High-Temperature Oxidation of High-Entropic Alloys: A Review
Abstract
1. Introduction
- (1)
- metals of the Fe subgroup and similar transition metals—Fe, Co, Ni, Cr, Mn, Cu;
- (2)
- transition refractory metals—Ti, Zr, Hf, Nb, Ta, Mo, W;
- (3)
- platinoids and Au;
- (4)
- light elements—Al, Li, Sc, Mg, Ti, Si;
- (5)
- lanthanide alloys.
2. Al-Co-Cr-Fe-Ni System
3. Mn-Co-Cr-Fe-Ni System
4. High-Entropy Alloys from Refractory Metals
5. HEAs Oxidation Products
6. Conclusions
- (1)
- Promising heat-resistant and corrosion-resistant HEAs can be found in alloys of the Al-Co-Cr-Fe-Ni system. The addition of other elements to this base alloy has a different effect on its properties: additions of Si, Ti, Y and Hf will increase the resistance of such alloys to high-temperature gas corrosion; Mo additions have a negative effect on corrosion resistance, since Mo oxides are characterized by low evaporation temperatures a and even at low concentrations of Mo, the presence of Mo oxides in the oxide layer can lead to a violation of the integrity of the scale and make it loose and porous; the introduction of Zr and Ta is not fully understood; the effect of Cu additions also requires additional research, but it is already obvious that at sufficiently high Cu concentrations (when its amount is comparable to the amounts of other elements forming a multicomponent base), the adhesion of the formed oxide layer with respect to the matrix surface is low; such scale peels off and breaks easily.
- (2)
- HEAs of the Mn-Co-Cr-Fe-Ni system are less resistant to high-temperature gas corrosion than alloys without Mn. The mechanism of oxidation of such alloys still needs to be studied; however, it is known that a multiphase, discontinuous scale is formed on such alloys, while the scale layers containing Mn oxide are porous, peel off easily, and do not interfere with the further oxidation of the alloy.
- (3)
- Studies of the resistance to high-temperature corrosion of HEAs based on refractory metals are contradictory. According to the literature, the alloys most resistant to high-temperature oxidation are the HEAs of the Al-Cr-Mo-Ta-Ti system. In the presence of Ta, Mo does not participate in the oxidation process and, therefore, there is no Mo oxide in the scale. In the absence of Al, oxides of refractory metals do not have sufficient protective properties.
- (4)
- The most important elements that contribute to an increase in the resistance of HEAs to oxidation are Al and Cr. Continuous films of Al2O3 or Cr2O3 with high adhesion to the alloy can be formed, which prevent the directional diffusion of oxygen into the alloy matrix. These same elements can react with other elements in the HEA (for example, Fe and Ni), which can lead to the formation of spinel films, which also increase resistance to oxidation. Elements such as Si and Y can also improve oxidation resistance.
- (5)
- The presence of refractory elements, such as W, Mo, Ta, Nb, V, Ti, Zr and Hf, can increase the strength of HEAs at high temperatures, but some of these elements (primarily Mo, W, and V) can accelerate the oxidation of alloys, primarily due to the formation of volatile oxides.
- (6)
- When Ti is one of the main elements of an alloy, it plays an important role in the formation of protective layers from CrTaO4, TiO2, Ti3O5 and ZrO2 oxides, while simultaneously reducing the number of oxides that reduce heat resistance (Nb2O5, Ta2O5).
- (7)
- The creation of alloys based on the basic composition Al-Co-Cr-Fe-Ni with the addition of refractory elements (for example, the combination of tantalum with molybdenum, hafnium or zirconium) is perspective trend for investigators.
- (8)
- Several gaps can be noted in the study of high-temperature oxidation of HEAs:
- (8.1)
- Thermodynamic modeling tools (for example, CALPHAD) are practically not used to predict high-temperature oxidation processes. The use of thermodynamic modeling techniques would optimize the search for compositions of corrosion-resistant HEAs.
- (8.2)
- All studies of high-temperature oxidation of HEAs are empirical. In the literature, there are no theoretical data on the mechanism of the formation of protective films on HEAs and the effect of additional elements on such mechanisms.
- (8.3)
- For the study of high-temperature gas corrosion HEAs, the authors of the presented articles proposed only one method - oxidation in air. However, for practical use, it would be beneficial to conduct tests in atmospheres of H2/H2O (water vapor), SO2/SO3 (typical for the oil and gas industry) or CO/CO2 (when engines are running in aircraft and rocketry mechanisms).
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yeh, J.-W.; Chen, S.-K.; Lin, S.-J.; Gan, J.-Y.; Chin, T.-S.; Shun, T.-T.; Tsau, C.-H.; Chang, S.-Y. Nanostructured High-Entropy Alloys with Multiple Principal Elements: Novel Alloy Design Concepts and Outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Yeh, J.-W.; Lin, S.-J.; Chin, T.-S.; Gan, J.-Y.; Chen, S.-K.; Shun, T.-T.; Tsau, C.-H.; Chou, S.-Y. Formation of simple crystal structures in Cu-Co-Ni-Cr-Al-Fe-Ti-V alloys with multiprincipal metallic elements. Met. Mater. Trans. A 2004, 35, 2533–2536. [Google Scholar] [CrossRef]
- Yeh, J.W.; Chen, Y.L.; Lin, S.J.; Chen, S.K. High-Entropy Alloys—A New Era of Exploitation. Mater. Sci. Forum 2007, 560, 1–9. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, Y.; Wang, F.; Wang, Y.; Chen, G. Effect of Cu addition on the microstructure and mechanical properties of AlCoCrFeNiTi0.5 solid-solution alloy. J. Alloys Compd. 2008, 466, 201–204. [Google Scholar] [CrossRef]
- Chen, Y.; Duval, T.; Hung, U.; Yeh, J.; Shih, H. Microstructure and electrochemical properties of high entropy alloys—A comparison with type-304 stainless steel. Corros. Sci. 2005, 47, 2257–2279. [Google Scholar] [CrossRef]
- Huang, Y.-S.; Chen, L.; Lui, H.-W.; Cai, M.-H.; Yeh, J.-W. Microstructure, hardness, resistivity and thermal stability of sputtered oxide films of AlCoCrCu0.5NiFe high-entropy alloy. Mater. Sci. Eng. A 2007, 457, 77–83. [Google Scholar] [CrossRef]
- Murty, B.S.; Yeh, J.-W.; Ranganathan, S.; Bhattacharjee, P.P. High-Entropy Alloys, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2019; p. 388. [Google Scholar]
- Gao, C.M.; Yeh, J.W.; Liaw, P.K.; Zhang, Y. High-Entropy Alloys: Fundamentals and Applications; Springer: Berlin/Heidelberg, Germany, 2016; p. 516. [Google Scholar] [CrossRef]
- Shi, Y.; Yang, B.; Liaw, P.K. Corrosion-resistant high-entropy alloys: A review. Metals 2017, 7, 43. [Google Scholar] [CrossRef]
- Miracle, D.; Senkov, O. A critical review of high entropy alloys and related concepts. Acta. Mater. 2017, 122, 448–511. [Google Scholar] [CrossRef]
- Moghaddam, A.O.; Shaburova, N.A.; Samodurova, M.N.; Abdollahzadeh, A.; Trofimov, E.A. Additive manufacturing of high entropy alloys: A practical review. J. Mater. Sci. Technol. 2021, 77, 131–162. [Google Scholar] [CrossRef]
- Moghaddam, A.O.; Trofimov, E.A. Toward expanding the realm of high entropy materials to platinum group metals: A review. J. Alloys Compd. 2021, 851, 156838. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, X.; Wang, W.; Liu, B.; Lv, Y.; Yang, W.; Xu, D.; Liu, Y. A review on fundamental of high entropy alloys with promising high–temperature properties. J. Alloys Compd. 2018, 760, 15–30. [Google Scholar] [CrossRef]
- Saunders, S.; Nicholls, J. Oxidation, Hot Corrosion and Protection of Metallic Materials. Physical Metallurgy, 4th ed.; Cahn, R.W., Hassen, P., Eds.; Elsevier Science: Amsterdam, The Netherlands, 1996; Chapter 14; pp. 1291–1361. [Google Scholar] [CrossRef]
- Stratton, P. Ellingham diagrams—Their use and misuse. Int. Heat Treat. Surf. Eng. 2013, 7, 70–73. [Google Scholar] [CrossRef]
- Xu, C.; Gao, W. Pilling-Bedworth ratio for oxidation of alloys. Mater. Res. Innov. 2000, 3, 231–235. [Google Scholar] [CrossRef]
- Azevedo, C. Selection of fuel cladding material for nuclear fission reactors. Eng. Fail. Anal. 2011, 18, 1943–1962. [Google Scholar] [CrossRef]
- Knaster, J.; Moeslang, A.; Muroga, T. Materials research for fusion. Nat. Phys. 2016, 12, 424–434. [Google Scholar] [CrossRef]
- Was, G.; Petti, D.; Ukai, S.; Zinkle, S. Materials for future nuclear energy systems. J. Nucl. Mater. 2019, 527, 151837. [Google Scholar] [CrossRef]
- Moghaddam, A.O.; Cabot, A.; Trofimov, E.A. Does the pathway for development of next generation nuclear materials straightly go through high-entropy materials? Int. J. Refract. Met. Hard Mater. 2021, 97, 105504. [Google Scholar] [CrossRef]
- Sims, C.T.; Hagel, W.C. (Eds.) The Superalloys; Wiley Interscience: Hoboken, NJ, USA, 1972; pp. 145–174. [Google Scholar]
- Beke, D.; Erdélyi, G. On the diffusion in high-entropy alloys. Mater. Lett. 2016, 164, 111–113. [Google Scholar] [CrossRef]
- Zhang, Y.; Zuo, T.T.; Tang, Z.; Gao, M.C.; Dahmen, K.A.; Liaw, P.K.; Lu, Z.P. Microstructures and properties of high-entropy alloys. Prog. Mater. Sci. 2014, 61, 1–93. [Google Scholar] [CrossRef]
- Tsai, K.-Y.; Tsai, M.-H.; Yeh, J.-W. Sluggish diffusion in Co–Cr–Fe–Mn–Ni high-entropy alloys. Acta Mater. 2013, 61, 4887–4897. [Google Scholar] [CrossRef]
- Huang, P.-K.; Yeh, J.-W.; Shun, T.-T.; Chen, S.-K. Multi-Principal-Element Alloys with Improved Oxidation and Wear Resistance for Thermal Spray Coating. Adv. Eng. Mater. 2004, 6, 74–78. [Google Scholar] [CrossRef]
- Liu, C.; Wang, H.; Zhang, S.; Tang, H.; Zhang, A. Microstructure and oxidation behavior of new refractory high entropy alloys. J. Alloys Compd. 2014, 583, 162–169. [Google Scholar] [CrossRef]
- Liu, Y.-X.; Cheng, C.-Q.; Shang, J.-L.; Wang, R.; Li, P.; Zhao, J. Oxidation behavior of high-entropy alloys AlxCoCrFeNi (x = 0.15, 0.4) in supercritical water and comparison with HR3C steel. Trans. Nonferr. Met. Soc. China 2015, 25, 1341–1351. [Google Scholar] [CrossRef]
- Lu, J.; Chen, Y.; Zhang, H.; Ni, N.; Li, L.; He, L.; Mu, R.; Zhao, X.; Guo, F. Y/Hf-doped AlCoCrFeNi high-entropy alloy with ultra oxidation and spallation resistance. Corros. Sci. 2020, 166, 108426. [Google Scholar] [CrossRef]
- Butler, T.M.; Weaver, M.L. Oxidation behavior of arc melted AlCoCrFeNi multi-component high-entropy alloys. J. Alloys Compd. 2016, 674, 229–244. [Google Scholar] [CrossRef]
- Butler, T.M.; Weaver, M.L. Influence of Annealing on the Microstructures and Oxidation Behaviors of Al8(CoCrFeNi)92, Al15(CoCrFeNi)85, and Al30(CoCrFeNi)70 High-Entropy Alloys. Metals 2016, 6, 222. [Google Scholar] [CrossRef]
- Butler, T.M.; Alfano, J.P.; Martens, R.L.; Weaver, M.L. High-Temperature Oxidation Behavior of Al-Co-Cr-Ni-(Fe or Si) Multicomponent High-Entropy Alloys. JOM 2015, 67, 246–259. [Google Scholar] [CrossRef]
- Dąbrowa, J.; Cieślak, G.; Stygar, M.; Mroczka, K.; Berent, K.; Kulik, T.; Danielewski, M. Influence of Cu content on high temperature oxidation behavior of AlCoCrCuxFeNi high entropy alloys (x = 0; 0.5; 1). Intermetallics 2017, 84, 52–61. [Google Scholar] [CrossRef]
- Daoud, H.M.; Manzoni, A.M.; Völkl, R.; Wanderka, N.; Glatzel, U. Oxidation Behavior of Al8Co17Cr17Cu8Fe17Ni33, Al23Co15Cr23Cu8Fe15Ni15, and Al17Co17Cr17Cu17Fe17Ni17 Compositionally Complex Alloys (High-Entropy Alloys) at Elevated Temperatures in Air. Adv. Eng. Mater. 2015, 17, 1134–1141. [Google Scholar] [CrossRef]
- Bartova, B.; Wiese, N.; Schryvers, D.; Chapman, J.; Ignacová, S. Microstructure of precipitates and magnetic domain structure in an annealed Co38Ni33Al29 shape memory alloy. Acta Mater. 2008, 56, 4470–4476. [Google Scholar] [CrossRef]
- Karaca, H.; Karaman, I.; Lagoudas, D.; Maier, H.; Chumlyakov, Y. Recoverable stress-induced martensitic transformation in a ferromagnetic CoNiAl alloy. Scr. Mater. 2003, 49, 831–836. [Google Scholar] [CrossRef]
- Pint, B.A. Optimization of Reactive-Element Additions to Improve Oxidation Performance of Alumina-Forming Alloys. J. Am. Ceram. Soc. 2003, 86, 686–695. [Google Scholar] [CrossRef]
- Nychka, J.A.; Clarke, D.R. Quantification of Aluminum Outward Diffusion during Oxidation of FeCrAl Alloys. Oxid. Met. 2005, 63, 325–352. [Google Scholar] [CrossRef]
- Zhao, C.; Zhou, Y.; Zou, Z.; Luo, L.; Zhao, X.; Guo, F.; Xiao, P. Effect of alloyed Lu, Hf and Cr on the oxidation and spallation behavior of NiAl. Corros. Sci. 2017, 126, 334–343. [Google Scholar] [CrossRef]
- Pint, B.; More, K.; Wright, I. Effect of Quaternary Additions on the Oxidation Behavior of Hf-Doped NiAl. Oxid. Met. 2003, 59, 257–283. [Google Scholar] [CrossRef]
- Pint, B.A. Experimental observations in support of the dynamic-segregation theory to explain the reactive-element effect. Oxid. Met. 1996, 45, 1–37. [Google Scholar] [CrossRef]
- Whittle, D.P.; Stringer, J. Improvements in high temperature oxidation resistance by additions of reactive elements or oxide dispersions. Philos. Trans. R. Soc. London. Ser. A Math. Phys. Sci. 1980, 295, 309–329. [Google Scholar] [CrossRef]
- Holcomb, G.R.; Tylczak, J.H.; Carney, C.S. Oxidation of CoCrFeMnNi High Entropy Alloys. JOM 2015, 67, 2326–2339. [Google Scholar] [CrossRef]
- Laplanche, G.; Volkert, U.F.; Eggeler, G.; George, E.P. Oxidation Behavior of the CrMnFeCoNi High-Entropy Alloy. Oxid. Met. 2016, 85, 629–645. [Google Scholar] [CrossRef]
- Rist, A.; Ancey-Moret, M.F.; Gatellier, C.; Riboud, P.V. Élaboration de la Fonte et de l’Acier—Données Thermodynamiques; Techniques de l’Inge´Nieur: Saint-Denis, France, 2016. [Google Scholar]
- Wild, R. High temperature oxidation of austenitic stainless steel in low oxygen pressure. Corros. Sci. 1977, 17, 87–104. [Google Scholar] [CrossRef]
- Païdassi, J.; Echeverria, A. Sur 1’ oxydation du manganese dans l’air aux température élevées. Acta Met. 1959, 7, 293–295. [Google Scholar] [CrossRef]
- Senkov, O.; Wilks, G.; Miracle, D.; Chuang, C.; Liaw, P. Refractory high-entropy alloys. Intermetallics 2010, 18, 1758–1765. [Google Scholar] [CrossRef]
- Senkov, O.; Scott, J.; Senkova, S.; Miracle, D.; Woodward, C. Microstructure and room temperature properties of a high-entropy TaNbHfZrTi alloy. J. Alloys Compd. 2011, 509, 6043–6048. [Google Scholar] [CrossRef]
- Senkov, O.N.; Senkova, S.V.; Dimiduk, D.M.; Woodward, C.; Miracle, D.B. Oxidation behavior of a refractory NbCrMo0.5Ta0.5TiZr alloy. J. Mater. Sci. 2012, 47, 6522–6534. [Google Scholar] [CrossRef]
- Senkov, O.; Senkova, S.; Woodward, C.; Miracle, D. Low-density, refractory multi-principal element alloys of the Cr–Nb–Ti–V–Zr system: Microstructure and phase analysis. Acta Mater. 2013, 61, 1545–1557. [Google Scholar] [CrossRef]
- Senkov, O.N.; Woodward, C.; Miracle, D.B. Microstructure and Properties of Aluminum-Containing Refractory High-Entropy Alloys. JOM 2014, 66, 2030–2042. [Google Scholar] [CrossRef]
- Senkov, O.; Senkova, S.; Woodward, C. Effect of aluminum on the microstructure and properties of two refractory high-entropy alloys. Acta Mater. 2014, 68, 214–228. [Google Scholar] [CrossRef]
- Juan, C.-C.; Tsai, M.-H.; Tsai, C.-W.; Lin, C.-M.; Wang, W.-R.; Yang, C.-C.; Chen, S.-K.; Lin, S.-J.; Yeh, J.-W. Enhanced mechanical properties of HfMoTaTiZr and HfMoNbTaTiZr refractory high-entropy alloys. Intermetallics 2015, 62, 76–83. [Google Scholar] [CrossRef]
- Gorr, B.; Azim, M.; Christ, H.-J.; Mueller, T.; Schliephake, D.; Heilmaier, M. Phase equilibria, microstructure, and high temperature oxidation resistance of novel refractory high-entropy alloys. J. Alloys Compd. 2015, 624, 270–278. [Google Scholar] [CrossRef]
- Butler, T.; Chaput, K.; Dietrich, J.; Senkov, O. High temperature oxidation behaviors of equimolar NbTiZrV and NbTiZrCr refractory complex concentrated alloys (RCCAs). J. Alloys Compd. 2017, 729, 1004–1019. [Google Scholar] [CrossRef]
- Senkov, O.; Woodward, C. Microstructure and properties of a refractory NbCrMo0.5Ta0.5TiZr alloy. Mater. Sci. Eng. A 2011, 529, 311–320. [Google Scholar] [CrossRef]
- Chen, H.; Kauffmann, A.; Gorr, B.; Schliephake, D.; Seemüller, C.; Wagner, J.; Christ, H.-J.; Heilmaier, M. Microstructure and mechanical properties at elevated temperatures of a new Al-containing refractory high-entropy alloy Nb-Mo-Cr-Ti-Al. J. Alloys Compd. 2016, 661, 206–215. [Google Scholar] [CrossRef]
- Gorr, B.; Mueller, F.; Christ, H.-J.; Chen, H.; Kauffmann, A.; Heilmaier, M. High temperature oxidation behavior of an equimolar refractory metal-based alloy 20Nb 20Mo 20Cr 20Ti 20Al with and without Si addition. J. Alloys Compd. 2016, 688, 468–477. [Google Scholar] [CrossRef]
- Gorr, B.; Müller, F.; Azim, M.; Christ, H.-J.; Müller, T.; Chen, H.; Kauffmann, A.; Heilmaier, M. High-Temperature Oxidation Behavior of Refractory High-Entropy Alloys: Effect of Alloy Composition. Oxid. Met. 2017, 88, 339–349. [Google Scholar] [CrossRef]
- Sheikh, S.; Gan, L.; Tsao, T.-K.; Murakami, H.; Shafeie, S.; Guo, S. Aluminizing for enhanced oxidation resistance of ductile refractory high-entropy alloys. Intermetallics 2018, 103, 40–51. [Google Scholar] [CrossRef]
- Jayaraj, J.; Thirathipviwat, P.; Han, J.; Gebert, A. Microstructure, mechanical and thermal oxidation behavior of AlNbTiZr high entropy alloy. Intermetallics 2018, 100, 9–19. [Google Scholar] [CrossRef]
- Yurchenko, N.; Panina, E.; Zherebtsov, S.; Salishchev, G.; Stepanov, N. Oxidation Behavior of Refractory AlNbTiVZr0.25 High-Entropy Alloy. Materials 2018, 11, 2526. [Google Scholar] [CrossRef]
- Chang, C.-H.; Titus, M.S.; Yeh, J.-W. Oxidation Behavior between 700 and 1300 °C of Refractory TiZrNbHfTa High-Entropy Alloys Containing Aluminum. Adv. Eng. Mater. 2018, 20, 1700948. [Google Scholar] [CrossRef]
- Waseem, O.A.; Auyeskhan, U.; Lee, H.M.; Ryu, H.J. A combinatorial approach for the synthesis and analysis of AlxCryMozNbTiZr high-entropy alloys: Oxidation behavior. J. Mater. Res. 2018, 33, 3226–3234. [Google Scholar] [CrossRef]
- Zhang, P.; Li, Y.; Chen, Z.; Zhang, J.; Shen, B. Oxidation response of a vacuum arc melted NbZrTiCrAl refractory high entropy alloy at 800–1200 °C. Vacuum 2019, 162, 20–27. [Google Scholar] [CrossRef]
- Müller, F.; Gorr, B.; Christ, H.-J.; Chen, H.; Kauffmann, A.; Heilmaier, M. Effect of microalloying with silicon on high temperature oxidation resistance of novel refractory high-entropy alloy Ta-Mo-Cr-Ti-Al. Mater. High Temp. 2018, 35, 168–176. [Google Scholar] [CrossRef]
- Xiao, Y.; Kuang, W.; Xu, Y.; Wu, L.; Gong, W.; Qian, J.; Zhang, Q.; He, Y. Microstructure and oxidation behavior of the CrMoNbTaV high-entropy alloy. J. Mater. Res. 2019, 34, 301–308. [Google Scholar] [CrossRef]
- Butler, T.; Chaput, K. Native oxidation resistance of Al20Nb30Ta10Ti30Zr10 refractory complex concentrated alloy (RCCA). J. Alloys Compd. 2019, 787, 606–617. [Google Scholar] [CrossRef]
- Waseem, O.A.; Ryu, H.J. Combinatorial synthesis and analysis of AlxTayVz-Cr20Mo20Nb20Ti20Zr10 and Al10CrMoxNbTiZr10 refractory high-entropy alloys: Oxidation behavior. J. Alloys Compd. 2020, 828, 154427. [Google Scholar] [CrossRef]
- Müller, F.; Gorr, B.; Christ, H.-J.; Müller, J.; Butz, B.; Chen, H.; Kauffmann, A.; Heilmaier, M. On the oxidation mechanism of refractory high entropy alloys. Corros. Sci. 2019, 159, 108161. [Google Scholar] [CrossRef]
- Cao, Y.-K.; Liu, Y.; Liu, B.; Zhang, W.-D.; Wang, J.-W.; Meng, D.U. Effects of Al and Mo on high temperature oxidation behavior of refractory high entropy alloys. Trans. Nonferr. Met. Soc. China 2019, 29, 1476–1483. [Google Scholar] [CrossRef]
- Kofstad, P. High-temperature oxidation of titanium. J. Less Common Met. 1967, 12, 449–464. [Google Scholar] [CrossRef]
- Šulhánek, P.; Drienovský, M.; Černičková, I.; Ďuriška, L.; Skaudžius, R.; Gerhátová, Ž.; Palcut, M. Oxidation of Al-Co Alloys at High Temperatures. Materials 2020, 13, 3152. [Google Scholar] [CrossRef]
- Manzoni, A.; Daoud, H.; Mondal, S.; van Smaalen, S.; Völkl, R.; Glatzel, U.; Wanderka, N. Investigation of phases in Al23Co15Cr23Cu8Fe15Ni16 and Al8Co17Cr17Cu8Fe17Ni33 high entropy alloys and comparison with equilibrium phases predicted by Thermo-Calc. J. Alloys Compd. 2013, 552, 430–436. [Google Scholar] [CrossRef]
- Ng, C.; Guo, S.; Luan, J.; Shi, S.; Liu, C. Entropy-driven phase stability and slow diffusion kinetics in an Al0.5CoCrCuFeNi high entropy alloy. Intermetallics 2012, 31, 165–172. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, F.; Chen, S.; Cao, W. Computational Thermodynamics Aided High-Entropy Alloy Design. JOM 2012, 64, 839–845. [Google Scholar] [CrossRef]
- Yao, H.; Qiao, J.; Gao, M.; Hawk, J.; Ma, S.; Zhou, H.; Zhang, Y. NbTaV-(Ti,W) refractory high-entropy alloys: Experiments and modeling. Mater. Sci. Eng. A 2016, 674, 203–211. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, C.; Chen, S.; Zhu, J.; Cao, W.; Kattner, U. An understanding of high entropy alloys from phase diagram calculations. Calphad 2014, 45, 1–10. [Google Scholar] [CrossRef]
- Guruvidyathri, K.; Kumar, K.C.H.; Yeh, J.W.; Murty, B.S. Topologically Close-packed Phase Formation in High Entropy Alloys: A Review of Calphad and Experimental Results. JOM 2017, 69, 2113–2124. [Google Scholar] [CrossRef]
- Ferrari, A.; Körmann, F. Surface segregation in Cr-Mn-Fe-Co-Ni high entropy alloys. Appl. Surf. Sci. 2020, 533, 147471. [Google Scholar] [CrossRef]
- Osei-Agyemang, E.; Balasubramanian, G. Surface oxidation mechanism of a refractory high-entropy alloy. npj Mater. Degrad. 2019, 3, 20. [Google Scholar] [CrossRef]
- Butler, T.M. Phase Stability and Oxidation Behavior of Al-Ni-Co-Cr-Fe Based High-Entropy Alloys. Ph.D. Thesis, University of Alabama, Tuscaloosa, AL, USA, 2016; pp. 118–120. [Google Scholar]
- Klein, L.; Zendegani, A.; Palumbo, M.; Fries, S.; Virtanen, S. First approach for thermodynamic modelling of the high temperature oxidation behaviour of ternary γ′-strengthened Co–Al–W superalloys. Corros. Sci. 2014, 89, 1–5. [Google Scholar] [CrossRef]
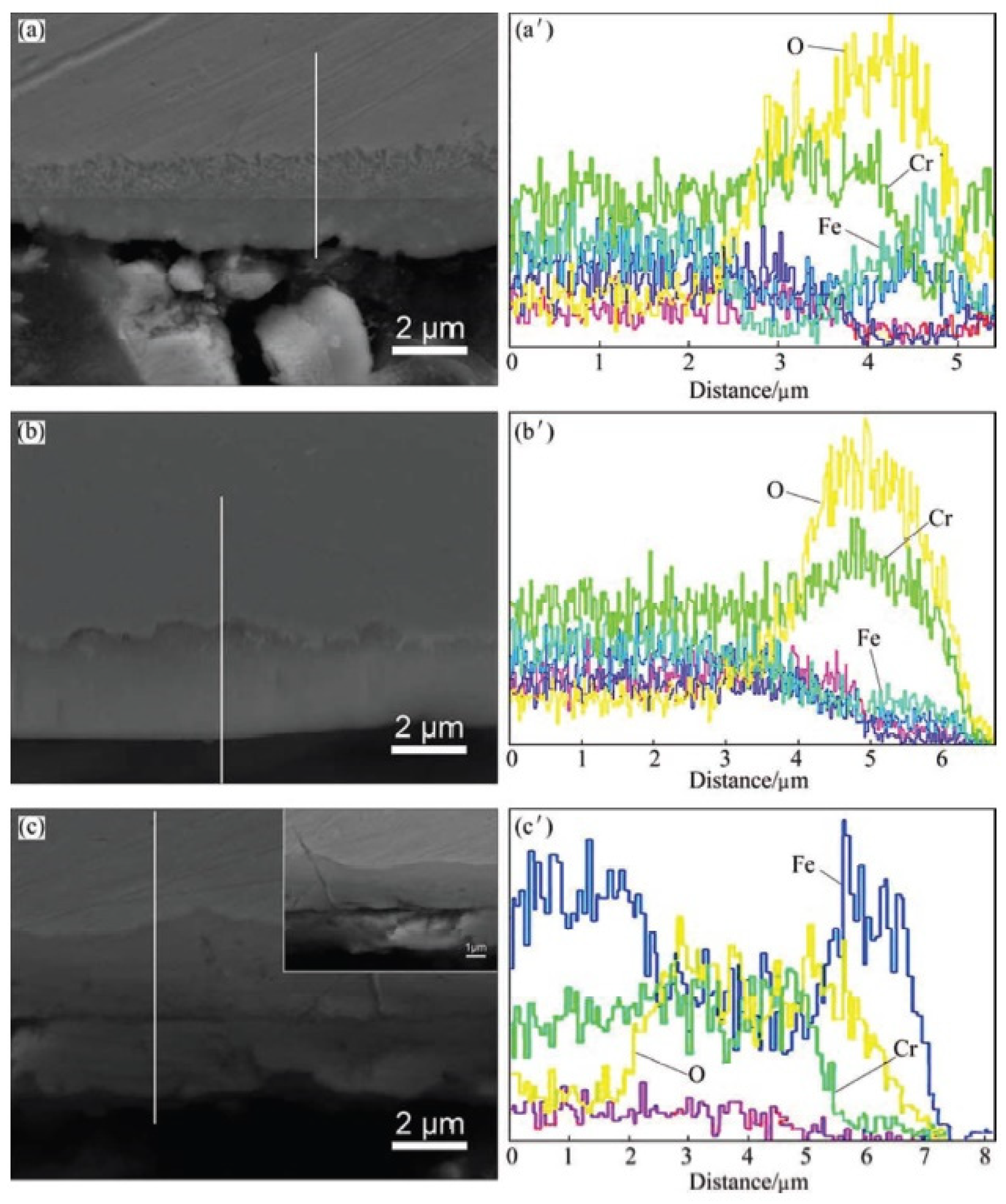

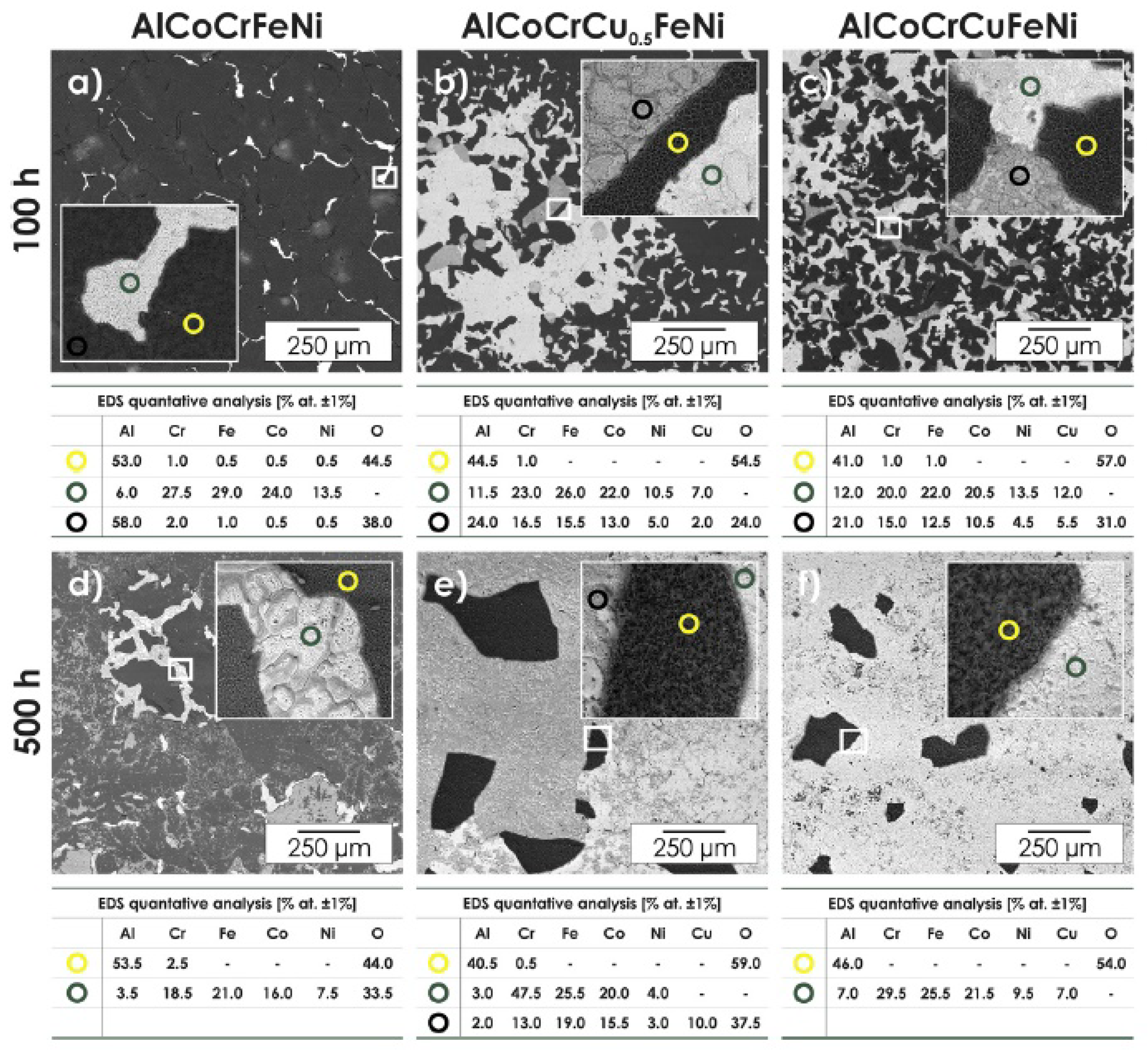
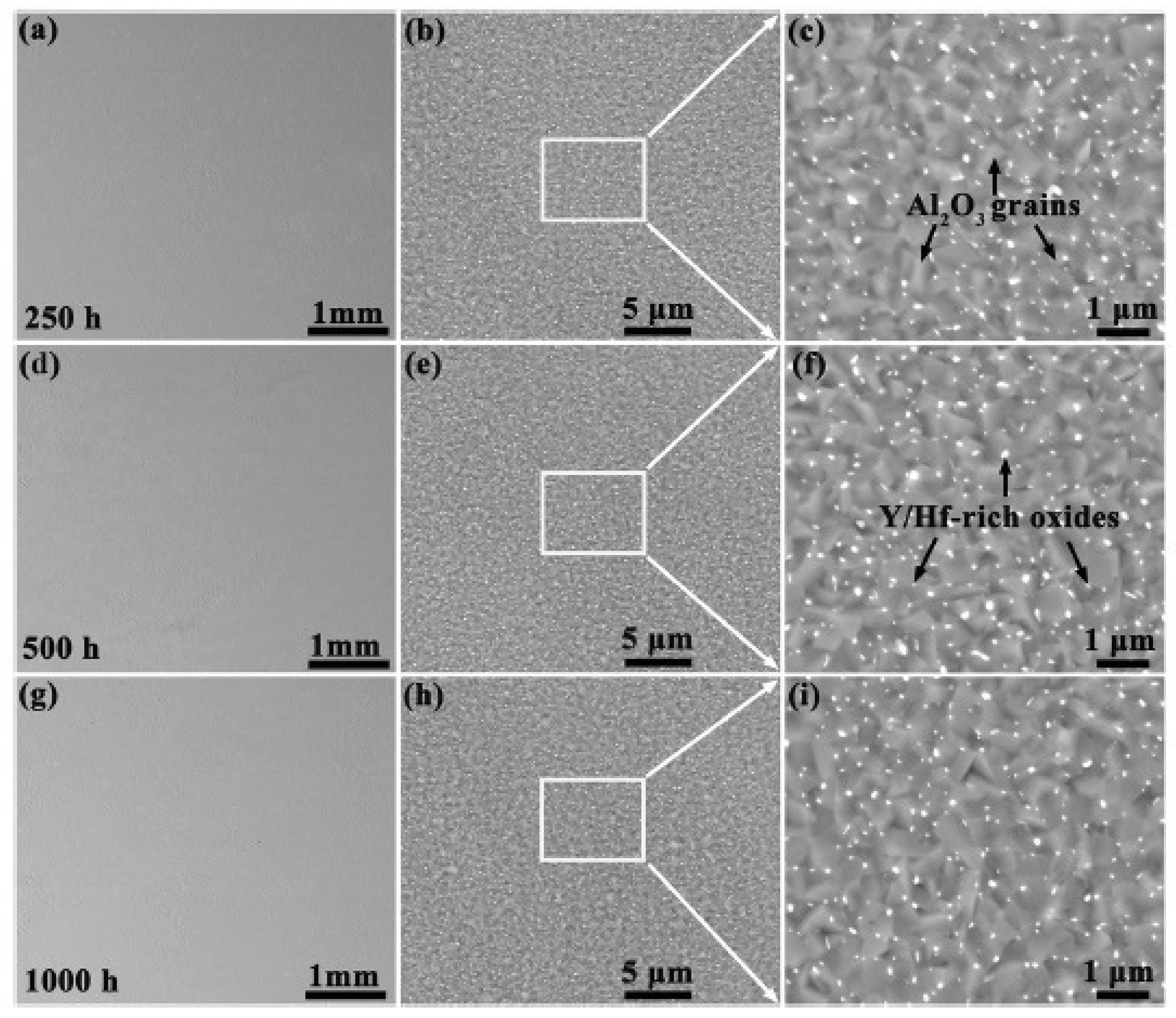
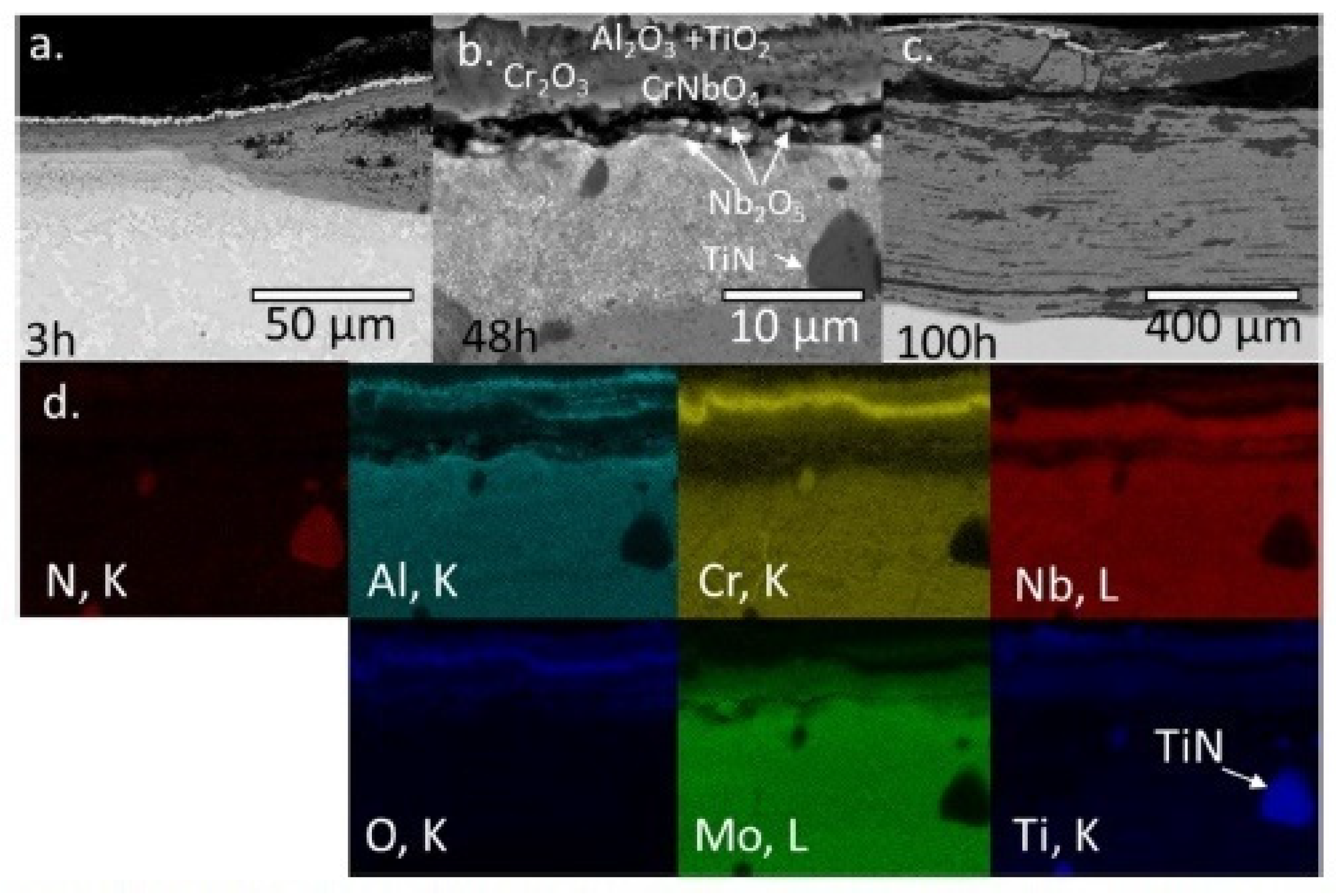

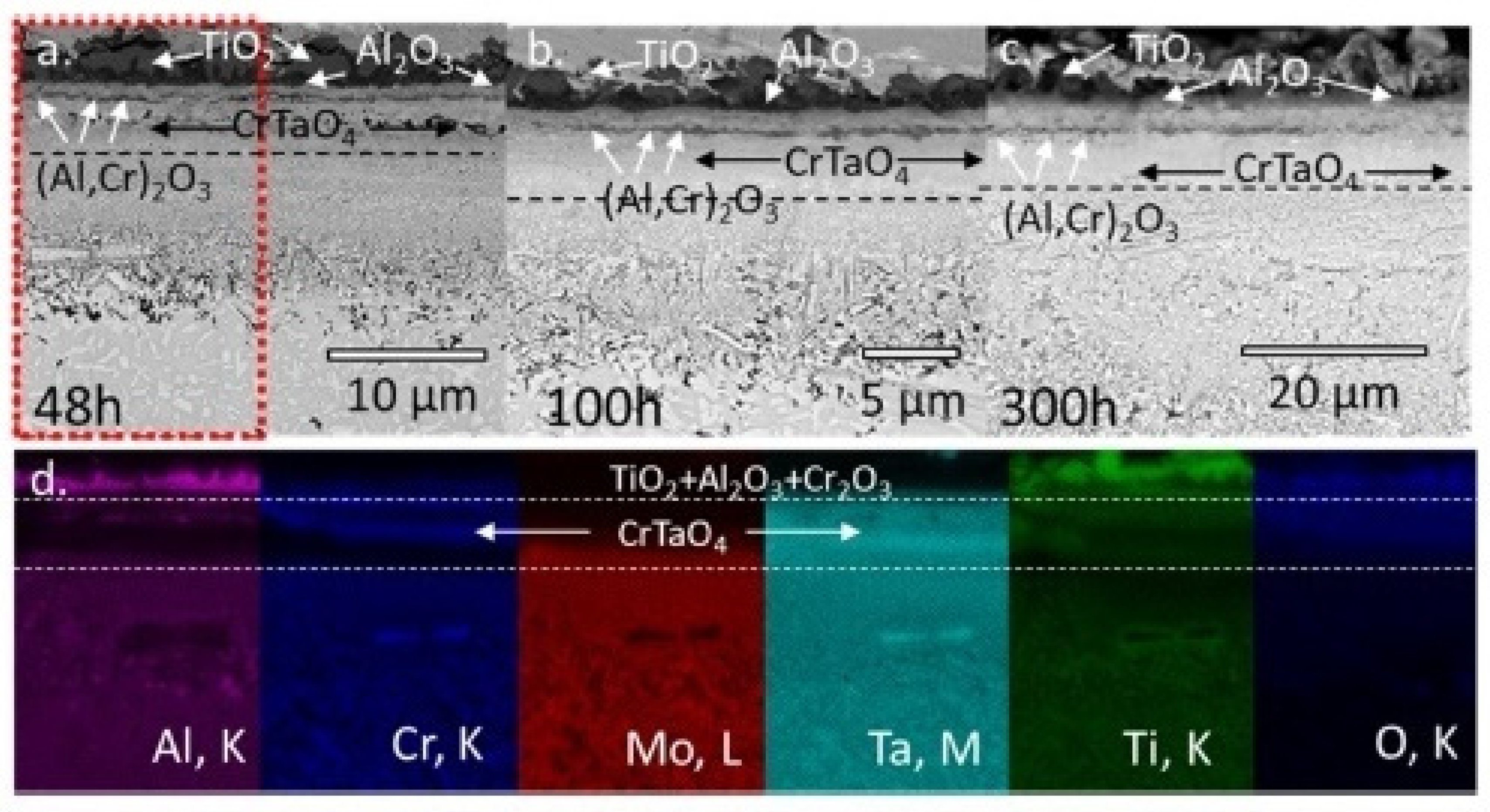
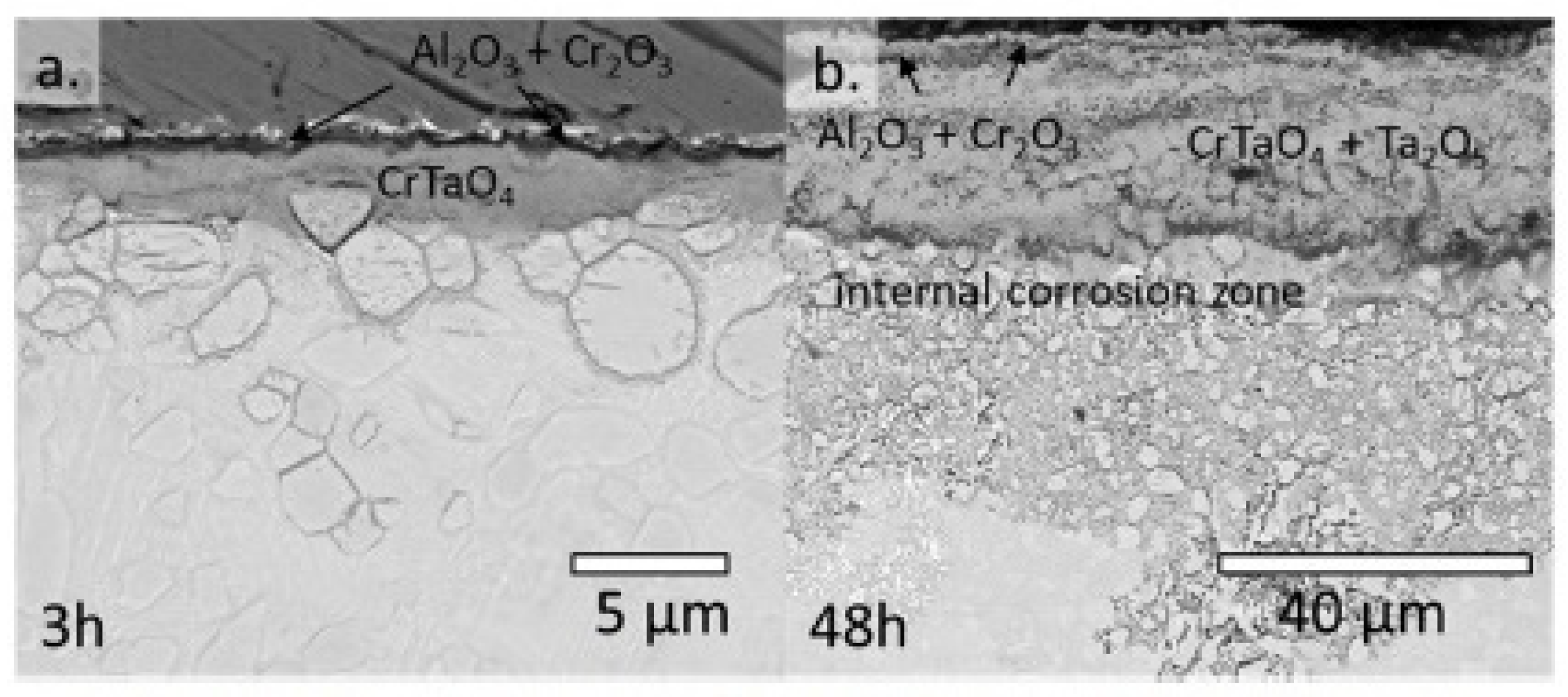
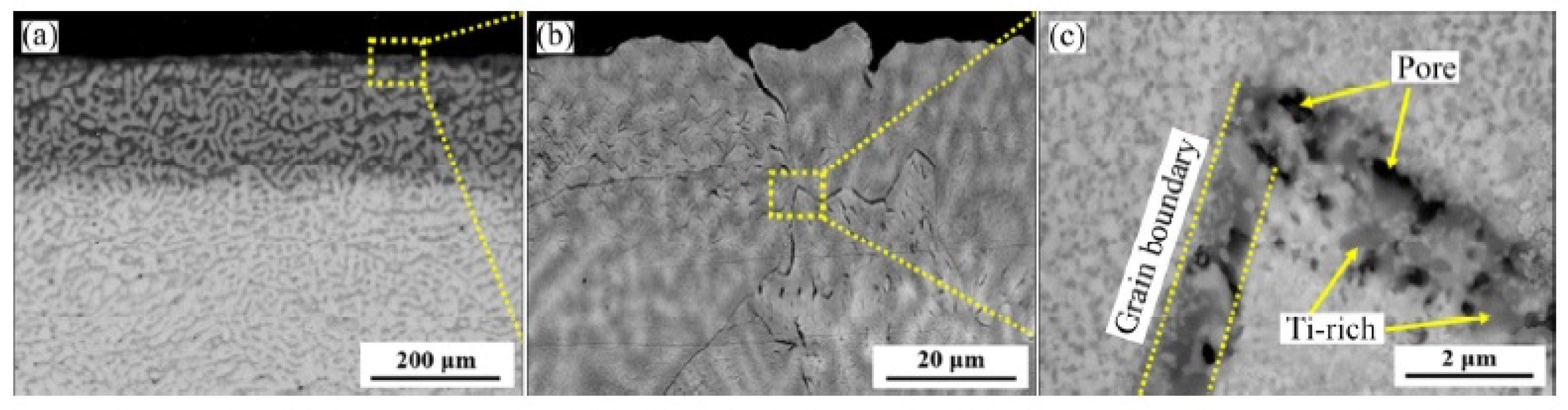
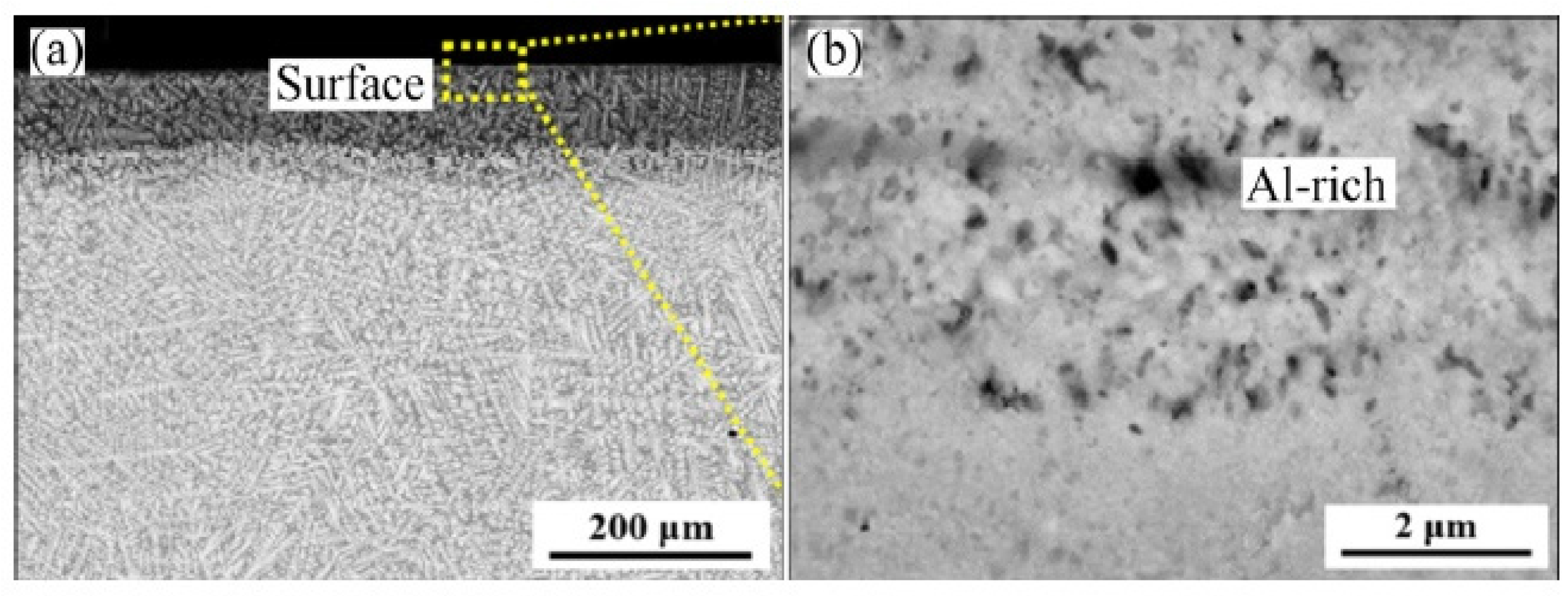
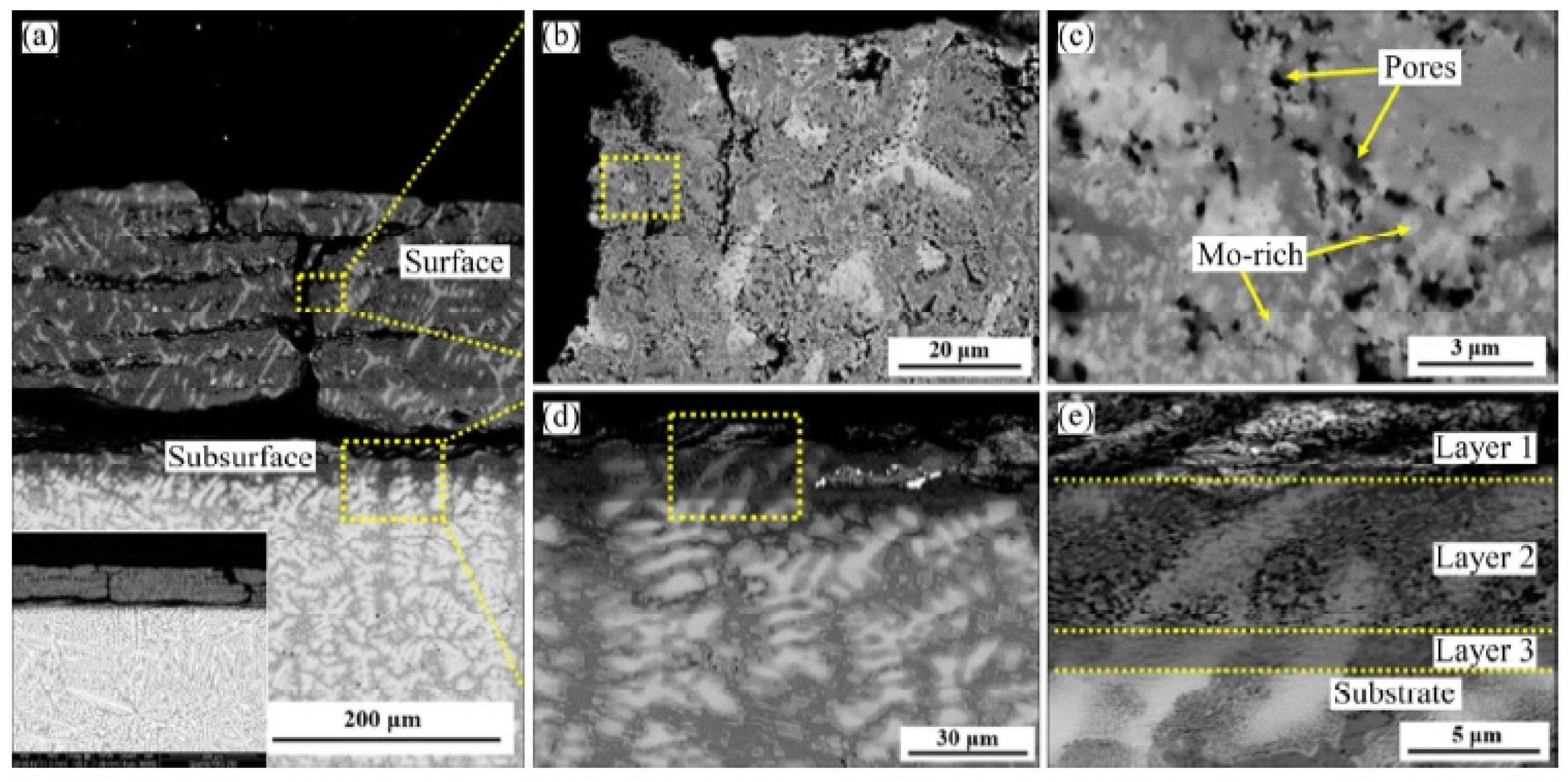
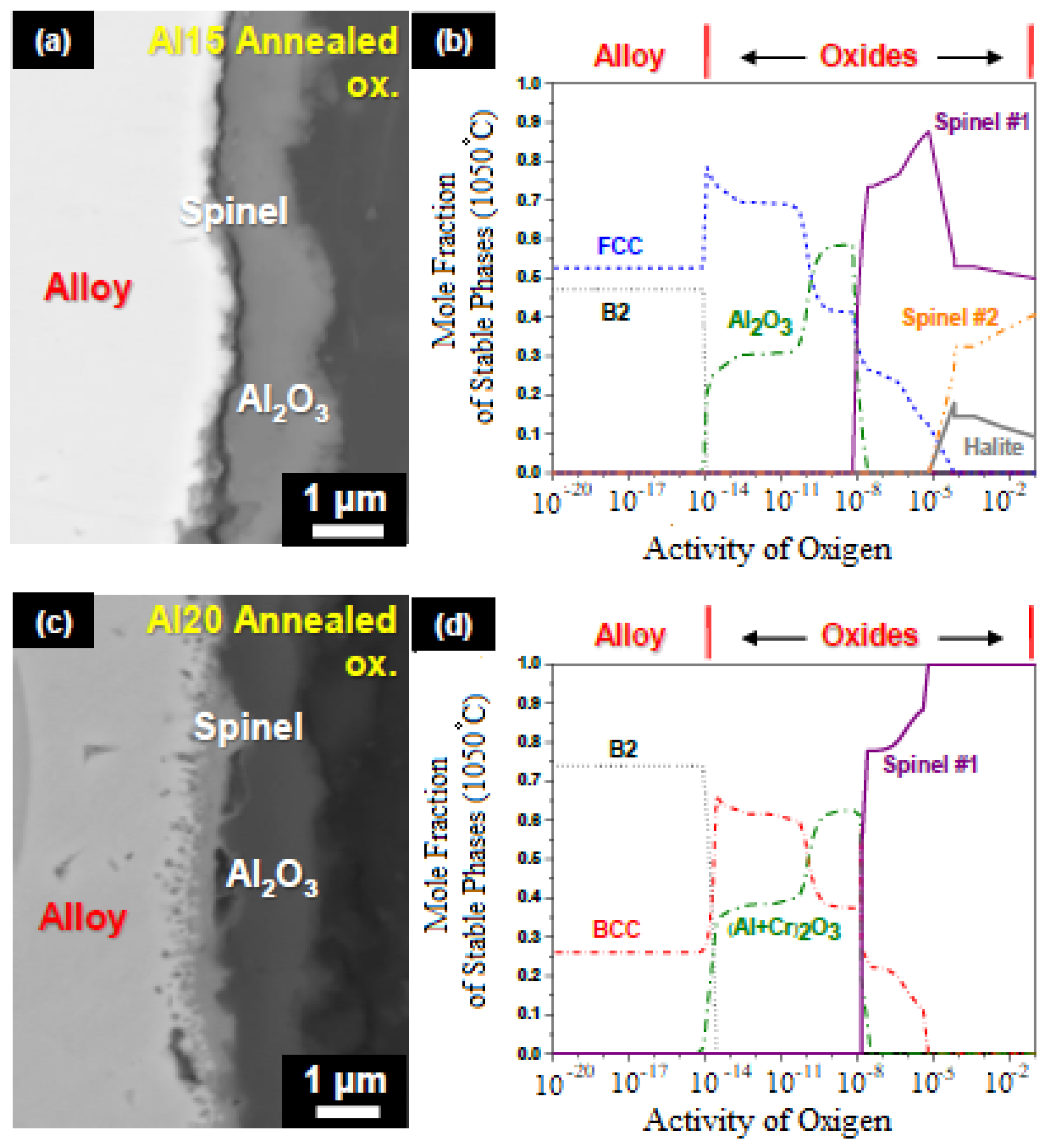
| Alloy | t, °C | Weight Gain (mg/cm2) | Source | Activation Energy, kJ/mol | |||
|---|---|---|---|---|---|---|---|
| 1 h | 5 h | 20 h | 50 h | ||||
| In descending order weight gain for 1 h | |||||||
| Al15(CoCrFeNi)85 | 1050 | 0.40 | 0.85 | 1.62 | 1.68 | [29] | – |
| Al20(NiCoCrFe)80 (annealed) | 1050 | 0.20 | 0.81 | 1.16 | 1.52 | [30] | – |
| Al8(CoCrFeNi)92 | 1050 | 0.18 | 0.82 | 1.50 | 1.70 | [29] | – |
| Al10(CoCrFeNi)90 | 1050 | 0.17 | 0.72 | 1.28 | 1.40 | [29] | – |
| Al12(CoCrFeNi)88 | 1050 | 0.15 | 0.63 | 1.08 | 1.12 | [29] | – |
| Al20(CoCrFeNi)80 | 1050 | 0.11 | 0.62 | 0.88 | 0.98 | [29] | – |
| Al15(CoCrFeNi)85 (annealed) | 1050 | 0.10 | 0.90 | 1.40 | 1.83 | [30] | – |
| Al8(CoCrFeNi)92 (annealed) | 1050 | 0.10 | 0.61 | 1.30 | 1.85 | [30] | – |
| Al12(CoCrFeNi)88 (annealed) | 1050 | 0.10 | 0.60 | 1.17 | 1.60 | [30] | – |
| Al30(CoCrFeNi)70 | 1050 | 0.07 | 0.45 | 0.50 | 0.49 | [29] | – |
| Al4Co5Cr5Ni5Si | 1050 | 0.06 | 0.35 | 0.40 | 0.43 | [31] | – |
| Al2Co4.5Cr4.5Ni4.5Fe4.5 | 1050 | 0.05 | 0.40 | 0.85 | 1.50 | [31] | – |
| Al3Co2Cr7Ni7Si | 1050 | 0.04 | 0.09 | 0.08 | 0.09 | [31] | – |
| Al30(CoCrFeNi)70 (annealed) | 1050 | 0.03 | 0.45 | 0.52 | 0.60 | [30] | – |
| In descending order weight gain for 20 h at 1000 °C | |||||||
| Al0.5CoCrCu0.5FeNi2 | 800 | – | – | 4.00 | 18.00 | [33] | 199 |
| 1000 | – | – | 23.00 | 40.00 | |||
| Al1.5CoCr1.5Cu0.5FeNi | 800 | – | – | 3.00 | 19.00 | [33] | 137 |
| 1000 | – | – | 10.00 | 17.00 | |||
| AlCoCrCuFeNi | 800 | – | – | 3.00 | 11.00 | [33] | 125 |
| 1000 | – | – | 9.00 | 10.00 | |||
| AlCoCrFeNi | 1000 | – | 0.27 | 0.50 | 0.72 | [32] | – |
| AlCoCrCu0.5FeNi | 1000 | – | 0.21 | 0.32 | 0.43 | [32] | – |
| AlCoCrCuFeNi | 1000 | – | 0.02 | 0.11 | 0.21 | [32] | – |
| In descending order weight gain for 1 h at 1100 °C | |||||||
| AlCoCrFeMo0.5NiSiTi | 500 | 0.9 | 1.2 | 1.7 | 2.3 | [25] | 35 |
| 800 | 0.3 | 1 | 2.2 | 3.7 | |||
| 1100 | 2.5 | 3.5 | 6.2 | 9 | |||
| AlCrFeMo0.5NiSiTi | 500 | 1.3 | 1.7 | 2.8 | 3 | [25] | 29 |
| 800 | 1 | 1.8 | 2.8 | 8 | |||
| 1100 | 2.2 | 3.1 | 4.9 | 7.1 | |||
| Alloy | Fe | Ni | Co | Mn | Cr | Nb | Al | other | Y | O | N | C | S |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| weight % | ppm | ||||||||||||
| HEA-1 | 24.85 | 25.89 | 26.00 | 0.51 | 22.66 | – | 0.07 | – | – | 14 | 67 | 82 | 135 |
| HEA-2 | 17.39 | 21.47 | 21.68 | 20.27 | 19.14 | – | – | – | – | 11 | 64 | 68 | 136 |
| HEA-3 | 17.18 | 22.15 | 21.45 | 19.91 | 19.17 | 0.11 | – | – | 1206 | 19 | 19 | 219 | 14 |
| HEA-4 | 31.01 | 17.86 | 17.63 | 16.25 | 17.15 | 0.09 | – | – | 418 | 14 | 51 | 174 | 4 |
| HEA-5 | 29.26 | 17.57 | 16.95 | 15.24 | 20.85 | 0.09 | – | – | 1033 | 3 | 15 | 159 | 11 |
| HEA-6 | 39.80 | 15.42 | 14.89 | 13.62 | 16.06 | 0.17 | – | – | 969 | 10 | 7 | 262 | <1 |
| HEA-7 | 46.86 | 12.88 | 12.54 | 11.72 | 15.80 | 0.16 | – | – | 357 | 5 | 29 | 303 | 15 |
| HEA-8 | 22.63 | 26.06 | 26.82 | 24.47 | – | – | – | – | 243 | 5 | 10 | 81 | 26 |
| 304H | 70.51 | 8.29 | 0.23 | 1.09 | 18.71 | – | 0.09 | 0.46 Si | – | – | – | 400 | 40 |
| 230 | 0.40 | 59.76 | 0.33 | 0.51 | 22.15 | – | 0.46 | 14.40 W | – | – | – | 1000 | – |
| 1.26 Mo | |||||||||||||
| 0.47 Si | |||||||||||||
| Alloy | t, °C | Weight Gain (mg/cm2) | Activation Energy, kJ/mol | Source | |||
|---|---|---|---|---|---|---|---|
| 5 h | 20 h | 50 h | 100 h | ||||
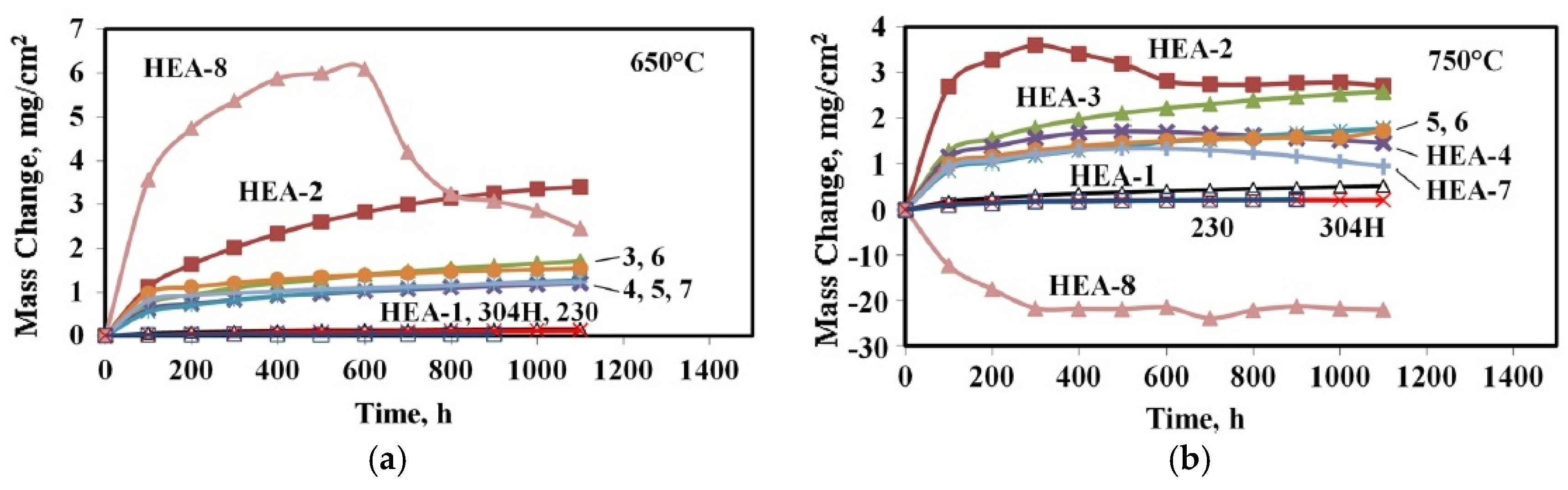
| Mn24Co26Fe23Ni26 (with addition of Y) (HEA-8) | 650 | – | – | 20 | 3.5 | – | [42] In descending order weight gain for 1 h at 650 °C |
| 750 | – | – | −6 | −13 | |||
| Mn20Co22Cr19Fe17Ni21 (HEA-2) | 650 | – | – | 0.5 | 1.2 | 111 | |
| 750 | – | – | 1.5 | 2.8 | |||
| Mn20Co22Cr19Fe17Ni22 (with addition of Y) (HEA-3) | 650 | – | – | 0.5 | 1.0 | 28 | |
| 750 | – | – | 0.8 | 1.3 | |||
| Mn14Co15Cr16Fe40Ni15 (with addition of Y) (HEA-6) | 650 | – | – | 0.5 | 0.9 | 77 | |
| 750 | – | – | 0.5 | 1.0 | |||
| Mn16Co18Cr17Fe31Ni17 (with addition of Y) (HEA-4) | 650 | – | – | 0.4 | 0.9 | 137 | |
| 750 | – | – | 0.7 | 1.1 | |||
| Mn15Co17Cr21Fe29Ni17 (with addition of Y) (HEA-5) | 650 | – | – | 0.4 | 0.5 | 56 | |
| 750 | – | – | 0.6 | 1.0 | |||
| Mn12Co12Cr16Fe47Ni13 (with addition of Y) (HEA-7) | 650 | – | – | 0.4 | 0.5 | 123 | |
| 750 | – | – | 0.5 | 1.0 | |||
| Mn0.5Co26Cr22Fe25Ni26 (HEA-1) | 650 | – | – | 0.10 | 0.15 | 162 | |
| 750 | – | – | 0.05 | 0.10 | |||
| MnCoCrFeNi | 600 | – | 0.10 | 0.20 | 0.35 | 130 | [43] |
| 700 | 0.10 | 0.35 | 0.65 | 1.05 | |||
| 800 | 0.25 | 0.75 | 1.40 | 2.25 | |||
| 900 | 0.60 | 1.25 | 2.15 | – | |||
| Alloy | t, °C | Weight Gain (mg/cm2) | Source | Activation Energy, kJ/mol | |||
|---|---|---|---|---|---|---|---|
| 1 h | 3 h | 20 h | 50 h | ||||
| CrMo0.5NbTa0.5TiZr | 1000 | 55 | 110 | – | – | [49] | – |
| NbTiVZr | 1000 | 30 | 100 | – | – | [55] | – |
| HfNbTaTiZr | 700 | 3 | 9 | 45 | 53 | [63] | 59 |
| 900 | 18 | 25 | 29 | 39 | |||
| 1000 | 17 | – | – | – | |||
| 1100 | 20.5 | 30 | 48 | 51 | |||
| 1300 | 25 | 75 | 225 | 250 | |||
| NbTa0.5TiZr | 1000 | 15 | 35 | 90 | – | [71] | – |
| Al0.5Cr0.5MoNbTiZr | 1000 | 13.38 | – | – | – | [64] | – |
| NbTiZrCr | 1000 | 13 | 25 | 60 | 83 | [55] | – |
| AlCrNbTiZr | 1000 | 10.66 | – | – | – | [64] | – |
| Cr1.5Mo0.5NbTiZr | 1000 | 9.4 | – | 39 | – | [64] | – |
| CrMoNbTiZr | 1000 | 8.77 | – | – | – | [64] | – |
| Al0.5HfNbTaTiZr | 700 | 3 | 4 | 8 | 10 | [63] | 132 |
| 900 | 6 | 10 | 11 | 12 | |||
| 1000 | 8.5 | – | – | – | |||
| 1100 | 12 | 17 | 25 | 38 | |||
| 1300 | 25 | 100 | 248 | 250 | |||
| AlMoNbTiZr | 1000 | 8.25 | – | – | – | [64] | – |
| Al1.5Mo0.5NbTiZr | 1000 | 7.29 | – | – | – | [64] | – |
| Al1.5Cr0.5NbTiZr | 1000 | 6.3 | – | 20 | – | [64] | – |
| Al0.5Cr1.5NbTiZr | 1000 | 6.1 | – | – | – | [64] | – |
| AlHfNbTaTiZr | 700 | 1 | 2 | 5 | 8 | [63] | 137 |
| 900 | 5 | 6 | 9 | 11 | |||
| 1000 | 6 | – | – | – | |||
| 1100 | 8 | 14.5 | 18.5 | 33 | |||
| 1300 | 25 | 49 | 177 | 250 | |||
| AlMo0.5NbTa0.5TiZr | 1000 | 5 | 17 | 75 | – | [71] | – |
| AlNb1.5Ta0.5Ti1.5Zr0.5 | 1000 | 5 | 10 | 25 | 38 | [68] | – |
| AlCr0.5Mo0.5NbTiZr | 1000 | 4.29 | – | 21 | – | [64] | – |
| AlCrNbTiZr | 800 | 3 | 5 | 8 | 10 | [65] | 180 |
| 1000 | 4 | 13 | 23 | 52 | |||
| 1200 | 5 | 17 | 90 | 185 | |||
| AlNbTa0.5TiZr | 1000 | 4 | 10 | 35 | – | [71] | – |
| AlNbTiZr | 1000 | 3.8 | – | – | – | [61] | – |
| Al0.5CrMo0.5NbTiZr | 1000 | 3.46 | – | – | – | [64] | – |
| CrMoNbTaV | 900 | 2 | 8 | 25 | – | [67] | 92 |
| 1000 | 3 | 13 | 42 | – | |||
| 1100 | 7.5 | 22 | 12 | – | |||
| Al10Cr24Mo8Nb24Ti24Zr10 (Mo-8) | 1000 | 3.0 | – | – | – | [69] | – |
| AlCrMoTiW | 1000 | 2.3 | 3.8 | 6.2 | – | [54] | – |
| Al10Cr25Mo4Nb25Ti25Zr10 (Mo-4) | 1000 | 2.0 | – | – | – | [69] | – |
| Al0.5Mo1.5NbTiZr | 1000 | 1.27 | – | – | – | [64] | – |
| AlCrMoTaTi | 1000 | 1.15 | 1.25 | 1.9 | 2.2 | [70] | – |
| Al10Cr27Nb27Ti27Zr10 (Mo-0) | 1000 | 1.0 | – | 17 | 24 | [69] | – |
| AlCrMoNbTi-1at%Si | 900 | 0.1 | 0.2 | 0.3 | 0.5 | [58] | 309 |
| 1000 | 0.5 | 1 | 2.2 | 5.9 | |||
| 1100 | 0.6 | 1.2 | 2.9 | 6.4 | |||
| AlCrMoNbTi | 900 | 0.1 | 0.2 | 0.3 | 0.4 | [58] | 395 |
| 1000 | 0.5 | 0.7 | 3.2 | 9 | |||
| 1100 | 0.7 | 0.9 | 5.5 | 8 | |||
| Cr0.5Mo1.5NbTiZr | 1000 | 0.5 | – | – | – | [64] | – |
| AlNbMoCr | 1000 | 0.3 | 0.6 | 14.3 | – | [70] | – |
| AlCrMoNbTi | 1000 | 0.3 | 0.6 | 3.1 | 12.5 | [70] | – |
| AlCrMoTaTi-1at%Si | 900 | 0.1 | 0.15 | 0.35 | 0.45 | [66] | 134 |
| 1000 | 0.25 | 0.4 | 0.8 | 1.3 | |||
| 1100 | 1 | 1.55 | 2.6 | 4 | |||
| HfNbTiZr | 1000 | 0.25 | – | – | – | [61] | – |
| AlCrMoTaTi | 900 | 0.1 | 0.15 | 0.2 | 0.25 | [66] | 284 |
| 1000 | 0.15 | 0.3 | 0.5 | 0.6 | |||
| 1100 | 0.55 | 1 | 2 | 3.05 | |||
| AlTaMoCr | 1000 | ~0 | 0.3 | 0.4 | 1.3 | [70] | – |
| Alloy | t, °C | Time, h | Oxidation Products | Source |
|---|---|---|---|---|
| AlCrMoTiW | 1000 | 40 | Cr2O3, Al2O3 | [54] |
| AlCrMoNbTi | 900–1100 | 48 | Cr2O3, Al2O3 | [58] |
| NbTiZrCr | 1000 | 100 | NbCrO4, ZrO2 | [55] |
| Al2Co4.5Cr4.5Fe4.5Ni4.5 | 1050 | 5–500 | Cr2O3, Al2O3, AlN | [31] |
| Al4Co5Cr5Ni5Si | 1050 | 5–500 | Cr2O3, Al2O3, AlN | [31] |
| Al3Co7Cr2Ni7Si | 1050 | 5–500 | Cr2O3, Al2O3, AlN | [31] |
| AlCoCrCuxFeNi (x = 0, 0.5 and 1.0 at.%) | 1000 | 100 500 | Al2O3, spinels with Cr and Co | [32] |
| Al0.5CoCrCu0.5FeNi2 | 800–1000 | 200 | Cr2O3, Al2O3, oxides of Fe and Ni | [33] |
| Al1.5CoCr1.5Cu0.5FeNi | 800–1000 | 200 | Cr2O3, Al2O3, oxides of Fe and Ni | [33] |
| AlCoCrCuFeNi | 800–1000 | 200 | Cr2O3, Al2O3, oxides of Fe and Ni | [33] |
| Alx(CoCrFeNi) (x = 8, 10, 12, 15, 20 and 30 at.%) | 1050 | 100 | Cr2O3, Al2O3, NiCr2O4, AlN | [29] |
| AlCoCrFeNi-0.02at%Y-0.02at%Hf | 1100 | 1–1000 | Al2O3, Y3Al5O12, HfO2 | [28] |
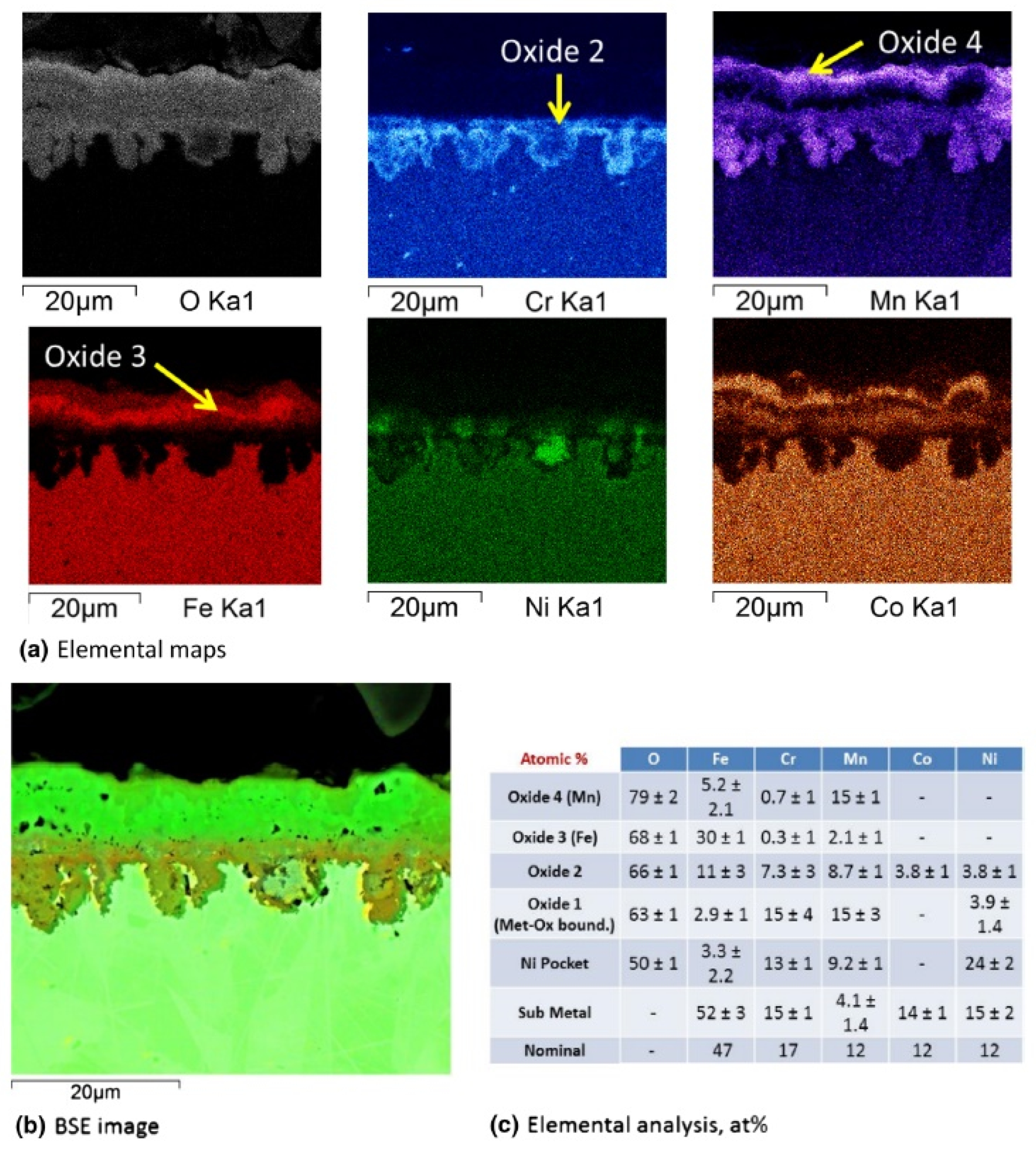
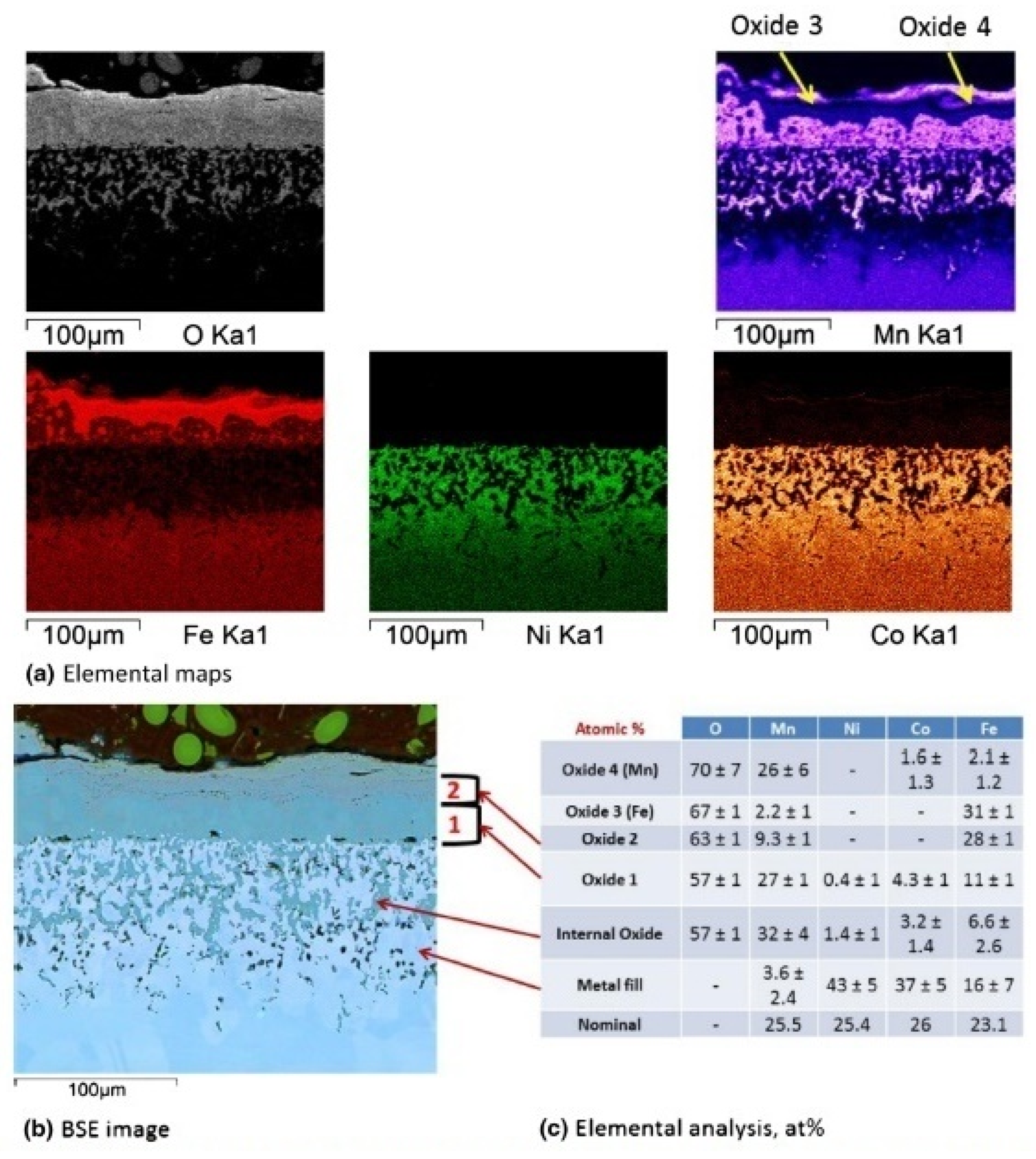
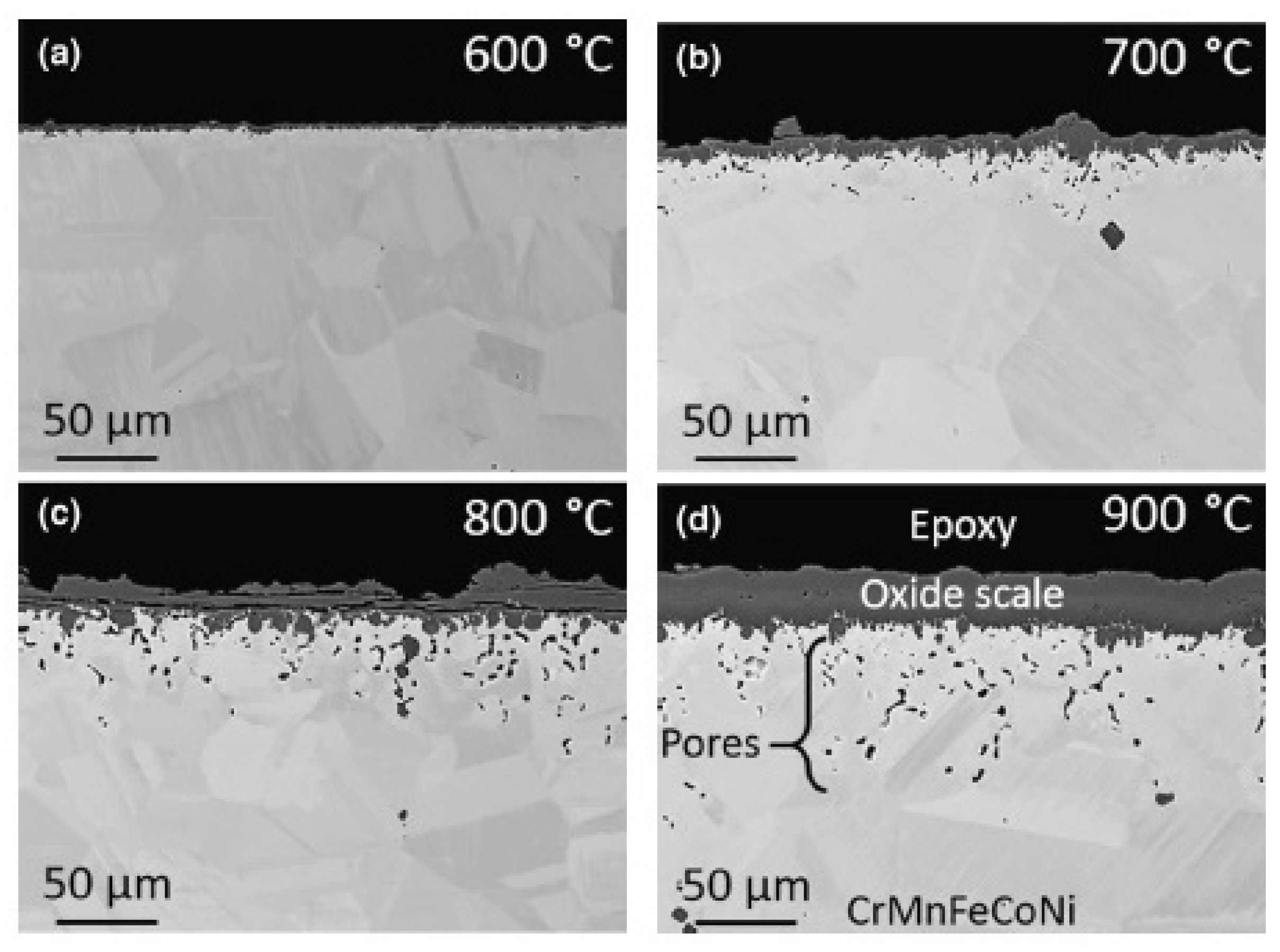
| MnCoCrFeNi (HEA 1-HEA 8) | 650–750 | 1100 | Cr2O3, oxides of Fe and Mn, MnCr2O4 | [42] |
| CoCrFeMnNi | 500–900 | 100 | α-Mn2O3, Mn3O4, Cr2O3 | [43] |
| AlCrMoTaTi | 900–1100 | 3–300 | Cr2O3, Al2O3, CrTaO4 | [70] |
| AlCrMoTa | 900–1100 | 3–300 | Cr2O3, Al2O3, CrTaO4 | [70] |
| AlCrMoNbTi | 900–1100 | 3–300 | Cr2O3, Al2O3, Nb2O5 | [70] |
| AlCrMoNb | 900–1100 | 3–300 | Cr2O3, Al2O3, Nb2O5 | [70] |
| AlCrMoNbTi | 1000–1100 | 48 | Al2O3, Cr2O3, TiO2 | [60] |
| AlCrMoTiW | 1000–1100 | 48 | Al2(WO4)3, Cr2O3, TiO2 | [59] |
| AlxCoCrFeNi (x = 0.15, 0.4 at.%) | 550–600 | 70 | Fe3O4, FeCr2O4, NiFe2O4 (Cr2O3) (Al2O3) | [27] |
| AlCrMoTaTi | 900–1100 | 48 | Al2O3, Cr2O3, TiO2, CrTaO4 | [66] |
| AlCrMoTaTi-1at%Si | 900–1100 | 48 | Al2O3, Cr2O3, TiO2, CrTaO4 | [66] |
| AlCrMoTaTi | 1000–1100 | 48 | Al2O3, Cr2O3, TiO2, CrTaO4 | [60] |
| NbTa0.5TiZr | 1000 | 10 | TiO2, Nb2O5, Ti3O5, ZrO2 | [71] |
| CrNbTi-10at%Al-10at%Zr-x at%Mo | 1000 | 1–50 | Cr2O3, Al2O3, AlTiO5, CrNbO4 | [69] |
| NbTiVZr | 1000 | 100 | TiO2, V2O5, TiNb2O7, Nb2Zr6O17 | [55] |
| AlNbTa0.5TiZr | 1000 | 10 | TiO2, Nb2O5, Ti3O5, ZrO2, Al2O3 | [71] |
| AlCrNbTiZr | 800–1200 | 5–50 | CrNbO4 ZrO2, TiO2, Al2O3, ZrNb2O7 | [65] |
| AlMo0.5NbTa0.5TiZr | 1000 | 10 | TiO2, Nb2O5, Ti3O5, ZrO2, Al2O3, MoO3 | [71] |
| AlNbTiZr | 600–1000 | 50 | AlNbO4, Ti2ZrO6 Al2O3, NbO, ZrO2, TiO2 | [61] |
| CrMoNbTaV | 900–1100 | 25 | Nb2O5, NbO2, CrTaO4, CrNbO4, Ta9VO25, Nb9VO25 | [67] |
| AlNbTiVZr0.25 | 600–900 | 100 | V2O5, VO2, TiO2, Nb2O5, TiNb2O7, AlNbO4, Nb2Zr6O17, ZrO2 | [62] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veselkov, S.; Samoilova, O.; Shaburova, N.; Trofimov, E. High-Temperature Oxidation of High-Entropic Alloys: A Review. Materials 2021, 14, 2595. https://doi.org/10.3390/ma14102595
Veselkov S, Samoilova O, Shaburova N, Trofimov E. High-Temperature Oxidation of High-Entropic Alloys: A Review. Materials. 2021; 14(10):2595. https://doi.org/10.3390/ma14102595
Chicago/Turabian StyleVeselkov, Sergey, Olga Samoilova, Nataliya Shaburova, and Evgeny Trofimov. 2021. "High-Temperature Oxidation of High-Entropic Alloys: A Review" Materials 14, no. 10: 2595. https://doi.org/10.3390/ma14102595
APA StyleVeselkov, S., Samoilova, O., Shaburova, N., & Trofimov, E. (2021). High-Temperature Oxidation of High-Entropic Alloys: A Review. Materials, 14(10), 2595. https://doi.org/10.3390/ma14102595







