Evaluation of Stresses on Implant, Bone, and Restorative Materials Caused by Different Opposing Arch Materials in Hybrid Prosthetic Restorations Using the All-on-4 Technique
Abstract
:1. Introduction
2. Materials and Methods
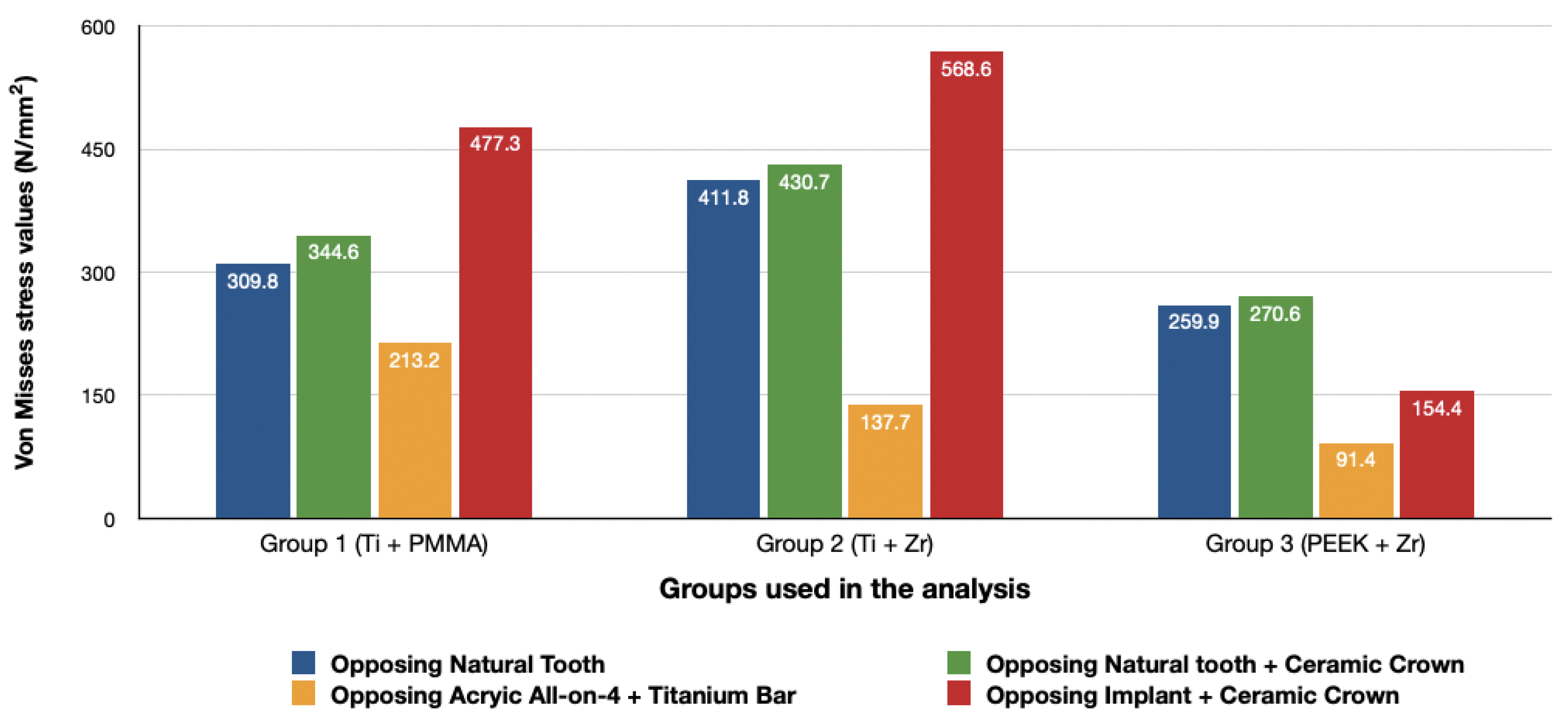
3. Results
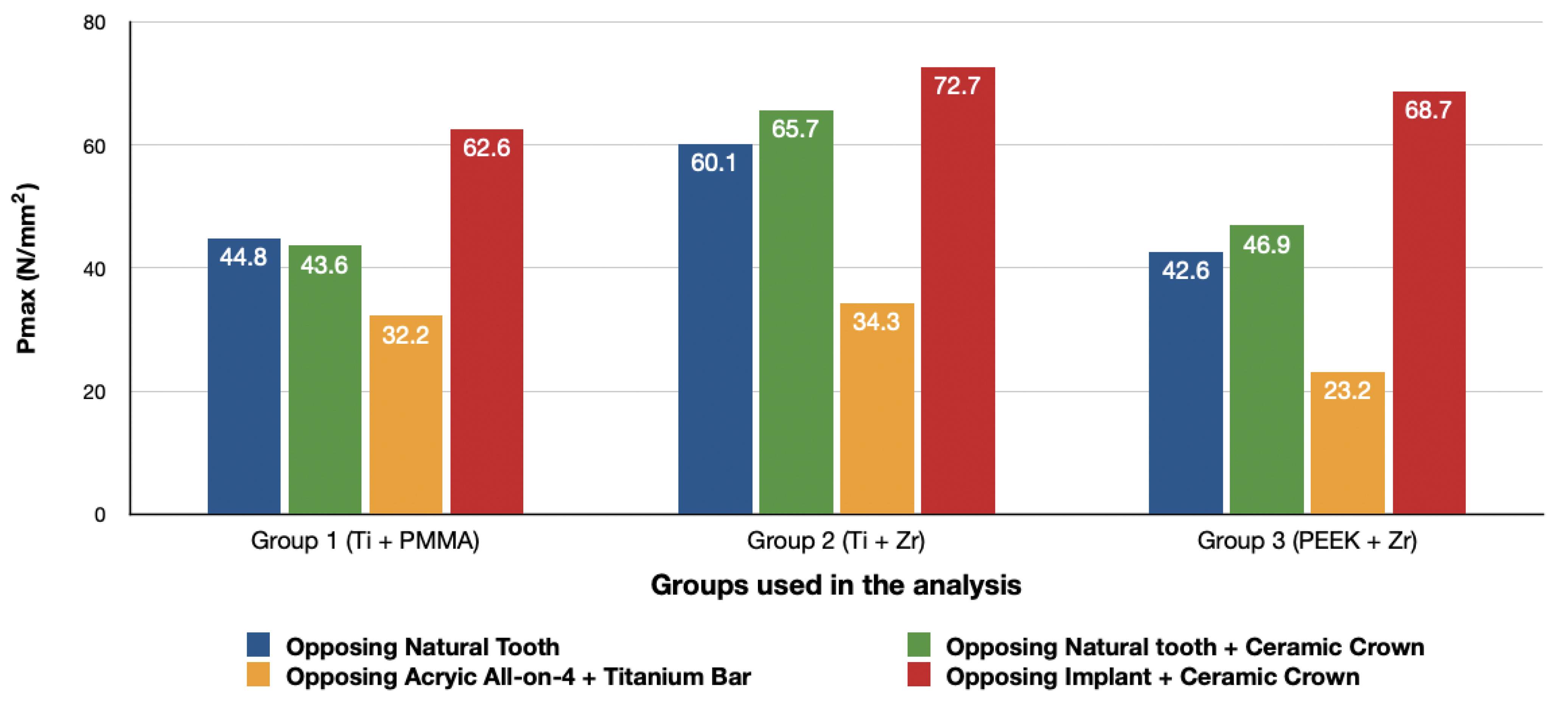
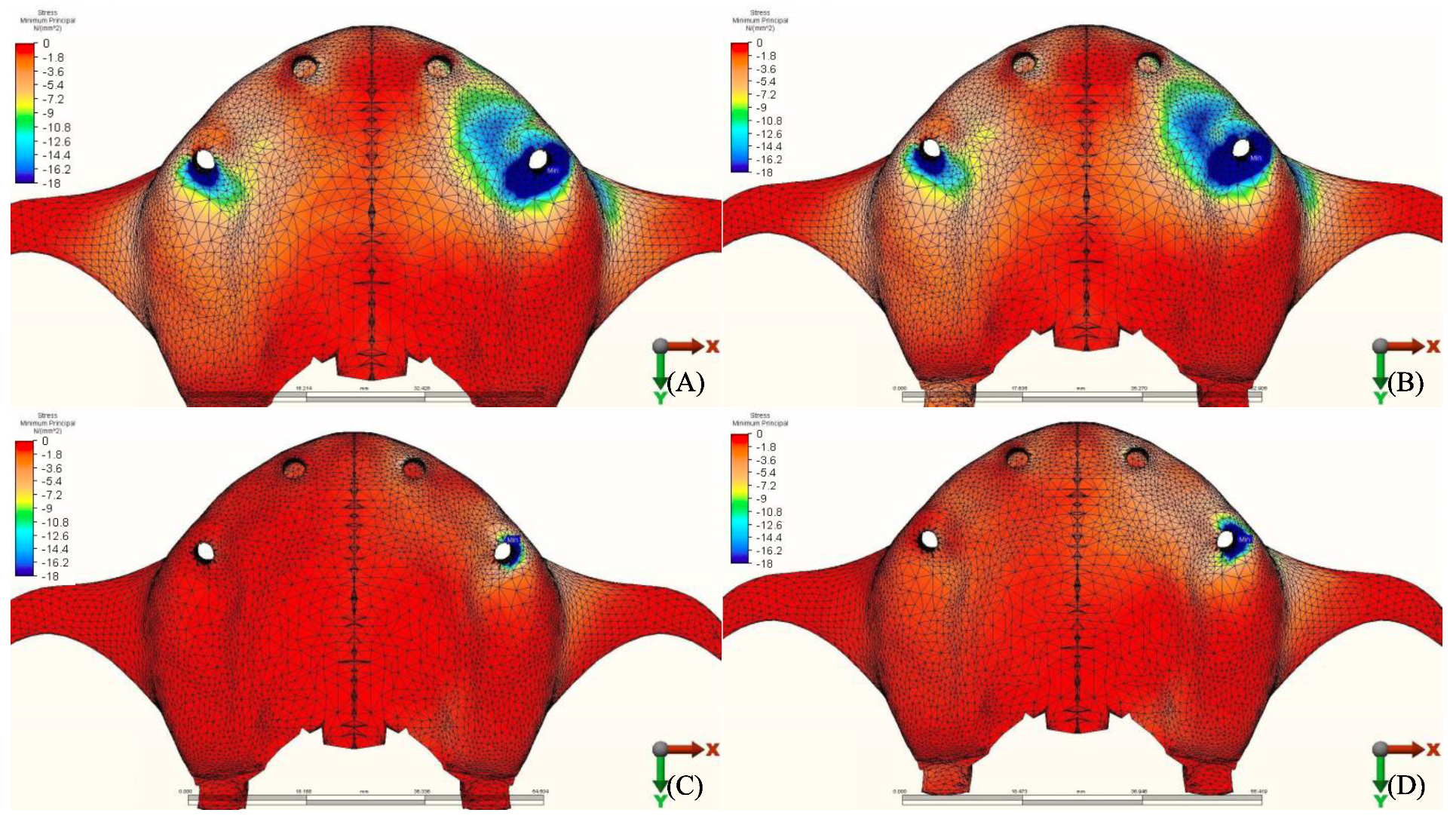
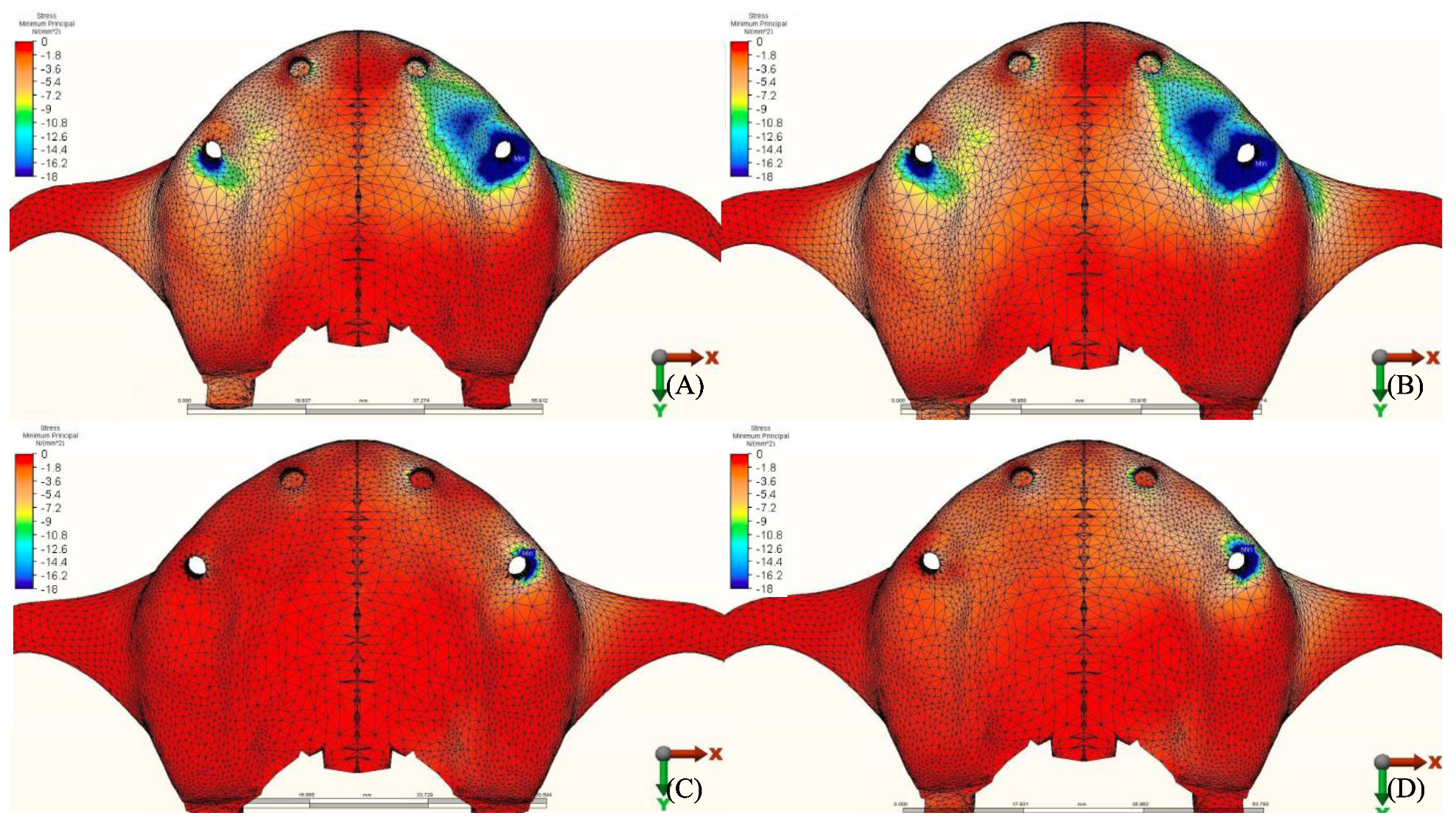
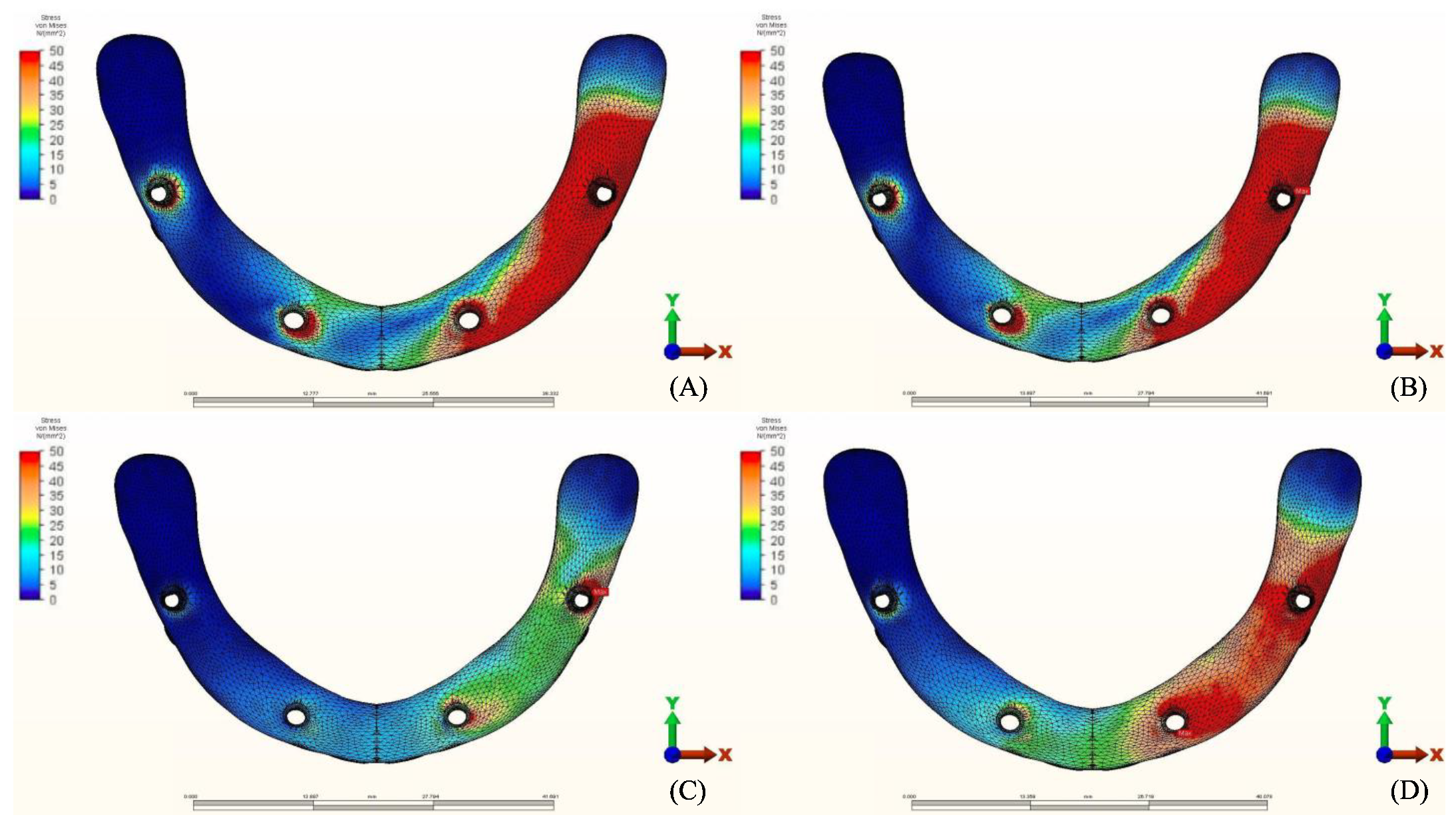
3.1. Stresses in Peri-Implant Cortical Bone
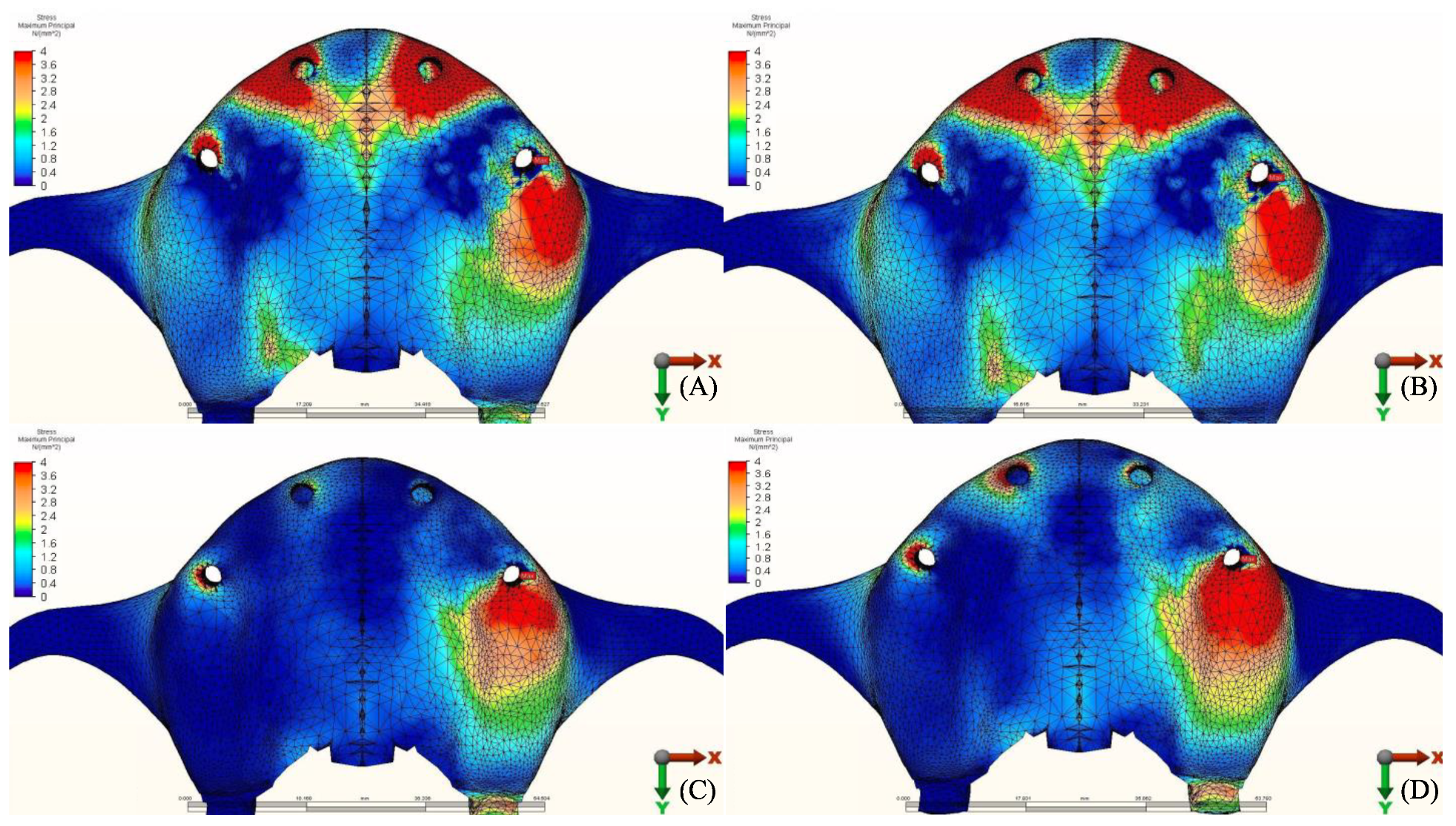
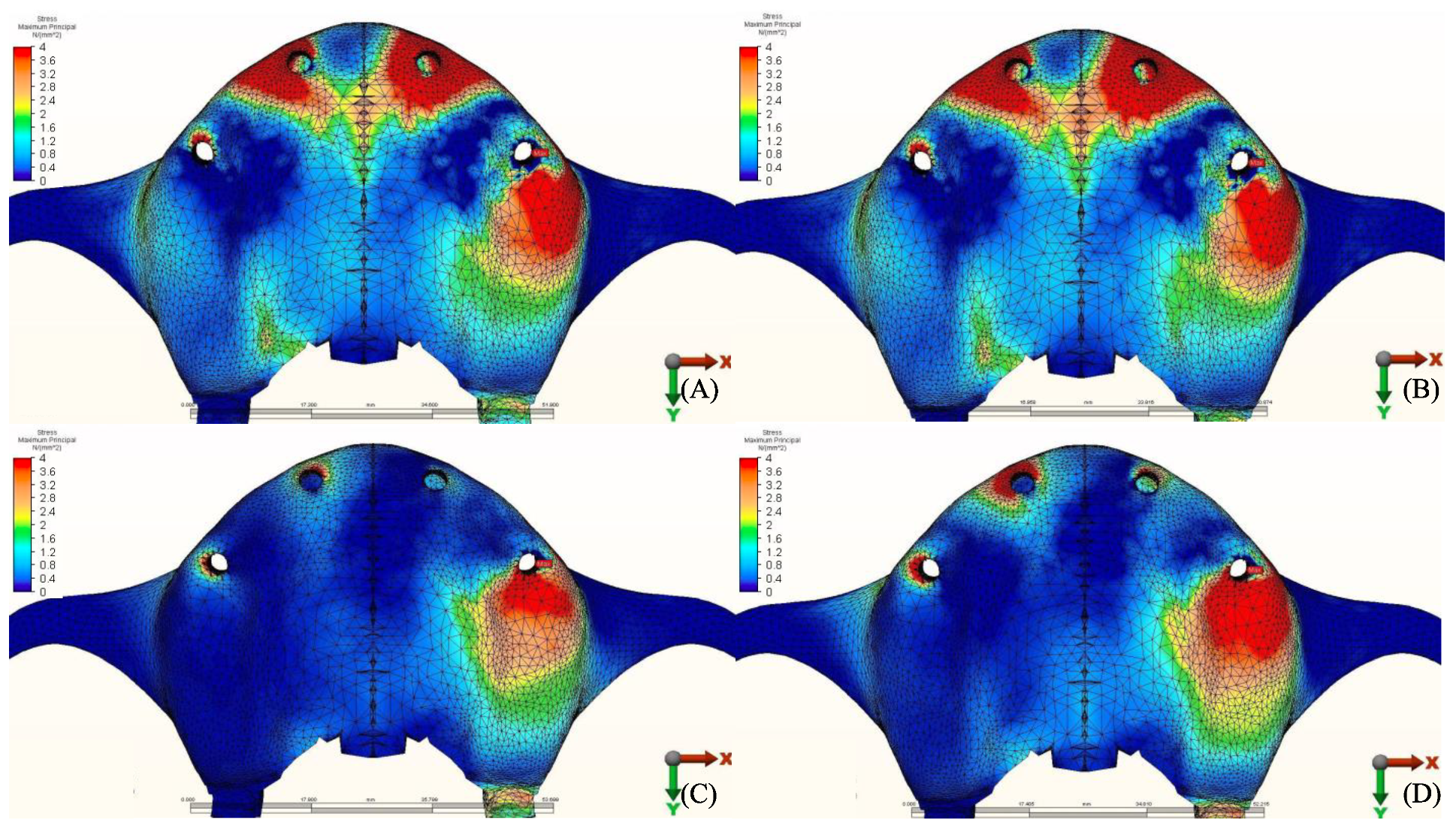
3.1.1. Maximum Principal Stresses (Pmax)
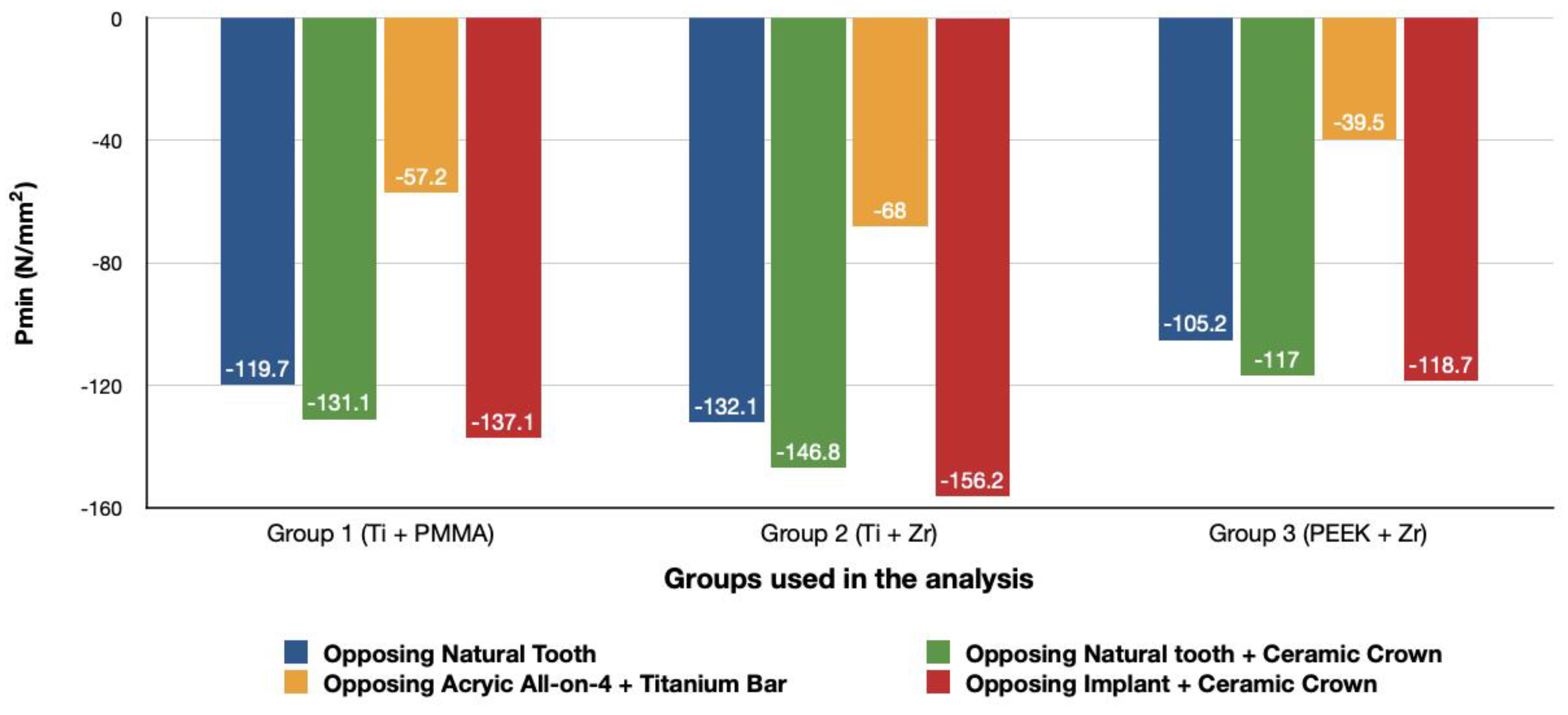
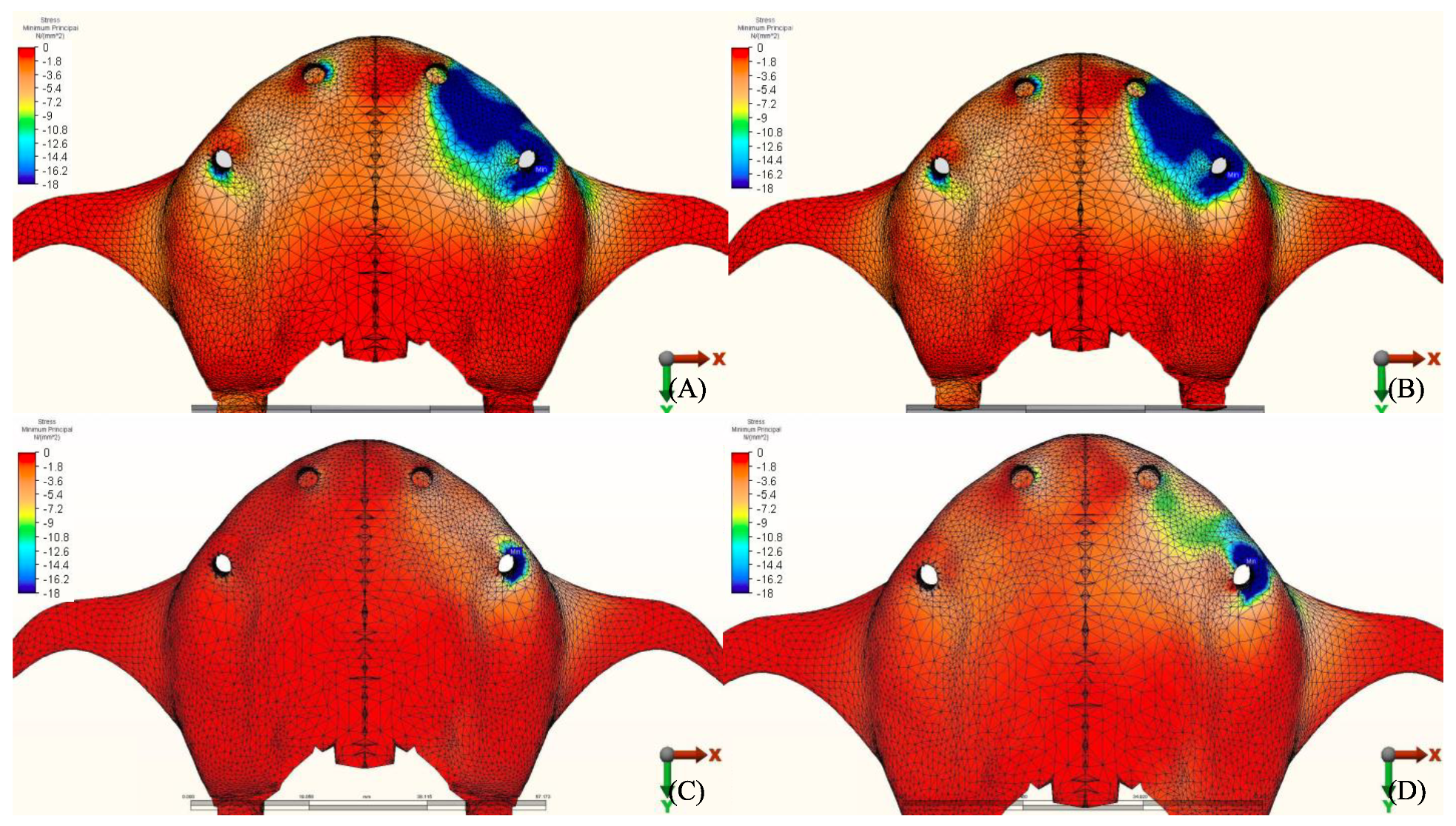
3.1.2. Minimum Principal Stresses (Pmin)
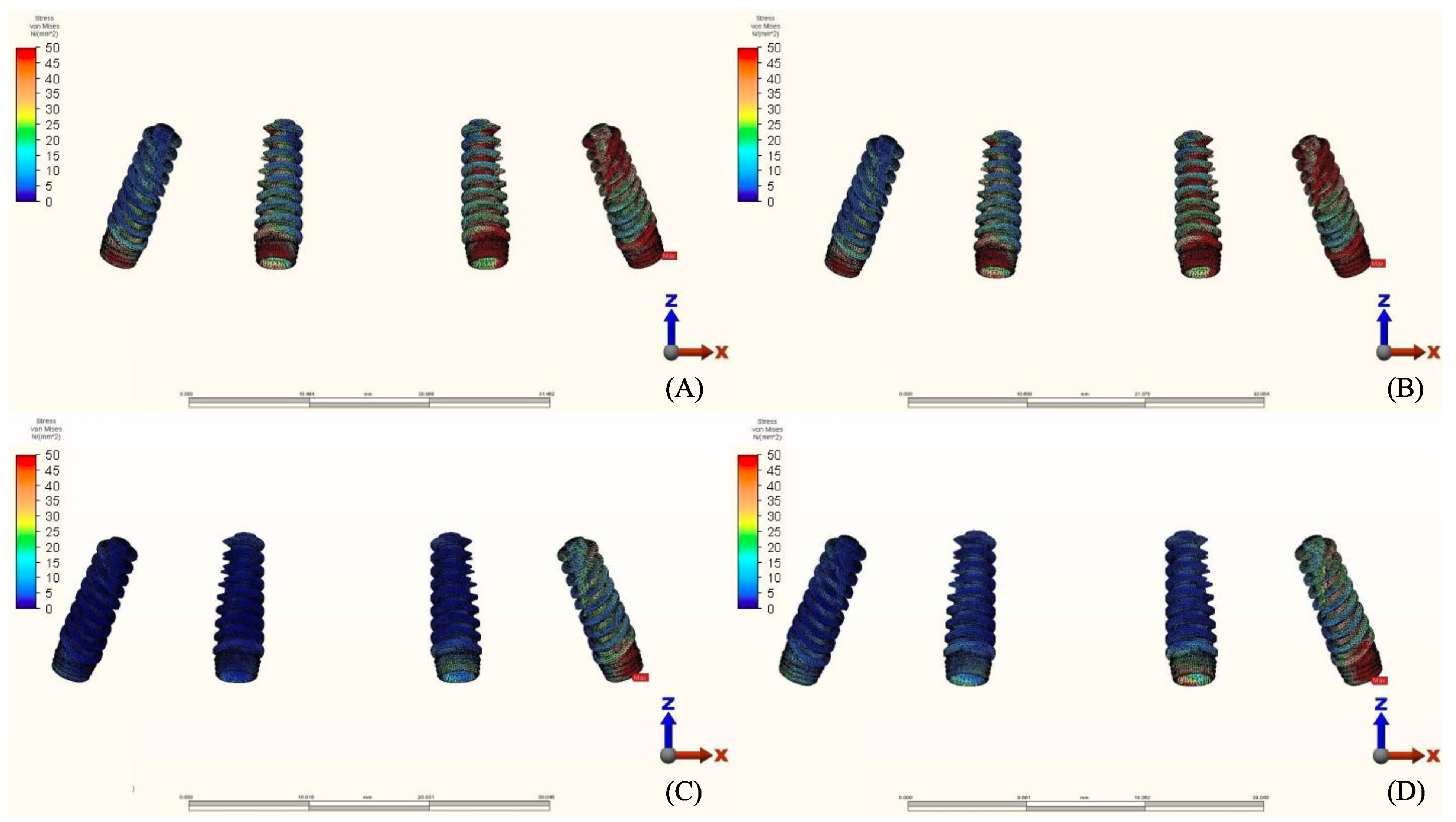
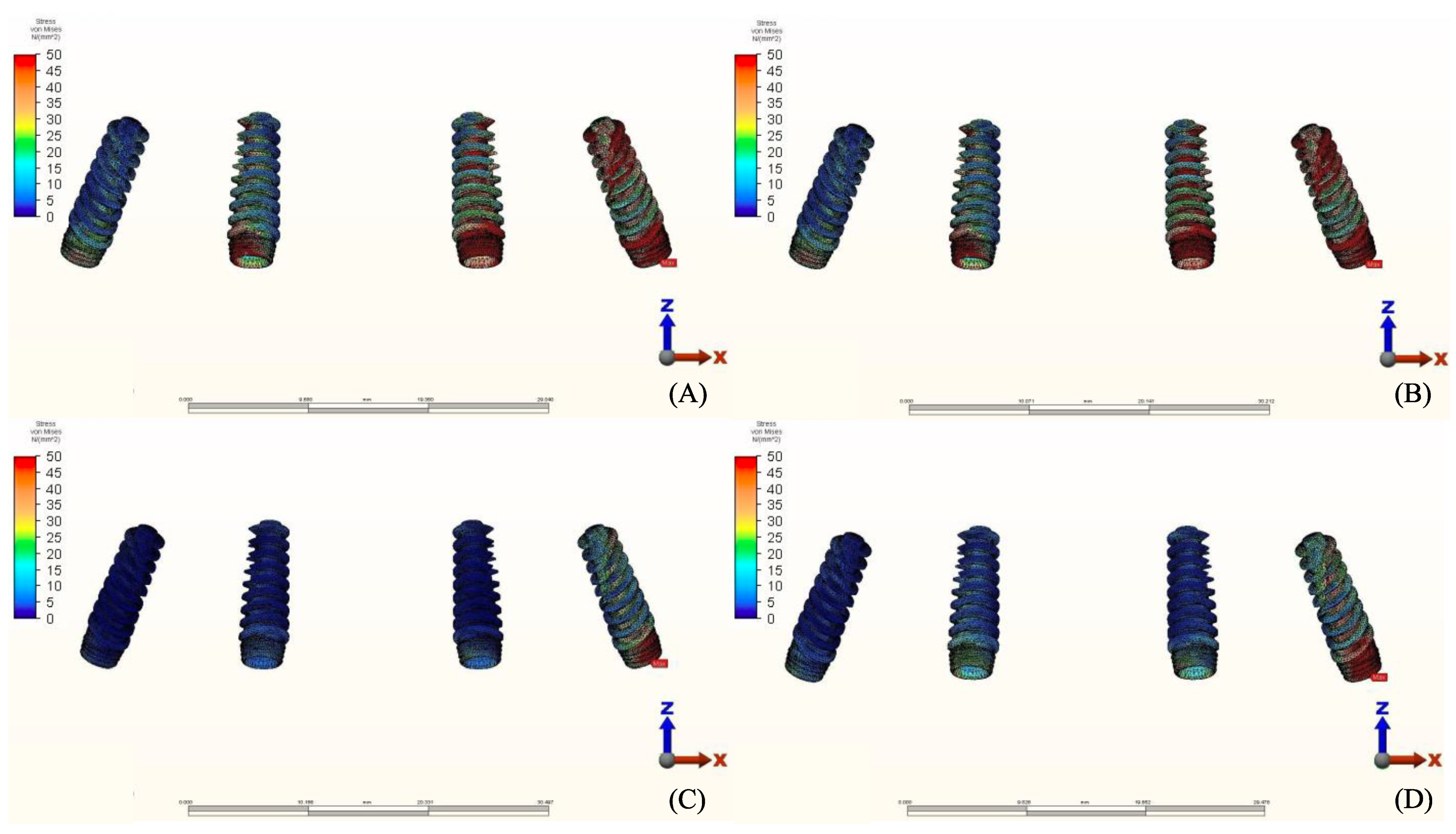
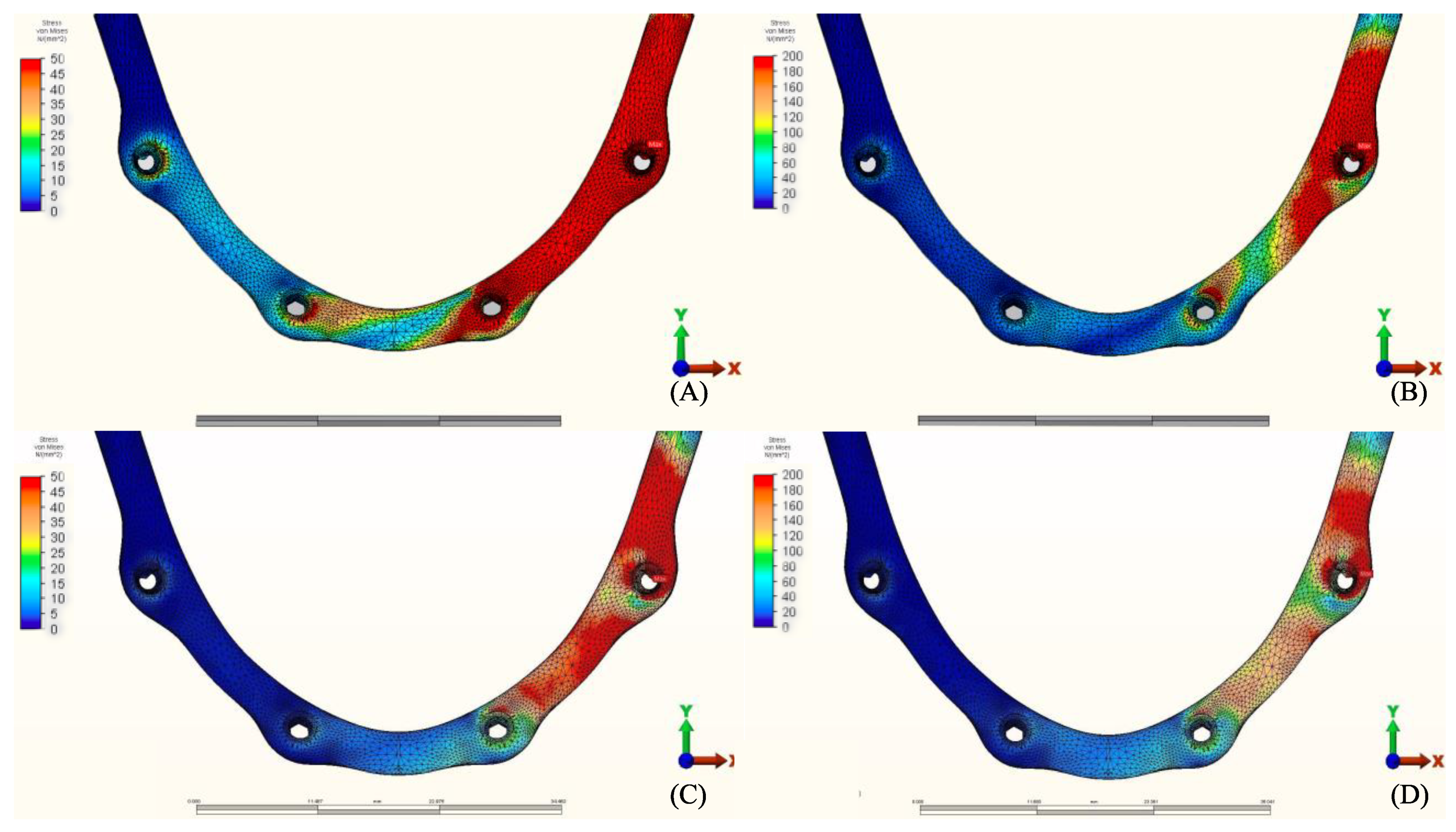
3.2. Stresses in Implants
3.3. Stresses in Framework
4. Discussion
5. Conclusions
- (1).
- The use of materials with a low modulus of elasticity such as acrylic and PEEK could reduce the amount of stresses transmitted to the bone.
- (2).
- The use of implant-supported ceramic prosthesis as an antagonist to another implant-supported full arch prosthesis increases the amount of stresses transmitted to the bone.
- (3).
- There is no difference in the amount of stresses transmitted when comparing natural tooth to tooth-supported ceramic restorations as an antagonist for a full arch implant-supported prosthesis.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Türker, N.; Büyükkaplan, U.S.; Sadowsky, S.J.; Özarsalan, M.M. Özarslan Finite Element Stress Analysis of Applied Forces to Implants and Supporting Tissues Using the “All-on-Four” Concept with Different Occlusal Schemes. J. Prosthodont. 2018, 28, 185–194. [Google Scholar] [CrossRef]
- Geringer, A.; Diebels, S.; Nothdurft, F.P. Influence of superstructure geometry on the mechanical behavior of zirconia implant abutments: A finite element analysis. Biomed. Tech. Eng. 2014, 59, 501–506. [Google Scholar] [CrossRef]
- Ferreira, M.B.; Barão, V.A.; Faverani, L.P.; Hipólito, A.C.; Assunção, W.G. The role of superstructure material on the stress distribution in mandibular full-arch implant-supported fixed dentures. A CT-based 3D-FEA. Mater. Sci. Eng. C 2014, 35, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Papaspyridakos, P.; Chen, C.-J.; Chuang, S.-K.; Weber, H.-P.; Gallucci, G. A systematic review of biologic and technical complications with fixed implant rehabilitations for edentulous patients. Int. J. Oral Maxillofac. Implant. 2012, 27, 102–110. [Google Scholar]
- Fischer, K.; Stenberg, T. Prospective 10-Year Cohort Study Based on a Randomized Controlled Trial (RCT) on Implant-Supported Full-Arch Maxillary Prostheses. Part 1: Sandblasted and Acid-Etched Implants and Mucosal Tissue. Clin. Implant. Dent. Relat. Res. 2011, 14, 808–815. [Google Scholar] [CrossRef] [PubMed]
- Priest, G.; Smith, J.; Wilson, M.G. Implant survival and prosthetic complications of mandibular metal-acrylic resin implant complete fixed dental prostheses. J. Prosthet. Dent. 2014, 111, 466–475. [Google Scholar] [CrossRef]
- Ventura, J.; Jiménez-Castellanos, E.; Romero, J.; Francisco, F. Tooth Fractures in Fixed Full-Arch Implant-Supported Acrylic Resin Prostheses: A Retrospective Clinical Study. Int. J. Prosthodont. 2016, 29, 161–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montero, J.; De Paula, C.M.; Albaladejo, A. The TORONTO PROSTHESIS, an appealing method for restoring patients candidates for hybrid overdentures: A case report. J. Clin. Exp. Dent. 2012, 4, e309–e312. [Google Scholar] [CrossRef]
- Maló, P.; Nobre, M.D.A.; Borges, J.; Almeida, R. Retrievable Metal Ceramic Implant-Supported Fixed Prostheses with Milled Titanium Frameworks and All-Ceramic Crowns: Retrospective Clinical Study with up to 10 Years of Follow-Up. J. Prosthodont. 2012, 21, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Maló, P.; De Sousa, S.T.; Nobre, M.D.A.; Guedes, C.M.; Almeida, R.; Torres, A.R.; Legatheaux, J.; Silva, A. Individual Lithium Disilicate Crowns in a Full-Arch, Implant-Supported Rehabilitation: A Clinical Report. J. Prosthodont. 2014, 23, 495–500. [Google Scholar] [CrossRef]
- Al-Mazedi, M.; Razzoog, M.E.; Yaman, P. Fixed maxillary and mandibular zirconia implant frameworks milled with anatomically contoured molars: A clinical report. J. Prosthet. Dent. 2014, 112, 1013–1016. [Google Scholar] [CrossRef]
- Pozzi, A.; Tallarico, M.; Barlattani, A. Monolithic Lithium Disilicate Full-Contour Crowns Bonded on CAD/CAM Zirconia Complete-Arch Implant Bridges With 3 to 5 Years of Follow-Up. J. Oral Implant. 2015, 41, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Bidra, A.S.; Rungruanganunt, P.; Gauthier, M. Clinical outcomes of full arch fixed implant-supported zirconia prostheses: A systematic review. Eur. J. Oral Implant. 2017, 1, 35–45. [Google Scholar]
- Zoidis, P.; Papathanasiou, I.; Polyzois, G. The Use of a Modified Poly-Ether-Ether-Ketone (PEEK) as an Alternative Framework Material for Removable Dental Prostheses. A Clinical Report. J. Prosthodont. 2016, 25, 580–584. [Google Scholar] [CrossRef]
- Suwannaroop, P.; Chaijareenont, P.; Koottathape, N.; Takahashi, H.; Arksornnukit, M. In Vitro wear resistance, hardness and elastic modulus of artificial denture teet. Dent. Mater. J. 2011, 30, 461–468. [Google Scholar] [CrossRef] [Green Version]
- Bhering, C.L.B.; Mesquita, M.F.; Kemmoku, D.T.; Noritomi, P.Y.; Consani, R.L.X.; Barão, V.A.R. Comparison between all-on-four and all-on-six treatment concepts and framework material on stress distribution in atrophic maxilla: A prototyping guided 3D-FEA study. Mater. Sci. Eng. C 2016, 69, 715–725. [Google Scholar] [CrossRef] [PubMed]
- Vinayagavel, K.; Thulasingam, C.; Sabarigirinathan, C.; Mythireyi, D. Comparative study on distribution of load and stress on natural tooth and periodontium in relation to different types of restorative crown materials-a photoelastic study. Int. J. Curr. Res. Rev. 2013, 5, 69. [Google Scholar]
- De Medeiros, R.-A.; Dos Santos, D.-M.; Pesqueira, A.-A.; Campaner, M.; Bitencourt, S.; Silva, E.; Goiato, M.-C.; Da Silva, E. Stress distribution in fixed mandibular prostheses fabricated by CAD/CAM and conventional techniques: Photoelastic and strain gauge analyses. J. Clin. Exp. Dent. 2019, 11, e807–e813. [Google Scholar] [CrossRef]
- Urdaneta, R.A.; Leary, J.; Panetta, K.M.; Chuang, S.K. The effect of opposing structures, natural teeth vs. implants on crestal bone levels surrounding single-tooth implants. Clin. Oral Implants Res. 2014, 25, 179–188. [Google Scholar] [CrossRef]
- Oh, J.-H.; Kim, Y.-S.; Lim, J.Y.; Choi, B.-H. Stress Distribution on the Prosthetic Screws in the All-on-4 Concept: A Three-Dimensional Finite Element Analysis. J. Oral Implant. 2020, 46, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Korioth, T.; Hannam, A. Mandibular forces during simulated tooth clenching. J. Orofac Pain 1994, 8, 178–189. [Google Scholar] [PubMed]
- Van Oers, R.F.; Ruimerman, R.; Tanck, E.; Hilbers, P.A.; Huiskes, R. A unified theory for osteonal and hemi-osteonal remodeling. Bone 2008, 42, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Duyck, J.; Vandamme, K. The effect of loading on peri-implant bone: A critical review of the literature. J. Oral Rehabil. 2014, 41, 783–794. [Google Scholar] [CrossRef]
- Albrektsson, T.; Chrcanovic, B.; Östman, P.-O.; Sennerby, L. Initial and long-term crestal bone responses to modern dental implants. Periodontol. 2000 2016, 73, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Naert, I.; Duyck, J.; Vandamme, K. Occlusal overload and bone/implant loss. Clin. Oral Implant. Res. 2012, 23, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Rubo, J.H.; Souza, E.A.C. Finite Element Analysis of Stress in Bone Adjacent to Dental Implants. J. Oral Implant. 2008, 34, 248–255. [Google Scholar] [CrossRef]
- Bhat, V.; Kelkar, K.C.; Hegde, C. Finite element analysis of the effect of framework materials at the bone–implant interface in the all-on-four implant system. Dent. Res. J. 2021, 18, 1. [Google Scholar] [CrossRef]
- Ciftçi, Y.; Canay, S. The effect of veneering materials on stress distribution in implant-supported fixed prosthetic restorations. Int. J. Oral Maxillofac. Implant. 2000, 15, 571–582. [Google Scholar]
- Erkmen, E.; Meriç, G.; Kurt, A.; Tunç, Y.; Eser, A. Biomechanical comparison of implant retained fixed partial dentures with fiber reinforced composite versus conventional metal frameworks: A 3D FEA study. J. Mech. Behav. Biomed. Mater. 2011, 4, 107–116. [Google Scholar] [CrossRef]
- Bijjargi, S.; Chowdhary, R. Stress dissipation in the bone through various crown materials of dental implant restoration: A 2-D finite element analysis. J. Investig. Clin. Dent. 2012, 4, 172–177. [Google Scholar] [CrossRef]
- Elsayyad, A.A.; Abbas, N.A.; AbdelNabi, N.M.; Osman, R.B. Biomechanics of 3-implant-supported and 4-implant-supported mandibular screw-retained prostheses: A 3D finite element analysis study. J. Prosthet. Dent. 2020, 124, 68.e1–68.e10. [Google Scholar] [CrossRef]
- Papaspyridakos, P.; Lal, K. Computer-assisted design/computer-assisted manufacturing zirconia implant fixed complete prostheses: Clinical results and technical complications up to 4 years of function. Clin. Oral Implant. Res. 2012, 24, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Sailer, I.; Pjetursson, B.E.; Zwahlen, M.; Hämmerle, C.H.F. A systematic review of the survival and complication rates of all-ceramic and metal-ceramic reconstructions after an observation period of at least 3 years. Part II: Fixed dental prostheses. Clin. Oral Implant. Res. 2007, 18, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Pjetursson, B.E.; Brägger, U.; Lang, N.P.; Zwahlen, M. Comparison of survival and complication rates of tooth-supported fixed dental prostheses (FDPs) and implant-supported FDPs and single crowns (SCs). Clin. Oral Implant. Res. 2007, 18, 97–113. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, J.; Triplett, R.G. Complications and Clinical Considerations of the Implant-Retained Zirconia Complete-Arch Prosthesis with Various Opposing Dentitions. Int. J. Oral Maxillofac. Implant. 2017, 32, 864–869. [Google Scholar] [CrossRef]
- Ishigaki, S.; Nakano, T.; Yamada, S.; Nakamura, T.; Takashima, F. Biomechanical stress in bone surrounding an implant under simulated chewing. Clin. Oral Implant. Res. 2003, 14, 97–102. [Google Scholar] [CrossRef]
- Magne, P.; Silva, M.; Oderich, E.; Boff, L.L.; Enciso, R. Damping behavior of implant-supported restorations. Clin. Oral Implant. Res. 2011, 24, 143–148. [Google Scholar] [CrossRef]
- Koosha, S.; Mirhashemi, F.S. An Investigation of Three types of Tooth Implant Supported Fixed Prosthesis Designs with 3D Finite Element Analysis. J. Dent. 2013, 10, 51–63. [Google Scholar]
- Park, S.-Y.; Kim, Y.-G.; Suh, J.-Y.; Lee, D.-H.; Lee, J.-M. Long-term outcomes of adjacent and antagonistic teeth after implant restoration: A focus on patient-related factors. J. Periodontal. Implant. Sci. 2021, 51, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Villefort, R.F.; Tribst, J.P.M.; Piva, A.M.D.O.D.; Borges, A.L.; Binda, N.C.; Ferreira, C.E.D.A.; Bottino, M.A.; Von Zeidler, S.L.V. Stress distribution on different bar materials in implant-retained palatal obturator. PLoS ONE 2020, 15, e0241589. [Google Scholar] [CrossRef]
- Tribst, J.P.M.; Dal Piva, A.M.d.O.; Lo Giudice, R.; Borges, A.L.S.; Bottino, M.A.; Epifania, E.; Ausiello, P. The Influence of Custom-Milled Framework Design for an Implant-Supported Full-Arch Fixed Dental Prosthesis: 3D-FEA Study. Int. J. Environ. Res. Public Health 2020, 17, 4040. [Google Scholar] [CrossRef] [PubMed]
- Piva, A.M.D.O.D.; Tribst, J.M.; De Morais, D.C.; Alonso, A.A.; Borges, A. Comparative three-dimensional finite element analysis of implant-supported fixed complete arch mandibular prostheses in two materials. J. Indian Prosthodont. Soc. 2017, 17, 255–260. [Google Scholar] [CrossRef]
- Lee, K.-S.; Shin, S.-W.; Lee, S.-P.; Kim, J.-E.; Kim, J.H.; Lee, J.-Y. Comparative Evaluation of a Four-Implant–Supported Polyetherketoneketone Framework Prosthesis: A Three-Dimensional Finite Element Analysis Based on Cone Beam Computed Tomography and Computer-Aided Design. Int. J. Prosthodont. 2017, 30, 581–585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sirandoni, D.; Leal, E.; Weber, B.; Noritomi, P.Y.; Fuentes, R.; Borie, E. Effect of Different Framework Materials in Implant-Supported Fixed Mandibular Prostheses: A Finite Element Analysis. Int. J. Oral Maxillofac. Implant. 2019, 34, e107–e114. [Google Scholar] [CrossRef]
- Shrikar, R.D.; Harshada, H.S. Finite Element Analysis: Basics and its applications in dentistry. Indian J. Dent. Sci. 2012, 4, 60–65. [Google Scholar]
- Lisiak-Myszke, M.; Marciniak, D.; Bieliński, M.; Sobczak, H.; Garbacewicz, Ł.; Drogoszewska, B. Application of Finite Element Analysis in Oral and Maxillofacial Surgery—A Literature Review. Materials 2020, 13, 3063. [Google Scholar] [CrossRef]
- Viceconti, M.; Zannoni, C.; Testi, D.; Petrone, M.; Perticoni, S.; Quadrani, P.; Taddei, F.; Imboden, S.; Clapworthy, G. The multimod application framework: A rapid application development tool for computer aided medicine. Comput. Methods Programs Biomed. 2007, 85, 138–151. [Google Scholar] [CrossRef] [PubMed]
- Commisso, M.S.; Martínez-Reina, J.; Ojeda, J.; Mayo, J. Finite element analysis of the human mastication cycle. J. Mech. Behav. Biomed. Mater. 2015, 41, 23–35. [Google Scholar] [CrossRef]
- Müller, F.; Hernandez, M.; Grütter, L.; Aracil-Kessler, L.; Weingart, D.; Schimmel, M. Masseter muscle thickness, chewing efficiency and bite force in edentulous patients with fixed and removable implant-supported prostheses: A cross-sectional multicenter study. Clin. Oral Implant. Res. 2011, 23, 144–150. [Google Scholar] [CrossRef] [Green Version]
- Gümrükçü, Z.; Korkmaz, Y.T.; Korkmaz, F.M. Biomechanical evaluation of implant-supported prosthesis with various tilting implant angles and bone types in atrophic maxilla: A finite element study. Comput. Biol. Med. 2017, 86, 47–54. [Google Scholar] [CrossRef]
- Bozyel, D.; Faruk, S.T. Biomechanical Behavior of All-on-4 and M-4 Configurations in an Atrophic Maxilla: A 3D Finite Element Method. Med. Sci. Monit. 2021, 27, e929908-1. [Google Scholar] [CrossRef]
- Kim, Y.; Oh, T.-J.; Misch, C.E.; Wang, H.-L. Occlusal considerations in implant therapy: Clinical guidelines with biomechanical rationale. Clin. Oral Implant. Res. 2005, 16, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Ozan, O.; Kurtulmus-Yilmaz, S. Biomechanical Comparison of Different Implant Inclinations and Cantilever Lengths in All-on-4 Treatment Concept by Three-Dimensional Finite Element Analysis. Int. J. Oral Maxillofac. Implant. 2018, 33, 64–71. [Google Scholar] [CrossRef] [PubMed]
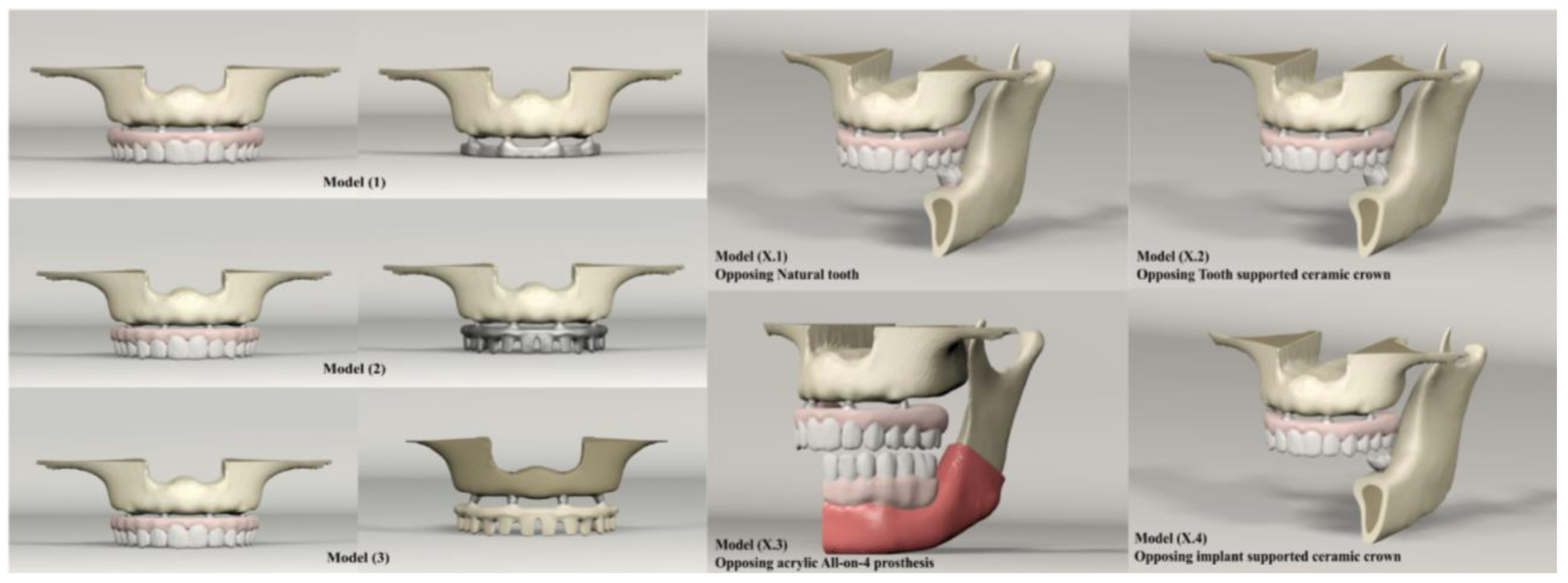
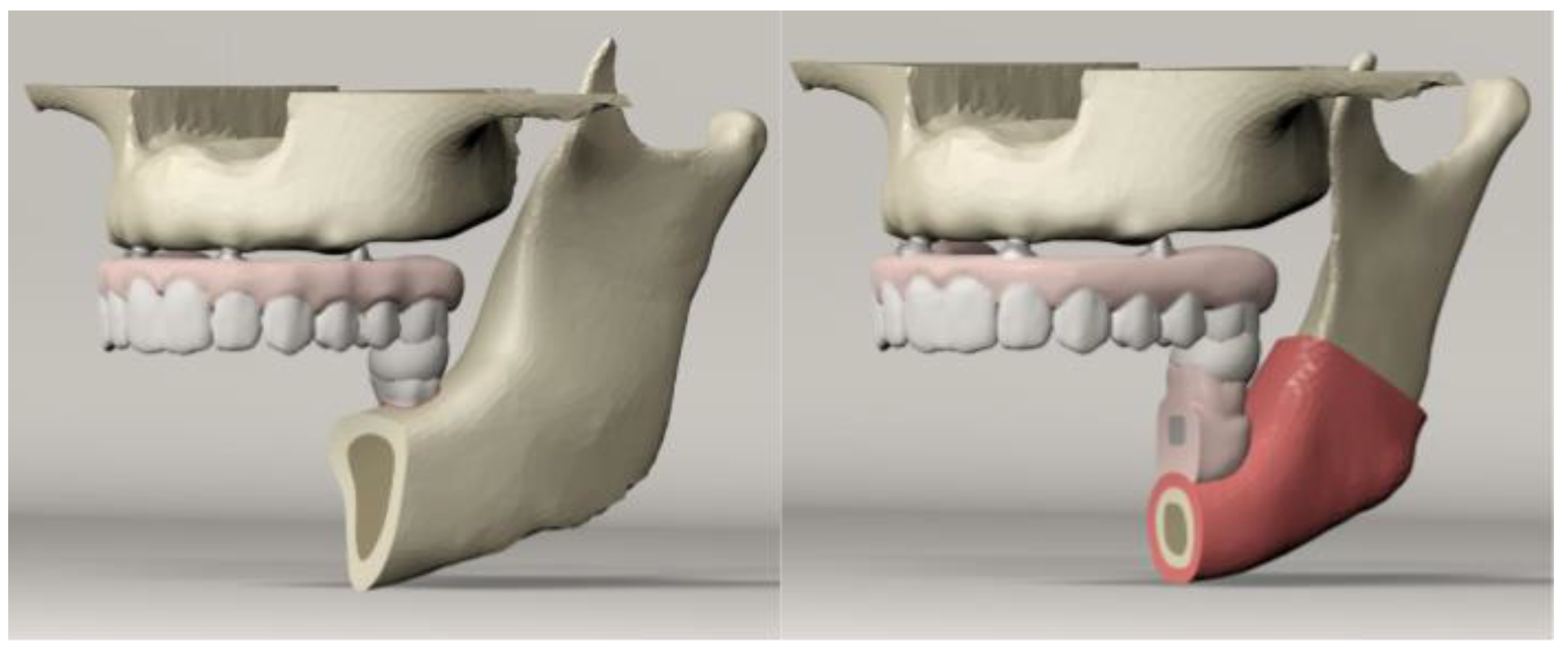
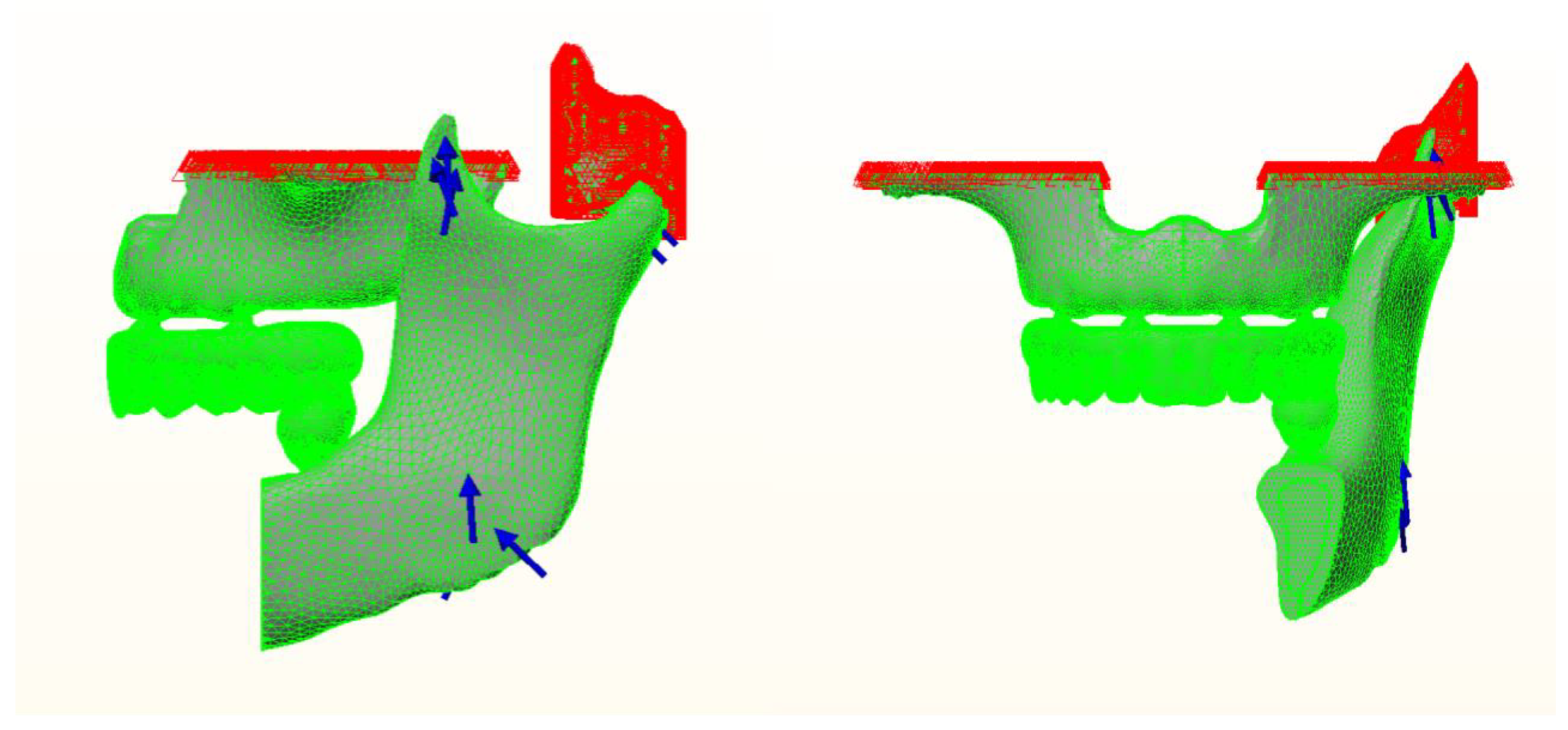




| Maxilla | Mandible | Number of Elements | Number of Nodes |
|---|---|---|---|
| Model 1: Titanium bar with acrylic teeth | 1.1 Natural tooth | 1,173,283 | 265,982 |
| 1.2 Full ceramic crown | 1,214,741 | 279,924 | |
| 1.3 Acrylic All-on-4 | 1,502,434 | 365,627 | |
| 1.4 Implant-supported ceramic crown | 1,347,938 | 299,558 | |
| Model 2: Titanium bar with resin composite gingiva and ceramic superstructure with zirconium (Toronto bridge) | 2.1 Natural tooth | 1,256,911 | 283,379 |
| 2.2 Full ceramic crown | 1,298,424 | 297,321 | |
| 2.3 Acrylic All-on-4 | 1,586,062 | 383,002 | |
| 2.4 Implant-supported ceramic crown | 1,431,626 | 316,955 | |
| Model 3: PEEK bar with composite resin gingiva and ceramic superstructure with zirconium (Toronto bridge) | 3.1 Natural tooth | 1,256,927 | 283,379 |
| 3.2 Full ceramic crown | 1,298,373 | 297,321 | |
| 3.3 Acrylic All-on-4 | 1,586,062 | 383,002 | |
| 3.4 Implant-supported ceramic crown | 1,431,566 | 316,955 |
| Material | Elastic Modulus | Poisson Ratio |
|---|---|---|
| Tempro-mandibular disk | 44.1 | 0.4 |
| Cortical bone | 13,700 | 0.3 |
| Spongy bone | 1370 | 0.3 |
| Periodontal ligament | 68.9 | 0.45 |
| Mucosa | 1 | 0.37 |
| Dentin | 18,600 | 0.32 |
| Enamel | 84,100 | 0.33 |
| Acrylic resin | 2200 | 0.31 |
| Titanium (Grade 4) | 105,000 | 0.37 |
| Titanium (Grade 5) | 114,000 | 0.33 |
| Zircon | 210,000 | 0.3 |
| Poly-Ether Ether Keton (PEEK) | 4100 | 0.4 |
| Composite (Gradia) | 50,000 | 0.3 |
| Feldspathic ceramic | 82,800 | 0.35 |
| Food stuff | 84.1 | 0.33 |
| Muscles | Node Number | Weighting Factor (Newton) |
|---|---|---|
| Right | ||
| Superficial masseter | 67 | 190.4 |
| Deep masseter | 38 | 81.6 |
| Medial pterygoid | 51 | 174.8 |
| Anterior temporalis | 43 | 158 |
| Middle temporalis | 18 | 95.6 |
| Posterior temporalis | 15 | 75.6 |
| Inferior lateral pterygoid | 5 | 66.9 |
| Superior lateral pterygoid | 4 | 28,7 |
| Anterior digastric | 8 | 40 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haroun, F.; Ozan, O. Evaluation of Stresses on Implant, Bone, and Restorative Materials Caused by Different Opposing Arch Materials in Hybrid Prosthetic Restorations Using the All-on-4 Technique. Materials 2021, 14, 4308. https://doi.org/10.3390/ma14154308
Haroun F, Ozan O. Evaluation of Stresses on Implant, Bone, and Restorative Materials Caused by Different Opposing Arch Materials in Hybrid Prosthetic Restorations Using the All-on-4 Technique. Materials. 2021; 14(15):4308. https://doi.org/10.3390/ma14154308
Chicago/Turabian StyleHaroun, Feras, and Oguz Ozan. 2021. "Evaluation of Stresses on Implant, Bone, and Restorative Materials Caused by Different Opposing Arch Materials in Hybrid Prosthetic Restorations Using the All-on-4 Technique" Materials 14, no. 15: 4308. https://doi.org/10.3390/ma14154308
APA StyleHaroun, F., & Ozan, O. (2021). Evaluation of Stresses on Implant, Bone, and Restorative Materials Caused by Different Opposing Arch Materials in Hybrid Prosthetic Restorations Using the All-on-4 Technique. Materials, 14(15), 4308. https://doi.org/10.3390/ma14154308





