Synthesis and Characterization of Electrospun Composite Scaffolds Based on Chitosan-Carboxylated Graphene Oxide with Potential Biomedical Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of electrospinning solutions
2.3. Electrospinning Process and Parameters
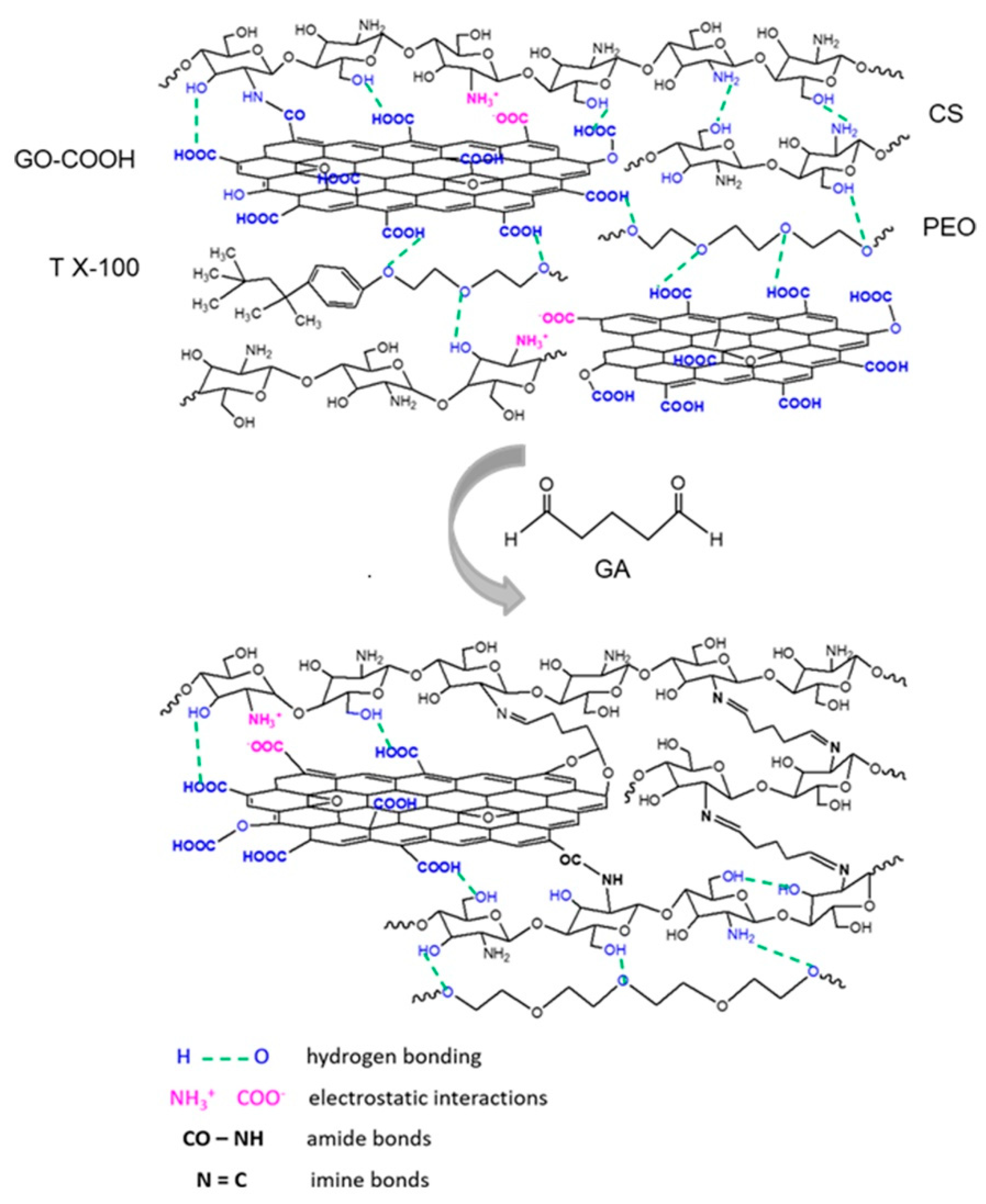
2.4. Crosslinking of Electrospun Nanofibrous Scaffolds
2.5. Characterization Methods
2.5.1. Hydrodynamic Diameter, Polydispersity Index and Diffusion
2.5.2. Structural Characterization
2.5.3. Morphological Characterization
2.5.4. Mechanical Analyses
2.5.5. DSC Investigations
2.5.6. Wettability Investigations
2.5.7. In Vitro Degradation
2.5.8. In Vitro Cytocompatibility and Cytotoxicity
2.5.9. Statistical Analyses
3. Results and Discussions
3.1. Hydrodynamic Studies
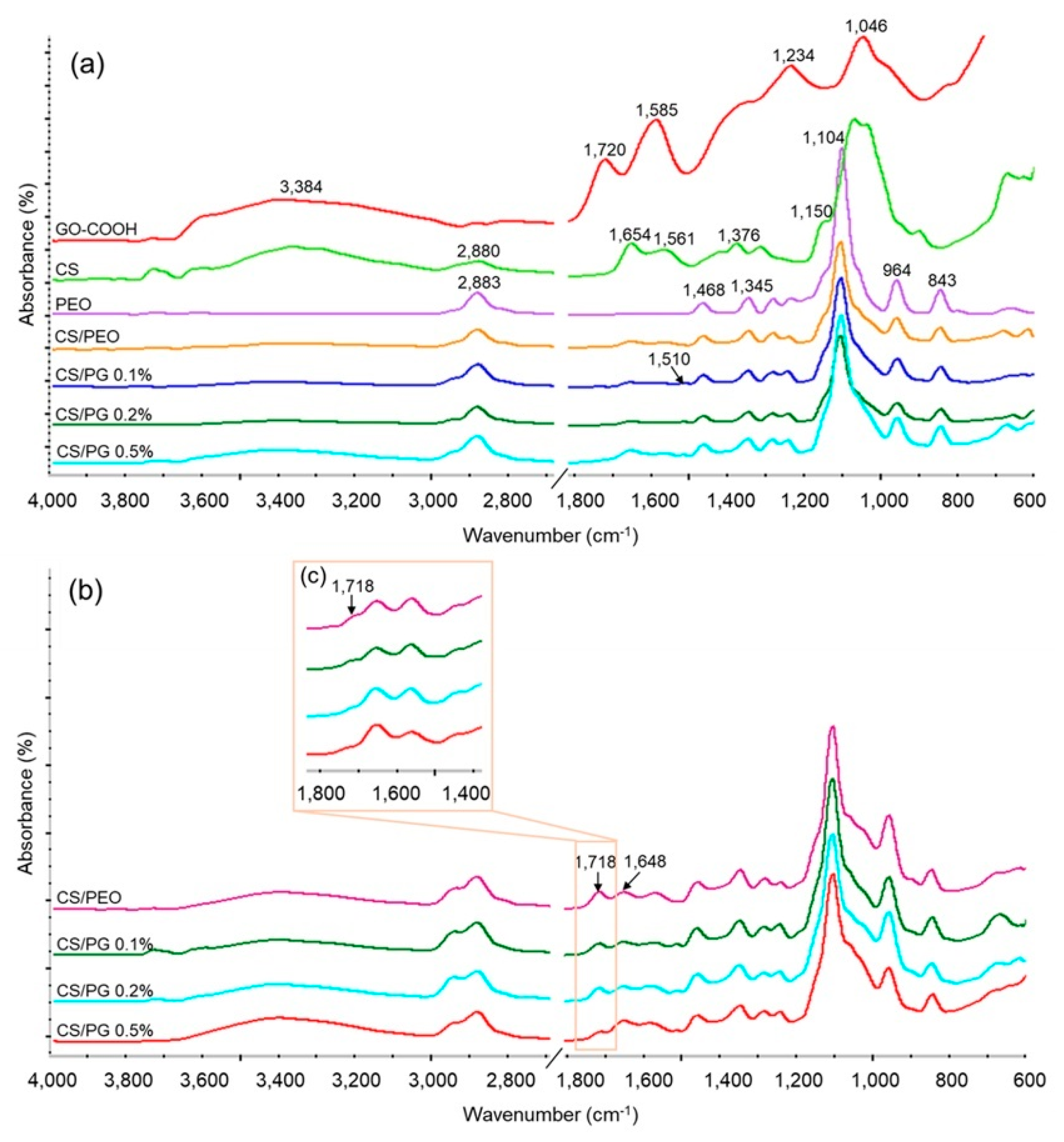
3.2. FTIR Studies
3.3. Raman Spectrometry Results
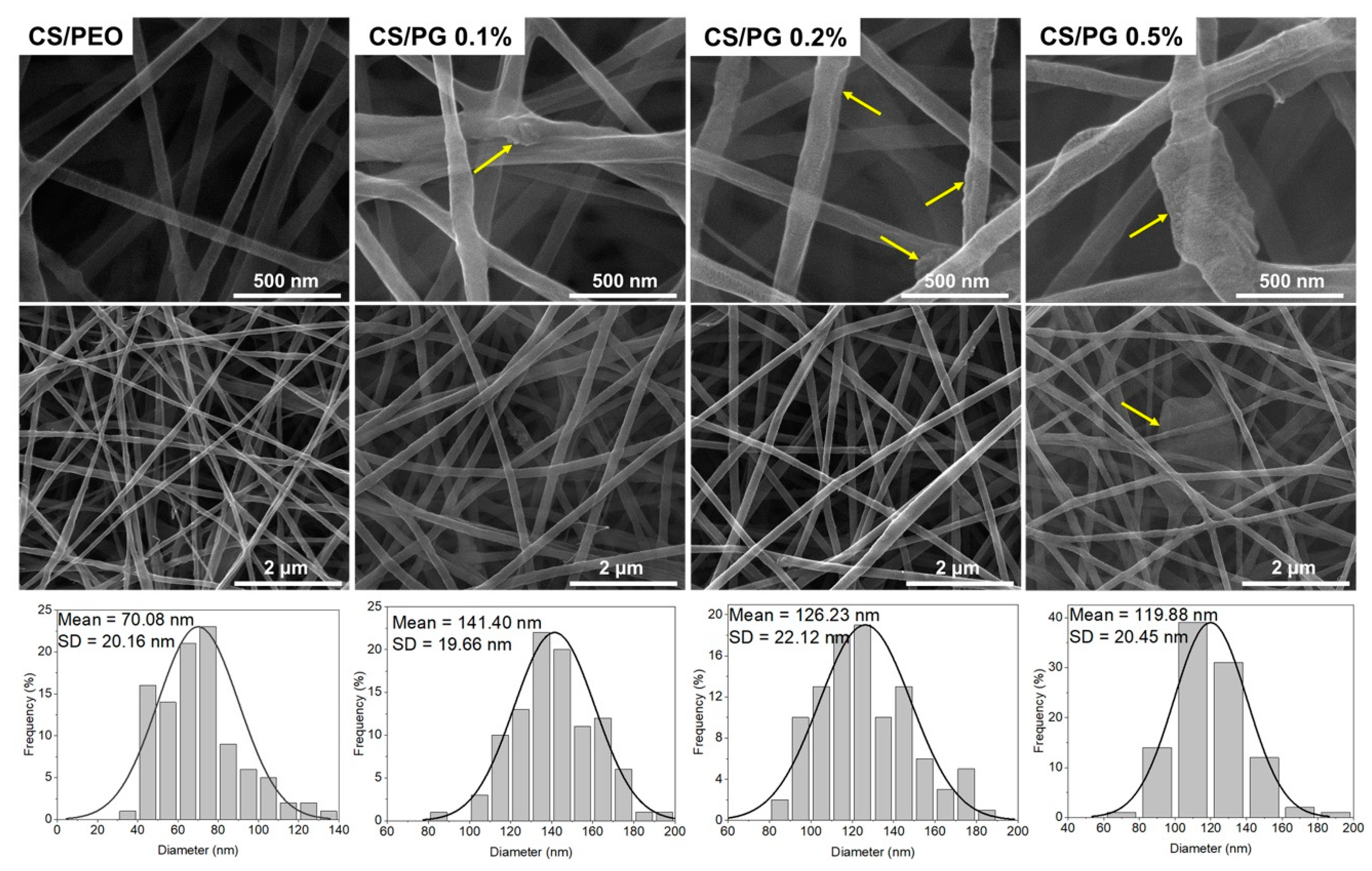
3.4. Morphology Investigation
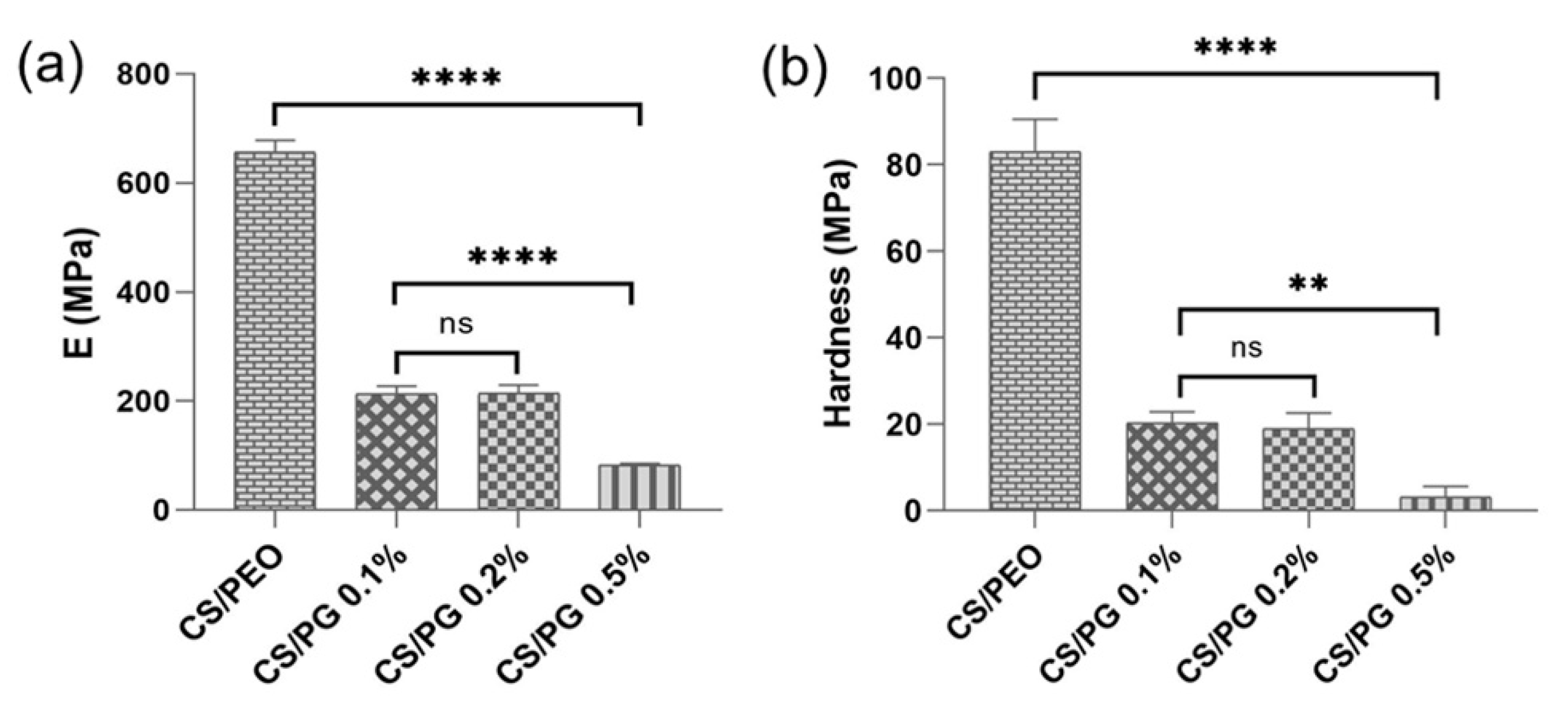
3.5. Mechanical Features Investigated by Nanoindentation
3.6. DSC Tests
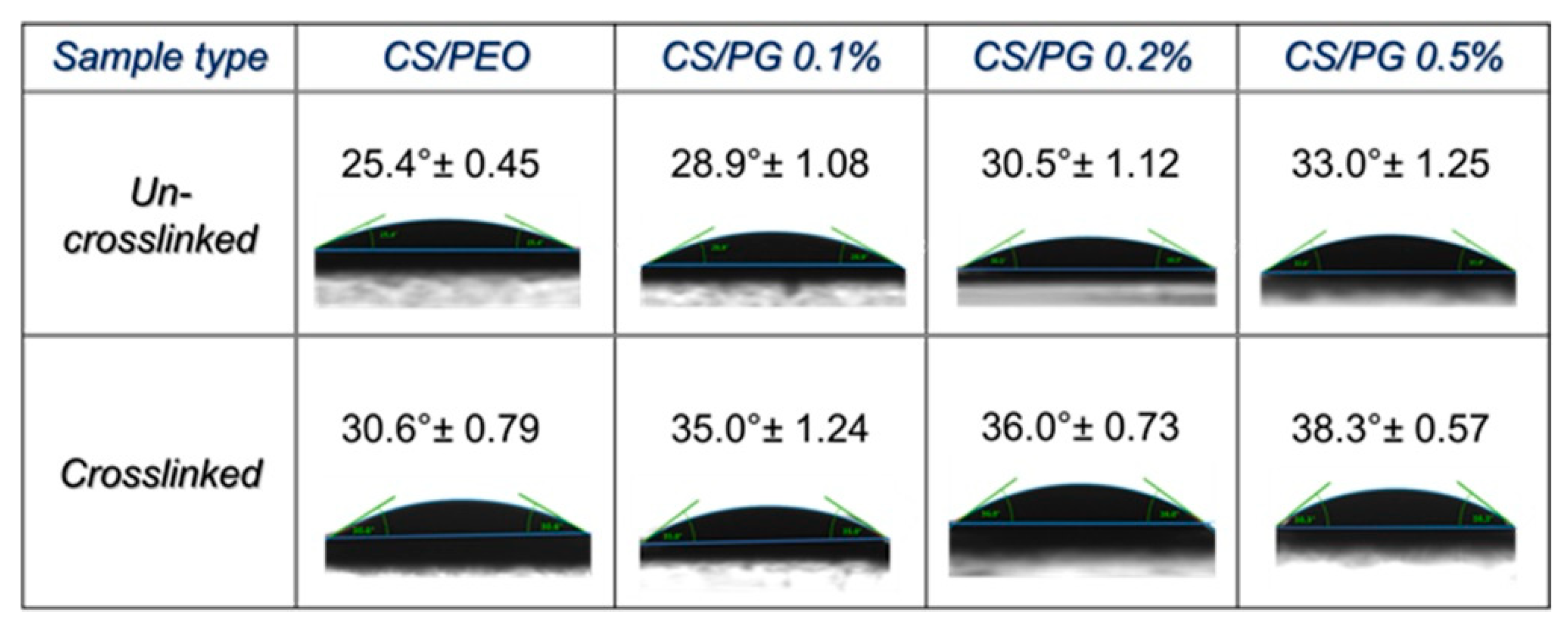
3.7. Wettability Properties
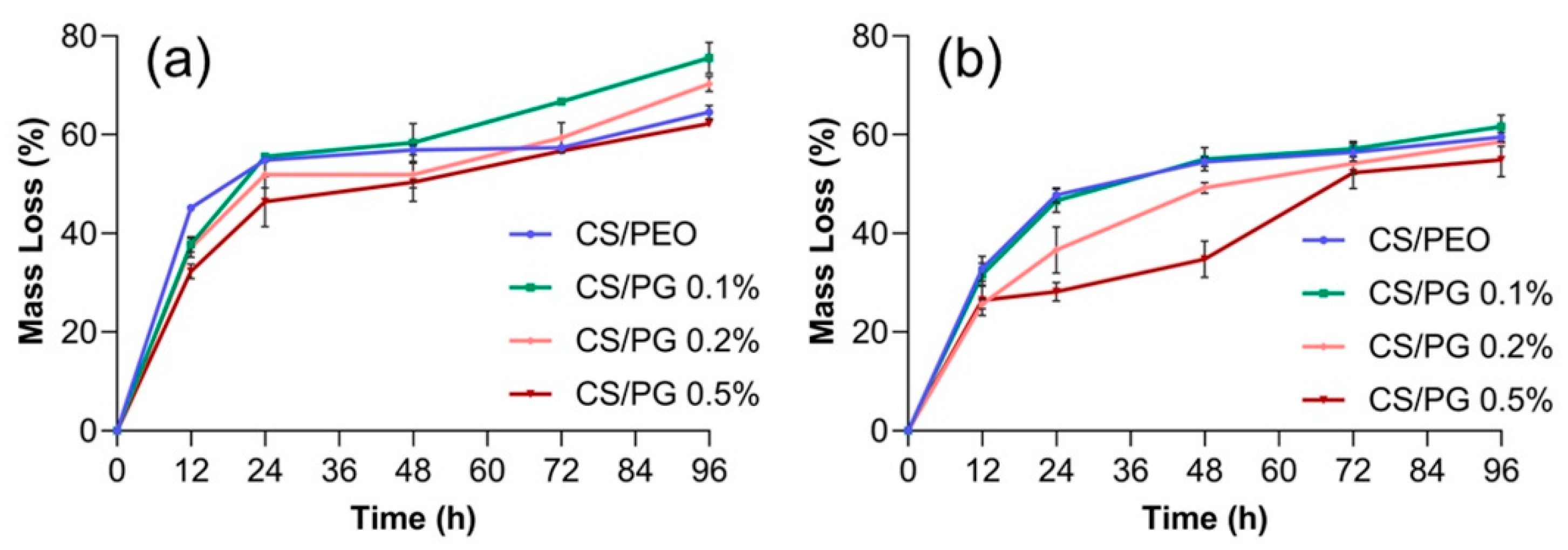
3.8. In Vitro Degradation Studies
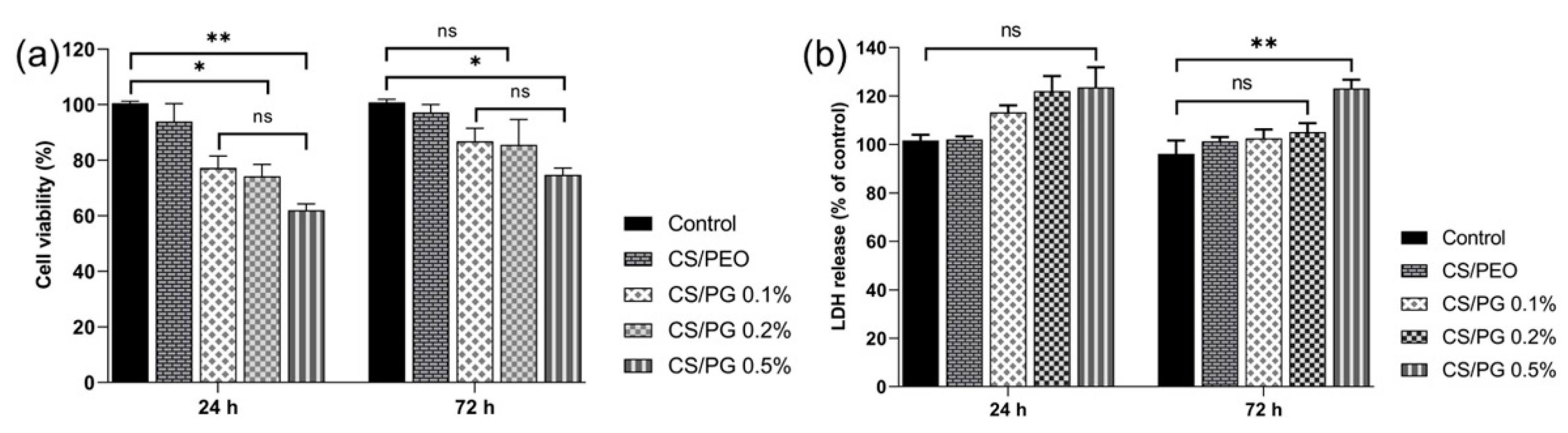
3.9. In Vitro Cytocompatibility and Cytotoxicity Assessment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kajdič, S.; Planinšek, O.; Gašperlin, M.; Kocbek, P. Electrospun Nanofibers for Customized Drug-Delivery Systems. J. Drug Deliv. Sci. Technol. 2019, 51, 672–681. [Google Scholar] [CrossRef]
- Ambekar, R.S.; Kandasubramanian, B. Advancements in Nanofibers for Wound Dressing: A Review. Eur. Polym. J. 2019, 117, 304–336. [Google Scholar] [CrossRef]
- Tao, F.; Cheng, Y.; Shi, X.; Zheng, H.; Du, Y.; Xiang, W.; Deng, H. Applications of Chitin and Chitosan Nanofibers in Bone Regenerative Engineering. Carbohydr. Polym. 2020, 230, 115658. [Google Scholar] [CrossRef] [PubMed]
- Perez-Puyana, V.; Rubio-Valle, J.F.; Jiménez-Rosado, M.; Guerrero, A.; Romero, A. Alternative Processing Methods of Hybrid Porous Scaffolds Based on Gelatin and Chitosan. J. Mech. Behav. Biomed. Mater. 2020, 102, 103472. [Google Scholar] [CrossRef] [PubMed]
- Perez-Puyana, V.; Rubio-Valle, J.; Jiménez-Rosado, M.; Guerrero, A.; Romero, A. Chitosan as a Potential Alternative to Collagen for the Development of Genipin-Crosslinked Scaffolds. React. Funct. Polym. 2020, 146, 104414. [Google Scholar] [CrossRef]
- Rajasree, S.R.; Gobalakrishnan, M.; Aranganathan, L.; Karthih, M. Fabrication and Characterization of Chitosan Based Collagen/ Gelatin Composite Scaffolds from Big Eye Snapper Priacanthus Hamrur Skin for Antimicrobial and Anti Oxidant Applications. Mater. Sci. Eng. C 2020, 107, 110270. [Google Scholar] [CrossRef]
- Prakash, J.; Prema, D.; Venkataprasanna, K.; Balagangadharan, K.; Selvamurugan, N.; Venkatasubbu, G.D. Nanocomposite Chitosan Film Containing Graphene oxide/hydroxyapatite/Gold for Bone Tissue Engineering. Int. J. Biol. Macromol. 2020, 154, 62–71. [Google Scholar] [CrossRef]
- Gierszewska, M.; Ostrowska-Czubenko, J. Chitosan-Based Membranes With Different Ionic Crosslinking Density for Pharmaceutical and Industrial Applications. Carbohydr. Polym. 2016, 153, 501–511. [Google Scholar] [CrossRef]
- Hamed, I.; Özogul, F.; Regenstein, J.M. Industrial Applications of Crustacean by-Products (chitin, Chitosan, and chitooligosaccharides): A Review. Trends Food Sci. Technol. 2016, 48, 40–50. [Google Scholar] [CrossRef]
- Huang, Y.-M.; Lin, Y.-C.; Chen, C.-Y.; Hsieh, Y.-Y.; Liaw, C.-K.; Huang, S.-W.; Tsuang, Y.-H.; Chen, C.-H.; Lin, F.-H. Thermosensitive Chitosan–Gelatin–Glycerol Phosphate Hydrogels as Collagenase Carrier for Tendon–Bone Healing in a Rabbit Model. Polymer 2020, 12, 436. [Google Scholar] [CrossRef]
- Palma, P.J.; Ramos, J.C.; Martins, J.B.; Diogenes, A.; Figueiredo, M.H.; Ferreira, P.; Viegas, C.; Santos, J.M. Histologic Evaluation of Regenerative Endodontic Procedures With the Use of Chitosan Scaffolds in Immature Dog Teeth With Apical Periodontitis. J. Endod. 2017, 43, 1279–1287. [Google Scholar] [CrossRef] [PubMed]
- Pakravan, M.; Heuzey, M.-C.; Ajji, A. A Fundamental Study of chitosan/PEO Electrospinning. Polymer 2011, 52, 4813–4824. [Google Scholar] [CrossRef]
- Zivanovic, S.; Li, J.; Davidson, P.M.; Kit, K. Physical, Mechanical, and Antibacterial Properties of Chitosan/PEO Blend Films. Biomacromolecules 2007, 8, 1505–1510. [Google Scholar] [CrossRef]
- Yang, S.; Lei, P.; Shan, Y.; Zhang, D. Preparation and Characterization of Antibacterial Electrospun chitosan/poly (vinyl alcohol)/Graphene Oxide Composite Nanofibrous Membrane. Appl. Surf. Sci. 2018, 435, 832–840. [Google Scholar] [CrossRef]
- Thien, D.V.H.; Hsiao, S.W.; Ho, M.H.; Li, C.H.; Shih, J.L. Electrospun chitosan/Hydroxyapatite Nanofibers for Bone Tissue Engineering. J. Mater. Sci. 2013, 48, 1640–1645. [Google Scholar] [CrossRef]
- Ardeshirzadeh, B.; Anaraki, N.A.; Irani, M.; Rad, L.R.; Shamshiri, S. Controlled Release of Doxorubicin from Electrospun PEO/chitosan/Graphene Oxide Nanocomposite Nanofibrous Scaffolds. Mater. Sci. Eng. C 2015, 48, 384–390. [Google Scholar] [CrossRef]
- Mendes, A.C.L.; Gorzelanny, C.; Halter, N.; Schneider, S.W.; Chronakis, I.S. Hybrid Electrospun Chitosan-Phospholipids Nanofibers for Transdermal Drug Delivery. Int. J. Pharm. 2016, 510, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Wei, X.; Kysar, J.W.; Hone, J. Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Ryan, A.J.; Kearney, C.J.; Shen, N.; Khan, U.; Kelly, A.G.; Probst, C.; Brauchle, E.; Biccai, S.; Garciarena, C.D.; Vega-Mayoral, V.; et al. Electroconductive Biohybrid Collagen/Pristine Graphene Composite Biomaterials With Enhanced Biological Activity. Adv. Mater. 2018, 30, e1706442. [Google Scholar] [CrossRef]
- Wang, K.; Ruan, J.; Song, H.; Zhang, J.; Wo, Y.; Guo, S.; Cui, D. Biocompatibility of Graphene Oxide. Nanoscale Res. Lett. 2010, 6, 8. [Google Scholar] [CrossRef]
- Yoon, H.H.; Bhang, S.H.; Kim, T.; Yu, T.; Hyeon, T.; Kim, B.-S. Dual Roles of Graphene Oxide in Chondrogenic Differentiation of Adult Stem Cells: Cell-Adhesion Substrate and Growth Factor-Delivery Carrier. Adv. Funct. Mater. 2014, 24, 6455–6464. [Google Scholar] [CrossRef]
- Zhou, M.; Lozano, N.; Wychowaniec, J.K.; Hodgkinson, T.; Richardson, S.M.; Kostarelos, K.; Hoyland, J.A. Graphene Oxide: A Growth Factor Delivery Carrier to Enhance Chondrogenic Differentiation of Human Mesenchymal Stem Cells in 3D Hydrogels. Acta Biomater. 2019, 96, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Nayak, T.R.; Andersen, H.; Makam, V.S.; Khaw, C.; Bae, S.; Xu, X.; Ee, P.-L.R.; Ahn, J.-H.; Hong, B.H.; Pastorin, G.; et al. Graphene for Controlled and Accelerated Osteogenic Differentiation of Human Mesenchymal Stem Cells. ACS Nano 2011, 5, 4670–4678. [Google Scholar] [CrossRef]
- Lasocka, I.; Szulc-Dąbrowska, L.; Skibniewski, M.; Skibniewska, E.; Strupiński, W.; Pasternak, I.; Kmieć, H.; Kowalczyk, P. Biocompatibility of Pristine Graphene Monolayer: Scaffold for Fibroblasts. Toxicol. Vitr. 2018, 48, 276–285. [Google Scholar] [CrossRef]
- Pattammattel, A.; Puglia, M.; Chakraborty, S.; Deshapriya, I.K.; Dutta, P.K.; Kumar, C.V. Tuning the Activities and Structures of Enzymes Bound to Graphene Oxide With a Protein Glue. Langmuir 2013, 29, 15643–15654. [Google Scholar] [CrossRef]
- Song, Y.; Qu, K.; Zhao, C.; Ren, J.; Qu, X. Graphene Oxide: Intrinsic Peroxidase Catalytic Activity and Its Application to Glucose Detection. Adv. Mater. 2010, 22, 2206–2210. [Google Scholar] [CrossRef]
- Kaur, P.; Shin, M.-S.; Sharma, N.; Kaur, N.; Joshi, A.; Chae, S.-R.; Park, J.-S.; Kang, M.-S.; Sekhon, S.S. Non-Covalent Functionalization of Graphene With poly(diallyl Dimethylammonium) Chloride: Effect of a Non-Ionic Surfactant. Int. J. Hydrogen Energy 2015, 40, 1541–1547. [Google Scholar] [CrossRef]
- Cao, L.; Zhang, F.; Wang, Q.; Wu, X. Fabrication of chitosan/Graphene Oxide Polymer Nanofiber and Its Biocompatibility for Cartilage Tissue Engineering. Mater. Sci. Eng. C 2017, 79, 697–701. [Google Scholar] [CrossRef]
- Liu, Y.; Park, M.; Shin, H.K.; Pant, B.; Choi, J.; Park, Y.W.; Lee, J.Y.; Park, S.-J.; Kim, H.-Y. Facile Preparation and Characterization of poly(vinyl alcohol)/chitosan/Graphene Oxide Biocomposite Nanofibers. J. Ind. Eng. Chem. 2014, 20, 4415–4420. [Google Scholar] [CrossRef]
- Sattaria, S.; Tehrani, A.D.; Adelia, M.; Soleimania, K.; Rashidipourb, M. Fabrication of New Generation of Co-Delivery Systems Based on Graphene-G-cyclodextrin/Chitosan Nanofiber. Int. J. Biol. Macromol. 2020, 156, 1126–1134. [Google Scholar] [CrossRef] [PubMed]
- Pavinatto, A.; Mercante, L.A.; Facure, M.H.M.; Pena, R.B.; Sanfelice, R.C.; Mattoso, L.H.C.; Correa, D.S. Ultrasensitive Biosensor Based on polyvinylpyrrolidone/chitosan/Reduced Graphene Oxide Electrospun Nanofibers for 17α—Ethinylestradiol Electrochemical Detection. Appl. Surf. Sci. 2018, 458, 431–437. [Google Scholar] [CrossRef]
- Mehdizadeh, B.; Maleknia, L.; Amirabadi, A.; Shabani, M. Glucose Sensing by a Glassy Carbon Electrode Modified With Glucose oxidase/chitosan/Graphene Oxide Nanofibers. Diam. Relat. Mater. 2020, 109, 108073. [Google Scholar] [CrossRef]
- Robinson, A.J.; Pérez-Nava, A.; Ali, S.C.; González-Campos, J.B.; Holloway, J.L.; Cosgriff-Hernandez, E.M. Comparative Analysis of Fiber Alignment Methods in Electrospinning. Matter 2021, 4, 821–844. [Google Scholar] [CrossRef]
- Alishahi, M.; Khorram, M.; Asgari, Q.; Davani, F.; Goudarzi, F.; Emami, A.; Arastehfar, A.; Zomorodian, K. Glucantime-Loaded Electrospun Core-Shell Nanofibers Composed of poly(ethylene oxide)/gelatin-poly(vinyl alcohol)/Chitosan As Dressing for Cutaneous Leishmaniasis. Int. J. Biol. Macromol. 2020, 163, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Baldino, L.; Cardea, S.; Reverchon, E. A Supercritical CO2 Assisted Electrohydrodynamic Process Used to Produce Microparticles and Microfibers of a Model Polymer. J. CO2 Util. 2019, 33, 532–540. [Google Scholar] [CrossRef]
- Zhao, L.; Guan, X.; Yu, B.; Ding, N.; Liu, X.; Ma, Q.; Yang, S.; Yilihamu, A.; Yang, S.-T. Carboxylated Graphene Oxide-Chitosan Spheres Immobilize Cu2+ in Soil and Reduce Its Bioaccumulation in Wheat Plants. Environ. Int. 2019, 133, 105208. [Google Scholar] [CrossRef] [PubMed]
- Biru, I.; Damian, C.; Gârea, S.; Iovu, H. Benzoxazine-Functionalized Graphene Oxide for Synthesis of New Nanocomposites. Eur. Polym. J. 2016, 83, 244–255. [Google Scholar] [CrossRef]
- Cheng, C.; Shen, L.; Yu, X.; Yang, Y.; Li, X.; Wang, X. Robust Construction of a Graphene Oxide Barrier Layer on a Nanofibrous Substrate Assisted by the Flexible poly(vinylalcohol) for Efficient Pervaporation Desalination. J. Mater. Chem. A 2017, 5, 3558–3568. [Google Scholar] [CrossRef]
- Geng, X.; Kwon, O.-H.; Jang, J. Electrospinning of Chitosan Dissolved in Concentrated Acetic Acid Solution. Biomaterials 2005, 26, 5427–5432. [Google Scholar] [CrossRef] [PubMed]
- Mya, K.Y.; Jamieson, A.M.; Sirivat, A. Interactions Between the Nonionic Surfactant and Polyacrylamide Studied by Light Scattering and Viscometry. Polymer 1999, 40, 5741–5749. [Google Scholar] [CrossRef]
- Shih, C.-J.; Lin, S.; Sharma, R.; Strano, M.S.; Blankschtein, D. Understanding the PH-Dependent Behavior of Graphene Oxide Aqueous Solutions: A Comparative Experimental and Molecular Dynamics Simulation Study. Langmuir 2012, 28, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Stetefeld, J.; McKenna, S.A.; Patel, T.R. Dynamic Light Scattering: A Practical Guide and Applications in Biomedical Sciences. Biophys. Rev. 2016, 8, 409–427. [Google Scholar] [CrossRef]
- Doi, M.; Edwards, S.F. The Theory of Polymer Dynamics; Oxford University Press: New York, NY, USA, 1988; Volume 73, pp. 1–391. [Google Scholar]
- He, D.; Peng, Z.; Gong, W.; Luo, Y.; Zhao, P.; Kong, L. Mechanism of a Green Graphene Oxide Reduction with Reusable Potassium Carbonate. RSC Adv. 2015, 5, 11966–11972. [Google Scholar] [CrossRef]
- Ghitman, J.; Stan, R.; Cecoltan, S.; Chifiriuc, M.C.; Iovu, H. Hybrid Nanocarriers Based on PLGA-Vegetable Oil: A Novel Approach for High Lipophilic Drug Delivery. J. Drug Deliv. Sci. Technol. 2018, 46, 162–172. [Google Scholar] [CrossRef]
- Silva, S.S.; Oliveira, J.M.; Benesch, J.; Caridade, S.G.; Mano, J.F.; Reis, R.R. Hybrid Biodegradable Membranes of Silane-Treated chitosan/Soy Protein for Biomedical Applications. J. Bioact. Compat. Polym. 2013, 28, 385–397. [Google Scholar] [CrossRef]
- Brako, F.; Raimi-Abraham, B.; Mahalingam, S.; Craig, D.Q.; Edirisinghe, M. Making Nanofibres of Mucoadhesive Polymer Blends for Vaginal Therapies. Eur. Polym. J. 2015, 70, 186–196. [Google Scholar] [CrossRef]
- Singh, A.; Sinsinbar, G.; Choudhary, M.; Kumar, V.; Pasricha, R.; Verma, H.; Singh, S.P.; Arora, K. Graphene Oxide-Chitosan Nanocomposite Based Electrochemical DNA Biosensor for Detection of Typhoid. Sens. Actuators B Chem. 2013, 185, 675–684. [Google Scholar] [CrossRef]
- Ingole, P.G.; Thakare, N.R.; Kim, K.; Bajaj, H.C.; Singh, K.; Lee, H. Preparation, Characterization and Performance Evaluation of Separation of Alcohol Using Crosslinked Membrane Materials. New J. Chem. 2013, 37, 4018–4024. [Google Scholar] [CrossRef]
- Sutirman, Z.A.; Sanagi, M.M.; Karim, K.J.A.; Ibrahim, W.A.W. Preparation of Methacrylamide-Functionalized Crosslinked Chitosan by Free Radical Polymerization for the Removal of Lead Ions. Carbohydr. Polym. 2016, 151, 1091–1099. [Google Scholar] [CrossRef] [PubMed]
- King, A.A.K.; Davies, B.R.; Noorbehesht, N.; Newman, P.; Church, T.L.; Harris, A.T.; Razal, J.M.; Minett, A.I. A New Raman Metric for the Characterisation of Graphene Oxide and Its Derivatives. Sci. Rep. 2016, 6, 19491. [Google Scholar] [CrossRef]
- Bîru, E.I.; Iovu, H. Graphene Nanocomposites Studied by Raman Spectroscopy. Raman Spectrosc. 2018, 179–201. [Google Scholar] [CrossRef]
- Wu, T.; Ding, M.; Shi, C.; Qiao, Y.; Wang, P.; Qiao, R.; Wang, X.; Zhong, J. Resorbable Polymer Electrospun Nanofibers: History, Shapes and Application for Tissue Engineering. Chin. Chem. Lett. 2020, 31, 617–625. [Google Scholar] [CrossRef]
- Yang, Y.; Rigdon, W.; Huang, X.; Li, X. Enhancing Graphene Reinforcing Potential in Composites by Hydrogen Passivation Induced Dispersion. Sci. Rep. 2013, 3, srep02086. [Google Scholar] [CrossRef] [PubMed]
- Atif, R.; Inam, F. Reasons and Remedies for the Agglomeration of Multilayered Graphene and Carbon Nanotubes in Polymers. Beilstein J. Nanotechnol. 2016, 7, 1174–1196. [Google Scholar] [CrossRef]
- Jalaja, K.; Sreehari, V.; Kumar, P.A.; Nirmala, R.J. Graphene Oxide Decorated Electrospun Gelatin Nanofibers: Fabrication, Properties and Applications. Mater. Sci. Eng. C 2016, 64, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Chhetri, S.; Kuila, T.; Murmu, N.C. Graphene Composites. In Graphene Technology: From Laboratory to Fabrication; John Wiley & Sons: Hoboken, NJ, USA, 2016; Chapter 3; pp. 63–111. [Google Scholar] [CrossRef]
- Xu, Q.; Zeng, M.; Feng, Z.; Yin, D.; Huang, Y.; Chen, Y.; Yan, C.; Li, R.; Gu, Y. Understanding the Effects of Carboxylated Groups of Functionalized Graphene Oxide on the Curing Behavior and Intermolecular Interactions of Benzoxazine Nanocomposites. RSC Adv. 2016, 6, 31484–31496. [Google Scholar] [CrossRef]
- Ramasamy, P.; Subhapradha, N.; Thinesh, T.; Selvin, J.; Selvan, K.M.; Shanmugam, V.; Shanmugam, A. Characterization of Bioactive Chitosan and Sulfated Chitosan from Doryteuthis Singhalensis (Ortmann, 1891). Int. J. Biol. Macromol. 2017, 99, 682–691. [Google Scholar] [CrossRef] [PubMed]
- Caramia, V.; Bayer, I.S.; Anyfantis, G.C.; Ruffilli, R.; Ayadi, F.; Martiradonna, L.; Cingolani, R.; Athanassiou, A. Tailoring the Morphology of poly(ethylene oxide)/silver Triflate Blends: From Crystalline to Self-Assembled Nanofibrillar Structures. Nanotechnology 2013, 24, 55602. [Google Scholar] [CrossRef]
- Huang, C.-L.; Wu, H.-H.; Jeng, Y.-C.; Liang, W.-Z. Electrospun Graphene Nanosheet-Filled Poly(Trimethylene Terephthalate) Composite Fibers: Effects of the Graphene Nanosheet Content on Morphologies, Electrical Conductivity, Crystallization Behavior, and Mechanical Properties. Polymer 2019, 11, 164. [Google Scholar] [CrossRef] [PubMed]


| Sample | V (mL) | CS/PEO (w/w) Ratio | c(GO-COOH) wt.% | c (Triton X-100) wt.% |
|---|---|---|---|---|
| CS/PEO | 5 | 3/7 | 0 | 1 |
| CS/PG 0.1% | 0.1 | |||
| CS/PG 0.2% | 0.2 | |||
| CS/PG 0.5% | 0.5 |
| Sample | d (nm) | PdI | D (µm2/s) |
|---|---|---|---|
| CS | 1108.00 ± 40.31 | 0.38 ± 0.01 | 0.45 ± 0.01 |
| PEO | 86.83 ± 16.55 | 0.30 ± 0.01 | 6.78 ± 0.18 |
| GO-COOH | 890.00 ± 35.73 | 0.66 ± 0.05 | 0.53 ± 0.02 |
| CS/PEO | 76.66 ± 2.95 | 0.45 ± 0.03 | 1.25 ± 0.03 |
| CS/PG 0.2% | 836.50 ± 13.42 | 0.40 ± 0.01 | 1.16 ± 0.01 |
| Sample | νD (cm−1) | νG (cm−1) | ID/IG (473 nm laser) |
|---|---|---|---|
| GO-COOH | 1360 | 1592 | 0.80 |
| US GO-COOH | 1358 | 1589 | 0.88 |
| CS/PG 0.1% | 1354 | 1589 | 0.93 |
| CS/PG 0.2% | 1356 | 1578 | 0.92 |
| CS/PG 0.5% | 1354 | 1601 | 0.84 |
| Sample | Tm (°C) | ΔHm (J/g) |
|---|---|---|
| CS/PEO | 63.00 ± 1.26 | 53.26 ± 1.06 |
| CS/PG 0.1% | 64.10 ± 2.56 | 58.51 ± 2.34 |
| CS/PG 0.2% | 65.10 ± 1.75 | 59.33 ± 1.60 |
| CS/PG 0.5% | 63.20 ± 2.84 | 61.67 ± 2.77 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cojocaru, E.; Ghitman, J.; Biru, E.I.; Pircalabioru, G.G.; Vasile, E.; Iovu, H. Synthesis and Characterization of Electrospun Composite Scaffolds Based on Chitosan-Carboxylated Graphene Oxide with Potential Biomedical Applications. Materials 2021, 14, 2535. https://doi.org/10.3390/ma14102535
Cojocaru E, Ghitman J, Biru EI, Pircalabioru GG, Vasile E, Iovu H. Synthesis and Characterization of Electrospun Composite Scaffolds Based on Chitosan-Carboxylated Graphene Oxide with Potential Biomedical Applications. Materials. 2021; 14(10):2535. https://doi.org/10.3390/ma14102535
Chicago/Turabian StyleCojocaru, Elena, Jana Ghitman, Elena Iuliana Biru, Gratiela Gradisteanu Pircalabioru, Eugeniu Vasile, and Horia Iovu. 2021. "Synthesis and Characterization of Electrospun Composite Scaffolds Based on Chitosan-Carboxylated Graphene Oxide with Potential Biomedical Applications" Materials 14, no. 10: 2535. https://doi.org/10.3390/ma14102535
APA StyleCojocaru, E., Ghitman, J., Biru, E. I., Pircalabioru, G. G., Vasile, E., & Iovu, H. (2021). Synthesis and Characterization of Electrospun Composite Scaffolds Based on Chitosan-Carboxylated Graphene Oxide with Potential Biomedical Applications. Materials, 14(10), 2535. https://doi.org/10.3390/ma14102535










