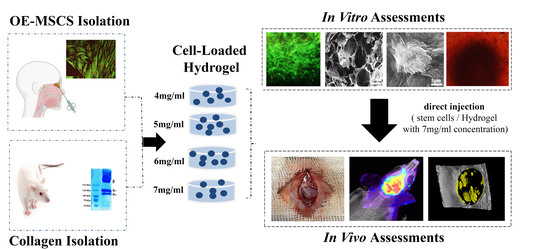
Human Olfactory Mucosa Stem Cells Delivery Using a Collagen Hydrogel: As a Potential Candidate for Bone Tissue Engineering
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Ethics
2.3. Experimental Studies
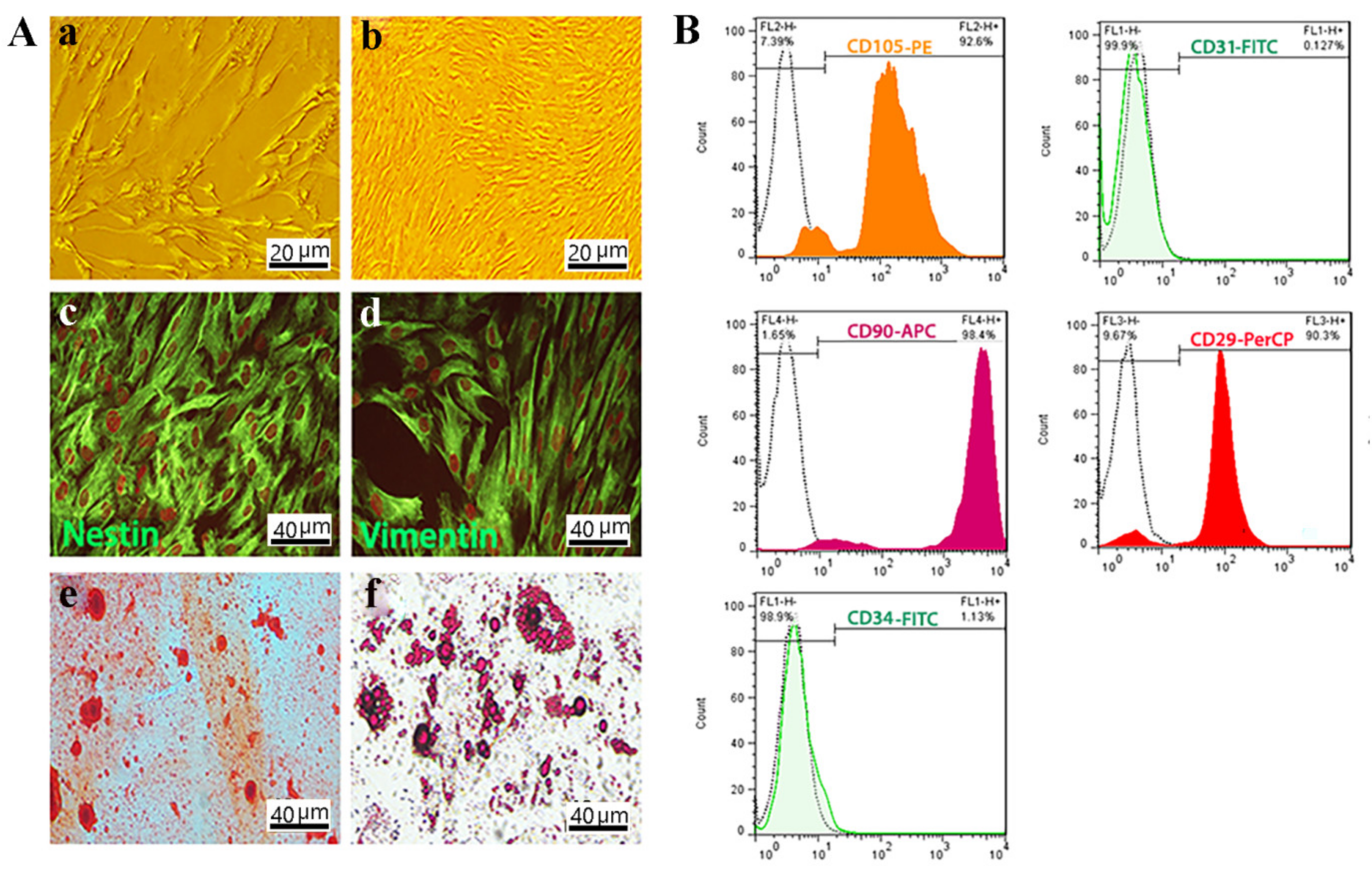
2.3.1. Isolation and Characterization of Cells
2.3.2. Collagen Extraction, Hydrogel Preparation, and Characterization
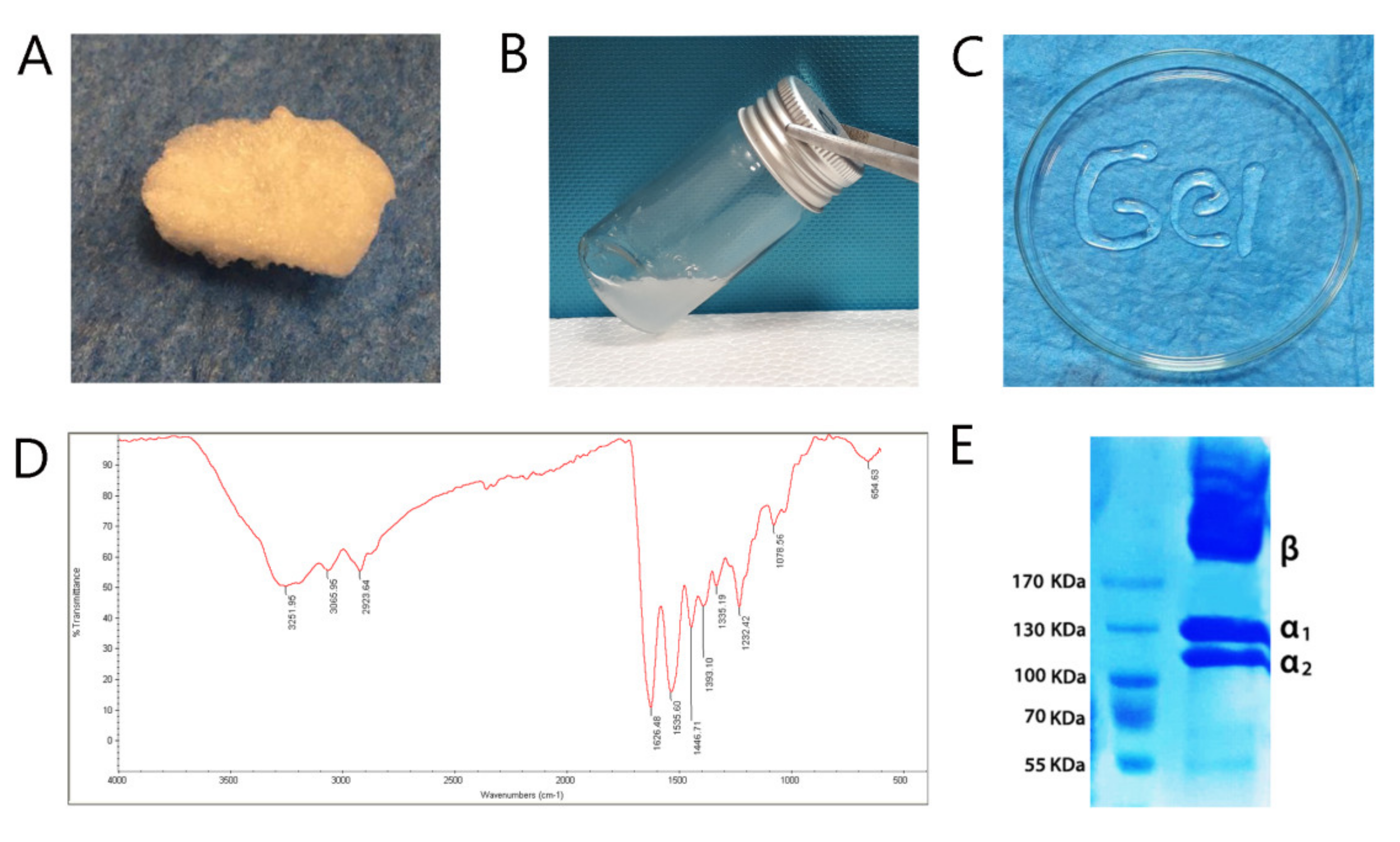
Rat Tail Collagen Type I Extraction
Fourier Transform Infrared Spectroscopy (FTIR)
Gel Electrophoresis
Hydrogel Preparation and OE-MSCs Encapsulation
Field Emission Scanning Electron Microscopy
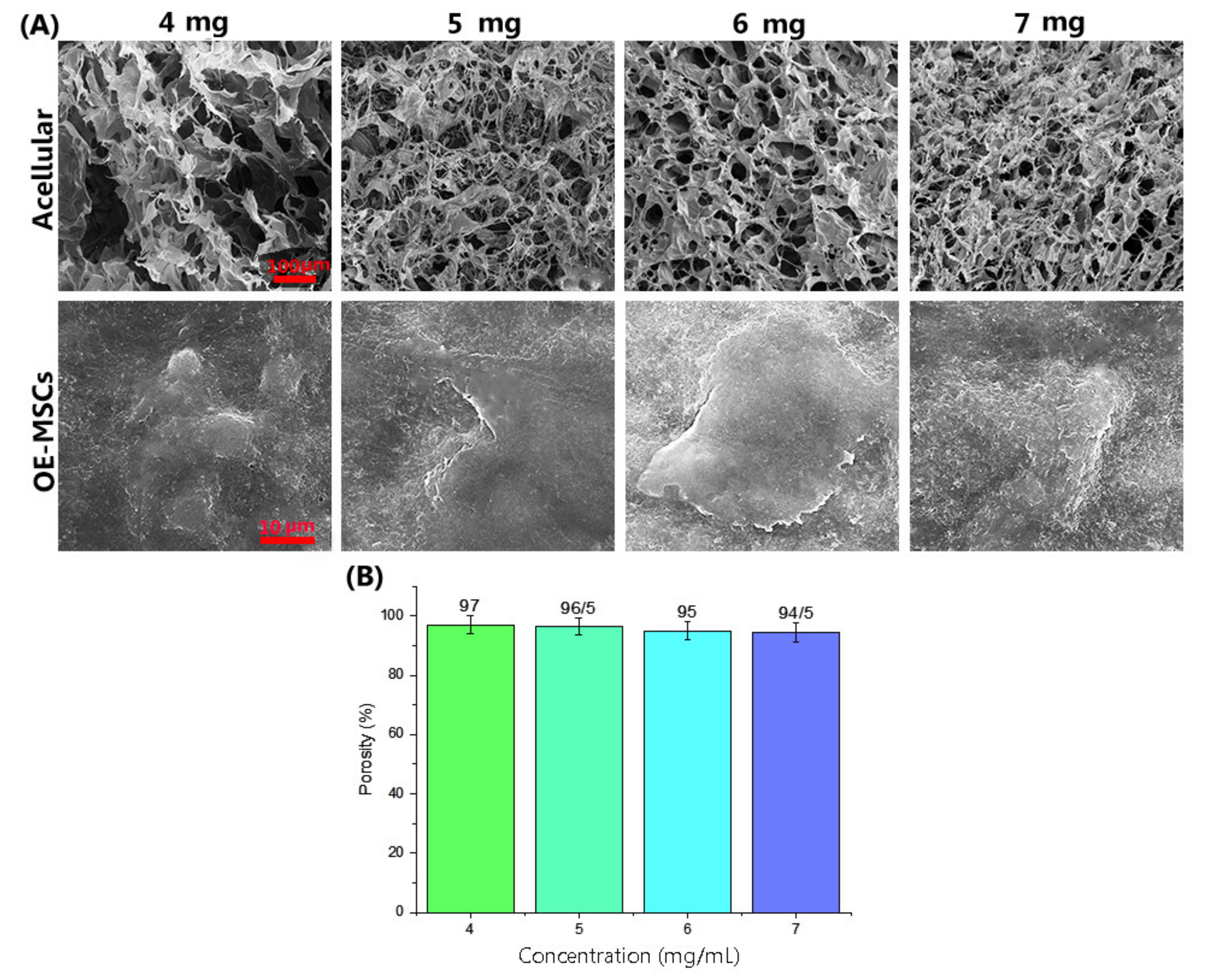
Porosity Assessment
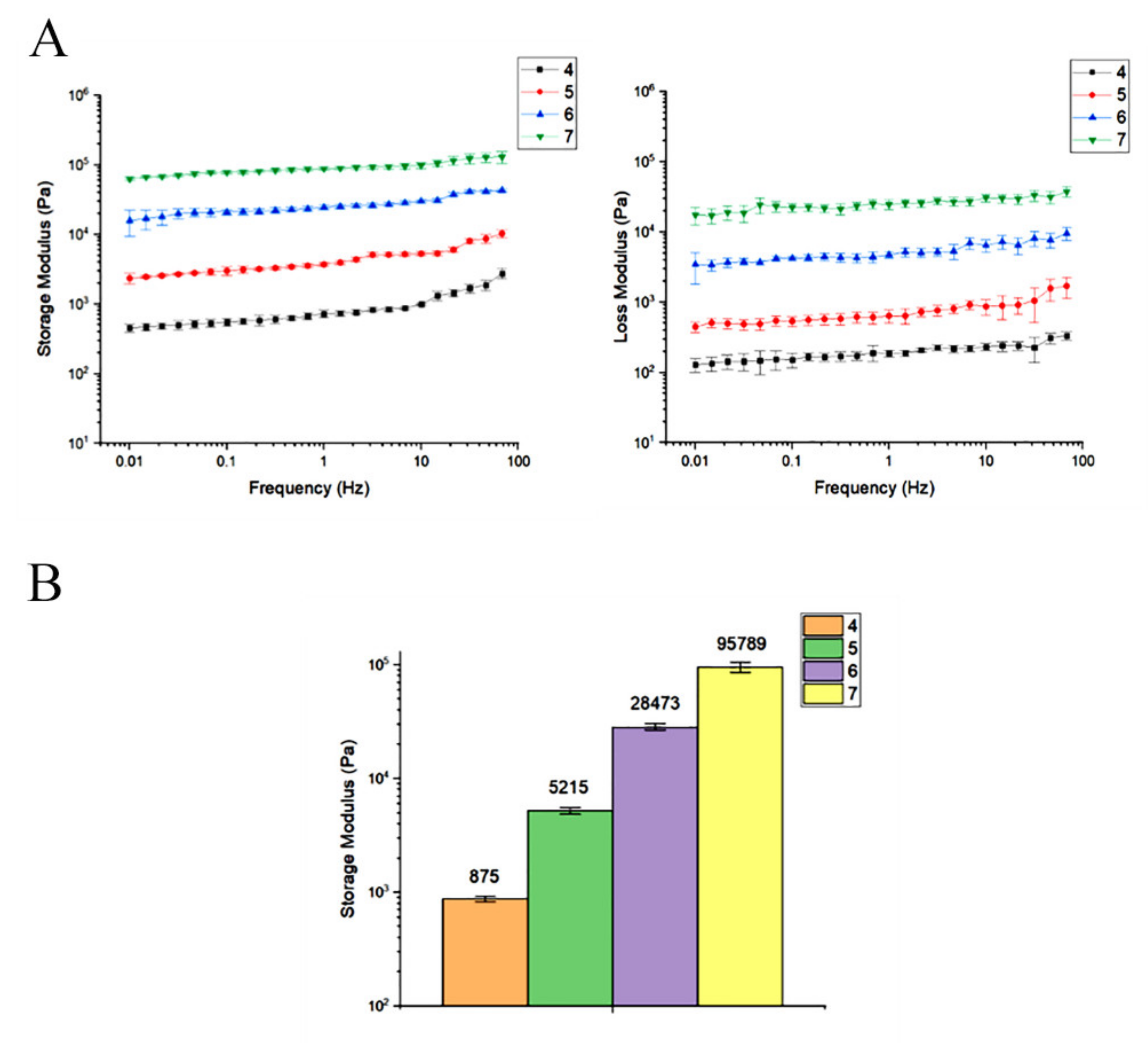
Rheological Analysis
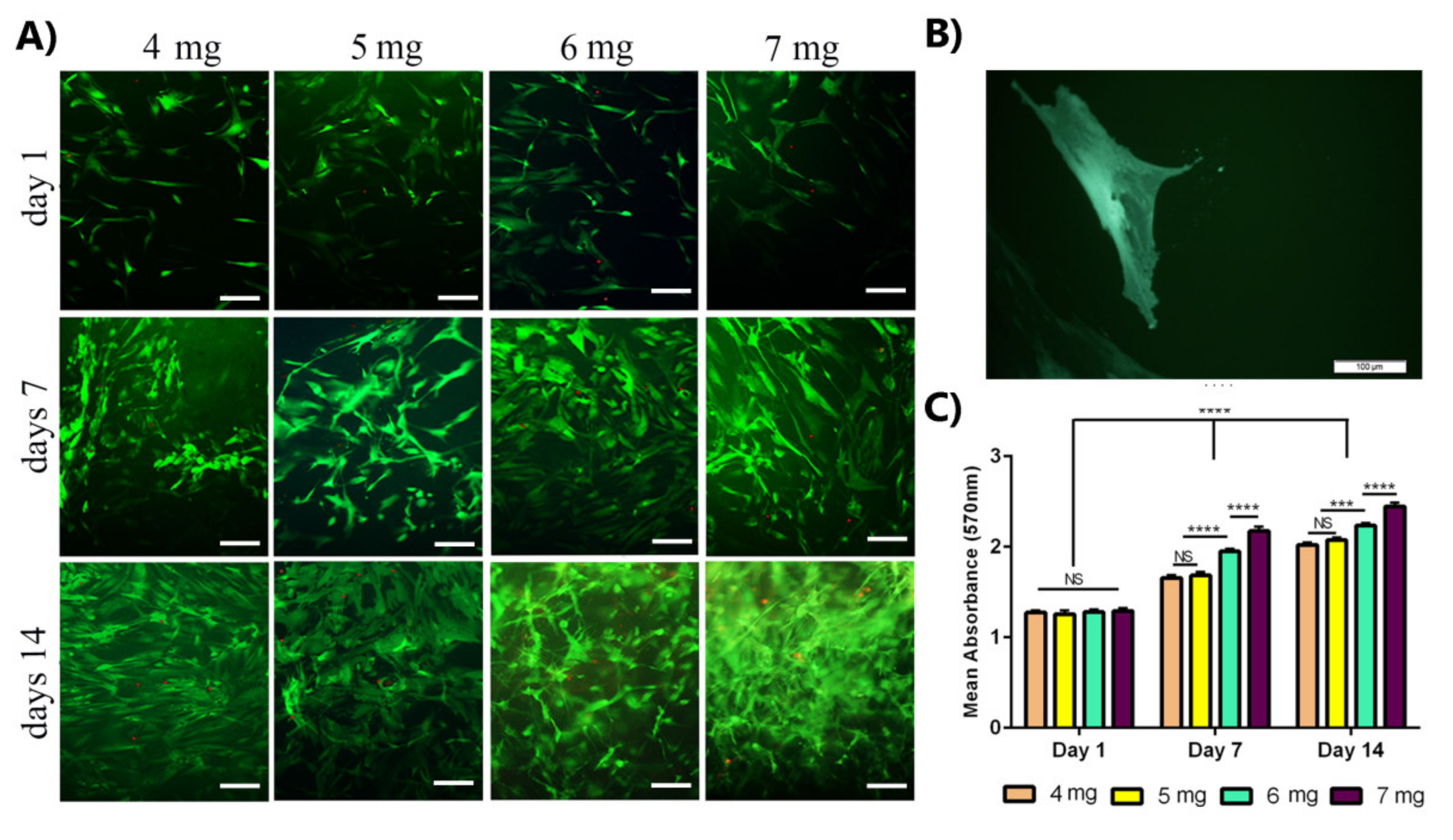
OE-MSCs Viability and Proliferation Assessment
Osteogenic Differentiation
ECM Mineralization
Gene Expression of Bone Markers
2.4. In Vivo Study
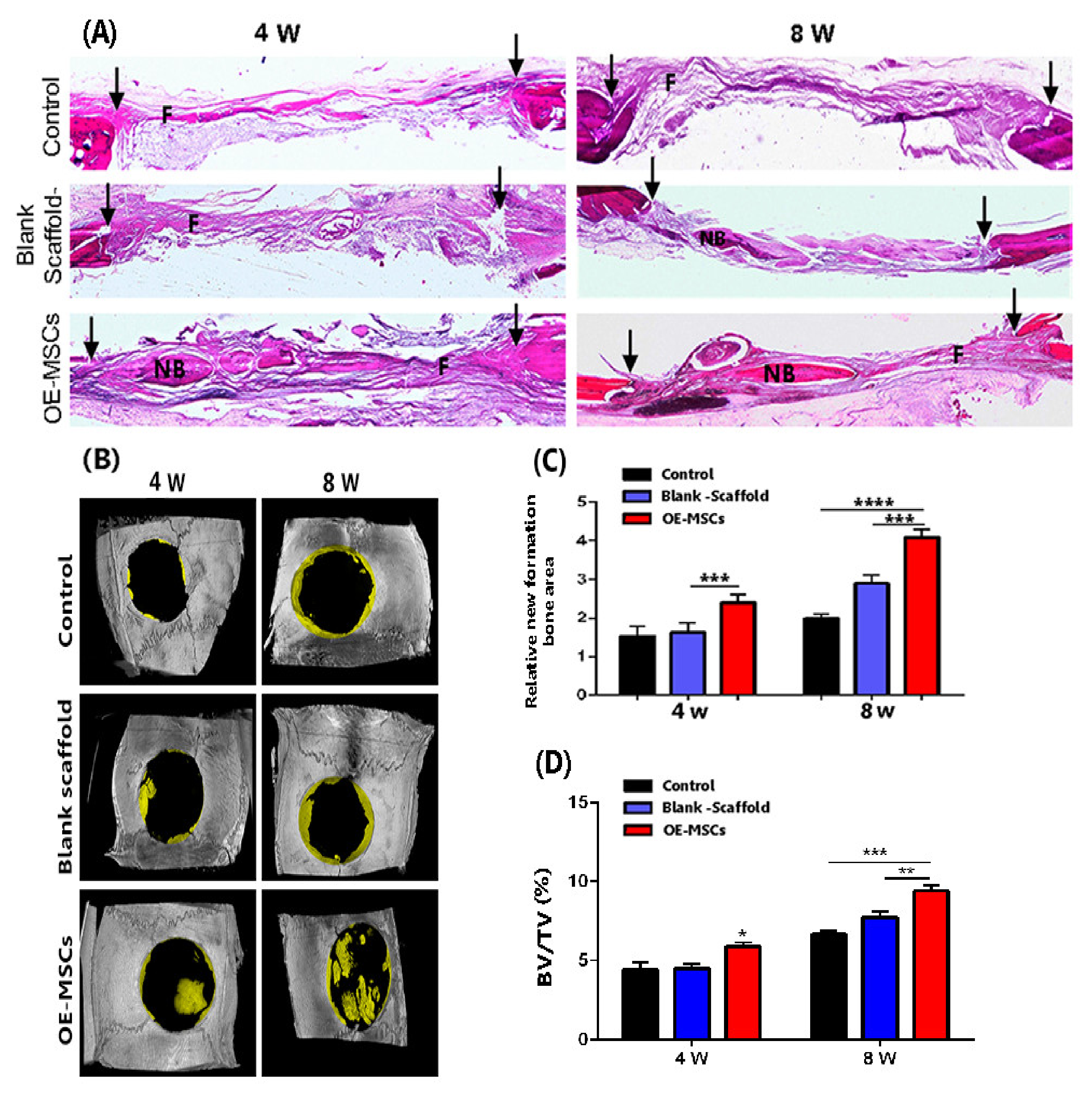
2.4.1. Calvarial Defect Model
2.4.2. OE-MSCs Labelling and Implantation
2.4.3. Optical Imaging
2.4.4. Micro-Computed Tomography
2.4.5. Hematoxylin and Eosin (H&E) Staining
2.5. Statistical Evaluation
3. Results
3.1. Collagen Type I Extracted from Rat Tail and Characterized Next Prepared Collagen Hydrogel
3.2. OE-MSCs Isolated from Human Olfactory Mucosa and Characterized
3.3. The Increase in the Concentration of Collagen Leads to Enhance Stem Cell Viability and Proliferation of OE-MSCs
3.4. Hydrogel Properties and Biocompatibility
3.5. Collagen Concentration of 7 mg/mL Has a Storage Modulus Comparable to the Other Concentrations
3.6. The Increase in the Concentration of Collagen Hydrogel Improved Osteogenic Differentiation of OE-MSCs
3.7. Encapsulated DiI-Labeled Human OE-MSCs in Collagen Hydrogel Were Traced in Rat Defection
3.8. The Highest Bone Healing Rate of Rat Calvarial Defection Observed in the OE-Mscs Group
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Simorgh, S.; Alizadeh, R.; Eftekharzadeh, M.; Haramshahi, S.M.A.; Milan, P.B.; Doshmanziari, M.; Ramezanpour, F.; Gholipourmalekabadi, M.; Seifi, M.; Moradi, F. Olfactory mucosa stem cells: An available candidate for the treatment of the Parkinson’s disease. J. Cell. Physiol. 2019, 234, 23763–23773. [Google Scholar] [CrossRef]
- Alizadeh, R.; Bagher, Z.; Kamrava, S.K.; Falah, M.; Hamidabadi, H.G.; Boroujeni, M.E.; Mohammadi, F.; Khodaverdi, S.; Zare-Sadeghi, A.; Olya, A. Differentiation of human mesenchymal stem cells (MSC) to dopaminergic neurons: A comparison between Wharton’s Jelly and olfactory mucosa as sources of MSCs. J. Chem. Neuroanat. 2019, 96, 126–133. [Google Scholar] [CrossRef]
- Rajan, N.; Habermehl, J.; Coté, M.-F.; Doillon, C.J.; Mantovani, D. Preparation of ready-to-use, storable and reconstituted type I collagen from rat tail tendon for tissue engineering applications. Nat. Protoc. 2006, 1, 2753. [Google Scholar] [CrossRef] [PubMed]
- Paola, C.M.; Camila, A.M.; Ana, C.; Marlon, O.; Diego, S.; Robin, Z.; Beatriz, G.; Cristina, C. Functional textile finishing of type I collagen isolated from bovine bone for potential healthtech. Heliyon 2019, 5, e01260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, J.; Li, Z.; Yuan, X.; Wang, P.; Liu, Y.; Wang, H. Extraction and isolation of type I, III and V collagens and their SDS-PAGE analyses. Trans. Tianjin Univ. 2011, 17, 111. [Google Scholar] [CrossRef]
- Jin, G.-Z.; Kim, H.-W. Effects of type I collagen concentration in hydrogel on the growth and phenotypic expression of rat chondrocytes. Tissue Eng. Regen. Med. 2017, 14, 383–391. [Google Scholar] [CrossRef]
- Bagheri, S.; Bagher, Z.; Hassanzadeh, S.; Simorgh, S.; Kamrava, S.K.; Nooshabadi, V.T.; Shabani, R.; Jalessi, M.; Khanmohammadi, M. Control of cellular adhesiveness in hyaluronic acid-based hydrogel through varying degrees of phenol moiety cross-linking. J. Biomed. Mater. Res. A 2021, 109, 649–658. [Google Scholar] [CrossRef]
- Iqbal, B.; Muhammad, N.; Jamal, A.; Ahmad, P.; Khan, Z.U.H.; Rahim, A.; Khan, A.S.; Gonfa, G.; Iqbal, J.; Rehman, I.U. An application of ionic liquid for preparation of homogeneous collagen and alginate hydrogels for skin dressing. J. Mol. Liq. 2017, 243, 720–725. [Google Scholar] [CrossRef]
- Jiankang, H.; Dichen, L.; Yaxiong, L.; Bo, Y.; Bingheng, L.; Qin, L. Fabrication and characterization of chitosan/gelatin porous scaffolds with predefined internal microstructures. Polymer 2007, 48, 4578–4588. [Google Scholar] [CrossRef]
- Hui, T.; Cheung, K.; Cheung, W.; Chan, D.; Chan, B. In vitro chondrogenic differentiation of human mesenchymal stem cells in collagen microspheres: Influence of cell seeding density and collagen concentration. Biomaterials 2008, 29, 3201–3212. [Google Scholar] [CrossRef]
- Karimi, S.; Bagher, Z.; Najmoddin, N.; Simorgh, S.; Pezeshki-Modaress, M. Alginate-magnetic short nanofibers 3D composite hydrogel enhances the encapsulated human olfactory mucosa stem cells bioactivity for potential nerve regeneration application. Int. J. Biol. Macromol. 2021, 167, 796–806. [Google Scholar] [CrossRef] [PubMed]
- Apinun, J.; Honsawek, S.; Kuptniratsaikul, S.; Jamkratoke, J.; Kanokpanont, S. Osteogenic differentiation of rat bone marrow-derived mesenchymal stem cells encapsulated in Thai silk fibroin/collagen hydrogel: A pilot study in vitro. Asian Biomed. 2019, 12, 273–279. [Google Scholar] [CrossRef] [Green Version]
- Souza, A.T.P.; Lopes, H.B.; Freitas, G.P.; Ferraz, E.P.; Oliveira, F.S.; Almeida, A.L.G.; Weffort, D.; Beloti, M.M.; Rosa, A.L. Role of embryonic origin on osteogenic potential and bone repair capacity of rat calvarial osteoblasts. J. Bone Miner. Metab. 2020, 1–10. [Google Scholar] [CrossRef]
- Delorme, B.; Nivet, E.; Gaillard, J.; Häupl, T.; Ringe, J.; Devèze, A.; Magnan, J.; Sohier, J.; Khrestchatisky, M.; Roman, F.S. The human nose harbors a niche of olfactory ectomesenchymal stem cells displaying neurogenic and osteogenic properties. Stem Cells Dev. 2010, 19, 853–866. [Google Scholar] [CrossRef] [PubMed]
- Haramshahi, S.M.A.; Bonakdar, S.; Moghtadaei, M.; Kamguyan, K.; Thormann, E.; Tanbakooei, S.; Simorgh, S.; Brouki-Milan, P.; Amini, N.; Latifi, N. Tenocyte-imprinted substrate: A topography-based inducer for tenogenic differentiation in adipose tissue-derived mesenchymal stem cells. Biomed. Mater. 2020, 15, 035014. [Google Scholar] [CrossRef]
- Liu, J.; Zheng, H.; Poh, P.S.; Machens, H.-G.; Schilling, A.F. Hydrogels for engineering of perfusable vascular networks. Int. J. Mol. Sci. 2015, 16, 15997–16016. [Google Scholar] [CrossRef] [PubMed]
- Hamidabadi, H.G.; Shafaroudi, M.M.; Seifi, M.; Bojnordi, M.N.; Behruzi, M.; Gholipourmalekabadi, M.; Shafaroudi, A.M.; Rezaei, N. Repair of critical-sized rat calvarial defects with three-dimensional hydroxyapatite-gelatin scaffolds and bone marrow stromal stem cells. Med. Arch. 2018, 72, 88. [Google Scholar] [CrossRef] [Green Version]
- Simorgh, S.; Alizadeh, R.; Shabani, R.; Karimzadeh, F.; Seidkhani, E.; Majidpoor, J.; Moradi, F.; Kasbiyan, H. Olfactory mucosa stem cells delivery via nasal route: A simple way for the treatment of Parkinson disease. Neurotox. Res. 2021, 39, 598–608. [Google Scholar] [CrossRef]
- Ramhormozi, P.; Mohajer Ansari, J.; Simorgh, S.; Nobakht, M. Bone Marrow-Derived Mesenchymal Stem Cells Combined With Simvastatin Accelerates Burn Wound Healing by Activation of the Akt/mTOR Pathway. J. Burn Care Res. 2020, 41, 1069–1078. [Google Scholar] [CrossRef]
- Abe, T.; Sumi, K.; Kunimatsu, R.; Oki, N.; Tsuka, Y.; Nakajima, K.; Tanimoto, K. Dynamic imaging of the effect of mesenchymal stem cells on osteoclast precursor cell chemotaxis for bone defects in the mouse skull. J. Dent. Sci. 2018, 13, 354–359. [Google Scholar] [CrossRef]
- Feldkamp, L.A.; Davis, L.C.; Kress, J.W. Practical cone-beam algorithm. J. Opt. Soc. Am. A 1984, 1, 612–619. [Google Scholar] [CrossRef] [Green Version]
- Hivechi, A.; Bahrami, S.H.; Siegel, R.A.; Siehr, A.; Sahoo, A.; Milan, P.B.; Joghataei, M.T.; Amoupour, M.; Simorgh, S. Cellulose nanocrystal effect on crystallization kinetics and biological properties of electrospun polycaprolactone. Mater. Sci. Eng. C 2021, 121, 111855. [Google Scholar] [CrossRef]
- Banfi, A.; Bianchi, G.; Notaro, R.; Luzzatto, L.; Cancedda, R.; Quarto, R. Replicative aging and gene expression in long-term cultures of human bone marrow stromal cells. Tissue Eng. 2002, 8, 901–910. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; Gobinet, C.; Feru, J.; Pasco, S.B.; Manfait, M.; Piot, O. Characterization of type I and IV collagens by Raman microspectroscopy: Identification of spectral markers of the dermo-epidermal junction. Int. J. Spectrosc. 2012, 27, 421–427. [Google Scholar] [CrossRef]
- Carvalho, A.M.; Marques, A.P.; Silva, T.H.; Reis, R.L. Evaluation of the potential of collagen from codfish skin as a biomaterial for biomedical applications. Mar. Drugs 2018, 16, 495. [Google Scholar] [CrossRef] [Green Version]
- Gelse, K.; Pöschl, E.; Aigner, T. Collagens—Structure, function, and biosynthesis. Adv. Drug Deliv. Rev. 2003, 55, 1531–1546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arabpour, Z.; Baradaran-Rafii, A.; Bakhshaiesh, N.L.; Ai, J.; Ebrahimi-Barough, S.; Esmaeili Malekabadi, H.; Nazeri, N.; Vaez, A.; Salehi, M.; Sefat, F. Design and characterization of biodegradable multi layered electrospun nanofibers for corneal tissue engineering applications. J. Biomed. Mater. Res. A 2019, 107, 2340–2349. [Google Scholar] [CrossRef]
- Dedhar, S.; Ruoslahti, E.; Pierschbacher, M.D. A cell surface receptor complex for collagen type I recognizes the Arg-Gly-Asp sequence. J. Cell Biol. 1987, 104, 585–593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazzoni, E.; D’Agostino, A.; Manfrini, M.; Maniero, S.; Puozzo, A.; Bassi, E.; Marsico, S.; Fortini, C.; Trevisiol, L.; Patergnani, S. Human adipose stem cells induced to osteogenic differentiation by an innovative collagen/hydroxylapatite hybrid scaffold. FASEB J. 2017, 31, 4555–4565. [Google Scholar] [CrossRef] [Green Version]
- Duan, W.; Haque, M.; Kearney, M.T.; Lopez, M.J. Collagen and hydroxyapatite scaffolds activate distinct osteogenesis signaling pathways in adult adipose-derived multipotent stromal cells. Tissue Eng. Part C Methods 2017, 23, 592–603. [Google Scholar] [CrossRef]
- Zhang, D.; Wu, X.; Chen, J.; Lin, K. The development of collagen based composite scaffolds for bone regeneration. Bioact. Mater. 2018, 3, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, A.; Zandi, M.; Pezeshki-Modaress, M.; Rajabi, S. Tough, hybrid chondroitin sulfate nanofibers as a promising scaffold for skin tissue engineering. Int. J. Biol. Macromol. 2019, 132, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Chen, H.; Zhang, Y.; Zan, Y.; Ni, T.; Liu, M.; Pei, R. Photo-crosslinkable, bone marrow-derived mesenchymal stem cells-encapsulating hydrogel based on collagen for osteogenic differentiation. Colloids Surf. B Biointerfaces 2019, 174, 528–535. [Google Scholar] [CrossRef] [PubMed]
- Ge, L.; Jiang, M.; Duan, D.; Wang, Z.; Qi, L.; Teng, X.; Zhao, Z.; Wang, L.; Zhuo, Y.; Chen, P. Secretome of olfactory mucosa mesenchymal stem cell, a multiple potential stem cell. Stem Cells Int. 2016, 2016, 1243659. [Google Scholar] [CrossRef] [Green Version]
- Chan, B.P.; Hui, T.; Yeung, C.; Li, J.; Mo, I.; Chan, G. Self-assembled collagen–human mesenchymal stem cell microspheres for regenerative medicine. Biomaterials 2007, 28, 4652–4666. [Google Scholar] [CrossRef]
- Chan, B.P.; Hui, T.Y.; Wong, M.Y.; Yip, K.H.K.; Chan, G.C.F. Mesenchymal stem cell–encapsulated collagen microspheres for bone tissue engineering. Tissue Eng. Part C Methods 2010, 16, 225–235. [Google Scholar] [CrossRef] [Green Version]


| Gene Name | Primer |
|---|---|
| RUNX2 | F GCCTCCAAGGTGGTAGCCC R CGTTACCCGCCATGAGAGTA |
| Collagen I (Col1) | F TCCGACCTCTCTCCTCTGAA R GAGTGGGGTTATGGAGGGAT |
| Osteopontin (OPN) | F GACCTGACATCCAGTACCC R GTTTCAGCACTCTGGTCATC |
| Osteocalcin (OC) | F GCAAAGGTGCAGCCTTTGTG RGGCTCCCAGCCATTGATACAG |
| Alkaline Phosphatase (ALP) | F GCACCTGCCTTACTAACTC RAGACACCCATCCCATCTC |
| Β-actin | F CTTCCTTCCTGGGCATG R GTC TTTGCGGATGTCCAC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simorgh, S.; Milan, P.B.; Saadatmand, M.; Bagher, Z.; Gholipourmalekabadi, M.; Alizadeh, R.; Hivechi, A.; Arabpour, Z.; Hamidi, M.; Delattre, C. Human Olfactory Mucosa Stem Cells Delivery Using a Collagen Hydrogel: As a Potential Candidate for Bone Tissue Engineering. Materials 2021, 14, 3909. https://doi.org/10.3390/ma14143909
Simorgh S, Milan PB, Saadatmand M, Bagher Z, Gholipourmalekabadi M, Alizadeh R, Hivechi A, Arabpour Z, Hamidi M, Delattre C. Human Olfactory Mucosa Stem Cells Delivery Using a Collagen Hydrogel: As a Potential Candidate for Bone Tissue Engineering. Materials. 2021; 14(14):3909. https://doi.org/10.3390/ma14143909
Chicago/Turabian StyleSimorgh, Sara, Peiman Brouki Milan, Maryam Saadatmand, Zohreh Bagher, Mazaher Gholipourmalekabadi, Rafieh Alizadeh, Ahmad Hivechi, Zohreh Arabpour, Masoud Hamidi, and Cédric Delattre. 2021. "Human Olfactory Mucosa Stem Cells Delivery Using a Collagen Hydrogel: As a Potential Candidate for Bone Tissue Engineering" Materials 14, no. 14: 3909. https://doi.org/10.3390/ma14143909
APA StyleSimorgh, S., Milan, P. B., Saadatmand, M., Bagher, Z., Gholipourmalekabadi, M., Alizadeh, R., Hivechi, A., Arabpour, Z., Hamidi, M., & Delattre, C. (2021). Human Olfactory Mucosa Stem Cells Delivery Using a Collagen Hydrogel: As a Potential Candidate for Bone Tissue Engineering. Materials, 14(14), 3909. https://doi.org/10.3390/ma14143909