3.1.1. Crystal Structure
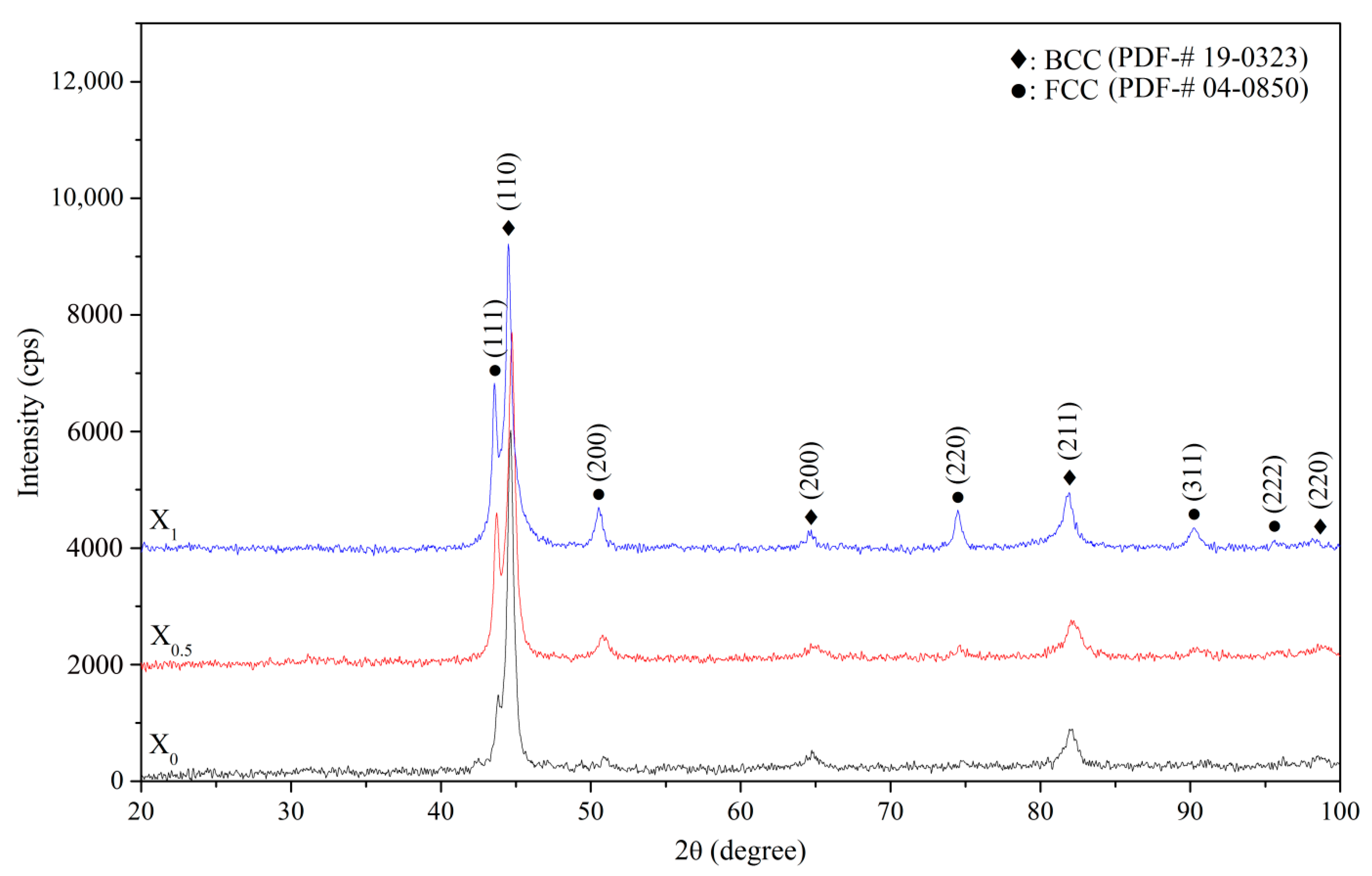
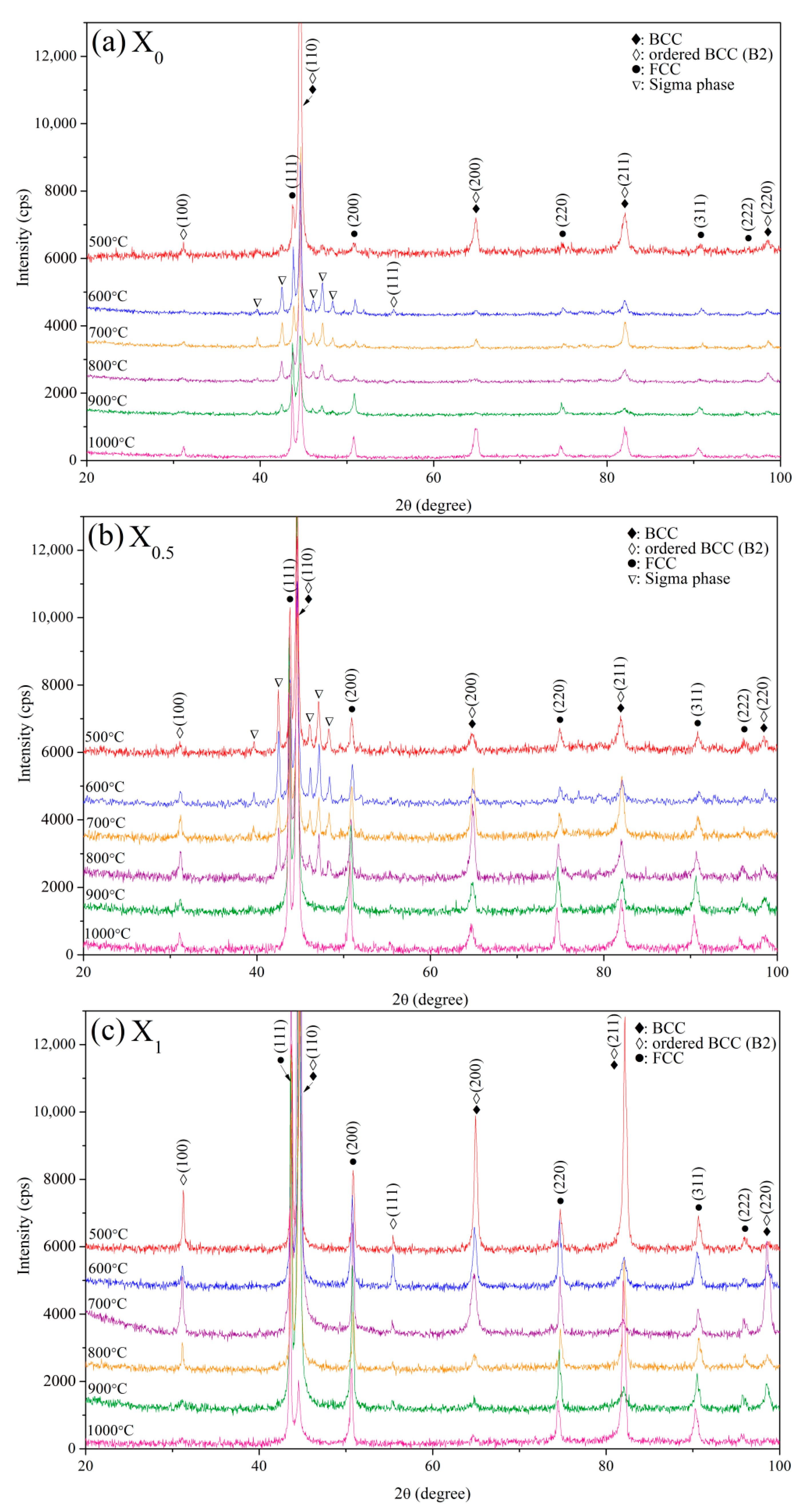
Figure 1 shows XRD patterns of the X
0, X
0.5, and X
1 as-cast alloys. Two phases were identified in the as-cast alloys, i.e., BCC (a = 2.865 Å) and FCC (a = 3.579 Å) phases. The BCC and FCC volume fractions were quantified by XRD with Cu K
α radiation via direct comparison [
21], which uses the integration of the most intense peaks for the BCC phase (characterized by the (110), (200), (211), and (220) planes) and FCC phase (characterized by the (111), (200), (220), and (311) planes). Origin™ software was used for integrating these peaks with a peak-fitting tool. The volume fractions of the FCC phase for X
0, X
0.5, and X
1 alloys were 7.7, 23.0, and 31.9%, respectively, indicating that compared with Co, Ni is a stronger FCC stabilizer. The FCC phase detected for the as-cast X
0 alloy is speculative, because the cooling rate (10
−1 to 10
−2 K/s) of the melt in the ceramic shell mold by vacuum induction melting is lower than that (10–20 K/s) of the water-cooled copper mold by vacuum arc re-melting, as reported in previous studies [
3,
4,
5,
6,
7,
8,
9,
10,
11,
12,
13,
14]. The fact that the FCC phase of as-cast AlCoCrFeNi is formed after homogenization at 1100 °C [
13] confirms this hypothesis.
3.1.2. Microstructure
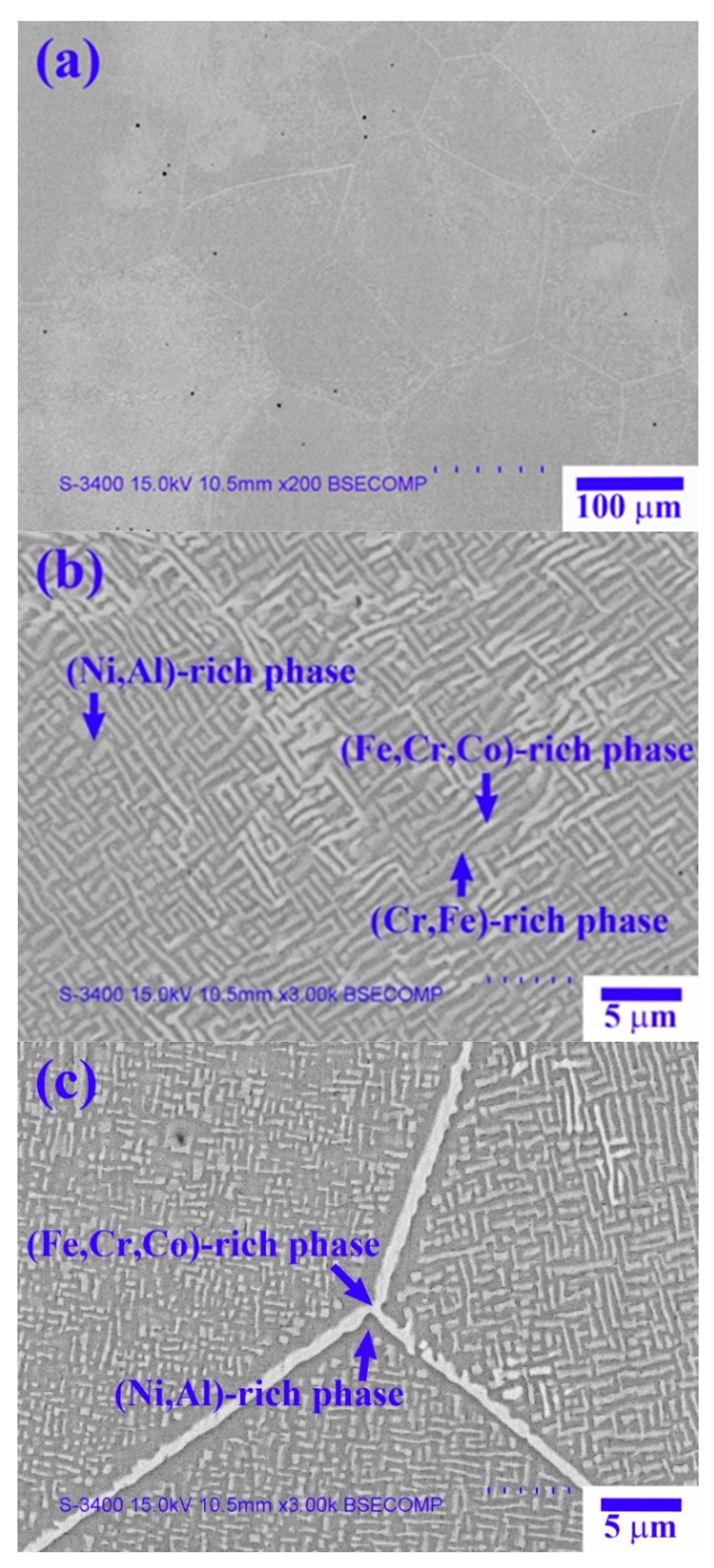
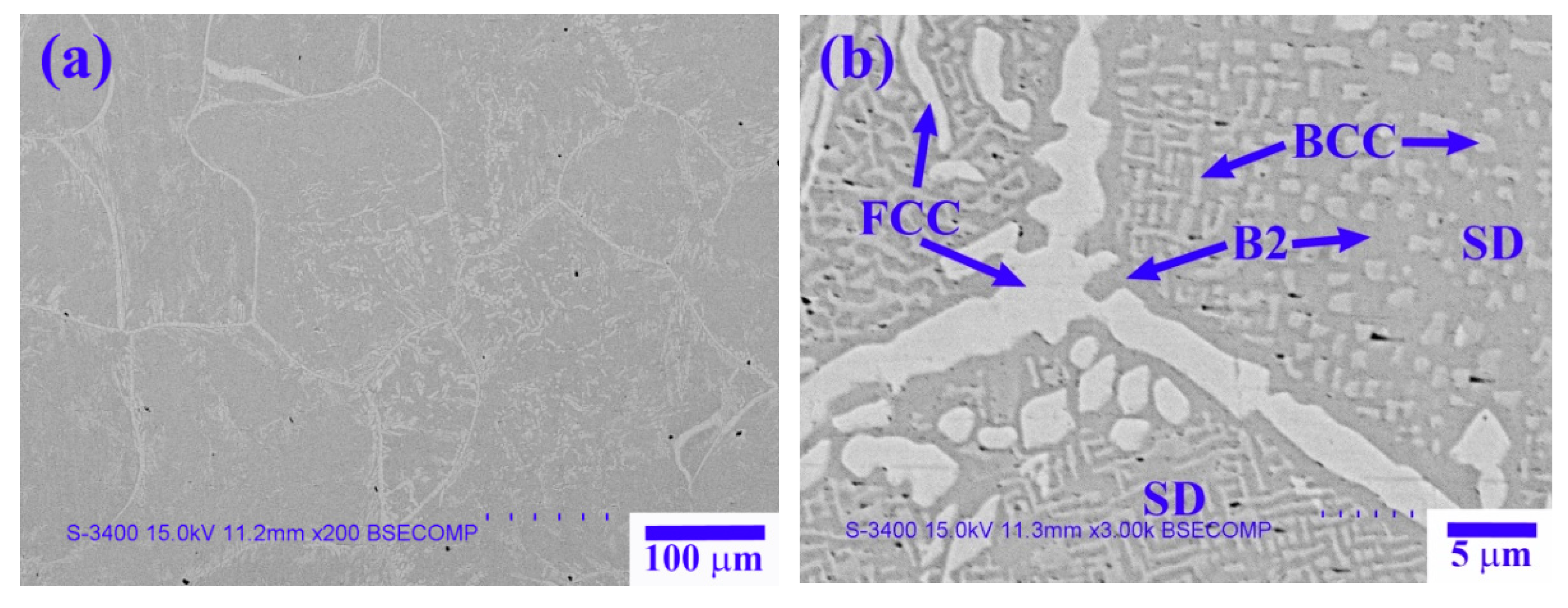
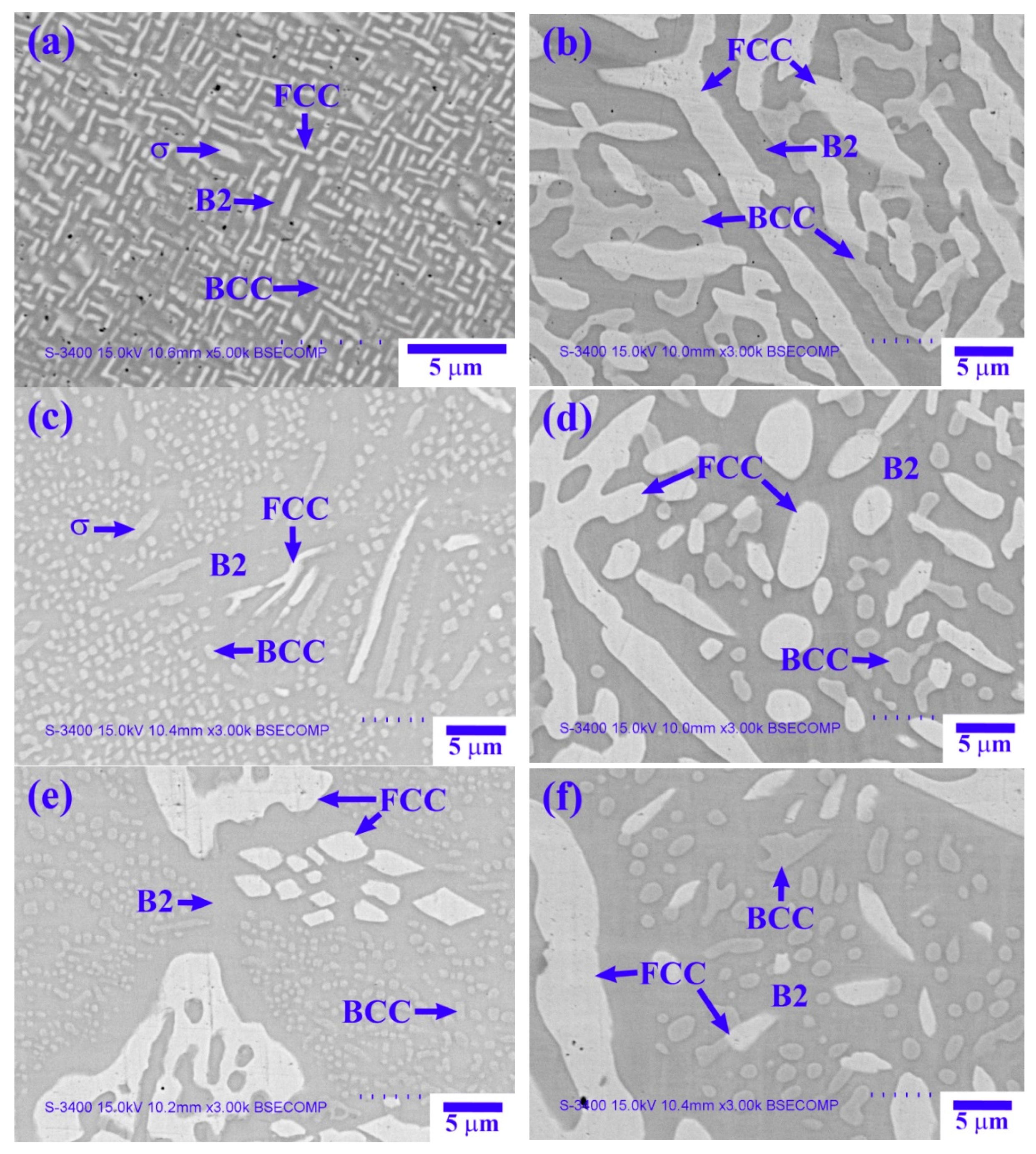
Figure 2 shows SEM-backscattered electron (BSE) images of the X
0 as-cast alloy.
Table 2 lists the chemical composition and volume fraction of the interior phases in
Figure 2, as analyzed by EDS and image analysis, respectively. The low-magnification image shown in
Figure 2a reveals the dendrite cell structure of the X
0 as-cast alloy, with a cell size of 100–250 µm.
Figure 2b shows the high-magnification image of the dendrite region, which has a spinodal decomposition (SD) structure comprising a dark-gray (Ni,Al)-rich matrix + light-gray wall-shaped (Cr,Fe)-rich phase [
13], as well as a bright wall-shaped (Fe,Cr,Co)-rich phase. The interdendrite region comprises a dark-gray (Ni,Al)-rich matrix and a bright boundary-like (Fe,Cr,Co)-rich phase (
Figure 2c).
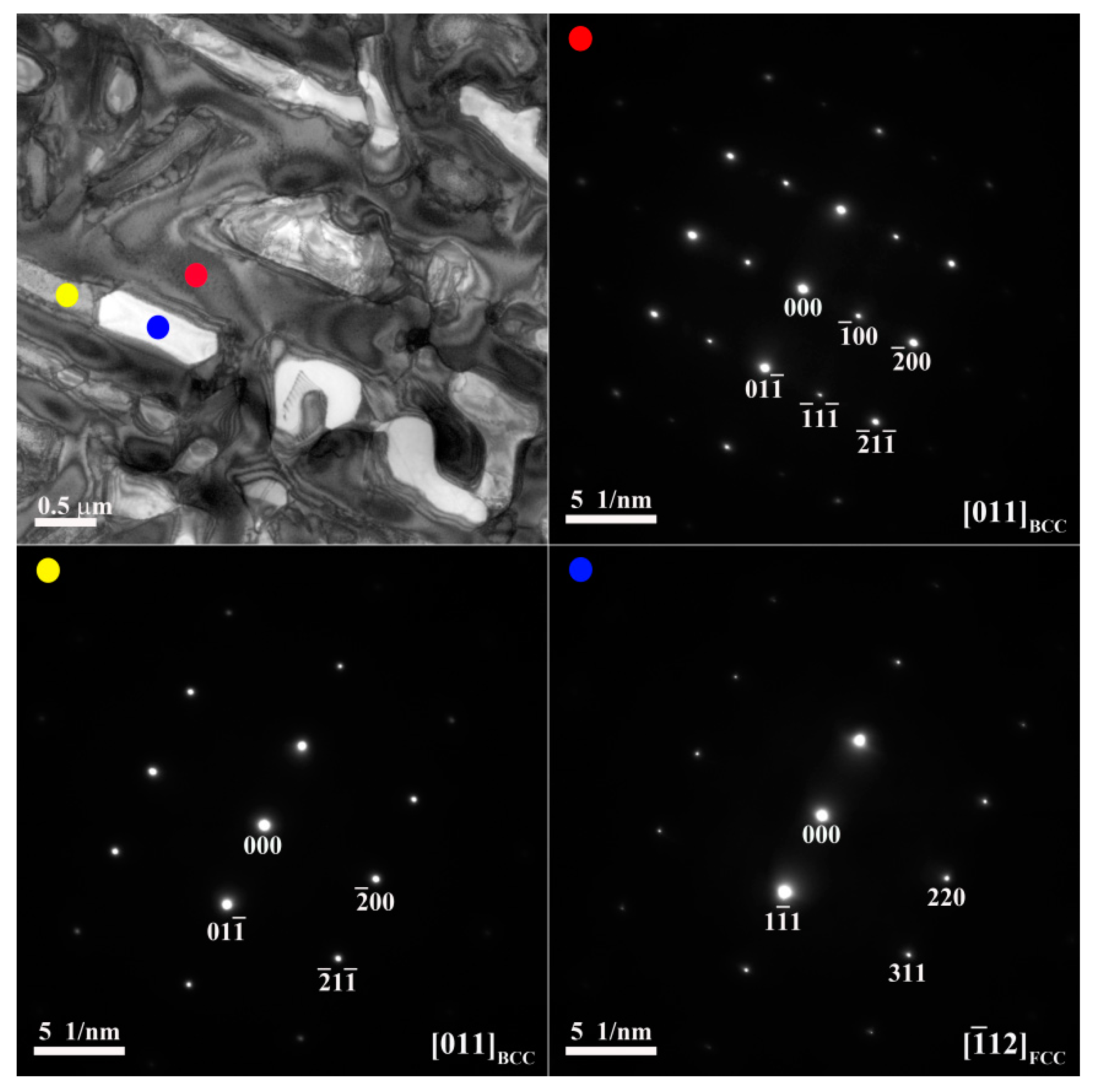
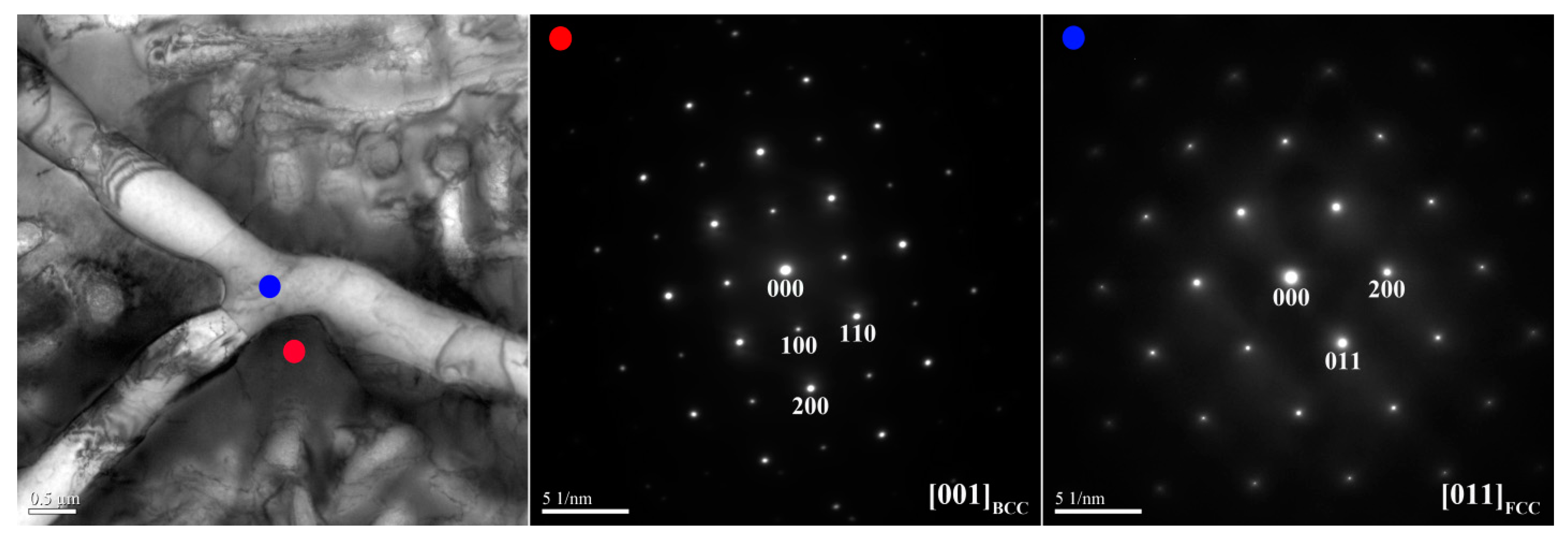
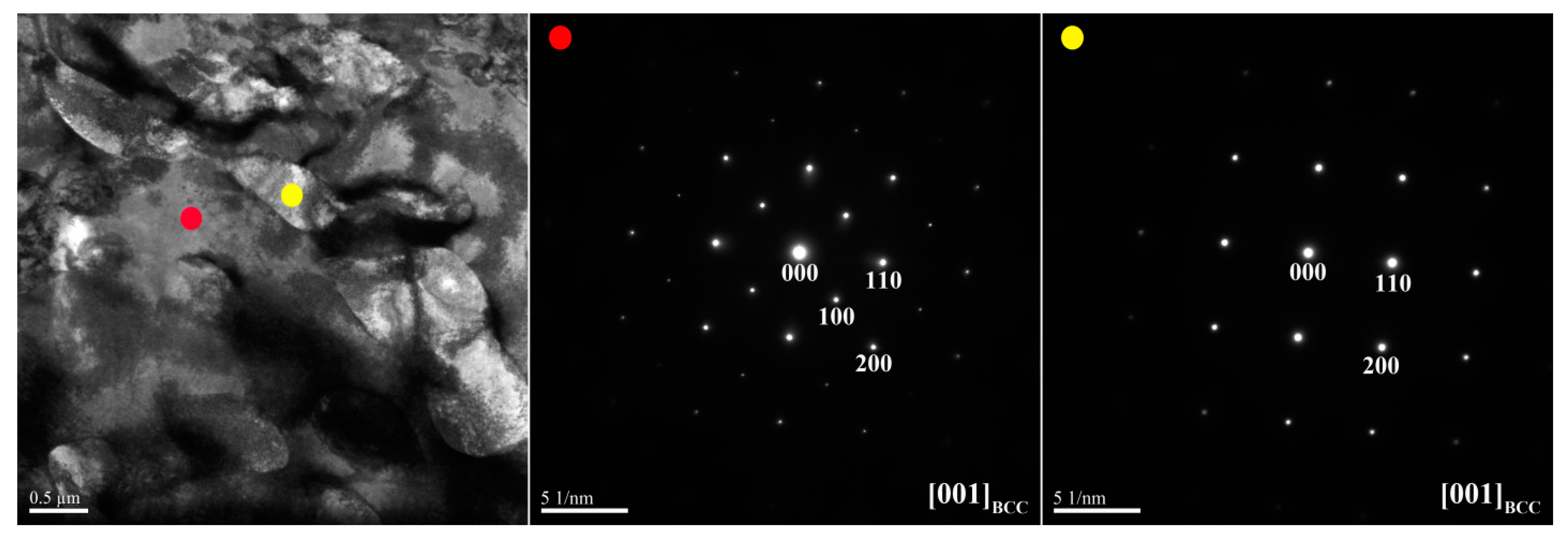
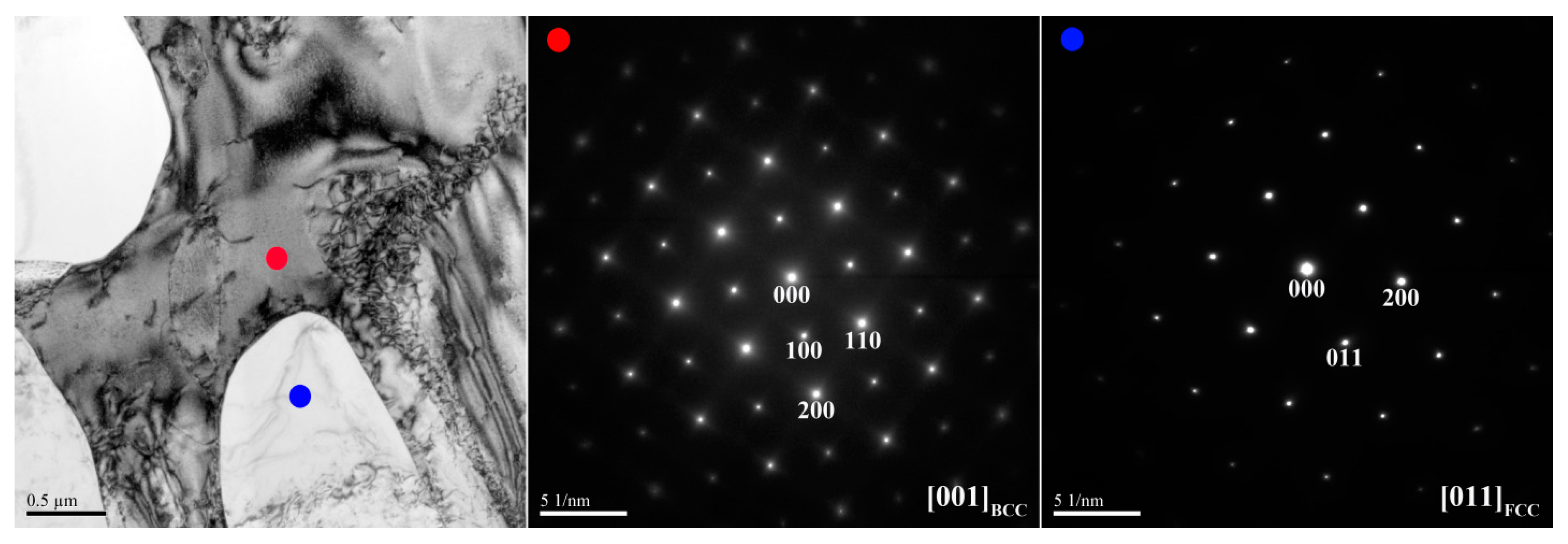
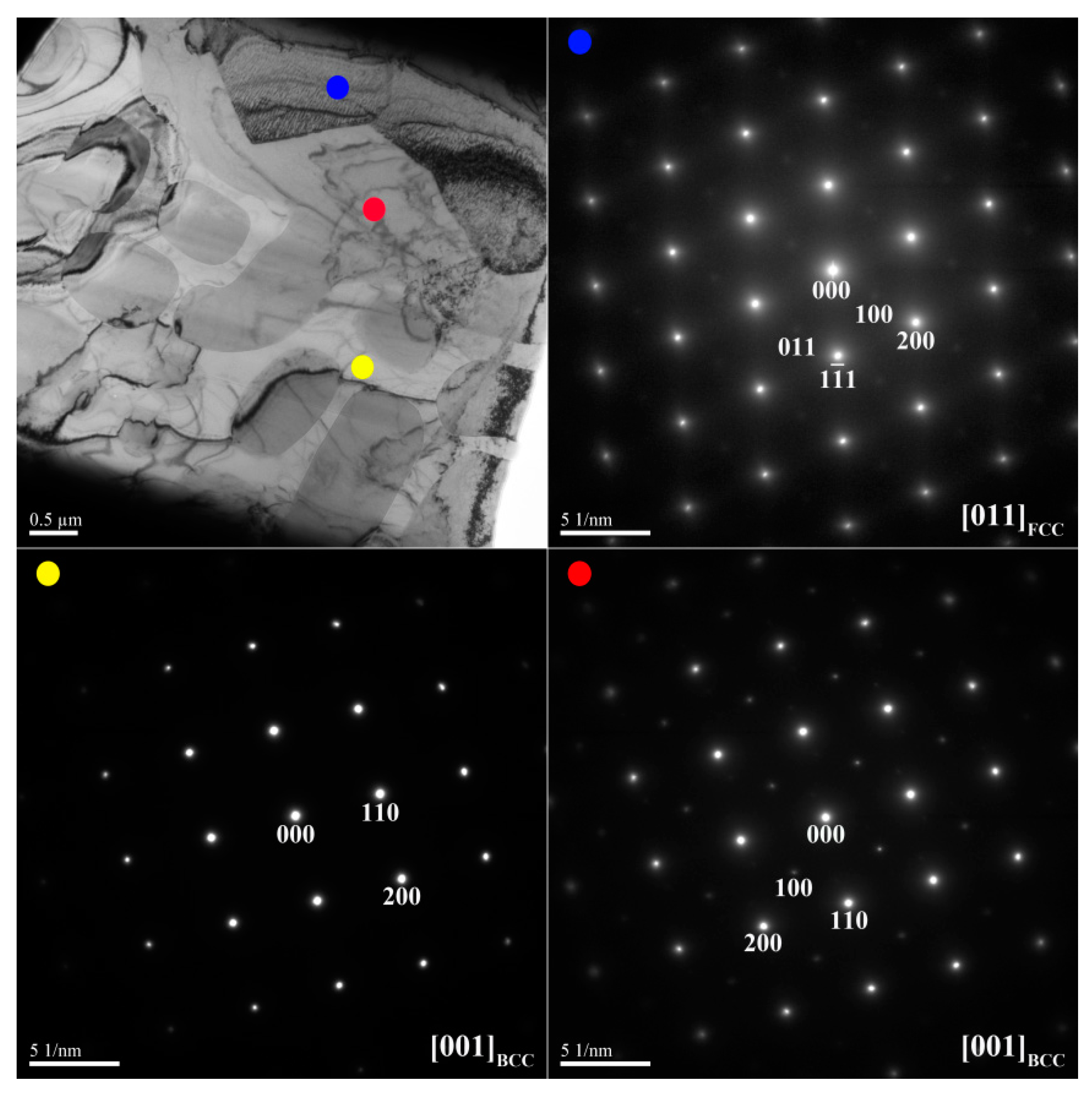
Figure 3 and
Figure 4 show TEM bright-field images and selected-area electron diffraction (SAED) patterns of the dendrite and interdendrite in the X
0 alloy, respectively.
Table 3 lists chemical compositions of the interior phases marked in
Figure 3 and
Figure 4. The SAED and EDS analysis revealed the presence of the dark-gray (Al,Ni)-rich ordered BCC (B2) phase, light-gray wall-shaped (Cr,Fe)-rich BCC phase, and bright, wall-shaped (Fe,Cr,Co)-rich FCC phase (
Figure 3 and
Figure 4). Notably, in the dendrite region, the BCC (yellow dot) and FCC (blue dot) phases formed in one wall-shaped structure (
Figure 3), indicating that the FCC phase transformed from the BCC phase because both phases are rich in Fe, Cr, and Co, and are deficient in Ni and Al.
Figure 5 shows SEM-BSE images of the X
0.5 as-cast alloy, and
Table 4 lists the chemical composition and volume fraction of the interior phases.
Figure 5a shows the microstructure of the X
0.5 as-cast alloy: a dendritic cell structure with a cell size of 120–300 µm is observed. The high-magnification image shown in
Figure 5b of the dendrite region indicated that it was constructed by an SD structure (dark-gray (Ni,Al)-rich B2 matrix + light-gray wall-shaped or spherical (Cr,Fe)-rich BCC phase) and a bright rod-like (Fe,Cr,Ni)-rich FCC phase. The interdendrite region comprises the dark-gray (Ni, Al)-rich B2 matrix and a bright boundary-like (Fe,Cr,Ni)-rich FCC phase (
Figure 5b). Compared with the X
0 as-cast alloy, the wall-shaped BCC phase exhibits spheroidization in the center region of the dendrite, and the FCC phase coarsens to a rod-like structure. In the interdendrite, the FCC phase is branched into the dendrite region. In addition, the replacement of Co with Ni permits the transformation of the (Fe,Cr,Co)-rich FCC phase in the X
0 alloy to the (Fe,Cr,Ni)-rich phase in the X
0.5 alloy.
Figure 6 and
Figure 7 show TEM bright-field images and SAED patterns of the X
0.5 as-cast alloy, respectively.
Table 5 lists the chemical composition of interior phases marked in
Figure 6 and
Figure 7.
Figure 6 shows the SD structure in the center region of the dendrite. This structure is a combination of the (Ni,Al)-rich B2 matrix and (Cr,Fe)-rich BCC phase.
Figure 7 shows the assembly of the interdendrite by the (Ni,Al)-rich B2 matrix and the (Fe,Cr,Ni)-rich FCC phase.
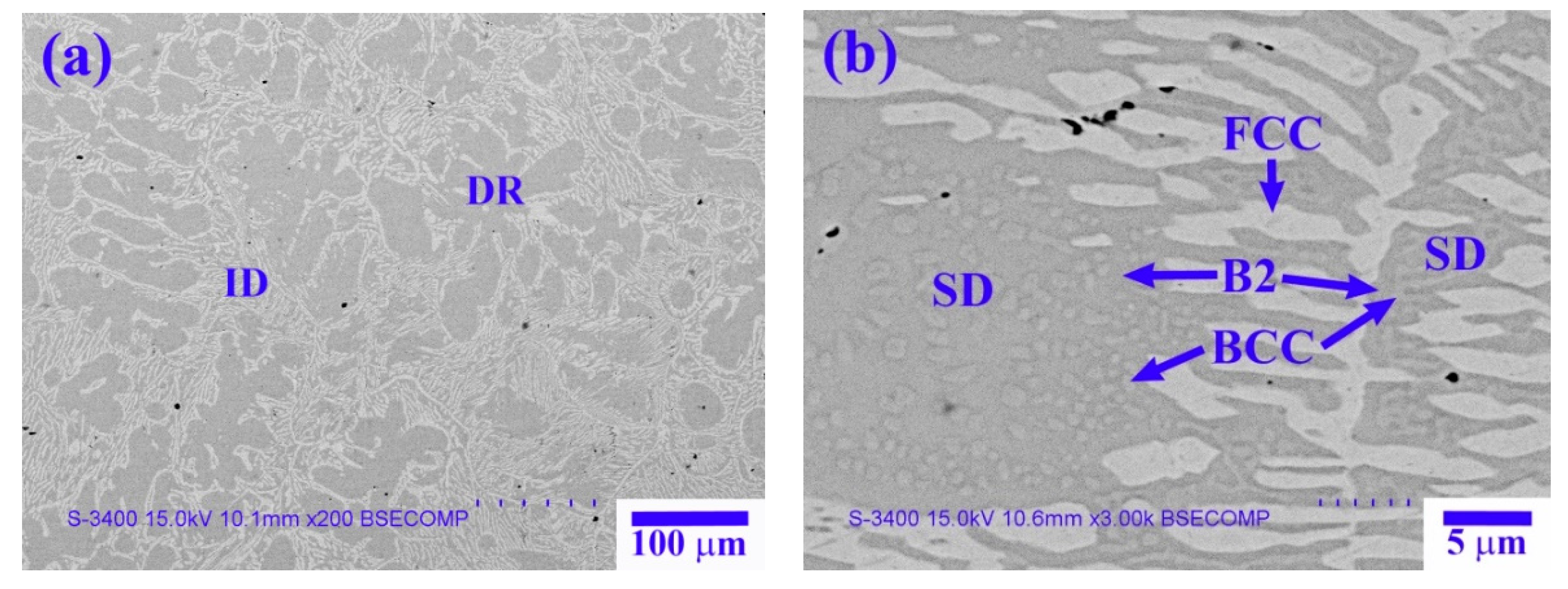
Figure 8 shows the microstructure of the X
1 as-cast alloy, and
Table 6 lists the chemical composition and volume fraction of the interior phases marked in
Figure 8. The X
1 as-cast alloy exhibited a dendritic structure (
Figure 8a). The high-magnification image of the SD structure shown in
Figure 8b reveals a dendrite region (DR) comprising a dark-gray (Ni,Al)-rich B2 matrix and a light-gray spherical (Cr,Fe)-rich BCC phase. The interdendrite (ID) region comprises a lamellar (Ni,Fe,Cr)-rich FCC phase and an SD structure between the FCC phase.
Figure 9 and
Figure 10 show TEM bright-field images and SAED patterns, respectively, of the X
1 as-cast alloy.
Table 7 lists chemical composition of the interior phases marked in
Figure 9 and
Figure 10. The SAED patterns and EDS results shown in
Figure 9 and
Figure 10 confirm that the dendrite comprises (Ni,Al)-rich B2 and (Cr,Fe)-rich BCC phases. The interdendrite comprises (Ni,Al)-rich B2 and (Ni,Fe,Cr)-rich FCC phases.
Comparison of the X
0.5 and X
1 as-cast alloys revealed that the chemical composition of the FCC phase in the interdendrite transformed from the (Fe,Cr,Ni)-rich phase in the X
0.5 alloy to the (Ni,Fe,Cr)-rich phase in the X
1 alloy because of the complete substitution of Co with Ni. Furthermore, the SAED pattern of the (Ni,Fe,Cr)-rich FCC phase shown in
Figure 10 reveals an ordered phase. With respect to the phase transformation theory [
22], in systems with strong chemical bonding between the atoms, ordered phases tend to be formed. The negative mixing enthalpy (ΔH
mix) between Ni and the other constitutional elements is thought to increase in comparison with that between those elements and Co [
23]; hence, the number of stronger Ni–Al, Ni–Cr, and Ni–Fe bonds increases, also further increasing with the substitution of Co with Ni in the alloy. Hence, the X
1 alloy exhibited an ordered FCC phase. The volume fractions of the FCC phase in X
0, X
0.5, and X
1 alloys calculated by image analysis were close to the results calculated by XRD peaks.
3.1.3. Mechanical Properties
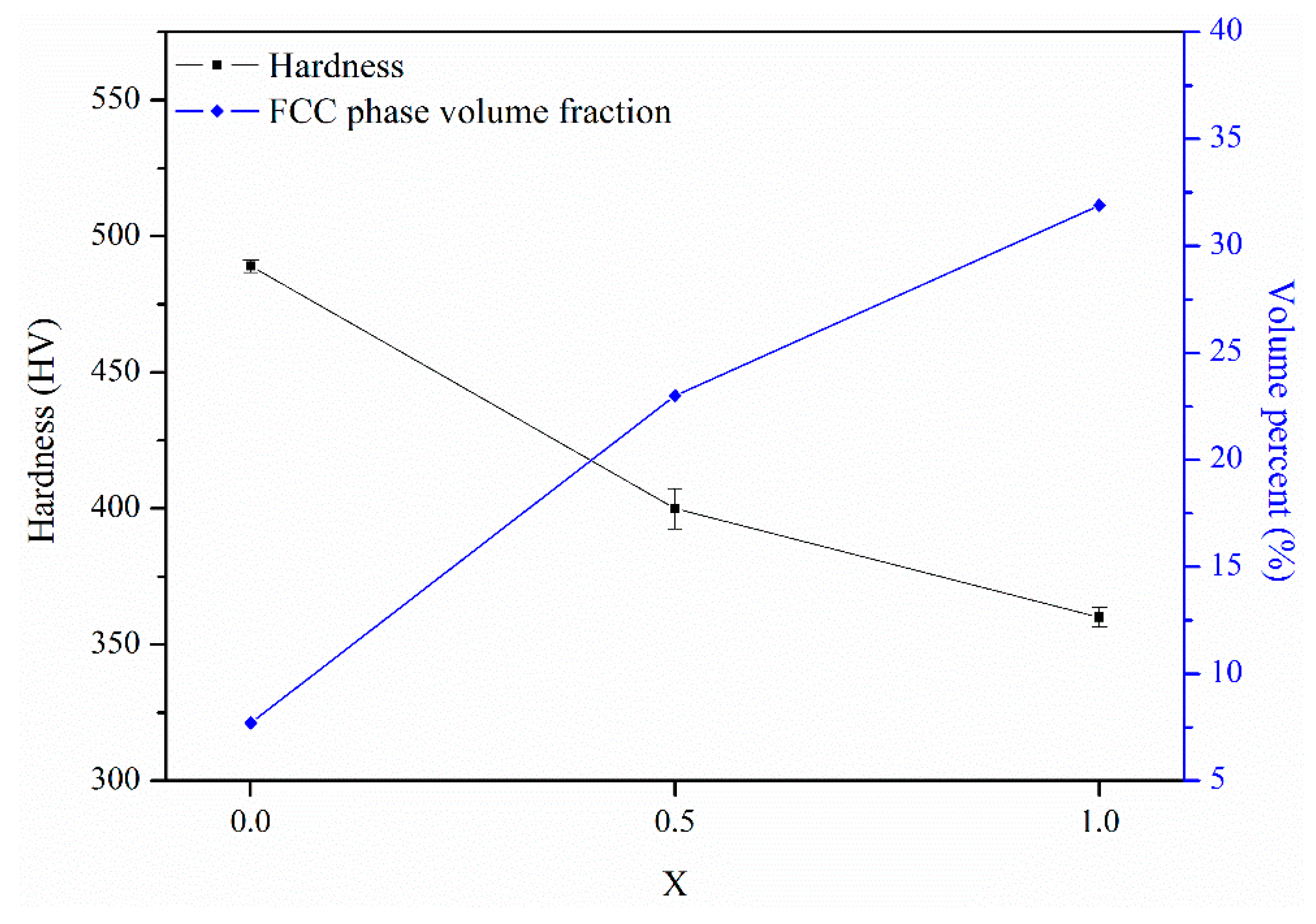
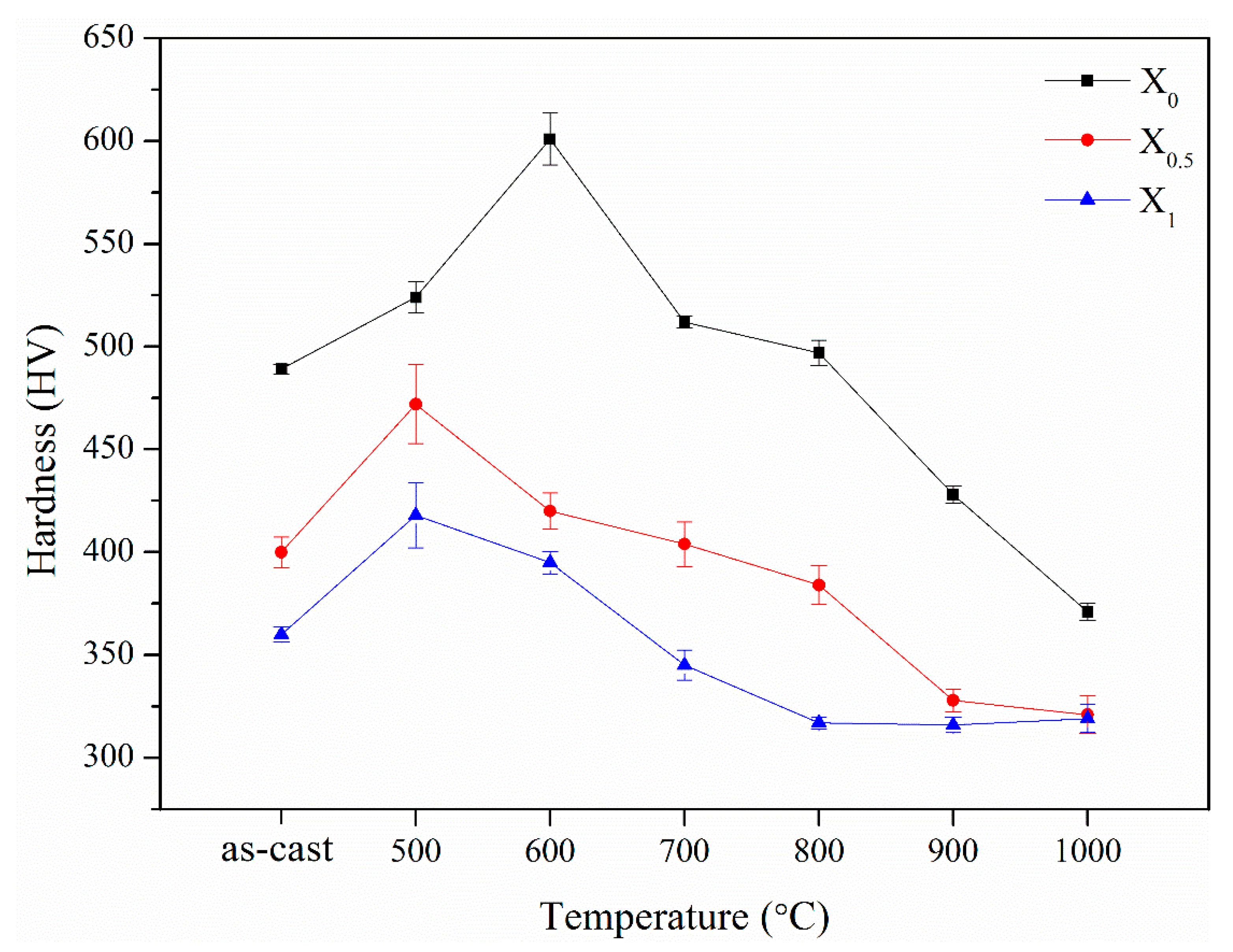
Figure 11 shows variations in the hardness and FCC phase volume fraction for the X
0, X
0.5, and X
1 as-cast alloys. From the substitution of Co with Ni, the hardness decreased by 25% from HV480 to HV360. Meanwhile, the FCC phase volume fraction increased from 7.7 to 31.9%. The decrease in the hardness was caused by the increase in the volume fraction of the soft FCC phase. From a previous report [
13], the hardness of as-cast AlCoCrFeNi alloy is HV484, which is close to the present X
0 alloy.
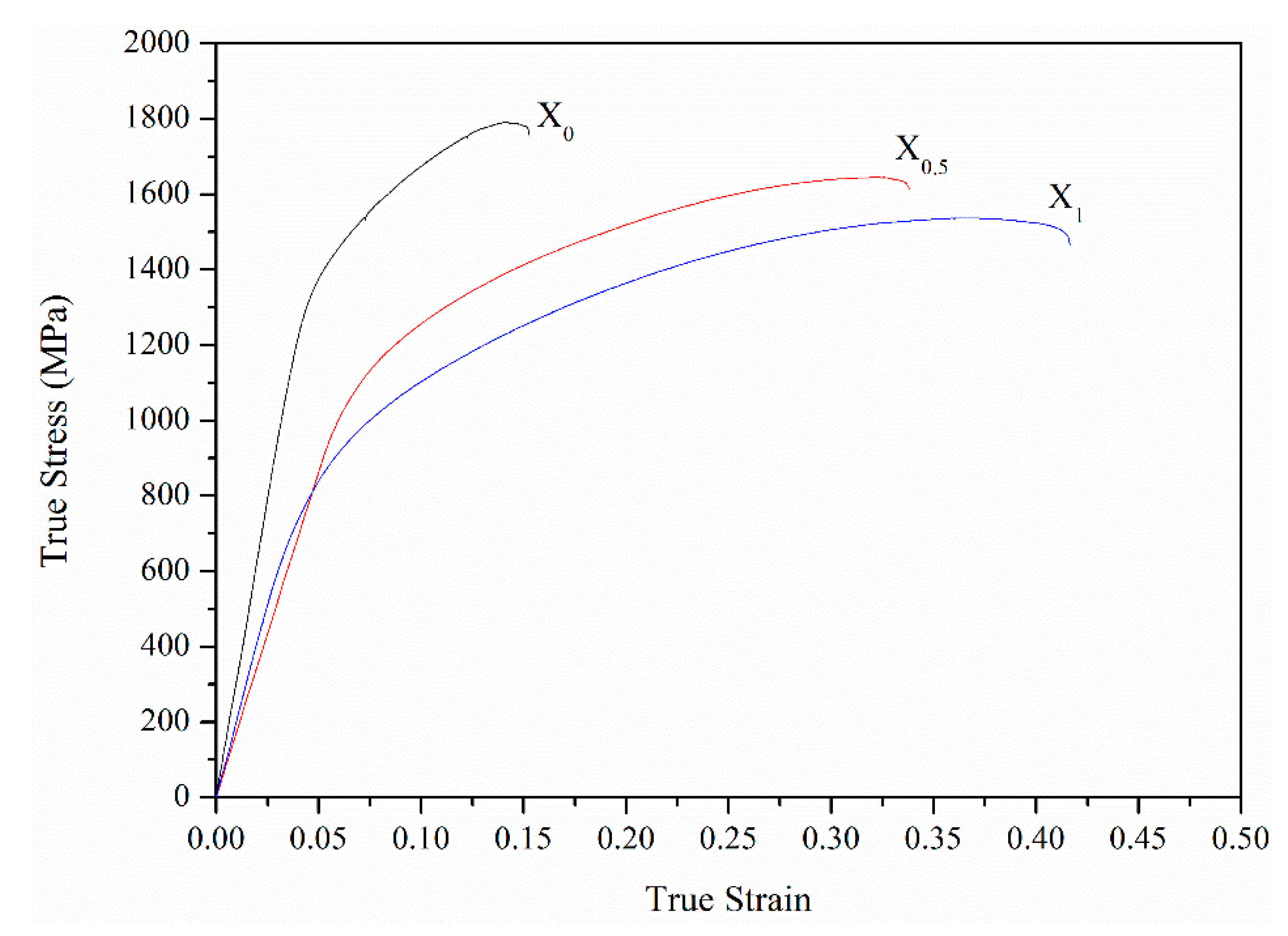
Figure 12 shows compressive true stress–strain curves of the X
0, X
0.5, and X
1 as-cast alloys, and
Table 8 lists yield stress (σ
y), compressive stress (σ
max), and fracture strain (ε
f) of the three alloys. From X
0 to X
1 alloys, the σ
y and σ
max decreased from 1202 and 1790 MPa to 693 and 1537 MPa, respectively. However, ε
f increased from 0.15 to 0.42. This result is also related to the increase in the amount of the soft FCC phase. Notably, from the X
0 to X
1 alloy, σ
max slightly decreased by 14%, but ε
f considerably increased by 180%, indicating that the substitution of Co with Ni can effectively improve the strength-ductility balance. Comparing the compressive properties of the X
0 alloy with AlCrFeCoNi alloy mentioned in the Introduction, the X
0 alloy exhibited lower σ
max and ε
f. We suggest that the worse cleanliness of the present alloy synthesized by the vacuum induction melting process under Al
2O
3 crucible than previously published AlCrFeCoNi alloy [
15] synthesized by vacuum arc re-melting process is the main reason.
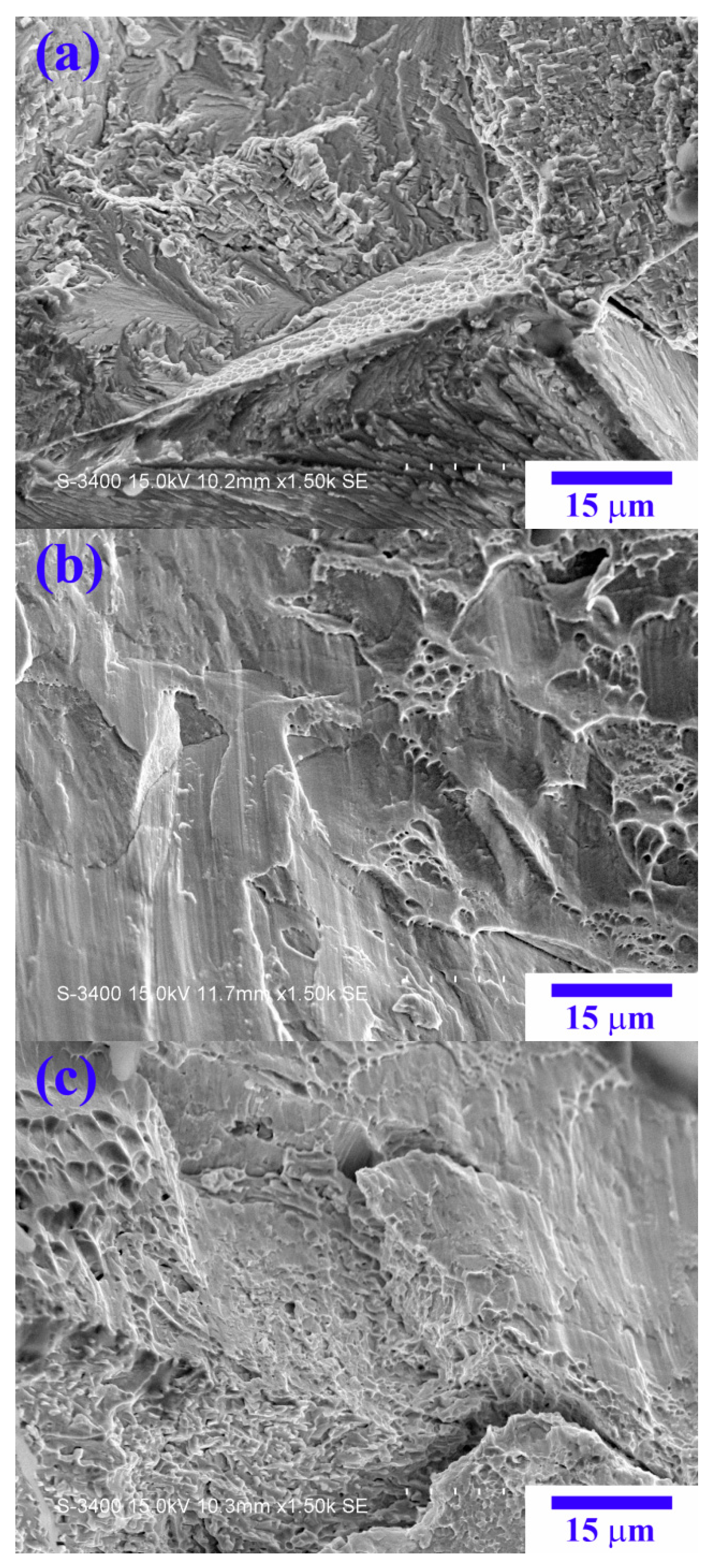
Figure 13 shows SEM images of the fracture surfaces after compression. From the X
0 to X
1 alloy, the amounts of the dimple structure clearly increase, indicative of the increase in the alloy ductility by the replacement of Co by Ni.