Electrical and Optical Properties of Co75Si15B10 Metallic Glass Nanometric Thin Films
Abstract
1. Introduction
2. Materials and Methods
2.1. Deposition of CoSiB TFMG and Co Thin Film
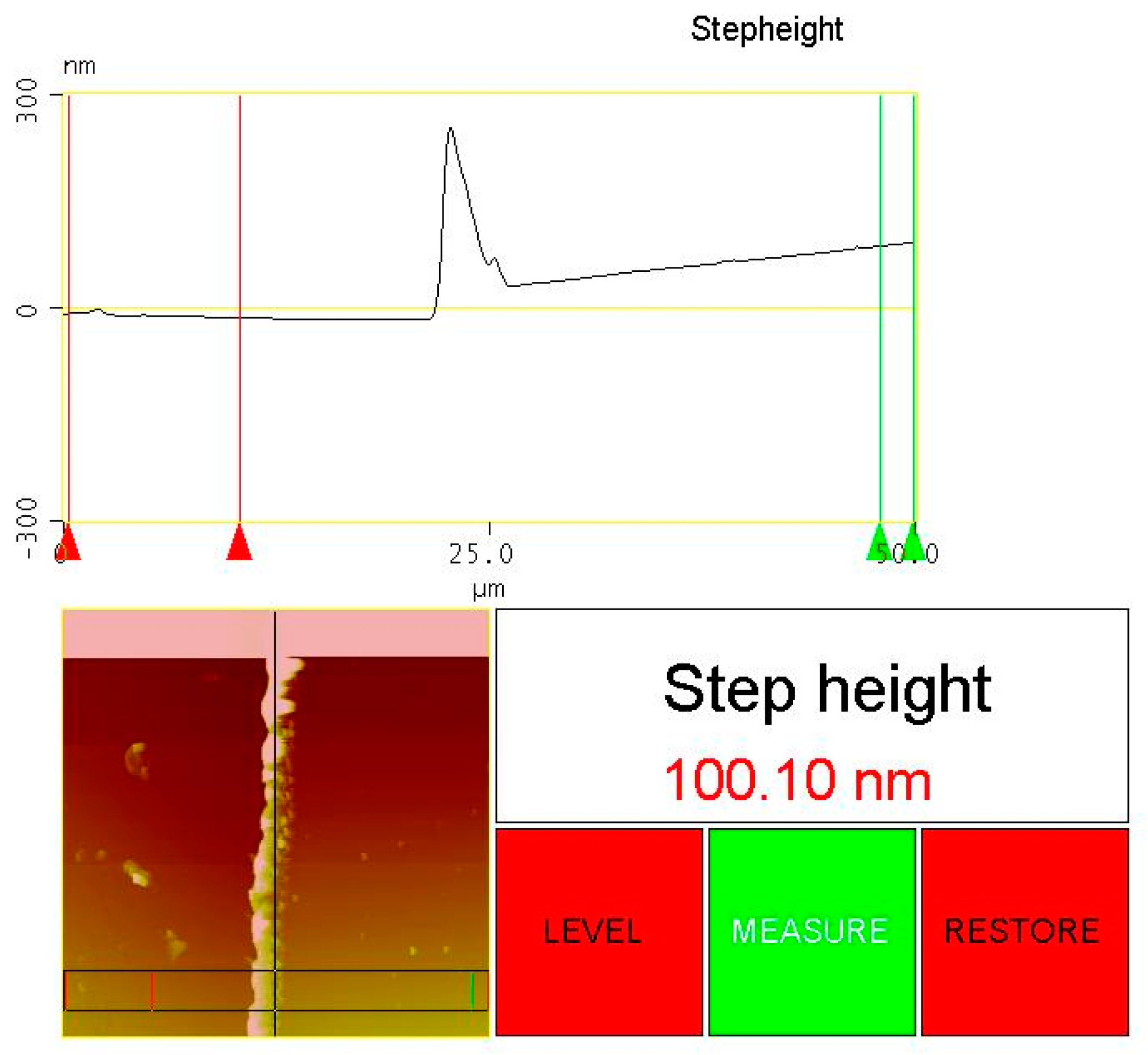
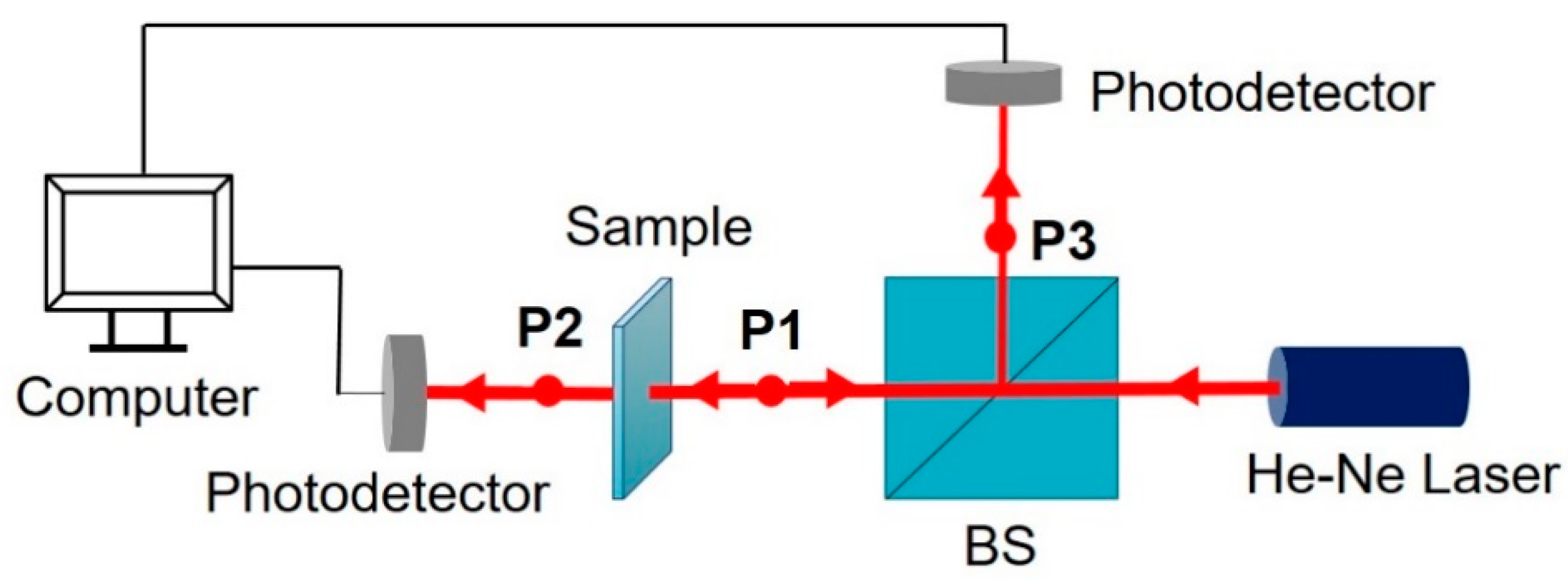
2.2. Characterization Methods
3. Results and Discussion
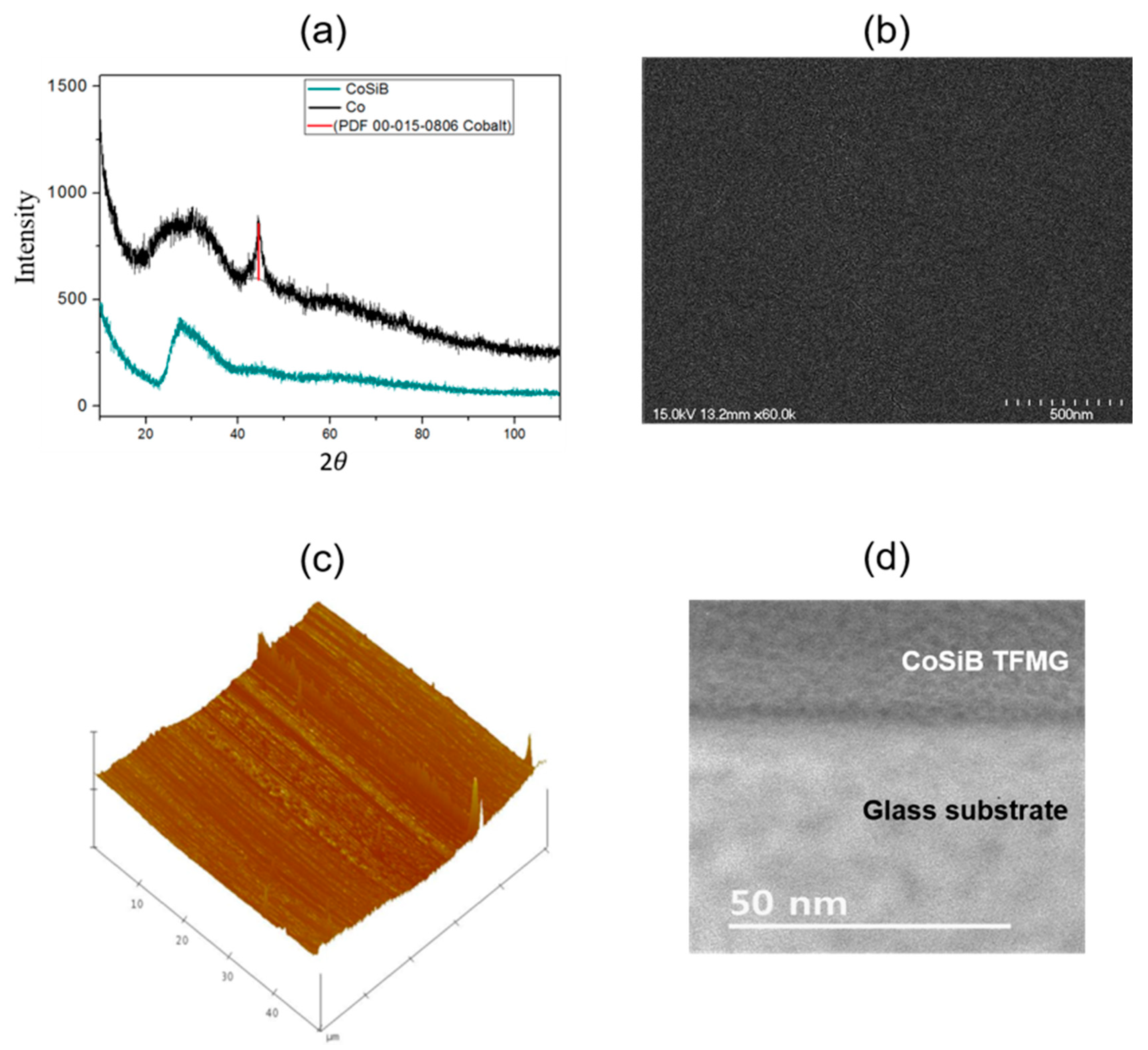
3.1. Structure Characterization
3.2. Characterization Using an LCR (Inductance, Capacitance, and Resistance) Meter
3.3. Hall Measurements at Room Temperature
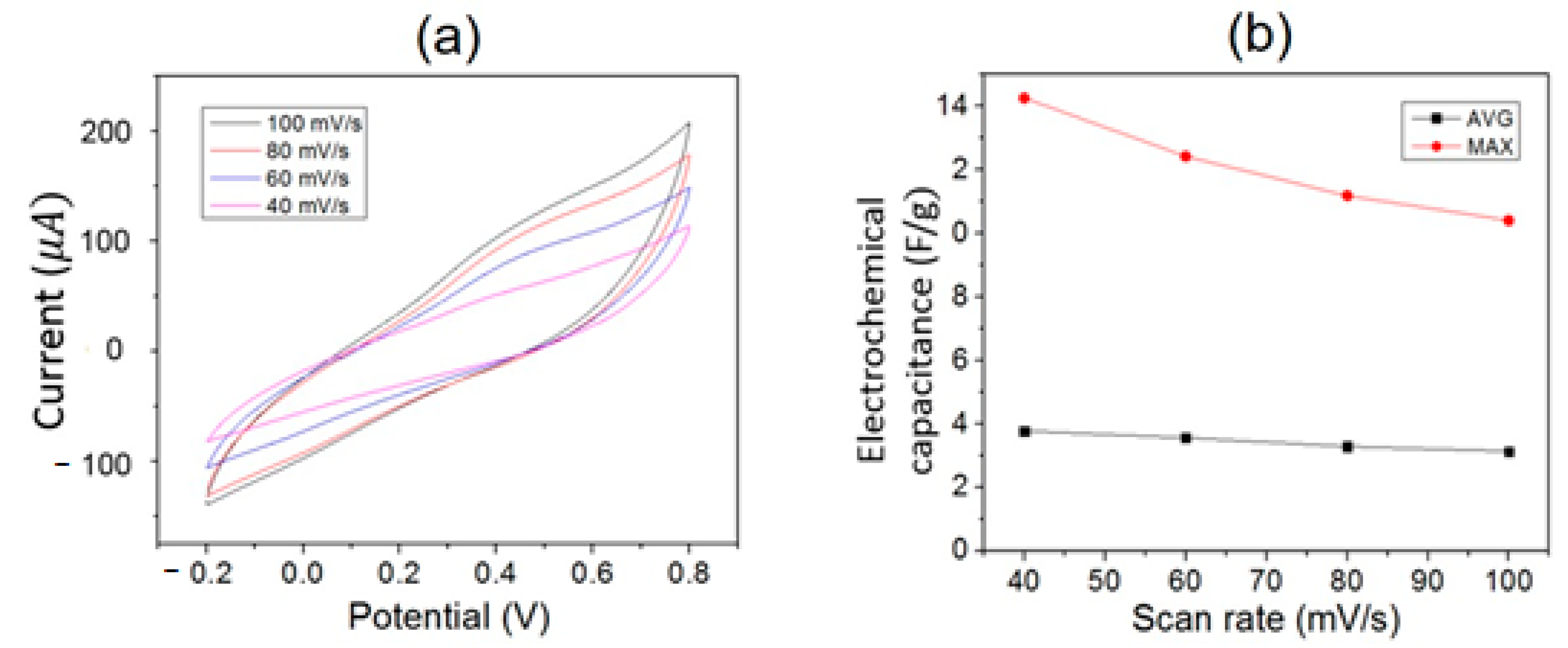
3.4. Electrochemical Measurements
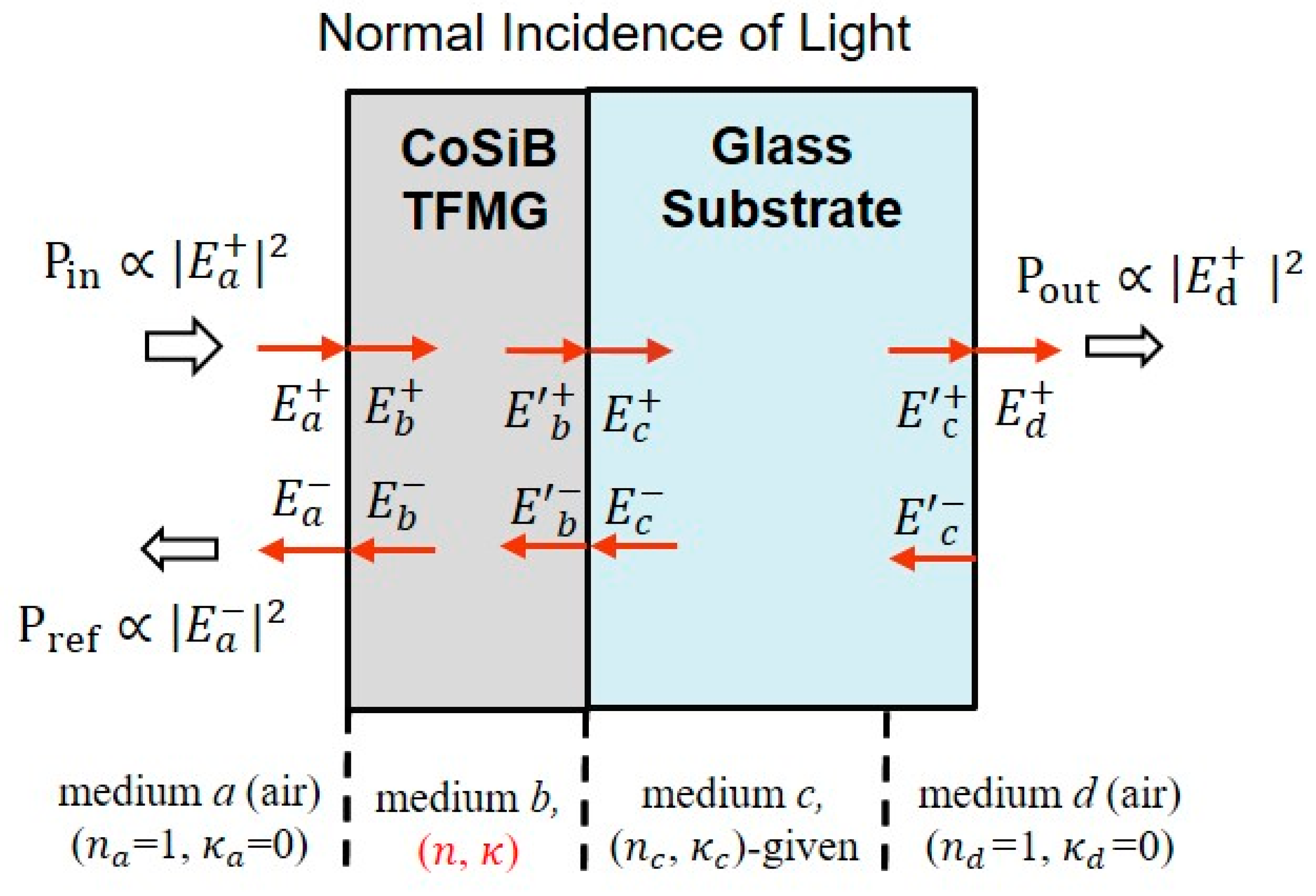
3.5. Optical Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, M.-H.; Suh, J.-Y.; Fleury, E.; Han, S.G.; Hong, K.T. Structural homogeneity and mass density of bulk metallic glasses revealed by their rough surfaces and ultra-small angle neutron scattering (USANS). Sci. Rep. 2018, 8, 12986. [Google Scholar] [CrossRef] [PubMed]
- Klement, W.; Willens, R.; Duwez, P. Non-crystalline Structure in Solidified Gold-Silicon Alloys. Nature 1960, 187, 869–870. [Google Scholar] [CrossRef]
- Liu, Y.; Hata, S.; Wada, K.; Shimokohbe, A. Thermal, Mechanical and Electrical Properties of Pd-Based Thin-Film Metallic Glass. Jpn. Appl. Phys. 2001, 40, 5382–5388. [Google Scholar] [CrossRef]
- Eckert, J.; Kubler, A.; Schultz, L. Mechanically alloyed Zr55Al10Cu30Ni5 metallic glass composites containing nanocrystalline W particles. J. Appl. Phys. 1999, 85, 7112. [Google Scholar] [CrossRef]
- Chen, M. A brief overview of bulk metallic glasses. NPG Asia Mater. 2011, 3, 82–90. [Google Scholar] [CrossRef]
- Johnson, W.L. Fundamental Aspects of Bulk Metallic Glass Formation in Multicomponent Alloys. Mater. Sci. Forum. 1996, 35, 225–227. [Google Scholar] [CrossRef]
- Jiang, Q.K.; Liu, P.; Ma, Y.; Cao, Q.P.; Wang, X.D.; Zhang, D.X.; Han, X.; Zhang, Z.; Jiang, J.Z. Super elastic strain limit in metallic glass film. Sci. Rep. 2012, 2, 852. [Google Scholar] [CrossRef]
- Xian, H.; Liu, M.; Wang, X.; Ye, F.; Wen, P.; Bai, H.; Liu, Y.; Wang, W. Flexible and stretchable metallic glass micro- and nano-structures of tunable properties. Nanotech. J. 2018, 8, 30. [Google Scholar] [CrossRef]
- DeCristofaro, N.J.; Stamatis, P.J.; Fish, G.E. Bulk Amorphous Metal Magnetic Components for Electric Motors. Patent No. 6675459, 13 January 2004. [Google Scholar]
- Martis, R.J.; Hanover, E.; Hasegawa, R.; Tatikola, S.V. Bulk Amorphous Metal Inductive Device. U.S. Patent 6737951, 18 May 2004. [Google Scholar]
- Chu, J.P.; Jang, J.; Huang, J.; Chou, H.; Yang, Y.; Ye, J.; Wang, Y.; Lee, J.; Liu, F.; Liaw, P.; et al. Thin film metallic glasses: Unique properties and potential applications. Thin Sol. Films J. 2012, 16, 520. [Google Scholar] [CrossRef]
- Liu, Y.; Hata, S.; Wada, K.; Shimokohbe, A. Thin Film Metallic Glasses: Fabrication and Property Test. In Proceedings of the Technical Digest. MEMS 2001. 14th IEEE International Conference on Micro Electro Mechanical Systems (Cat. No.01CH37090), Interlaken, Switzerland, 25 January 2001; pp. 102–105. [Google Scholar] [CrossRef]
- Chu, J.P.; Huang, J.C.; Jang JS, C.; Wang, Y.C.; Liaw, P.K. Thin film metallic glasses: Preparations, properties, and applications. JOM J. 2010, 62, 19–24. [Google Scholar] [CrossRef]
- Chu, J.P.; Kacha, B.T.; Yu, C.C.; Lee, C.M. Thin film metallic glasses in optoelectronic, magnetic, and electronic applications: A recent update. Curr. Opin. Solid State Mater. Sci. 2015, 19, 95–106. [Google Scholar]
- Sakurai, J.; Seiichi, H.; Shimokohbe, A. Novel fabrication method of metallic glass thin films using carousel-type sputtering system. Proc. SPIE-Int. Soc. Opt. Eng. 2005, 5650, 260–267. [Google Scholar] [CrossRef]
- Ketov, S.; Joksimovic, R.; Xie, G.; Trifonov, A.; Kurihara, K.; Louzguine-Luzgin, D. Formation of nanostructured metallic glass thin films upon sputtering. Heliyon J. 2017, 1, 3. [Google Scholar] [CrossRef] [PubMed]
- Zeman, P.; Zítek, M.; Zuzjakova, S.; Cerstvý, R. Amorphous Zr-Cu thin-film alloys with metallic glass behavior. J. Alloys Compd. 2017, 696, 1298–1306. [Google Scholar] [CrossRef]
- Glushko, O.; Mühlbacher, M.; Gammer, C.; Cordill, M.J.; Mitterer, C.; Eckert, J. Exceptional fracture resistance of ultrathin metallic glass films due to an intrinsic size effect. Sci. Rep. 2019, 9, 8281. [Google Scholar] [CrossRef]
- Zhang, B.; Yang, X.; Cao, G.; Liu, G.; Wei, Z.; Zong, H.; Yin, Y.; Bala, H. Characteristic studies on the ZrCuAlSi multi-component thin-film metallic glasses fabricated by pulsed laser deposition. Appl. Phys. 2020, A126, 453. [Google Scholar] [CrossRef]
- Pookat, G.; Thomas, H.; Thomas, S.; Al-Harthi, S.H.; Raghavan, L.; Al-Omari, I.A.; Sakthikumar, D.; Ramanujan, R.V.; Anantharaman, M.R. Evolution of structural and magnetic properties of Co–Fe based metallic glass thin films with thermal annealing. Surf. Coat. Technol. J. 2013, 236, 246–251. [Google Scholar] [CrossRef]
- Habib, K.; Caceres, P.G. Properties and structures of Co-based metallic glasses. Scanning J. 1995, 17, 254–262. [Google Scholar] [CrossRef]
- O’Handley, R.C.; Boudreaux, D.S. Magnetic properties of transition metal-metalloid glasses. Phys. Status Solidi 1978, 45, 607–615. [Google Scholar] [CrossRef]
- Kikuchi, M.; Fujimori, H.; Obi, Y.; Masumoto, T. New Amorphous Ferromagnets with Low Coercive Force. Jpn. J. Appl. Phys. 1975, 14, 1077. [Google Scholar] [CrossRef]
- Jung, S.; Han, B.; Yim, H.; Kim, N.; Cho, J.; You, C. Effects of rapid thermal annealing on the magnetic properties of CoSiB/Pd multilayers with perpendicular anisotropy. J. Korean Phys. Soc. 2014, 65, 65–69. [Google Scholar] [CrossRef]
- Hwang, J.; Park, J.; Yim, H.; Kim, T.; Shin, D.; Lee, S. Perpendicular Magnetic Anisotropy in Amorphous Ferromagnetic CoSiB/Pt Multilayers. J. Nanosci. Nanotech. 2011, 11, 856–859. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, S.; Li, F.; Jiang, W.; Zhang, Z.; Chen, Z. Thickness and Temperature Dependent Out-of-Plane Anisotropy of Amorphous CoSiB Thin Films. Phys. Status Solidi B 2018, 255, 1. [Google Scholar] [CrossRef]
- Behl, W.; Toni, J. Anodic oxidation of cobalt in potassium hydroxide electrolytes. J. Electroanal. Chem. 1971, 31, 63. [Google Scholar] [CrossRef]
- Pedrotti, F. Introduction to Optics, 3rd ed.; Cambridge University Press: Cambridge, UK, 2017; p. 477. [Google Scholar]
- Saleh, B.; Teich, M. Fundamentals of Photonics, 2nd ed.; WILEY: Hoboken, NJ, USA, 2009. [Google Scholar]
- Hecht, E. Optics, 5th ed.; Pearson Education Inc.: London, UK, 2017. [Google Scholar]

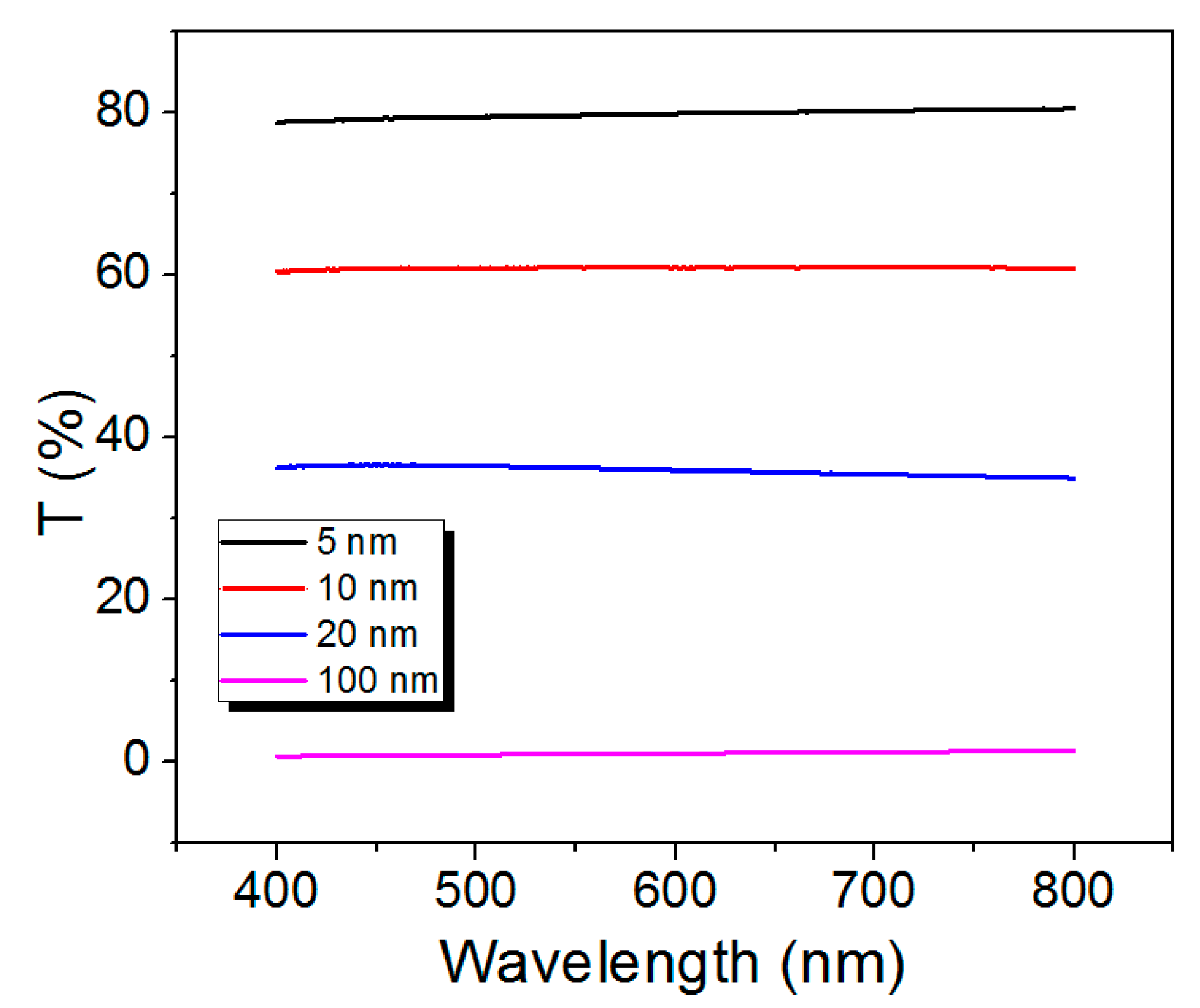
| Thickness [nm] | Sample | Carrier Concentration [/] | Mobility [/Vs] | Resistivity [ ] | Hall Coefficient [/C] |
|---|---|---|---|---|---|
| 100 | CoSiB | 1.107 | 241.3 | ||
| Co | 2.145 | 73.30 | |||
| 200 | CoSiB | 242.9 | |||
| Co | 119.2 | ||||
| 300 | CoSiB | 252.2 | |||
| Co | 87.0 |
| Thickness (nm) | R | T | A | |||
|---|---|---|---|---|---|---|
| CoSiB | 100 | 0.4651 | 0.0001 | 0.5348 | 13.94 +1.12i | |
| 200 | 0.4496 | 0.0011 | 0.5493 | 16.40 + 2.52i | ||
| 300 | 0.4474 | 0.0111 | 0.5415 | 15.23 + 0.15i |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shauyenova, D.; Jung, S.; Yang, H.; Yim, H.; Ju, H. Electrical and Optical Properties of Co75Si15B10 Metallic Glass Nanometric Thin Films. Materials 2021, 14, 162. https://doi.org/10.3390/ma14010162
Shauyenova D, Jung S, Yang H, Yim H, Ju H. Electrical and Optical Properties of Co75Si15B10 Metallic Glass Nanometric Thin Films. Materials. 2021; 14(1):162. https://doi.org/10.3390/ma14010162
Chicago/Turabian StyleShauyenova, Danagul, Sol Jung, Haneul Yang, Haein Yim, and Heongkyu Ju. 2021. "Electrical and Optical Properties of Co75Si15B10 Metallic Glass Nanometric Thin Films" Materials 14, no. 1: 162. https://doi.org/10.3390/ma14010162
APA StyleShauyenova, D., Jung, S., Yang, H., Yim, H., & Ju, H. (2021). Electrical and Optical Properties of Co75Si15B10 Metallic Glass Nanometric Thin Films. Materials, 14(1), 162. https://doi.org/10.3390/ma14010162





