Effects of Graphite on Electrically Conductive Cementitious Composite Properties: A Review
Abstract
:1. Introduction
2. Inherent Properties of Graphite
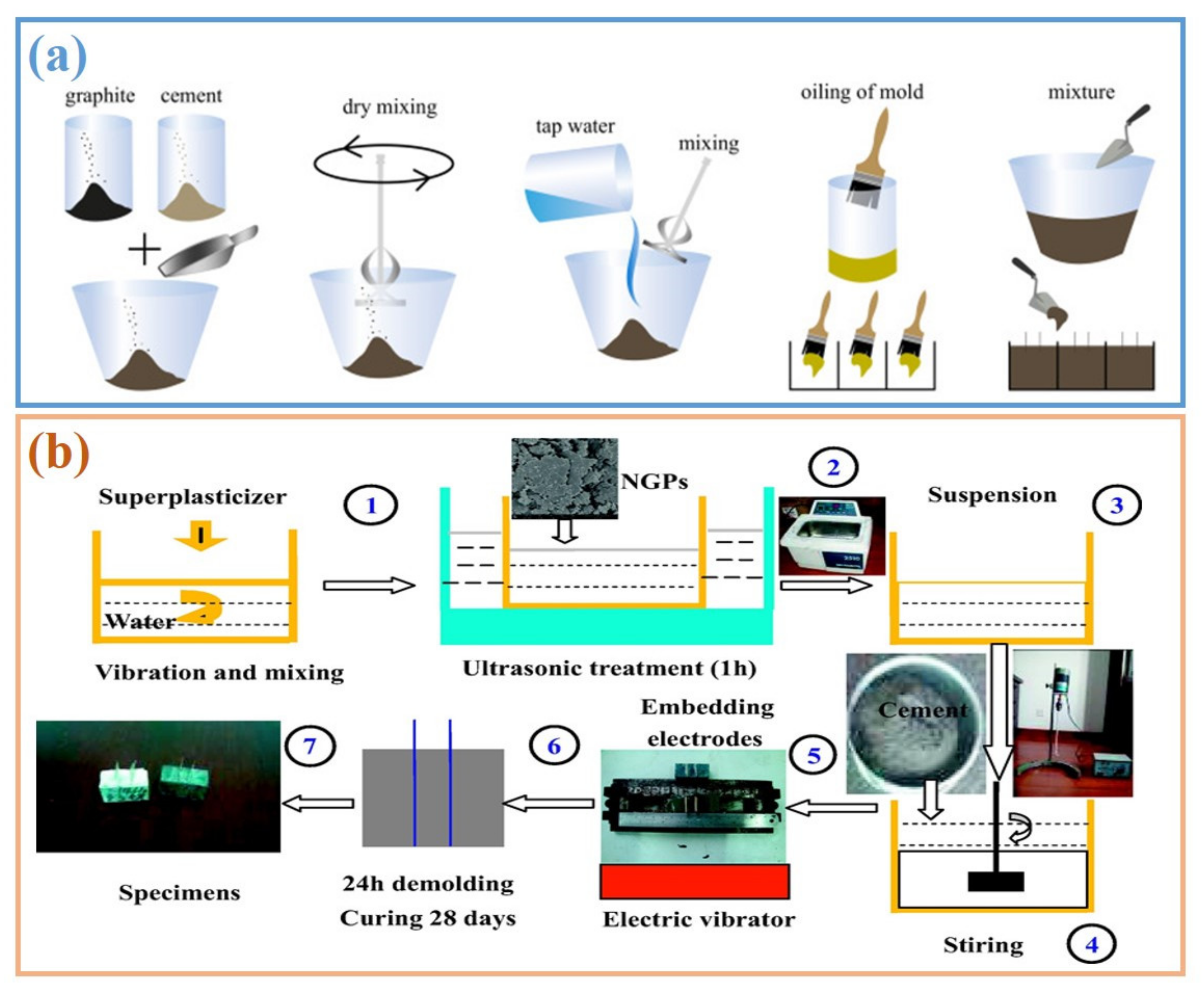
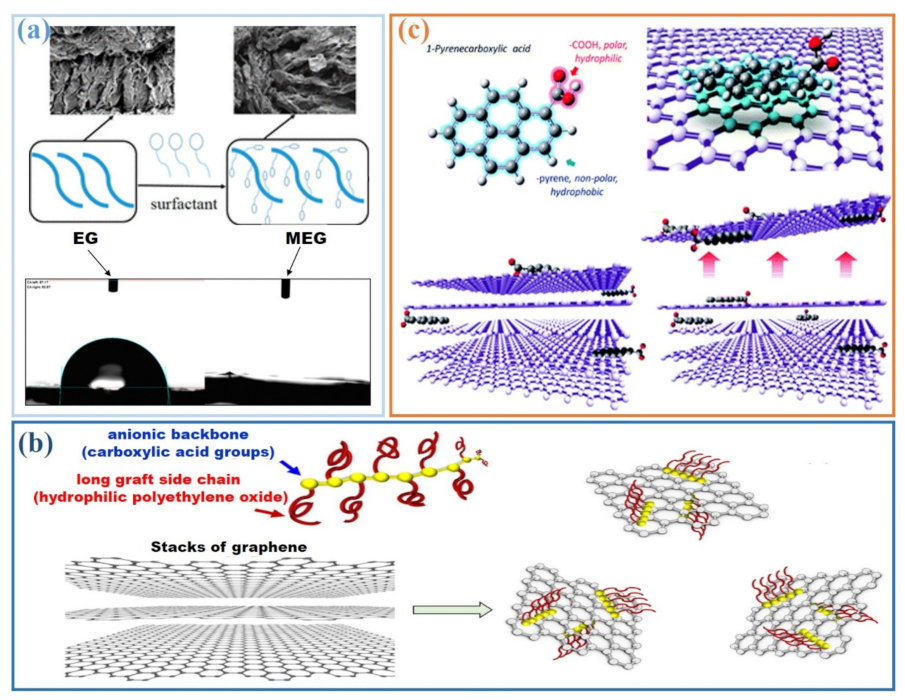
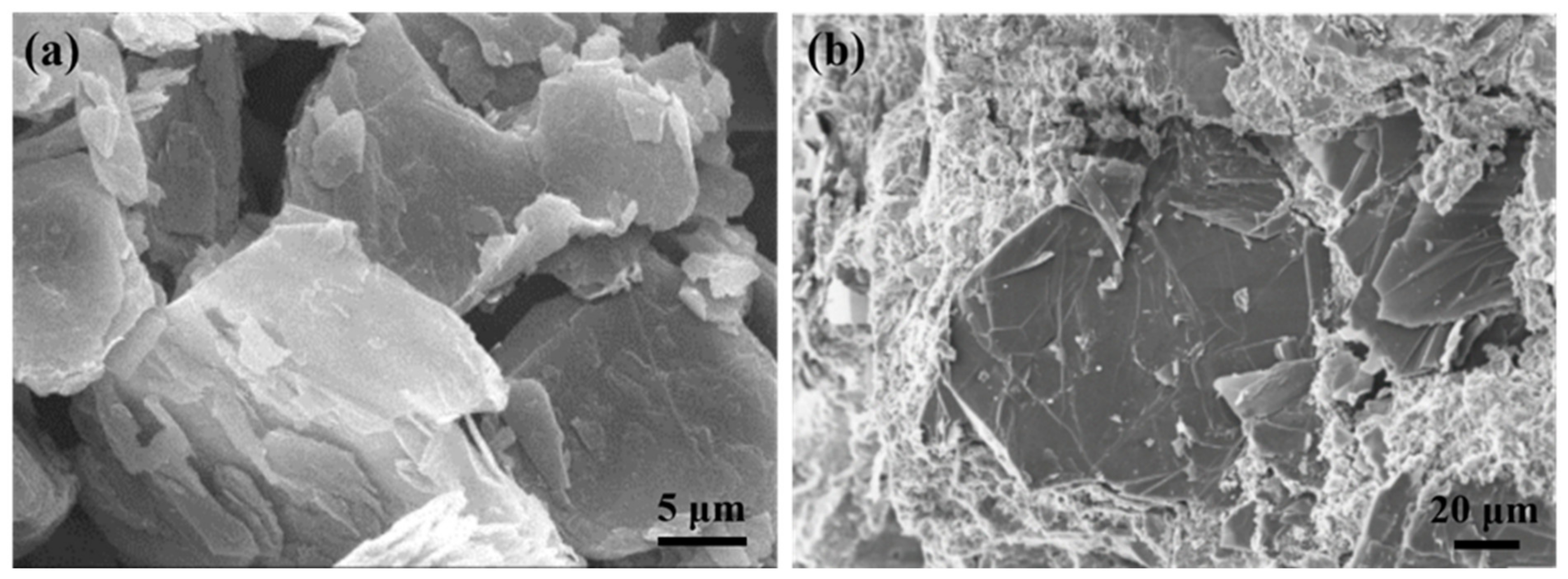
3. Dispersion of Graphite in Cement-Based Materials
4. Workability of Graphite in Cement-Based Materials
| Matrix | Graphite Content | w/c | Method | Changes in Fluidity/Slump | Refs. |
|---|---|---|---|---|---|
| Paste | 10, 20, 30 and 40 (wt%) a | 0.45 | Rheology measurement | Increasing graphite fineness led to a dramatic reduction in fluidity. Viscosity increased progressively as graphite content increased. | [3] |
| Concrete | 0.23, 0.68, 1.13, and 1.58 (vol%) b | 0.57 | Slump tests | The effect of graphite on slump increased as the replacement level increased. The use of 7 vol% replacement resulted in 33% reduction in slump. | [18] |
| Paste | 1, 2, 3 and 4 (wt%) a | 0.5 | Mini-cone test | Spread diameters decreased with increase in graphite addition. | [44] |
| Mortar | 0.01, 0.1, and 0.2 (wt%) a | 0.18 | Rheology measurement | Nano-graphite thickened the cementitious admixture. | [66] |
| Paste | 10, 15,20, and 30 (wt%) a | 0.4 | Flow diameter test | Cement paste flow diameter decreased from 25.5 cm to 9.5 cm after 30% graphite addition. | [67] |
| Mortar | 10, 20, 30 (wt%) a | 0.7 | Flow diameter test | The flow diameter of 25.4 cm for plain cement mortar reduced to 12.5 cm when graphite was increased to a 30% weight. | [67] |
| Mortar | Graphite nanoplatelets water paste | - | Rheology measurement | A higher shear stress to start flowing and a slightly higher plastic viscosity were observed. Workability decreased due to the reduction of free water in the paste and an increase in friction among the particles. | [68] |
5. Effect of Graphite on Cement Hydration
6. Effects of Graphite on Cementitious Composite Mechanical Performance
| Matrix | w/c | Graphite Size (μm) | Compressive Strength | Ref. | |
|---|---|---|---|---|---|
| Graphite Content | Increase/Reduction (%)/d | ||||
| Paste | 0.45 | 2000 | 10 (wt%) a | −47/2d, −39/7d, and −45/28d | [3] |
| 150 | −28/2d, −32/7d, and −36/28d | ||||
| 44 | −12/2d, −21/7d, and −12/28d | ||||
| 2000 | 20 (wt%) a | −51/2d, −50/7d, and −56/28d | |||
| 150 | −31/2d, −40/7d, and −42/28d | ||||
| 44 | −21/2d, −20/7d, and −35/28d | ||||
| Concrete | 0.57 | Few microns | 0.23 (vol%) b | −6/28d | [18] |
| 0.68 (vol%) | −17/28d | ||||
| 1.13 (vol%) | −22/28d | ||||
| 1.58 (vol%) | −30/28d | ||||
| Concrete | 0.38 | 30 | 2.5 (wt%) a | +10.1/28d | [30] |
| 5 (wt%) | +7.8/28d | ||||
| 7.5 (wt%) | +0.5/28d | ||||
| Paste | 0.53 | 12 (d90) | 5 (wt%) a | −28/21d | [64] |
| 0.55 | 10 (wt%) | −39/21d | |||
| 0.60 | 20 (wt%) | −61/21d | |||
| 0.65 | 30 (wt%) | −72/21d | |||
| 0.70 | 40 (wt%) | −83/21d | |||
| 0.75 | 50 (wt%) | −93/21d | |||
| 0.80 | 60 (wt%) | −95/21d | |||
| 0.85 | 70 (wt%) | −97/21d | |||
| 0.90 | 80 (wt%) | −98/21d | |||
| Concrete | 0.30 | 11 (d50) | 5 (wt%) a | −12/7d | [75] |
| 10 (wt%) | −38/7d | ||||
| 15 (wt%) | −49/7d | ||||
| 20 (wt%) | −52/7d | ||||
| Mortar | 0.4 | 30 | 0.5 (wt%) a | +1.2/28d | [76] |
| 1.0 (wt%) | −5.5/28d | ||||
| 2.0 (wt%) | −10.1/28d | ||||
| 3.0 (wt%) | −18.9/28d | ||||
| Concrete | 0.59 0.801.0 1.2 | 1–5000 | 5.0 (wt%) c 10 (wt%) 15 (wt%) 20 (wt%) | −82.5/28d −91.9/28d −96.2/28d −99.4/28d | [77] |
7. Effects of Graphite on Cementitious Composite Durability
8. Electrical Properties of Graphite-Based ECCCs
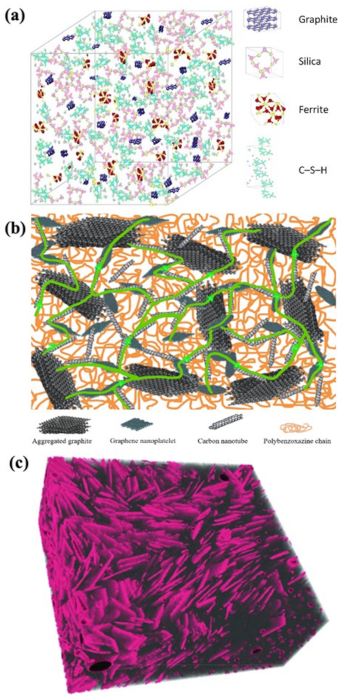
8.1. Conductive Mechanisms
- (1)
- Conductive pathway theory: when some conductive fillers are in contact with each other, the conductive pathway can be formed to allow current to pass through the cementitious matrix [90].
- (2)
- Tunnelling effect theory: in a cementitious matrix, partially conductive fillers are distributed in the form of isolated particles or small aggregates. When these isolated particles and small aggregates are surrounded by a thin layer of hydration products, the electrons can hop across the thin layer into adjacent conductive particles [91]. This phenomenon is the so-called tunnelling effect, where electrons can be activated by thermal vibration and electron transition.
- (3)
- Field emission theory: when there is a strong internal electric field among conductive fillers, an electric field emission current can be generated as electrons pass through the electronic barrier formed by the thin cementitious layer [92].
- (1)
- Through the cement-based matrix: the electrical transport behavior of the cement-based composites is mainly affected by cement matrix system when the conductive component is lower than the percolation threshold value [94]. Electrical resistance is closely related to water consumption. Han et al. [95] found that electrical resistance decreases as water content increases, thus enhancing ionic conduction and ultimately improving electrical conductivity. Frattini et al. [64] reported that hardened cement paste with a relatively low added graphite content behaves as an insulator; the order of magnitude of its conductivity is approximately 10−5 S/m.
- (2)
- Through composite conductive pathway: the composite conductive pathway is composed of a conductive component and cement matrix, so there is a synergic effect between the cementitious matrix and conductive fillers. The filler–matrix interface and C-S-H gel surface may be conductive insofar as improving the charge transfer mechanism [88]. Once graphite content reaches a certain level, the conductivity of GC composite pastes is of the order of magnitude from 10−5 to 1 S/m [64].
- (3)
- Through the conductive network: once the conductive components in a cementitious composite form a conductive network, conductive fillers dominate electrical transport in the material. A higher conductive component content forms more continuous conductive pathways. A certain level of graphite content can bring the magnitude order of conductivity in GC composite pastes to between 1 and 10 S/m [64]. However, the conductive filler content should be controlled within a certain range to prevent the degradation of other concrete mixture properties.
8.2. Resistivity Testing Methods
- (1)
- Specimen size: when small-size specimens are used, the discreteness and error of test values increase due to the inhomogeneity of the materials. Large specimen sizes are recommended for resistivity tests to improve uniformity and ensure the veracity of test data [97].
- (2)
- Specimen treatment: fresh samples can be cured in molds for 24 h, then demolded and cured for 28 days (20 °C and 95% RH). After curing, such samples are usually processed in an oven for treatment to eliminate any polarization effect during resistivity measurements [98,99] and to minimize the influence of moisture and pore solution on the resulting volume resistivity data [100]. However, there is no universal standard for sample treatments. Samples may also be placed in an oven at 60 °C for three days followed by 95 °C for another three days [98], into an 105 °C oven for 24 h [100], or held overnight at 80 °C to eliminate free water [101].
- (3)
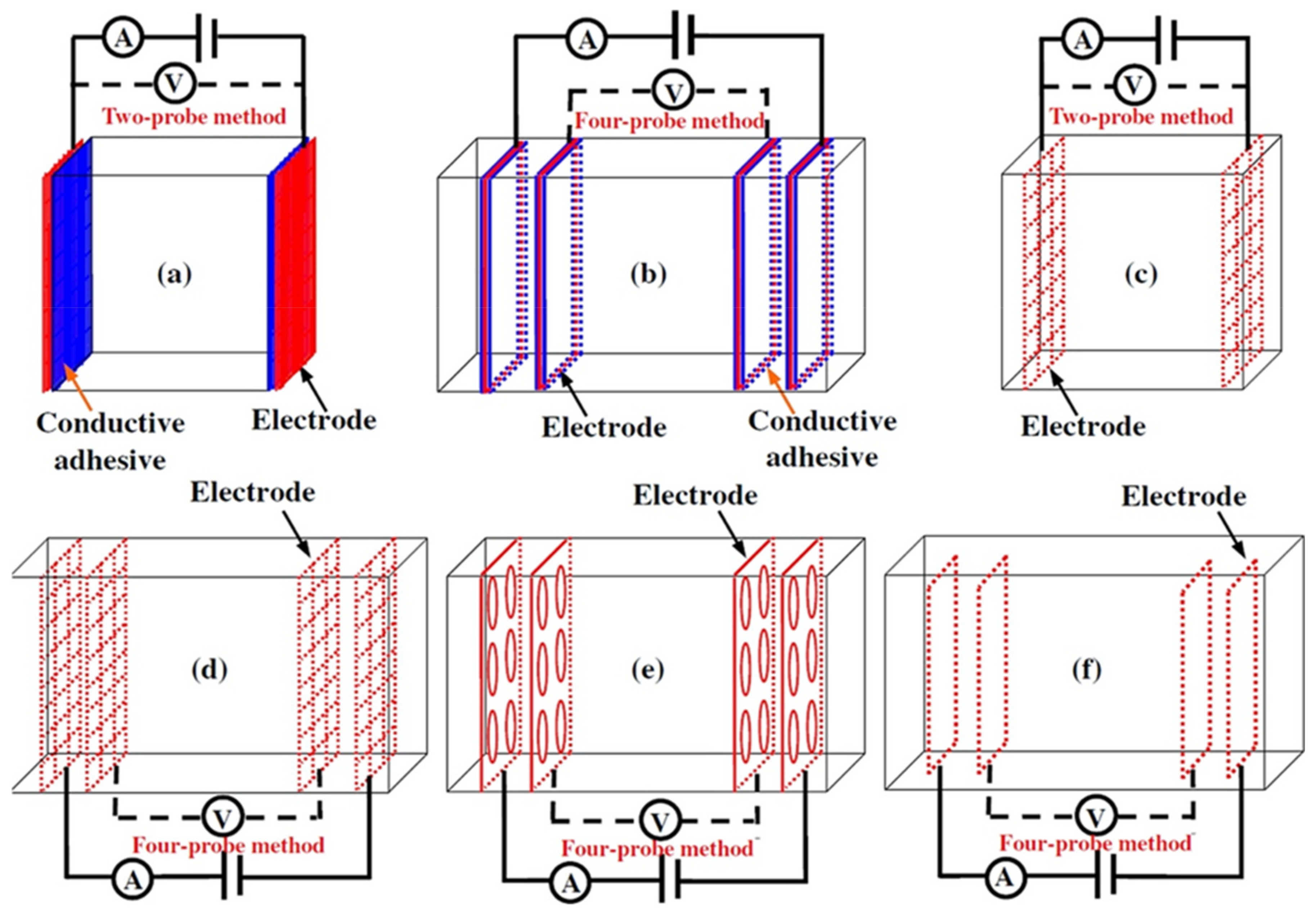
- Test method: resistivity test methods include the two-probe method and four-probe method [8] (Figure 10). The 4-probe method has generally shown higher accuracy, as the 2-probe method may introduce contact resistance that results in error. The 2-probe method is more commonly used due to its relative convenience. However, the 4-probe method is recommended for the sake of accuracy [86].
- (4)
- Test power supply: the supply voltage used to measure the resistivity of cementitious composites must fall within the resistivity stable region. Alternating current (AC) is recommended to measure the electrical resistance of the samples, as this can resolve the technical difficulties and problems (e.g., polarization effects) associated with direct current (DC) measurements [8,101].
- (5)
- Test instrument: a high precision desktop digital multimeter is typically used in resistivity tests to reduce the influence of the test instrument on the resulting data.
| Matrix | w/c | Specimen Size | Test Method | Test Power Supply | Test Instrument | Equation | Ref. |
|---|---|---|---|---|---|---|---|
| Paste | 0.45 | 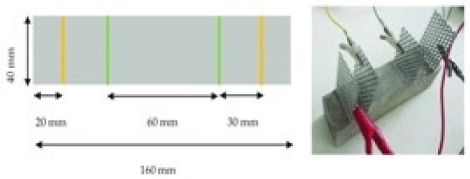 40 mm × 40 mm × 160 mm | 4-probe method | 10 V (DC) | - | - | [3] |
| Concrete | 0.44 | 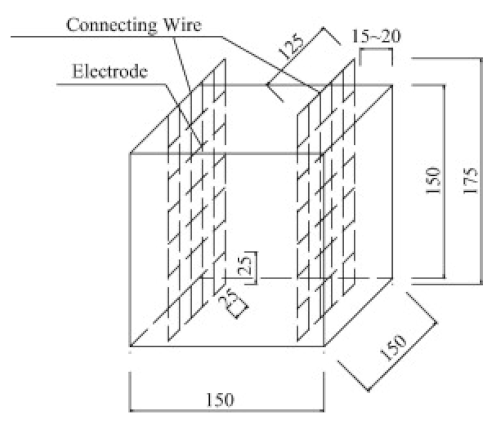 150 mm × 150 mm × 150 mm | 2-probe method | 50 Hz (AC) | Digital multimeter | - | [45] |
| Mortar | 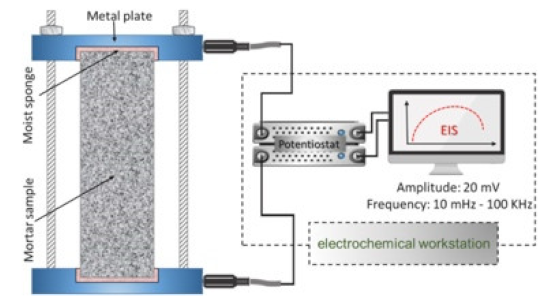 40 mm × 40 mm × 160 mm | Uniaxial two-point electrode method | 20 mV 10 mKz-100 kHz (AC) | EIS tests (VMP3) | ρ = RS/L | [68] | |
| Concrete | 0.28 |  100 mm × 100 mm × 400 mm | 4-probe method | - | Digital multimeter | ρ = 100 × UA/IL | [87] |
| Paste | 0.4 |  110 mm × 15 mm × 15 mm | 2-probe method | 1000 Hz(AC) | Resistivity meter | ρ = RAcosθ/L | [98] |
| Paste | 0.5 |  203.2 mm × 25.4 mm × 25.4 mm | 2-probe method | DC | Digital multimeter and DC Hipot Tester | ρ = RS/L | [100] |
| Paste | 0.45 |  20 mm × 20 mm × 75 mm | 2-probe method | 300 mV 200 KHz (AC) | - | - | [101] |
| Concrete | 0.43 | 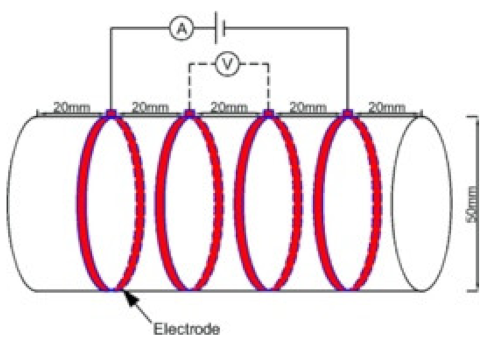 50 mm × 100 mm | 4-probe method | DC | Digital multimeter | ρ = 2παV/I | [102] |
| Paste | 0.4 |  770 mm × 55 mm × 42 mm | Non-contact electrical resistivity test | - | Cement and Concrete Resistivity-III | - | [103] |
| Mortar | 0.35 |  40 mm × 40 mm × 160 mm | 2-probe method | AC | Digital multimeter | ρ = RS/L | [104] |
| Paste | 0.35 | 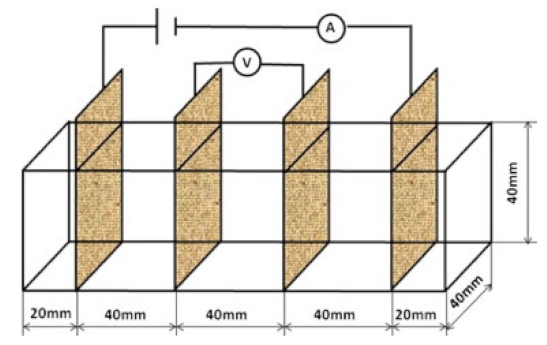 40 mm × 40 mm × 160 mm | 4-probe method | DC | Digital multimeter | - | [105] |
9. Methods to Improve Graphite-Based ECCC Properties
10. Concluding Remarks and Future Research Directions
- (1)
- The dispersion of graphite in the cement matrix is a notable technical limitation. The surfaces of graphite are hydrophobic and atomically smooth, thus encouraging mutual bonding to each other (i.e., agglomeration) in aqueous solutions (e.g., fresh cement mixtures).
- (2)
- The properties of a fully fabricated ECCC are dependent on the quality of the filler dispersion. The size and dispersion of a given filler are more important than its conductivity. This dispersion may require further treatments such as surfactant addition to improve the final properties, or graphite may need further functionalization to achieve the desired properties.
- (3)
- The ECCC is a heterogeneous material which has poor workability that restricts its wider application in engineering practice. A reduction in fluidity due to the inter-particle friction with cement particles, as well as the low hydrophilicity of graphite, cause a large amount of water to be entrapped in agglomerated graphite particles. The mixture design, water content, addition of any water reducing agents, graphite content, and fineness should be adjusted to ensure sufficient flowability without sacrificing functionality.
- (4)
- Graphite does not directly participate in cement hydration; rather, graphite particles act as inert conductive fillers. Graphite has a large specific surface area which can provide nucleation sites for hydration product precipitation. A large amount of hydration products is generated near the graphite sheets, which may improve the compatibility of graphite as a cement composite additive.
- (5)
- The key parameter of an ECCC is its electrical conductivity. Electrical conductivity increases as graphite addition increases, but compressive strength decreases simultaneously. The cementitious matrix–graphite particle interface has a significant effect on compressive strength. The graphite content supplied to a cementitious system must be properly adjusted to minimize any adverse effects on the mechanical properties of the material.
- (6)
- The addition of graphite in the matrix increases its porosity. Graphene-based materials as fillers not only create a physical barrier, but also form tortuous network paths that ultimately reduce the permeability of the composite.
- (7)
- The high surface area of fillers allows them to efficiently control the propagation of microcracks in cementitious composite materials. The layered structure of graphite further allows it to entrap ions which can protect the matrix.
- (8)
- The long-term performance (e.g., freeze–thaw resistance, shrinkage, sulfate resistance, steel corrosion resistance) of graphite-based cementitious composites has not yet been reported. To effectively utilize graphite in future engineering practice, in-depth research on other properties of ECCCs with graphite are yet needed.
- (9)
- The ECCC is a percolation system with complex conduction mechanisms that have attracted a great deal of research attention. The electrically conductive mechanisms of cementitious composites need further research in regard to their transport and electrical conductivity properties.
- (10)
- Currently, there is no strict standard or specification for ECCC conductivity testing. Electrical resistivity is the primary index of ECCCs, which determines its performance and application value. A standardized test method for ECCC electrical resistivity is of great significance in terms of the material’s potential application in engineering practice.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haddad, A.S.; Chung, D. Decreasing the electric permittivity of cement by graphite particle incorporation. Carbon 2017, 122, 702–709. [Google Scholar] [CrossRef]
- Wen, S.; Chung, D. The role of electronic and ionic conduction in the electrical conductivity of carbon fiber reinforced cement. Carbon 2006, 44, 2130–2138. [Google Scholar] [CrossRef]
- Papanikolaou, I.; Litina, C.; Zomorodian, A.; Al-Tabbaa, A. Effect of Natural Graphite Fineness on the Performance and Electrical Conductivity of Cement Paste Mixes for Self-Sensing Structures. Materials 2020, 13, 5833. [Google Scholar] [CrossRef]
- Zhu, P.; Li, H.; Ling, Q.; Asghar, H.K.; Gang, L.; Jun, W.Z.; Frank, C.; Dan, L.; Wen, H.D.; Ming, C.W. Mechanical Properties and Microstructure of a Graphene Oxide-Cement Composite. Cem. Concr. Compos. 2015, 58, 140–147. [Google Scholar]
- Yoo, D.Y.; You, I.; Lee, S.J. Electrical Properties of Cement-Based Composites with Carbon Nanotubes, Graphene, and Graphite Nanofibers. Sensors 2017, 17, 1064. [Google Scholar] [CrossRef] [PubMed]
- Alireza, S.; Ali, A.; Halil, C.; Sunghwan, K.; Sajed, S.S.M.; Kasthurirangan, G.; Taylor, P.C.; Hesham, A. Carbon Fiber-Based Electrically Conductive Concrete for Salt-Free Deicing of Pavements. J. Clean. Prod. 2018, 203, 799–809. [Google Scholar]
- Zhang, J.; Xu, L.; Zhao, Q. Investigation of carbon fillers modified electrically conductive concrete as grounding electrodes for transmission towers: Computational model and case study. Constr. Build. Mater. 2017, 145, 347–353. [Google Scholar] [CrossRef]
- Wang, L.N.; Farhad, A. A Review on Material Design, Performance, and Practical Application of Electrically Conductive Cementitious Composites. Constr. Build. Mater. 2019, 229, 116892. [Google Scholar] [CrossRef]
- Cao, J.; Chung, D. Carbon fiber reinforced cement mortar improved by using acrylic dispersion as an admixture. Cem. Concr. Res. 2001, 31, 1633–1637. [Google Scholar] [CrossRef]
- Peyvandi, A.A.; Soroushian, P.; Balachandra, A.M.; Sobolev, K. Enhancement of the durability characteristics of concrete nanocomposite pipes with modified graphite nanoplatelets. Constr. Build. Mater. 2013, 47, 111–117. [Google Scholar] [CrossRef]
- Sassani, A.; Ceylan, H.; Kim, S.; Gopalakrishnan, K.; Arabzadeh, A.; Taylor, P.C. Influence of mix design variables on engineering properties of carbon fiber-modified electrically conductive concrete. Constr. Build. Mater. 2017, 152, 168–181. [Google Scholar] [CrossRef] [Green Version]
- Sassani, A.; Ceylan, H.; Kim, S.; Arabzadeh, A.; Taylor, P.C.; Gopalakrishnan, K. Development of Carbon Fiber-modified Electrically Conductive Concrete for Implementation in Des Moines International Airport. Case Stud. Constr. Mater. 2018, 8, 277–291. [Google Scholar] [CrossRef]
- Mohammed, A.G.; Ozgur, G.; Sevkat, E. Electrical resistance heating for deicing and snow melting applications: Experimental study. Cold Reg. Sci. Technol. 2019, 160, 128–138. [Google Scholar] [CrossRef]
- Tuan, C.Y.; Yehia, S. Evaluation of Electrically Conductive Concrete Containing Carbon Products for Deicing. ACI Mater. J. 2004, 101, 287–293. [Google Scholar]
- Chung, D. Electromagnetic interference shielding effectiveness of carbon materials. Carbon 2001, 39, 279–285. [Google Scholar] [CrossRef]
- Qin, Z.; Wang, Y.; Mao, X.; Xie, X. Development of Graphite Electrically Conductive Concrete and Application in Grounding Engineering. New Build. Mater. 2009, 11, 46–48. [Google Scholar]
- Bertolini, L.; Bolzoni, F.M.; Pastore, T.; Pedeferri, P. Effectiveness of a conductive cementitious mortar anode for cathodic protection of steel in concrete. Cem. Concr. Res. 2004, 34, 681–694. [Google Scholar] [CrossRef]
- El-Dieb, A.S.; El-Ghareeb, M.A.; Abdel-Rahman, M.A.; Nasr, E.S.A. Multifunctional electrically conductive concrete using different fillers. J. Build. Eng. 2018, 15, 61–69. [Google Scholar] [CrossRef]
- Sun, M.-Q.; Liew, R.; Zhang, M.-H.; Li, W. Development of cement-based strain sensor for health monitoring of ultra high strength concrete. Constr. Build. Mater. 2014, 65, 630–637. [Google Scholar] [CrossRef]
- Chiarello, M.; Zinno, R. Electrical conductivity of self-monitoring CFRC. Cem. Concr. Compos. 2005, 27, 463–469. [Google Scholar] [CrossRef]
- Chen, P.-W.; Chung, D.D.L. Carbon fiber reinforced concrete as an electrical contact material for smart structures. Smart Mater. Struct. 1993, 2, 181–188. [Google Scholar] [CrossRef]
- Chung, D.D.L. Electrically Conductive Cement-Based Materials. Adv. Cem. Res. 2004, 16, 167–176. [Google Scholar] [CrossRef]
- Jara, A.; Betemariam, A.; Woldetinsae, G.; Kim, J.Y. Purification, application and current market trend of natural graphite: A review. Int. J. Min. Sci. Technol. 2019, 29, 671–689. [Google Scholar] [CrossRef]
- Lee, C.; Wei, X.; Kysar, J.W.; Hone, J. Measurement of the Elastic Properties and Intrinsic Strength of Monolayer Graphene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef]
- Balandin, A.A. Thermal properties of graphene and nanostructured carbon materials. Nat. Mater. 2011, 10, 569–581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geim, A.K.; Novoselov, K.S. The Rise of Graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef]
- Zheng, W.; Wong, S.-C. Electrical conductivity and dielectric properties of PMMA/expanded graphite composites. Compos. Sci. Technol. 2003, 63, 225–235. [Google Scholar] [CrossRef]
- Sachdev, V.K.; Sharma, S.K.; Bhattacharya, S.; Patel, K.; Mehra, N.C.; Gupta, V.; Tandon, R.P. Electromagnetic Shielding Performance of Graphite in Cement Matrix for Applied Application. Adv. Mater. Lett. 2015, 6, 965–972. [Google Scholar] [CrossRef]
- Scogings, A. Global Graphite Market Set for Change. Aust. Paydirt 2016, 1, 42. [Google Scholar]
- Chen, M.; Gao, P.; Geng, F.; Zhang, L.; Liu, H. Mechanical and smart properties of carbon fiber and graphite conductive concrete for internal damage monitoring of structure. Constr. Build. Mater. 2017, 142, 320–327. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Sachdev, V.K.; Chatterjee, R.; Tandon, R.P. Decisive properties of graphite-filled cement composites for device application. Appl. Phys. A 2008, 92, 417–420. [Google Scholar] [CrossRef]
- Han, M.; Muhammad, Y.; Wei, Y.; Zhu, Z.; Huang, J.; Li, J. A review on the development and application of graphene based materials for the fabrication of modified asphalt and cement. Constr. Build. Mater. 2021, 285, 122885. [Google Scholar] [CrossRef]
- Wenk, H.R.; Bulakh, A. Minerals: Their Constitution and Origin; Cambridge University Press: Cambridge, UK, 2016. [Google Scholar]
- Gust, W.H. Phase transition and shock-compression parameters to 120 GPa for three types of graphite and for amorphous carbon. Phys. Rev. B 1980, 22, 4744–4756. [Google Scholar] [CrossRef]
- Solfiti, E.; Berto, F. Mechanical properties of flexible graphite: Review. Procedia Struct. Integr. 2020, 25, 420–429. [Google Scholar] [CrossRef]
- Wallace, P.R. The Band Theory of Graphite. Phys. Rev. 1947, 71, 622–634. [Google Scholar] [CrossRef]
- Nakamizo, M.; Honda, H.; Inagaki, M. Raman spectra of ground natural graphite. Carbon 1978, 16, 281–283. [Google Scholar] [CrossRef]
- Chung, D.D.L. Review Graphite. J. Mater. Sci. 2002, 37, 1475–1489. [Google Scholar] [CrossRef]
- Caragiu, M.; Finberg, S. Alkali metal adsorption on graphite: A review. J. Phys. Condens. Matter 2005, 17, R995–R1024. [Google Scholar] [CrossRef]
- Kavanagh, A.; Schlögl, R. The morphology of some natural and synthetic graphites. Carbon 1988, 26, 23–32. [Google Scholar] [CrossRef]
- Chung, D.D.L. A review of exfoliated graphite. J. Mater. Sci. 2015, 51, 554–568. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, Y.; Guo, F.; Liu, X.; Wang, Y. Friction Characteristics of Impregnated and Non-Impregnated Graphite against Cemented Carbide under Water Lubrication. J. Mater. Sci. Technol. 2017, 33, 1203–1209. [Google Scholar] [CrossRef]
- Chen, G.-H.; Wu, D.-J.; Weng, W.-G.; Yan, W.-L. Dispersion of graphite nanosheets in a polymer matrix and the conducting property of the nanocomposites. Polym. Eng. Sci. 2001, 41, 2148–2154. [Google Scholar] [CrossRef]
- Wang, D.; Wang, Q.; Huang, Z. Investigation on the poor fluidity of electrically conductive cement-graphite paste: Experiment and simulation. Mater. Des. 2019, 169, 107679. [Google Scholar] [CrossRef]
- Wu, J.; Liu, J.; Yang, F. Three-phase composite conductive concrete for pavement deicing. Constr. Build. Mater. 2015, 75, 129–135. [Google Scholar] [CrossRef]
- Zhang, H.; Xing, F.; Cui, H.-Z.; Chen, D.-Z.; Ouyang, X.; Xu, S.-Z.; Wang, J.-X.; Huang, Y.-T.; Zuo, J.-D.; Tang, J.-N. A novel phase-change cement composite for thermal energy storage: Fabrication, thermal and mechanical properties. Appl. Energy 2016, 170, 130–139. [Google Scholar] [CrossRef]
- Banthia, N.; Djeridane, S.; Pigeon, M. Electrical resistivity of carbon and steel micro-fiber reinforced cements. Cem. Concr. Res. 1992, 22, 804–814. [Google Scholar] [CrossRef]
- Helmy, A.K.; Ferreiro, E.A.; de Bussetti, S.G. The water/graphitic-carbon interaction energy. Appl. Surf. Sci. 2007, 253, 4966–4969. [Google Scholar] [CrossRef]
- Gopalakrishnan, K.; Birgisson, B.; Taylor, P.; Attoh-Okine, N.O. (Eds.) Nanotechnology in Civil Infrastructure; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar] [CrossRef] [Green Version]
- Birgin, H.B.; D’Alessandro, A.; Laflamme, S.; Ubertini, F. Smart Graphite–Cement Composite for Roadway-Integrated Weigh-In-Motion Sensing. Sensors 2020, 20, 4518. [Google Scholar] [CrossRef]
- Sun, S.; Han, B.; Jiang, S.; Yu, X.; Wang, Y.; Li, H.; Ou, J. Nano graphite platelets-enabled piezoresistive cementitious composites for structural health monitoring. Constr. Build. Mater. 2017, 136, 314–328. [Google Scholar] [CrossRef] [Green Version]
- Dresel, A.; Teipel, U. Influence of the wetting behavior and surface energy on the dispersibility of multi-wall carbon nanotubes. Colloids Surf. A Physicochem. Eng. Asp. 2016, 489, 57–66. [Google Scholar] [CrossRef]
- Liebscher, M.; Dinh, T.T.; Schröfl, C.; Mechtcherine, V. Dispersion of different carbon-based nanofillers in aqueous suspension by polycarboxylate comb-type copolymers and their influence on the early age properties of cementitious matrices. Constr. Build. Mater. 2020, 241, 118039. [Google Scholar] [CrossRef]
- Parveen, S.; Rana, S.; Fangueiro, R. A Review on Nanomaterial Dispersion, Microstructure, and Mechanical Properties of Carbon Nanotube and Nanofiber Reinforced Cementitious Composites. J. Nanomater. 2013, 2013, 710175. [Google Scholar] [CrossRef]
- Luo, J.; Duan, Z.; Li, H. The influence of surfactants on the processing of multi-walled carbon nanotubes in reinforced cement matrix composites. Phys. Status Solidi A 2009, 206, 2783–2790. [Google Scholar] [CrossRef]
- Kaur, R.; Kothiyal, N. Positive synergistic effect of superplasticizer stabilized graphene oxide and functionalized carbon nanotubes as a 3-D hybrid reinforcing phase on the mechanical properties and pore structure refinement of cement nanocomposites. Constr. Build. Mater. 2019, 222, 358–370. [Google Scholar] [CrossRef]
- Li, G.Y.; Wang, P.M.; Zhao, X. Mechanical behavior and microstructure of cement composites incorporating surface-treated multi-walled carbon nanotubes. Carbon 2005, 43, 1239–1245. [Google Scholar] [CrossRef]
- Peyvandi, A.A.; Soroushian, P.; Abdol, N.; Balachandra, A.M. Surface-modified graphite nanomaterials for improved reinforcement efficiency in cementitious paste. Carbon 2013, 63, 175–186. [Google Scholar] [CrossRef]
- Zhou, Y.; Sun, W.; Ling, Z.; Fang, X.; Zhang, Z. Hydrophilic Modification of Expanded Graphite to Prepare a High-Performance Composite Phase Change Block Containing a Hydrate Salt. Ind. Eng. Chem. Res. 2017, 56, 14799–14806. [Google Scholar] [CrossRef]
- Wang, B.; Pang, B. Mechanical property and toughening mechanism of water reducing agents modified graphene nanoplatelets reinforced cement composites. Constr. Build. Mater. 2019, 226, 699–711. [Google Scholar] [CrossRef]
- Du, H.; Pang, S.D. Dispersion and stability of graphene nanoplatelet in water and its influence on cement composites. Constr. Build. Mater. 2018, 167, 403–413. [Google Scholar] [CrossRef]
- An, X.; Simmons, T.; Shah, R.; Wolfe, C.; Lewis, K.M.; Washington, M.; Nayak, S.K.; Talapatra, S.; Kar, S. Stable Aqueous Dispersions of Noncovalently Functionalized Graphene from Graphite and their Multifunctional High-Performance Applications. Nano Lett. 2010, 10, 4295–4301. [Google Scholar] [CrossRef]
- Kozbial, A.; Li, Z.; Sun, J.; Gong, X.; Zhou, F.; Wang, Y.; Xu, H.; Liu, H.; Li, L. Understanding the intrinsic water wettability of graphite. Carbon 2014, 74, 218–225. [Google Scholar] [CrossRef] [Green Version]
- Frattini, D.; Accardo, G.; Ferone, C.; Cioffi, R. Fabrication and Characterization of Graphite-Cement Compositesfor Microbial Fuel Cells Applications. Mater. Res. Bull. 2017, 88, 188–199. [Google Scholar] [CrossRef]
- Collins, F.; Lambert, J.; Duan, W.H. The Influences of Admixtures on The Dispersion, Workability, and Strength of Carbon Nanotube–OPC Paste Mixtures. Cem. Concr. Compos. 2012, 34, 201–207. [Google Scholar] [CrossRef]
- Chougan, M.; Marotta, E.; Lamastra, F.R.; Vivio, F.; Montesperelli, G.; Ianniruberto, U.; Ghaffar, S.H.; Al-Kheetan, M.J.; Bianco, A. High performance cementitious nanocomposites: The effectiveness of nano-Graphite (nG). Constr. Build. Mater. 2020, 259, 119687. [Google Scholar] [CrossRef]
- Medina, N.F.; Barbero-Barrera, M.D.M.; Jové-Sandoval, F. Improvement of the Mechanical and Physical Properties of Cement Pastes and Mortars through The Addition Isostatic Graphite. Constr. Build. Mater. 2018, 189, 898–905. [Google Scholar] [CrossRef]
- Lamastra, F.R.; Chougan, M.; Marotta, E.; Ciattini, S.; Ghaffar, S.H.; Caporali, S.; Vivio, F.; Montesperelli, G.; Ianniruberto, U.; Al-Kheetan, M.J.; et al. Toward a better understanding of multifunctional cement-based materials: The impact of graphite nanoplatelets (GNPs). Ceram. Int. 2021, 47, 20019–20031. [Google Scholar] [CrossRef]
- Zhao, L.; Guo, X.; Song, L.; Song, Y.; Dai, G.; Liu, J. An intensive review on the role of graphene oxide in cement-based materials. Constr. Build. Mater. 2020, 241, 117939. [Google Scholar] [CrossRef]
- Nishikawa, T.; Takatsu, M.; Daimon, M. Fracture behavior of hardened cement paste incorporating mineral additions. Cem. Concr. Res. 1995, 25, 1218–1224. [Google Scholar] [CrossRef]
- Yuan, H.-W.; Lu, C.-H.; Xu, Z.-Z.; Ni, Y.-R.; Lan, X.-H. Mechanical and thermal properties of cement composite graphite for solar thermal storage materials. Sol. Energy 2012, 86, 3227–3233. [Google Scholar] [CrossRef]
- Zel, I.Y.; Kenessarin, M.; Kichanov, S.; Balasoiu, M.; Kozlenko, D.; Nazarov, K.; Nicu, M.; Ionascu, L.; Dragolici, A.; Dragolici, F. Spatial distribution of graphite in cement materials used for radioactive waste conditioning: An approach to analysis of neutron tomography data. Cem. Concr. Compos. 2021, 119, 103993. [Google Scholar] [CrossRef]
- Bi, L.; Long, G.; Ma, C.; Wu, J.; Xie, Y. Effect of phase change composites on hydration characteristics of steam-cured cement paste. Constr. Build. Mater. 2020, 274, 122030. [Google Scholar] [CrossRef]
- Wu, S.; Mo, L.; Shui, Z.; Chen, Z. Investigation of the conductivity of asphalt concrete containing conductive fillers. Carbon 2005, 43, 1358–1363. [Google Scholar] [CrossRef]
- Cordon, H.C.F.; Tadini, F.B.; Akiyama, G.A.; De Andrade, V.O.; Da Silva, R.C. Development of electrically conductive concrete. Cerâmica 2020, 66, 88–92. [Google Scholar] [CrossRef]
- Dong, W.; Li, W.; Wang, K.; Shah, S.P. Physicochemical and Piezoresistive properties of smart cementitious composites with graphene nanoplates and graphite plates. Constr. Build. Mater. 2021, 286, 122943. [Google Scholar] [CrossRef]
- Shen, G.; Dong, F.Q. Study on Graphite Electrically Conductive Concrete. Concrete 2004, 2, 21–24. [Google Scholar]
- Mohammed, A.; Sanjayan, J.; Duan, W.H.; Nazari, A. Incorporating graphene oxide in cement composites: A study of transport properties. Constr. Build. Mater. 2015, 84, 341–347. [Google Scholar] [CrossRef]
- Hou, P.K.; Cheng, X.; Qian, J.S.; Zhang, R.; Cao, W.; Shah, S.P. Characteristics of Surface-Treatment of Nano-Sio2 on The Transport Properties of Hardened Cement Pasts with Different Water-To-Cement Ratios. Cem. Concr. Compos. 2015, 55, 26–33. [Google Scholar] [CrossRef]
- Compton, O.C.; Kim, S.; Pierre, C.; Torkelson, J.M.; Nguyen, S. Crumpled Graphene Nanosheets as Highly Effective Barrier Property Enhancers. Adv. Mater. 2010, 22, 4759–4763. [Google Scholar] [CrossRef]
- Yanturina, R.; Trofimov, B.; Ahmedjanov, R. The Influence of Graphite-Containing Nano-Additives on Thermo-Frost Resistance of Concrete. Procedia Eng. 2017, 206, 869–874. [Google Scholar] [CrossRef]
- Sedaghat, A.; Ram, M.K.; Zayed, A.; Kamal, R.; Shanahan, N. Investigation of Physical Properties of Graphene-Cement Composite for Structural Applications. Open J. Compos. Mater. 2014, 4, 12–21. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Q.; Han, B.; Cui, X.; Yu, X.; Ou, J. Graphene-Engineered Cementitious Composites: Small Makes a Big Impact. Nanomater. Nanotechnol. 2017, 7, 1–18. [Google Scholar] [CrossRef]
- Tang, Z.; Li, Z.; Qian, J.; Wang, K. Experimental Study on Deicing Performance of Carbon Fiber Reinforced Conductive Concrete. J. Mater. Sci. Technol. 2005, 21, 113–117. [Google Scholar]
- Cañón, A.; Garcés, P.; Climent, M.; Carmona, J.; Zornoza, E. Feasibility of electrochemical chloride extraction from structural reinforced concrete using a sprayed conductive graphite powder–cement paste as anode. Corros. Sci. 2013, 77, 128–134. [Google Scholar] [CrossRef]
- Xin, T.; Hu, H. Test and Study on Electrical Property of Conductive Concrete. Procedia Earth Planet. Sci. 2012, 5, 83–87. [Google Scholar]
- Sun, J.; Lin, S.; Zhang, G.; Sun, Y.; Zhang, J.; Chen, C.; Morsy, A.M.; Wang, X. The effect of graphite and slag on electrical and mechanical properties of electrically conductive cementitious composites. Constr. Build. Mater. 2021, 281, 122606. [Google Scholar] [CrossRef]
- Sun, S.; Ding, S.; Han, B.; Dong, S.; Yu, X.; Zhou, D.; Ou, J. Multi-layer graphene-engineered cementitious composites with multifunctionality/intelligence. Compos. Part B Eng. 2017, 129, 221–232. [Google Scholar] [CrossRef]
- Fan, X.; Fang, D.; Sun, M.; Li, Z. Piezoresistivity of carbon fiber graphite cement-based composites with CCCW. J. Wuhan Univ. Technol. Sci. Ed. 2011, 26, 339–343. [Google Scholar] [CrossRef]
- Tang, Z.Q.; Li, Z.Q.; Hou, Z.F.; Liang, X.D. Properties Analysis on Electrically Conductive Concrete Road Material and Conductive Additive Selection. Concrete 2002, 4, 28–31. [Google Scholar]
- Rajagopal, S.M. Studies on Electrical Conductivity of Insulator Conductor Composites. Appl. Phys. 1978, 49, 461–469. [Google Scholar] [CrossRef]
- Carmonn, A.F. Conducting Filled Polymers. Solid State Commu. 1989, 157, 461–469. [Google Scholar] [CrossRef]
- Witpathomwong, S.; Okhawilai, M.; Jubsilp, C.; Karagiannidis, P.; Rimdusit, S. Highly filled graphite/graphene/carbon nanotube in polybenzoxazine composites for bipolar plate in PEMFC. Int. J. Hydrog. Energy 2020, 45, 30898–30910. [Google Scholar] [CrossRef]
- Dong, S.; Li, L.; Ashour, A.; Dong, X.; Han, B. Self-assembled 0D/2D nano carbon materials engineered smart and multifunctional cement-based composites. Constr. Build. Mater. 2020, 272, 121632. [Google Scholar] [CrossRef]
- Han, B.-G.; Ou, J.-P. Humidity sensing property of cements with added carbon. New Carbon Mater. 2008, 23, 382–384. [Google Scholar] [CrossRef]
- Dehghanpour, H.; Yilmaz, K.; Afshari, F.; Ipek, M. Electrically conductive concrete: A laboratory-based investigation and numerical analysis approach. Constr. Build. Mater. 2020, 260, 119948. [Google Scholar] [CrossRef]
- Chen, B.; Wu, K.R.; Yao, W. Studies on Electrical Conductivity of Fiber Reinforced Concrete and Its Application. Concrete 2002, 7, 23–27. [Google Scholar]
- Li, X.; Wang, L.; Liu, Y.; Li, W.; Dong, B.; Duan, W.H. Dispersion of graphene oxide agglomerates in cement paste and its effects on electrical resistivity and flexural strength. Cem. Concr. Compos. 2018, 92, 145–154. [Google Scholar] [CrossRef]
- Konsta-Gdoutos, M.S.; Aza, C.A. Self sensing carbon nanotube (CNT) and nanofiber (CNF) cementitious composites for real time damage assessment in smart structures. Cem. Concr. Compos. 2014, 53, 162–169. [Google Scholar] [CrossRef]
- Arabzadeh, A.; Sassani, A.; Ceylan, H.; Kim, S.; Gopalakrishnan, K.; Taylor, P.C. Comparison between cement paste and asphalt mastic modified by carbonaceous materials: Electrical and thermal properties. Constr. Build. Mater. 2019, 213, 121–130. [Google Scholar] [CrossRef]
- Lavagna, L.; Musso, S.; Ferro, G.; Pavese, M. Cement-based composites containing functionalized carbon fibers. Cem. Concr. Compos. 2018, 88, 165–171. [Google Scholar] [CrossRef]
- Dong, W.; Huang, Y.; Lehane, B.; Aslani, F.; Ma, G. Mechanical and electrical properties of concrete incorporating an iron-particle contained nano-graphite by-product. Constr. Build. Mater. 2020, 270, 121377. [Google Scholar] [CrossRef]
- Li, W.; Li, X.; Jian, S.; Ming, Y.; Hui, W.; Shah, S.P. Effects of Graphene Oxide on Early-Age Hydration and Electrical Resistivity of Portland Cement Paste. Constr. Build. Mater. 2017, 136, 506–514. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, M.; Wang, W. Ohmic heating curing of electrically conductive carbon nanofiber/cement-based composites to avoid frost damage under severely low temperature. Compos. Part A Appl. Sci. Manuf. 2018, 115, 236–246. [Google Scholar] [CrossRef]
- Xie, N.; Shi, X.; Feng, D.; Kuang, B.; Li, H. Percolation backbone structure analysis in electrically conductive carbon fiber reinforced cement composites. Compos. Part B Eng. 2012, 43, 3270–3275. [Google Scholar] [CrossRef]
- Tabatabaei, M.; Taleghani, A.D.; Alem, N. Nanoengineering of cement using graphite platelets to refine inherent microstructural defects. Compos. Part B Eng. 2020, 202, 108277. [Google Scholar] [CrossRef]


| Carbon Material | State | Bulk Density (g/cm3) | Specific Surface Area (m2/g) | Conductivity (S/cm) | Dispersion | Cost |
|---|---|---|---|---|---|---|
| CNFs | Fiber | 0.06–2.1 | 13–200 | 10–104 | Aggregates easily | High |
| CFs | Fiber | 1.5–2.0 | 10–50 | 10−1–103 | Aggregates easily | Medium |
| Graphene | Powder | 1–2.5 | 120–575 | 103 | Relatively easier dispersion | High |
| GP | Powder | 1.9–2.3 | 10–35 | 104 | Relatively easier dispersion | Low |
| CB | Powder | 0.4–2.0 | 20–250 | 10 | Aggregates easily | Low |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, T.; Wang, Q. Effects of Graphite on Electrically Conductive Cementitious Composite Properties: A Review. Materials 2021, 14, 4798. https://doi.org/10.3390/ma14174798
Luo T, Wang Q. Effects of Graphite on Electrically Conductive Cementitious Composite Properties: A Review. Materials. 2021; 14(17):4798. https://doi.org/10.3390/ma14174798
Chicago/Turabian StyleLuo, Ting, and Qiang Wang. 2021. "Effects of Graphite on Electrically Conductive Cementitious Composite Properties: A Review" Materials 14, no. 17: 4798. https://doi.org/10.3390/ma14174798
APA StyleLuo, T., & Wang, Q. (2021). Effects of Graphite on Electrically Conductive Cementitious Composite Properties: A Review. Materials, 14(17), 4798. https://doi.org/10.3390/ma14174798







