Microstructure and Compression Properties of VSS-V3B2 Eutectic Alloys in the V-Si-B System
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
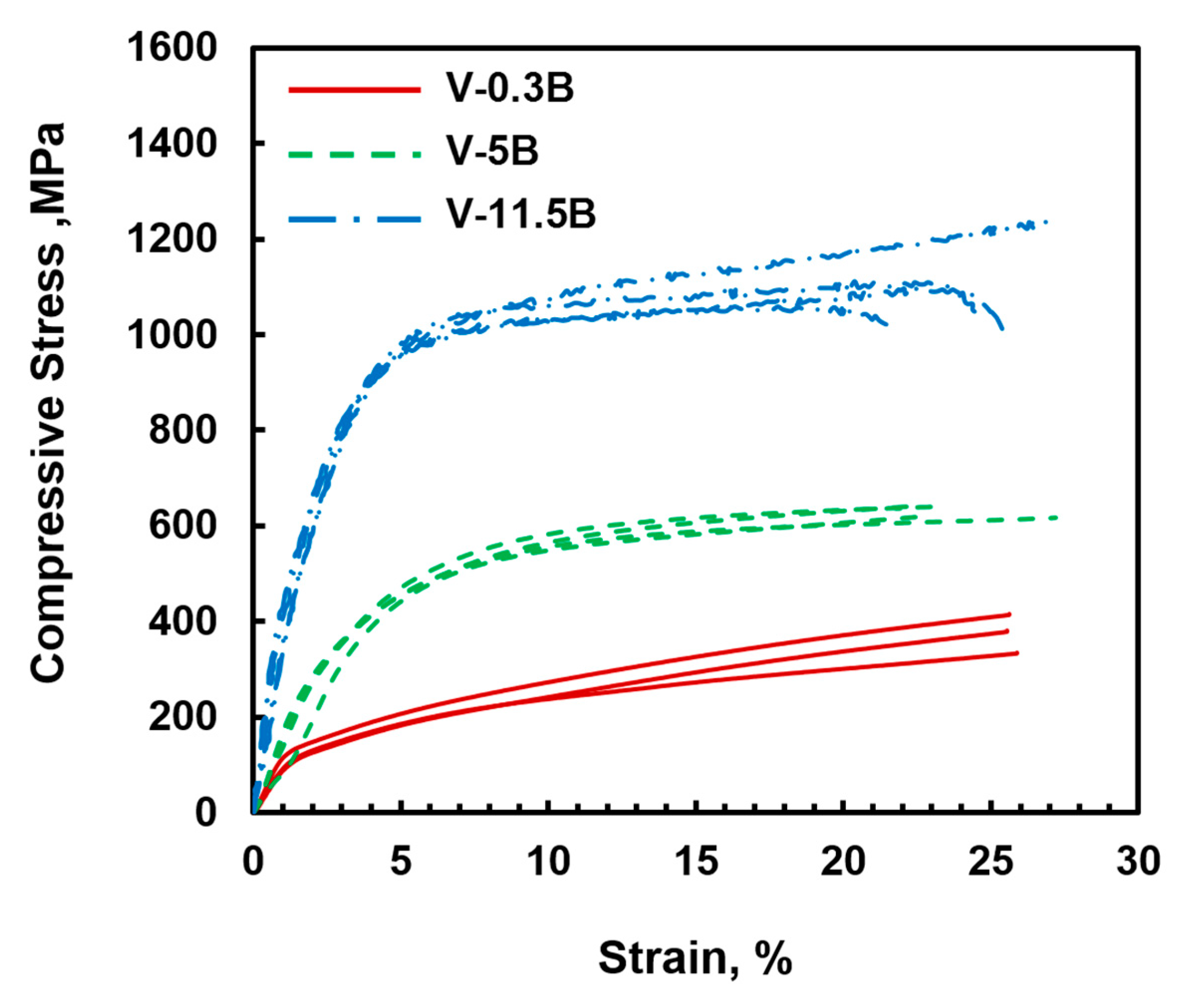
3.1. Binary VSS-V3B2 Alloys
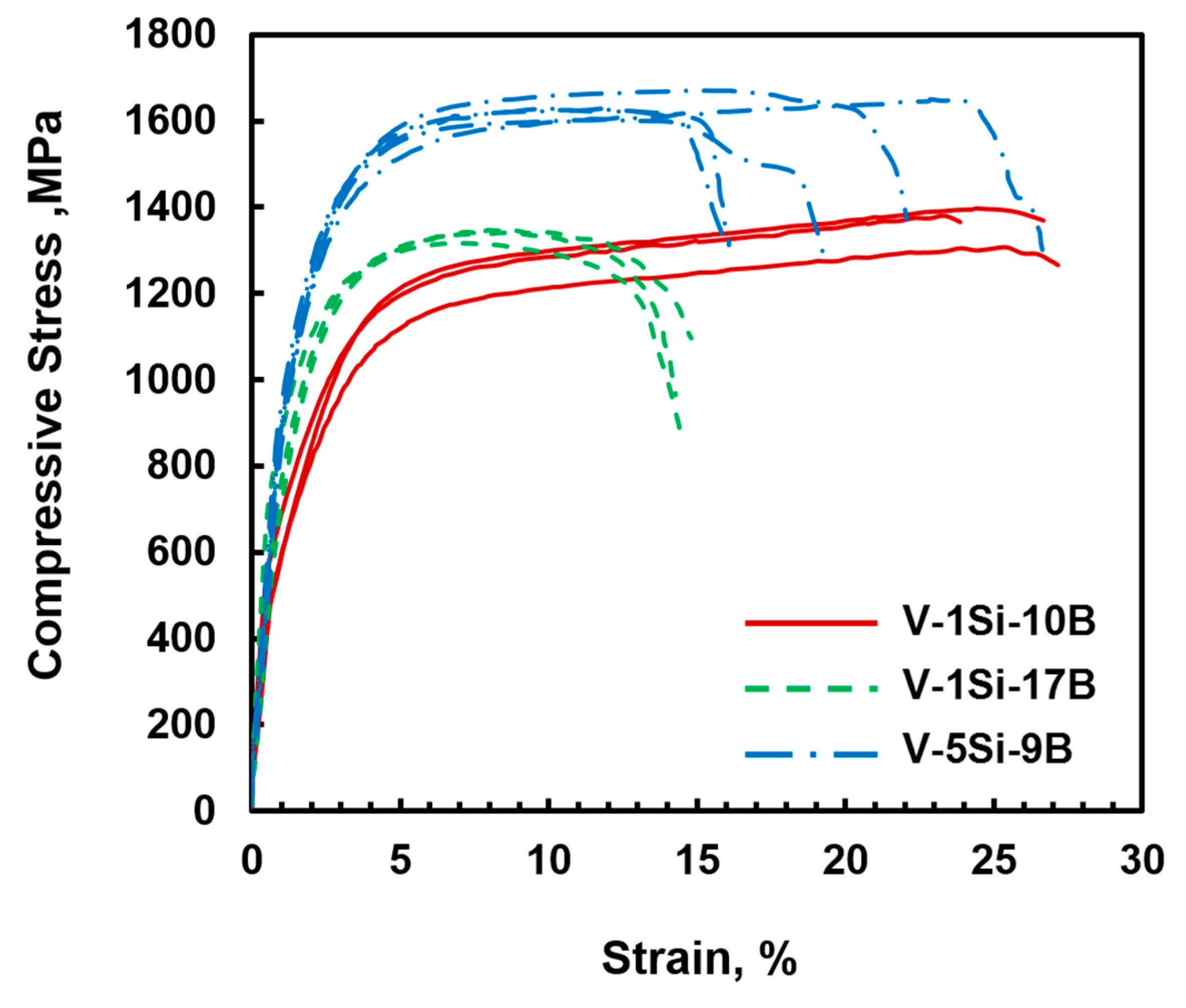
3.2. VSS-V3B2 Alloys with Si Additions
4. Summary and Conclusions
- The binary V-B alloys show great ductility up to 25% total compressive strain in room temperature compression tests.
- In contrast to silicon [21], boron seems to have almost no effect on solid solution strengthening which can be attributed to its negligible solubility in the VSS phase. The contribution of Si dissolution can be estimated to be 300 MPa for 1 at.% Si and approximately 600 MPa for 5 at.% Si. It cannot be excluded that oxygen contamination in the VSS phase could have affected the alloys response.
- The VSS phase controls the deformability in the VSS-V3B2 eutectic microstructure of the V(-Si)-B alloys, whereas the intermetallic V3B2 phase acts as second phase strengthener.
- By comparing the results within this work with compression strength data from the current literature, the contribution of V3B2 second phase hardening in near-eutectic VSS-V3B2 alloys can be estimated to be 400 MPa.
- With increasing silicon additions from 1 to 5 at.%, the eutectic composition is shifted to lower boron concentrations. Compared to the calculated liquidus projection of the V-Si-B system [18], the present experimental results also suggest a much larger binary eutectic trough than reported recently by da Silva et al. [18]. Thus, more experimental work is recommended to clarify this question.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Smith, J.F. The Si-V (Silicon-Vanadium) system. Bull. Alloy Phase Diagrams 1981, 2, 42–48. [Google Scholar] [CrossRef]
- Krüger, M.; Bolbut, V.; Gang, F.; Hasemann, G. Microstructure Variations and Creep Properties of Novel High Temperature V-Si-B Materials. JOM 2016, 68, 2811–2816. [Google Scholar] [CrossRef]
- Schmelzer, J.; Baumann, T.; Dieck, S.; Krüger, M. Hardening of V-Si alloys during high energy ball milling. Powder Technol. 2016, 294, 493–497. [Google Scholar] [CrossRef]
- Krüger, M. Innovative Metallische Hochtemperaturwerkstoffe. Habilitation Thesis, Otto-Von-Guericke University Magdeburg, Magdeburg, Germany, 2017. [Google Scholar]
- Tsakiropoulos, P. On Nb Silicide Based Alloys: Alloy Design and Selection. Materials 2018, 11, 844. [Google Scholar] [CrossRef] [PubMed]
- Bewlay, B.P.; Jackson, M.R.; Subramanian, P.R.; Zhao, J.-C. A review of very-high-temperature Nb-silicide-based composites. Metall. Mater. Trans. A 2003, 34, 2043–2052. [Google Scholar] [CrossRef]
- Jain, P.; Kumar, K.S. Dissolved Si in Mo and its effects on the properties of Mo-Si-B alloys. Scripta Mater. 2010, 62, 1–4. [Google Scholar] [CrossRef]
- Rioult, F.A.; Imhoff, S.D.; Sakidja, R.; Perepezko, J.H. Transient oxidation of Mo-Si-B alloys: Effect of the microstructure size scale. Acta Mater. 2009, 57, 4600–4613. [Google Scholar] [CrossRef]
- Wenderoth, M.; Völkl, R.; Vorberg, S.; Yamabe-Mitarai, Y.; Harada, H.; Glatzel, U. Microstructure, oxidation resistance and high-temperature strength of γ′ hardened Pt-base alloys. Intermetallics 2007, 15, 539–549. [Google Scholar] [CrossRef]
- Chaia, N.; Bouizi, Y.; Mathieu, S.; Vilasi, M. Isothermal and cyclic oxidation behavior of hot-pressed MSi2 compounds (with M = V, Ti, Cr). Intermetallics 2015, 65, 35–41. [Google Scholar] [CrossRef]
- Williams, J.; Akinc, M. Oxidation behavior of V5Si3 based materials. Intermetallics 1998, 6, 269–275. [Google Scholar] [CrossRef]
- Chaia, N.; Mathieu, S.; Rouillard, F.; Vilasi, M. The ability of silicide coating to delay the catastrophic oxidation of vanadium under severe conditions. J. Nucl. Mater. 2015, 457, 124–129. [Google Scholar] [CrossRef]
- Chaia, N.; Mathieu, S.; Cozzika, T.; Rouillard, F.; Desgranges, C.; Courouau, J.L.; Petitjean, C.; David, N.; Vilasi, M. An overview of the oxidation performance of silicide diffusion coatings for vanadium-based alloys for generation IV reactors. Corros. Sci. 2013, 66, 285–291. [Google Scholar] [CrossRef]
- Krüger, M. High temperature compression strength and oxidation of a V-9Si-13B alloy. Scripta Mater. 2016, 121, 75–78. [Google Scholar] [CrossRef]
- Nowotny, H.; Dimakopoulou, E.; Kudielka, H. Untersuchungen in den Dreistoffsystemen: Molybdän-Silizium-Bor, Wolfram-Silizium-Bor und in dem System: VSi2-TaSi2. Mon. Chem. 1957, 88, 180–192. [Google Scholar] [CrossRef]
- Kudielka, H.; Nowotny, H.; Findeisen, G. Untersuchungen in den Systemen: V-B, Nb-B, V-Si-B und Ta-B-Si. Mon. Chem. 1957, 88, 1048–1055. [Google Scholar] [CrossRef]
- Nunes, C.A.; De Lima, B.B.; Coelho, G.C.; Suzuki, P.A. Isothermal section of the V-Si-B system at 1600 °C in the V-VSi2-VB region. J. Phase Equilibria Diffus. 2009, 30, 345–350. [Google Scholar] [CrossRef]
- Da Silva, A.A.A.P.; Chaia, N.; Ferreira, F.; Coelho, G.C.; Fiorani, J.M.; David, N.; Vilasi, M.; Nunes, C.A. Thermodynamic modeling of the V-Si-B system. Calphad 2017, 59, 199–206. [Google Scholar] [CrossRef]
- Hasemann, G. Experimental study of the liquidus surface in the V-rich portion of the V-Si-B system. J. Alloys Compd. 2020, 824, 153843. [Google Scholar] [CrossRef]
- Henshall, G.A.; Sturm, M.J.; Bewlay, B.P.; Sutliff, J.A. Ductile-phase toughening in V-V3Si in situ composites. Metall. Mater. Trans. A 1997, 28, 2555–2564. [Google Scholar] [CrossRef]
- Hasemann, G.; Müller, C.; Krüger, M. Room temperature plastic deformability in V-rich V-Si-B alloys. Acta Mater. 2019, 175, 140–147. [Google Scholar] [CrossRef]
- Hasemann, G.; Krüger, M.; Palm, M.; Stein, F. Microstructures of ternary eutectic refractory Me-Si-B (Me = Mo, V) alloy systems. Mater. Sci. Forum 2018, 941, 827–832. [Google Scholar] [CrossRef]
- Spear, K.E.; Liao, P.K.; Smith, J.F. The B-V (Boron-Vanadium) system. Phase Equilibria 1987, 8, 447–454. [Google Scholar] [CrossRef]
- De Lima, B.B.; Nunes, C.A.; Coelho, G.C.; Suzuki, P.A.; Rogl, P. Evaluation of the Invariant Reactions of the V-B System. J. Phase Equilibria Diffus. 2004, 25, 134–139. [Google Scholar] [CrossRef]
- Bei, H.; George, E.P.; Kenik, E.A.; Pharr, G.M. Microstructures and mechanical properties of V-V3Si eutectic composites. Z. Metallkd. 2004, 95, 505–512. [Google Scholar] [CrossRef]
- Rietveld, H.M. A Profile Refinement Method for Nuclear and Magnetic Structures. J. Appl. Crystallogr. 1969, 2, 65–71. [Google Scholar] [CrossRef]
- Riabov, A.B.; Yartys, V.A.; Hauback, B.C.; Guegan, P.W.; Wiesinger, G.; Harris, I.R. Hydrogenation behaviour, neutron diffraction studies and microstructural characterisation of boron oxide-doped Zr–V alloys. J. Alloys Compd. 1999, 293, 93–100. [Google Scholar] [CrossRef]
- James, W.J.; Straumanis, M.E. Lattice parameter and expansion coefficient of vanadium. J. Electrochem. Soc. 1960, 107, 69. [Google Scholar] [CrossRef]
- Rawn, C.J.; Schneibel, J.H.; Hoffmann, C.M.; Hubbard, C.R. The crystal structure and thermal expansion of Mo5SiB2. Intermetallics 2001, 9, 209–216. [Google Scholar] [CrossRef]
- Touzani, R.S.; Becker, J.; Krüger, M. Site preference of V and its influence on the elastic properties in the boride series VxMo5–xSiB2 as studied by first principles density functional theory. J. Alloys Compd. 2019, 819, 153041. [Google Scholar] [CrossRef]
- Dinnebier, R.E.; Leineweber, A.; Evans, J.S.O. Rietveld Refinement: Practical Powder Diffraction Pattern Analysis Using Topas; De Gruyter: Berlin, Germany; Boston, MA, USA, 2019. [Google Scholar]
- Sahm, P.R.; Egry, I.; Volkmann, T. Schmelze, Erstarrung, Grenzflächen: Eine Einführung in die Physik und Technologie Flüssiger und Fester Metalle; Springer: Berlin/Heidelberg, Germany, 1999; pp. 164–207. [Google Scholar]
- Kurz, W.; Sahm, P.R. Gerichtet Erstarrte Eutektische Werkstoffe: Herstellung, Eigenschaften und Anwendung von In-Situ-Verbundwerkstoffen; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 1975; pp. 100–122. [Google Scholar]
- Lukas, H.; Fries, S.G.; Sundman, B. Computational Thermodynamics: The Calphad Method; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]

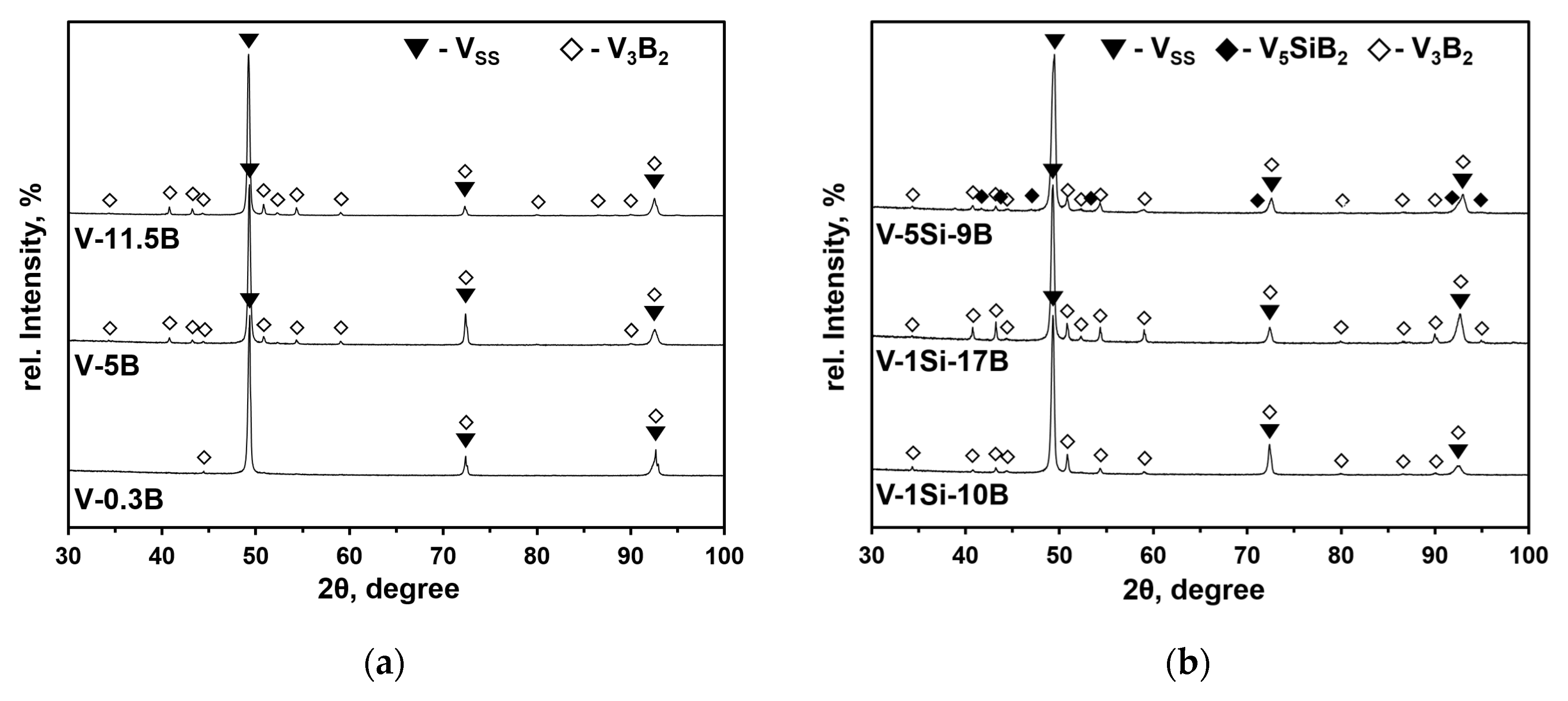


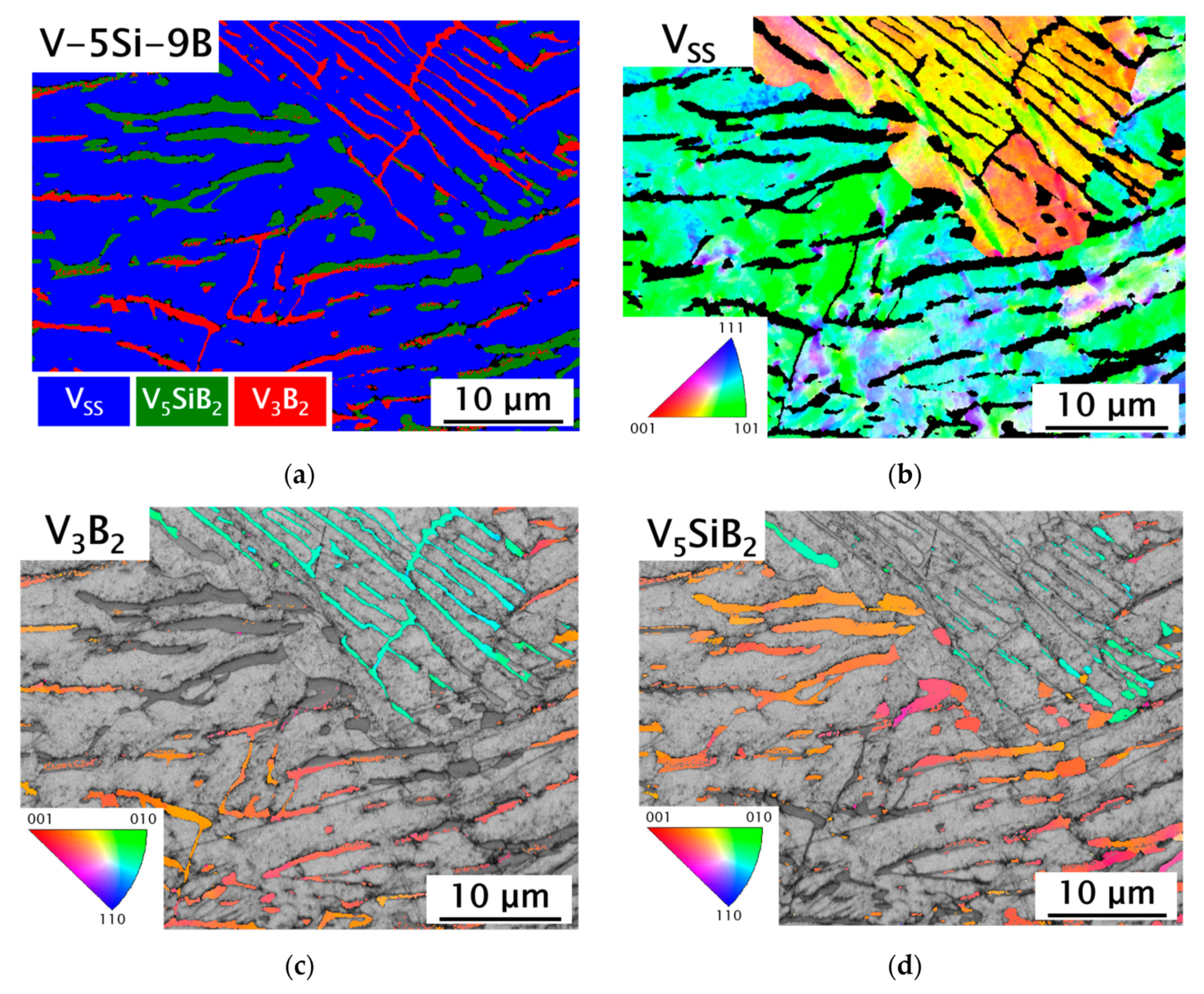
| No. | Nominal Composition | Si [at.%] | B [at.%] | Oxygen [wt.ppm] | Primary Phase | VSS [vol.%] | V3B2 [vol.%] | V5SiB2 [vol.%] |
|---|---|---|---|---|---|---|---|---|
| 1 | V-0.3B | - | 0.3 | 700 ± 50 | VSS | 97.5 | 2.5 | - |
| 2 | V-5B | - | 5.2 | 700 ± 170 | VSS | 87.0 | 13.0 | - |
| 3 | V-11.5B | - | 11.5 | 1420 ± 300 | V3B2 | 78.5 | 21.5 | - |
| 4 | V-1Si-10B | 1.1 | 9.9 | 1480 ± 610 | V3B2 | 80.0 | 20.0 | - |
| 5 | V-1Si-17B | 1.0 | 17.3 | 930 ± 60 | V3B2 | 58.5 | 41.5 | - |
| 6 | V-5Si-9B | 5.2 | 8.3 | 1080 ± 250 | V3B2 | 78.0 | 14.0 | 8.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Müller, C.; Hasemann, G.; Regenberg, M.; Betke, U.; Krüger, M. Microstructure and Compression Properties of VSS-V3B2 Eutectic Alloys in the V-Si-B System. Materials 2020, 13, 2100. https://doi.org/10.3390/ma13092100
Müller C, Hasemann G, Regenberg M, Betke U, Krüger M. Microstructure and Compression Properties of VSS-V3B2 Eutectic Alloys in the V-Si-B System. Materials. 2020; 13(9):2100. https://doi.org/10.3390/ma13092100
Chicago/Turabian StyleMüller, Christopher, Georg Hasemann, Maximilian Regenberg, Ulf Betke, and Manja Krüger. 2020. "Microstructure and Compression Properties of VSS-V3B2 Eutectic Alloys in the V-Si-B System" Materials 13, no. 9: 2100. https://doi.org/10.3390/ma13092100
APA StyleMüller, C., Hasemann, G., Regenberg, M., Betke, U., & Krüger, M. (2020). Microstructure and Compression Properties of VSS-V3B2 Eutectic Alloys in the V-Si-B System. Materials, 13(9), 2100. https://doi.org/10.3390/ma13092100






