Evaluation of the Mechanical and Biocidal Properties of Lapacho from Tabebuia Plant as a Biocomposite Material
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
- PLA-type 2003D (Cargill Down LLC, Minnetonka, MN, USA) with a melt flow rate of 4.2 g/10 min. (2.16 kg; at 190 °C) and a density ρ of 1.24 g/cm3 was used as a materials matrix.
- Cortex Lapacho fibers (Posadas, Argentina/Brazil) with a fiber length of 20 mm in the amount of 1–10 wt% were melt-compounded with the PLA matrix.
- Enzyme Proteinase K from Tritirachium album (Blirt, Poland), a buffer of 0.1 M Tris HCl and sodium azide (NaN3), was used for enzymatic degradation tests.
2.2. Processing
2.3. Measurements
3. Results
3.1. Mechanical Properties
3.2. Material Structure
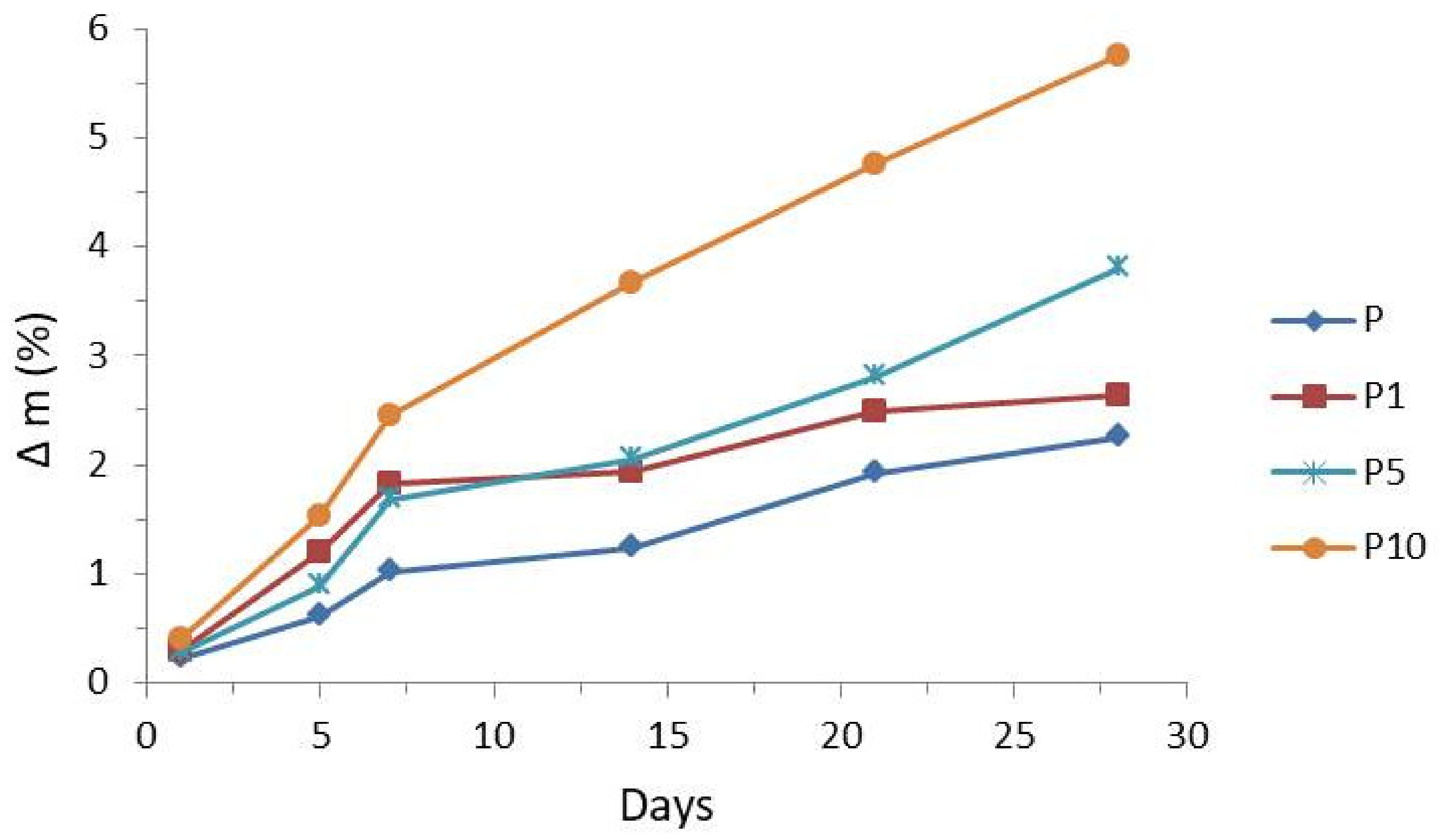
3.3. Enzymatic Degradation Testing
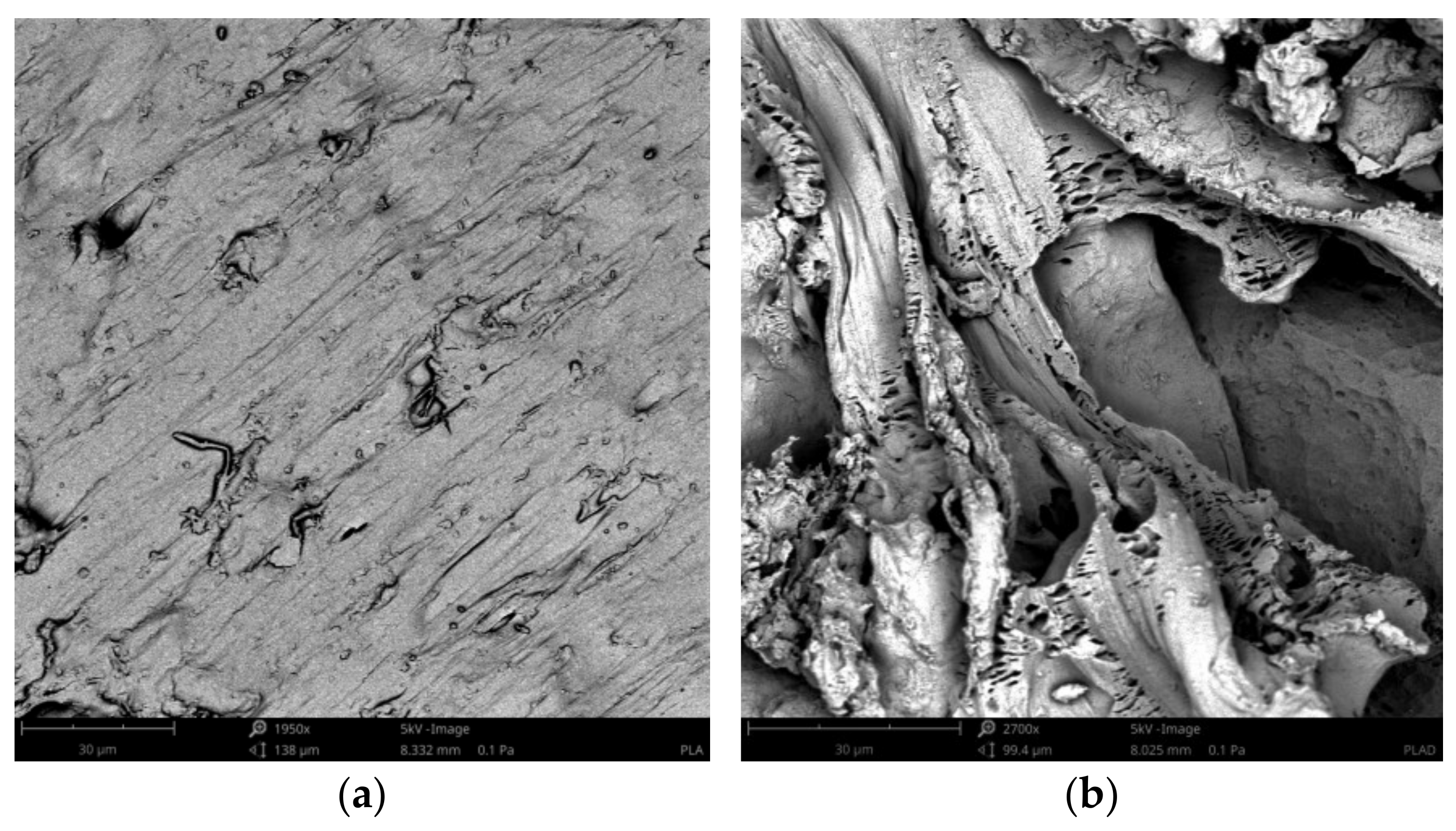
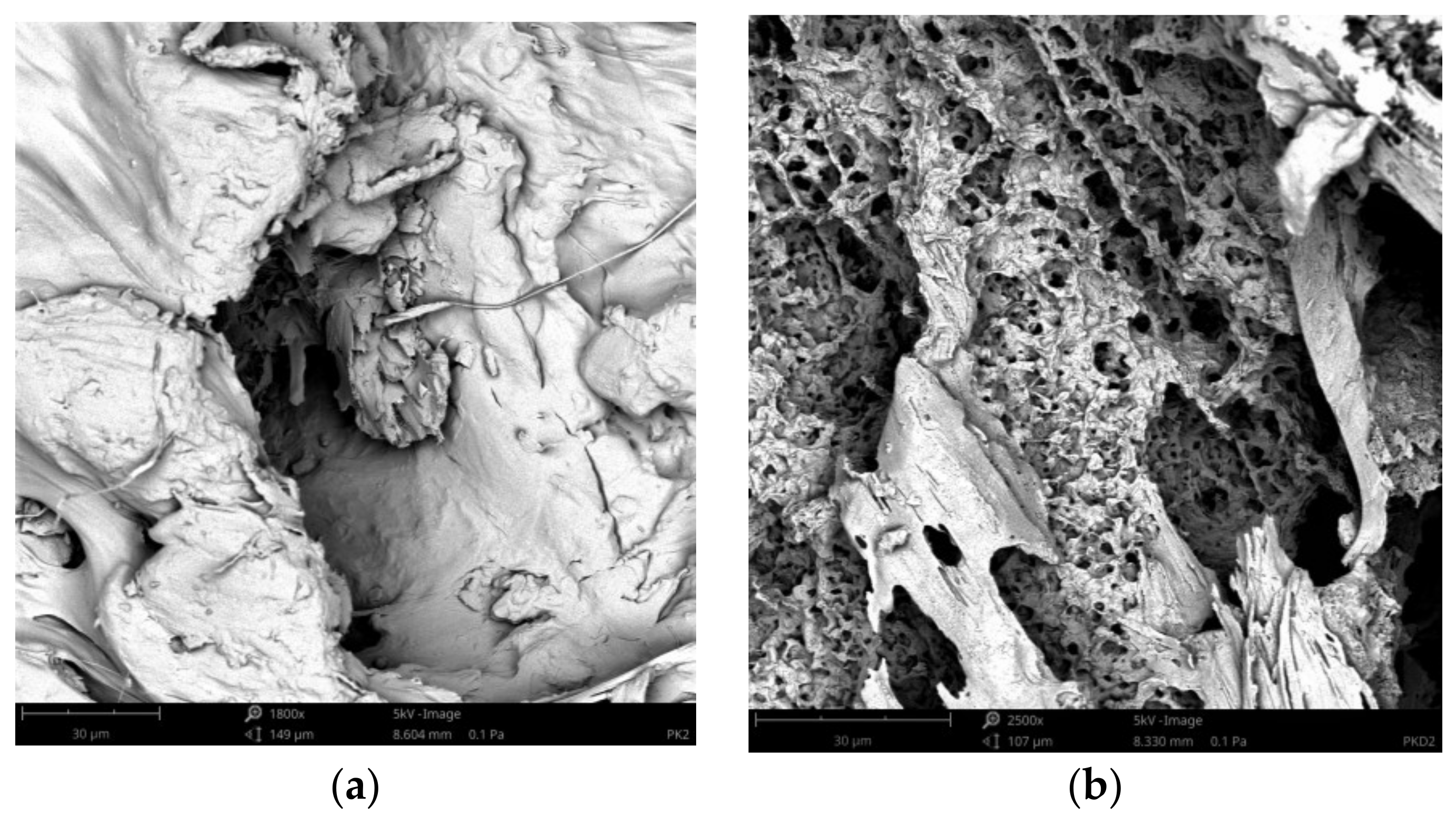
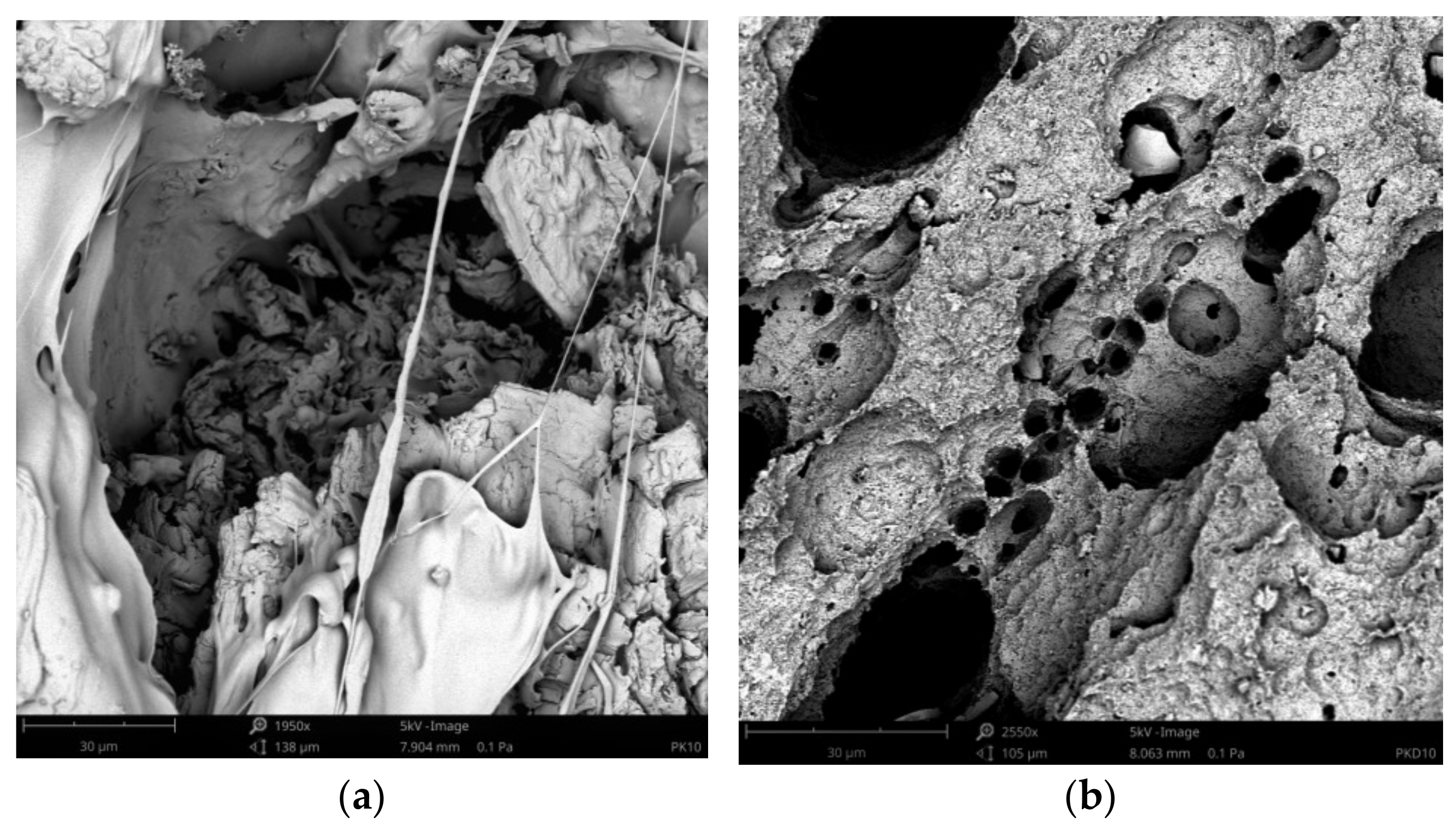
3.4. Surface Morphology Changes
3.5. Antimicrobial Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saini, P.; Arora, M.; Kumar, M.N.V.R. Poly(lactic acid) blends in biomedical applications. Adv. Drug Deliv. Rev. 2016, 107, 47–59. [Google Scholar] [CrossRef]
- Castro-Aguirre, E.; Iñiguez-Franco, F.; Samsudin, H.; Fang, X.; Auras, R. Poly(lactic acid)—Mass production, processing, industrial applications, and end of life. Adv. Drug Deliv. Rev. 2016, 107, 333–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Auras, R.; Harte, B.; Selke, S. An overview of polylactides as packaging materials. Macromol. Biosci. 2004, 4, 835–864. [Google Scholar] [CrossRef]
- Auras, R.A.; Lim, L.T.; Selke, S.E.M.; Tsuji, H. Poly(Lactic Acid): Synthesis, Structures, Properties, Processing and Applications; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010. [Google Scholar]
- Gupta, A.P.; Kumar, V. New emerging trends in synthetic biodegradable polymers—Polylactide: A critique. Eur. Polym. J. 2007, 43, 4053–4074. [Google Scholar] [CrossRef]
- Tokiwa, Y.; Calabia, B.P. Biodegradability and biodegradation of poly(lactide). Appl. Microbiol. Biotechnol. 2006, 72, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Rasal, R.M.; Janorkar, A.V.; Hirt, D.E. Poly(lactic acid) modifications. Prog. Polym. Sci. (Oxf.) 2010, 35, 338–356. [Google Scholar] [CrossRef]
- Sanjay, M.R.; Madhu, P.; Jawaid, M.; Senthamaraikannan, P.; Senthil, S.; Pradeep, S. Characterization and properties of natural fiber polymer composites: A comprehensive review. J. Clean. Prod. 2018, 172, 566–581. [Google Scholar] [CrossRef]
- Singh, T.; Gangil, B.; Patnaik, A.; Biswas, D.; Fekete, G. Agriculture waste reinforced corn starch-based biocomposites: Effect of rice husk/walnut shell on physicomechanical, biodegradable and thermal properties. Mater. Res. Express 2019, 6, 045702. [Google Scholar] [CrossRef]
- Joseph, B.; Sagarika, V.K.; Sabu, C.; Kalarikkal, N.; Thomas, S. Cellulose nanocomposites: Fabrication and biomedical applications. J. Bioresour. Bioprod. 2020, 5, 223–237. [Google Scholar] [CrossRef]
- Faruk, O.; Bledzki, A.K.; Fink, H.P.; Sain, M. Biocomposites reinforced with natural fibers: 2000–2010. Prog. Polym. Sci. 2012, 37, 1552–1596. [Google Scholar] [CrossRef]
- Faruk, O.; Sain, M. Biofiber reinforced polymer composites for structural applications. In Developments in Fiber-Reinforced Polymer (FRP) Composites for Civil Engineering; Woodhead Publishing: Sawston, UK, 2013; pp. 18–53. ISBN 978-0-85709-234-2. [Google Scholar]
- Mohammed, L.; Ansari, M.N.M.; Pua, G.; Jawaid, M.; Islam, M.S. A Review on Natural Fiber Reinforced Polymer Composite and Its Applications. Int. J. Polym. Sci. 2015, 2015, 243947. [Google Scholar] [CrossRef] [Green Version]
- Mehta, G.; Mohanty, A.K.; Thayer, K.; Misra, M.; Drzal, L.T. Novel biocomposites sheet molding compounds for low cost housing panel applications. J. Polym. Environ. 2005, 13, 169–175. [Google Scholar] [CrossRef]
- Li, X.; Tabil, L.G.; Panigrahi, S. Chemical treatments of natural fiber for use in natural fiber-reinforced composites: A review. J. Polym. Environ. 2007, 15, 25–33. [Google Scholar] [CrossRef]
- Lee, B.H.; Kim, H.J.; Yu, W.R. Fabrication of long and discontinuous natural fiber reinforced polypropylene biocomposites and their mechanical properties. Fibers Polym. 2009, 10, 83–90. [Google Scholar] [CrossRef]
- Cao, Y.; Wu, Y.Q. Evaluation of statistical strength of bamboo fiber and mechanical properties of fiber reinforced green composites. J. Cent. South Univ. Technol. 2008, 15, 564–567. [Google Scholar] [CrossRef]
- Kozłowski, R. Types, Properties and Factors Affecting Breeding and Cultivation. In Handbook of Natural Fibres, 2nd ed.; Woodhead Publishing: Sawston, UK; Cambridge, UK, 2020; Volume 1, ISBN 9780128183984. [Google Scholar]
- Kozłowski, R. Processing and Applications. In Handbook of Natural Fibres; Woodhead Publishing: Sawston, UK; Cambridge, UK, 2020; Volume 2, ISBN 9780128187821. [Google Scholar]
- Gurunathan, T.; Mohanty, S.; Nayak, S.K. A review of the recent developments in biocomposites based on natural fibres and their application perspectives. Compos. Part A Appl. Sci. Manuf. 2015, 77, 1–25. [Google Scholar] [CrossRef]
- Moliner, C.; Finocchio, E.; Arato, E.; Ramis, G.; Lagazzo, A. Influence of the degradation medium on water uptake, morphology, and chemical structure of Poly(Lactic Acid)-Sisal bio-composites. Materials 2020, 13, 3974. [Google Scholar] [CrossRef]
- Verma, D.; Senal, I. Natural fiber-reinforced polymer composites. In Biomass, Biopolymer-Based Materials, and Bioenergy: Construction, Biomedical, and other Industrial Applications; Woodhead Publishing: Sawston, UK, 2019; pp. 103–122. ISBN 9780081024263. [Google Scholar]
- Das, S.K.; Chakraborty, S.; Naskar, S.; Rajabalaya, R. Techniques and methods used for the fabrication of bionanocomposites. Bionanocomposites Tissue Eng. Regen. Med. 2021, 17–43. [Google Scholar] [CrossRef]
- Khairnar, Y.; Hansora, D.; Hazra, C.; Kundu, D.; Tayde, S.; Tonde, S.; Naik, J.; Chatterjee, A. Cellulose bionanocomposites for sustainable planet and people: A global snapshot of preparation, properties, and applications. Carbohydr. Polym. Technol. Appl. 2021, 2, 100065. [Google Scholar] [CrossRef]
- Ates, B.; Koytepe, S.; Ulu, A.; Gurses, C.; Thakur, V.K. Chemistry, structures, and advanced applications of nanocomposites from biorenewable resources. Chem. Rev. 2020, 120, 9304–9362. [Google Scholar] [CrossRef]
- Siqueira, G.; Bras, J.; Dufresne, A. Cellulosic bionanocomposites: A review of preparation, properties and applications. Polymers 2010, 2, 728–765. [Google Scholar] [CrossRef] [Green Version]
- Azmana, M.; Mahmood, S.; Hilles, A.R.; Rahman, A.; Arifin, M.A.B.; Ahmed, S. A review on chitosan and chitosan-based bionanocomposites: Promising material for combatting global issues and its applications. Int. J. Biol. Macromol. 2021, 185, 832–848. [Google Scholar] [CrossRef]
- Cabañas-Romero, L.V.; Valls, C.; Valenzuela, S.V.; Roncero, M.B.; Pastor, F.I.J.; Diaz, P.; Martínez, J. Bacterial Cellulose-Chitosan Paper with Antimicrobial and Antioxidant Activities. Biomacromolecules 2020, 21, 1568–1577. [Google Scholar] [CrossRef] [PubMed]
- Akram, N.; Anjum, M.N.; Malik, F.Z.; Rehman, S.; Hafeez, I.; Murtaza, M.A.; Arshad, M.U. Bionanocomposites in food industry. Bionanocomposites 2020, 421–456. [Google Scholar] [CrossRef]
- Sucinda, E.F.; Majid, M.S.A.; Ridzuan, M.J.M.; Cheng, E.M.; Alshahrani, H.A.; Mamat, N. Development and characterisation of packaging film from Napier cellulose nanowhisker reinforced polylactic acid (PLA) bionanocomposites. Int. J. Biol. Macromol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Chi, C. Development of pullulan/carboxylated cellulose nanocrystal/tea polyphenol bionanocomposite films for active food packaging. Int. J. Biol. Macromol. 2021, 186, 405–413. [Google Scholar] [CrossRef]
- Stepczyńska, M. Surface Modification by Low Temperature Plasma: Sterilization of Biodegradable Materials. Plasma Process. Polym. 2016, 13, 1078–1086. [Google Scholar] [CrossRef]
- Stepczyńska, M. Research of biocidal effect of corona discharges on poly(lactic acid) packaging films. J. Food Eng. 2014, 126, 56–61. [Google Scholar] [CrossRef]
- Paterno, E. Ricerche sull’acido lapachico. Gazz Chim It 1882, 12, 337–392. [Google Scholar]
- Grolig, J.; Wagner, R. Naphthoquinones. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, Germany, 2005. [Google Scholar]
- Eyong, K.; Kumar, P.; Kuete, V. Semisynthesis and antitumoral activity of 2-acetylfuranonaphthoquinone and other naphthoquinone derivatives from lapachol. Bioorg. Med. Chem. Lett. 2008, 18, 5387–5390. [Google Scholar] [CrossRef]
- Suryanegara, L.; Nakagaito, A.Y.H. The effect of crystallization of PLA on the thermal and mechanical properties of microfibrillated cellulose-reinforced PLA composites. Compos. Sci. Technol. 2009, 69, 1187–1192. [Google Scholar] [CrossRef]
- Xanthos, M. Functional Fillers for Plastics, 1st ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2005. [Google Scholar]
- Shebani, A.N.; van Reenen, A.J.; Meincken, M. The effect of wood extractives on the thermal stability of different wood-LLDPE composites. Thermochim. Acta 2009, 481, 52–56. [Google Scholar] [CrossRef]
- Rytlewski, P.; Stepczyńska, M.; Gohs, U.; Malinowski, R.; Budner, B.; Żenkiewicz, M. Flax fibres reinforced polylactide modified by ionizing radiation. Ind. Crop. Prod. 2018, 112, 716–723. [Google Scholar] [CrossRef]
- Rytlewski, P.; Stepczyńska, M.; Moraczewski, K.; Malinowski, R.; Jagodziński, B.; Żenkiewicz, M. Mechanical properties and biodegradability of flax fiber-reinforced composite of polylactide and polycaprolactone. Polim. Polym. 2018, 63, 603–610. [Google Scholar] [CrossRef]
- Wielage, B.; Lampke, T.; Marx, G.; Nestler, K.; Starke, D. Thermogravimetric and differential scanning calorimetric analysis of natural fibres and polypropylene. Thermochim. Acta 1999, 337, 169–177. [Google Scholar] [CrossRef]
- Gröndahl, M.; Teleman, A.G.P. Effect of acetylation on the material properties of glucuronoxylanfrom aspen wood. Carbohydr. Polym. 2003, 52, 359–366. [Google Scholar] [CrossRef]
- Meszaros, E.; Jakab, E.; Varhegyi, G. TG/MS, Py-GC/MS and THM-GC/MS study of the composition andthermal behavior of extractive components ofRobinia pseudoacacia. J. Anal. Appl. Pyrolysis 2007, 79, 61–70. [Google Scholar] [CrossRef]
- Marcovich, N.E.; Villar, M.A. Thermal and mechanical characterization of linear low-density polyethylene/wood flour composites. J. Appl. Polym. Sci. 2003, 90, 2775–2784. [Google Scholar] [CrossRef]
- Nunez, A.J.; Kenny, J.M.; Reboredo, M.M.; Aranguren, M.I.; Marcovich, N.E. Thermal and dynamic mechanical characterization of polypropylene-woodflour composites. Polym. Eng. Sci. 2002, 42, 733–742. [Google Scholar] [CrossRef]
- Kim, H.; Kim, S.; Kim, H.Y.H. Thermal properties of bio-flour-filled polyolefin composites withdifferent compatibilizing agent type and content. Thermochim. Acta 2006, 451, 181–188. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- McKendry, P. Energy production from biomass (part 1): Overview of biomass. Biores. Technol. 2002, 83, 37–46. [Google Scholar] [CrossRef]
- Rao, T.R.; Sharma, A. Pyrolysis rates of biomass materials. Energy 1998, 23, 973–978. [Google Scholar] [CrossRef]
- Stepczyńska, M.; Rytlewski, P. Enzymatic degradation of flax-fibers reinforced polylactide. Int. Biodeterior. Biodegrad. 2018, 126, 160–166. [Google Scholar] [CrossRef]

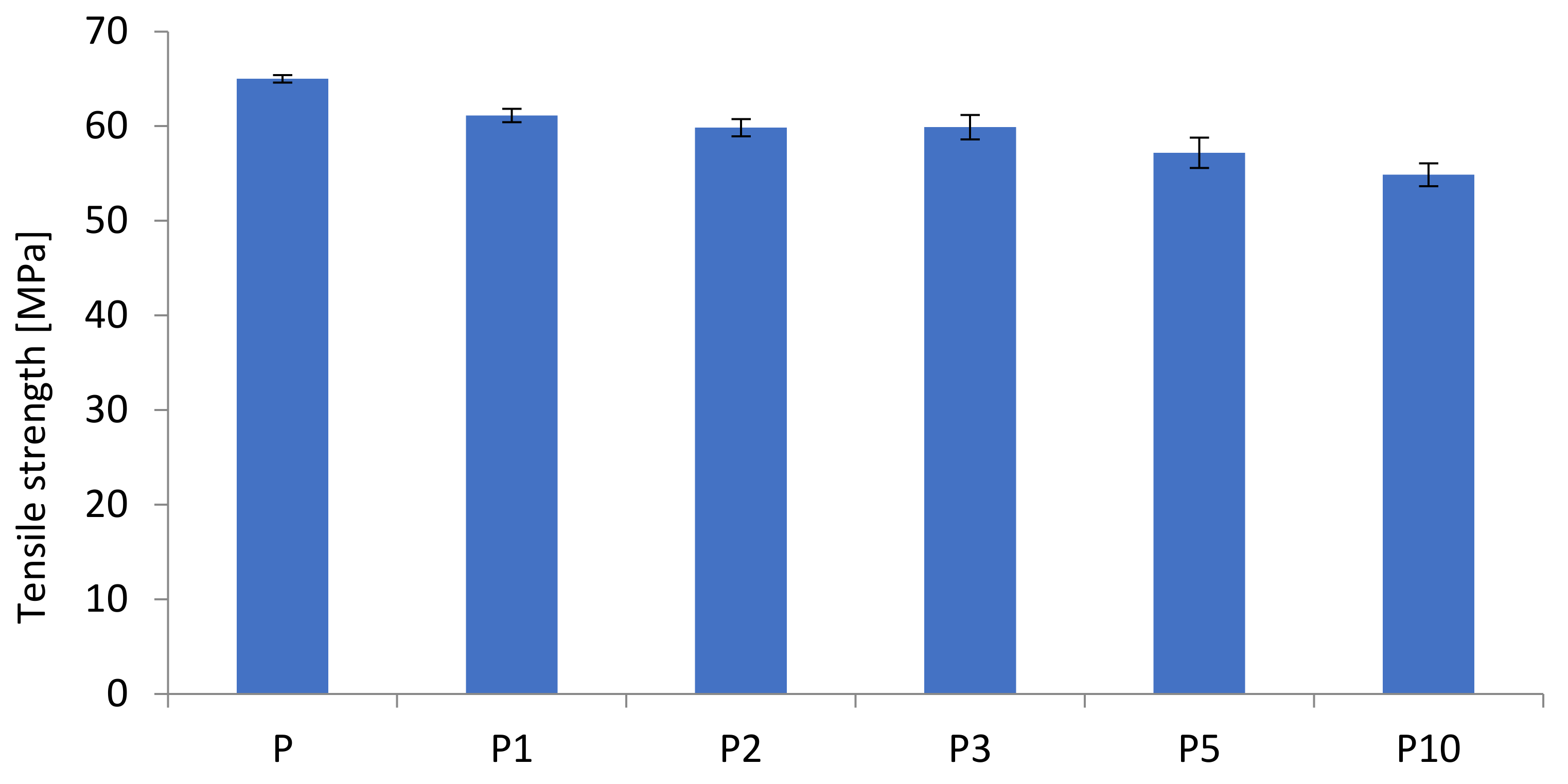
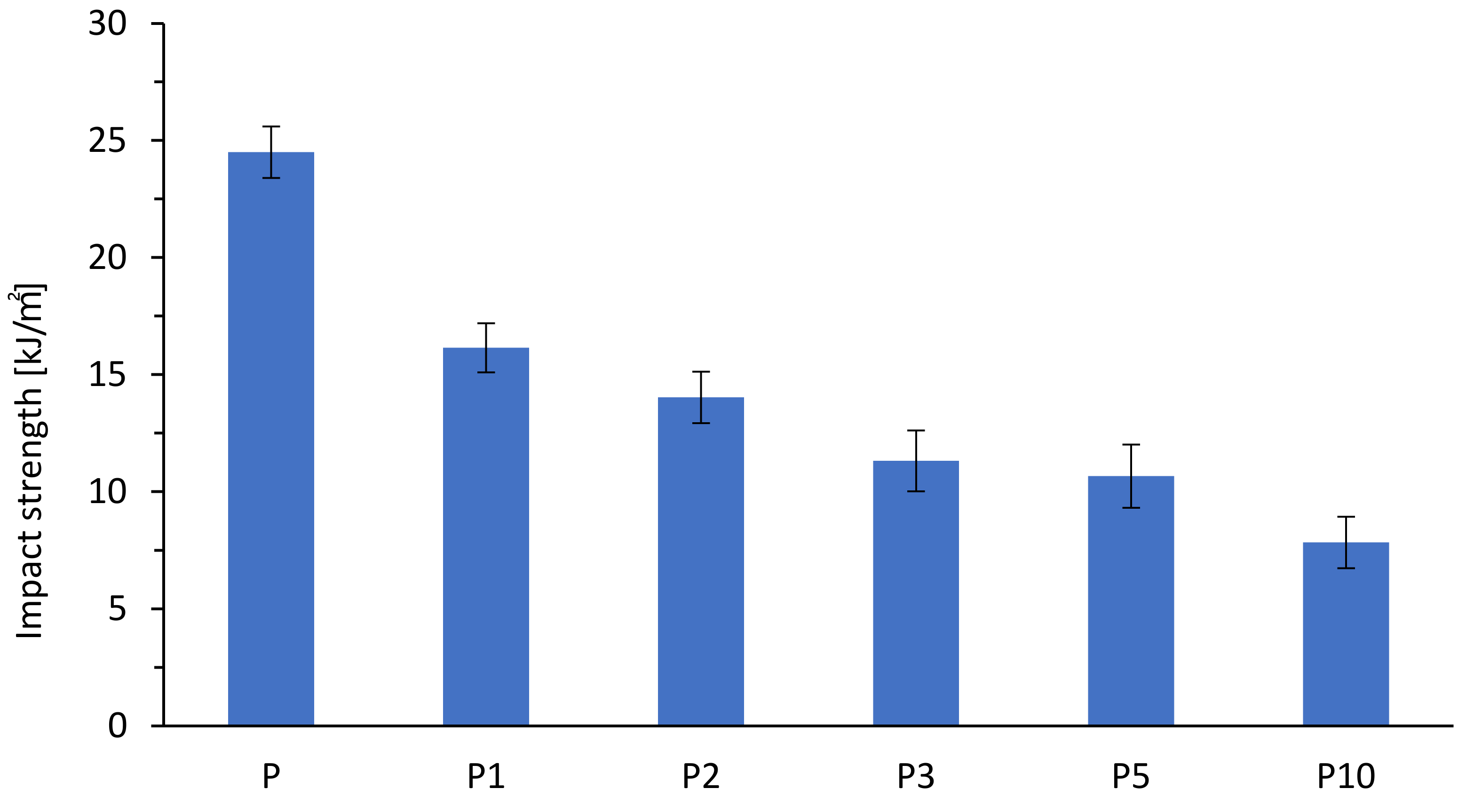
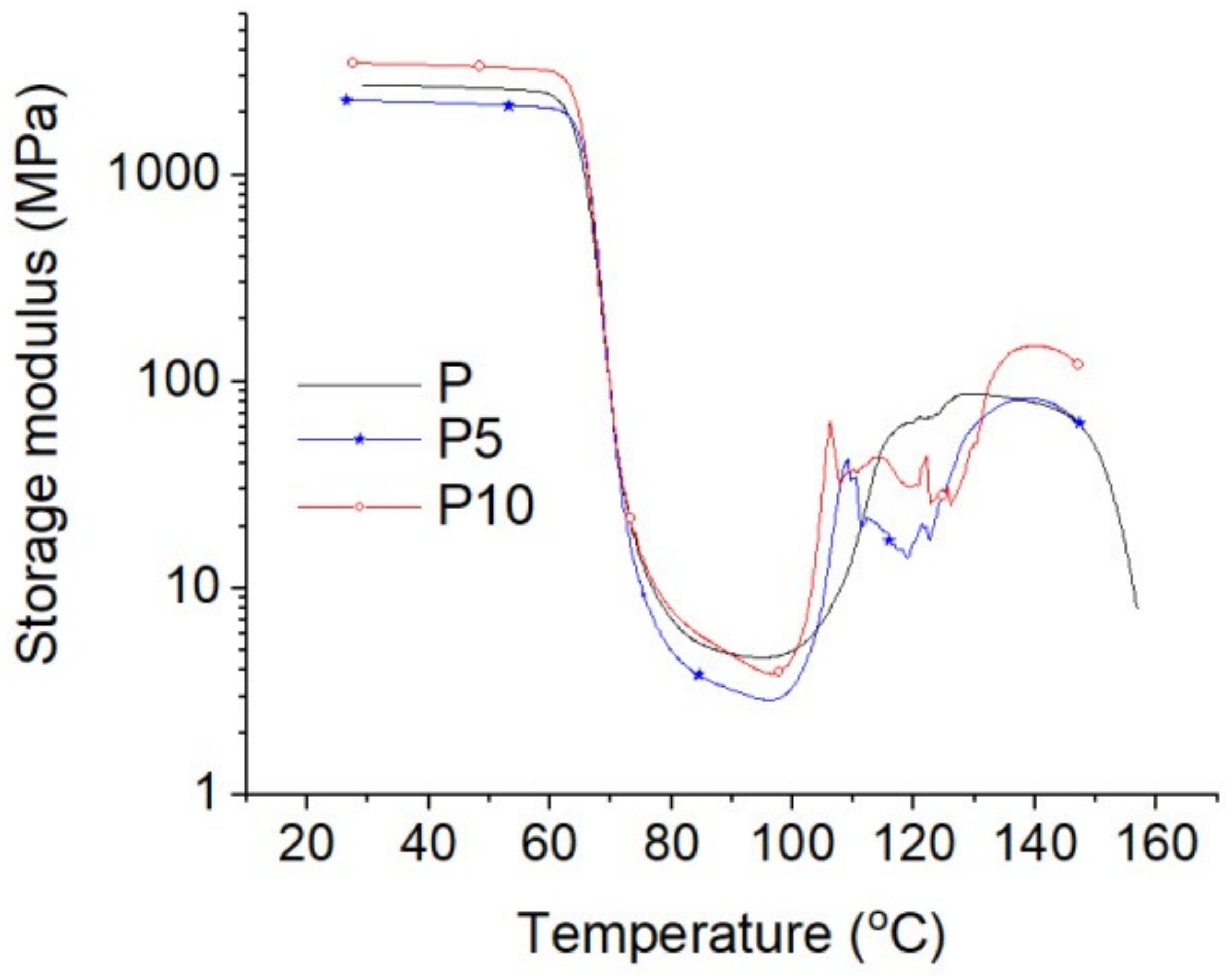

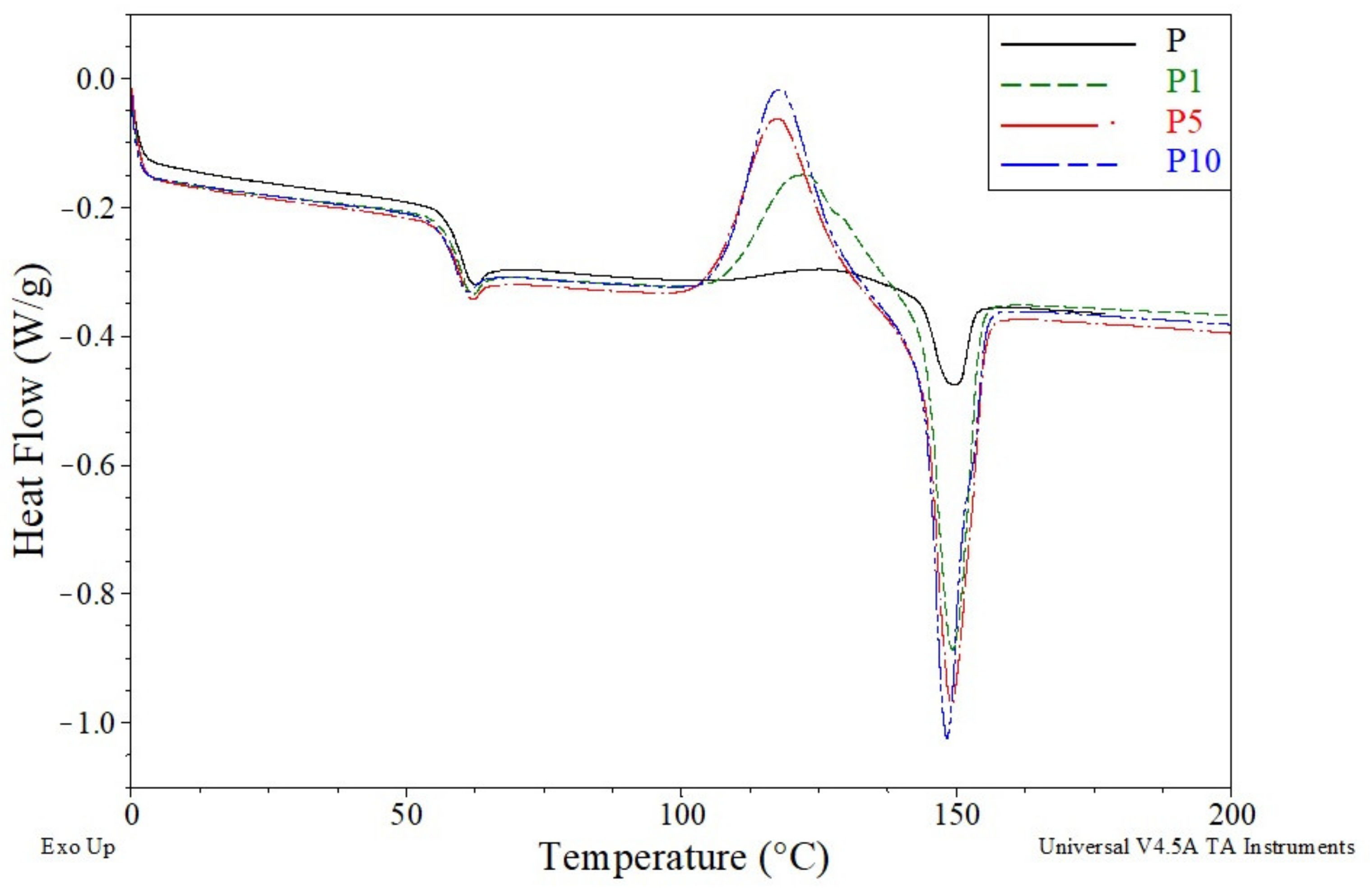

| Sample | Tensile Strength (MPa) | Elongation at Break (%) | E-Modulus (MPa) |
|---|---|---|---|
| P | 65.0 ± 0.4 | 4.58 ± 0.17 | 2050 ± 200 |
| P1 | 61.1 ± 0.7 | 3.89 ± 0.23 | 1992.58 ± 190 |
| P2 | 59.8 ± 0.9 | 3.72 ± 0.15 | 2107.93 ± 144 |
| P3 | 59.9 ± 1.3 | 3.51 ± 0.15 | 2264.97 ± 48 |
| P5 | 57.2 ± 1.6 | 3.19 ± 0.32 | 2275.01 ± 75 |
| P10 | 54.9 ± 1.2 | 2.87 ± 0.24 | 2458.80 ± 47 |
| Sample | Tg (°C) | Tcc (°C) | ΔHcc (J/g) | Tm (°C) | ΔHm (J/g) |
|---|---|---|---|---|---|
| P | 60.2 | 127.1 | 4.4 | 149.6 | 5.2 |
| P1 | 59.8 | 122.8 | 20.4 | 149.3 | 21.0 |
| P2 | 59.9 | 121.2 | 22.4 | 148.9 | 23.1 |
| P3 | 59.8 | 119.6 | 23.1 | 148.6 | 23.8 |
| P5 | 59.8 | 117.9 | 26.7 | 149.2 | 27.5 |
| P10 | 58.8 | 118.2 | 26.9 | 148.3 | 28.1 |
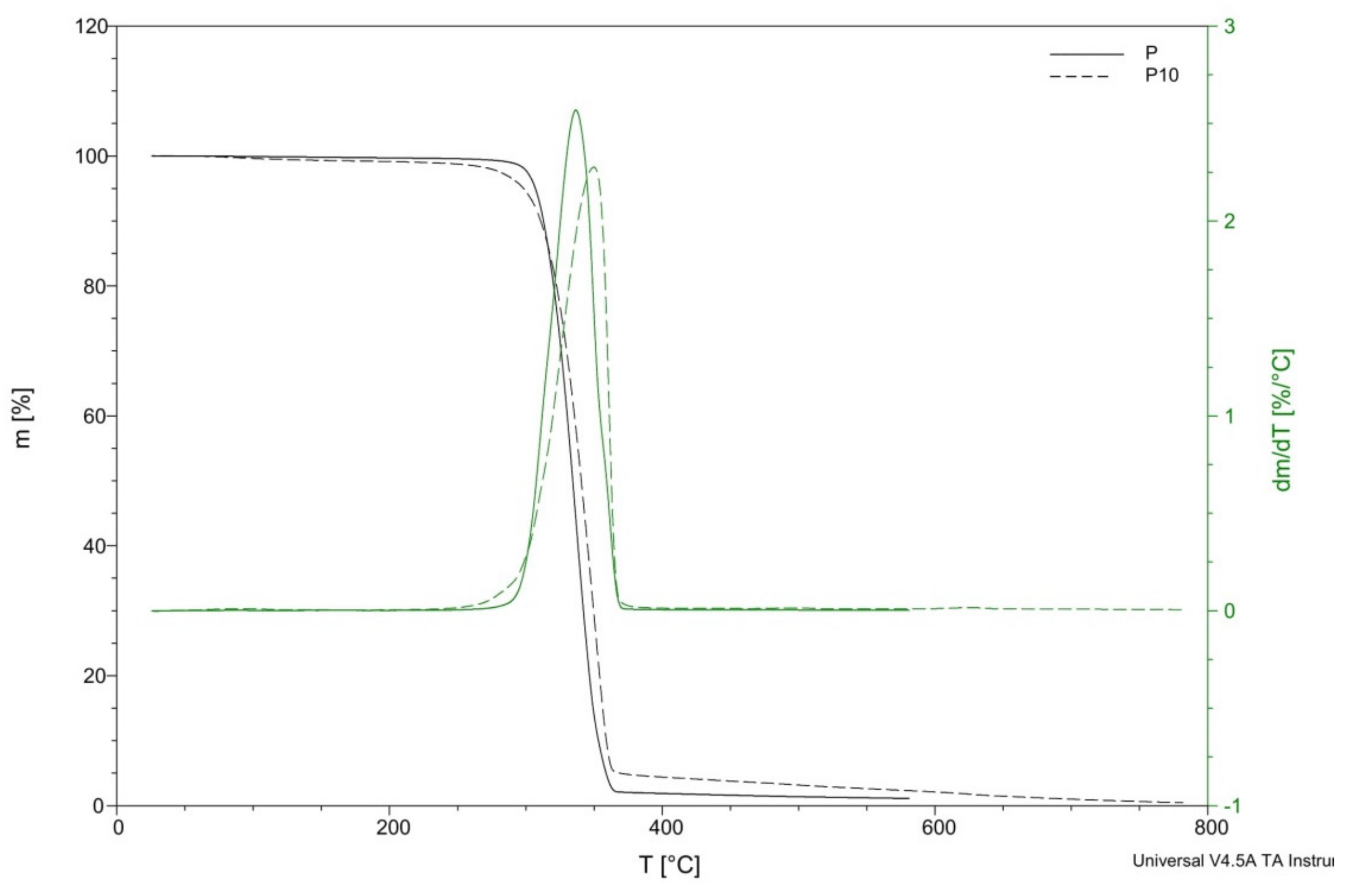
| Sample | Td (°C) | Td/dt (°C) | T5% (°C) | T25% (°C) | T50% (°C) | T95% (°C) | Residue at 450 °C (%) |
|---|---|---|---|---|---|---|---|
| P | 315.68 | 337.32 | 306.94 | 323.25 | 334.36 | 358.78 | 1.6 |
| P1 | 328.99 | 356.62 | 312.45 | 335.69 | 348.08 | 367.51 | 1.36 |
| P2 | 325.14 | 355.62 | 306.28 | 330.95 | 344.16 | 363.43 | 1.42 |
| P3 | 316.48 | 351.86 | 301.63 | 324.66 | 338.35 | 359.97 | 1.66 |
| P5 | 311.91 | 344.86 | 284.07 | 317.34 | 333.84 | 357.53 | 3.2 |
| P10 | 321.74 | 349.71 | 298.30 | 326.45 | 340.63 | 368.96 | 3.8 |
| Tested Bacteria * | Cortex Content (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 5 | 10 | ||||||
| ×106 (jtk/mL) | R | ×106 (jtk/mL) | R | ×106 (jtk/mL) | R | ×106 (jtk/mL) | R | ×106 (jtk/mL) | R | |
| Escherichia coli (E. coli) | 1.1 | 0 | 1.2 | 0 | 1.0 | 0 | 1.0 | 0 | 0.3 | 1 |
| Staphylococcus aureus (S. aureus) | 1.0 | 0 | 0.9 | 1 | 0.9 | 1 | 0.9 | 1 | 0.1 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stepczyńska, M.; Pawłowska, A.; Moraczewski, K.; Rytlewski, P.; Trafarski, A.; Olkiewicz, D.; Walczak, M. Evaluation of the Mechanical and Biocidal Properties of Lapacho from Tabebuia Plant as a Biocomposite Material. Materials 2021, 14, 4241. https://doi.org/10.3390/ma14154241
Stepczyńska M, Pawłowska A, Moraczewski K, Rytlewski P, Trafarski A, Olkiewicz D, Walczak M. Evaluation of the Mechanical and Biocidal Properties of Lapacho from Tabebuia Plant as a Biocomposite Material. Materials. 2021; 14(15):4241. https://doi.org/10.3390/ma14154241
Chicago/Turabian StyleStepczyńska, Magdalena, Alona Pawłowska, Krzysztof Moraczewski, Piotr Rytlewski, Andrzej Trafarski, Daria Olkiewicz, and Maciej Walczak. 2021. "Evaluation of the Mechanical and Biocidal Properties of Lapacho from Tabebuia Plant as a Biocomposite Material" Materials 14, no. 15: 4241. https://doi.org/10.3390/ma14154241
APA StyleStepczyńska, M., Pawłowska, A., Moraczewski, K., Rytlewski, P., Trafarski, A., Olkiewicz, D., & Walczak, M. (2021). Evaluation of the Mechanical and Biocidal Properties of Lapacho from Tabebuia Plant as a Biocomposite Material. Materials, 14(15), 4241. https://doi.org/10.3390/ma14154241








