Degradation Monitoring of HDPE Material in CO2-Saturated NaCl Environment through Electrochemical Impedance Spectroscopy Technique
Abstract
1. Introduction
2. Materials and Methods
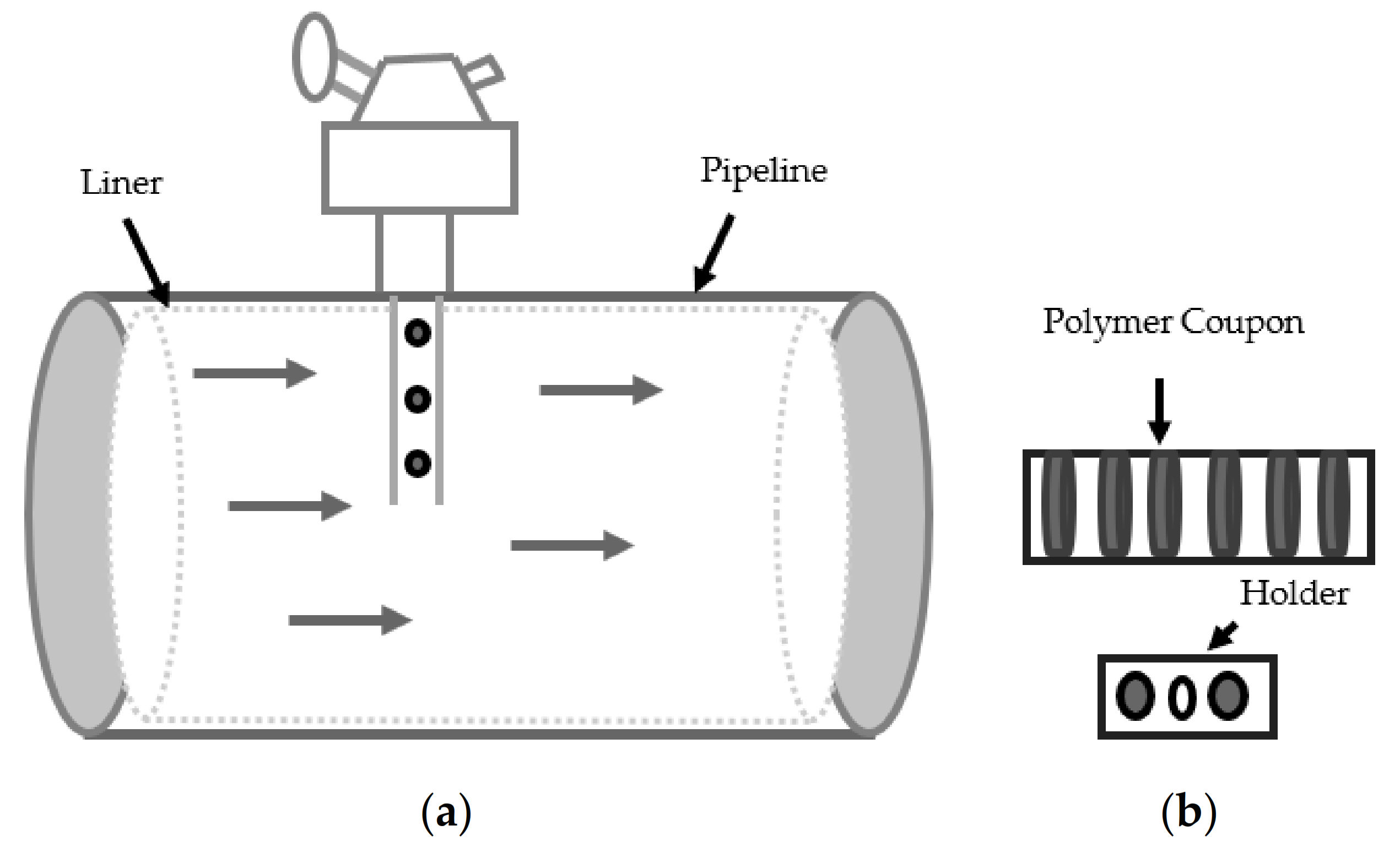
2.1. Sample Preparation
2.2. Degradation/Aging of HDPE
2.3. Characterization Techniques
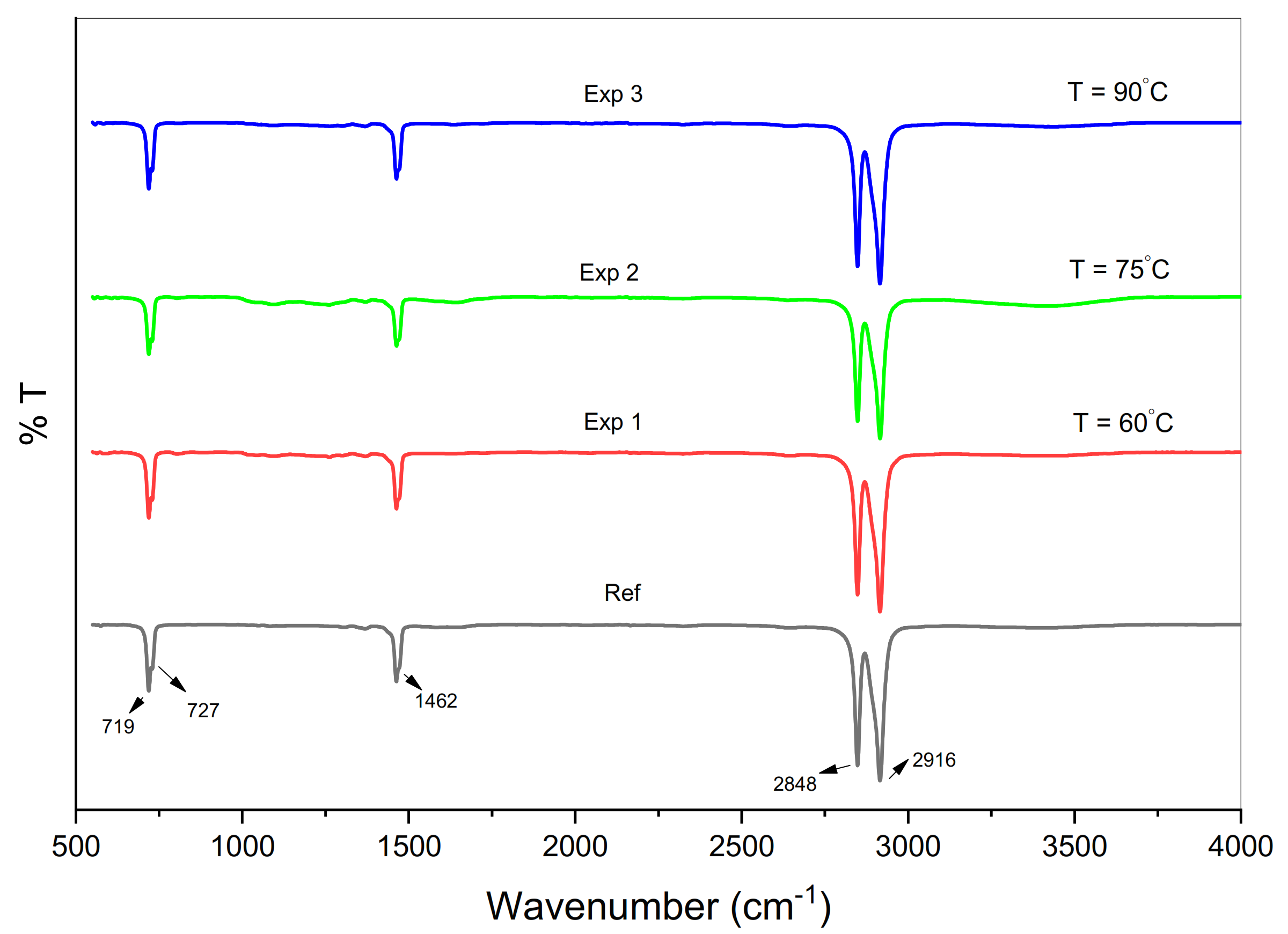
2.3.1. Fourier Transform Infrared Spectroscopy (FTIR)
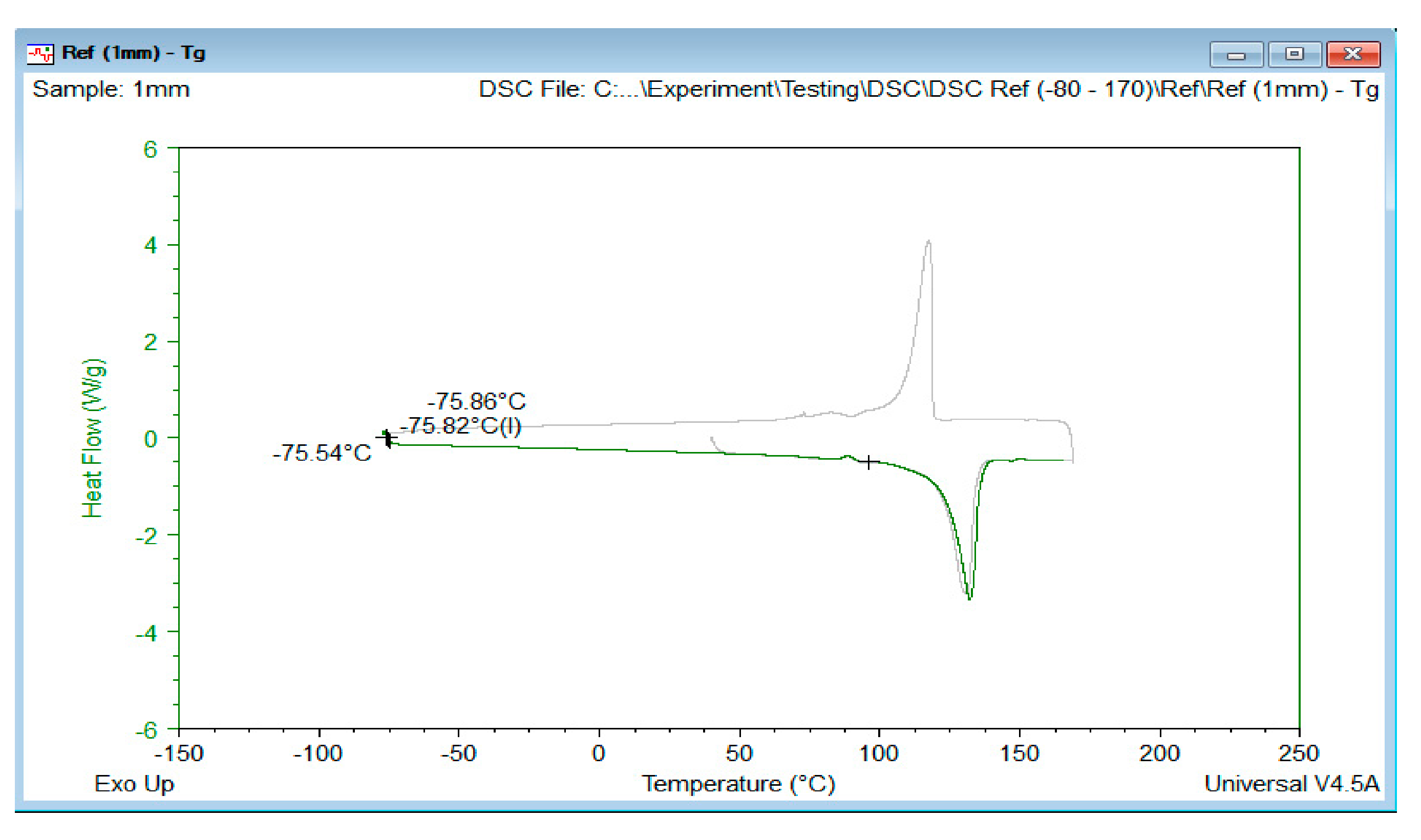
2.3.2. Differential Scanning Calorimetry (DSC)
2.3.3. Thermogravimetric Analysis (TGA)
2.3.4. Field Emission Scanning Electron Microscopy (FESEM)
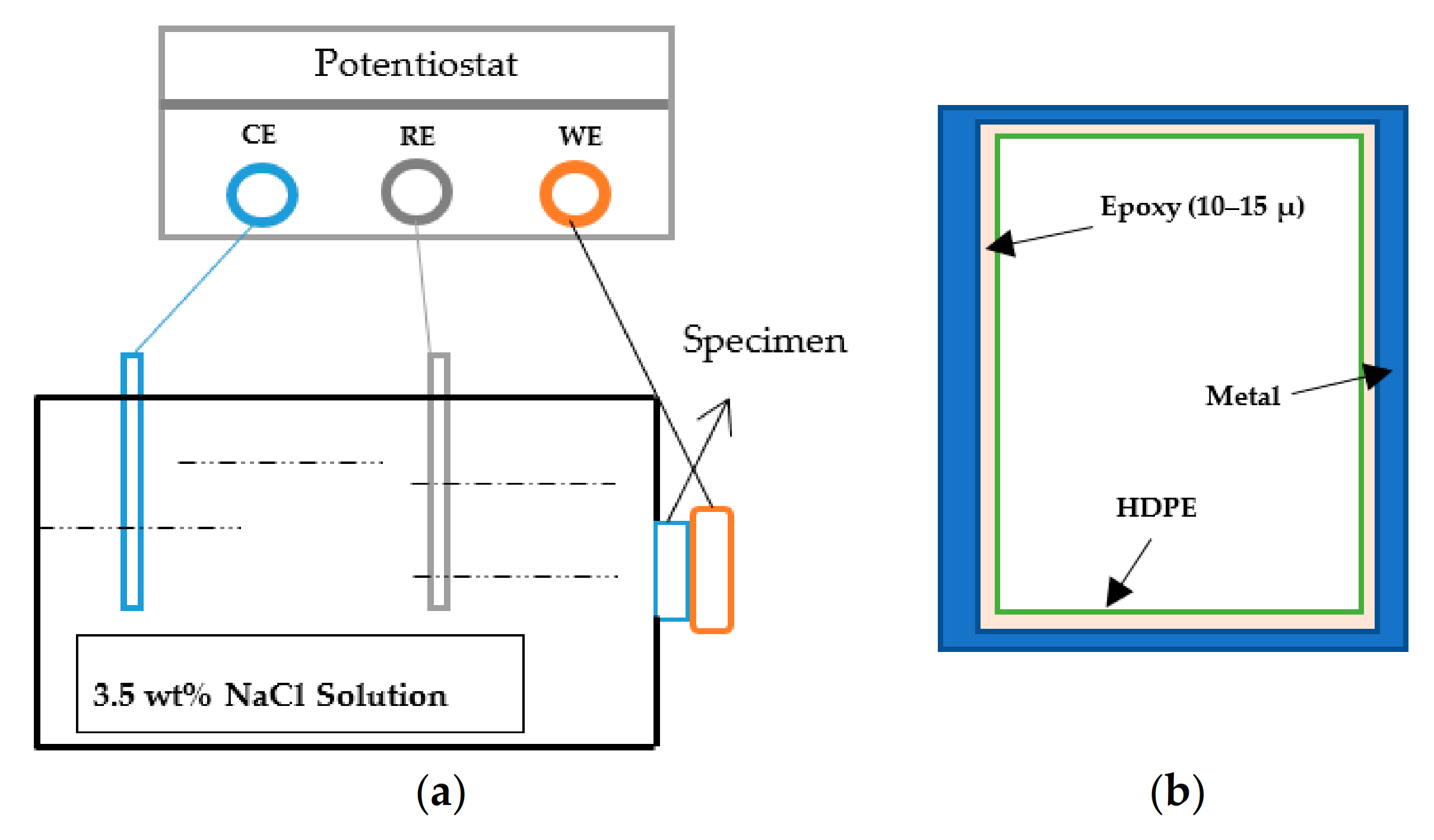
2.3.5. Electrochemical Impedance Spectroscopy (EIS)
3. Results and Discussion
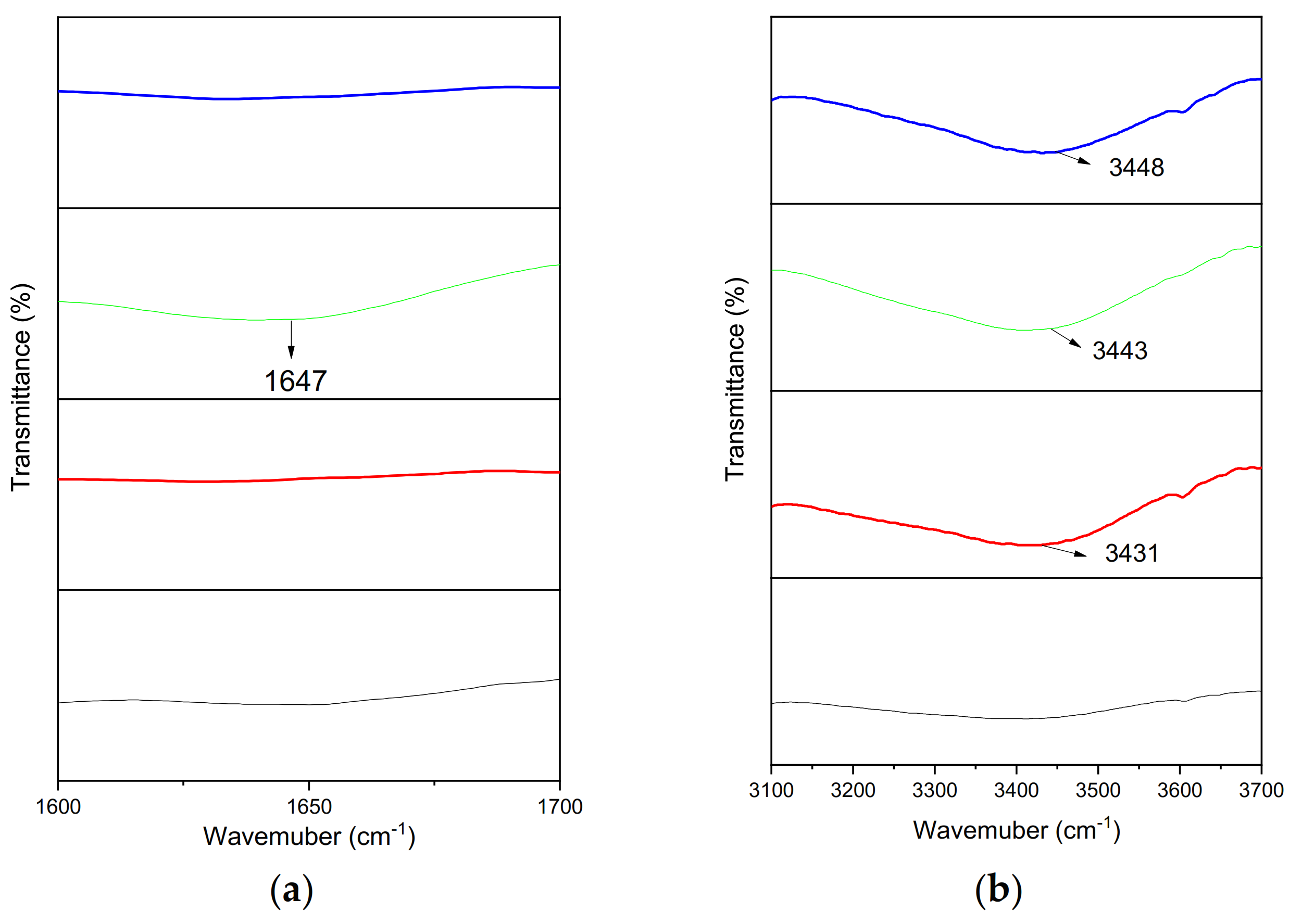
3.1. Structural Modifications and Chemical Composition
3.1.1. Chain Scission
3.1.2. Degradation Products
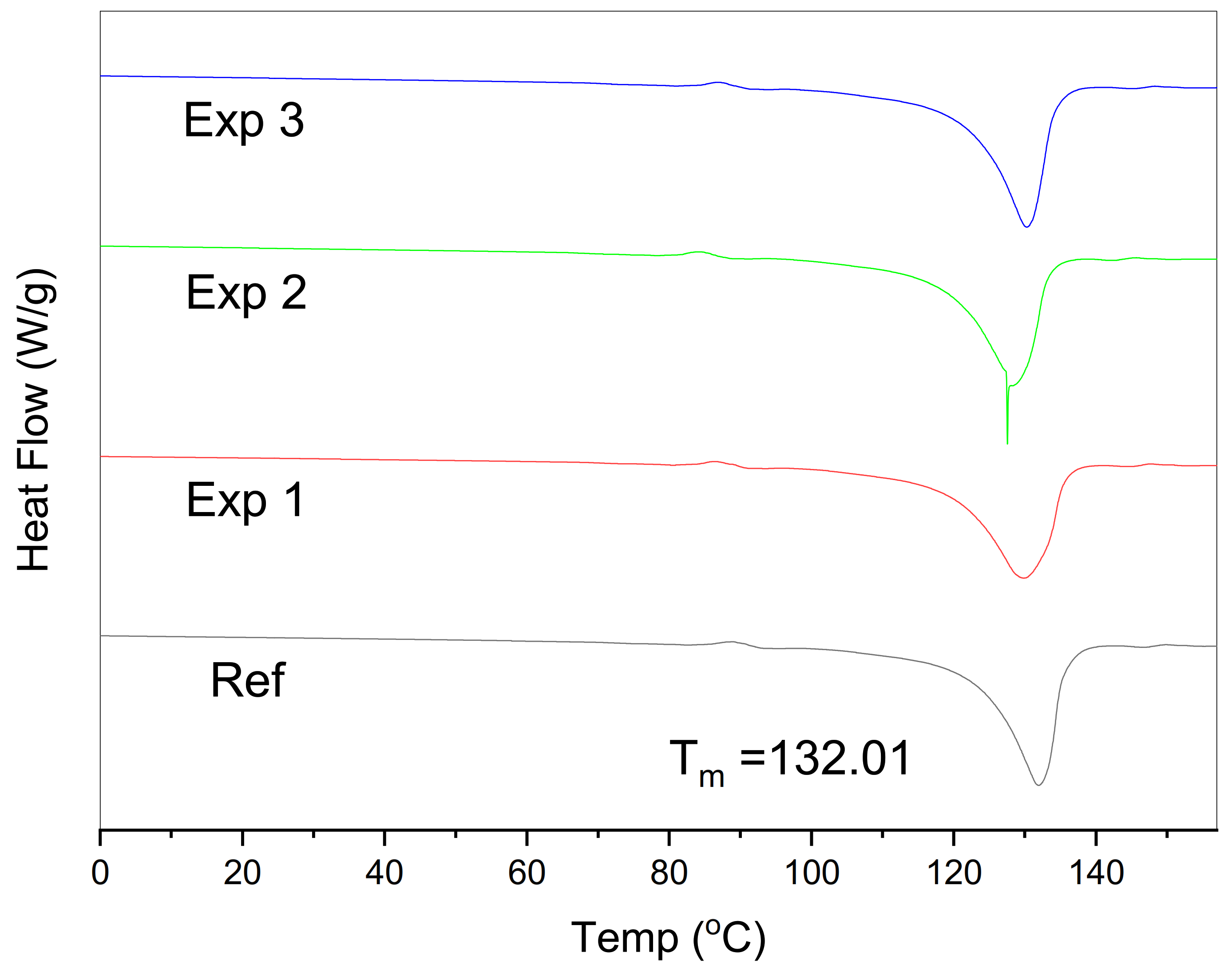
3.2. Thermal and Crystallinity Analysis
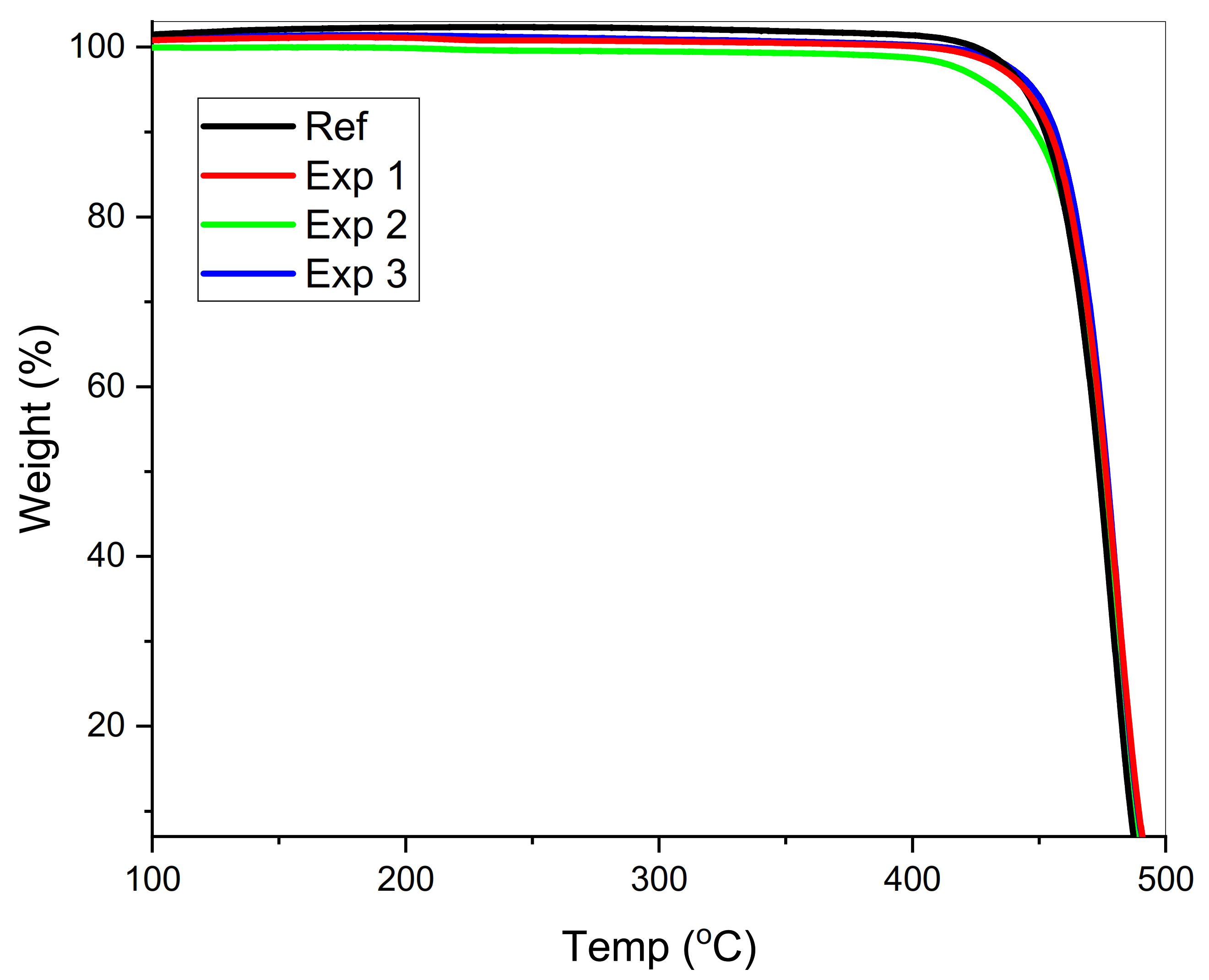
3.3. Thermal Stability Analysis
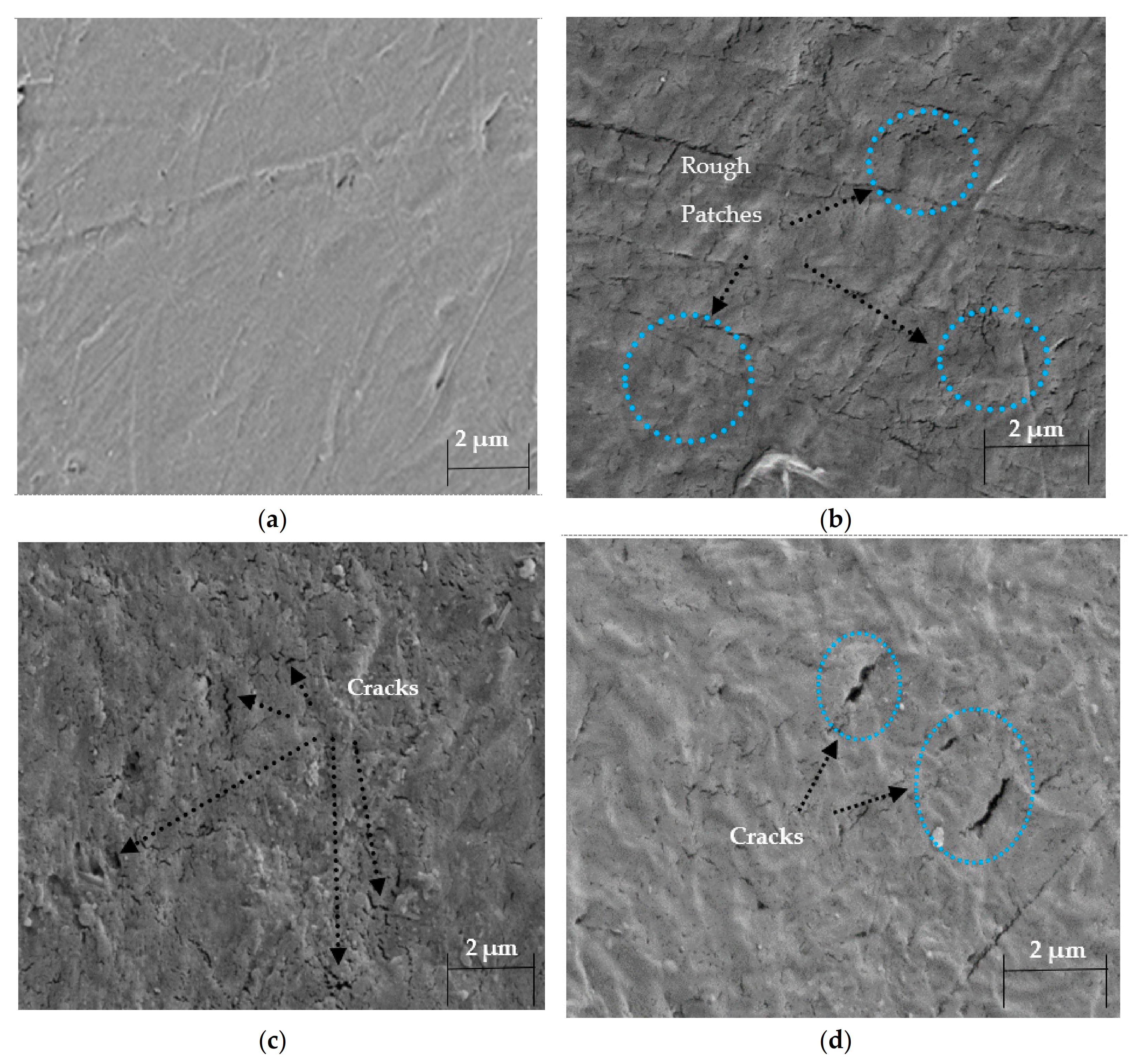
3.4. Morphological Properties
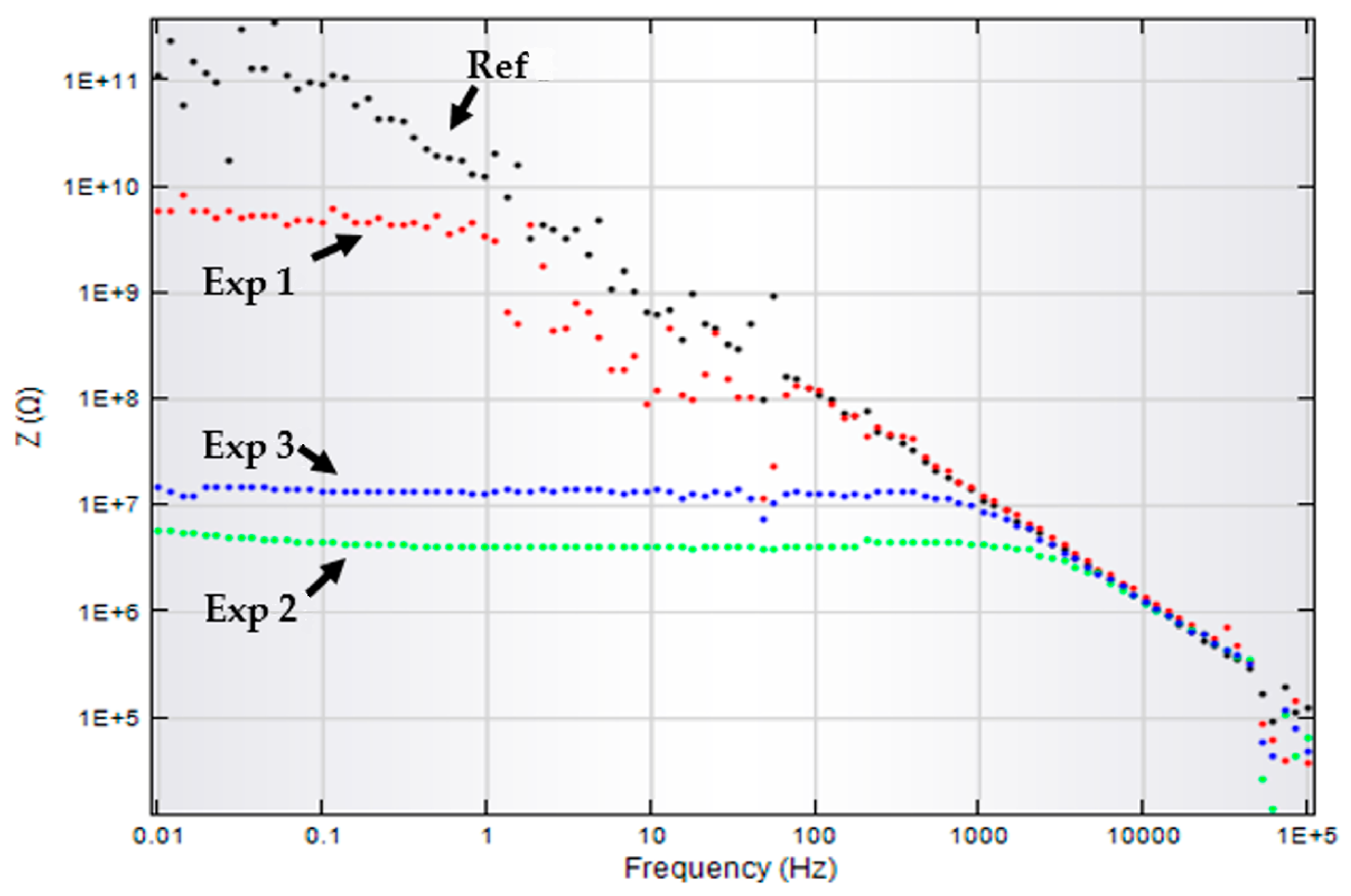
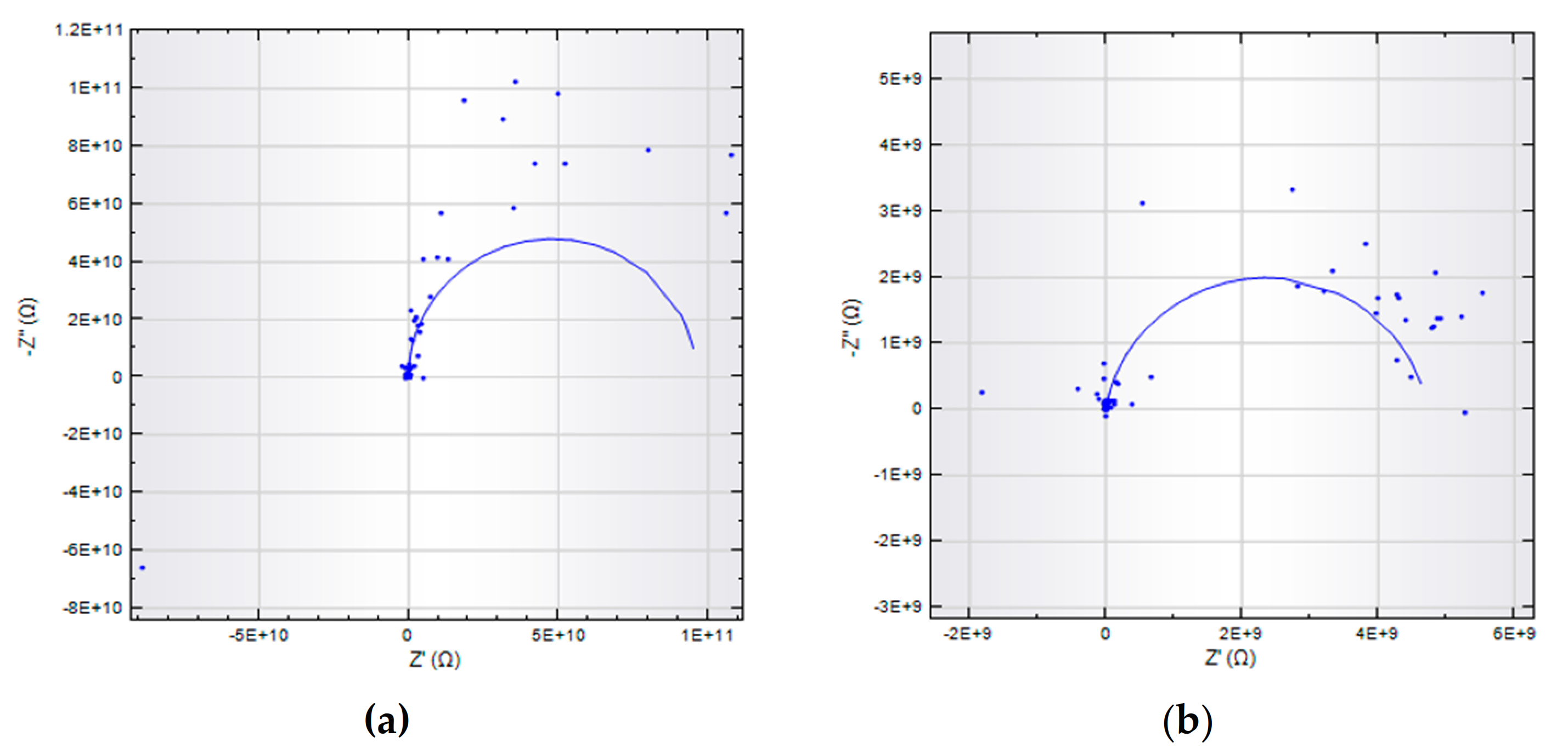
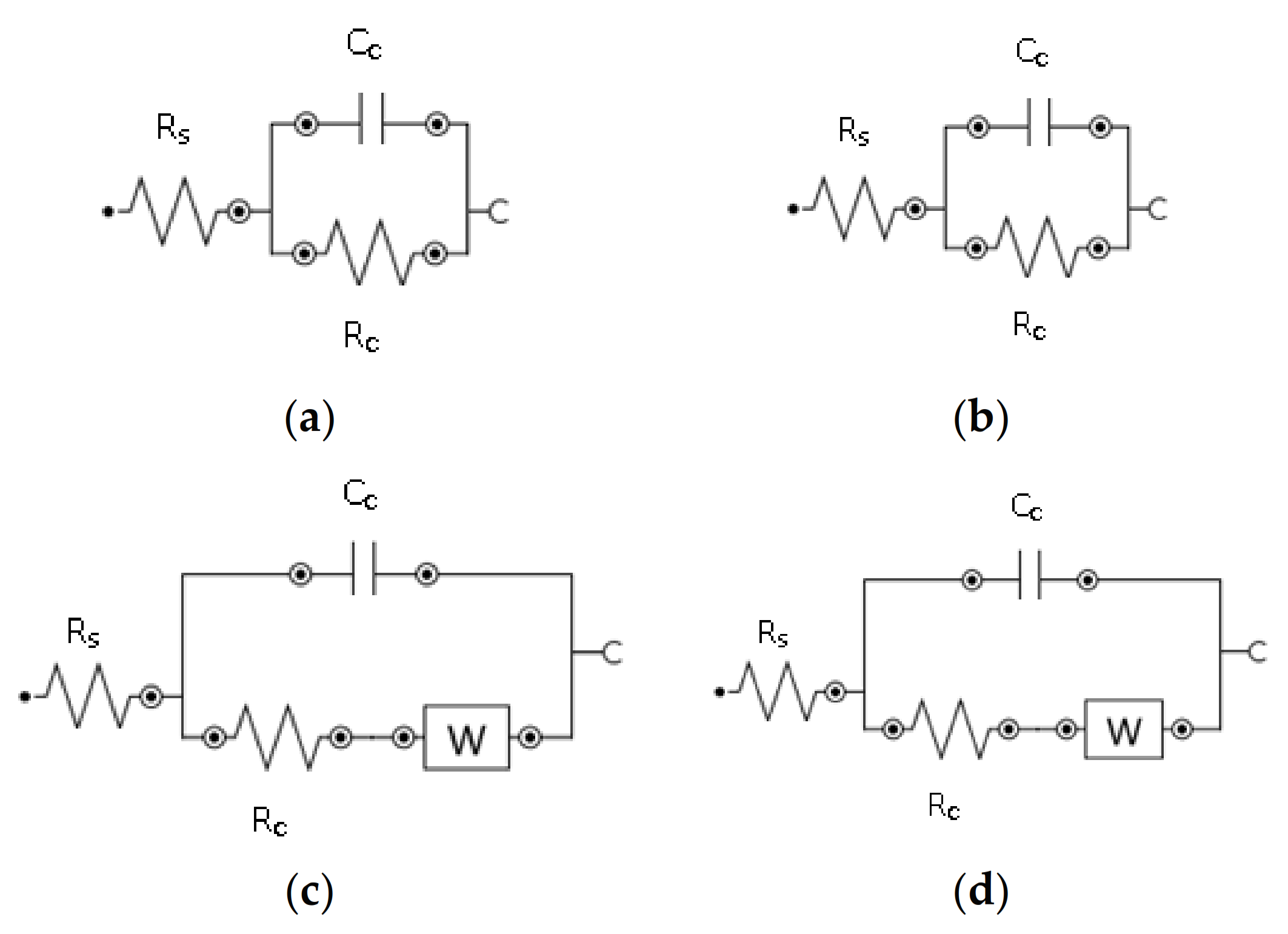
3.5. Impedance Analysis
Dielectric Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A



References
- Samimi, A. Causes of Increased Corrosion in Oil and Gas Pipelines in the Middle East. Int. J. Basic Appl. Sci. 2013, 1, 572–577. [Google Scholar]
- Popoola, L.T.; Grema, A.S.; Latinwo, G.K.; Gutti, B.; Balogun, A.S. Corrosion problems during oil and gas production and its mitigation. Int. J. Ind. Chem. 2013, 4, 35. [Google Scholar] [CrossRef]
- Ghali, E.; Sastri, V.S.; Elboujdaini, M. Corrosion Prevention and Protection: Practical Solutions; John Wiley & Sons: New York, NY, USA, 2007. [Google Scholar]
- Papavinasam, S. Corrosion Control in the Oil and Gas Industry; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Groysman, A. Corrosion Problems and Solutions in Oil Refining and Petrochemical Industry; Springer: Cham, Switzerland, 2017. [Google Scholar]
- Savino, V.; Mehdi, M.; Al-Dossary, A. Thermoplastic Liners for Rehabilitation of Oil Flowline and Water Injection Lines, Integrity and Service Life. p. 8. Available online: www.ndt.net/article/mendt2009/papers/Vincenzo-1 (accessed on 5 April 2021).
- Esaklul, K.A.; Mason, J. Nonmetallics applications in oil and gas production (pipes, liners, rehabilitations). In Trends in Oil and Gas Corrosion Research and Technologies; Elsevier: Amsterdam, The Netherlands, 2017; pp. 627–660. [Google Scholar]
- Davis, R.; Snider, B. Economically Mitigating Downhole Corrosion and Wear Failures with Thermoplastic Liners; NACE International: New Orleans, LA, USA, 2017. [Google Scholar]
- Schweitzer, P.A. Mechanical and Corrosion-Resistant Properties of Plastics and Elastomers; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar]
- Ritums, J.E.; Mattozzi, A.; Gedde, U.W.; Hedenqvist, M.S.; Bergman, G.; Palmlöf, M. Mechanical properties of high-density polyethylene and crosslinked high-density polyethylene in crude oil and its components. J. Polym. Sci. Part B Polym. Phys. 2006, 44, 641–648. [Google Scholar] [CrossRef]
- Szklarz, K.E.; Baron, J.J. Learnings from Thermoplastic Liner Failures in Sour Gas Pipeline Service and Replacement Liner Design and Installation; NACE International: Houston, TX, USA, 2004. [Google Scholar]
- Zakaria, N.; Merican, Z.; Hamza, M. Performance and Critical Issues of Polymer Liners in Pipeline Industry: A review. Mater. Today Proc. 2019, 16, 2389–2397. [Google Scholar] [CrossRef]
- Brogden, S.; Lu, L.; Dowe, A.; Messina, N.; Robinson, J. The use of Engineering Polymers for Internal Corrosion Protection of Hydrocarbon Pipelines. In Proceedings of the MERL Oilfield Engineering with Polymers; AMI: London, UK, 2012. [Google Scholar]
- Mierzwa-Hersztek, M.; Gondek, K.; Kopeć, M. Degradation of polyethylene and biocomponent-derived polymer materials: An overview. J. Polym. Environ. 2019, 27, 600–611. [Google Scholar] [CrossRef]
- Celina, M.C. Review of polymer oxidation and its relationship with materials performance and lifetime prediction. Polym. Degrad. Stab. 2013, 98, 2419–2429. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Kusakabe, K.; Morooka, S. Effect of temperature on the gas permeation properties of NaY-type zeolite formed on the inner surface of a porous support tube. Chem. Eng. Sci. 2001, 56, 4273–4281. [Google Scholar] [CrossRef]
- Perez, E.V.; Balkus Jr, K.J.; Ferraris, J.P.; Musselman, I.H. Instrument for gas permeation measurements at high pressure and high temperature. Rev. Sci. Instrum. 2013, 84, 065107. [Google Scholar] [CrossRef]
- Khalid, H.U.; Ismail, M.C.; Nosbi, N. Permeation Damage of Polymer Liner in Oil and Gas Pipelines. Rev. Polym. 2020, 12, 2307. [Google Scholar] [CrossRef]
- Torres, A.H.U.; d’Almeida, J.R.M.; Habas, J.-P. Aging of HDPE pipes exposed to diesel lubricant. Polym.-Plast. Technol. Eng. 2011, 50, 1594–1599. [Google Scholar] [CrossRef]
- Grabmayer, K.; Wallner, G.M.; Beißmann, S.; Braun, U.; Steffen, R.; Nitsche, D.; Röder, B.; Buchberger, W.; Lang, R.W. Accelerated aging of polyethylene materials at high oxygen pressure characterized by photoluminescence spectroscopy and established aging characterization methods. Polym. Degrad. Stab. 2014, 109, 40–49. [Google Scholar] [CrossRef]
- Grabmann, M.K.; Wallner, G.M.; Grabmayer, K.; Nitsche, D.; Lang, R.W. Aging behavior and lifetime assessment of polyolefin liner materials for seasonal heat storage using micro-specimen. Sol. Energy 2018, 170, 988–990. [Google Scholar] [CrossRef]
- Chen, G.; Yang, Y.; Zhou, C.; Zhou, Z.; Yan, D. Thermal-oxidative aging performance and life prediction of polyethylene pipe under cyclic and constant internal pressure. J. Appl. Polym. Sci. 2019, 136, 47766. [Google Scholar] [CrossRef]
- Flaconnèche, B.; Martin, J.; Klopffer, M.H. Permeability, diffusion and solubility of gases in polyethylene, polyamide 11 and poly (vinylidene fluoride). Oil Gas Sci. Technol. 2001, 56, 261–278. [Google Scholar] [CrossRef]
- Menon, N.C.; Kruizenga, A.M.; Alvine, K.J.; San Marchi, C.; Nissen, A.; Brooks, K. Behaviour of Polymers in High Pressure Environments as Applicable to the Hydrogen Infrastructure; American Society of Mechanical Engineers Digital Collection: New York, NY, USA, 2016. [Google Scholar]
- Mason, J.F.; Stanley, M.; Ponda, A.; Demicoli, D. Case Study: Engineered Polyamide 12 (PA12) Pipeline Liner for Management of Sour Gas Corrosion at Elevated Temperatures; NACE International: Houston, TX, USA, 2017. [Google Scholar]
- Zhou, J.; Chen, X. Compatibility study of high-density polyethylene with ethanol–gasoline and biodiesel. J. Elastomers Plast. 2019, 53, 0095244319891206. [Google Scholar] [CrossRef]
- Durbin, T.D.; Karavalakis, G.; Norbeck, J.M.; Park, C.S.; Castillo, J.; Rheem, Y.; Bumiller, K.; Yang, J.; Van, V.; Hunter, K. Material compatibility evaluation for elastomers, plastics, and metals exposed to ethanol and butanol blends. Fuel 2016, 163, 248–259. [Google Scholar] [CrossRef]
- El-Sherik, A. Trends in Oil and Gas Corrosion Research and Technologies: Production and Transmission; Woodhead Publishing: Cambridge, UK, 2017. [Google Scholar]
- Technology, F. Flexible Pipe Polymer Monitoring with Coupons. Available online: http://www.kks.com.au/wp-content/uploads/2014/01/Force-Technology-Pipe-Monitoring-flexible-pipe-polymer-monitoring-with-coupons.pdf (accessed on 24 August 2020).
- Kranbuehl, D.E. Method to Predict the End-Point, Replacement Time and to Monitor Changes in that Time Using pre Aged Witness Coupons. Google Patents US20050247103A1, 17 August 2009. [Google Scholar]
- Wolodko, J.; Fotty, B.; Perras, T. Application of Non-Metallic Materials in Oil Sands Operations; NACE International: Houston, TX, USA, 2016; pp. 1–14. [Google Scholar]
- Muren, J.; Caveny, K.; Eriksen, M.; Viko, N.G.; MÜLler-Allers, J.; JØRgen, K. Un-Bonded Flexible Risers-Recent Field Experience and Actions for Increased Robustness; 4subsea: Asker, Norway, 2013; Volume 2, pp. 1–78. [Google Scholar]
- Lu, F.; Song, B.; He, P.; Wang, Z.; Wang, J. Electrochemical impedance spectroscopy (EIS) study on the degradation of acrylic polyurethane coatings. RSC Adv. 2017, 7, 13742–13748. [Google Scholar] [CrossRef]
- Tang, Y.; Cao, J.; Qu, S.; Quan, L.; Zhao, X.; Zuo, Y. Degradation of a High Build Epoxy Primer/Polyurethane Composite Coatings under Cyclic Wet–dry Conditions. Int. J. Electrochem. Sci. 2018, 13, 3874–3887. [Google Scholar] [CrossRef]
- Garcia, E.; Torres, J.; Rebolledo, N.; Arrabal, R.; Sanchez, J. Corrosion of Steel Rebars in Anoxic Environments. Part I: Electrochemical Measurements. Materials 2021, 14, 2491. [Google Scholar] [CrossRef]
- Yang, Y.; Akid, R. Electrochemical investigation of the corrosion of different microstructural phases of X65 pipeline steel under saturated carbon dioxide conditions. Materials 2015, 8, 2635–2649. [Google Scholar] [CrossRef]
- Bozzini, B.; Gianoncelli, A.; Kourousias, G.; Boniardi, M.; Casaroli, A.; Dal Zilio, S.; Hussain, R.; Abyaneh, M.K.; Kiskinova, M.; Mele, C. The role of chromium in the corrosion performance of cobalt-and cobalt-nickel based hardmetal binders: A study centred on X-ray absorption microspectroscopy. Int. J. Refract. Met. Hard Mater. 2020, 92, 105320. [Google Scholar] [CrossRef]
- Mele, C.; Bilotta, A.; Bocchetta, P.; Bozzini, B. Characterization of the particulate anode of a laboratory flow Zn–air fuel cell. J. Appl. Electrochem. 2017, 47, 877–888. [Google Scholar] [CrossRef]
- Jung, M.R.; Horgen, F.D.; Orski, S.V.; Rodriguez, V.; Beers, K.L.; Balazs, G.H.; Jones, T.T.; Work, T.M.; Brignac, K.C.; Royer, S.-J. Validation of ATR FT-IR to identify polymers of plastic marine debris, including those ingested by marine organisms. Mar. Pollut. Bull. 2018, 127, 704–716. [Google Scholar] [CrossRef]
- Prasad, S.G.; Lal, C.; Sahu, K.R.; Saha, A.; De, U. Spectroscopic Investigation of Degradation Reaction Mechanism in γ-Rays Irradiation of HDPE. Biointerface Res. Appl. Chem. 2021, 11, 9405–9419. [Google Scholar] [CrossRef]
- Fakirov, S.; Krasteva, B. On the glass transition temperature of polyethylene as revealed by microhardness measurements. J. Macromol. Sci. Part B 2000, 39, 297–301. [Google Scholar] [CrossRef]
- Whelton, A.J.; Dietrich, A.M. Critical considerations for the accelerated ageing of high-density polyethylene potable water materials. Polym. Degrad. Stab. 2009, 94, 1163–1175. [Google Scholar] [CrossRef]
- Blaine, R.L. Thermal Applications Note; Polymer Heats of Fusion: New Castle, DE, USA, 2002. [Google Scholar]
- Weon, J.-I. Effects of thermal ageing on mechanical and thermal behaviors of linear low density polyethylene pipe. Polym. Degrad. Stab. 2010, 95, 14–20. [Google Scholar] [CrossRef]
- Hamzah, M.; Khenfouch, M.; Rjeb, A.; Sayouri, S.; Houssaini, D.S.; Darhouri, M.; Srinivasu, V.V. Surface Chemistry Changes and Microstructure Evaluation of Low Density Nanocluster Polyethylene under Natural Weathering: A Spectroscopic Investigation; IOP Publishing: London, UK, 2018; p. 012010. [Google Scholar]
- Charles, J. Qualitative analysis of high density polyethylene using FTIR spectroscopy. Asian J. Chem. 2009, 21, 4477. [Google Scholar]
- Kong, L.; Fan, X.; Ding, N.; Ding, H.; Shao, X.; Li, H.; Qi, D.; Liu, Q.; Xu, Y.; Ge, P. Experimental and Simulation Investigation on Failure Mechanism of a Polyethylene Elbow Liner Used in an Oilfield Environment. J. Fail. Anal. Prev. 2020, 20, 2129–2136. [Google Scholar] [CrossRef]
- Nandiyanto, A.B.D.; Oktiani, R.; Ragadhita, R. How to read and interpret FTIR spectroscope of organic material. Indones. J. Sci. Technol. 2019, 4, 97–118. [Google Scholar] [CrossRef]
- Singh, R.K.; Ruj, B.; Sadhukhan, A.K.; Gupta, P. A TG-FTIR investigation on the co-pyrolysis of the waste HDPE, PP, PS and PET under high heating conditions. J. Energy Inst. 2020, 93, 1020–1035. [Google Scholar] [CrossRef]
- Brandon, J.; Goldstein, M.; Ohman, M.D. Long-term aging and degradation of microplastic particles: Comparing in situ oceanic and experimental weathering patterns. Mar. Pollut. Bull. 2016, 110, 299–308. [Google Scholar] [CrossRef]
- Guo, S.-M.; Yang, Z.-G.; Tang, X.-Y.; Zuo, Y.-T. Safety assessment of high density polyethylene pipe with thermal damages. Plast. Rubber Compos. 2017, 46, 173–183. [Google Scholar] [CrossRef]
- Bellamy, L. The Infra-Red Spectra of Complex Molecules; Springer Science Business Media: Berlin, Germany, 2013. [Google Scholar]
- Pagès, P. Characterization of Polymer Materials Using FT-IR and DSC Techniques; Universidade da Coruña: Coruña, Spain, 2005. [Google Scholar]
- Shirkavand, M.J.; Azizi, H.; Ghasemi, I.; Karabi, M. Effect of Molecular Structure Parameters on Crystallinity and Environmental Stress Cracking Resistance of High-Density Polyethylene/TiO2 Nanocomposites. Adv. Polym. Technol. 2018, 37, 770–777. [Google Scholar] [CrossRef]
- Mendes, L.C.; Rufino, E.S.; De Paula, F.O.C.; Torres, A.C., Jr. Mechanical, thermal and microstructure evaluation of HDPE after weathering in Rio de Janeiro City. Polym. Degrad. Stab. 2003, 79, 371–383. [Google Scholar] [CrossRef]
- Davidson, R.G. Polymer degradation studies by FTIR. In Progress in Pacific Polymer Science 2; Springer: Berlin, Germany, 1992; pp. 101–111. [Google Scholar]
- Hadjiivanov, K. Identification and characterization of surface hydroxyl groups by infrared spectroscopy. Adv. Catal. 2014, 57, 99–318. [Google Scholar]
- Khanam, P.N.; AlMaadeed, M.A.A. Processing and characterization of polyethylene-based composites. Adv. Manuf. Polym. Compos. Sci. 2015, 1, 63–79. [Google Scholar] [CrossRef]
- Cuadri, A.A.; Martín-Alfonso, J.E. The effect of thermal and thermo-oxidative degradation conditions on rheological, chemical and thermal properties of HDPE. Polym. Degrad. Stab. 2017, 141, 11–18. [Google Scholar] [CrossRef]
- Tantipattarakul, S.; Vaughan, A.S.; Andritsch, T. Ageing behaviour of a polyethylene blend: Influence of chemical defects and morphology on charge transport. High Volt. 2020, 5, 270–279. [Google Scholar] [CrossRef]
- Méndez-Hernández, M.L.; Tena-Salcido, C.S.; Sandoval-Arellano, Z.; González-Cantú, M.C.; Mondragón, M.; Rodríguez-González, F.J. The effect of thermoplastic starch on the properties of HDPE/TPS blends during UV-accelerated aging. Polym. Bull. 2011, 67, 903–914. [Google Scholar] [CrossRef]
- Shrivastava, A. Introduction to Plastics Engineering; William Andrew: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Teymouri, Y.; Adams, A.; Blümich, B. Impact of Exposure Conditions on the Morphology of Polyethylene by Compact NMR; Wiley Online Library: New York, NY, USA, 2018; p. 1600156. [Google Scholar]
- Khajehpour-Tadavani, S.; Nejabat, G.-R.; Mortazavi, M.-M. Changes in crystallinity of HDPE films containing different amounts of an oxo-biodegradable additive due to UVC exposure. Polyolefins J. 2019, 7, 25–32. [Google Scholar]
- Ansaloni, L.; Alcock, B.; Peters, T.A. Effects of CO2 on polymeric materials in the CO2 transport chain: A review. Int. J. Greenh. Gas Control. 2020, 94, 102930. [Google Scholar] [CrossRef]
- McKeen, L.W. Permeability Properties of Plastics and Elastomers; William Andrew: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Li, D.; Zhou, L.; Wang, X.; He, L.; Yang, X. Effect of crystallinity of polyethylene with different densities on breakdown strength and conductance property. Materials 2019, 12, 1746. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Liu, Y.; Yu, J.; Wang, K. Thermal oxidation products and kinetics of polyethylene composites. Polym. Degrad. Stab. 2006, 91, 1651–1657. [Google Scholar] [CrossRef]
- Fu, A.; Zhao, B.; Yuan, J.; Yin, C. Lab Research and Filed Experience of HDPE-Lined Tubing Used in Nitrogen Injection Well; NACE International: Houston, TX, USA, 2019. [Google Scholar]
- Bredács, M.; Frank, A.; Bastero, A.; Stolarz, A.; Pinter, G. Accelerated aging of polyethylene pipe grades in aqueous chlorine dioxide at constant concentration. Polym. Degrad. Stab. 2018, 157, 80–89. [Google Scholar] [CrossRef]
- University, K. Using Electrochemical Impedance Spectroscopy (EIS) for Evaluating Coating Performance in the Laboratory. Available online: https://kta.com/kta-university/using-electrochemical-impedance/ (accessed on 17 March 2021).
- GAMRY Instruments. EIS of Organic Coatings and Paints. Available online: https://www.gamry.com/application-notes/EIS/eis-of-organic-coatings-and-paints/ (accessed on 7 May 2021).
- Joshi, J.H.; Kanchan, D.K.; Joshi, M.J.; Jethva, H.O.; Parikh, K.D. Dielectric relaxation, complex impedance and modulus spectroscopic studies of mix phase rod like cobalt sulfide nanoparticles. Mater. Res. Bull. 2017, 93, 63–73. [Google Scholar] [CrossRef]
- Marín-Genescà, M.; García-Amorós, J.; Mujal-Rosas, R.; Massagués, L.; Colom, X. Study and Characterization of the Dielectric Behavior of Low Linear Density Polyethylene Composites Mixed with Ground Tire Rubber Particles. Polymers 2020, 12, 1075. [Google Scholar] [CrossRef]
- Aziz, S.B.; Marf, A.S.; Dannoun, E.; Brza, M.A.; Abdullah, R.M. The Study of the Degree of Crystallinity, Electrical Equivalent Circuit, and Dielectric Properties of Polyvinyl Alcohol (PVA)-Based Biopolymer Electrolytes. Polymers 2020, 12, 2184. [Google Scholar] [CrossRef]
- Misra, M.; Kumar, S.K. Using time–temperature superposition for determining dielectric loss in functionalized polyethylenes. ACS Macro Lett. 2017, 6, 200–204. [Google Scholar] [CrossRef]
- Mikhaĭlov, G.P.; Sazhin, B.I. Effect of Crystallization of Polymers on Dielectric Loss. Rubber Chem. Technol. 1960, 33, 741–747. [Google Scholar] [CrossRef]


| Material | Density (g/cm3) | Melting Temperature (°C) | Glass Transition (°C) | Dielectric Constant | Dielectric Strength (MV/m) |
|---|---|---|---|---|---|
| HDPE | 0.95 | 125–130 | −78 | 2.3–2.4 | 18.9–160 |
| Gas | MW (g/mol) | Density (kg/m3) | Critical Temperature (°C) | Crystal Structure | Purity (%) |
|---|---|---|---|---|---|
| CO2 | 44.01 | 1.977 | 31.1 | Trigonal | 99.9 |
| Material | Parameters | Characterization | |
|---|---|---|---|
| HDPE | - | - | FTIR Evaluation of the chemical changes within the structure |
| Pressure | 70 bars | DSC Evaluation of crystallinity and melting temperature | |
| Temperature | 60, 75, 90 °C | TGA Evaluation of thermal stability and mass loss | |
| Duration | 21 days | FESEM Evaluation of morphological properties | |
| - | - | EIS Evaluation of impedance and dielectric properties | |
| Wavenumber (cm−1) | Assigned Vibrational Bands/Functional Groups | |||
|---|---|---|---|---|
| Reference | Exp 1 | Exp 2 | Exp 3 | |
| 556 | 558 | C-Cl bending vibration [47] | ||
| 719 | 719 | 719 | 719 | CH2 rocking vibration (amorphous phase) [19,48] |
| 727 | 728 | 729 | 729 | CH2 rocking vibration (crystalline phase) [49,50] |
| 1041 | 1042 | C-O stretching (alcohol)/O-H deformation in alcohol [51] | ||
| 1082 | 1086 | 1089 | C-O stretching (alcohol) [52] | |
| 1100 | 1105 | C-O stretching vibration (alcohol) [53,54] | ||
| 1262 | 1262 | 1262 | CH3 vibration [52,53] | |
| 1368 | 1368 | 1368 | CH2 wagging (amorphous) [55] | |
| 1463 | 1463 | 1463 | 1463 | CH2 scissoring (amorphous phase) 45,46] |
| 1647 | O-H bending (water absorption) [48] | |||
| 2848 | 2848 | 2848 | 2848 | CH2 symmetric stretching [51] |
| 2916 | 2916 | 2916 | 2916 | Asymmetric stretching of CH2 [51] |
| 3431 | 3443 | 3448 | Hydroxyl group [19,20] | |
| Sample | Melting Temp (Tm) | Melting Enthalpy | Glass Transition (Tg) | Crystallinity (%Xc) a |
|---|---|---|---|---|
| Ref | 132.01 | 177.6 | −75.82 | 60.6 |
| Exp 1 | 130.24 | 173.8 | −76.67 | 59.3 |
| Exp 2 | 129.01 | 168.6 | −77.13 | 57.5 |
| Exp 3 | 129.85 | 170.9 | −76.39 | 58.3 |
| Characteristic Temperatures | Ref | Exp 1 | Exp 2 | Exp 3 |
|---|---|---|---|---|
| T5% (°C) | 446.86 | 444.36 | 432.95 | 439.59 |
| T10% (°C) | 455.32 | 452.82 | 448.41 | 451.71 |
| Samples | Rp (MΩ.cm−2) | Cc (pF.cm−2) | Zw (μΩ.cm−2) |
|---|---|---|---|
| Ref | 95.5 | 17.4 | |
| Exp 1 | 16.7 | 32.9 | |
| Exp 2 | 3.83 | 78.7 | 1.62 |
| Exp 3 | 12.8 | 23.6 | 1.68 |
| Parameters | Ref () | Exp 1 () | Exp 2 () | Exp 3 () |
|---|---|---|---|---|
| Frequency (Hz) | ||||
| 34.3043 | 0.0025 | 0.0075 | 5.67 | 0.157 |
| 6.73416 | 2.27 × 10−18 | 3.23 × 10−17 | 28.12 | 8.72 × 10−16 |
| 1.32193 | 2.80 × 10−18 | 8.34 × 10−17 | 142.25 | 4.21 × 10−15 |
| 0.00999989 | 0.567 | 12.845 | 12811 | 1.215× 10−5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khalid, H.U.; Ismail, M.C.; Nosbi, N. Degradation Monitoring of HDPE Material in CO2-Saturated NaCl Environment through Electrochemical Impedance Spectroscopy Technique. Materials 2021, 14, 2823. https://doi.org/10.3390/ma14112823
Khalid HU, Ismail MC, Nosbi N. Degradation Monitoring of HDPE Material in CO2-Saturated NaCl Environment through Electrochemical Impedance Spectroscopy Technique. Materials. 2021; 14(11):2823. https://doi.org/10.3390/ma14112823
Chicago/Turabian StyleKhalid, Hafiz Usman, Mokhtar Che Ismail, and Norlin Nosbi. 2021. "Degradation Monitoring of HDPE Material in CO2-Saturated NaCl Environment through Electrochemical Impedance Spectroscopy Technique" Materials 14, no. 11: 2823. https://doi.org/10.3390/ma14112823
APA StyleKhalid, H. U., Ismail, M. C., & Nosbi, N. (2021). Degradation Monitoring of HDPE Material in CO2-Saturated NaCl Environment through Electrochemical Impedance Spectroscopy Technique. Materials, 14(11), 2823. https://doi.org/10.3390/ma14112823






