Influence of SiC Sludge on the Microstructure of Geopolymers
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Methods
2.2. Analysis
3. Results
3.1. Mechanical Characteristics of the SiCSMGs
3.2. Setting Time of the SiCSMGs
3.3. Compressive Strength Development of the SiCSMGs
3.4. FTIR Spectroscopy of the SiCSMGs
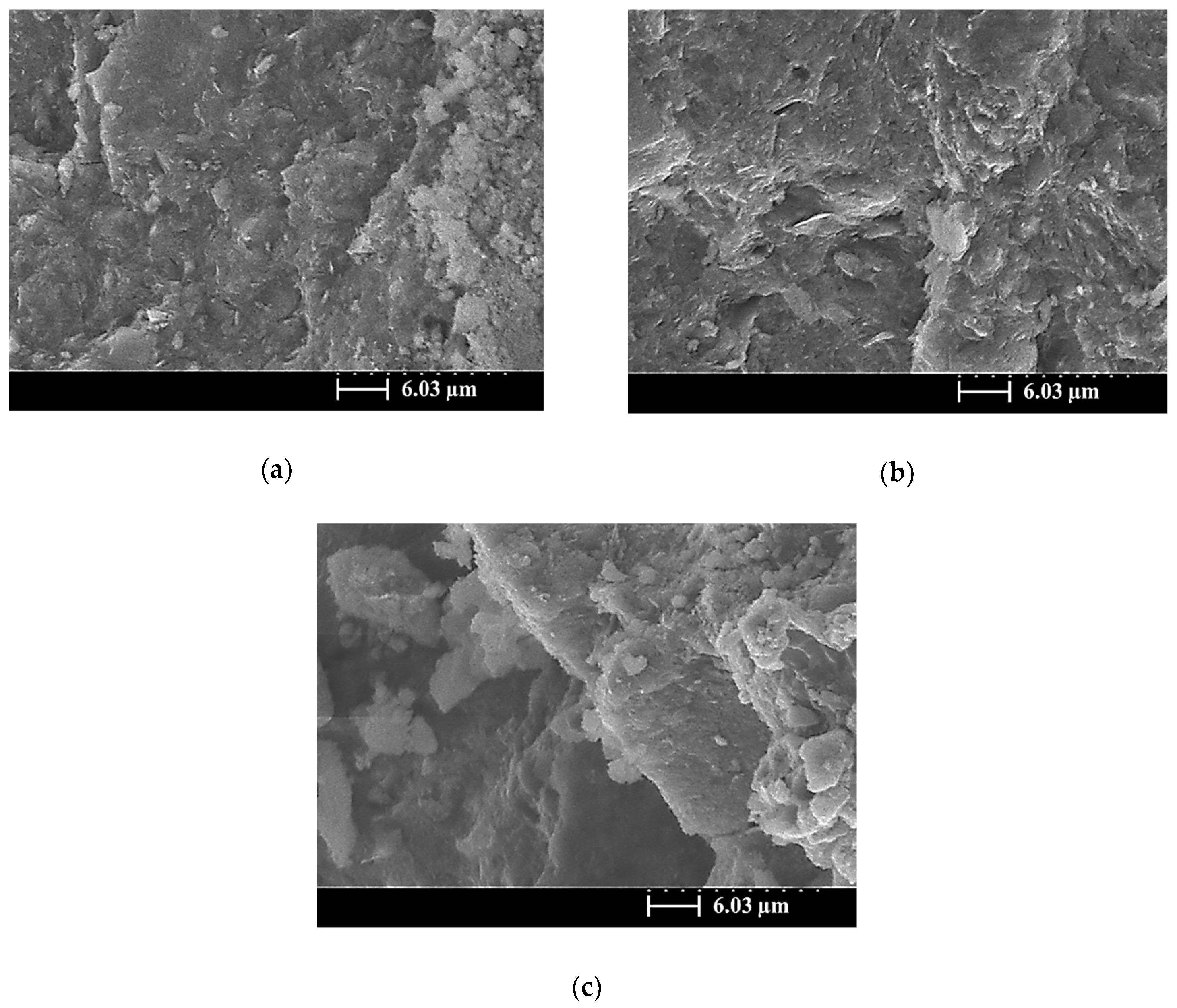
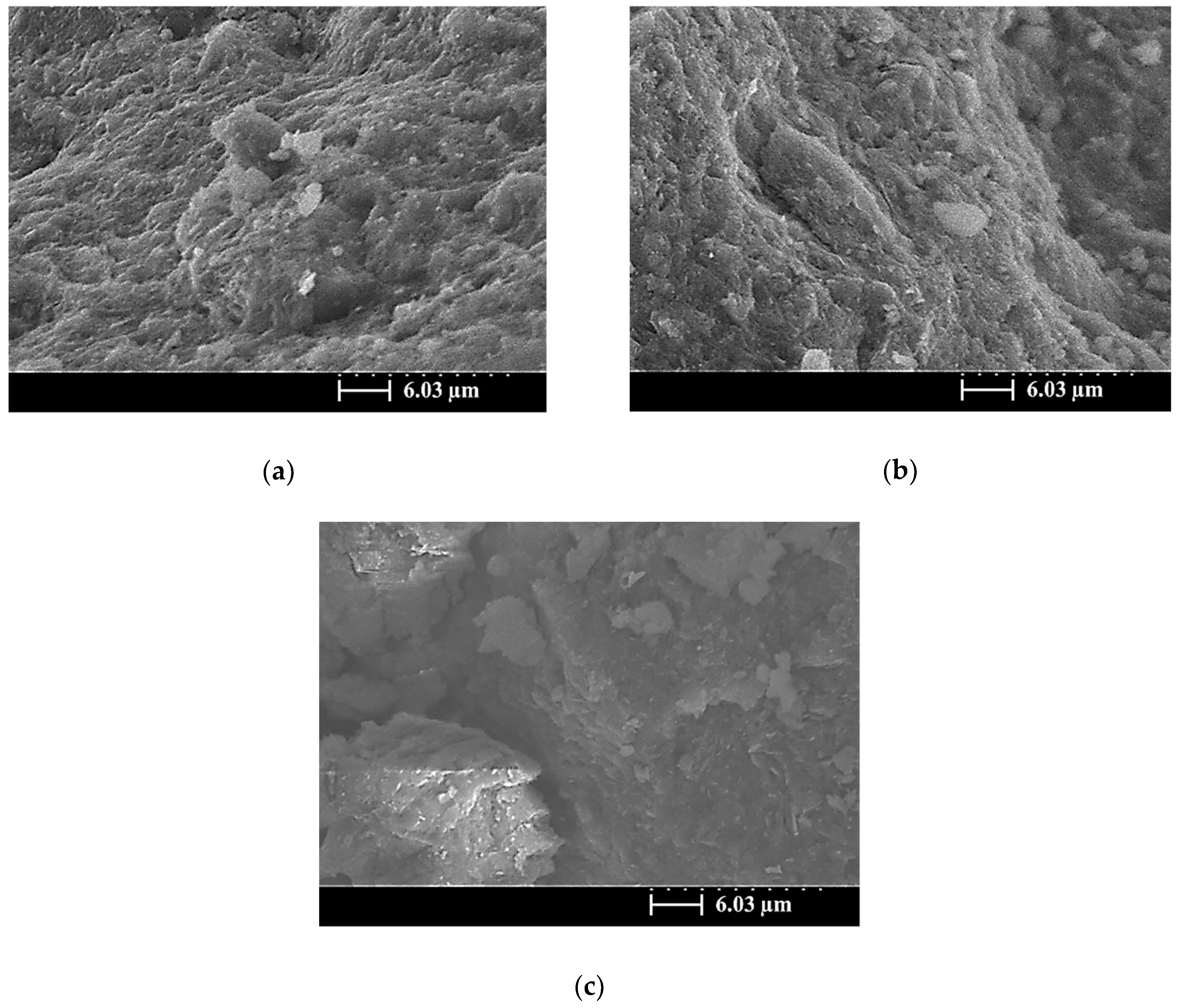
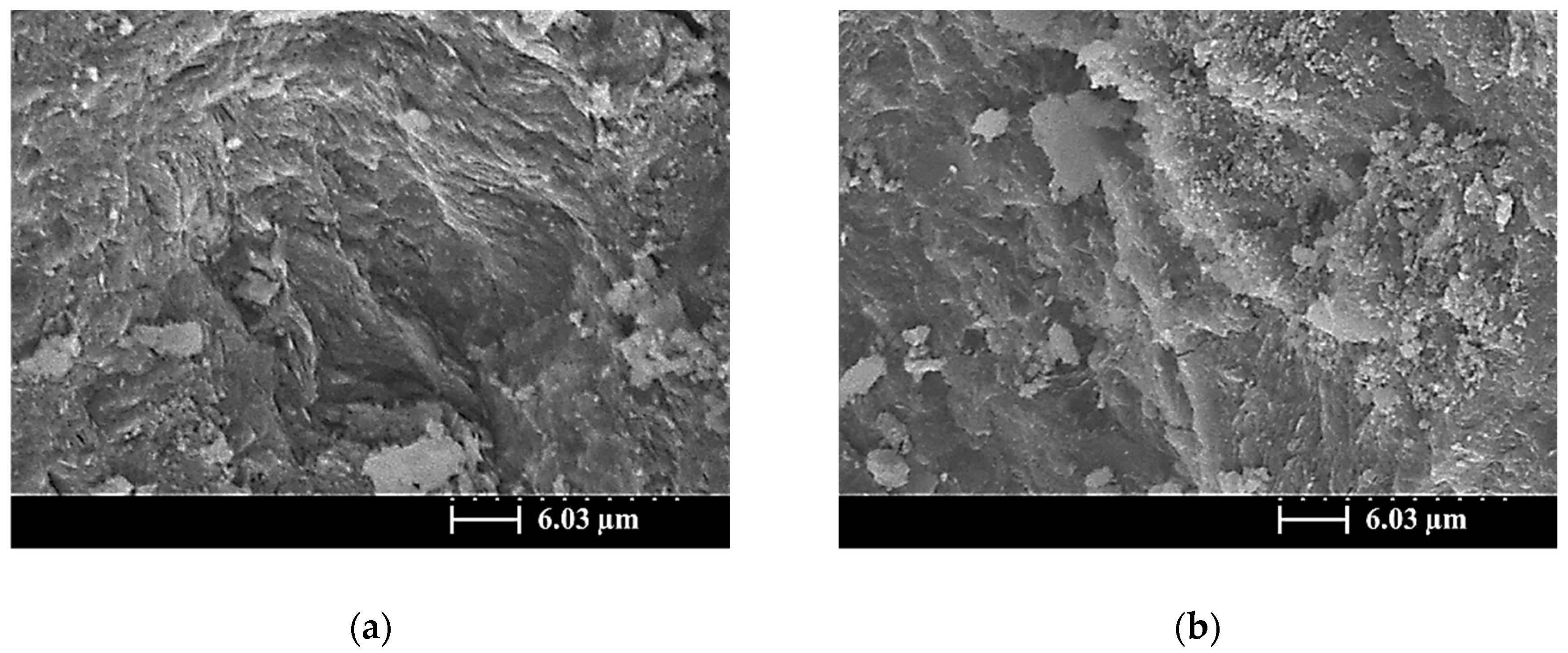
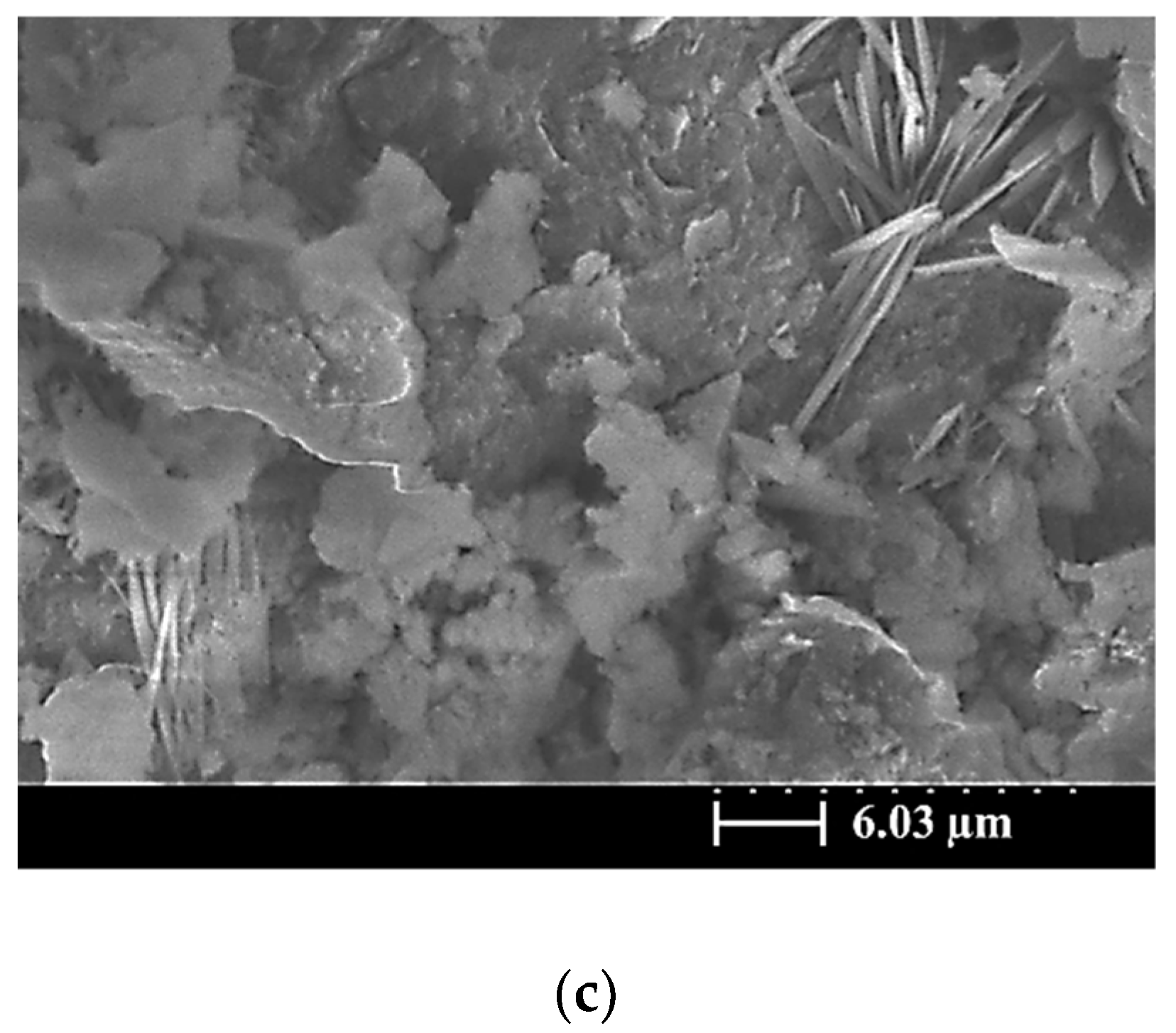
3.5. SEM Images of the SiCSMGs
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, M.H.; Gao, L.; Zhou, J.H.; Wang, M. Application of reaction sintering tothe manufacturing of a spacecraft combustion chamber of SiC ceramics. J. Mater. Process. Technol. 2002, 129, 408–411. [Google Scholar] [CrossRef]
- Zapata-Solvas, E.; Bonilla, S.; Wilshaw, P.R.; Todd, R.I. Preliminary investi-gation of flash sintering of SiC. J. Eur. Cream. Soc. 2013, 33, 2811–2816. [Google Scholar] [CrossRef]
- Eblagon, F.; Ehrle, B.; Graule, T.; Kuebler, J. Development of siliconnitride/silicon carbide composites for wood-cutting tools. J. Eur. Cream. Soc. 2007, 27, 419–428. [Google Scholar] [CrossRef]
- Flinders, M.; Ray, D.; Anderson, A.; Cutler, R.A. High-toughness siliconcarbide as armor. J. Am. Cream. Soc. 2005, 88, 2217–2226. [Google Scholar] [CrossRef]
- Lo, K.W.; Lin, K.L.; Cheng, T.W.; Zhang, B.X. The influence of sapphire substrate silicon carbide sludge on structural properties of metakaolin-based geopolymers. Environ. Prog. Sustain. Energy 2019, 13305. [Google Scholar] [CrossRef]
- He, P.G.; Wang, M.R.; Fu, S.; Jia, D.C.; Yan, S.; Yuan, J.K.; Xu, J.H.; Wang, P.F.; Zhou, Y. Effects of Si/Al ratio on the structure and properties of metakaolin based geopolymer. Ceram. Int. 2016, 42, 14416–14422. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, L.; Liu, M.; Mu, C.; Liang, Y.N.; Hu, X. Role of alkali cation in compressive strength of metakaolin based Geopolymers. Ceram. Int. 2017, 43, 3811–3817. [Google Scholar] [CrossRef]
- Pangdaeng, S.; Phoo-ngernkham, T.; Sata, V.; Chindaprasirt, P. Influence of curing conditions on properties of high calcium fly ash geopolymer containing Portland cement as additive. Mater. Des. 2014, 53, 269–274. [Google Scholar] [CrossRef]
- Yan, D.; Chen, S.; Zeng, Q.; Xu, S.; Li, H. Correlating the elastic properties of metakaolin-based geopolymer with its composition. Mater. Des. 2016, 95, 306–318. [Google Scholar] [CrossRef]
- Yusuf, M.O.; Johari, M.A.M.; Ahmad, Z.A.; Maslehuddin, M. Effects of H2O/Na2O molar ratio on the strength of alkaline activated ground blast furnace slag-ultrafine palm oil fuel ash-based concrete. Mater. Des. 2014, 56, 158–164. [Google Scholar] [CrossRef]
- Lahoti, M.; Wong, K.K.; Yang, E.H.; Tan, K.H. Effects of Si/Al molar ratio on strength endurance and volume stability of metakaolin geopolymers subject to elevated temperature. Ceram. Int. 2018, 44, 5726–5734. [Google Scholar] [CrossRef]
- Zhang, D.W.; Wang, D.M.; Xie, F.Z. Microrheology of fresh geopolymer pastes with different NaOH amountsat room temperature. Constr. Build. Mater. 2019, 207, 284–290. [Google Scholar] [CrossRef]
- Hu, Y.; Liang, S.; Yang, J.K.; Chen, Y.; Ye, N.; Ke, Y.; Tao, S.Y.; Xiao, K.K.; Hu, J.P.; Hou, H.J.; et al. Role of Fe species in geopolymer synthesized from alkali-thermal pretreated Fe-rich Bayer red mud. Constr. Build. Mater. 2019, 200, 398–407. [Google Scholar] [CrossRef]
- Wang, Y.G.; Liu, X.M.; Zhang, W.; Li, Z.P.; Zhang, Y.L.; Li, Y.; Ren, Y.U. Effects of Si/Al ratio on the efflorescence and properties of fly ash based geopolymer. J. Clean. Prod. 2020, 244, 118852. [Google Scholar] [CrossRef]
- Hamdi, N.; Messaoud, I.B.; Srasra, E. Production of geopolymer binders using clay minerals and industrial wastes. C. R. Chimie. 2019, 22, 220–226. [Google Scholar] [CrossRef]
- Abolpour, B.; Afsahi, M.M.; Hosseini, S.G. Statistical analysis of the effective factors on the 28 days compressive strength and setting time of the concrete. J. Adv. Res. 2015, 6, 699–709. [Google Scholar] [CrossRef]
- ASTM C39/C39M-20, Standard Test Method for Compressive Strength of Cylindrical Concrete Specimens; ASTM International: West Conshohocken, PA, USA, 2020.
- Heah, C.Y.; Kamarudin, H.; Bakri, A.M.M.A.; Bnhussain, M.; Luqman, M.; Khairul, N.I.; Ruzaidi, C.M.; Liew, Y.M. Study on solids-to-liquid and alkaline activator ratios on kaolin-based geopolymers. Constr. Build. Mater. 2012, 35, 912–922. [Google Scholar] [CrossRef]
- Zuhua, Z.; Xiao, Y.; Huajun, Z.; Yue, C. Role of water in the synthesis of calcined kaolin-based geopolymer. Appl. Clay Sci. 2009, 43, 218–223. [Google Scholar] [CrossRef]
- Panias, D.; Giannopoulou, I.P.; Perraki, T. Effect of synthesis parameters on the mechanical properties of fly ash-based geopolymers. Colloid. Surface. A. 2007, 301, 246–254. [Google Scholar] [CrossRef]
- Lin, K.L.; Shiu, H.S.; Shie, J.L.; Cheng, T.W.; Hwang, C.L. Effect of composition on characteristics of thin film transistor liquid crystal display (TFT-LCD) waste glass-metakaolin-based geopolymers. Constr. Build. Mater. 2012, 36, 501–507. [Google Scholar] [CrossRef]
- Brylewska, K.; Rożek, P.; Król, M.; Mozgawa, W. The influence of dealumination/desilication on structural properties of metakaolin-based geopolymers. Ceram. Int. 2018, 44, 12853–12861. [Google Scholar] [CrossRef]
- Bagheri, A.; Nazari, A.; Hajimohammadi, A.; Sanjayan, J.G.; Rajeev, P.; Nikzad, M.; Ngo, T.; Mendis, P. Microstructural study of environmentally friendly boroaluminosilicate geopolymers. J. Clean. Prod. 2018, 189, 805–812. [Google Scholar] [CrossRef]
- Kong, D.L.Y.; Sanjayan, J.G.; Sagoe-Crentsil, K. Comparative performance of geopolymers made with metakaolin and fly ash after exposure to elevated temperatures. Cem. Concr. Res. 2007, 37, 1583–1589. [Google Scholar] [CrossRef]
- Lo, K.W.; Lin, K.L.; Cheng, T.W.; Shiu, H.S. Effect of alkali activation thin film transistor-liquid crystal display waste glass on the mechanical behavior of geopolymers. Constr. Build. Mater. 2018, 162, 724–731. [Google Scholar] [CrossRef]
- Yaghoubi, M.; Arulrajah, A.; Disfani, M.M.; Horpibulsuk, S.; Darmawan, S.; Wang, J. Impact of field conditions on the strength development of a geopolymer stabilized marine clay. Appl. Clay Sci. 2019, 167, 33–42. [Google Scholar] [CrossRef]
- Henon, J.; Alzina, A.; Absi, J.; Smith, D.S.; Rossignol, S. Porosity control of cold consolidated geomaterial foam: Temperature effect. Ceram. Int. 2012, 38, 77–84. [Google Scholar] [CrossRef]
- Bergamonti, L.; Taurino, R.; Cattani, L.; Ferretti, D.; Bondioli, F. Lightweight hybrid organic-inorganic geopolymers obtained using polyurethane waste. Constr. Build. Mater. 2018, 185, 285–292. [Google Scholar] [CrossRef]
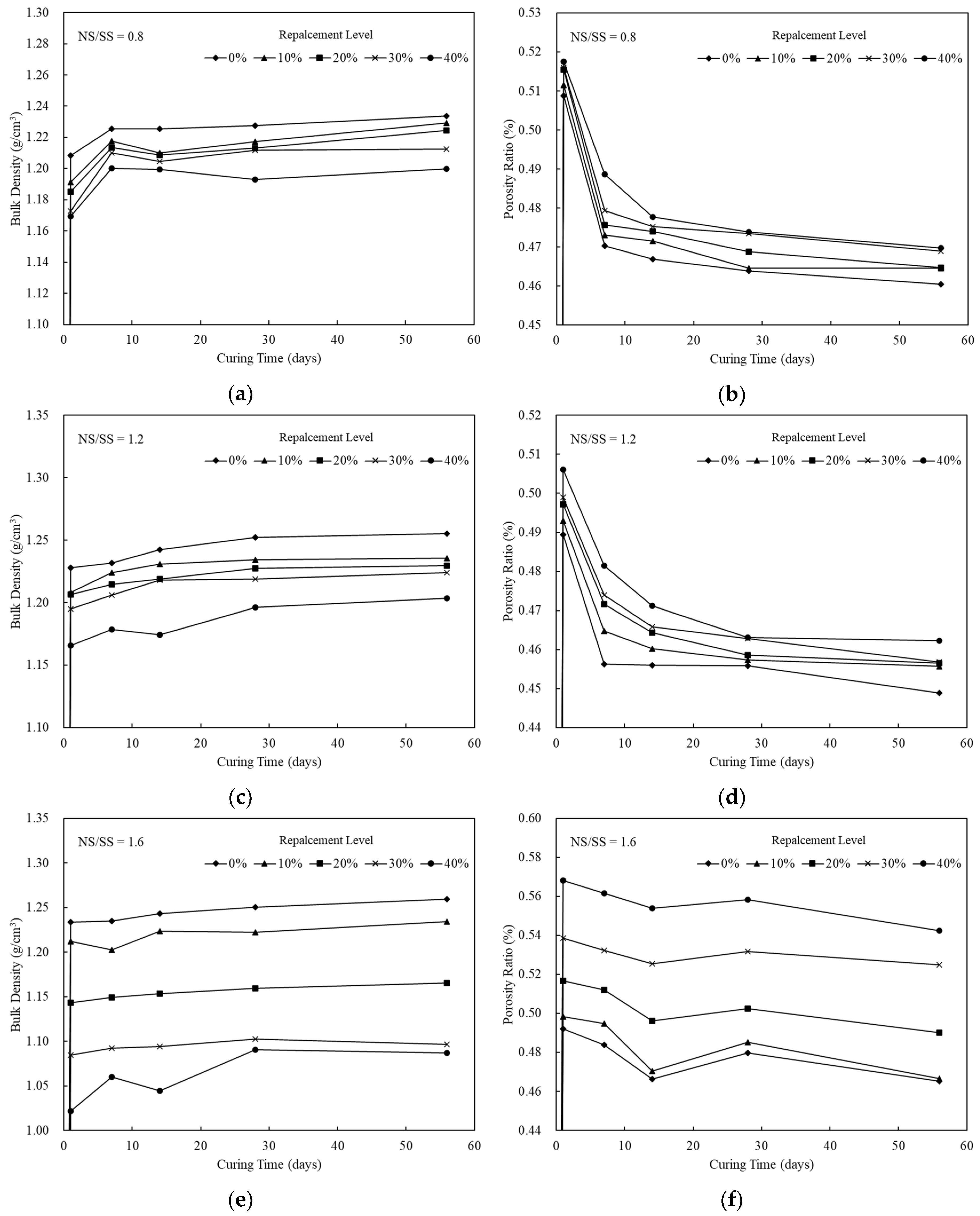
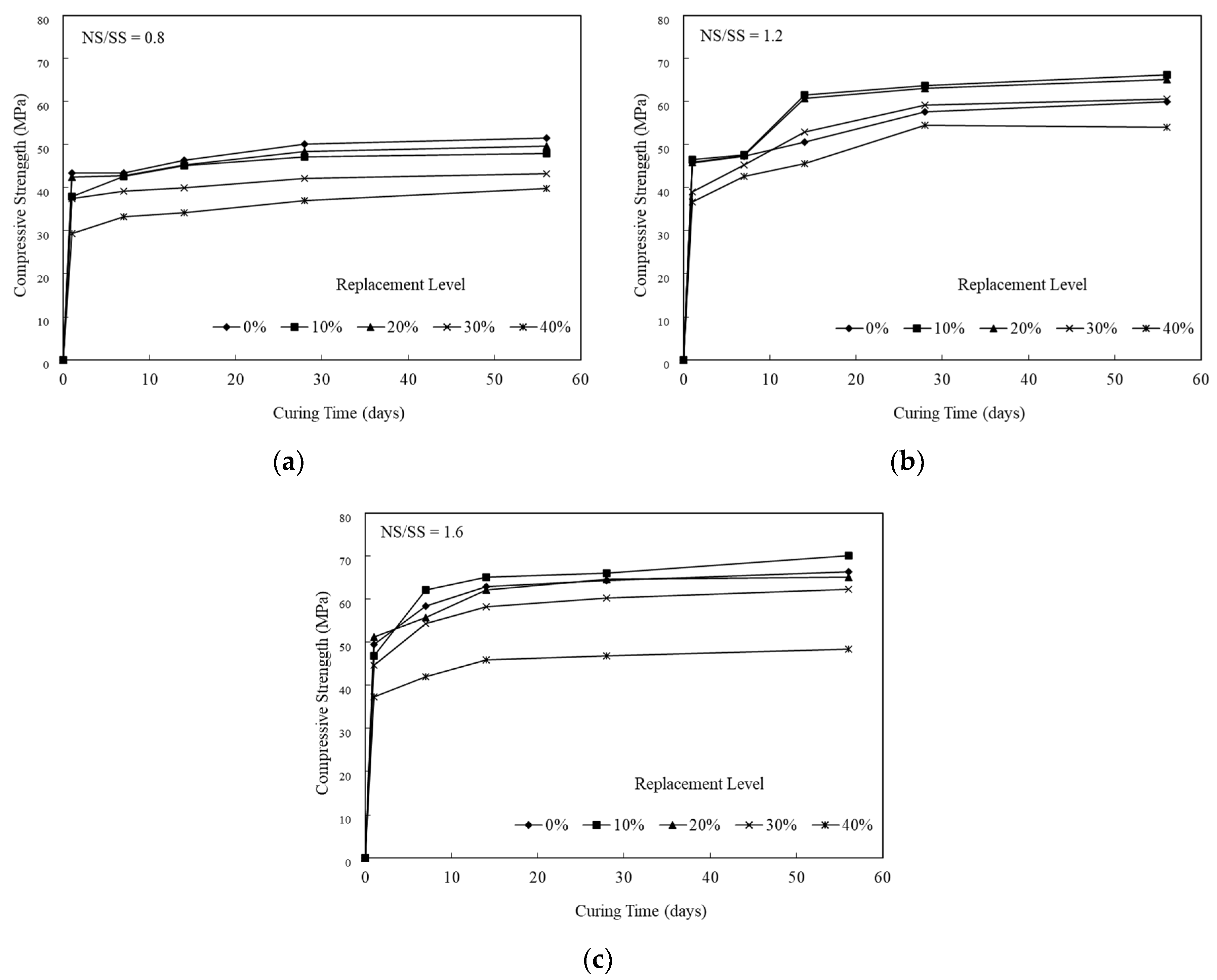
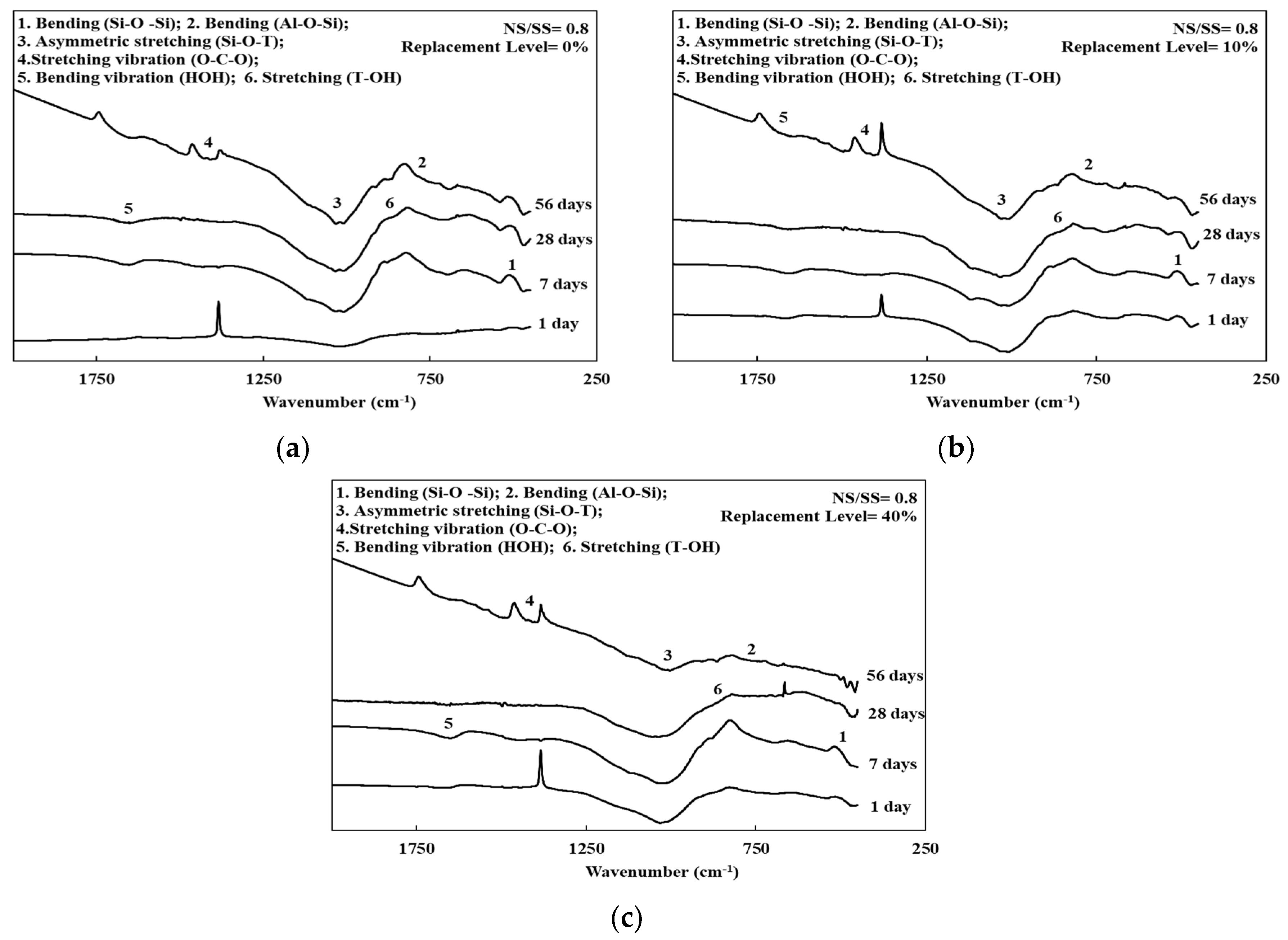
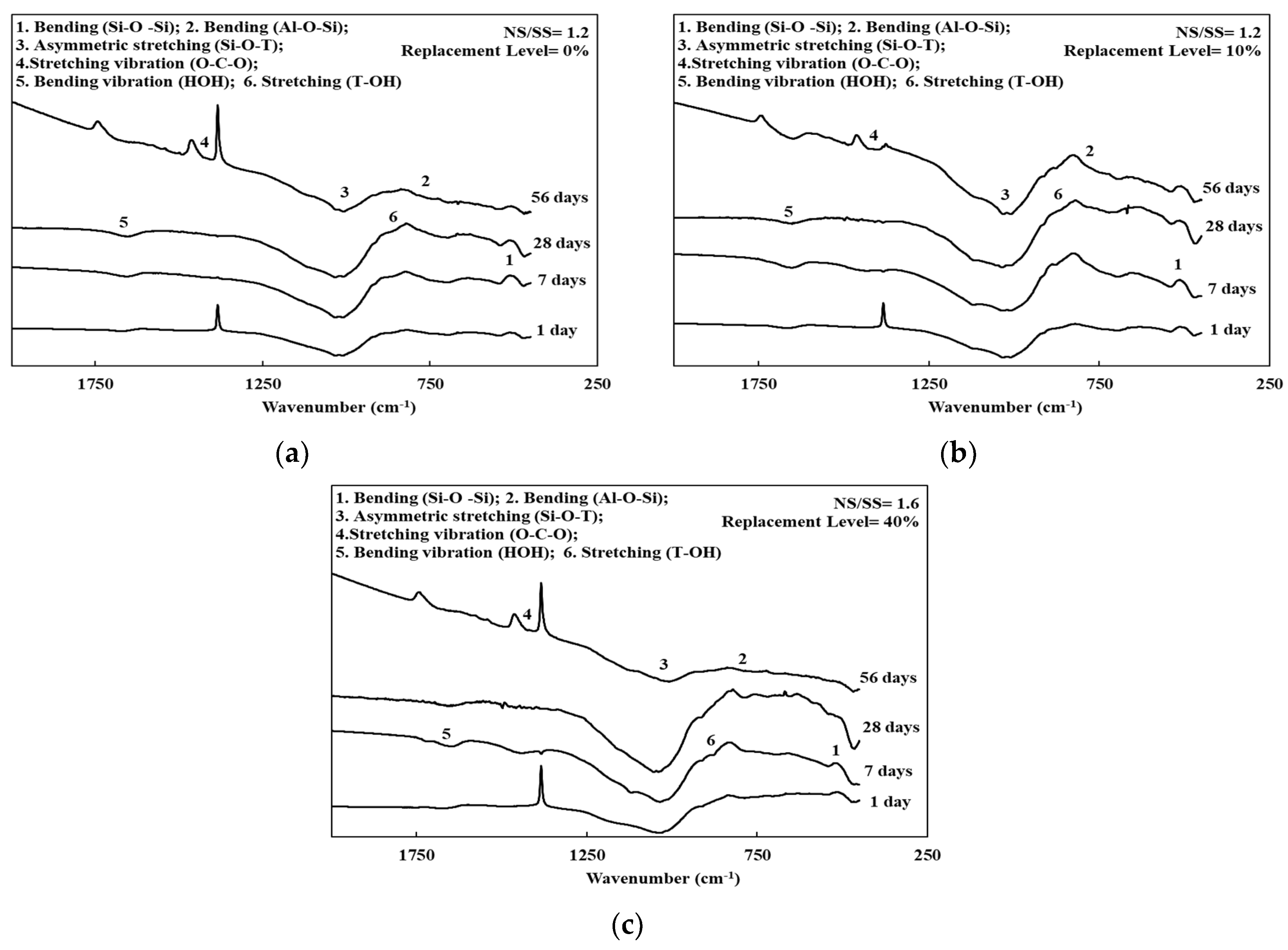

| Composition | SiCS | MK | Kaolinite |
|---|---|---|---|
| Si (%) | 51.35 ± 0.01 | 24.21 ± 0.01 | 25.10 ± 0.01 |
| Al (%) | 0.42 ± 0.01 | 22.77 ± 0.01 | 20.06 ± 0.01 |
| Fe (%) | 0.41 ± 0.01 | 0.91 ± 0.01 | 0.62 ± 0.01 |
| Ca (%) | 0.06 ± 0.01 | 0.18 ± 0.01 | 0.14 ± 0.01 |
| Mg (%) | N.D. 1 | N.D. 1 | N.D. 1 |
| S (%) | 0.02 ± 0.01 | N.D. 1 | N.D. 1 |
| Na (%) | N.D. 1 | 0.03 ± 0.01 | 0.03 ± 0.01 |
| K (%) | 0.01 ± 0.01 | 0.27 ± 0.01 | 0.28 ± 0.01 |
| C (%) | 6.90 ± 0.01 | N.D. 1 | N.D. 1 |
| NS/SS Ratio | Replacement (%) | Initial Setting (min) | Final Setting (min) |
|---|---|---|---|
| 0.8 | 0 | 41 | 90 |
| 10 | 74 | 105 | |
| 20 | 83 | 120 | |
| 30 | 96 | 135 | |
| 40 | 132 | 210 | |
| 1.2 | 0 | 65 | 120 |
| 10 | 89 | 150 | |
| 20 | 125 | 180 | |
| 30 | 128 | 180 | |
| 40 | 145 | 210 | |
| 1.6 | 0 | 101 | 150 |
| 10 | 134 | 180 | |
| 20 | 158 | 225 | |
| 30 | 192 | 255 | |
| 40 | 221 | 285 |
| Characteristics Vibrations | Wavenumber (cm–1) |
|---|---|
| Bending vibration (H–O–H) | 1642 |
| Stretching vibration (O–C–O) | 1460 |
| Asymmetric stretching (Si–O–T) (T = Al or Si) | 900–1300 |
| Stretching (T–OH) (T = Al or Si) | 860 |
| Bending (Al–O–Si) | 700 |
| Bending (Si–O–Si) | 470 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, K.-L.; Lo, K.-W.; Cheng, T.-W.; Lin, W.-T.; Lin, Y.-W. Influence of SiC Sludge on the Microstructure of Geopolymers. Materials 2020, 13, 2203. https://doi.org/10.3390/ma13092203
Lin K-L, Lo K-W, Cheng T-W, Lin W-T, Lin Y-W. Influence of SiC Sludge on the Microstructure of Geopolymers. Materials. 2020; 13(9):2203. https://doi.org/10.3390/ma13092203
Chicago/Turabian StyleLin, Kae-Long, Kang-Wei Lo, Ta-Wui Cheng, Wei-Ting Lin, and Ya-Wen Lin. 2020. "Influence of SiC Sludge on the Microstructure of Geopolymers" Materials 13, no. 9: 2203. https://doi.org/10.3390/ma13092203
APA StyleLin, K.-L., Lo, K.-W., Cheng, T.-W., Lin, W.-T., & Lin, Y.-W. (2020). Influence of SiC Sludge on the Microstructure of Geopolymers. Materials, 13(9), 2203. https://doi.org/10.3390/ma13092203







