Effect of Titanium and Zirconium Oxide Microparticles on Pro-Inflammatory Response in Human Macrophages under Induced Sterile Inflammation: An In Vitro Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Titanium, Zirconium and Glass Microparticles
2.3. Macrophage Differentiation and Particle/LPS Stimulation
2.4. Cell Viability Assay
2.5. Gene Expression Analysis
2.6. Statistical Analysis
3. Results
3.1. Effect of Particles on Cell Viability
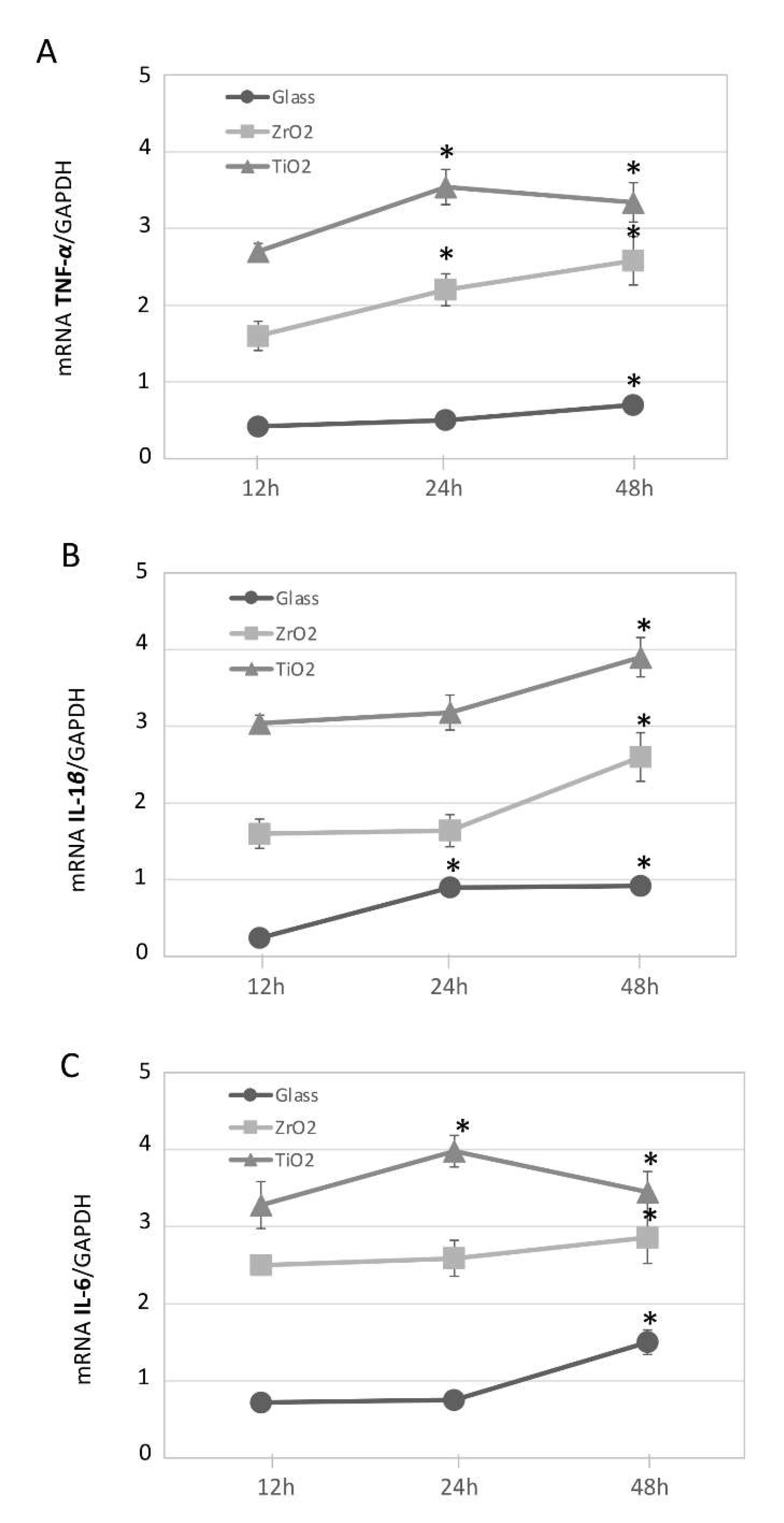
3.2. Particle-Induced Pro-Inflammatory Gene Expression in Macrophage Cultures Over Time
3.3. Particle and Sterile LPS Inflammation-Induced Pro-Inflammatory Gene Expression
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Branemark, P.I.; Adell, R.; Breine, U.; Hansson, B.O.; Lindstrom, J.; Ohlsson, A. Intraosseous anchorage of dental prostheses. I. Experimental studies. Scand. J. Plast. Reconstr. Surg. 1969, 3, 81–100. [Google Scholar] [CrossRef]
- Branemark, P.I.; Hansson, B.O.; Adell, R.; Breine, U.; Lindström, J.; Hallén, O.; Ohman, A. Osseointegrated implants in the treatment of the edentulous jaw. Experience from a 10-year period. Scand. J. Plast. Reconstr. Surg. Suppl. 1977, 16, 1–132. [Google Scholar]
- Albrektsson, T.; Branemark, P.I.; Hansson, H.A.; Lindstrom, J. Osseointegrated titanium implants. Requirements for ensuring a long-lasting, direct bone to implant anchorage in man. Acta Orthop. Scand. 1981, 52, 155–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adell, R.; Eriksson, B.; Lekholm, U.; Branemark, P.I.; Jemt, T. Long-term follow-up study of osseointegrated implants in the treatment of totally edentulous jaws. Int. J. Oral Maxillofac. Implant. 1990, 5, 347–359. [Google Scholar]
- Lindquist, L.W.; Carlsson, G.E.; Jemt, T. A prospective 15-year follow-up study of mandibular fixed prostheses supported by osseointegrated implants. Clinical results and marginal bone loss. Clin. Oral Implant. Res. 1996, 7, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Pjetursson, B.E.; Tan, K.; Lang, N.P.; Bragger, U.; Egger, M.; Zwahlen, M. A systematic review of the survival and complication rates of fixed partial dentures (FPDs) after an observation period of at least 5 years. Clin. Oral Implant. Res. 2004, 15, 625–642. [Google Scholar] [CrossRef] [PubMed]
- Berglundh, T.; Armitage, G.; Araujo, M.G.; Avila-Ortiz, G.; Blanco, J.; Camargo, P.M.; Chen, S.; Cochran, D.; Derks, J.; Figuero, E.; et al. Peri-implant diseases and conditions: Consensus report of workgroup 4 of the 2017 world workshop on the classification of periodontal and Peri-implant diseases and conditions. J. Clin. Periodontol. 2018, 45, 286–291. [Google Scholar] [CrossRef] [Green Version]
- Albrektsson, T.; Isidor, F. Consensus Report of Session-IV. In Proceedings of the First European Workshop on Periodontology, Thurgau, Switzerland, 1–4 February 1993; Lang, N.P., Karring, T., Eds.; Quintessence Publishing: London, UK, 1994; pp. 365–369. [Google Scholar]
- Roos-Jansaker, A.M.; Lindahl, C.; Renvert, H.; Renvert, S. Nine- to fourteen-year follow- up of implant treatment. Part II: Presence of peri-implant lesions. J. Clin. Periodontol. 2006, 33, 290–295. [Google Scholar] [CrossRef]
- Fransson, C.; Lekholm, U.; Jemt, T.; Berglundh, T. Prevalence of subjects with progressive bone loss at implants. Clin. Oral Implant. Res. 2005, 16, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Koldsland, O.C.; Scheie, A.A.; Aass, A.M. Prevalence of peri-implantitis related to severity of the disease with different degrees of bone loss. J. Periodontol. 2010, 81, 231–238. [Google Scholar] [CrossRef]
- Renvert, S.; Roos-Jansaker, A.M.; Lindahl, C.; Renvert, H.; Rutger Persson, G. Infection at titanium implants with or without a clinical diagnosis of inflammation. Clin. Oral Implant. Res. 2007, 18, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Rinke, S.; Ohl, S.; Ziebolz, D.; Lange, K.; Eickholz, P. Prevalence of periimplant disease in partially edentulous patients: A practice-based cross-sectional study. Clin. Oral Implant. Res. 2011, 22, 826–833. [Google Scholar] [CrossRef]
- Zitzmann, N.U.; Abrahamsson, I.; Berglundh, T.; Lindhe, J. Soft tissue reactions to plaque formation at implant abutments with different surface topography. An experimental study in dogs. J. Clin. Periodontol. 2002, 29, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Mombelli, A.; Decaillet, F. The characteristics of biofilms in peri-implant disease. J. Clin. Periodontol. 2011, 38, 203–213. [Google Scholar] [CrossRef] [Green Version]
- Charalampakis, G.; Belibasakis, G.N. Microbiome of peri-implant infections: Lessons from conventional, molecular and metagenomic analyses. Virulence 2015, 6, 183–187. [Google Scholar] [CrossRef] [Green Version]
- Belibasakis, G.N. Microbiological and immuno-pathological aspects of periimplant diseases. Arch. Oral Biol. 2014, 59, 66–72. [Google Scholar] [CrossRef] [Green Version]
- Yu, T.; Acharya, A.; Mattheos, N.; Li, S.; Ziebolz, D.; Schmalz, G.; Haak, R.; Schmidt, J.; Sun, Y. Molecular mechanisms linking peri-implantitis and type 2 diabetes mellitus revealed by transcriptomic analysis. PeerJ 2019, 7, e7124. [Google Scholar] [CrossRef] [PubMed]
- Atieh, M.A.; Alsabeeha, N.H.; Faggion, C.M., Jr.; Duncan, W.J. The frequency of periimplant diseases: A systematic review and meta-analysis. J. Periodontol. 2013, 84, 1586–1598. [Google Scholar]
- Senna, P.; Antoninha Del Bel Cury, A.; Kates, S.; Meirelles, L. Surface damage on dental implants with release of loose particles after insertion into bone. Clin. Implant. Dent. Relat. Res. 2015, 17, 681–692. [Google Scholar] [CrossRef] [Green Version]
- Fretwurst, T.; Nelson, K.; Tarnow, D.P.; Wang, H.L.; Giannobile, W.V. Is metal particle release associated with Peri-implant bone destruction? An emerging concept. J. Dent. Res. 2018, 97, 259–265. [Google Scholar] [CrossRef]
- Kuroda, P.A.B.; de Freitas Quadros, F.; Sousa, K.D.S.J.; Donato, T.A.G.; de Araújo, R.O.; Grandini, C.R. Preparation, structural, microstructural, mechanical and cytotoxic b. characterization of as-cast Ti-25Ta-Zr alloys. J. Mater. Sci. Mater. Med. 2020, 31, 19. [Google Scholar] [CrossRef]
- Jawed, S.F.; Rabadia, C.D.; Liu, Y.J.; Wang, L.Q.; Qin, P.; Li, Y.H.; Zhang, X.H.; Zhang, L.C. Strengthening mechanism and corrosion resistance of beta-type Ti-Nb-Zr-Mn alloys. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 110, 110728. [Google Scholar] [CrossRef] [PubMed]
- Sicilia, A.; Cuesta, S.; Coma, G.; Arregui, I.; Guisasola, C.; Ruiz, E.; Maestro, A. Titanium allergy in dental implant patients: A clinical study on 1500 consecutive patients. Clin. Oral Implants. Res. 2008, 19, 823–835. [Google Scholar] [CrossRef] [PubMed]
- Muller, K.; Valentine-Thon, E. Hypersensitivity to titanium: Clinical and laboratory evidence. Neuro Endocrinol. Lett. 2006, 27, 31–35. [Google Scholar]
- Weingart, D.; Steinemann, S.; Schilli, W.; Strub, J.; Hellerich, U.; Assenmacher, J.; Simpson, J. Titanium deposition in regional lymph nodes after insertion of titanium screw implants in maxillofacial region. Int. J. Oral Maxillofac. Surg. 1994, 23, 450–452. [Google Scholar] [CrossRef]
- Toumelin-Chemla, F.; Rouelle, F.; Burdairon, G. Corrosive properties of fluoride-containing odontologic gels against titanium. J. Dent. 1996, 24, 109–115. [Google Scholar] [CrossRef]
- Sanon, C.; Chevalier, J.; Douillard, T.; Cattani-Lorente, M.; Scherrer, S.S.; Gremillard, L. A new testing protocol for zirconia dental implants. Dent. Mater. 2015, 31, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Lughi, V.; Sergo, V. Low temperature degradation-aging-of zirconia: A critical review of the relevant aspects in dentistry. Dent. Mater. 2010, 26, 807–820. [Google Scholar] [CrossRef] [PubMed]
- Noronha Oliveira, M.; Schunemann, W.V.H.; Mathew, M.T.; Henriques, B.; Magini, R.S.; Teughels, W.; Souza, J.C.M. Can degradation products released from dental implants affect peri-implant tissues? J. Periodontal Res. 2018, 53, 1–11. [Google Scholar] [CrossRef]
- Mareci, D.; Chelariu, R.; Gordin, D.M.; Ungureanu, G.; Gloriant, T. Comparative corrosion study of Ti-ta alloys for dental applications. Acta Biomater. 2009, 5, 3625–3639. [Google Scholar] [CrossRef]
- Sikora, C.L.; Alfaro, M.F.; Yuan, J.C.; Barao, V.A.; Sukotjo, C.; Mathew, M.T. Wear and corrosion interactions at the titanium/zirconia Interface: Dental implant application. J. Prosthodont. 2018, 27, 842–852. [Google Scholar] [CrossRef]
- Stimmelmayr, M.; Edelhoff, D.; Guth, J.F.; Erdelt, K.; Happe, A.; Beuer, F. Wear at the titanium- titanium and the titanium-zirconia implant-abutment interface: A comparative in vitro study. Dent. Mater. 2012, 28, 1215–1220. [Google Scholar] [CrossRef] [PubMed]
- Barao, V.A.; Mathew, M.T.; Assuncao, W.G.; Yuan, J.C.; Wimmer, M.A.; Sukotjo, C. Stability of cp Ti and Ti-6Al-4V alloy for dental implants as a function of saliva pH-an electrochemical study. Clin. Oral Implant. Res. 2012, 23, 1055–1062. [Google Scholar] [CrossRef]
- Olmedo, D.G.; Nalli, G.; Verdú, S.; Paparella, M.L.; Cabrini, R.L. Exfoliative cytology and titanium dental implants: A pilot study. J. Periodontol. 2013, 84, 78–83. [Google Scholar] [CrossRef]
- Olmedo, D.G.; Paparella, M.L.; Spielberg, M.; Brandizzi, D.; Guglielmotti, M.B.; Cabrini, R.L. Oral mucosa tissue response to titanium cover screws. J. Periodontol. 2012, 83, 973–980. [Google Scholar] [CrossRef] [PubMed]
- Wilson, T.G., Jr.; Valderrama, P.; Burbano, M.; Blansett, J.; Levine, R.; Kessler, H.; Rodrigues, D.C. Foreign bodies associated with peri-implantitis human biopsies. J. Periodontol. 2015, 86, 9–15. [Google Scholar] [CrossRef]
- Okuda-Shimazaki, J.; Takaku, S.; Kanehira, K.; Sonezaki, S.; Taniguchi, A. Effects of titanium dioxide nanoparticle aggregate size on gene expression. Int. J. Mol. Sci. 2010, 11, 2383–2392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, K.; Hou, Y.; Hu, Y.; Zhao, L.; Luo, Z.; Shi, Y.; Lai, M.; Yang, W.; Liu, P. Correlation of the cytotoxicity of TiO2 nanoparticles with different particle sizes on a sub-200-nm scale. Small 2011, 7, 3026–3031. [Google Scholar] [CrossRef]
- Irshad, M.; Scheres, N.; Crielaard, W.; Loos, B.G.; Wismeijer, D.; Laine, M.L. Influence of titanium on in vitro fibroblast-Porphyromonas gingivalis interaction in peri-implantitis. J. Clin. Periodontol. 2013, 40, 841–849. [Google Scholar] [CrossRef] [PubMed]
- Pettersson, M.; Kelk, P.; Belibasakis, G.N.; Bylund, D.; Molin Thoren, M.; Johansson, A. Titanium ions form particles that activate and execute interleukin-1beta release from lipopolysaccharide-primed macrophages. J. Periodontal Res. 2017, 52, 21–32. [Google Scholar] [CrossRef] [Green Version]
- Olmedo, D.G.; Tasat, D.R.; Evelson, P.; Rebagliatti, R.; Guglielmotti, M.B.; Cabrini, R.L. In vivo comparative biokinetics and biocompatibility of titanium and zirconium microparticles. J. Biomed. Mater. Res. Part A 2011, 98, 604–613. [Google Scholar] [CrossRef]
- Ishii, K.J.; Koyama, S.; Nakagawa, A.; Coban, C.; Akira, S. Host innate immune receptors and beyond: Making sense of microbial infections. Cell Host Microbe 2008, 3, 352–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dostert, C.; Petrilli, V.; Van Bruggen, R.; Steele, C.; Mossman, B.T.; Tschopp, J. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science 2008, 320, 674–677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hornung, V.; Bauernfeind, F.; Halle, A.; Samstad, E.O.; Kono, H.; Rock, K.L.; Fitzgerald, K.A.; Latz, E. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat. Immunol. 2008, 9, 847–856. [Google Scholar] [CrossRef]
- Hornung, V.; Ablasser, A.; Charrel-Dennis, M.; Bauernfeind, F.; Horvath, G.; Caffrey, D.R.; Latz, E.; Fitzgerald, K.A. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature 2009, 458, 514–518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanneganti, T.D.; Lamkanfi, M.; Kim, Y.G.; Chen, G.; Park, J.H.; Franchi, L.; Vandenabeele, P.; Núñez, G. Pannexin-1-mediated recognition of bacterial molecules activates the cryopyrin inflammasome independent of Toll-like receptor signaling. Immunity 2007, 26, 433–443. [Google Scholar] [CrossRef]
- Brunner, T.J.; Grass, R.N.; Stark, W.J. Glass and bioglass nanopowders by flame synthesis. Chem. Commun. 2006, 7, 1384–1386. [Google Scholar] [CrossRef]
- Jäger, F.; Mohn, D.; Attin, T.; Tauböck, T.T. Polymerization and shrinkage stress formation of experimental resin composites doped with nano- vs. micron-sized bioactive glasses. Dent. Mater. J. 2021, 40, 110–115. [Google Scholar] [CrossRef]
- Schoenenberger, A.D.; Schipanski, A.; Malheiro, V.; Kucki, M.; Snedeker, J.G.; Wick, P.; Maniura-Weber, K. Macrophage Polarization by Titanium Dioxide (TiO2) Particles: Size Matters. ACS Biomater. Sci. Eng. 2016, 2, 908–919. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Hartlieb, E.; Rothmund, L.; Waschke, J.; Wu, X.; Van Landuyt, K.L.; Milz, S.; Michalke, B.; Hickel, R.; Reichl, F.X.; et al. Intracellular uptake and toxicity of three different titanium particles. Dent. Mater. 2015, 31, 734–744. [Google Scholar] [CrossRef]
- Nkamgueu, E.M.; Adnet, J.J.; Bernard, J.; Zierold, K.; Kilian, L.; Jallot, E.; Benhayoune, H.; Bonhomme, P. In vitro effects of zirconia and alumina particles on human blood monocyte-derived macrophages: X-ray microanalysis and flow cytometric studies. J. Biomed. Mater. Res. 2000, 52, 587–594. [Google Scholar] [CrossRef]
- Day, R.M.; Boccaccini, A.R. Effect of particulate bioactive glasses on human macrophages and monocytes in vitro. J. Biomed. Mater. Res. A 2005, 73, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Cabal, B.; Alou, L.; Cafini, F.; Couceiro, R.; Sevillano, D.; Esteban-Tejeda, L.; Guitián, F.; Torrecillas, R.; Moya, J.S. A new biocompatible and antibacterial phosphate free glass-ceramic for medical applications. Sci. Rep. 2014, 4, 5440. [Google Scholar] [CrossRef] [PubMed]
- Trindade, R.; Albrektsson, T.; Tengvall, P.; Wennerberg, A. Foreign body reaction to biomaterials: On mechanisms for buildup and breakdown of osseointegration. Clin. Implant. Dent. Relat. Res. 2016, 18, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Pajarinen, J.; Kouri, V.P.; Jamsen, E.; Li, T.F.; Mandelin, J.; Konttinen, Y.T. The response of macrophages to titanium particles is determined by macrophage polarization. Acta Biomater. 2013, 9, 9229–9240. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramenzoni, L.L.; Flückiger, L.B.; Attin, T.; Schmidlin, P.R. Effect of Titanium and Zirconium Oxide Microparticles on Pro-Inflammatory Response in Human Macrophages under Induced Sterile Inflammation: An In Vitro Study. Materials 2021, 14, 4166. https://doi.org/10.3390/ma14154166
Ramenzoni LL, Flückiger LB, Attin T, Schmidlin PR. Effect of Titanium and Zirconium Oxide Microparticles on Pro-Inflammatory Response in Human Macrophages under Induced Sterile Inflammation: An In Vitro Study. Materials. 2021; 14(15):4166. https://doi.org/10.3390/ma14154166
Chicago/Turabian StyleRamenzoni, Liza L., Laura B. Flückiger, Thomas Attin, and Patrick R. Schmidlin. 2021. "Effect of Titanium and Zirconium Oxide Microparticles on Pro-Inflammatory Response in Human Macrophages under Induced Sterile Inflammation: An In Vitro Study" Materials 14, no. 15: 4166. https://doi.org/10.3390/ma14154166
APA StyleRamenzoni, L. L., Flückiger, L. B., Attin, T., & Schmidlin, P. R. (2021). Effect of Titanium and Zirconium Oxide Microparticles on Pro-Inflammatory Response in Human Macrophages under Induced Sterile Inflammation: An In Vitro Study. Materials, 14(15), 4166. https://doi.org/10.3390/ma14154166






