Evaluation of the Feasibility of NaCaPO4-Blended Zirconia as a New CAD/CAM Material for Dental Restoration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Sample Characteristics
2.3. Statistical Methods
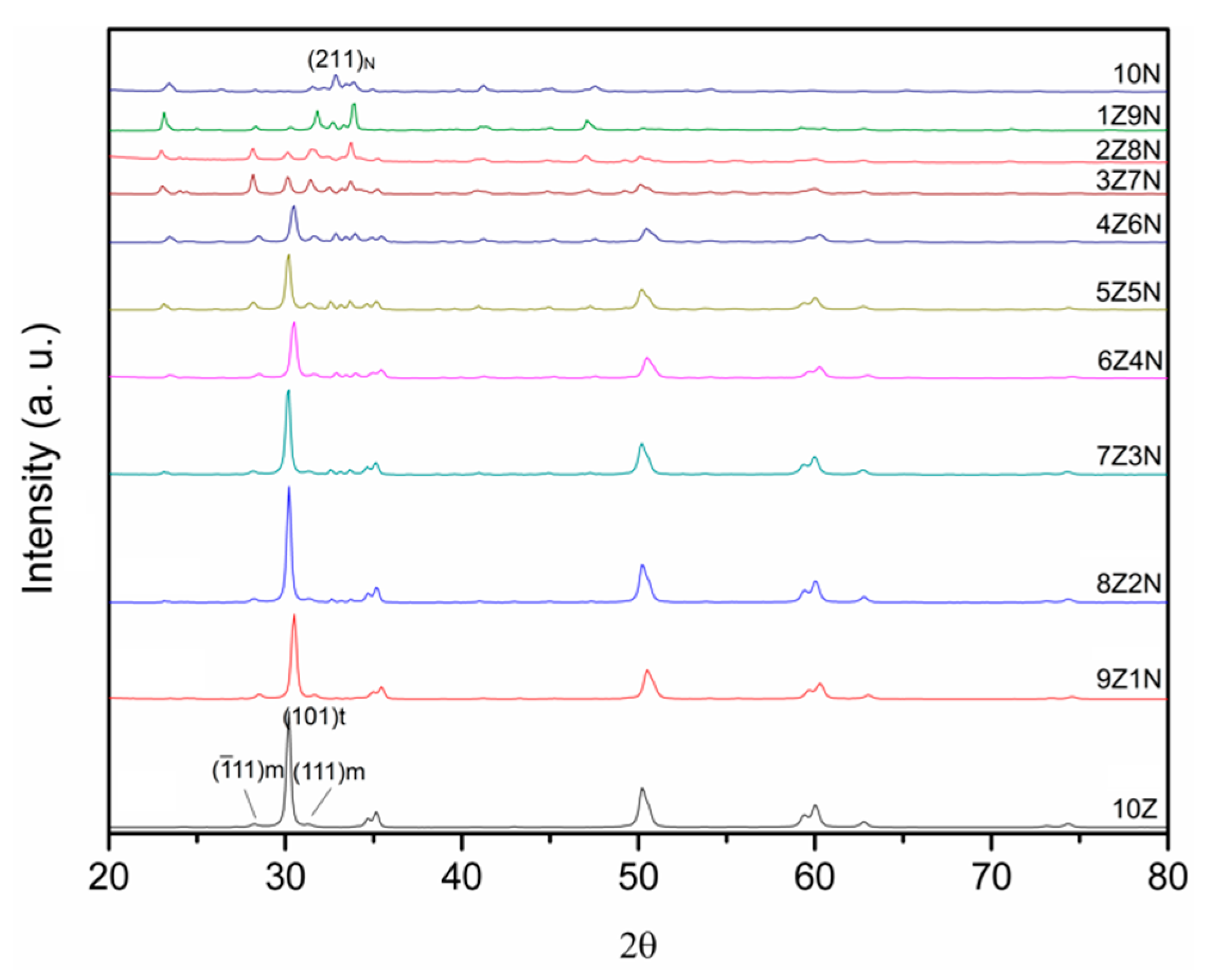
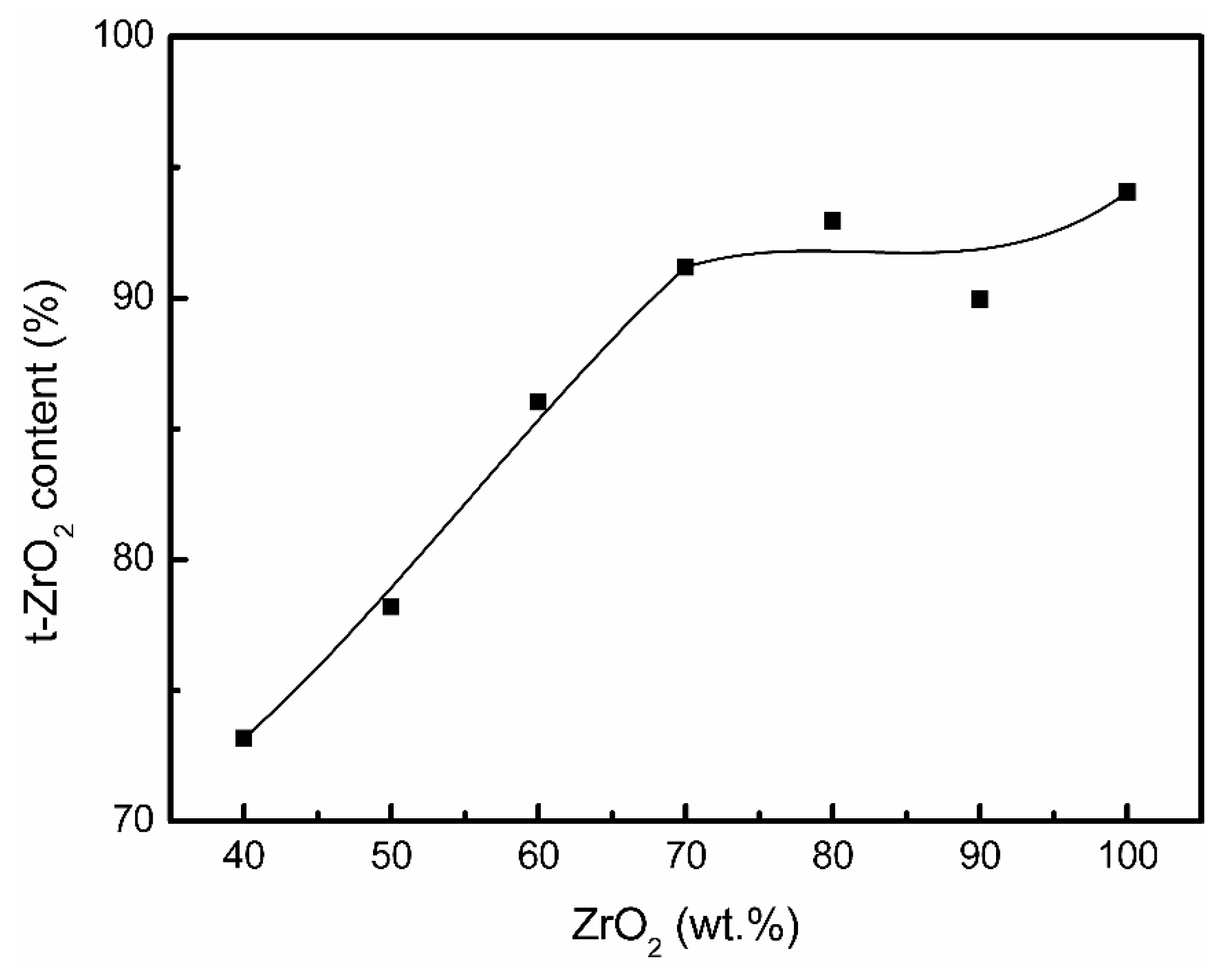
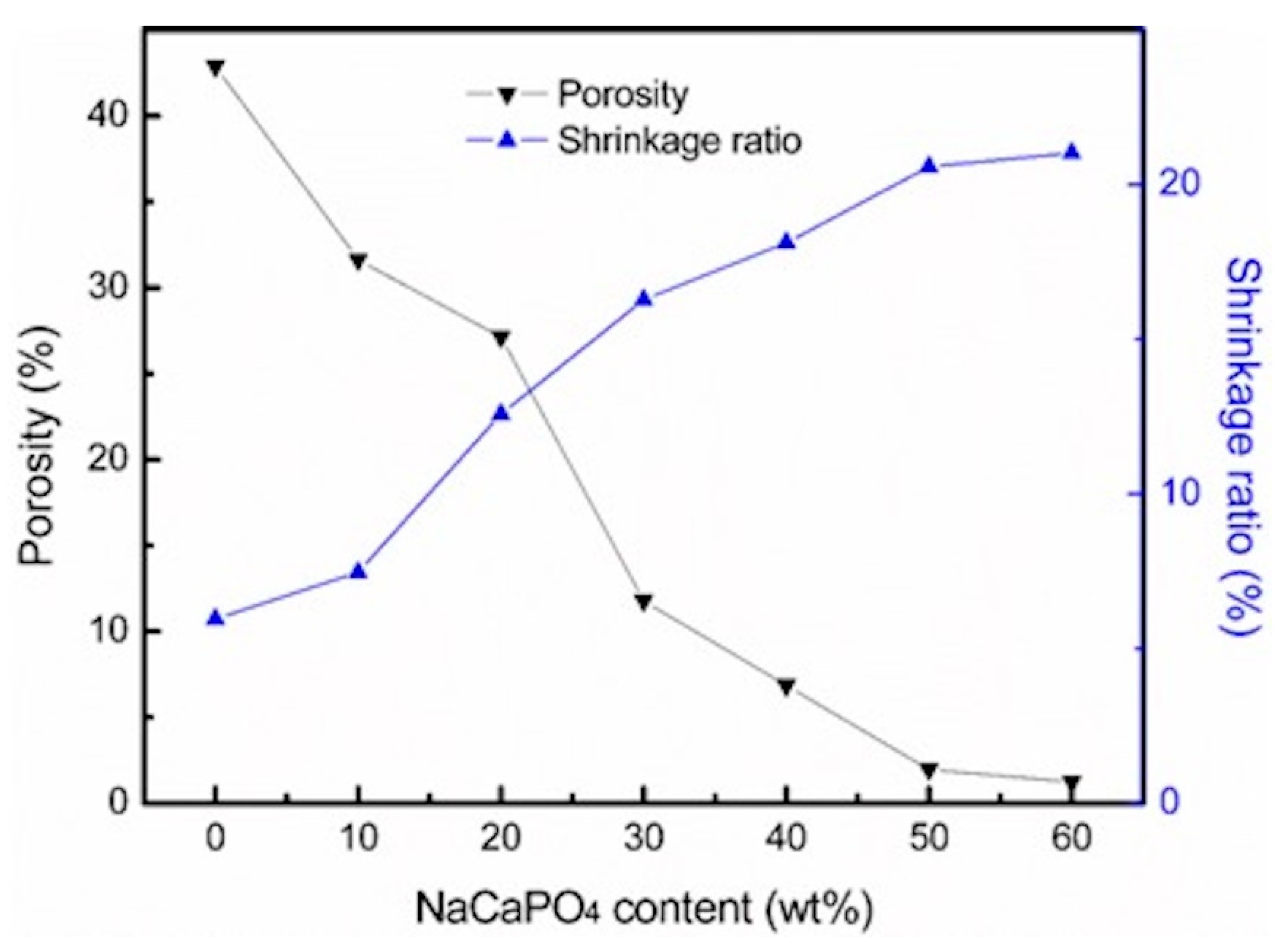
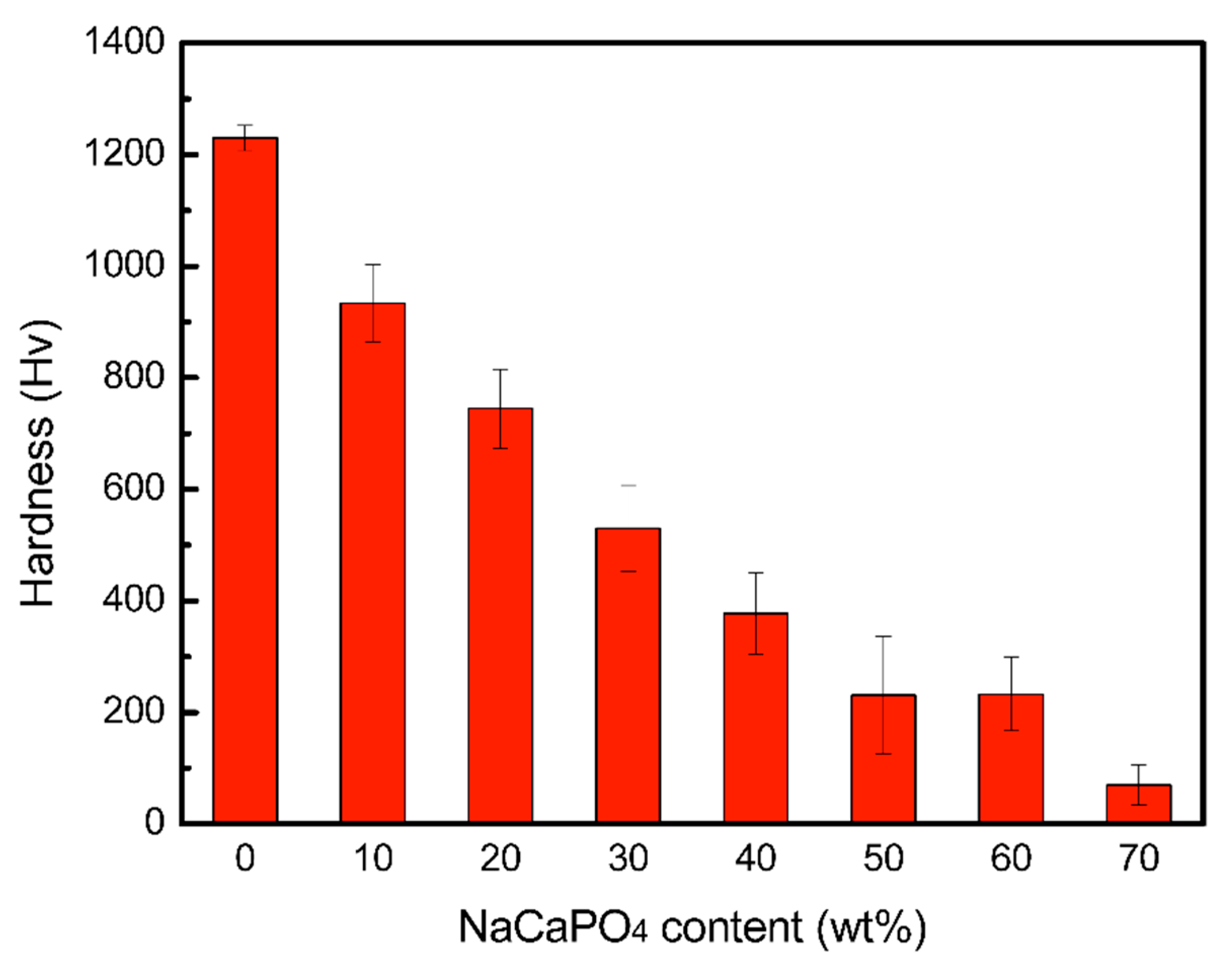
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Grech, J.; Antunes, E. Zirconia in dental prosthetics: A literature review. J. Mater. Sci. Technol. 2019, 18, 4956–4964. [Google Scholar] [CrossRef]
- Elias, C.N.; dos Santos, H.E.S.; Garbossa, M.; dos Santos, C. Mechanical properties of zirconia Y-TZP core veneered for dentistry applications. J. Ceram. Sci. Technol. 2017, 8, 525–530. [Google Scholar]
- Aboushahba, M.; Katamish, H.; Elagroudy, M. Evaluation of hardness and wear of surface treated zirconia on enamel wear. An in-vitro study. Future Dent. J. 2018, 4, 76–83. [Google Scholar] [CrossRef]
- Zarifah, N.A.; Matori, K.A.; Sidek, H.A.A.; Wahab, Z.A.; Mohd Salleh, M.A.; Zainuddin, N.; Khiri, M.Z.A.; Farhana, N.S.; Omar, N.A.S. Effect of hydroxyapatite reinforced with 45S5 glass on physical, structural and mechanical properties. Procedia Chem. 2016, 19, 30–37. [Google Scholar] [CrossRef] [Green Version]
- Curran, D.J.; Fleming, T.J.; Towler, M.R.; Hampshire, S. Mechanical properties of hydroxyapatite–zirconia compacts sintered by two different sintering methods. J. Mater. Sci. Mater. Med. 2010, 21, 1109–1120. [Google Scholar] [CrossRef]
- Kong, Y.; Yang, Z.; Zhang, G.; Yuan, Q. Friction and wear characteristics of mullite, ZTM and TZP ceramics. Wear 1998, 218, 159–166. [Google Scholar] [CrossRef]
- Casellas, D.; Nagl, M.M.; Llanes, L.; Anglada, M. Fracture toughness of alumina and ZTA ceramics: Microstructural coarsening effects. J. Mater. Process. Technol. 2003, 143–144, 148–152. [Google Scholar] [CrossRef]
- Towler, M.R.; Gibson, I.R. The effect of low levels of zirconia addition on the mechanical properties of hydroxyapatite. J. Mater. Sci. Lett. 2001, 20, 1719–1722. [Google Scholar] [CrossRef]
- Shih, W.J.; Chen, Y.F.; Wang, M.C.; Hon, M.H. Crystal growth and morphology of the nanosized hydroxyapatite powders synthesized from CaHPO4·2H2O and CaCO3 by hydrolysis method. J. Cryst. Growth 2004, 270, 211–218. [Google Scholar] [CrossRef]
- Hung, I.M.; Shih, W.J.; Hon, M.H.; Wang, M.C. The properties of sintered calcium phosphate with [Ca]/[P] = 1.50. Int. J. Mol. Sci. 2012, 13, 13569–13586. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.C.; Shih, W.J.; Hung, I.M.; Chen, H.T.; Hon, M.H.; Huang, H.H. Characterization of calcium phosphate apatite with variable Ca/P ratios sintered at low temperature. Ceram. Int. 2015, 41, 1223–1233. [Google Scholar] [CrossRef]
- Suchanek, W.; Yashima, M.; Kakihana, M.; Yoshimura, M. β-Rhenanite (β-NaCaPO4) as weak interphase for hydroxyapatite ceramics. J. Eur. Ceram. Soc. 1988, 18, 1923–1929. [Google Scholar] [CrossRef]
- Kangasniemi, I.M.O.; Vedel, E.; de Blick-Hogerworst, J.; Yli-Urpo, A.U.; de Groot, K. Dissolution and scanning electron microscopic studies of Ca, P particle-containing bioactive glasses. J. Biomed. Mater. Res. 1993, 27, 1225–1233. [Google Scholar] [CrossRef]
- Nair, G.B.; Tamboli, S.; Dhoble, S.J.; Swart, H.C. Structural and luminescence properties of thermally stable cool-white light emitting NaCaPO4:Dy3+ phosphor. Optik 2020, 219, 1–11. [Google Scholar] [CrossRef]
- Yang, Z.; Yang, G.; Wang, S.; Tian, J.; Li, X.N.; Guo, Q.L.; Fu, G.S. A novel green-emitting phosphor NaCaPO4:Eu2+ for white LEDs. Mater. Lett. 2008, 62, 1884–1886. [Google Scholar] [CrossRef]
- Jalota, S.; Bhaduri, S.B.; Tas, A.C. A new rhenanite (β-NaCaPO4) and hydroxyapatite biphasic biomaterial for skeletal repair. J. Biomed. Mater. Res. B Appl. Biomater. 2007, 80, 304–316. [Google Scholar] [CrossRef]
- Lan, T.H.; Wang, C.H.; Chen, K.K.; Wang, M.C.; Lee, H.E. Milling properties of low temperature sintered zirconia blocks for dental use. Mater. Sci. Eng. C 2017, 73, 692–699. [Google Scholar] [CrossRef]
- Craig, R.G.; Peyton, F.A. The microhardness of enamel and dentin. J. Dent. Res. 1958, 37, 661–668. [Google Scholar] [CrossRef]
- El-Ghannam, A.; Ning, C.Q.; Mehta, J. Cyclosilicate nanocomposite: A novel resorbable bioactive tissue engineering scaffold for BMP and bone-marrow cell delivery. J. Biomed. Mater. Res. A 2004, 71A, 377–390. [Google Scholar] [CrossRef]
- Evis, Z. Reactions in hydroxyapatite–zirconia composites. Ceram. Int. 2007, 33, 987–991. [Google Scholar] [CrossRef]
- Sato, T.; Shimada, M. Transformation of yttria-doped tetragonal ZrO2 polycrystals by annealing in water. J. Am. Ceram. Soc. 1985, 68, 356. [Google Scholar] [CrossRef]
- Skovgaard, M.; Almdal, K.; van Lelieveld, A. Phase stabilizing effects of phosphates and sulfates on noncrystalline metastable tetragonal zirconia. J. Mater. Sci. 2010, 45, 6271–6274. [Google Scholar] [CrossRef]
- Mörmann, W.H.; Stawarczyk, B.; Ender, A.; Sener, B.; Attin, T.; Mehl, A. Wear characteristics of current aesthetic dental restorative CAD/CAM materials: Two-body wear, gloss retention, roughness and Martens hardness. J. Mech. Behav. Biomed. 2013, 20, 113–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stober, T.; Bermejo, J.L.; Schwindling, F.S.; Schmitter, M. Clinical assessment of enamel wear caused by monolithic zirconia crowns. J. Oral. Rehabil. 2016, 43, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Gemalmaz, D.; Kukrer, D. In vivo and in vitro evaluation of marginal fit of class II ceromer inlays. J. Oral. Rehabil. 2006, 33, 436–442. [Google Scholar] [CrossRef]
- Goujat, A.; Abouelleil, H.; Colon, P.; Jeannin, C.; Pradelle, N.; Seux, D.; Grosgogeat, B. Mechanical properties and internal fit of 4 CAD-CAM block materials. J. Prosthet. Dent. 2018, 119, 384–389. [Google Scholar] [CrossRef] [PubMed]
- Boitelle, P.; Mawussi, B.; Tapie, L.; Fromentin, O. A systematic review of CAD/CAM fit restoration evaluations. J. Oral. Rehabil. 2014, 41, 853–874. [Google Scholar] [CrossRef]
- Hamza, T.A.; Sherif, R.M. In vitro evaluation of marginal discrepancy of monolithic zirconia restorations fabricated with different CAD-CAM systems. J. Prosthet. Dent. 2017, 117, 762–766. [Google Scholar] [CrossRef]

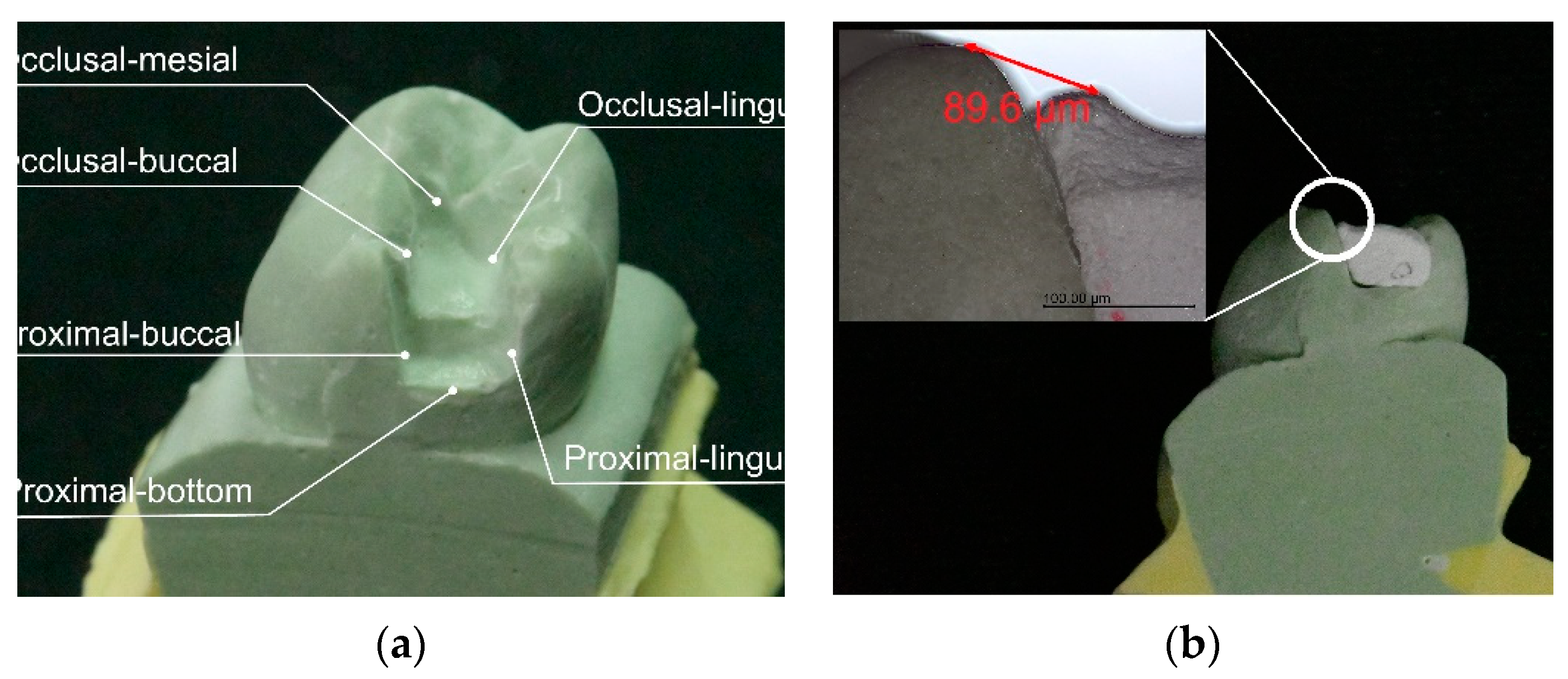
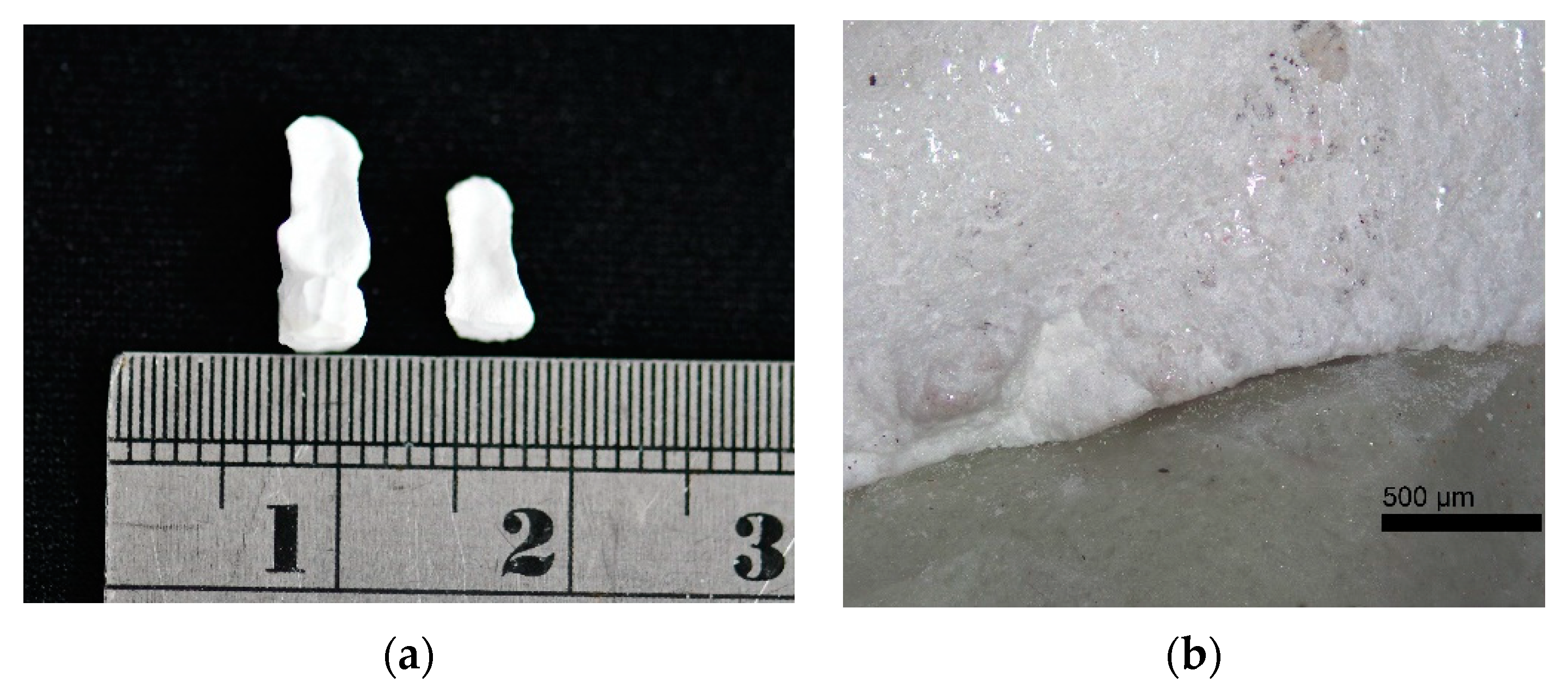
| Sample | Mean ± SD (mm) | p-Value * |
|---|---|---|
| 7Z3N | 0.90 ± 0.96 | p < 0.001 |
| 6Z4N | 7.16 ± 10.65 |
| Position | Mean ± SD (mm) | p-Value * (MC) |
|---|---|---|
| Occlusal–lingual | 0.74 ± 0.44 | p = 0.067 |
| Occlusal–buccal | 1.41 ± 1.55 | |
| Occlusal–mesial | 1.10 ± 0.98 | |
| Proximal–bottom | 0.93 ± 1.01 | |
| Proximal–lingual | 0.51 ± 0.58 | |
| Proximal–buccal | 0.70 ± 0.55 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lan, T.-H.; Chen, Y.-F.; Wang, Y.-Y.; Chou, M.M.C. Evaluation of the Feasibility of NaCaPO4-Blended Zirconia as a New CAD/CAM Material for Dental Restoration. Materials 2021, 14, 3819. https://doi.org/10.3390/ma14143819
Lan T-H, Chen Y-F, Wang Y-Y, Chou MMC. Evaluation of the Feasibility of NaCaPO4-Blended Zirconia as a New CAD/CAM Material for Dental Restoration. Materials. 2021; 14(14):3819. https://doi.org/10.3390/ma14143819
Chicago/Turabian StyleLan, Ting-Hsun, Yu-Feng Chen, Yen-Yun Wang, and Mitch M. C. Chou. 2021. "Evaluation of the Feasibility of NaCaPO4-Blended Zirconia as a New CAD/CAM Material for Dental Restoration" Materials 14, no. 14: 3819. https://doi.org/10.3390/ma14143819






