Synthesis and Na+ Ion Conductivity of Stoichiometric Na3Zr2Si2PO12 by Liquid-Phase Sintering with NaPO3 Glass
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
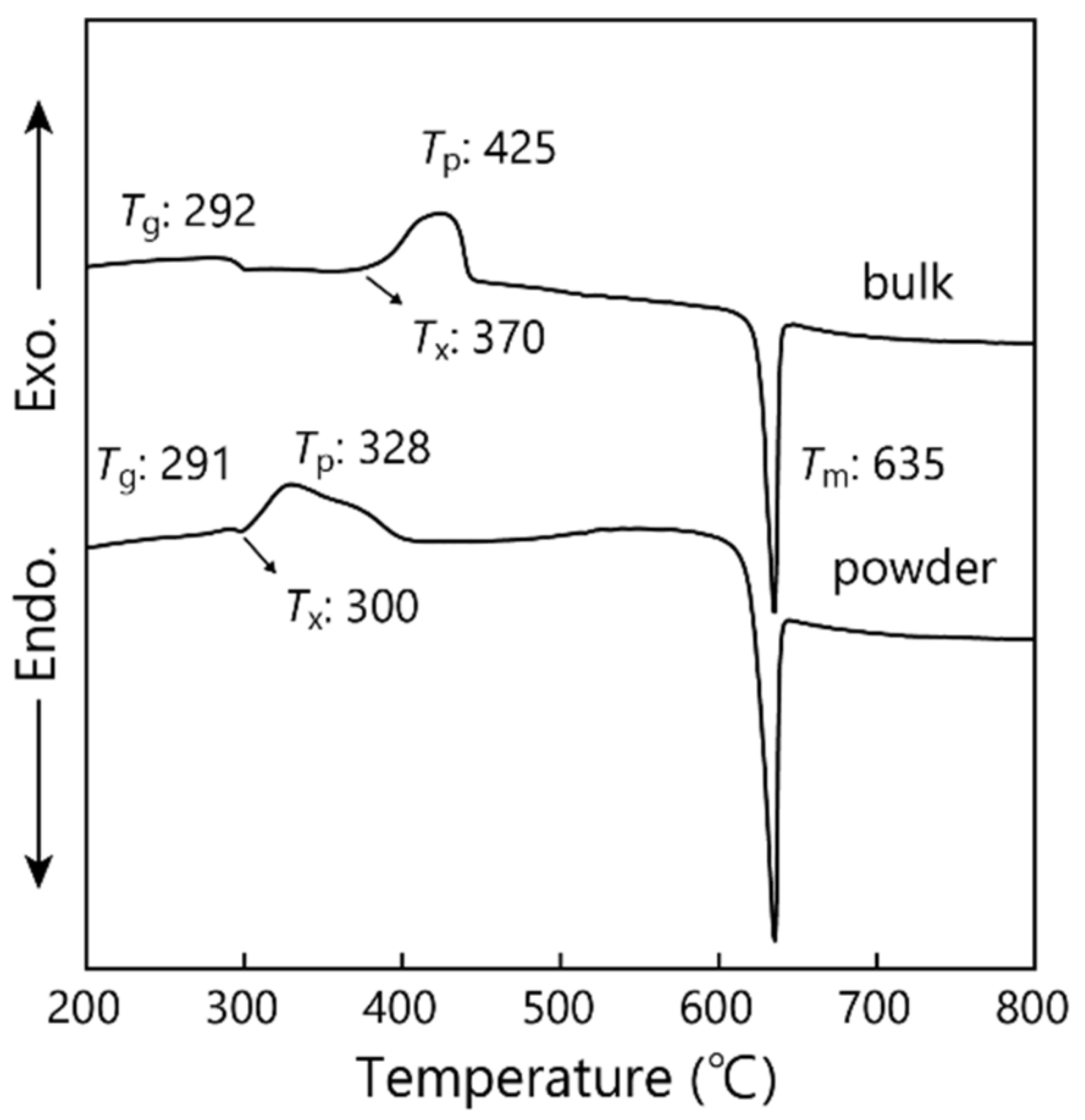
3.1. Thermal Properties of NaPO3 Glass
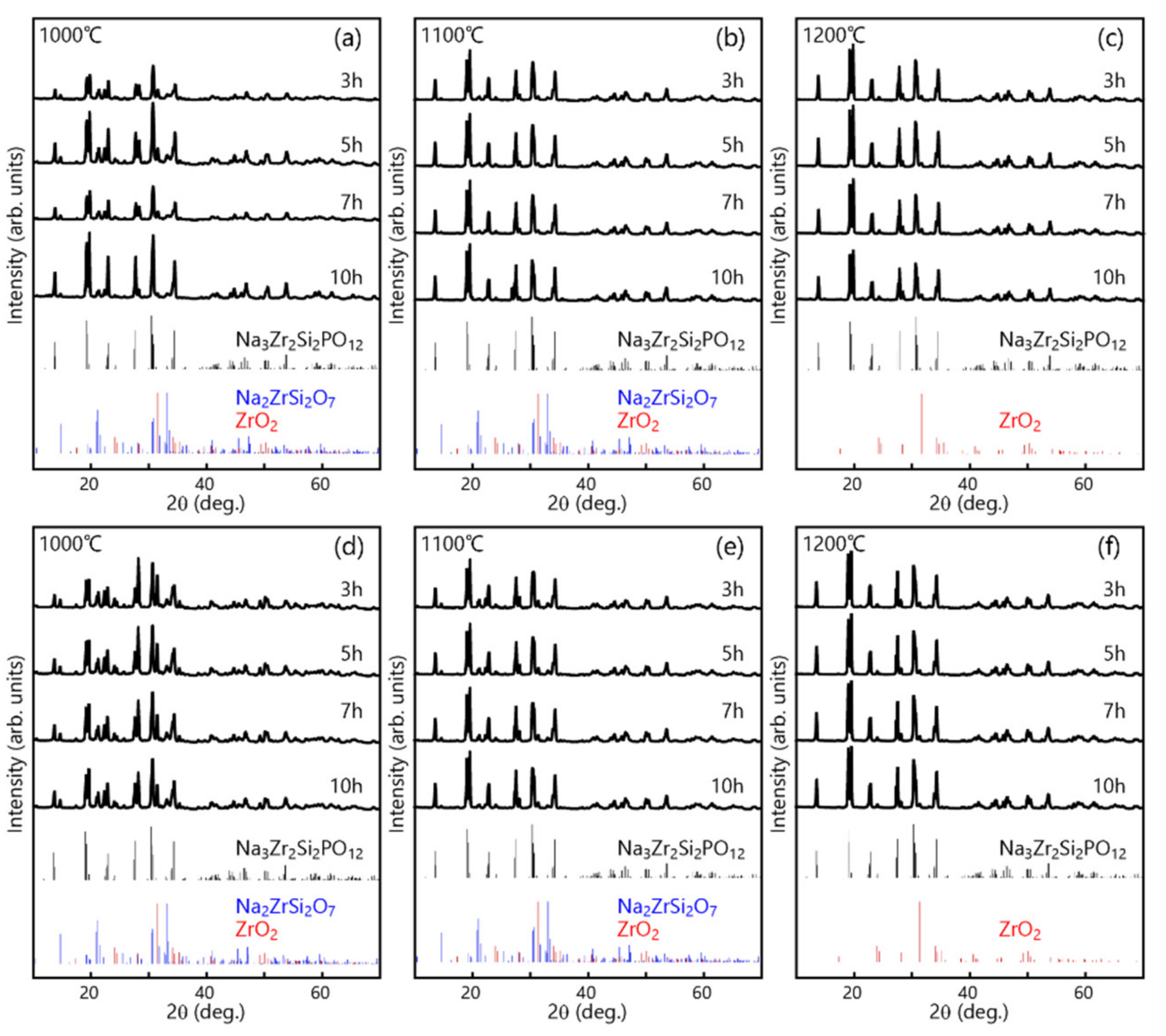
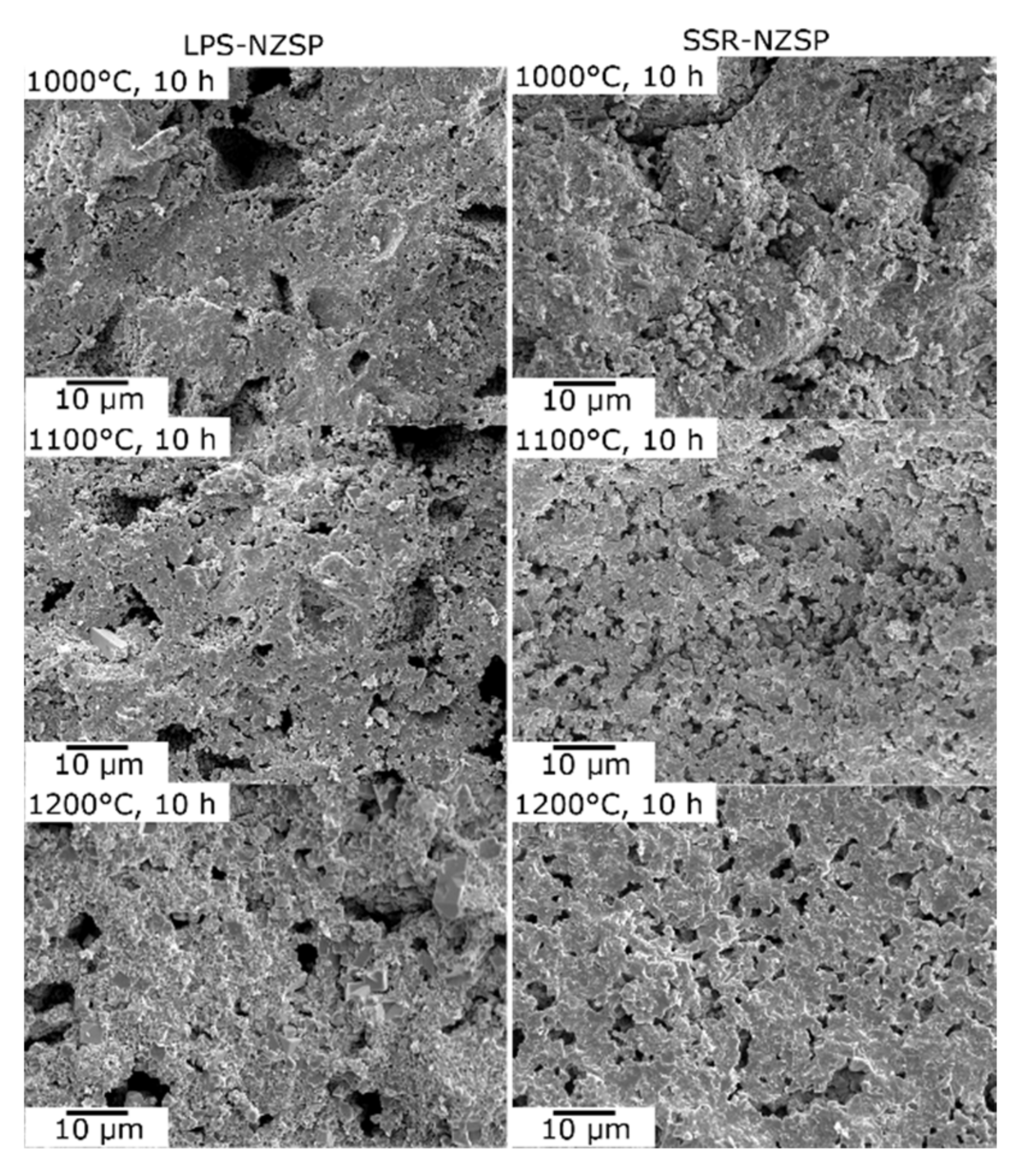
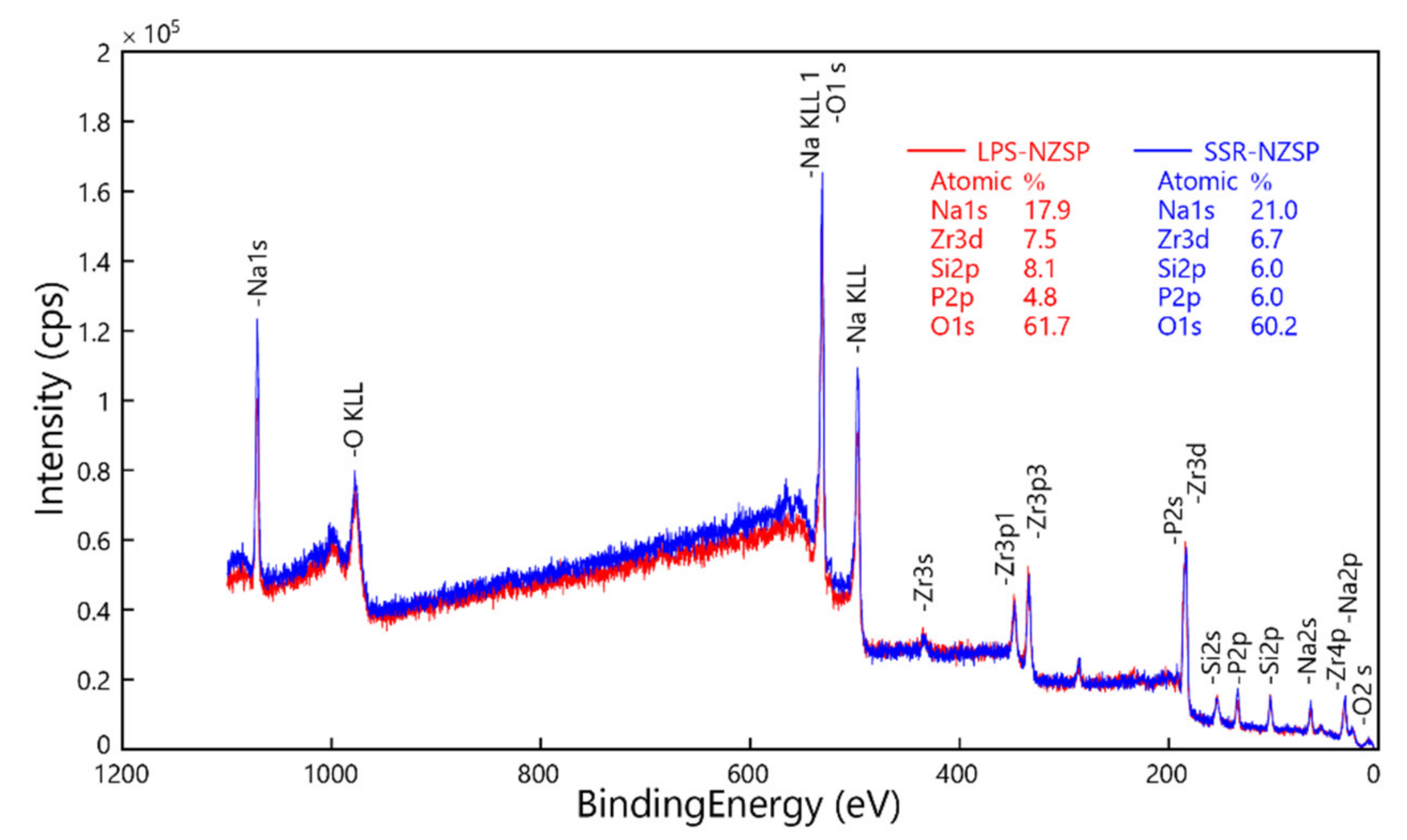
3.2. Formation and Microstructure of Na3Zr2Si2PO12
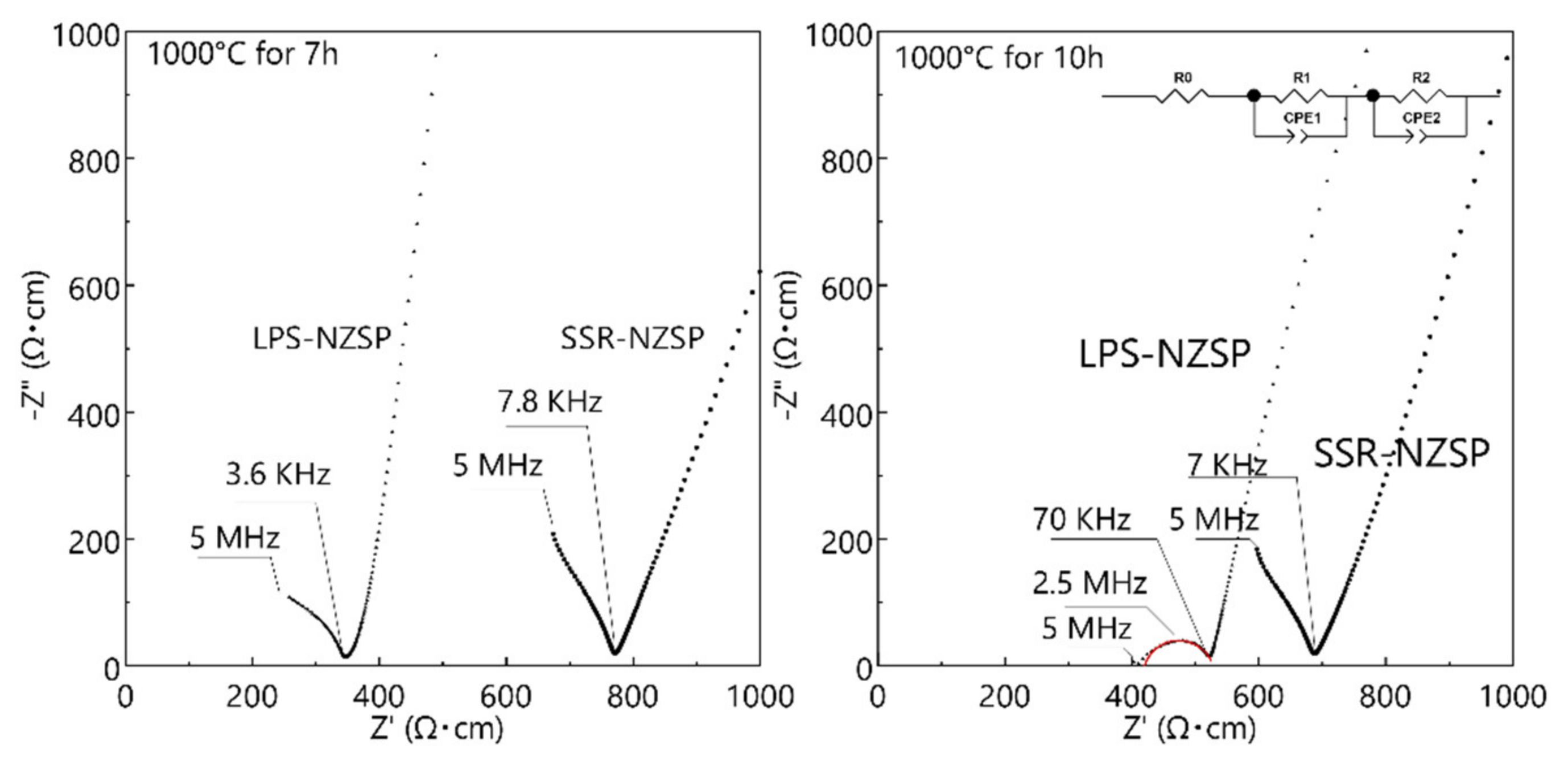
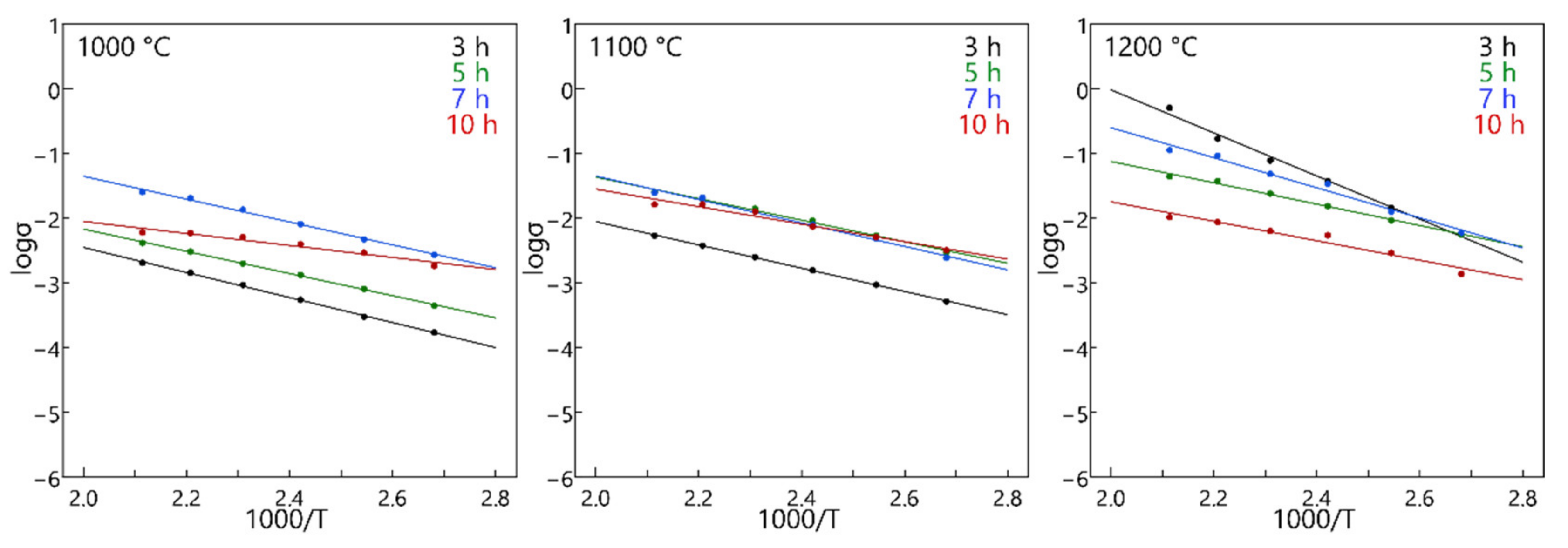
3.3. Electrical Conductivity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Takada, K. Progress and prospective of solid-state lithium batteries. Acta Mater. 2013, 61, 759–770. [Google Scholar] [CrossRef]
- Kato, Y.; Hori, S.; Saito, T.; Suzuki, K.; Hirayama, M.; Mitsui, A.; Yonemura, M.; Iba, H.; Kanno, R. High-power all-solid-state batteries using sulfide superionic conductors. Nat. Energy 2016, 1, 16030. [Google Scholar] [CrossRef]
- Tatsumisago, M.; Nagao, M.; Hayashi, A. Recent development of sulfide solid electrolytes and interfacial modification for all-solid-state rechargeable lithium batteries. J. Asian Ceram. Soc. 2013, 1, 17–25. [Google Scholar] [CrossRef] [Green Version]
- Tatsumisago, M.; Hayashi, A. Sulfide Glass-Ceramic Electrolytes for All-Solid-State Lithium and Sodium Batteries. Int. J. Appl. Glass Sci. 2014, 5, 226–235. [Google Scholar] [CrossRef]
- Hayashi, A.; Noi, K.; Sakuda, A.; Tatsumisago, M. Superionic glass-ceramic electrolytes for room-temperature rechargeable sodium batteries. Nat. Commun. 2012, 3, 856. [Google Scholar] [CrossRef]
- Lalère, F.; Leriche, J.B.; Courty, M.; Boulineau, S.; Viallet, V.; Masquelier, C.; Seznec, V. An all-solid state NASICON sodium battery operating at 200 °C. J. Power Sources 2014, 247, 975–980. [Google Scholar] [CrossRef]
- Lan, T.; Tsai, C.-L.; Tietz, F.; Wei, X.-K.; Heggen, M.; Dunin-Borkowski, R.E.; Wang, R.; Xiao, Y.; Ma, Q.; Guillon, O. Room-temperature all-solid-state sodium batteries with robust ceramic interface between rigid electrolyte and electrode materials. Nano Energy 2019, 65, 104040. [Google Scholar] [CrossRef]
- Yamauchi, H.; Ikejiri, J.; Sato, F.; Oshita, H.; Honma, T.; Komatsu, T. Pressureless all-solid-state sodium-ion battery consisting of sodium iron pyrophosphate glass-ceramic cathode and β″-alumina solid electrolyte composite. J. Am. Ceram. Soc. 2019, 102, 6658–6667. [Google Scholar] [CrossRef]
- Yamauchi, H.; Ikejiri, J.; Tsunoda, K.; Tanaka, A.; Sato, F.; Honma, T.; Komatsu, T. Enhanced rate capabilities in a glass-ceramic-derived sodium all-solid-state battery. Sci. Rep. 2020, 10, 9453. [Google Scholar] [CrossRef]
- Honma, T.; Togashi, T.; Ito, N.; Komatsu, T. Fabrication of Na2FeP2O7 glass-ceramics for sodium ion battery. J. Ceram. Soc. Jpn. 2012, 120, 344–346. [Google Scholar] [CrossRef] [Green Version]
- Honma, T.; Ito, N.; Togashi, T.; Sato, A.; Komatsu, T. Triclinic Na2−xFe1+x/2P2O7/C glass-ceramics with high current density performance for sodium ion battery. J. Power Sources 2013, 227, 31–34. [Google Scholar] [CrossRef]
- Ji, Y.; Honma, T.; Komatsu, T. Crystallization of the Na2FexNi1−xP2O7 Glass and Ability of Cathode for Sodium-Ion Batteries. Front. Mater. 2020, 7. in press. [Google Scholar] [CrossRef] [Green Version]
- Tanabe, M.; Honma, T.; Komatsu, T. Unique crystallization behavior of sodium manganese pyrophosphate Na2MnP2O7 glass and its electrochemical properties. J. Asian Ceram. Soc. 2017, 5, 209–215. [Google Scholar] [CrossRef] [Green Version]
- Tanabe, M.; Honma, T.; Komatsu, T. Crystallization behavior and electrochemical properties of Na2FeyMn1−yP2O7 glass. J. Non Cryst. Solids 2018, 501, 153–158. [Google Scholar] [CrossRef]
- Akatsuka, C.; Honma, T.; Komatsu, T. Surface crystallization tendency of Na2FeP2O7 glass. J. Ceram. Soc. Jpn. 2018, 126, 563–567. [Google Scholar] [CrossRef] [Green Version]
- Kato, A.; Nagao, M.; Sakuda, A.; Hayashi, A.; Tatsumisago, M. Evaluation of young’s modulus of Li2S-P2S5-P2O5 oxysulfide glass solid electrolytes. J. Ceram. Soc. Jpn. 2014, 122, 552–555. [Google Scholar] [CrossRef] [Green Version]
- Goodenough, J.B.; Hong, H.-P.; Kafalas, J.A. Fast Na+-ion transport in skeleton structures. Mater. Res. Bull. 1976, 11, 203–220. [Google Scholar] [CrossRef]
- Naqash, S.; Ma, Q.; Tietz, F.; Guillon, O. Na3Zr2(SiO4)2(PO4) prepared by a solution-assisted solid state reaction. Solid State Ionics 2017, 302, 83–91. [Google Scholar] [CrossRef]
- Naqash, S.; Sebold, D.; Tietz, F.; Guillon, O. Microstructure–conductivity relationship of Na3Zr2(SiO4)2(PO4) ceramics. J. Am. Ceram. Soc. 2019, 102, 1057–1070. [Google Scholar] [CrossRef]
- Bouquin, O.; Perthuis, H.; Colomban, P. Low-temperature sintering and optimal physical properties: A challenge-the NASICON ceramics case. J. Mater. Sci. Lett. 1985, 4, 956–959. [Google Scholar] [CrossRef]
- Höland, W.; Beall, G. Glass-Ceramic Technology; Wiley: Hoboken, NJ, USA, 2019; ISBN 9781119423690. [Google Scholar]
- Deubener, J.; Allix, M.; Davis, M.J.; Duran, A.; Höche, T.; Honma, T.; Komatsu, T.; Krüger, S.; Mitra, I.; Müller, R.; et al. Updated definition of glass-ceramics. J. Non Cryst. Solids 2018, 501, 3–10. [Google Scholar] [CrossRef]
- Agea-Blanco, B.; Reinsch, S.; Müller, R. Sintering and foaming of barium silicate glass powder compacts. Front. Mater. 2016, 3, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Blaeß, C.; Müller, R.; Poologasundarampillai, G.; Brauer, D.S. Sintering and concomitant crystallization of bioactive glasses. Int. J. Appl. Glass Sci. 2019, 10, 449–462. [Google Scholar] [CrossRef]
- Goel, A.; Ferrari, A.M.; Kansal, I.; Pascual, M.J.; Barbieri, L.; Bondioli, F.; Lancellotti, I.; Ribeiro, M.J.; Ferreira, J.M.F. Sintering and crystallization behavior of CaMgSi2O6-NaFeSi2O6 based glass-ceramics. J. Appl. Phys. 2009, 106. [Google Scholar] [CrossRef] [Green Version]
- Goel, A.; Reddy, A.A.; Pascual, M.J.; Gremillard, L.; Malchere, A.; Ferreira, J.M.F. Sintering behavior of lanthanide-containing glass-ceramic sealants for solid oxide fuel cells. J. Mater. Chem. 2012, 22, 10042. [Google Scholar] [CrossRef]
- Honma, T.; Okamoto, M.; Togashi, T.; Ito, N.; Shinozaki, K.; Komatsu, T. Electrical conductivity of Na2O–Nb2O5–P2O5 glass and fabrication of glass–ceramic composites with NASICON type Na3Zr2Si2PO12. Solid State Ionics 2015, 269, 19–23. [Google Scholar] [CrossRef]
- Suzuki, K.; Noi, K.; Hayashi, A.; Tatsumisago, M. Low temperature sintering of Na1+xZr2SixP3−xO12 by the addition of Na3BO3. Scr. Mater. 2018, 145, 67–70. [Google Scholar] [CrossRef]
- Oh, J.A.S.; He, L.; Plewa, A.; Morita, M.; Zhao, Y.; Sakamoto, T.; Song, X.; Zhai, W.; Zeng, K.; Lu, L. Composite NASICON (Na3Zr2Si2PO12) Solid-State Electrolyte with Enhanced Na+ Ionic Conductivity: Effect of Liquid Phase Sintering. ACS Appl. Mater. Interfaces 2019, 11, 40125–40133. [Google Scholar] [CrossRef]
- Shao, Y.; Zhong, G.; Lu, Y.; Liu, L.; Zhao, C.; Zhang, Q.; Hu, Y.S.; Yang, Y.; Chen, L. A novel NASICON-based glass-ceramic composite electrolyte with enhanced Na-ion conductivity. Energy Storage Mater. 2019, 23, 514–521. [Google Scholar] [CrossRef]
- Grady, Z.M.; Tsuji, K.; Ndayishimiye, A.; Hwan-Seo, J.; Randall, C.A. Densification of a Solid-State NASICON Sodium-Ion Electrolyte Below 400 °C by Cold Sintering With a Fused Hydroxide Solvent. ACS Appl. Energy Mater. 2020, 3, 4356–4366. [Google Scholar] [CrossRef]
- Okubo, K.; Wang, H.; Hayashi, K.; Inada, M.; Enomoto, N.; Hasegawa, G.; Osawa, T.; Takamura, H. A dense NASICON sheet prepared by tape-casting and low temperature sintering. Electrochim. Acta 2018, 278, 176–181. [Google Scholar] [CrossRef]
- Hoppe, U.; Delevoye, L.; Montagne, L.; Zimmermann, M.V.; Hannon, A.C. Structure of Nb2O5–NaPO3 glasses by X-ray and neutron diffraction. Phys. Chem. Chem. Phys. 2013, 15, 8520–8528. [Google Scholar] [CrossRef]
- Bhide, A.; Hariharan, K. Sodium ion transport in NaPO3-Na2SO4 glasses. Mater. Chem. Phys. 2007, 105, 213–221. [Google Scholar] [CrossRef]
- Rousselot, C. Correlations between structure and conductivity in NaPO3-TiO2 glasses. Solid State Ionics 1992, 58, 71–76. [Google Scholar] [CrossRef]
- Langar, A.; Sdiri, N.; Elhouichet, H.; Ferid, M. Conductivity and dielectric behavior of NaPO3–ZnO–V2O5 glasses. J. Alloys Compd. 2014, 590, 380–387. [Google Scholar] [CrossRef]
- Sokolov, I.A.; Murin, I.V.; Kriyt, V.E.; Pronkin, A.A. Structure and electric properties of Na2SO4-NaPO3 glasses. Russ. J. Electrochem. 2011, 47, 410–415. [Google Scholar] [CrossRef]
- Sokolov, I.A.; Murin, I.V.; Kriyt, V.E.; Pronkin, A.A. Structure and electrical conductivity of glasses in the Na2O-Na2SO4-P2O5 system. Glas. Phys. Chem. 2011, 37, 351–361. [Google Scholar] [CrossRef]
- Doebelin, N.; Kleeberg, R. Profex: A graphical user interface for the Rietveld refinement program BGMN. J. Appl. Crystallogr. 2015, 48, 1573–1580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voronkov, A.A.; Shumyatskaya, N.G.; Pyatenko, Y.A.; Voronkov, A.A.; Shumyatskaya, N.G.; Pyatenko, Y.A. Crystal Structure of a New Natural Modification of Na2Zr(Si2O7). J. Struct. Chem. 1970, 11, 932–933. [Google Scholar] [CrossRef]
- Nikolova, R.P.; Fujiwara, K.; Nakayama, N.; Kostov-Kytin, V. Crystal structure of a new small-pore zirconosilicate Na2ZrSi2O7·H2O and its relation to stoichiometrically and topologically similar compounds. Solid State Sci. 2009, 11, 382–388. [Google Scholar] [CrossRef]
- Petrova, N.R.; Noriaki, N.; Snejana, B.; Petr, B.; Vladislav, K.-K. Temperature-induced phase transformations of the small-pore zirconosilicate Na2ZrSi2O7·H2O. Solid State Sci. 2011, 13, 1187–1190. [Google Scholar] [CrossRef]
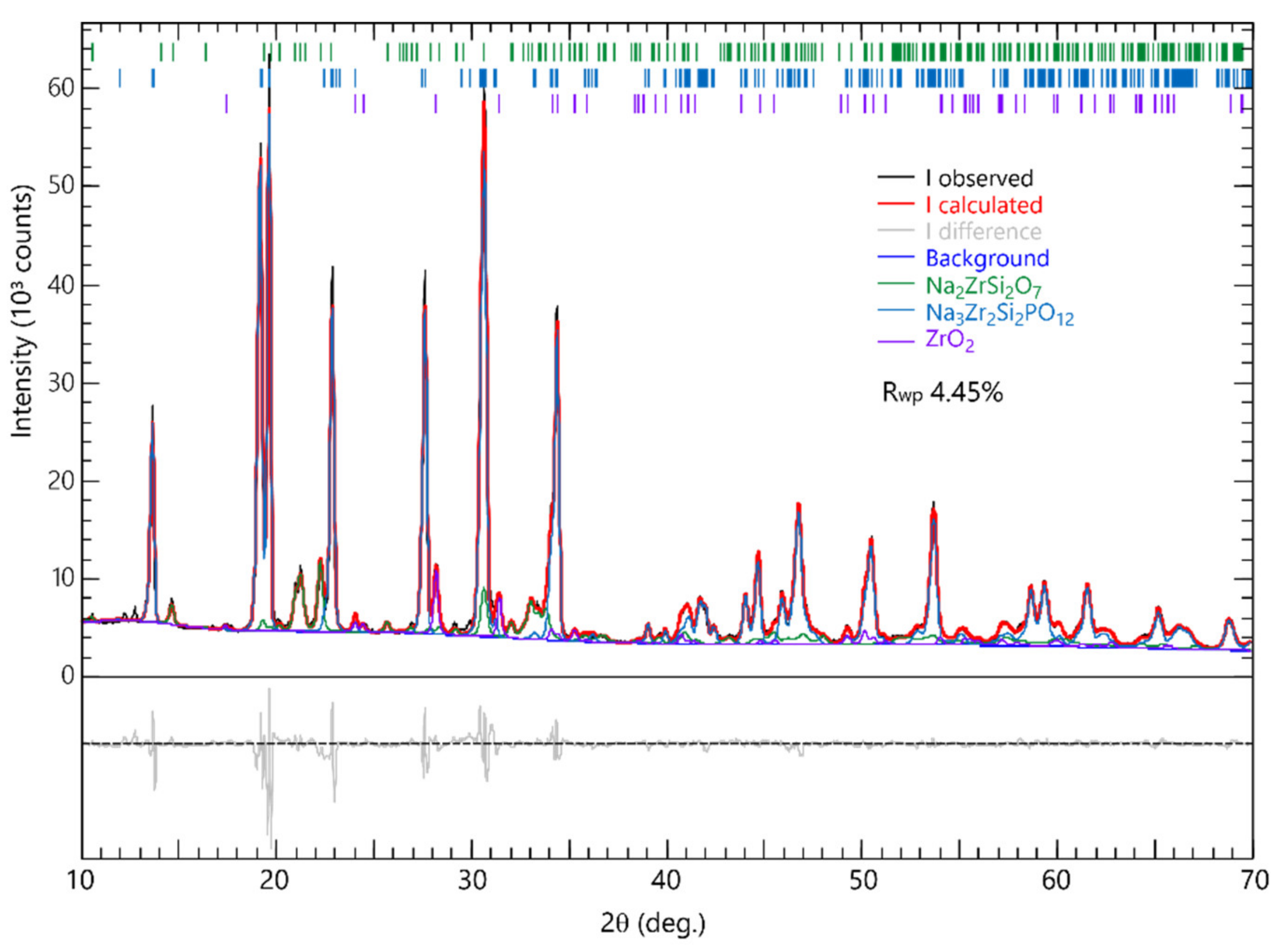



| Heat Treatment Condition | a (nm) | b (nm) | c (nm) | Beta (Deg.) |
|---|---|---|---|---|
| 1000 °C for 3 h | 1.5604 (1) | 0.9003 (4) | 0.9244 (3) | 124.25 (2) |
| 1000 °C for 5 h | 1.5617 (1) | 0.9015 (2) | 0.9247 (3) | 124.23 (4) |
| 1000 °C for 7 h | 1.5641 (1) | 0.9012 (1) | 0.9241 (2) | 124.20 (4) |
| 1000 °C for 10 h | 1.5652 (1) | 0.9018 (4) | 0.9233 (2) | 124.15 (3) |
| 1100 °C for 3 h | 1.5647 (4) | 0.9043 (4) | 0.9227 (4) | 123.76 (3) |
| 1100 °C for 5 h | 1.5650 (1) | 0.9046 (4) | 0.9227 (1) | 123.74 (3) |
| 1100 °C for 7 h | 1.5648 (1) | 0.9046 (4) | 0.9228 (2) | 123.73 (1) |
| 1100 °C for 10 h | 1.5653 (1) | 0.9055 (4) | 0.9220 (2) | 123.72 (2) |
| 1200 °C for 3 h | 1.5647 (1) | 0.9051 (4) | 0.9223 (4) | 123.69 (3) |
| 1200 °C for 5 h | 1.5644 (1) | 0.9049 (3) | 0.9224 (2) | 123.67 (4) |
| 1200 °C for 7 h | 1.5642 (1) | 0.9047 (3) | 0.9224 (3) | 123.65 (1) |
| 1200 °C for 10 h | 1.5644 (1) | 0.9049 (3) | 0.9217 (4) | 123.68 (2) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, Y.; Honma, T.; Komatsu, T. Synthesis and Na+ Ion Conductivity of Stoichiometric Na3Zr2Si2PO12 by Liquid-Phase Sintering with NaPO3 Glass. Materials 2021, 14, 3790. https://doi.org/10.3390/ma14143790
Ji Y, Honma T, Komatsu T. Synthesis and Na+ Ion Conductivity of Stoichiometric Na3Zr2Si2PO12 by Liquid-Phase Sintering with NaPO3 Glass. Materials. 2021; 14(14):3790. https://doi.org/10.3390/ma14143790
Chicago/Turabian StyleJi, Yongzheng, Tsuyoshi Honma, and Takayuki Komatsu. 2021. "Synthesis and Na+ Ion Conductivity of Stoichiometric Na3Zr2Si2PO12 by Liquid-Phase Sintering with NaPO3 Glass" Materials 14, no. 14: 3790. https://doi.org/10.3390/ma14143790
APA StyleJi, Y., Honma, T., & Komatsu, T. (2021). Synthesis and Na+ Ion Conductivity of Stoichiometric Na3Zr2Si2PO12 by Liquid-Phase Sintering with NaPO3 Glass. Materials, 14(14), 3790. https://doi.org/10.3390/ma14143790






