Electroless Plating of High-Performance Composite Pd Membranes with EDTA-Free Bath
Abstract
:1. Introduction
2. Materials and Methods
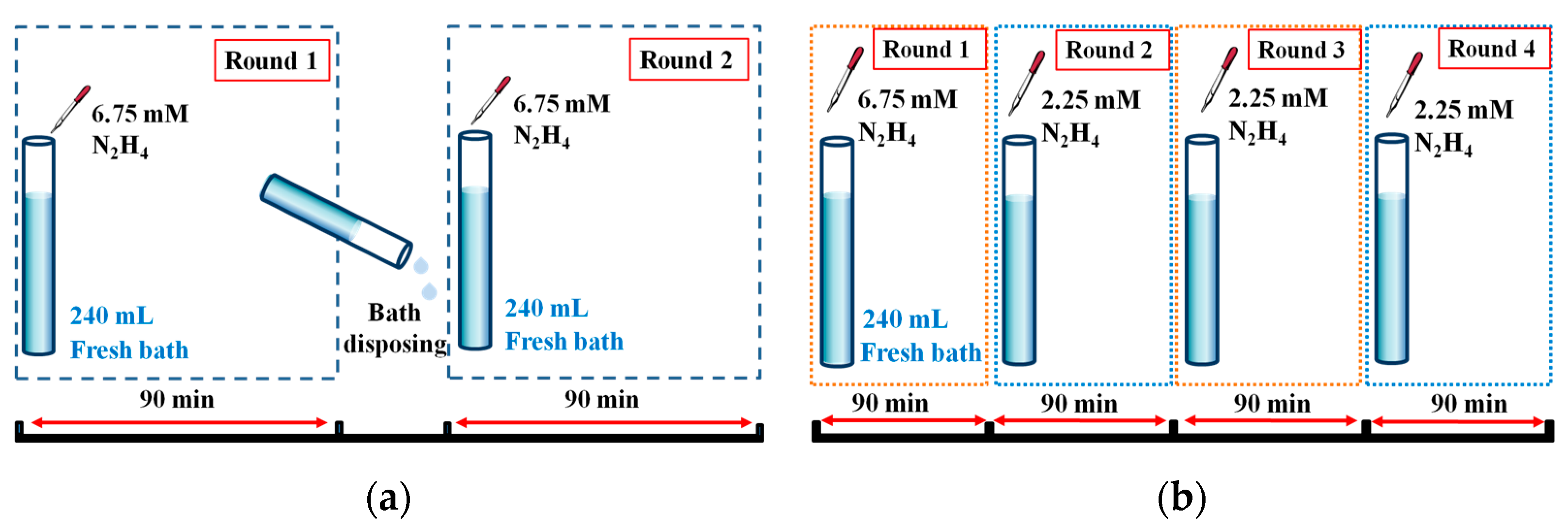
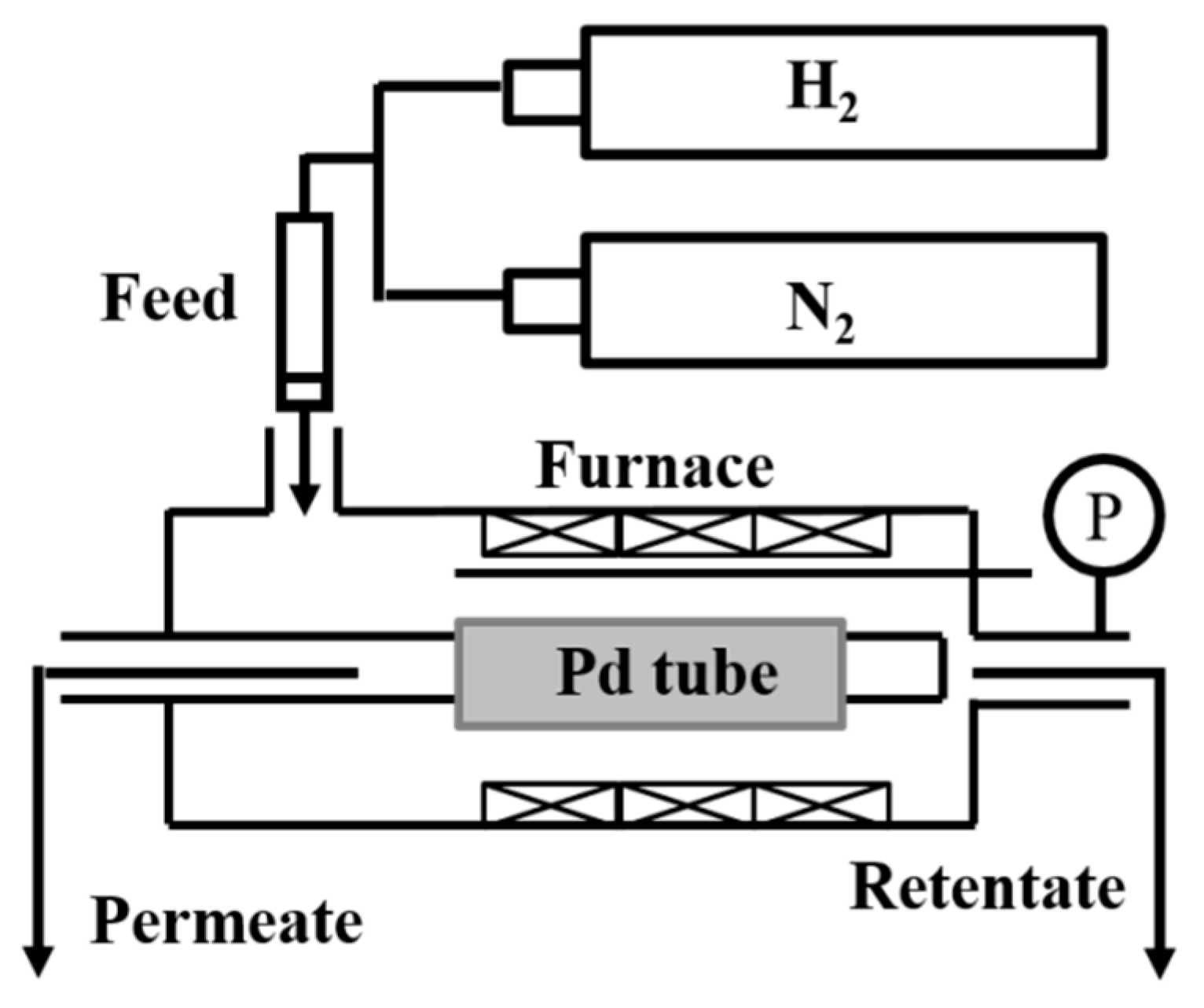
2.1. Membrane Preparation
2.2. Characterization
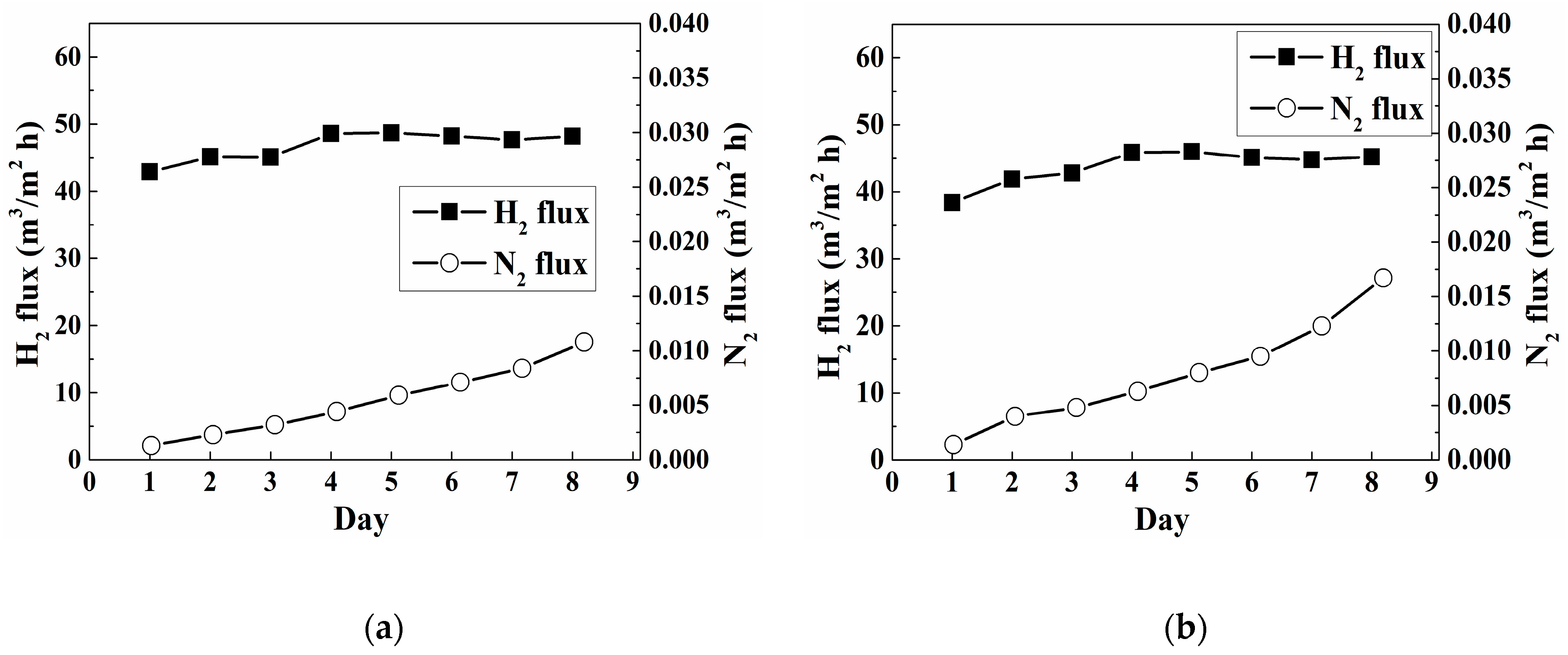
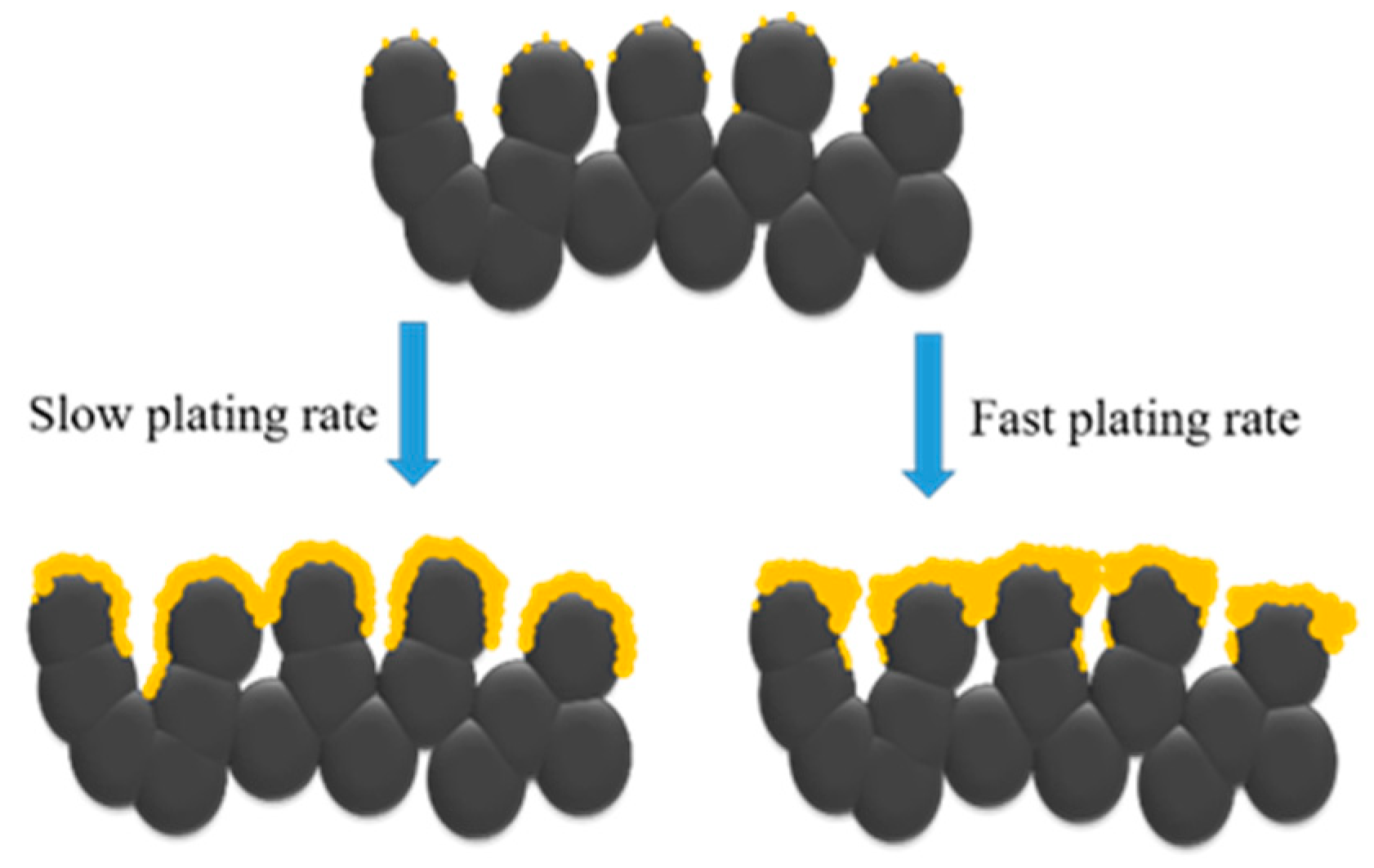
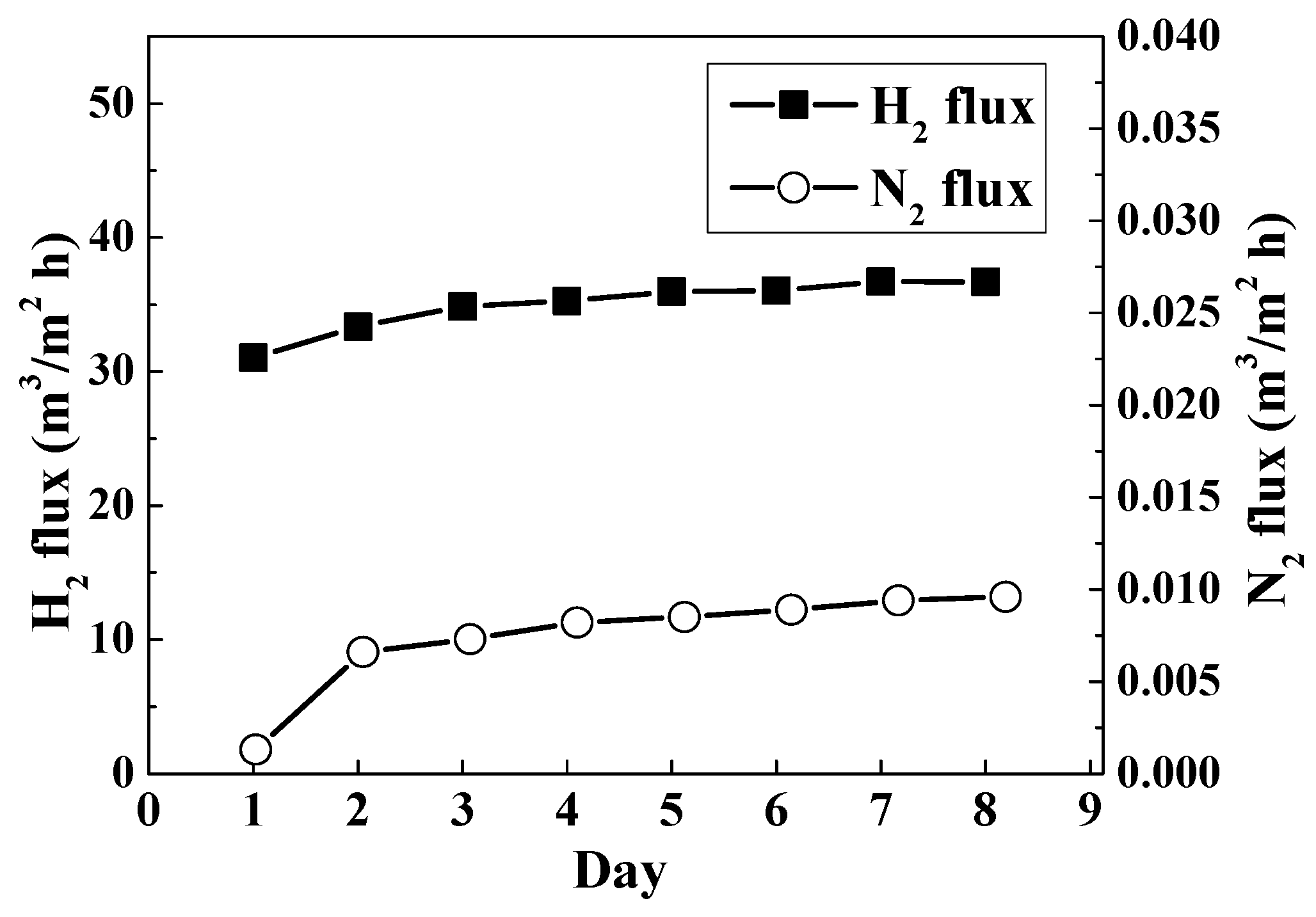
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Solomon, B.D.; Banerjee, A. A global survey of hydrogen energy research, development and policy. Energy Policy 2006, 34, 781–792. [Google Scholar] [CrossRef]
- Kalbasi, R.; Jahangiri, M.; Tahmasebi, A. Comprehensive Investigation of Solar-Based Hydrogen and Electricity Production in Iran. Int. J. Photoenery 2021, 2021, 6627491. [Google Scholar] [CrossRef]
- Calado, G.; Castro, R. Hydrogen production from offshore wind parks: Current situation and future perspectives. Appl. Sci. 2021, 11, 5561. [Google Scholar] [CrossRef]
- El-Emam, R.S.; Ozcan, H. Comprehensive review on the techno-economics of sustainable large-scale clean hydrogen production. J. Clean. Prod. 2019, 220, 593–609. [Google Scholar] [CrossRef]
- Wang, M.; Wang, G.; Sun, Z.; Zhang, Y.; Xu, D. Review of renewable energy-based hydrogen production processes for sustainable energy innovation. Glob. Energy Interconnect 2019, 2, 436–443. [Google Scholar] [CrossRef]
- International Energy Agency. Available online: https://www.iea.org/reports/the-future-of-hydrogen (accessed on 22 August 2021).
- Du, Z.; Liu, C.; Zhai, J.; Guo, X.; Xiong, Y.; Su, W.; He, G. A Review of Hydrogen Purification Technologies for Fuel Cell Vehicles. Catalysts 2021, 11, 393. [Google Scholar] [CrossRef]
- Shu, J.; Grandjean, B.P.A.; Vanneste, A.; Kaliaguine, S. Catalytic palladium-based membrane reactors: A review. Can. J. Chem. Eng. 1991, 69, 1036–1060. [Google Scholar] [CrossRef]
- Gallucci, F.; Fernandez, E.; Corengia, P.; Annaland, M.V.S. Recent advances on membranes and membrane reactors for hydrogen production. Chem. Eng. Sci. 2013, 92, 40–66. [Google Scholar] [CrossRef]
- Shu, J.; Grandjean, B.P.A.; Ghali, E.; Kaliaguine, S. Simultaneous deposition of Pd and Ag on porous stainless steel by electroless plating. J. Membr. Sci. 1993, 77, 181–195. [Google Scholar] [CrossRef]
- Uemiya, S.; Kude, Y.; Sugino, K.; Sato, N.; Matsuda, T.; Kikuchi, E. A palladium/porous-glass composite membrane for hydrogen separation. Chem. Lett. 1988, 10, 1687–1690. [Google Scholar] [CrossRef]
- Jayaraman, V.; Lin, Y.S.; Pakala, M.; Lin, R.Y. Fabrication of ultrathin metallic membranes on ceramic supports by sputter deposition. J. Membr. Sci. 1995, 99, 89–100. [Google Scholar] [CrossRef]
- Xomeritakis, G.; Lin, Y.S. CVD synthesis and gas permeation properties of thin palladium/alumina membranes. AIChE J. 1998, 44, 174–183. [Google Scholar] [CrossRef]
- Chen, S.C.; Tu, G.C.; Hung, C.C.Y.; Huang, C.A.; Rei, M.H. Preparation of palladium membrane by electroplating on AISI 316L porous stainless steel supports and its use for methanol steam reformer. J. Membr. Sci. 2008, 314, 5–14. [Google Scholar] [CrossRef]
- Mardilovich, P.P.; She, Y.; Ma, Y.H.; Rei, M.H. Defect-free palladium membranes on porous stainless-steel support. AlChE J. 1998, 44, 310–322. [Google Scholar] [CrossRef]
- Chi, Y.H.; Uan, J.Y.; Lin, M.C.; Lin, Y.L.; Huang, J.H. Preparation of a novel Pd/layered double hydroxide composite membrane for hydrogen filtration and characterization by thermal cycling. Int. J. Hydrogen Energy 2013, 38, 13734–13741. [Google Scholar] [CrossRef]
- Chi, Y.H.; Lin, J.J.; Lin, Y.L.; Yang, C.C.; Huang, J.H. Influence of the rotation rate of porous stainless steel tubes on electroless palladium deposition. J. Membr. Sci. 2015, 475, 259–265. [Google Scholar] [CrossRef]
- Martinez-Diaz, D.; Martínez del Monte, D.; García-Rojas, E.; Alique, D.; Calles, J.A.; Sanz, R. Comprehensive permeation analysis and mechanical resistance of electroless pore-plated Pd-membranes with ordered mesoporous ceria as intermediate layer. Sep. Purif. Technol. 2021, 258, 118066. [Google Scholar] [CrossRef]
- Martinez-Diaz, D.; Sanz, R.; Carrero, A.; Calles, J.A.; Alique, D. Effective H2 separation through electroless pore-plated Pd membranes containing graphite lead barriers. Membranes 2020, 10, 410. [Google Scholar] [CrossRef]
- Zhang, D.; Zhao, J.; Yang, P.; Chen, Y.; Fan, Y. Preparation of high stability Pd/Ceramic/Ti-Al alloy composite membranes by electroless plating. Front. Chem. 2020, 8, 202. [Google Scholar] [CrossRef] [Green Version]
- Volpe, M.; Inguanta, R.; Piazza, S.; Sunseri, C. Optimized bath for electroless deposition of palladium on amorphous alumina membranes. Surf. Coat. Technol. 2006, 200, 5800–5806. [Google Scholar] [CrossRef]
- Roa, F.; Way, J.D. The effect of air exposure on palladium–copper composite membranes. Appl. Surf. Sci. 2005, 240, 85–104. [Google Scholar] [CrossRef]
- Orakwe, I.; Shehu, I.; Gobina, E. Preparation and characterization of palladium ceramic alumina membrane for hydrogen permeation. Int. J. Hydrogen Energy 2019, 44, 9914–9921. [Google Scholar] [CrossRef]
- Gade, S.K.; Thoen, P.M.; Way, J.D. Unsupported palladium alloy foil membranes fabricated by electroless plating. J. Membr. Sci. 2008, 316, 112–118. [Google Scholar] [CrossRef]
- Ryi, S.K.; Xu, N.; Li, A.W.; Lim, C.J.; Grace, J.R. Electroless Pd membrane deposition on alumina modified porous Hastelloy substrate with EDTA-free bath. Int. J. Hydrogen Energy 2010, 35, 2328–2335. [Google Scholar] [CrossRef]
- Thoen, P.M.; Roa, F.; Way, J.D. High flux palladium–copper composite membranes for hydrogen separations. Desalination 2006, 193, 224–229. [Google Scholar] [CrossRef]
- Mardilovich, I.P.; Engwall, E.; Ma, Y.H. Dependence of hydrogen flux on the pore size and plating surface topology of asymmetric Pd-porous stainless steel membranes. Desalination 2002, 144, 85–89. [Google Scholar] [CrossRef]
- Yeung, K.L.; Christiansen, S.C.; Varma, A. Palladium composite membranes by electroless plating technique Relationships between plating kinetics, film microstructure and membrane performance. J. Membr. Sci. 1999, 159, 107–122. [Google Scholar] [CrossRef]
- Felix, E.M.; Muench, F.; Ensinger, W. Green plating of high aspect ratio gold nanotubes and their morphology-dependent performance in enzyme-free peroxide sensing. RSC Adv. 2014, 4, 24504–24510. [Google Scholar] [CrossRef]

| Components | Amount |
|---|---|
| PdCl2 | 3.2 g/L |
| HCl | 4 mL/L |
| NH4OH | 320 mL/L |
| N2H4 Conc. (mM) | Bath Volume (mL) | Plating Yield a (%) | Pd Thickness (μm) | H2 Permeance b (mol/m2 s Pa0.5) | N2 Flux c (mol/m2 s) | Selectivity d [H2/N2] |
|---|---|---|---|---|---|---|
| 4.5 | 120 | 33 | 2.2 | # e | 9.6 × 10−1 | # e |
| 4.5 | 180 | 31 | 3.0 | # e | 2.0 × 10−3 | # e |
| 4.5 | 240 | 33 | 4.3 | 5.9 × 10−3 | 8.5 × 10−5 | 1.1 × 102 |
| 6.75 | 120 | 55 | 3.6 | 6.8 × 10−3 | 1.9 × 10−3 | 1.7 × 102 |
| 6.75 | 180 | 51 | 5.0 | 4.4 × 10−3 | 6.0 × 10−6 | 1.6 × 104 |
| 6.75 | 240 | 46 | 6.0 | 3.4 × 10−3 | 1.3 × 10−5 | 5.9 × 103 |
| 9 | 120 | 86 | 5.5 | 3.4 × 10−3 | 5.8 × 10−5 | 8.6 × 102 |
| 9 | 180 | 60 | 5.8 | 3.1 × 10−3 | 9.6 × 10−6 | 5.0 × 103 |
| 9 | 240 | 47 | 6.1 | 3.3 × 10−3 | 7.2 × 10−6 | 8.1 × 103 |
| 13.5 | 120 | 93 | 6.0 | 3.3 × 10−3 | 2.2 × 10−5 | 1.3 × 103 |
| 13.5 | 180 | 66 | 6.4 | 2.9 × 10−3 | 2.4 × 10−6 | 1.8 × 104 |
| 13.5 | 240 | 56 | 7.2 | 2.6 × 10−3 | 4.8 × 10−6 | 1.1 × 104 |
| 18 | 120 | 96 | 6.2 | 2.8 × 10−3 | 1.7 × 10−3 | 83 |
| 18 | 180 | 84 | 8.2 | 2.2 × 10−3 | 1.2 × 10−4 | 3.0 × 102 |
| 18 | 240 | 68 | 8.9 | 2.0 × 10−3 | 3.8 × 10−5 | 1.5 × 103 |
| N2H4 Addition | Thickness | Yield | H2 Permeance | N2 Flux c | Selectivity c |
|---|---|---|---|---|---|
| μm | % | mol/m2 s Pa0.5 | mol/m2 s | H2/N2 | |
| One-Time a | 6.00 | 46 | 5.9 × 10−3 | 1.3 × 10−5 | 5.9 × 103 |
| Sequential b | 6.00 | 93 | 3.0 × 10−3 | 1.2 × 10−6 | 6.8 × 104 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.-Y.; Chi, Y.-H.; Huang, J.-H. Electroless Plating of High-Performance Composite Pd Membranes with EDTA-Free Bath. Materials 2021, 14, 4894. https://doi.org/10.3390/ma14174894
Wang J-Y, Chi Y-H, Huang J-H. Electroless Plating of High-Performance Composite Pd Membranes with EDTA-Free Bath. Materials. 2021; 14(17):4894. https://doi.org/10.3390/ma14174894
Chicago/Turabian StyleWang, Jun-Yi, Yen-Hsun Chi, and Jin-Hua Huang. 2021. "Electroless Plating of High-Performance Composite Pd Membranes with EDTA-Free Bath" Materials 14, no. 17: 4894. https://doi.org/10.3390/ma14174894
APA StyleWang, J.-Y., Chi, Y.-H., & Huang, J.-H. (2021). Electroless Plating of High-Performance Composite Pd Membranes with EDTA-Free Bath. Materials, 14(17), 4894. https://doi.org/10.3390/ma14174894





