On the Disintegration of A1050/Ni201 Explosively Welded Clads Induced by Long-Term Annealing
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
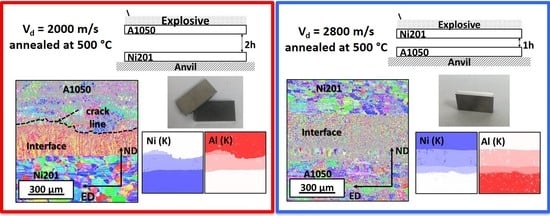
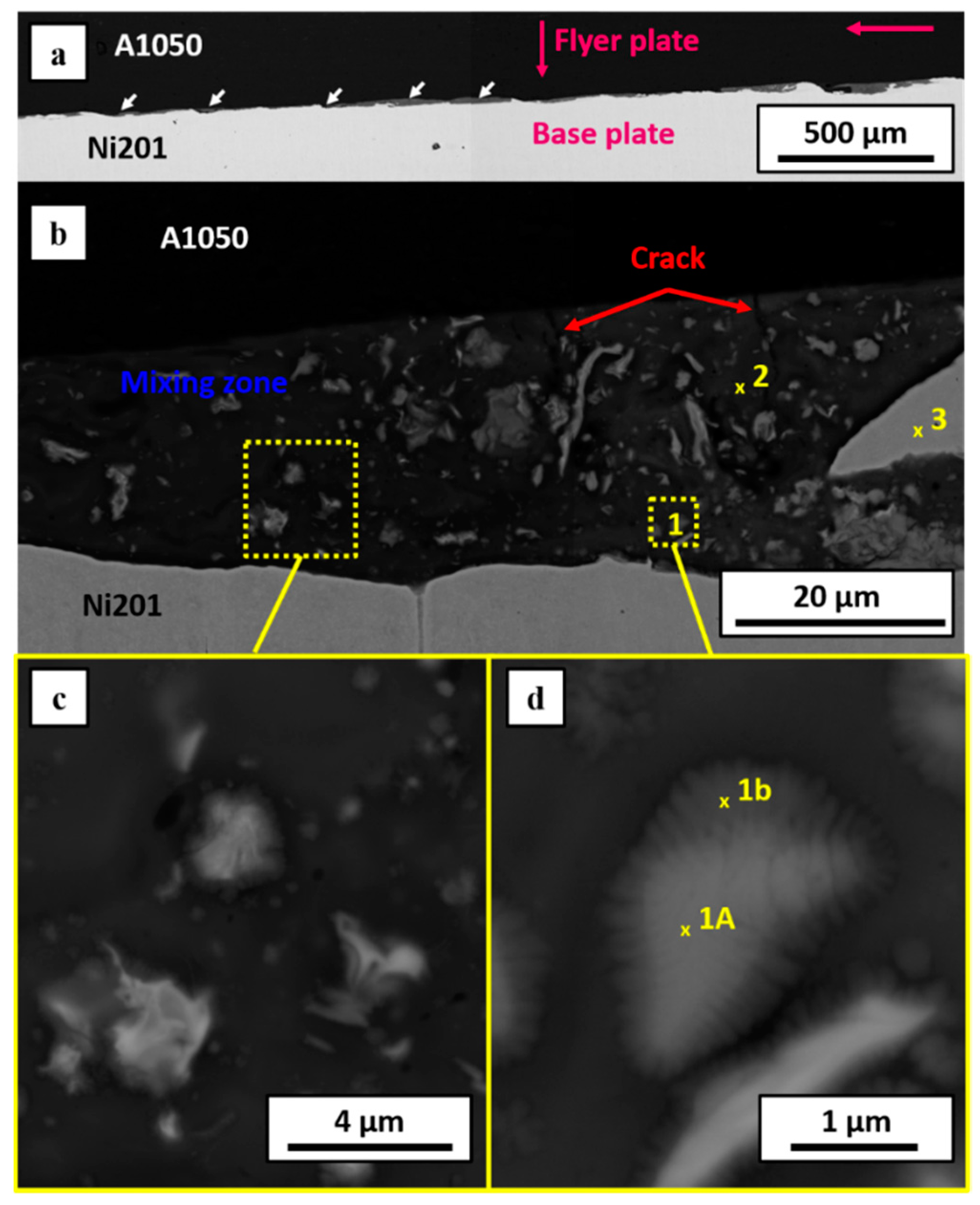
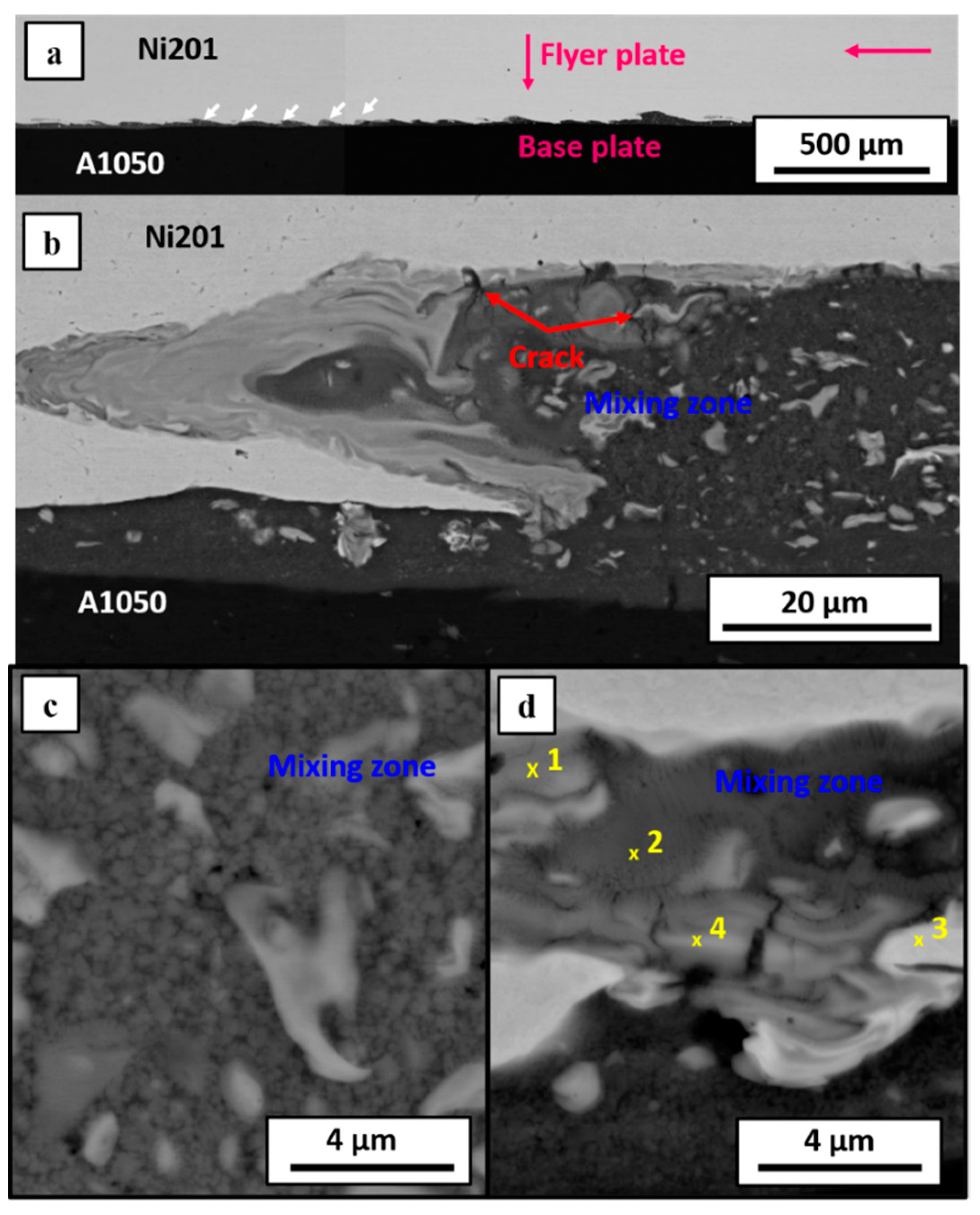
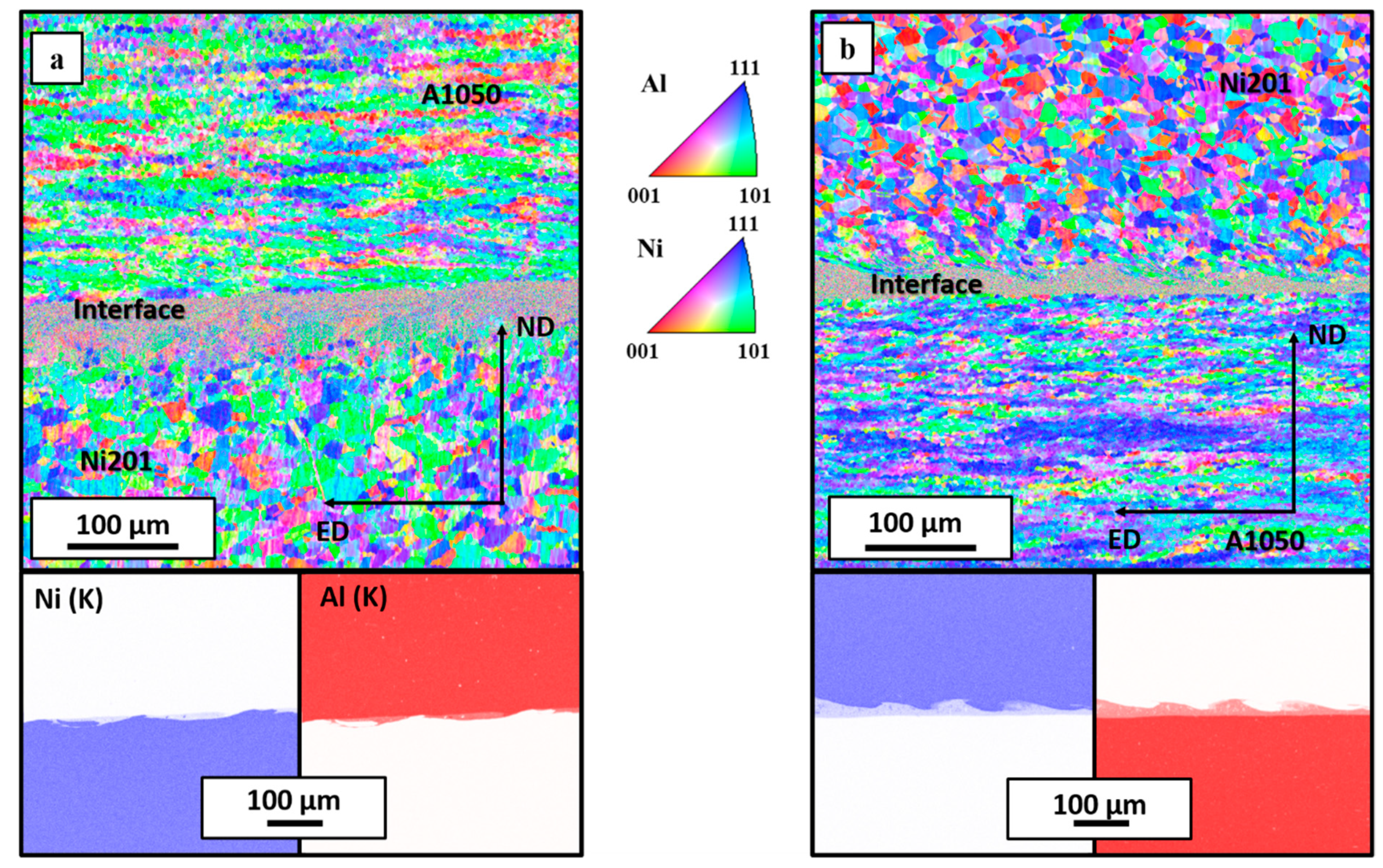
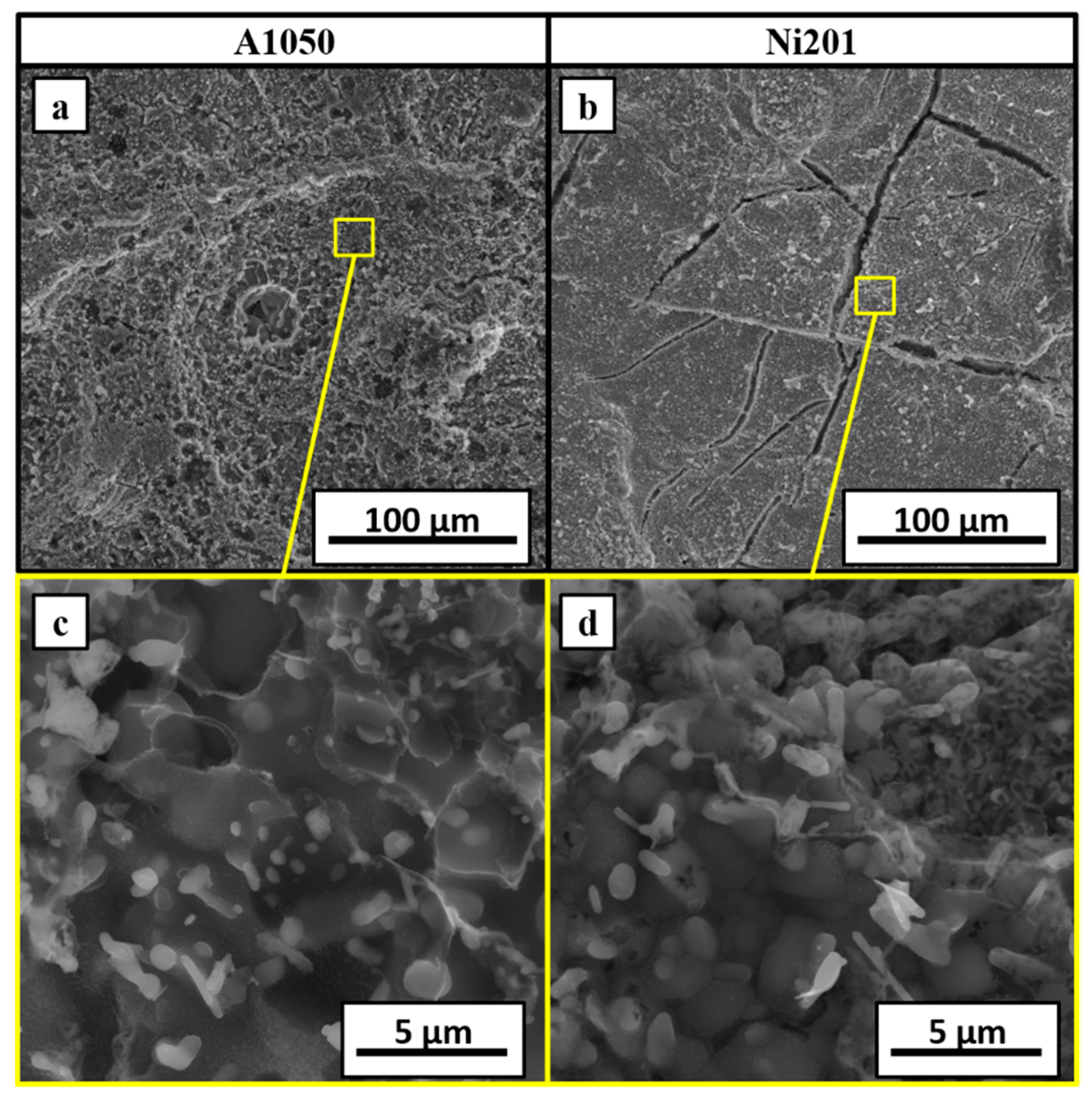
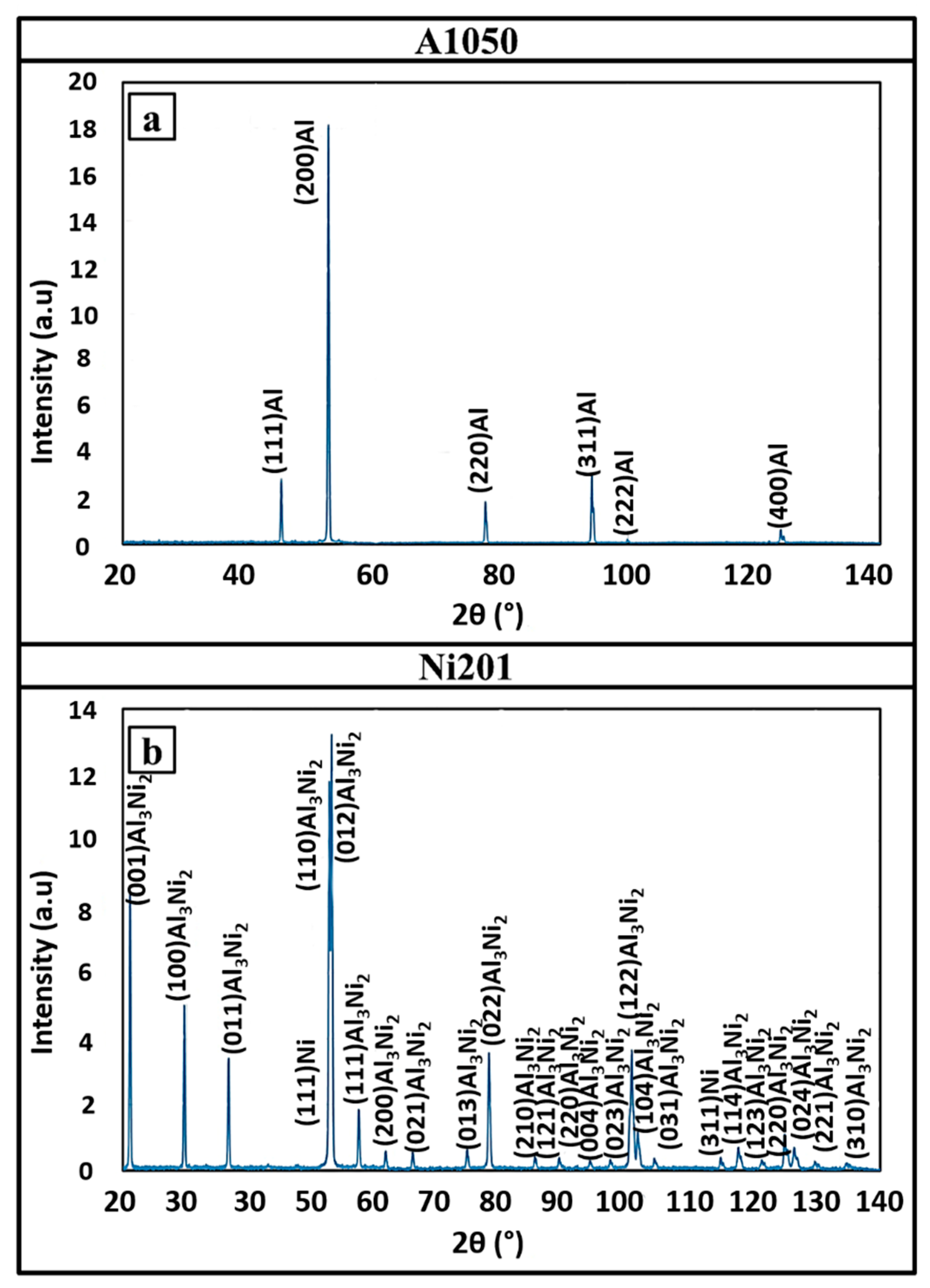
3.1. Microstructural Characterization after Welding
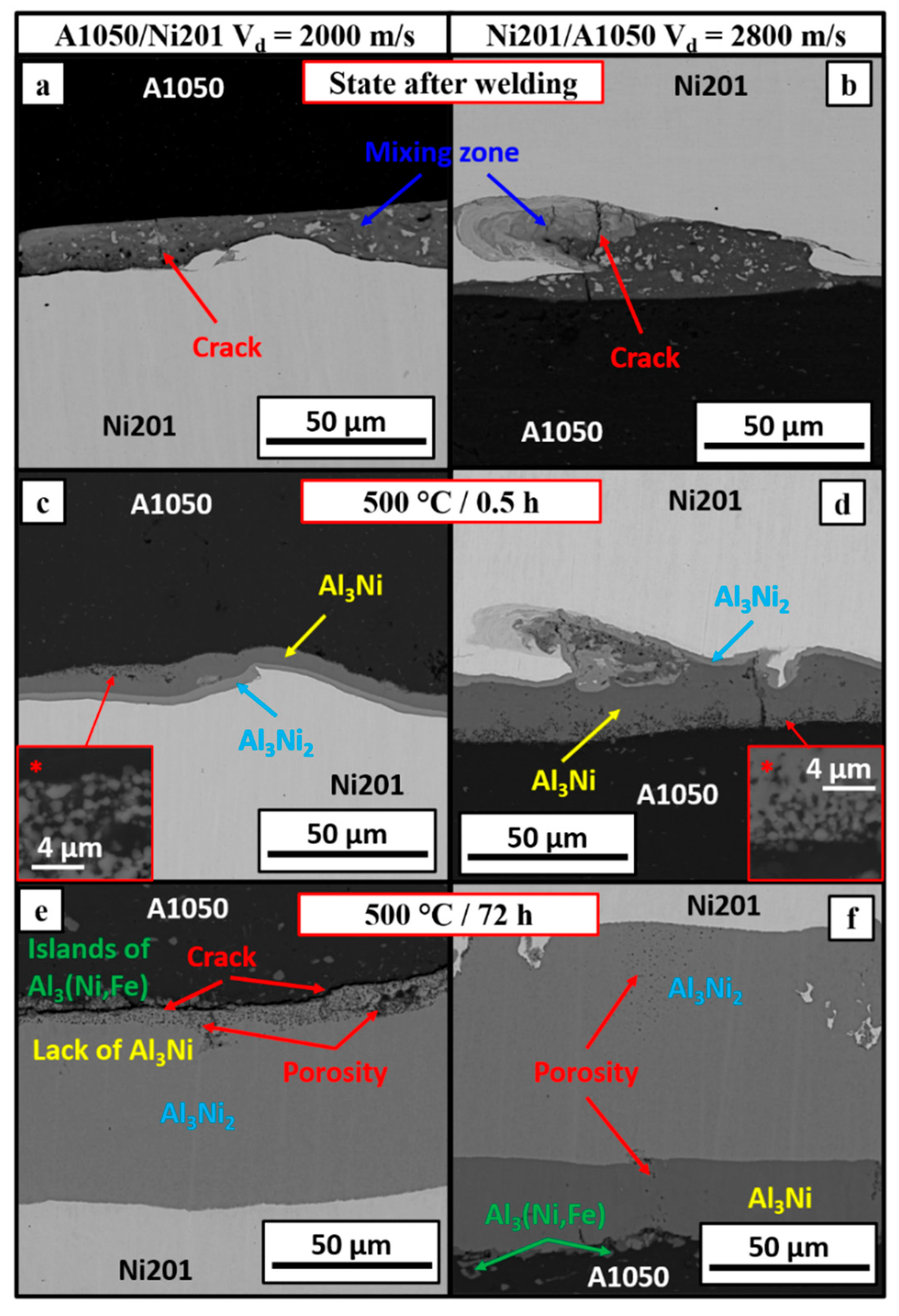
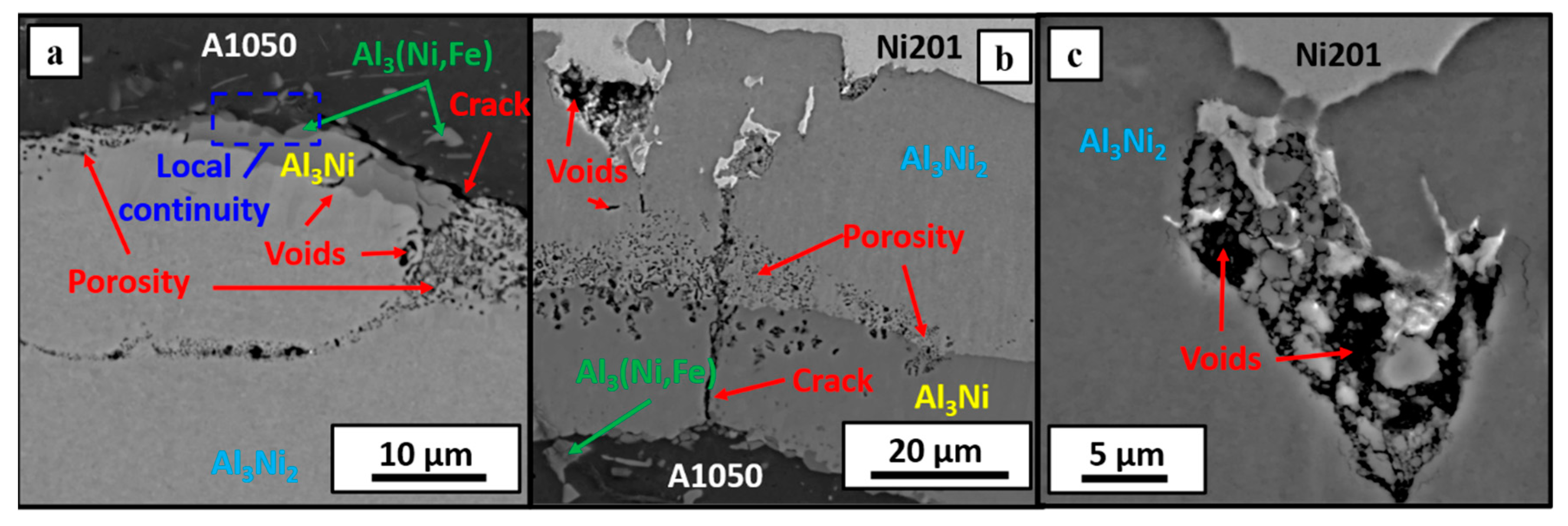
3.2. Microstructure Characterization after Welds’ Annealing
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Faraji, G.; Kim, H.S.; Kashi, H.T. Severe Plastic Deformation Methods for Sheets. In Severe Plastic Deformation; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 113–129. [Google Scholar]
- Lenard, J.G. Severe Plastic Deformation—Accumulative Roll Bonding. In Primer on Flat Rolling, 2nd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2013; pp. 303–322. [Google Scholar]
- Gerland, M.; Presles, H.N.; Guin, J.P.; Bertheau, D. Explosive cladding of a thin Ni-film to an aluminium alloy. Mater. Sci. Eng. A 2000, 280, 311–319. [Google Scholar] [CrossRef]
- Kaya, Y. Microstructural, Mechanical and Corrosion Investigations of Ship Steel-Aluminum Bimetal Composites Produced by Explosive Welding. Metals 2018, 8, 544. [Google Scholar] [CrossRef]
- Fronczek, D.M.; Chulist, R.; Litynska-Dobrzynska, L.; Szulc, Z.; Zieba, P.; Wojewoda-Budka, J. Microstructure Changes and Phase Growth Occurring at the Interface of the Al/Ti Explosively Welded and Annealed Joints. J. Mater. Eng. Perform. 2016, 25, 3211–3217. [Google Scholar] [CrossRef]
- Honarpisheh, M.; Asemabadi, M.; Sedighi, M. Investigation of annealing treatment on the interfacial properties of explosive-welded Al/Cu/Al multilayer. Mater. Des. 2012, 37, 122–127. [Google Scholar] [CrossRef]
- Fronczek, D.M.; Chulist, R.; Litynska-Dobrzynska, L.; Kac, S.; Schell, N.; Kania, Z.; Szulc, Z.; Wojewoda-Budka, J. Microstructure and kinetics of intermetallic phase growth of three-layered A1050/AZ31/A1050 clads prepared by explosive welding combined with subsequent annealing. Mater. Des. 2017, 130, 120–130. [Google Scholar] [CrossRef]
- Bataev, I.A.; Ogneva, T.S.; Bataev, A.A.; Mali, V.I.; Esikov, M.A.; Lazurenko, D.V.; Guo, Y.; Jorge Junior, A.M. Explosively welded multilayer Ni-Al composites. Mater. Des. 2015, 88, 1082–1087. [Google Scholar] [CrossRef]
- Prazmowski, M.; Paul, H. The effect of stand-off distance on the structure and properties of zirconium—Carbon steel bimetal produced by explosion welding. Arch. Metall. Mater. 2012, 57, 1201–1210. [Google Scholar] [CrossRef][Green Version]
- Topolski, K.; Wieciński, P.; Szulc, Z.; Gałka, A.; Garbacz, H. Progress in the characterization of explosively joined Ti/Ni bimetals. Mater. Des. 2014, 63, 479–487. [Google Scholar] [CrossRef]
- Paul, H.; Skuza, W.; Chulist, R.; Miszczyk, M.; Gałka, A.; Prazmowski, M.; Pstrus, J. The Effect of Interface Morphology on the Electro-Mechanical Properties of Ti/Cu Clad Composites Produced by Explosive Welding. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2020, 51, 750–766. [Google Scholar] [CrossRef]
- Grignon, F.; Benson, D.; Vecchio, K.S.; Meyers, M.A. Explosive welding of aluminum to aluminum: Analysis, computations and experiments. Int. J. Impact Eng. 2004, 30, 1333–1351. [Google Scholar] [CrossRef]
- Findik, F.; Yilmaz, R.; Somyurek, T. The effects of heat treatment on the microstructure and microhardness of explosive welding. Sci. Res. Essays 2011, 6, 4141–4151. [Google Scholar] [CrossRef]
- Linnert, G.E. Welding Metallurgy, 4th ed.; American Welding Society: Columbia, SC, USA, 1994; ISBN 9780871714572. [Google Scholar]
- Joshi, J.R.; Potta, M.; Adepu, K.; Reddy Gankidi, M.; Kumar Katta, R. Influence of Welding Techniques on Heat Affected Zone Softening of Dissimilar Metal Maraging Steel and High Strength Low Alloy Steel Gas Tungsten Arc Weldments. Trans. Indian Inst. Met. 2016, 70, 69–81. [Google Scholar] [CrossRef]
- Shiran, M.K.G.; Khalaj, G.; Pouraliakbar, H.; Jandaghi, M.R.; Dehnavi, A.S.; Bakhtiari, H. Multilayer Cu/Al/Cu explosive welded joints: Characterizing heat treatment effect on the interface microstructure and mechanical properties. J. Manuf. Process. 2018, 35, 657–663. [Google Scholar] [CrossRef]
- Fronczek, D.M.; Chulist, R.; Litynska-Dobrzynska, L.; Lopez, G.A.; Wierzbicka-Miernik, A.; Schell, N.; Szulc, Z.; Wojewoda-Budka, J. Microstructural and Phase Composition Differences Across the Interfaces in Al/Ti/Al Explosively Welded Clads. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2017, 48, 4154–4165. [Google Scholar] [CrossRef]
- Kahraman, N.; Gülenç, B.; Findik, F. Joining of titanium/stainless steel by explosive welding and effect on interface. J. Mater. Process. Technol. 2005, 169, 127–133. [Google Scholar] [CrossRef]
- Durgutlu, A.; Okuyucu, H.; Gulenc, B. Investigation of effect of the stand-off distance on interface characteristics of explosively welded copper and stainless steel. Mater. Des. 2008, 29, 1480–1484. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Y. High-Velocity Impact Welding Process: A Review. Metals 2019, 9, 144. [Google Scholar] [CrossRef]
- Kwiecien, I.; Bobrowski, P.; Janusz-Skuza, M.; Wierzbicka-Miernik, A.; Szulc, Z.; Wojewoda-Budka, J. Microstructure of the interface zone after explosive welding and further annealing of A1050/Ni201 clads using various joining conditions. J. Mater. Sci. 2020, 55, 9163–9172. [Google Scholar] [CrossRef]
- Mori, D.; Kasada, R.; Konishi, S.; Morizono, Y.; Hokamoto, K. Underwater explosive welding of tungsten to reduced-activation ferritic steel F82H. Fusion Eng. Des. 2014, 89, 1086–1090. [Google Scholar] [CrossRef]
- Hokamoto, K.; Manikandan, P.; Mori, A. Some trials on underwater explosive welding of thin W plate onto copper substrate. Mater. Sci. Forum. 2010, 638–642, 1041–1046. [Google Scholar] [CrossRef]
- Durgutlu, A.; Gülenç, B.; Findik, F. Examination of copper/stainless steel joints formed by explosive welding. Mater. Des. 2005, 26, 497–507. [Google Scholar] [CrossRef]
- Zareie Rajani, H.R.; Akbari Mousavi, S.A.A. The effect of explosive welding parameters on metallurgical and mechanical interfacial features of Inconel 625/plain carbon steel bimetal plate. Mater. Sci. Eng. A 2012, 556, 454–464. [Google Scholar] [CrossRef]
- Loureiro, A.; Mendes, R.; Ribeiro, J.B.; Leal, R.M. Effect of explosive ratio on explosive welding quality of copper to aluminium. Cienc. Tecnol. Mater. 2017, 29, e46–e50. [Google Scholar] [CrossRef]
- Guo, X.; Ma, Y.; Jin, K.; Wang, H.; Tao, J.; Fan, M. Effect of Stand-Off Distance on the Microstructure and Mechanical Properties of Ni/Al/Ni Laminates Prepared by Explosive Bonding. J. Mater. Eng. Perform. 2017, 26, 4235–4244. [Google Scholar] [CrossRef]
- Skuza, W.; Paul, H.; Berent, K.; Prazmowski, M.; Bobrowski, P. Microstructure and mechanical properties of Ti/Cu clads manufactured by explosive bonding at different stand-off distances. Key Eng. Mater. 2016, 716, 464–471. [Google Scholar] [CrossRef]
- Crossland, B.; Williams, J.D. Explosive welding. Metall. Rev. 1970, 144, 79–100. [Google Scholar] [CrossRef]
- Zhang, H.; Jiao, K.X.; Zhang, J.L.; Liu, J. Comparisons of the microstructures and micro-mechanical properties of copper/steel explosive-bonded wave interfaces. Mater. Sci. Eng. A 2019, 756, 430–441. [Google Scholar] [CrossRef]
- Nash, P. Phase Diagrams of Binary Nickel Alloys; ASM International, Materials Park: Novelty, OH, USA, 1991. [Google Scholar]
- Ogneva, T.S.; Lazurenko, D.V.; Bataev, I.A.; Mali, V.I.; Esikov, M.A.; Bataev, A.A. Formation of intermetallics at the interface of explosively welded Ni-Al multilayered composites during annealing. IOP Conf. Ser. Mater. Sci. Eng. 2016, 124, 012132. [Google Scholar] [CrossRef]
- Jung, S.B.; Minamino, Y.; Yamane, T.; Saji, S. Reaction diffusion and formation of Al3Ni and Al3Ni2 phases in the Al-Ni system. J. Mater. Sci. Lett. 1993, 12, 1684–1686. [Google Scholar] [CrossRef]
- Kwiecien, I.; Bobrowski, P.; Wierzbicka-Miernik, A.; Litynska-Dobrzynska, L.; Wojewoda-Budka, J. Growth kinetics of the selected intermetallic phases in Ni/Al/Ni system with various nickel substrate microstructure. Nanomaterials 2019, 9, 134. [Google Scholar] [CrossRef]
- Lopez, G.A.; Sommadossi, S.; Zieba, P.; Gust, W.; Mittemeijer, E.J. Kinetic behaviour of diffusion-soldered Ni/Al/Ni interconnections. Mater. Chem. Phys. 2003, 78, 459–463. [Google Scholar] [CrossRef]
- Tumminello, S.; Sommadossi, S. Growth kinetics of intermetallic phases in transient liquid phase bonding process (TLPB) in Al/Ni system. Defect Diffus. Forum. 2012, 323-325, 465–470. [Google Scholar] [CrossRef]
- Brunelli, K.; Peruzzo, L.; Dabalà, M. The effect of prolonged heat treatments on the microstructural evolution of Al/Ni intermetallic compounds in multi layered composites. Mater. Chem. Phys. 2015, 149, 350–358. [Google Scholar] [CrossRef]
- Yang, C.; Hu, Y.; Shen, R.; Ye, Y.; Wang, S.; Hua, T. Fabrication and performance characterization of Al/Ni multilayer energetic films. Appl. Phys. A Mater. Sci. Process. 2014, 114, 459–464. [Google Scholar] [CrossRef]
- Sameyshcheva, T.S.; Bataev, I.A.; Bataev, A.A.; Yartsev, P.S.; Polyakov, I.A. Metallic—Intermetallic composites produced by vacuum casting and annealing of Ni and Al. In Proceedings of the 2012 7th—International Forum on Strategic Technology (IFOST), Tomsk, Russia, 18–21 September 2012; pp. 1–4. [Google Scholar] [CrossRef]
- Kwiecien, I.; Bobrowski, P.; Janusz-Skuza, M.; Wierzbicka-Miernik, A.; Tarasek, A.; Szulc, Z.; Wojewoda-Budka, J. Interface Characterization of Ni/Al Bimetallic Explosively Welded Plate Manufactured with Application of Exceptionally High Detonation Speed. J. Mater. Eng. Perform. 2020, 29, 6286–6294. [Google Scholar] [CrossRef]
- Fronczek, D.M.; Wojewoda-Budka, J.; Chulist, R.; Sypien, A.; Korneva, A.; Szulc, Z.; Schell, N.; Zieba, P. Structural properties of Ti/Al clads manufactured by explosive welding and annealing. Mater. Des. 2016, 91, 80–89. [Google Scholar] [CrossRef]
- Shiran, M.R.K.G.; Bakhtiari, H.; Mousavi, S.A.A.A.; Khalaj, G.; Mirhashemi, S.M. Effect of stand-off distance on the mechanical and metallurgical properties of explosively bonded 321 austenitic stainless steel—1230 aluminum alloy tubes. Mater. Res. 2017, 20, 291–302. [Google Scholar] [CrossRef]
- Carvalho, G.H.S.F.L.; Galvao, I.; Mendes, R.; Leal, R.M.; Loureiro, A. Weldability of aluminium-copper in explosive welding. Int. J. Adv. Manuf. Technol. 2019, 103, 3211–3221. [Google Scholar] [CrossRef]
- Mousavi, A.A.A.; Al-Hassani, S.T.S. Numerical and experimental studies of the mechanism of the wavy interface formations in explosive/impact welding. J. Mech. Phys. Solids 2005, 53, 2501–2528. [Google Scholar] [CrossRef]
- Ufrgs, L.; Alia, B.; Alley, R.; Apblett, W.; Baeslack, W.; Ballis, W.; Bampton, C.; Banerjee, P.; Banker, J.; Bartifay, R.; et al. ASM Handbook. In ASM: Welding, Brazing and Soldering; ASM International, Metals Park: Novelty, OH, USA, 1993. [Google Scholar]
- Paul, H.; Morgiel, J.; Baudin, T.; Brisset, F.; Prazmowski, M.; Miszczyk, M. Characterization of explosive weld joints by TEM and SEM/EBSD. Arch. Metall. Mater. 2014, 59, 1129–1136. [Google Scholar] [CrossRef]
- Fang, Z.; Shi, C.; Shi, H.; Sun, Z. Influence of Explosive Ratio on Morphological and Structural Properties of Ti/Al Clads. Metals 2019, 9, 119. [Google Scholar] [CrossRef]
- Carvalho, G.H.S.F.L.; Galvão, I.; Mendes, R.; Leal, R.M.; Loureiro, A. Explosive welding of aluminium to stainless steel using carbon steel and niobium interlayers. J. Mater. Process. Technol. 2020, 283, 116707. [Google Scholar] [CrossRef]
- Bina, M.H.; Dehghani, F.; Salimi, M. Effect of heat treatment on bonding interface in explosive welded copper/stainless steel. Mater. Des. 2013, 45, 504–509. [Google Scholar] [CrossRef]
- Akbari Mousavi, S.A.A.; Sartangi, P.F. Effect of post-weld heat treatment on the interface microstructure of explosively welded titanium-stainless steel composite. Mater. Sci. Eng. A 2008, 494, 329–336. [Google Scholar] [CrossRef]
- Fronczek, D.M.; Chulist, R.; Szulc, Z.; Wojewoda-Budka, J. Growth kinetics of TiAl3 phase in annealed Al/Ti/Al explosively welded clads. Mater. Lett. 2017, 198, 160–163. [Google Scholar] [CrossRef]
- Cubiotti, G.; Krasovskii, E.E.; Slobodyan, O.V.; Kucherenko, Y.N.; Antonov, V. The electronic structure of Al3Ni. J. Phys. Condens. Matter 1995, 7, 4865–4874. [Google Scholar] [CrossRef]
- Dong, C. The Al3Ni2 Structure as Approximant of Quasicrystals. J. Phys. I 1995, 5, 1625–1634. [Google Scholar] [CrossRef]
- Ding, Z.; Hu, Q.; Lu, W.; Sun, S.; Xia, M.; Li, J. In situ observation on the formation of intermetallics compounds at the interface of liquid Al/solid Ni. Scr. Mater. 2017, 130, 214–218. [Google Scholar] [CrossRef]
- Wolczynski, W.; Okane, T.; Senderowski, C.; Kania, B.; Zasada, D.; Janczak-Rusch, J. Meta-stable conditions of diffusion brazing. Arch. Metall. Mater. 2011, 56, 311–324. [Google Scholar] [CrossRef]
- Arbo, S.M.; Bergh, T.; Holmedal, B.; Vullum, P.E.; Westermann, I. Relationship between Al-Ni intermetallic Phases and Bond Strength in Roll Bonded Steel-Aluminum Composites with Nickel Interlayers. Metals 2019, 9, 827. [Google Scholar] [CrossRef]
- Castleman, L.; Seigle, L.L. Layer Growth During Interdiffusion in the Alumium-Nickel Alloy System. Trans. Metall. Soc. Aime 1958, 212, 589–596. [Google Scholar]
- Janssen, M.M.P.; Rieck, G.D. Reaction diffusion and Kirkendall-effect in the nickel-aluminum system. Trans. Metall. Soc. Aime 1967, 239, 1372–1385. [Google Scholar]
- Lopez, G.A.; Sommadossi, S.; Gust, W.; Mittemeijer, E.J.; Zieba, P. Phase Characterization of diffusion Soldered Ni/Al/Ni Intercononnections. Interface Sci. 2002, 10, 13–19. [Google Scholar] [CrossRef]
- Wierzbicka-Miernik, A.; Miernik, K.; Wojewoda-Budka, J.; Szyszkiewicz, K.; Filipek, R.; Litynska-Dobrzynska, L.; Kodentsov, A.; Zieba, P. Growth kinetics of the intermetallic phase in diffusion-soldered (Cu-5 at.%Ni)/Sn/(Cu-5 at.%Ni) interconnections. Mater. Chem. Phys. 2013, 142, 682–685. [Google Scholar] [CrossRef]



| Alloy | Ni | Cu | Fe | Mn | C | Si | S | ||
| Ni 201 | 99.0 | 0.25 | 0.40 | 0.35 | 0.02 | 0.35 | 0.01 | ||
| Alloy | Al | Fe | Si | Zn | Ti | Mg | Mn | Cu | Other |
| A1050 | 99.50 | 0.40 | 0.25 | 0.07 | 0.05 | 0.05 | 0.05 | 0.05 | 0.03 |
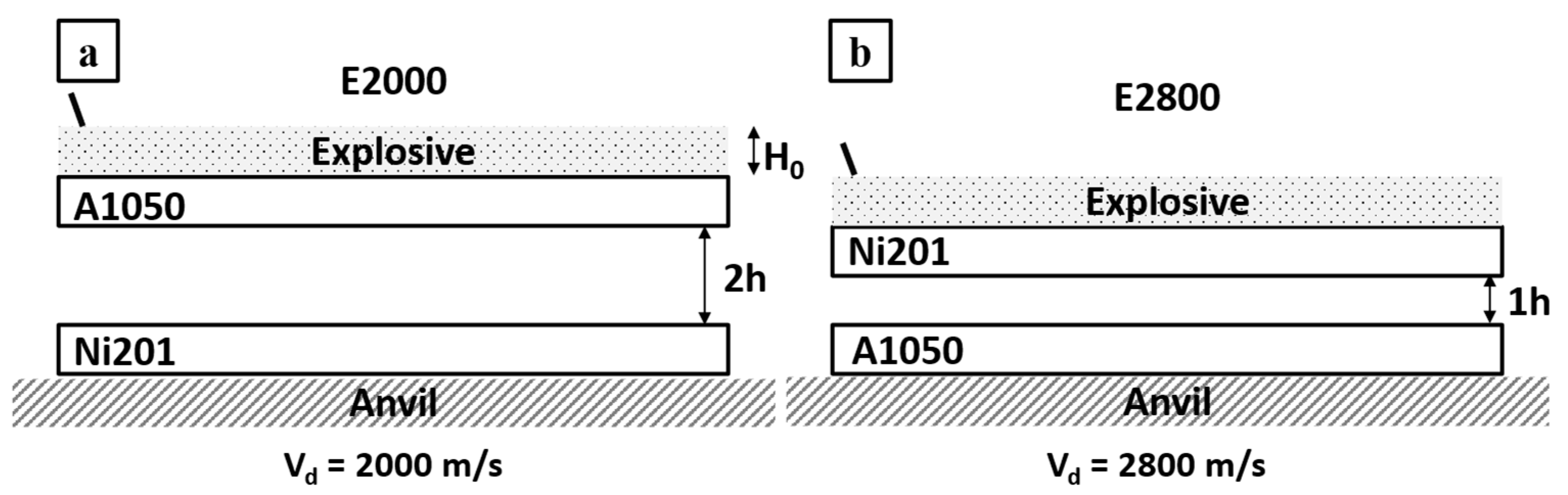
| Samples Name | Flyer Plate | Base Plate | Thickness of Flyer Plate | Thickness of Base Plate | Detonation Velocity | Stand of Distance | Temp. of Heat Treatment | Time of Heat Treatment |
|---|---|---|---|---|---|---|---|---|
| E2000 | A1050 | Ni201 | 1 mm | 1 mm | 2000 m/s | 2h | 500 °C | 0.5–168 h |
| E2800 | Ni201 | A1050 | 1 mm | 1 mm | 2800 m/s | 1h | 500 °C | 0.5–168 h |
| Point | Average Content of Element in at. % | ||
|---|---|---|---|
| Al | Ni | Phase | |
| 1a | 74.4 ± 1.5 | 25.6 ± 0.5 | Al3Ni |
| 1b | 81.2 ± 1.6 | 18.8 ± 0.4 | (Al) + Al3Ni |
| 2 | 86.0 ± 1.7 | 14.0 ± 0.3 | (Al) + Al3Ni |
| 3 | 0.3 ± 0.3 | 99.7 ± 2.0 | (Ni) |
| Point | Average Content of Element in at. % | ||
|---|---|---|---|
| Al | Ni | Phase | |
| 1 | 52.8 ± 1.15 | 47.2 ± 0.9 | AlNi |
| 2 | 71.9 ± 1.4 | 28.1 ± 0.6 | Al3Ni |
| 3 | 0.3 ± 0.3 | 99.7 ± 1.9 | Ni |
| 4 | 59.7 ± 1.2 | 40.3 ± 0.8 | Al3Ni2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwiecien, I.; Wierzbicka-Miernik, A.; Szczerba, M.; Bobrowski, P.; Szulc, Z.; Wojewoda-Budka, J. On the Disintegration of A1050/Ni201 Explosively Welded Clads Induced by Long-Term Annealing. Materials 2021, 14, 2931. https://doi.org/10.3390/ma14112931
Kwiecien I, Wierzbicka-Miernik A, Szczerba M, Bobrowski P, Szulc Z, Wojewoda-Budka J. On the Disintegration of A1050/Ni201 Explosively Welded Clads Induced by Long-Term Annealing. Materials. 2021; 14(11):2931. https://doi.org/10.3390/ma14112931
Chicago/Turabian StyleKwiecien, Izabella, Anna Wierzbicka-Miernik, Maciej Szczerba, Piotr Bobrowski, Zygmunt Szulc, and Joanna Wojewoda-Budka. 2021. "On the Disintegration of A1050/Ni201 Explosively Welded Clads Induced by Long-Term Annealing" Materials 14, no. 11: 2931. https://doi.org/10.3390/ma14112931
APA StyleKwiecien, I., Wierzbicka-Miernik, A., Szczerba, M., Bobrowski, P., Szulc, Z., & Wojewoda-Budka, J. (2021). On the Disintegration of A1050/Ni201 Explosively Welded Clads Induced by Long-Term Annealing. Materials, 14(11), 2931. https://doi.org/10.3390/ma14112931