Some Aspects of the Thermochemical Route for the Valorization of Plastic Wastes, Part I: Reduction of Iron Oxides by Polyvinyl Chloride (PVC)
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Physicochemical Characterization of the Experiment Specimens
3.2. Treatment of the PVC and Clay Samples at Different Temperatures
3.3. Treatment of (Hematite + PVC + Clay) Mixtures in Various Conditions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Plastics—The Facts. 2019. Available online: https://www.plasticseurope.org/application/files/9715/7129/9584/FINAL_web_version_Plastics_the_facts2019_14102019.pdf (accessed on 15 October 2020).
- OECD. Improving Plastics Management: Trends, Policy Responses, and the Role of International Co-Operation and Trade; OECD Environment Policy Papers No. 12; OECD Publishing: Paris, France, 2018; Available online: https://www.oecd-ilibrary.org/environment/improving-plastics-management_c5f7c448-en (accessed on 14 July 2021).
- Khoo, K.S.; Ho, L.Y.; Lim, H.R.; Leong, H.Y.; Chew, K.W. Plastic waste associated with the COVID-19 pandemic: Crisis or opportunity? J. Hazard. Mater. 2021, 417, 126108. [Google Scholar] [CrossRef] [PubMed]
- Dąbrowska, J.; Sobota, M.; Świąder, M.; Borowski, P.; Moryl, A.; Stodolak, R.; Kucharczak, E.; Zięba, Z.; Kazak, J.K. Marine Waste—Sources, Fate, Risks, Challenges and Research Needs. Int. J. Environ. Res. Public Health 2021, 18, 433. [Google Scholar] [CrossRef] [PubMed]
- Miandad, R.; Barakat, M.; Aburiazaiza, A.S.; Rehan, M.; Ismail, I.; Nizami, A. Effect of plastic waste types on pyrolysis liquid oil. Int. Biodeterior. Biodegrad. 2017, 119, 239–252. [Google Scholar] [CrossRef]
- Kumar, A.; Samadder, S.; Kumar, N.; Singh, C. Estimation of the generation rate of different types of plastic wastes and possible revenue recovery from informal recycling. Waste Manag. 2018, 79, 781–790. [Google Scholar] [CrossRef]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Hoffmann, G.; Schirmer, M.; Chen, G.; Rotter, V.S. Chlorine characterization and thermal behavior in MSW and RDF. J. Hazard. Mater. 2010, 178, 489–498. [Google Scholar] [CrossRef]
- Kanari, N.; Allain, E.; Shallari, S.; Diot, F.; DiLiberto, S.; Patisson, F.; Yvon, J. Thermochemical Route for Extraction and Recycling of Critical, Strategic and High-Value Elements from By-Products and End-of-Life Materials, Part II: Processing in Presence of Halogenated Atmosphere. Materials 2020, 13, 4203. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wei, R.; Wang, X.; He, J.; Wang, J. Pyrolysis and Combustion of Polyvinyl Chloride (PVC) Sheath for New and Aged Cables via Thermogravimetric Analysis-Fourier Transform Infrared (TG-FTIR) and Calorimeter. Materials 2018, 11, 1997. [Google Scholar] [CrossRef] [PubMed]
- Niu, L.; Xu, J.; Yang, W.; Ma, J.; Zhao, J.; Kang, C.; Su, J. Study on the Synergetic Fire-Retardant Effect of Nano-Sb2O3 in PBT Matrix. Materials 2018, 11, 1060. [Google Scholar] [CrossRef]
- Rani, M.; Marchesi, C.; Federici, S.; Rovelli, G.; Alessandri, I.; Vassalini, I.; Ducoli, S.; Borgese, L.; Zacco, A.; Bilo, F.; et al. Miniaturized Near-Infrared (MicroNIR) Spectrometer in Plastic Waste Sorting. Materials 2019, 12, 2740. [Google Scholar] [CrossRef]
- Hermosillo-Nevárez, J.J.; Bustos-Terrones, V.; Bustos-Terrones, Y.A.; Uriarte-Aceves, P.M.; Rangel-Peraza, J.G. Feasibility Study on the Use of Recycled Polymers for Malathion Adsorption: Isotherms and Kinetic Modeling. Materials 2020, 13, 1824. [Google Scholar] [CrossRef] [PubMed]
- Mun, S.-Y.; Hwang, C.-H. Experimental and Numerical Studies on Major Pyrolysis Properties of Flame Retardant PVC Cables Composed of Multiple Materials. Materials 2020, 13, 1712. [Google Scholar] [CrossRef] [PubMed]
- Kaczorek-Chrobak, K.; Fangrat, J. PVC-Based Copper Electric Wires under Various Fire Conditions: Toxicity of Fire Effluents. Materials 2020, 13, 1111. [Google Scholar] [CrossRef] [PubMed]
- Sekine, Y.; Fukuda, K.; Kato, K.; Adachi, Y.; Matsuno, Y. CO2 reduction potentials by utilizing waste plastics in steel works. Int. J. Life Cycle Assess. 2009, 14, 122–136. [Google Scholar] [CrossRef]
- Trinkel, V.; Kieberger, N.; Bürgler, T.; Rechberger, H.; Fellner, J. Influence of waste plastic utilisation in blast furnace on heavy metal emissions. J. Clean. Prod. 2015, 94, 312–320. [Google Scholar] [CrossRef]
- Bürgler, T.; Kieberger, N. Erfahrungen mit der Altkunststoff-Verwertung im Hochofenprozess. Available online: https://www.vivis.de/wp-content/uploads/EaA9/2012_EaA_99_108_Buergler.pdf (accessed on 15 July 2021).
- Ahmed, H. New Trends in the Application of Carbon-Bearing Materials in Blast Furnace Iron-Making. Minerals 2018, 8, 561. [Google Scholar] [CrossRef]
- Sahajwalla, V.; Zaharia, M.; Rahman, M.F.; Khanna, R.; Saha-Chaudhury, N.; O’Kane, P.; Dicker, J.; Skidmore, C.; Knights, D. Recycling Rubber Tyres and Waste Plastics in EAF Steelmaking. Steel Res. Int. 2011, 82, 566–572. [Google Scholar] [CrossRef]
- Dankwah, J.R.; Koshy, P.; Saha-Chaudhury, N.M.; O’Kane, P.; Skidmore, C.; Knights, D.; Sahajwalla, V. Reduction of FeO in EAF Steelmaking Slag by Metallurgical Coke and Waste Plastics Blends. ISIJ Int. 2011, 51, 498–507. [Google Scholar] [CrossRef]
- Fick, G.; Mirgaux, O.; Neau, P.; Patisson, F. Using Biomass for Pig Iron Production: A Technical, Environmental and Economical Assessment. Waste Biomass Valorization 2014, 5, 43–55. [Google Scholar] [CrossRef]
- Echterhof, T. Review on The Use of Alternative Carbon Sources in EAF Steelmaking. Metals 2021, 11, 222. [Google Scholar] [CrossRef]
- Patisson, F.; Mirgaux, O. Hydrogen Ironmaking: How it Works. Metals 2020, 10, 922. [Google Scholar] [CrossRef]
- Lv, B.; Zhao, G.; Li, N.; Liang, C. Dechlorination and oxidation for waste poly(vinylidene chloride) by hydrothermal catalytic oxidation on Pd/AC catalyst. Polym. Degrad. Stab. 2009, 94, 1047–1052. [Google Scholar] [CrossRef]
- Yoshioka, T.; Kameda, T.; Ieshige, M.; Okuwaki, A. Dechlorination behaviour of flexible poly(vinyl chloride) in NaOH/EG solution. Polym. Degrad. Stab. 2008, 93, 1822–1825. [Google Scholar] [CrossRef]
- Li, T.; Zhao, P.; Lei, M.; Li, Z. Understanding Hydrothermal Dechlorination of PVC by Focusing on the Operating Conditions and Hydrochar Characteristics. Appl. Sci. 2017, 7, 256. [Google Scholar] [CrossRef]
- Castro, A.; Soares, D.; Vilarinho, C.; Castro, F. Kinetics of thermal de-chlorination of PVC under pyrolytic conditions. Waste Manag. 2012, 32, 847–851. [Google Scholar] [CrossRef] [PubMed]
- Cao, Q.; Yuan, G.; Yin, L.; Chen, D.; He, P.; Wang, H. Morphological characteristics of polyvinyl chloride (PVC) dechlorination during pyrolysis process: Influence of PVC content and heating rate. Waste Manag. 2016, 58, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Huang, Q.; Sun, K.; Chi, Y.; Yan, J. Co-pyrolysis characteristics and kinetic analysis of organic food waste and plastic. Bioresour. Technol. 2018, 249, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Albrecht, J.W.; Kolapkar, S.S.; Zinchik, S.; Bar-Ziv, E. Chlorine Removal from U.S. Solid Waste Blends through Torrefaction. Appl. Sci. 2020, 10, 3337. [Google Scholar] [CrossRef]
- Kanari, N.; Allain, E.; Shallari, S.; Diot, F.; DiLiberto, S.; Patisson, F.; Yvon, J. Thermochemical Route for Extraction and Recycling of Critical, Strategic and High Value Elements from By-Products and End-of-Life Materials, Part I: Treatment of a Copper By-Product in Air Atmosphere. Materials 2019, 12, 1625. [Google Scholar] [CrossRef] [PubMed]
- Menad, N.; Wavrer, P.; Seron, A.; Save, M.; Silvente, E.; Russo, P.; Quatravaux, T.; Kanari, N.; Diot, F.; Yvon, J. Recyclage de la Fraction Organique Issue des Résidus de Broyage Automobiles; Rapport Final du Projet “REFORBA”—n° ANR-10-ECOT-013-01: Orléans, France, 2012; 30p. [Google Scholar]
- Gasparini, E.; Tarantino, S.; Ghigna, P.; Riccardi, M.P.; Cedillo-González, E.I.; Siligardi, C.; Zema, M. Thermal dehydroxylation of kaolinite under isothermal conditions. Appl. Clay Sci. 2013, 80–81, 417–425. [Google Scholar] [CrossRef]
- Kanari, N.; Diot, F.; Gauthier, C.; Yvon, J. Use of residual materials for synthesis of lightweight granulates by thermal treatment process. Appl. Clay Sci. 2016, 123, 259–271. [Google Scholar] [CrossRef]
- Roine, A. Outokumpu HSC Chemistry for Windows; Version 3.0; Outokumpu Research: Pori, Finland, 1997. [Google Scholar]
- Kanari, N.; Allain, E.; Gaballah, I. Reactions of wüstite and hematite with different chlorinating agents. Thermochim. Acta 1999, 335, 79–86. [Google Scholar] [CrossRef][Green Version]
- Kanari, N.; Mishra, D.; Filippov, L.; Diot, F.; Mochón, J.; Allain, E. Kinetics of hematite chlorination with Cl2 and Cl2+O2: Part I. Chlorination with Cl2. Thermochim. Acta 2010, 497, 52–59. [Google Scholar] [CrossRef]
- Kanari, N.; Mishra, D.; Filippov, L.; Diot, F.; Mochón, J.; Allain, E. Kinetics of hematite chlorination with Cl2 and Cl2+O2. Part II. Chlorination with Cl2+O2. Thermochim. Acta 2010, 506, 34–40. [Google Scholar] [CrossRef]
- Kanari, N.; Menad, N.-E.; Ostrosi, E.; Shallari, S.; Diot, F.; Allain, E.; Yvon, J. Thermal Behavior of Hydrated Iron Sulfate in Various Atmospheres. Metals 2018, 8, 1084. [Google Scholar] [CrossRef]
- Kanari, N.; Allain, E.; Filippov, L.; Shallari, S.; Diot, F.; Patisson, F. Reactivity of Low-Grade Chromite Concentrates towards Chlorinating Atmospheres. Materials 2020, 13, 4470. [Google Scholar] [CrossRef] [PubMed]
- Marques, V.; Tulyaganov, D.; Agathopoulos, S.; Gataullin, V.; Kothiyal, G.; Ferreira, J. Low temperature synthesis of anorthite based glass-ceramics via sintering and crystallization of glass-powder compacts. J. Eur. Ceram. Soc. 2006, 26, 2503–2510. [Google Scholar] [CrossRef]
- Valderrama, D.M.A.; Cuaspud, J.A.G.; Roether, J.A.; Boccaccini, A.R. Development and Characterization of Glass-Ceramics from Combinations of Slag, Fly Ash, and Glass Cullet without Adding Nucleating Agents. Materials 2019, 12, 2032. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Li, B.; Guo, H.; Yang, W.; Li, S.; Guo, J. Thermodynamic Study on Reduction of Iron Oxides by H2 + CO + CH4 + N2 Mixture at 900 °C. Energies 2020, 13, 5053. [Google Scholar] [CrossRef]
- Ubando, A.T.; Chen, W.; Show, P.; Ong, H.C. Kinetic and thermodynamic analysis of iron oxide reduction by graphite for CO2 mitigation in chemical-looping combustion. Int. J. Energy Res. 2020, 44, 3865–3882. [Google Scholar] [CrossRef]
- Manchili, S.K.; Wendel, J.; Hryha, E.; Nyborg, L. Analysis of Iron Oxide Reduction Kinetics in the Nanometric Scale Using Hydrogen. Nanomaterials 2020, 10, 1276. [Google Scholar] [CrossRef] [PubMed]
- Hamadeh, H.; Mirgaux, O.; Patisson, F. Detailed Modeling of the Direct Reduction of Iron Ore in a Shaft Furnace. Materials 2018, 11, 1865. [Google Scholar] [CrossRef] [PubMed]
- Béchara, R.; Hamadeh, H.; Mirgaux, O.; Patisson, F. Optimization of the Iron Ore Direct Reduction Process through Multiscale Process Modeling. Materials 2018, 11, 1094. [Google Scholar] [CrossRef] [PubMed]

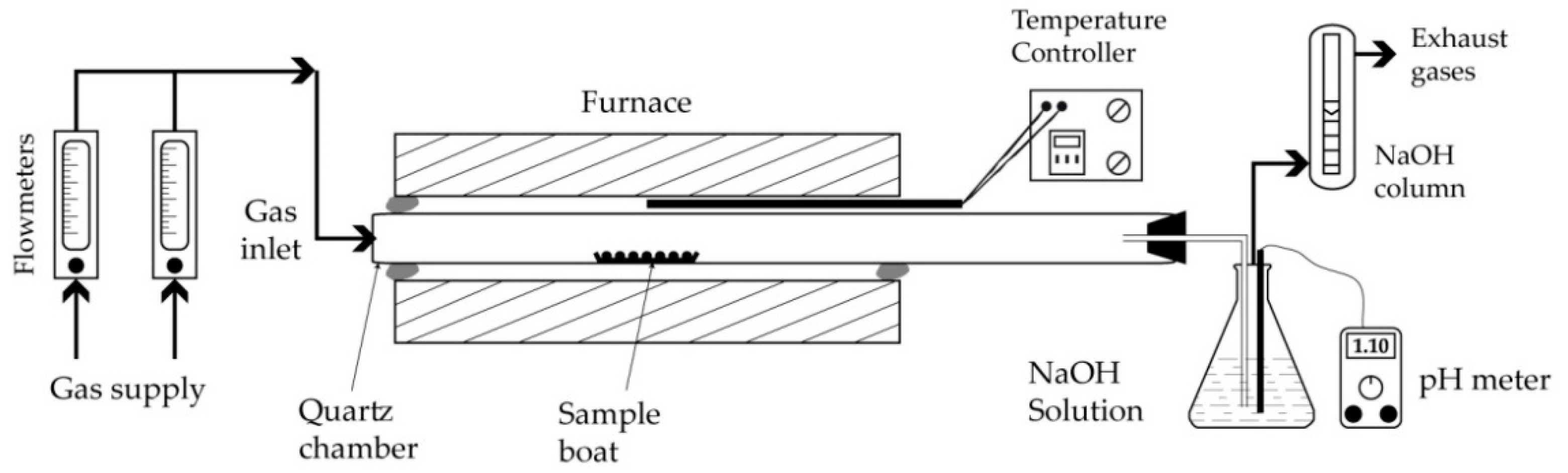
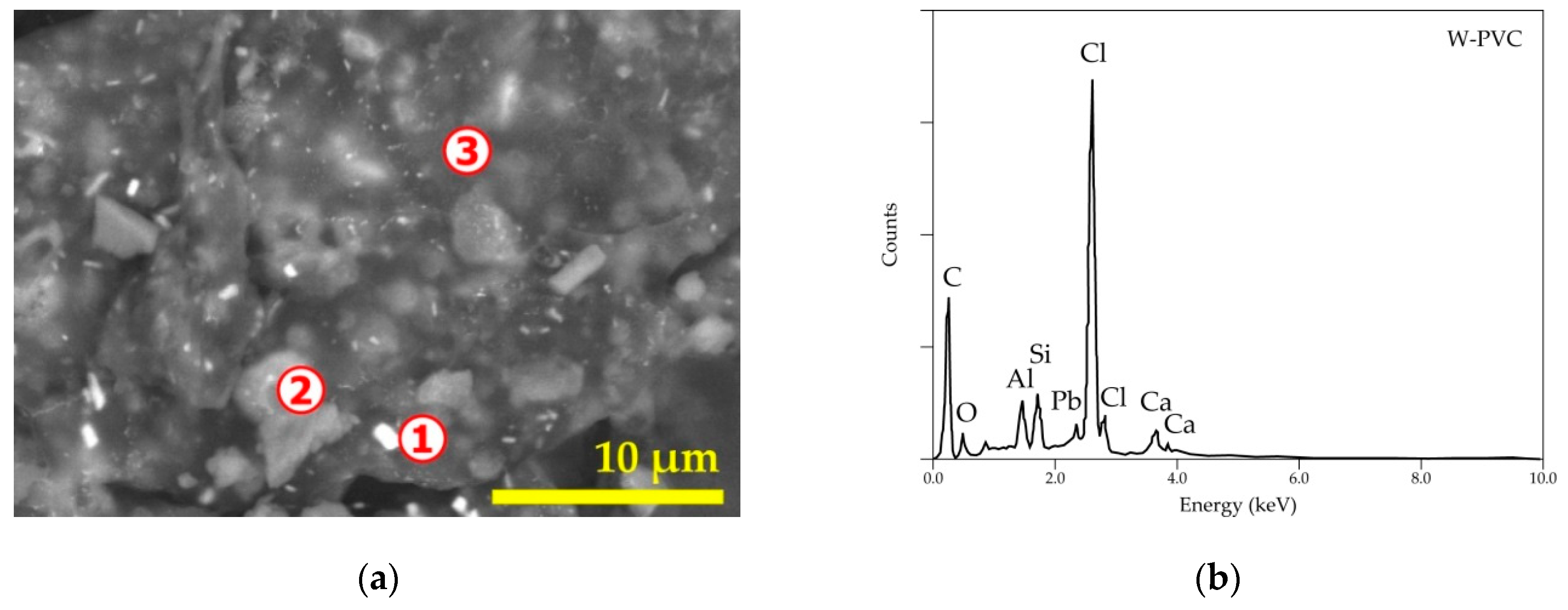
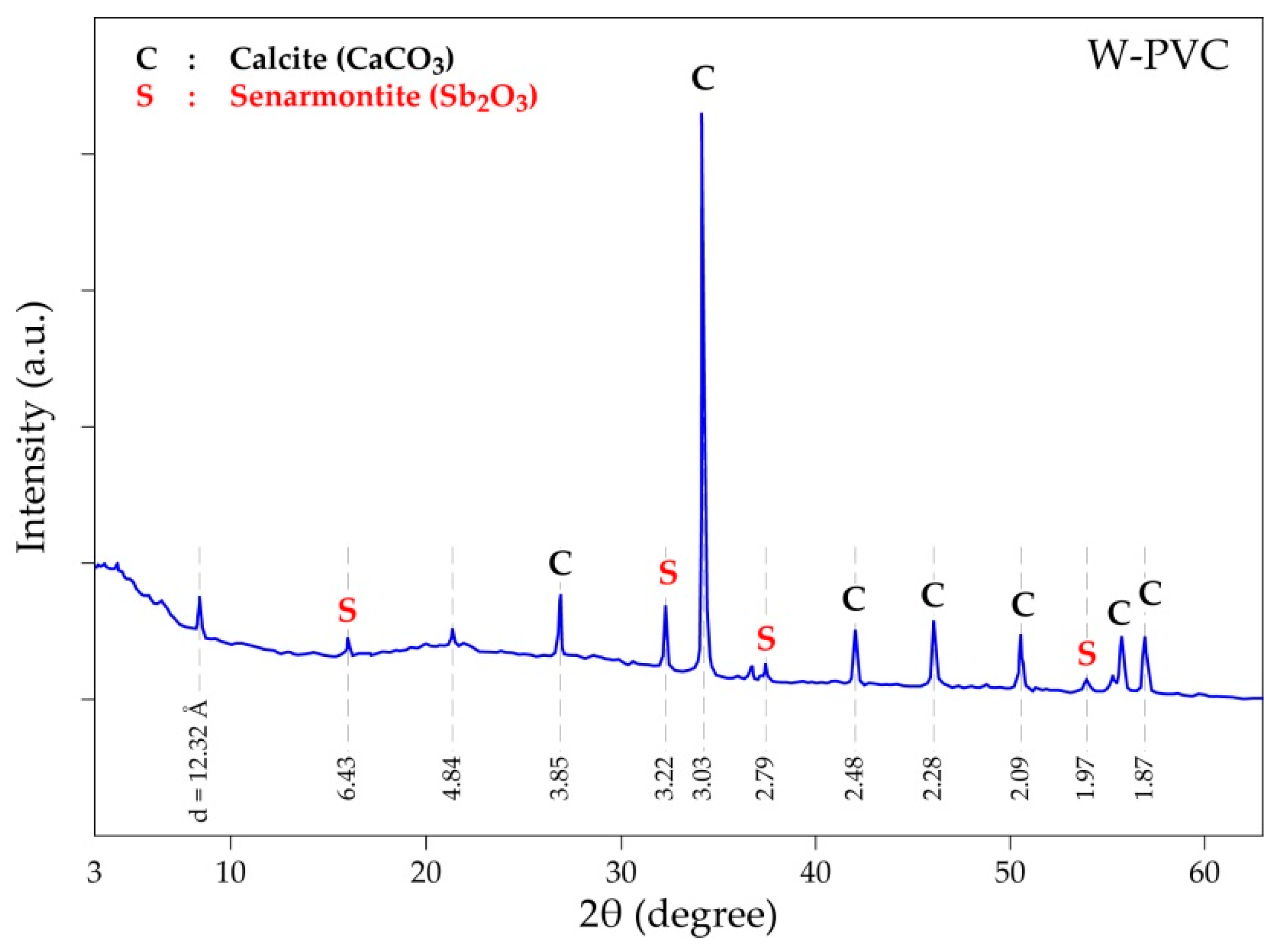
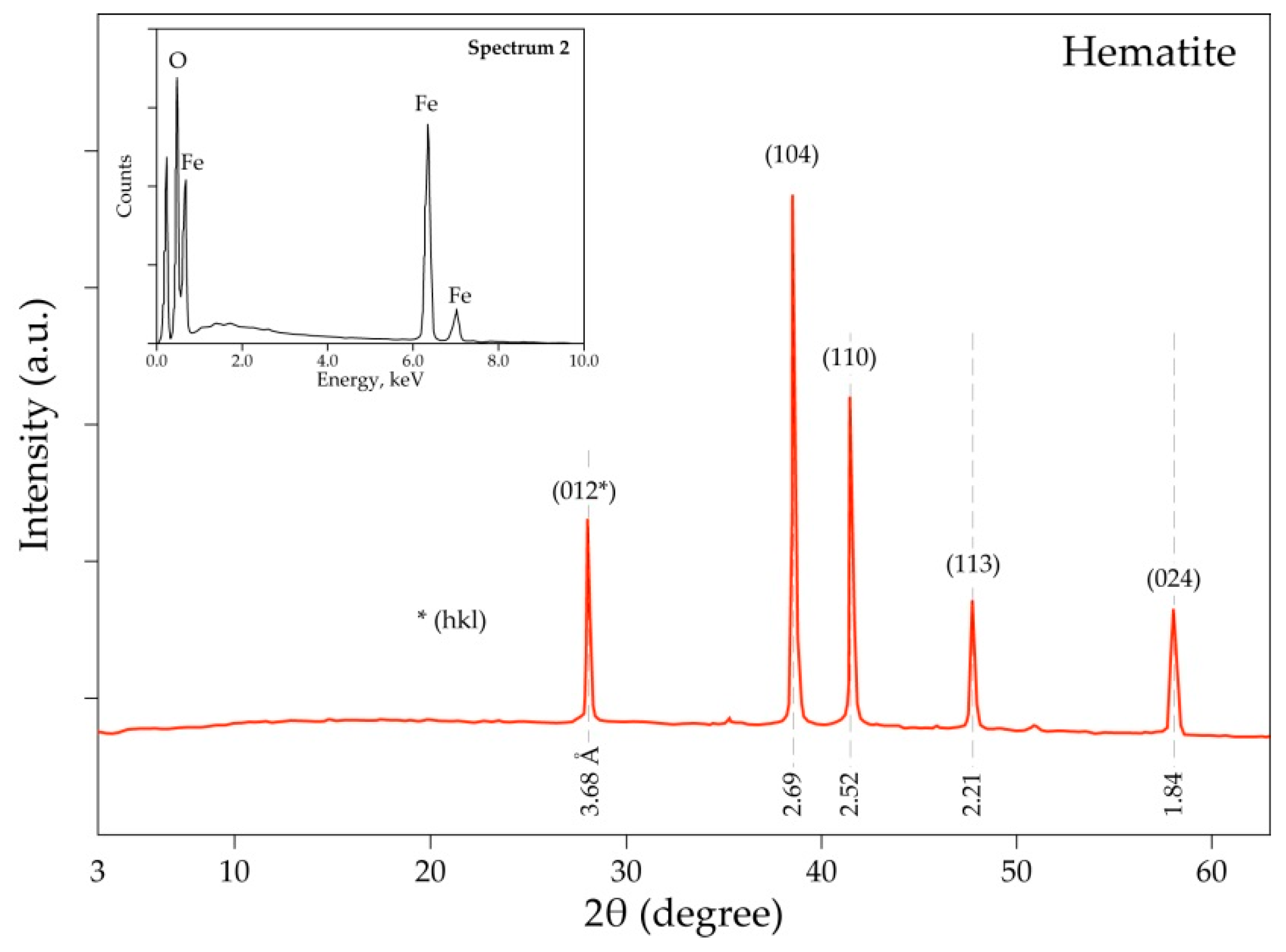
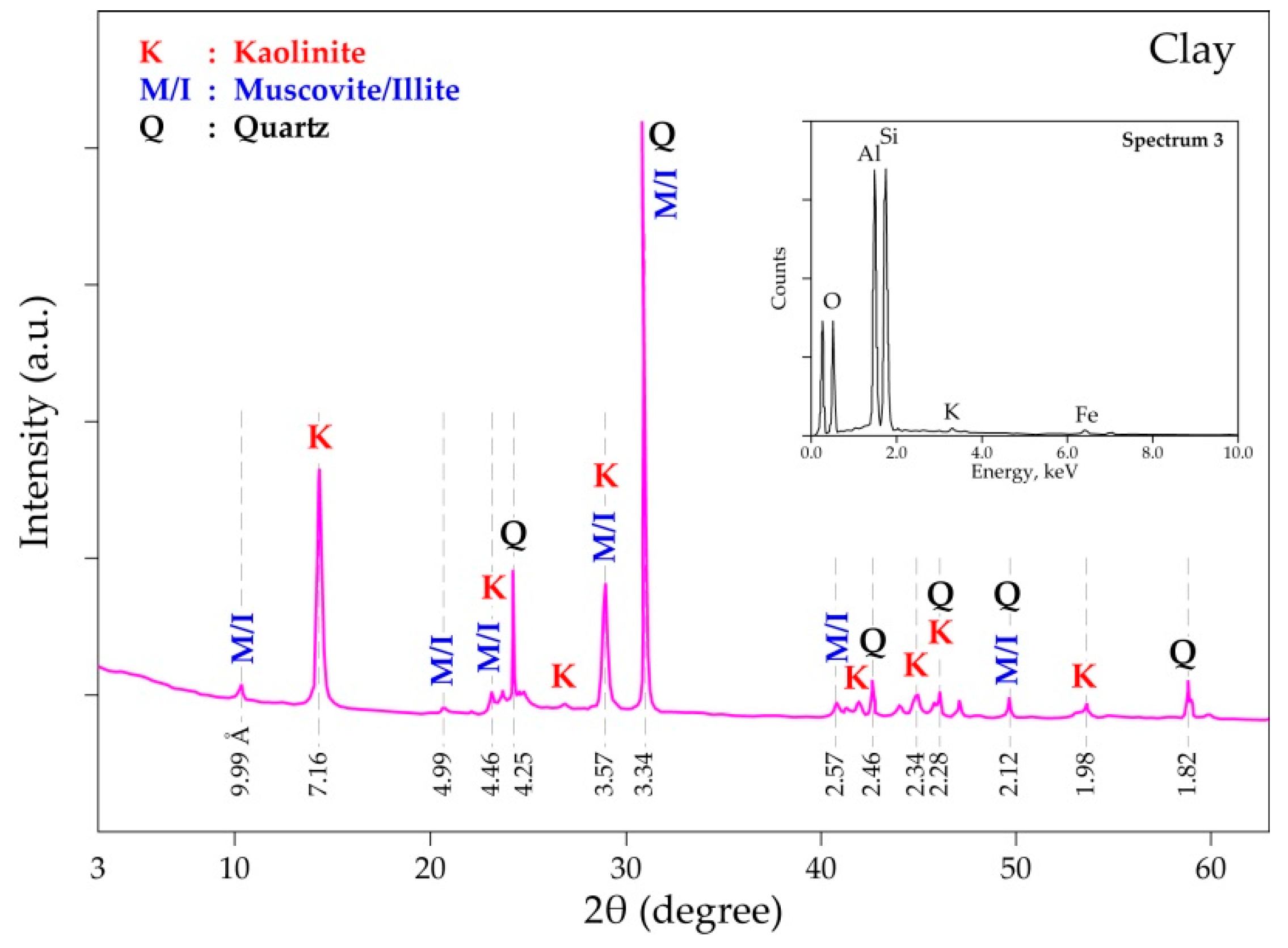
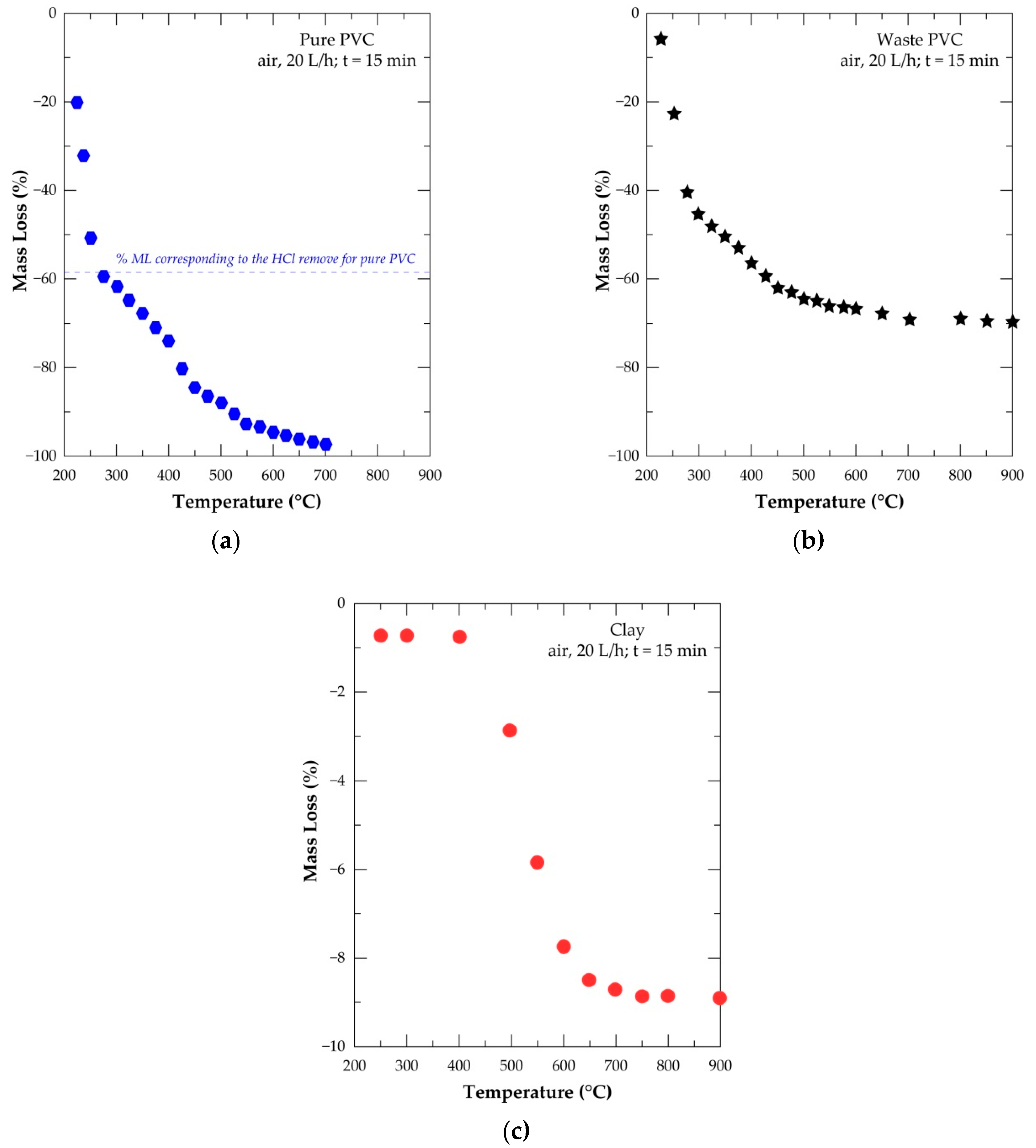
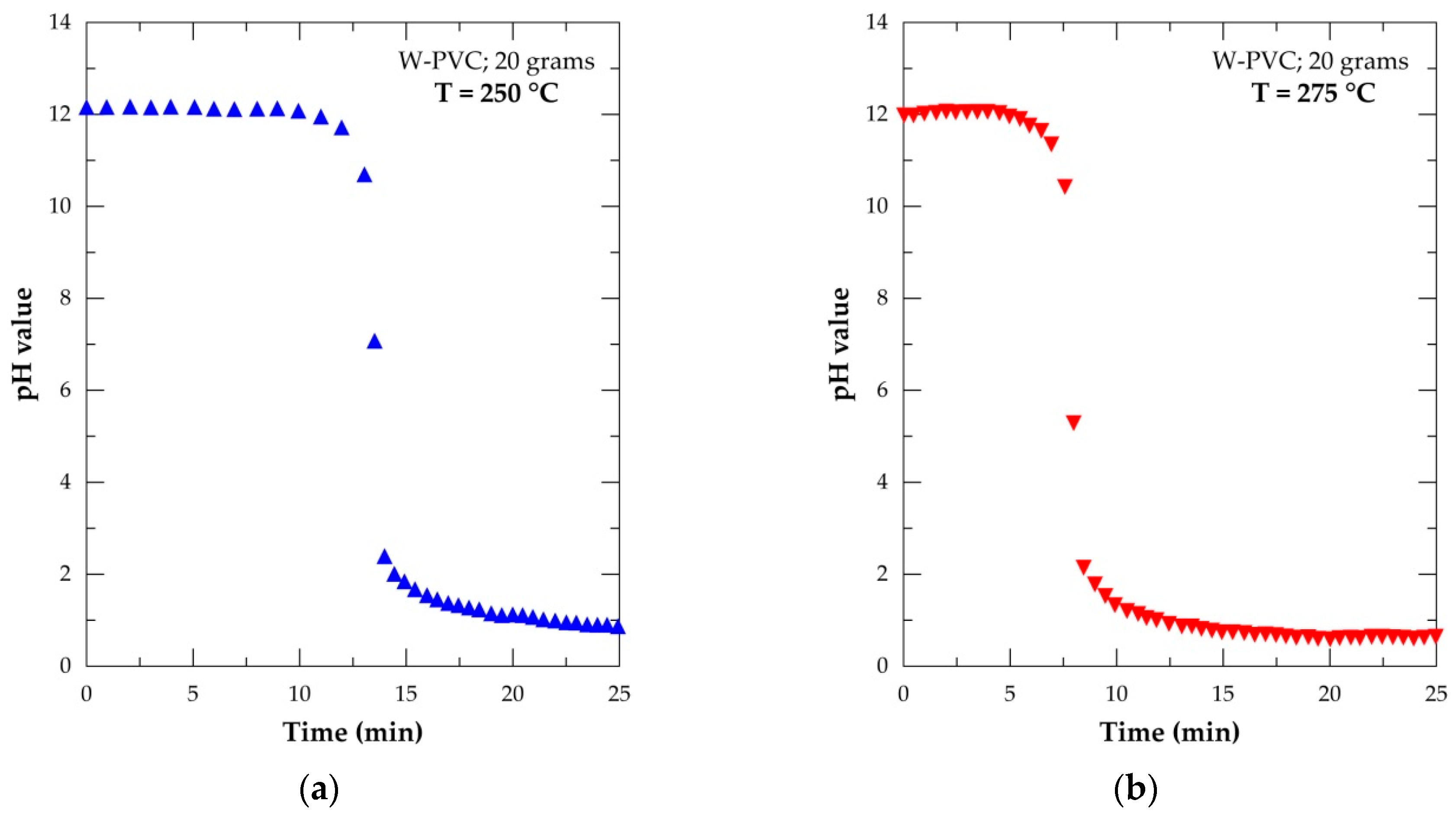

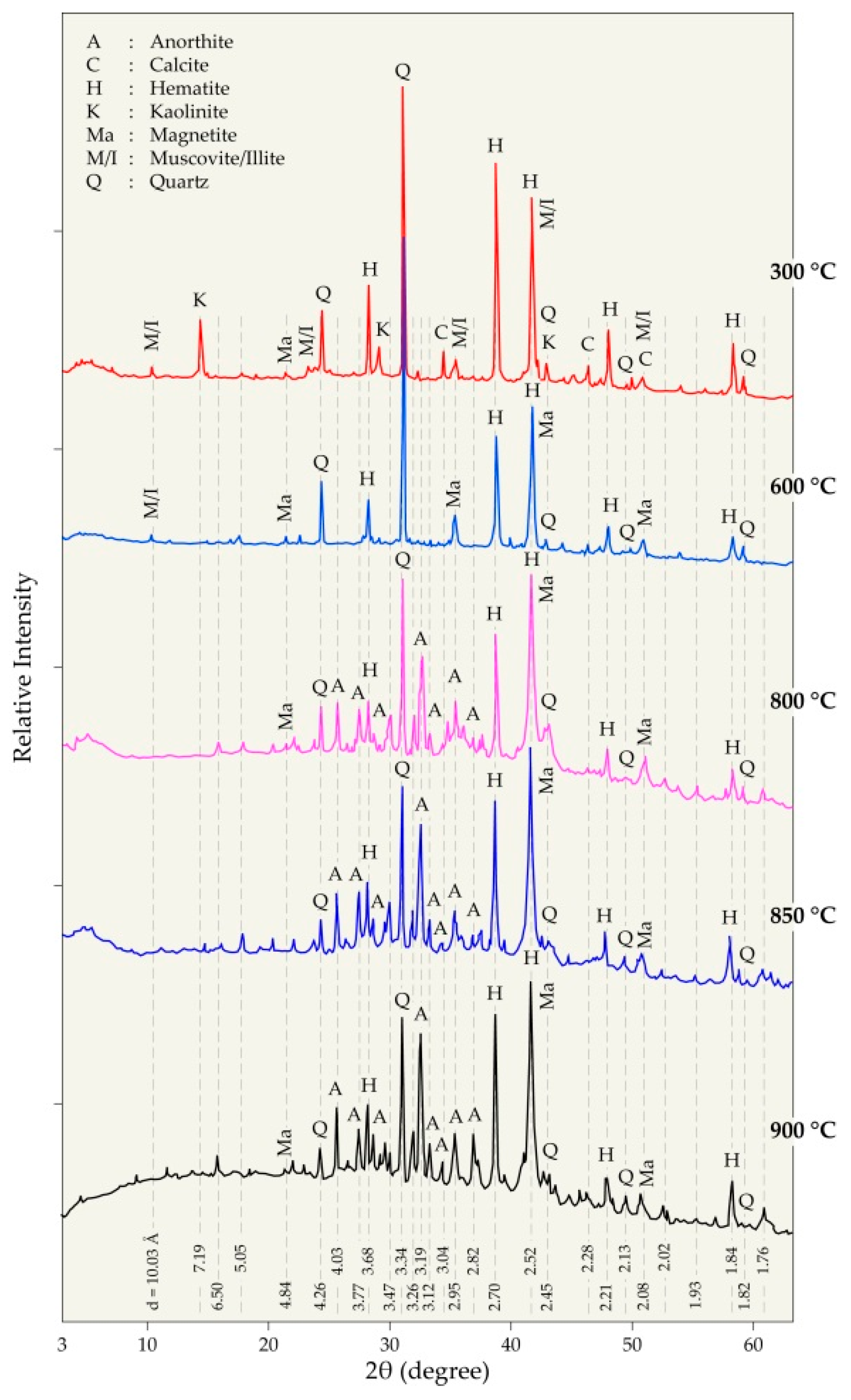

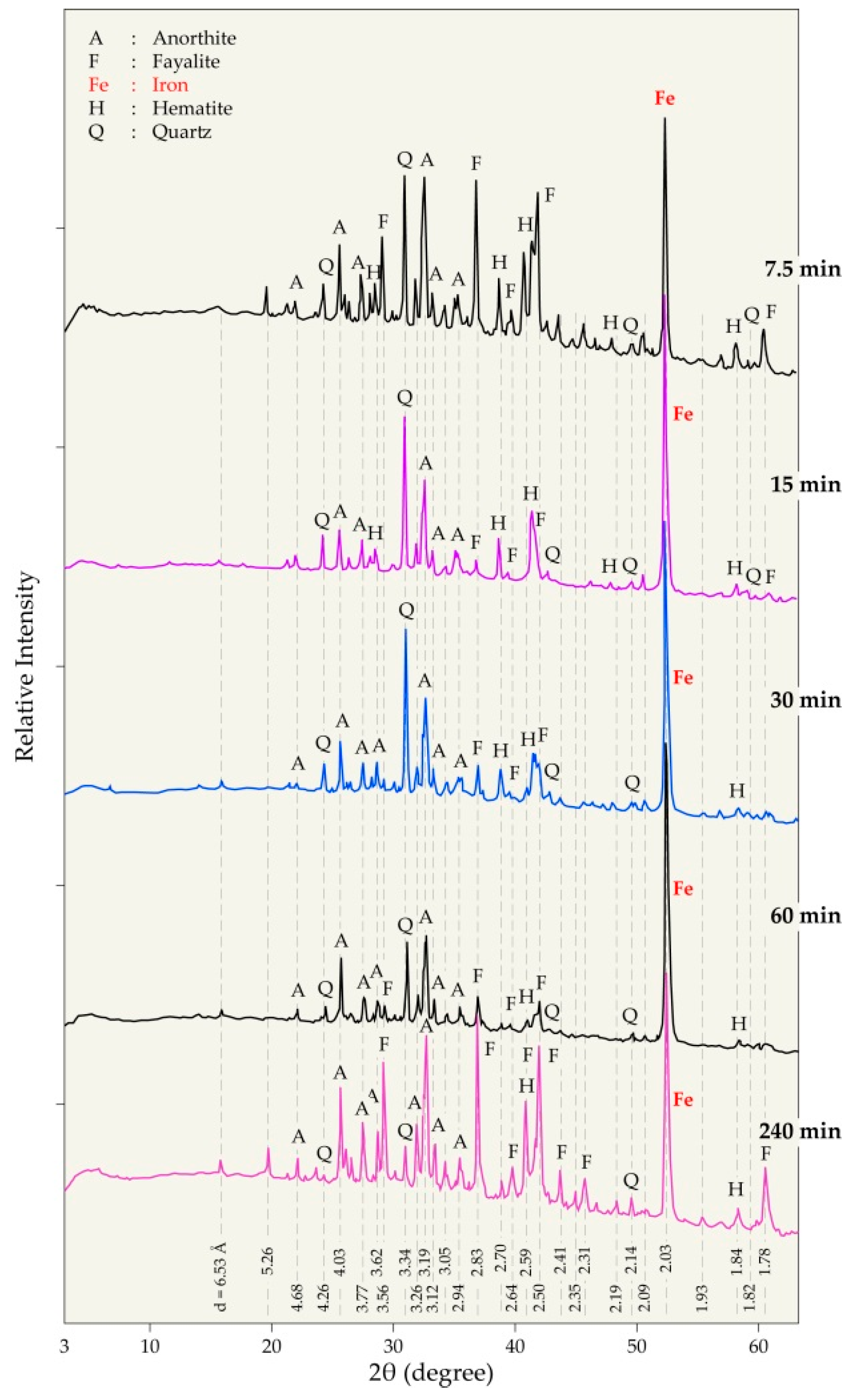
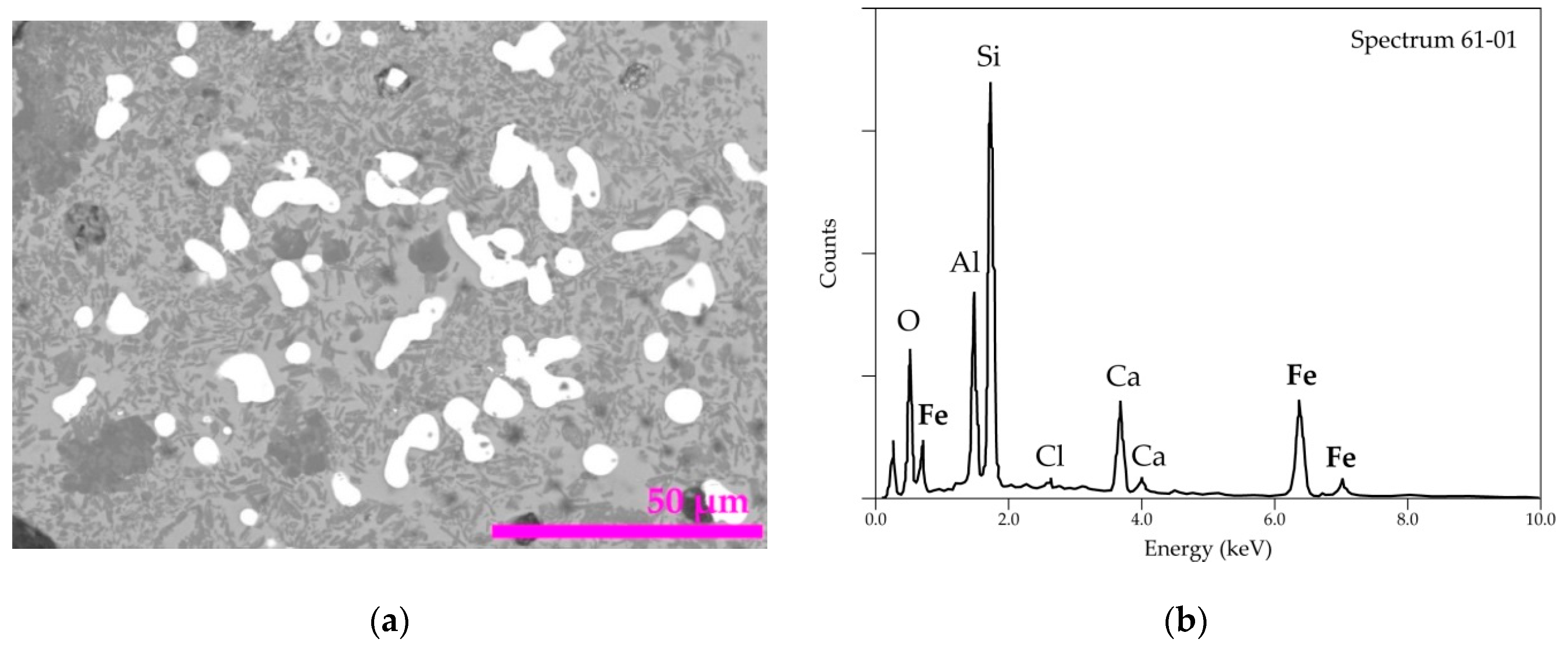
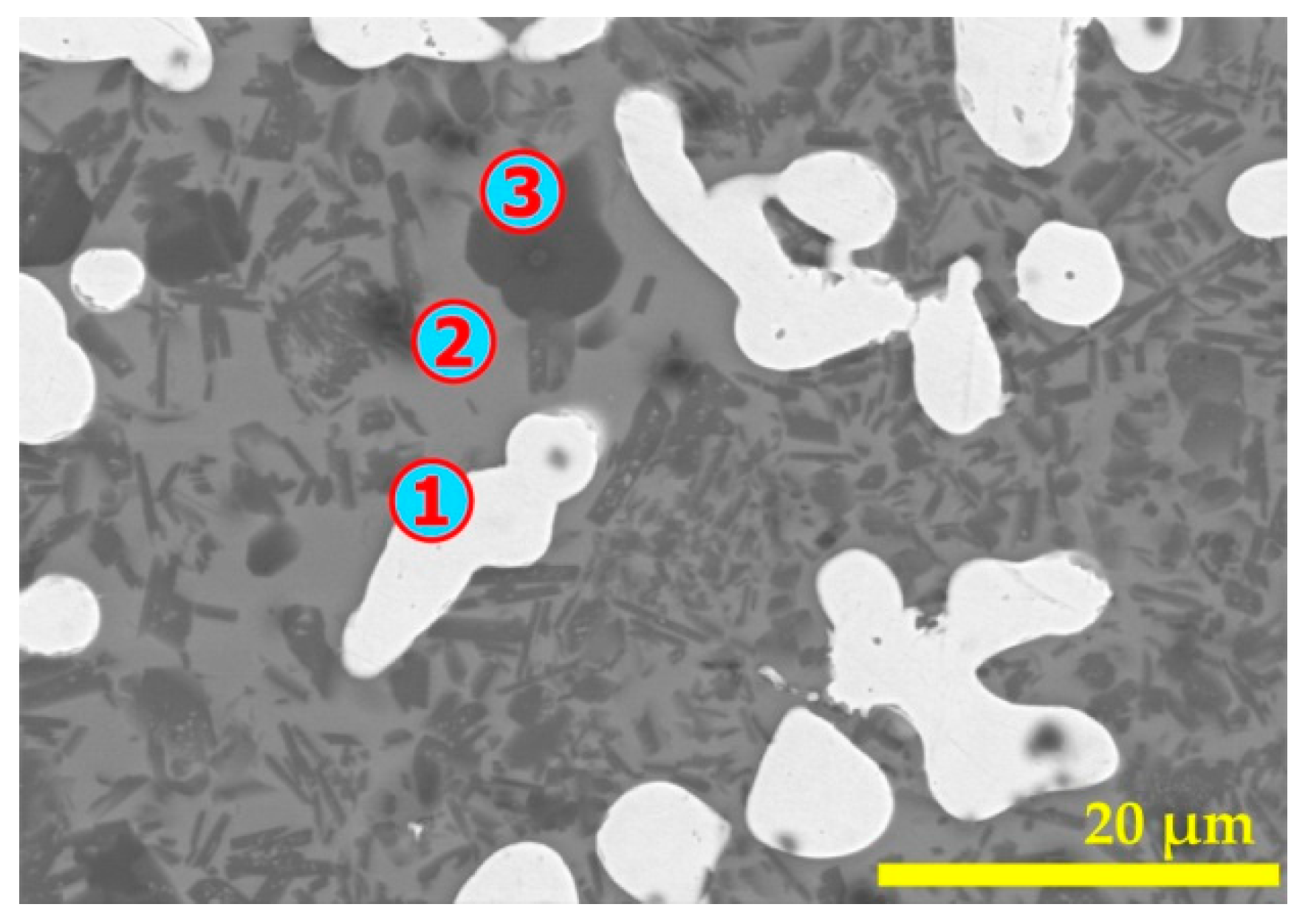
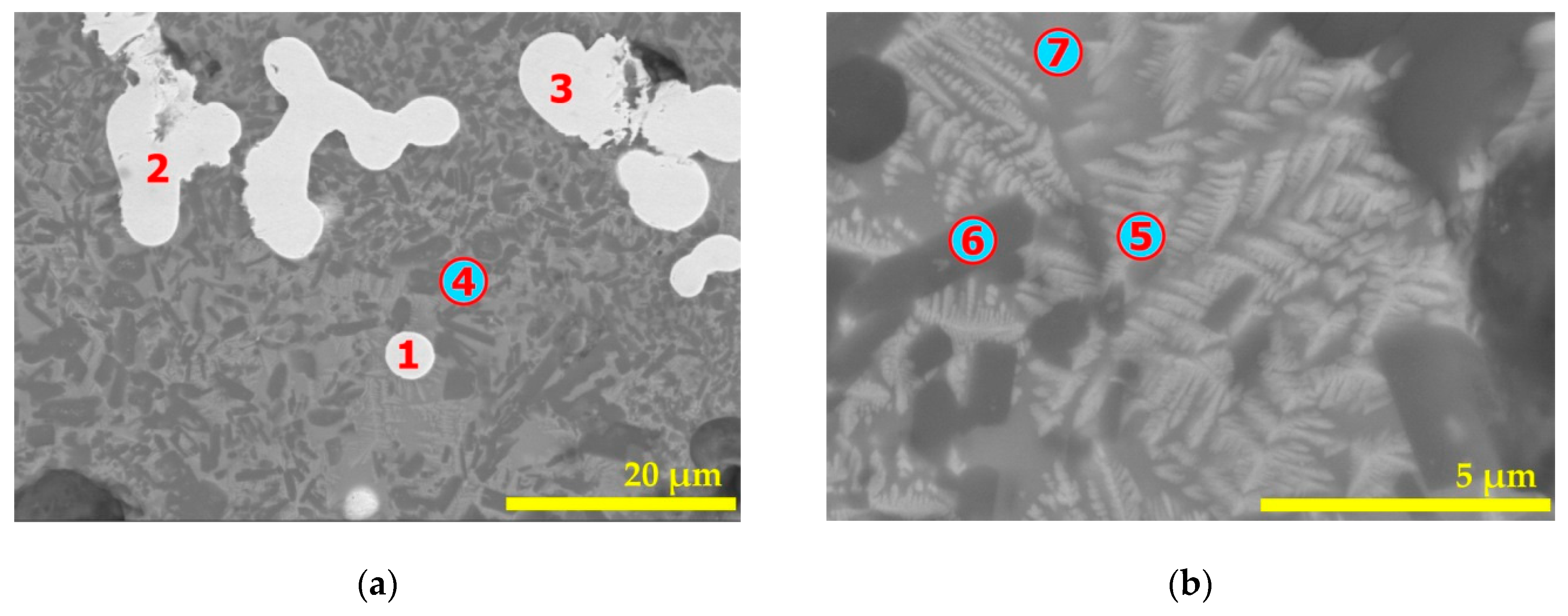
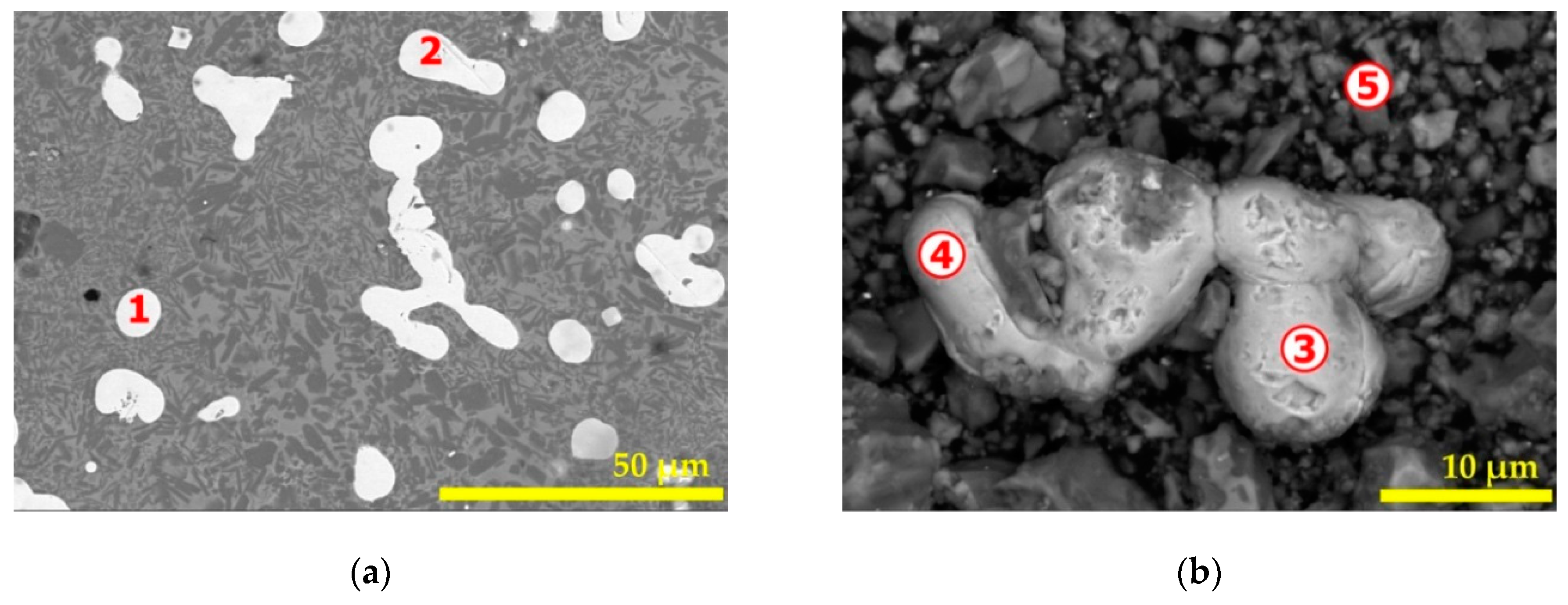
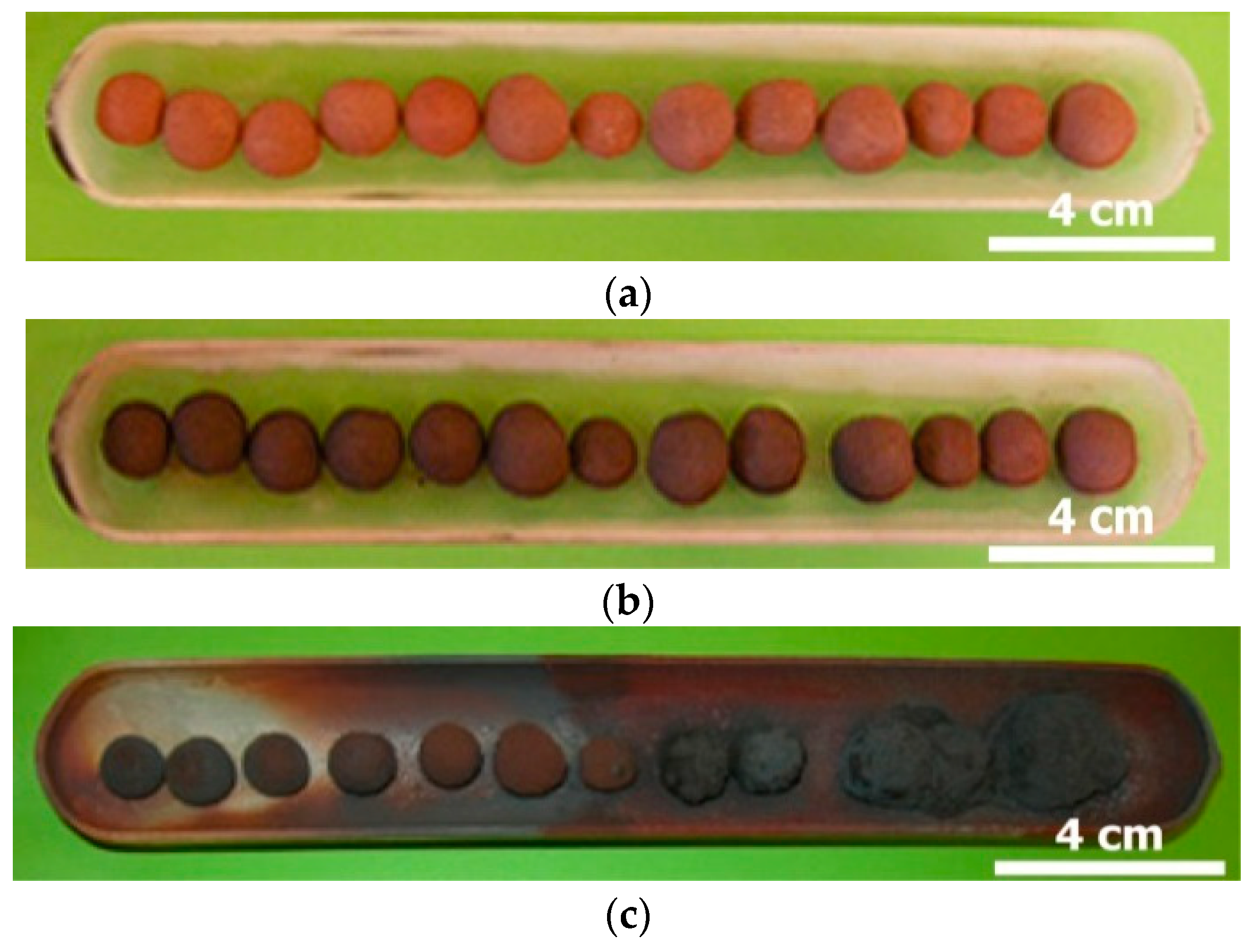
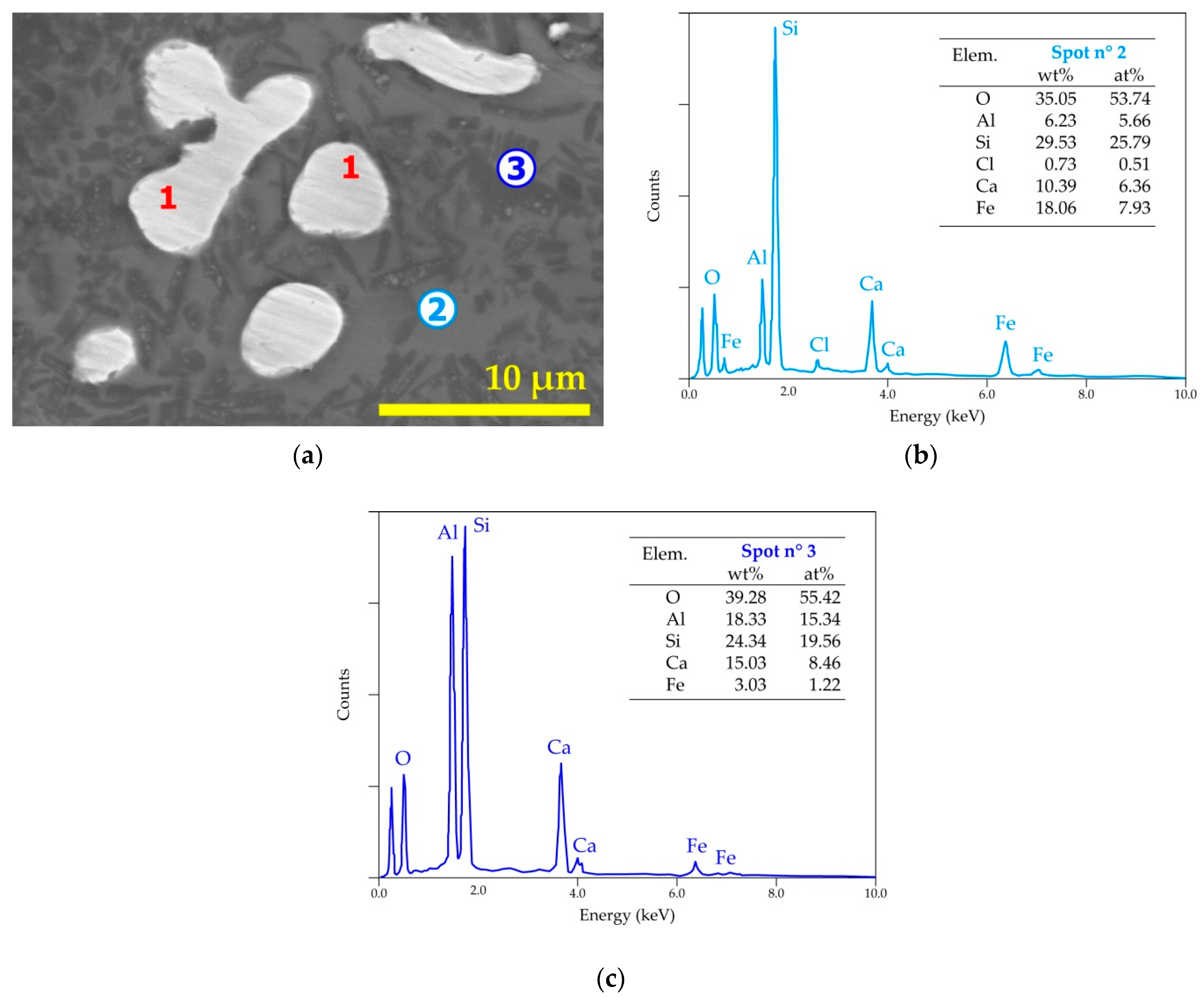
| Elements | Spot n° 1 | Spot n° 2 | Spot n° 3 | |||
|---|---|---|---|---|---|---|
| - | 1 wt% | 1 at% | wt% | at% | wt% | at% |
| O | 7.35 | 22.26 | 55.74 | 75.13 | 7.64 | 17.88 |
| Al | 2.18 | 3.92 | 0.60 | 0.48 | 5.27 | 7.32 |
| Si | 2.38 | 4.11 | 0.73 | 0.56 | 5.61 | 7.47 |
| Cl | 40.52 | 55.40 | 10.45 | 6.36 | 56.83 | 60.01 |
| Ca | 2.03 | 2.45 | 32.48 | 17.48 | 1.73 | 1.62 |
| Sb | 7.32 | 2.92 | - | - | 12.31 | 3.78 |
| Pb | 38.22 | 8.94 | - | - | 10.61 | 1.92 |
| Elements | Spot n° 1 | Spot n° 2 | Spot n° 3 | |||
|---|---|---|---|---|---|---|
| 1 wt% | 1 at% | wt% | at% | wt% | at% | |
| O | - | - | 36.97 | 57.68 | 48.30 | 62.11 |
| Al | - | - | 4.51 | 4.17 | 0.78 | 0.59 |
| Si | - | - | 23.91 | 21.25 | 50.93 | 37.30 |
| Cl | - | - | 0.92 | 0.65 | - | - |
| Ca | - | - | 6.72 | 4.19 | - | - |
| Fe | 100.00 | 100.00 | 26.97 | 12.06 | - | - |
| Elements | Spot n° 1,2,3 | Spot n° 4 | Spot n° 5 | Spot n° 6 | Spot n° 7 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 wt% | 1 at% | wt% | at% | wt% | at% | wt% | at% | wt% | at% | |
| O | - | - | 40.05 | 57.03 | 34.03 | 55.91 | 42.47 | 59.19 | 35.51 | 56.00 |
| Al | - | - | 16.35 | 13.81 | 3.31 | 3.22 | 12.58 | 10.40 | 4.42 | 4.14 |
| Si | - | - | 22.71 | 18.43 | 22.61 | 21.16 | 26.67 | 21.17 | 24.36 | 21.88 |
| Cl | - | - | - | - | - | - | - | - | 0.75 | 0.54 |
| Ca | - | - | 13.76 | 7.82 | 4.63 | 3.04 | 12.33 | 6.86 | 9.28 | 5.84 |
| Fe | 100.00 | 100.00 | 7.13 | 2.91 | 35.42 | 16.67 | 5.95 | 2.38 | 25.68 | 11.60 |
| Elements | Spot n° 1,2 | Spot n° 3 | Spot n° 4 | Spot n° 5 | ||||
|---|---|---|---|---|---|---|---|---|
| 1 wt% | 1 at% | wt% | at% | wt% | at% | wt% | at% | |
| O | - | - | 3.15 | 9.91 | 3.17 | 10.01 | 30.89 | 49.36 |
| Al | - | - | 0.69 | 1.29 | 1.08 | 2.02 | 11.09 | 10.51 |
| Si | - | - | 2.43 | 4.35 | 1.36 | 2.46 | 25.81 | 23.49 |
| Ca | - | - | - | - | - | - | 10.52 | 6.71 |
| Fe | 100.00 | 100.00 | 93.73 | 84.45 | 94.39 | 85.51 | 21.70 | 9.93 |
| Chemical Reactions | ΔrH°1000 °C (kJ) | ΔrG°1000 °C (kJ) | Equation Number |
|---|---|---|---|
| 3 Fe2O3(s) + C(s) → 2 Fe3O4(s) + CO(g) | 125.70 | −171.97 | (4) |
| Fe3O4(s) + C(s) → 3 FeO(s) + CO(g) | 181.76 | −56.08 | (5) |
| FeO(s) + C(s) → Fe(s) + CO(g) | 152.09 | −43.28 | (6) |
| 3 Fe2O3(s) + CO(g) → 2 Fe3O4(s) + CO2(g) | −42.05 | −119.70 | (7) |
| Fe3O4(s) + CO(g) → 3 FeO(s) + CO2(g) | 14.016 | −3.81 | (8) |
| FeO(s) + CO(g) → Fe(s) + CO2(g) | −15.65 | 8.99 | (9) |
| 2 C(s) + O2(g) → 2 CO(g) | −227.42 | −448.39 | (10) |
| C(s) + O2(g) → CO2(g) | −395.17 | −396.12 | (11) |
| C(s) + CO2(g) → 2 CO(g) | 167.74 | −52.27 | (12) |
| C(s) + H2O(g) → CO(g) + H2(g) | 135.55 | −46.69 | (13) |
| CO(g) + H2O(g) → CO2(g) + H2(g) | −32.20 | 5.58 | (14) |
| 3 Fe2O3(s) + H2(g) → 2 Fe3O4(s) + H2O(g) | −9.85 | −125.27 | (15) |
| Fe3O4(s) + H2(g) → 3 FeO(s) + H2O(g) | 46.21 | −9.40 | (16) |
| FeO(s) + H2(g) → Fe(s) + H2O(g) | 16.54 | 3.42 | (17) |
| 1/2 CaO(s) +SiO2(s) + 1/2 Al2O3(s) → 1/2 CaAl2Si2O8(s) | −58.26 | −66.95 | (18) |
| 2 FeO(s) + SiO2(s) → Fe2SiO4(s) | −40.04 | −13.62 | (19) |
| 2 Fe2SiO4(s) + CaO(s) + Al2O3(s) → CaAl2Si2O8(s) + 4 FeO(s) | −37.67 | −92.80 | (20) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanari, N.; Menad, N.-E.; Filippov, L.O.; Shallari, S.; Allain, E.; Patisson, F.; Yvon, J. Some Aspects of the Thermochemical Route for the Valorization of Plastic Wastes, Part I: Reduction of Iron Oxides by Polyvinyl Chloride (PVC). Materials 2021, 14, 4129. https://doi.org/10.3390/ma14154129
Kanari N, Menad N-E, Filippov LO, Shallari S, Allain E, Patisson F, Yvon J. Some Aspects of the Thermochemical Route for the Valorization of Plastic Wastes, Part I: Reduction of Iron Oxides by Polyvinyl Chloride (PVC). Materials. 2021; 14(15):4129. https://doi.org/10.3390/ma14154129
Chicago/Turabian StyleKanari, Ndue, Nour-Eddine Menad, Lev O. Filippov, Seit Shallari, Eric Allain, Fabrice Patisson, and Jacques Yvon. 2021. "Some Aspects of the Thermochemical Route for the Valorization of Plastic Wastes, Part I: Reduction of Iron Oxides by Polyvinyl Chloride (PVC)" Materials 14, no. 15: 4129. https://doi.org/10.3390/ma14154129
APA StyleKanari, N., Menad, N.-E., Filippov, L. O., Shallari, S., Allain, E., Patisson, F., & Yvon, J. (2021). Some Aspects of the Thermochemical Route for the Valorization of Plastic Wastes, Part I: Reduction of Iron Oxides by Polyvinyl Chloride (PVC). Materials, 14(15), 4129. https://doi.org/10.3390/ma14154129








