuk, O.V.; Avdeev, M.V.; Ivankov, O.I.; Bulavin, L.A.; Aksenov, V.L. Features of colloidal aggregation in tetraethoxysilane-water-ethanol ternary mixtures by small-angle neutron scattering. J. Surf. Investig. X-Ray Synchrotron Neutron Tech. 2019, 13, 1122–1125. [Google Scholar] [CrossRef]
- Tomchuk, O.V.; Bulavin, L.A.; Pipich, V.; Ryukhtin, V.; Ivankov, O.I.; Aksenov, V.L.; Avdeev, M.V. Fractal aggregation in silica sols in basic tetraethoxysilane/ethanol/water solutions by small-angle neutron scattering. J. Mol. Liq. 2020, 304, 112736. [Google Scholar] [CrossRef]
- Fagadar-Cosma, E.; Dudás, Z.; Birdeanu, M.; Almásy, L. Hybrid organic — silica nanomaterials based on novel A3B mixed substituted porphyrin. Mater. Chem. Phys. 2014, 148, 143–152. [Google Scholar] [CrossRef]
- Lázár, I.; Forgács, A.; Horváth, A.; Király, G.; Nagy, G.; Len, A.; Dudás, Z.; Papp, V.; Balogh, Z.; Moldován, K.; et al. Mechanism of hydration of biocompatible silica-casein aerogels probed by NMR and SANS reveal backbone rigidity. Appl. Surf. Sci. 2020, 531, 147232. [Google Scholar] [CrossRef]
- Margaca, F.M.A.; Miranda Salvado, I.M.; Teixeira, J. Small angle neutron scattering study of silica gels: Influence of pH. J. Non-Cryst. Solids 1999, 258, 70–77. [Google Scholar] [CrossRef]
- Wu, C.-M.; Lin, S.-Y. Preparation and fractal-structure characterization of monolithic silica aerogel with a short-chain Ionic liquid as the solvent. Trans. Mater. Res. Soc. Jpn. 2012, 37, 123–126. [Google Scholar] [CrossRef][Green Version]
- Dudás, Z.; Fagadar-Cosma, E.; Len, A.; Románszki, L.; Almásy, L.; Vlad-Oros, B.; Dascalu, D.; Krajnc, A.; Kriechbaum, M.; Kuncser, A. Improved optical and morphological properties of vinyl-substituted hybrid silica materials incorporating a Zn-metalloporphyrin. Materials 2018, 11, 565. [Google Scholar] [CrossRef]
- Becauge, G. Small-angle scattering from polymeric mass fractals of arbitrary mass-fractal dimension. J. Appl. Cryst. 1996, 29, 134–146. [Google Scholar] [CrossRef]
- Chal, B.; Roiban, L.; Masenelli-Varlot, K.; Baeza, G.P.; Yrieix, B.; Foray, G. 3D multi-scale quantification of industrially relevant ultra-porous silicas by low-dose electron tomography combined with SANS. J. Non-Cryst. Solids 2021, 557, 120577. [Google Scholar] [CrossRef]
- Teixeira, J. Small-angle scattering by fractal systems. J. Appl. Cryst. 1988, 21, 781–785. [Google Scholar] [CrossRef]
- Karout, A.; Pierre, A.C. Porous texture of silica aerogels made with ionic liquids as gelation catalysts. J. Sol-Gel Sci. Technol. 2009, 49, 364–372. [Google Scholar] [CrossRef]
- Karout, A.; Pierre, A.C. Silica gelation catalysis by ionic liquids. Catal. Commun. 2009, 10, 359–361. [Google Scholar] [CrossRef]
- Fox, D.M.; Awad, W.H.; Gilman, J.W.; Maupin, P.H.; De Long, H.C.; Trulove, P.C. Flammability, thermal stability, and phase change characteristics of several trialkylimidazolium salts. Green Chem. 2003, 5, 724–727. [Google Scholar] [CrossRef]
- Kosmulski, M.; Gustafsson, J.; Rosenholm, J.B. Thermal stability of low temperature ionic liquids revisited. Thermochim. Acta 2004, 412, 47–53. [Google Scholar] [CrossRef]
- Song, Y.; Liu, L.; Zhu, X.; Wang, X.; Jia, H.; Xiao, X.; Yu, H.; Yang, X. Physicochemical properties of ionic liquids based on imidazolium/pyrrolidinium cations and maleate/phthalate anions. Solid State Ion. 2008, 179, 516–521. [Google Scholar] [CrossRef]
- Kärnä, M.; Lahtinen, M.; Valkonen, J. Preparation and characterization of new low melting ammonium-based ionic liquids with ether functionality. J. Mol. Struct. 2009, 922, 64–76. [Google Scholar] [CrossRef]
- Valkenburg, M.E.V.; Vaughn, R.L.; Williams, M.; Wilkes, J.S. Thermochemistry of ionic liquid heat-transfer fluids. Thermochim. Acta 2005, 425, 181–188. [Google Scholar] [CrossRef]
- Del Sesto, R.E.; McCleskey, T.M.; Macomber, C.; Ott, K.C.; Koppisch, A.T.; Baker, G.A.; Burrell, A.K. Limited thermal stability of imidazolium and pyrrolidinium ionic liquids. Thermochim. Acta 2009, 491, 118–120. [Google Scholar] [CrossRef]
- Ngo, H.; LeCompte, K.; Hargens, L.; McEwen, A. Thermal properties of imidazolium ionic liquids. Thermochim. Acta 2000, 357, 97–102. [Google Scholar] [CrossRef]
- Ozdemir, S.; Varlikli, C.; Oner, I.; Ocakoglu, K.; Icli, S. The synthesis of 1,8-naphthalimide groups containing imidazolium salts/ionic liquids using I−, PF6−, TFSI− anions and their photophysical, electrochemical and thermal properties. Dyes Pigm. 2010, 86, 206–216. [Google Scholar] [CrossRef]
- Hsieh, Y.N.; Kuei, C.H.; Chou, Y.K.; Liu, C.C.; Leu, K.L.; Yang, T.H.; Wang, M.Y.; Ho, W.Y. Facile synthesis of polymerized ionic liquids with high thermal stability. Tetrahedron Lett. 2010, 51, 3666–3669. [Google Scholar] [CrossRef]
- Almásy, L.; Turmine, M.; Perera, A. Structure of aqueous solutions of ionic liquid 1-butyl-3-methylimidazolium tetrafluoroborate by small-angle neutron scattering. J. Phys. Chem. B 2008, 112, 2382–2387. [Google Scholar] [CrossRef]
- Bowers, J.; Butts, C.P.; Martin, P.J.; Vergara-Gutierrez, M.C.; Heenan, R.K. Aggregation behavior of aqueous solutions of ionic liquids. Langmuir 2004, 20, 2191–2198. [Google Scholar] [CrossRef] [PubMed]
- Sastry, N.V.; Vaghela, N.M.; Macwan, P.M.; Soni, S.S.; Aswal, V.K.; Gibaud, A. Aggregation behavior of pyridinium based ionic liquids in water—surface tension, 1H NMR chemical shifts, SANS and SAXS measurements. J. Colloid Interface Sci. 2012, 371, 52–61. [Google Scholar] [CrossRef] [PubMed]
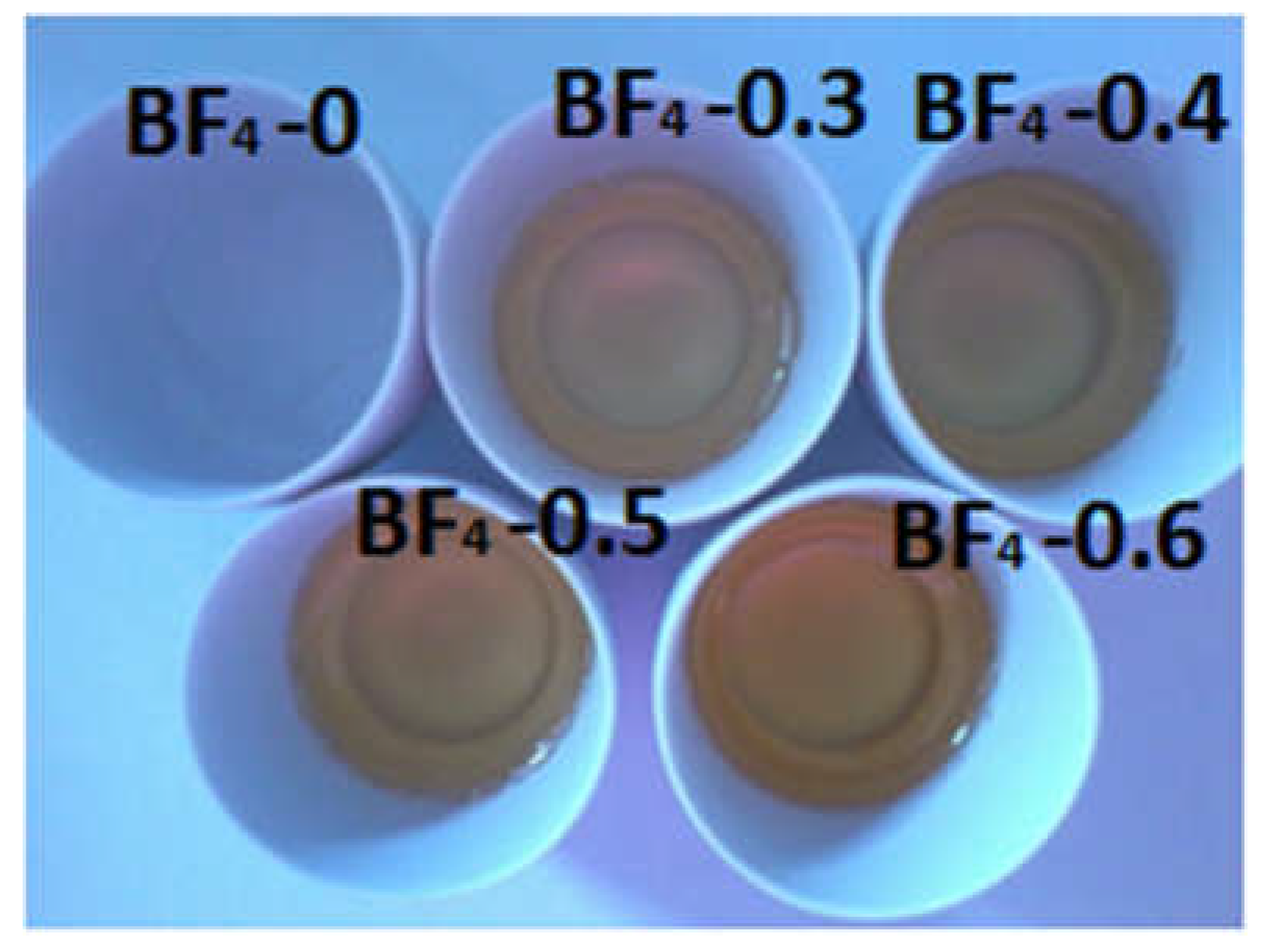

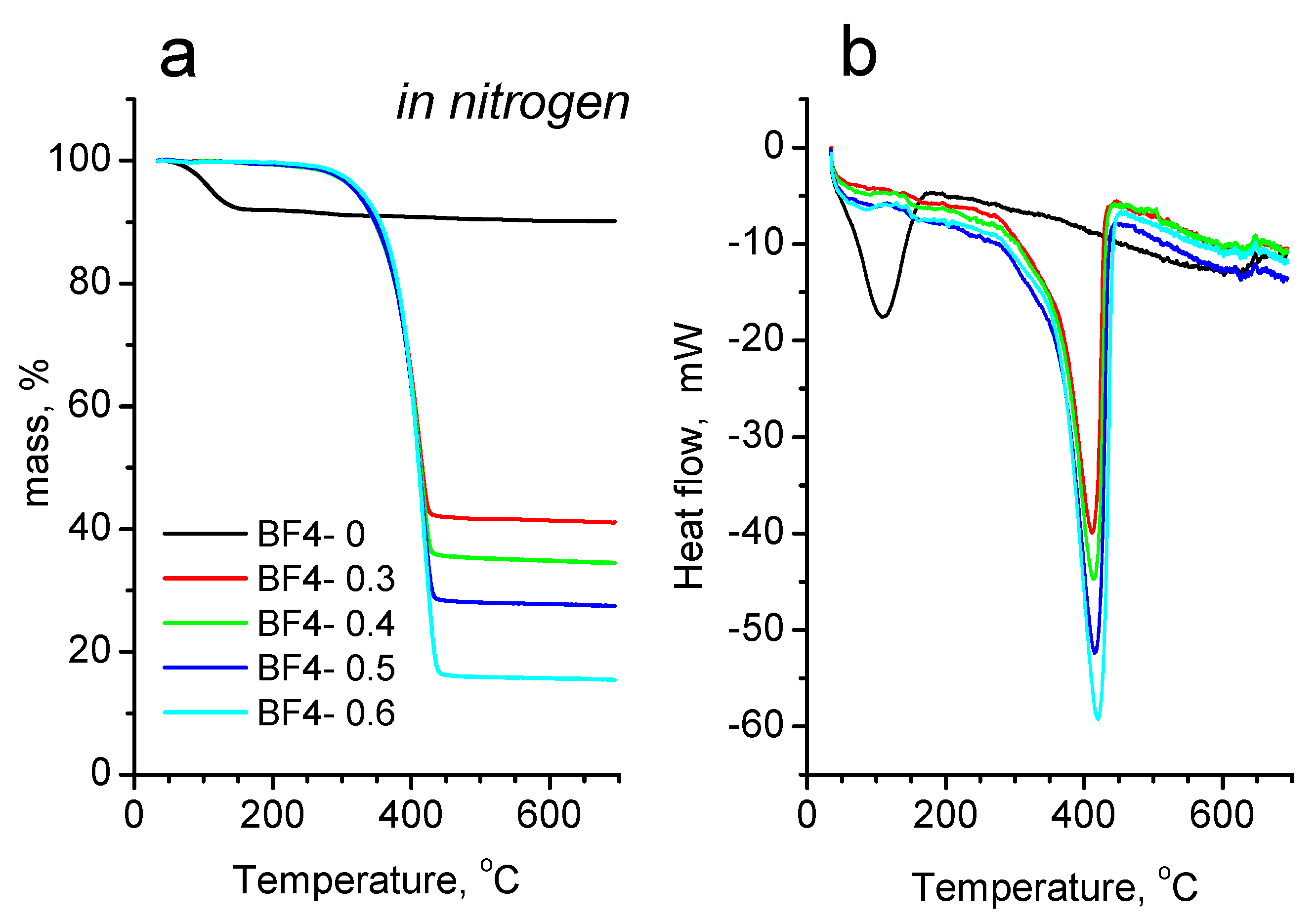
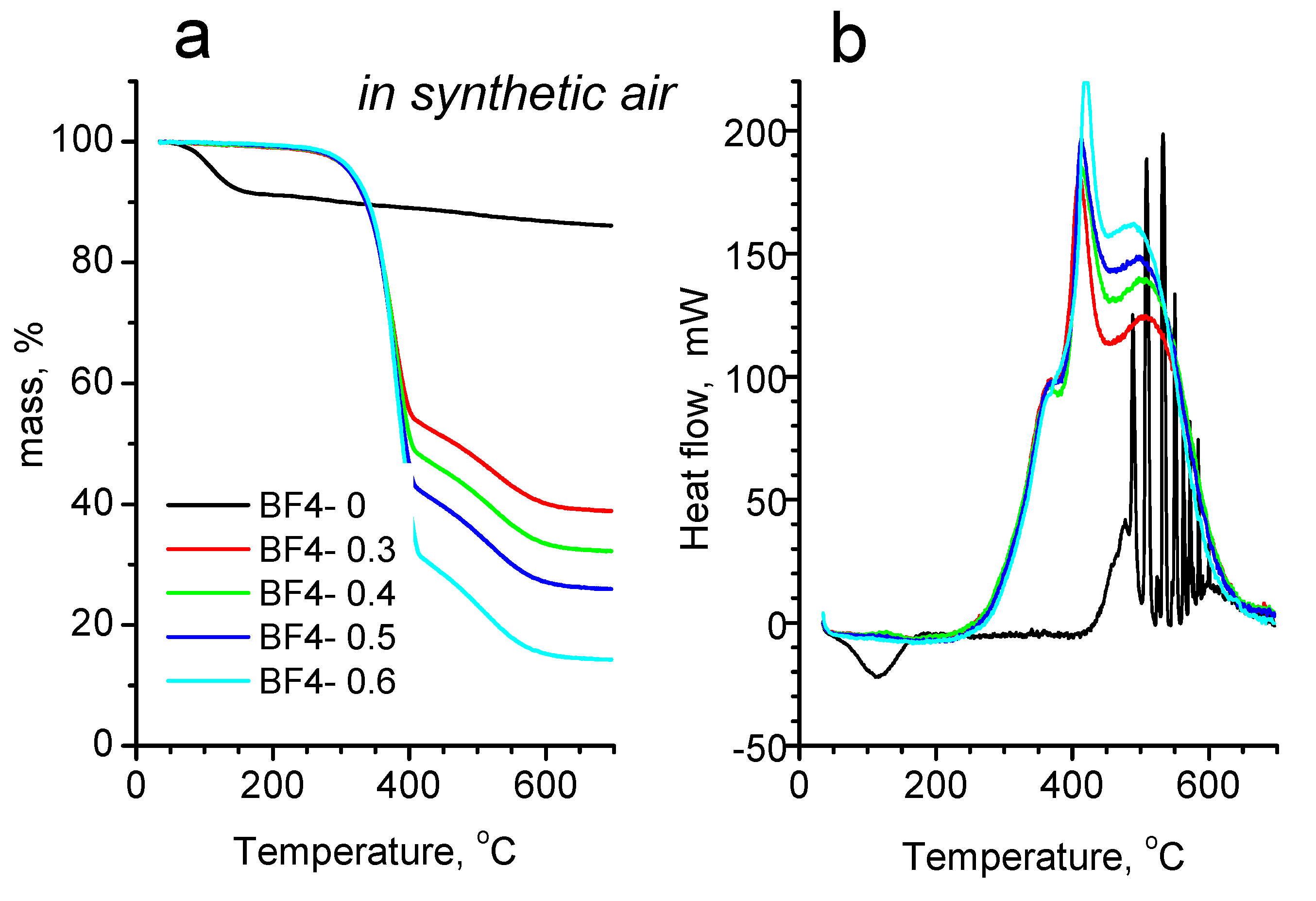
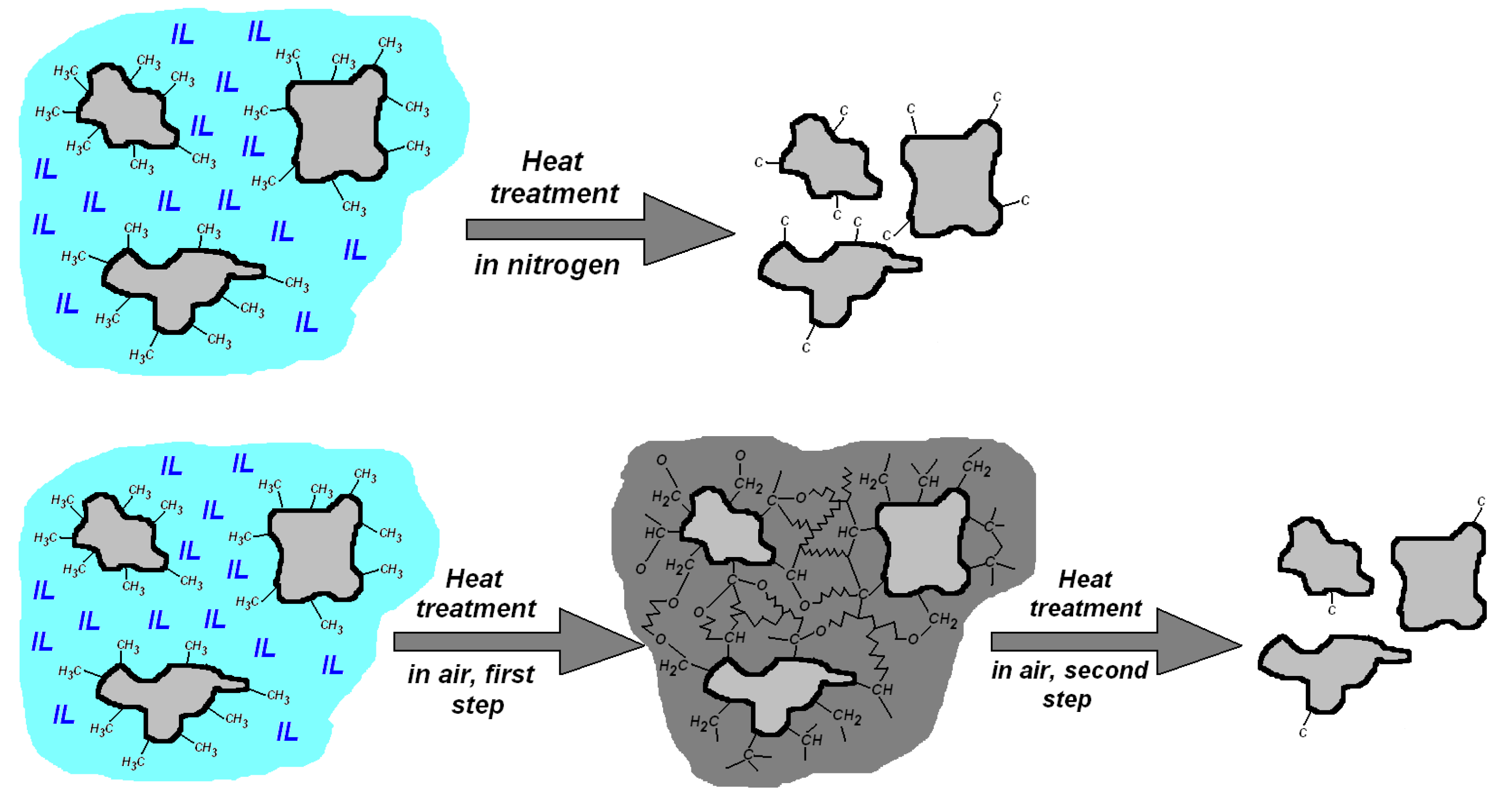

| Sample | IL/Si Molar Ratio | IL [g] | TMOS [g] | MTMS [g] |
|---|---|---|---|---|
| BF4-0 | 0 | 0 | 4.95 | 0.55 |
| BF4-0.3 | 0.3 | 2.60 | 4.95 | 0.55 |
| BF4-0.4 | 0.4 | 3.46 | 4.95 | 0.55 |
| BF4-0.5 | 0.5 | 4.33 | 4.95 | 0.55 |
| BF4-0.6 | 0.6 | 5.20 | 4.95 | 0.55 |
| Band Positions | Assignments | |
|---|---|---|
| BF4-0.3, BF4-0.6 (Xerogels) | BF4-0.3, BF4-0.6 (Heated Samples) | |
| 3479 cm−1, 3553 cm−1 | O-H stretching bands of hydrogen-bonded water molecules and SiO-H stretching of surface silanols hydrogen-bonded to molecular water (SiO-H…H2O) [35]. | |
| 3194–3081 cm−1 | Changes with thermal treatment. Intense in xerogels and decreases in the heated samples. | OH stretching vibrations [36]. |
| 2965–2977 cm−1 and 2878–2879 cm−1 | Disappear after thermal treatment. | Stretching vibrations of alkyl chains [41]. |
| 2324–1981 cm−1 | 2324–1981 cm−1 | Vibrations of organic residue, molecular water and silica network [38]. |
| 1636–1637 cm−1 | - | The adsorbed water deformation vibration [35,36,37,38]. |
| 1635 cm−1 | 1635 cm−1 | Vibrations of SiO2 network and molecular water [38]. |
| 1277–1279 cm−1 | Disappear after thermal treatment. | Symmetric CH3 bending band from Si–CH3 [37,42]. |
| 1057–1024 cm−1 | Shifted to higher wave numbers after calcination, due to elimination of the IL and strengthening of the silica matrix [26]. | Si-O-Si asymmetric stretching vibrations [35]. |
| 1507 cm−1 and 1468 cm−1 | Shifted to 1383 cm−1. | Pyridinium group vibrations [46].The band at 1383 cm−1 is present also in the bulk IL and is generally assigned to stretching vibrations of C-H bond [41] and C-C bond [47]. This shows that some IL still remains entrapped in the silica matrix, after the one hour thermal treatment. |
| 1468 cm−1 | Disappear after thermal treatment. | C-H stretching vibrations of the alkyl chain [14]. |
| 800 cm−1 | 800 cm−1 | Symmetric stretching vibrations of Si-O-Si [35,40]. |
| 765 cm−1 | - | Characteristic band of BF4 group [43] (overlaps with the symmetric stretching vibrations of Si-O-Si appearing at 800 cm−1 and 680 cm−1). |
| 687 cm−1 | Almost disappears since the CH3 groups from the silica matrix were also eliminated. | Symmetric stretching vibration of the C-Si bond (reported at 676 cm−1 in [45]). |
| 680 cm−1 | 680 cm−1 | Symmetric stretching vibrations of Si-O-Si [35,40]. |
| Sample | Specific Surface Area [m2/g] | Mean Pore Width [nm] | Total Pore Volume [cm3/g] |
|---|---|---|---|
| BF4-0 | 206 | 2.4 | 1.02 × 10−1 |
| BF4-0.3 | 11.3 | 6.1 | 2.2 × 10−2 |
| BF4-0.4 | 4.1 | 7.6 | 5.0 × 10−3 |
| BF4-0.5 | 3.2 | 5.3 | 4.5 × 10−3 |
| BF4-0.6 | 1.1 | 2.1 | 6.9 ×10−3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Putz, A.-M.; Almásy, L.; Horváth, Z.E.; Trif, L. Butyl-Methyl-Pyridinium Tetrafluoroborate Confined in Mesoporous Silica Xerogels: Thermal Behaviour and Matrix-Template Interaction. Materials 2021, 14, 4918. https://doi.org/10.3390/ma14174918
Putz A-M, Almásy L, Horváth ZE, Trif L. Butyl-Methyl-Pyridinium Tetrafluoroborate Confined in Mesoporous Silica Xerogels: Thermal Behaviour and Matrix-Template Interaction. Materials. 2021; 14(17):4918. https://doi.org/10.3390/ma14174918
Chicago/Turabian StylePutz, Ana-Maria, László Almásy, Zsolt Endre Horváth, and László Trif. 2021. "Butyl-Methyl-Pyridinium Tetrafluoroborate Confined in Mesoporous Silica Xerogels: Thermal Behaviour and Matrix-Template Interaction" Materials 14, no. 17: 4918. https://doi.org/10.3390/ma14174918
APA StylePutz, A.-M., Almásy, L., Horváth, Z. E., & Trif, L. (2021). Butyl-Methyl-Pyridinium Tetrafluoroborate Confined in Mesoporous Silica Xerogels: Thermal Behaviour and Matrix-Template Interaction. Materials, 14(17), 4918. https://doi.org/10.3390/ma14174918








