Synthesis and Evaluation of Zwitterionic Surfactants Bearing Benzene Ring in the Hydrophobic Tail
Abstract
1. Introduction
2. Materials and Synthesis
2.1. Materials
2.2. Determination of Structure
2.3. Solubility and Salt Tolerance Tests
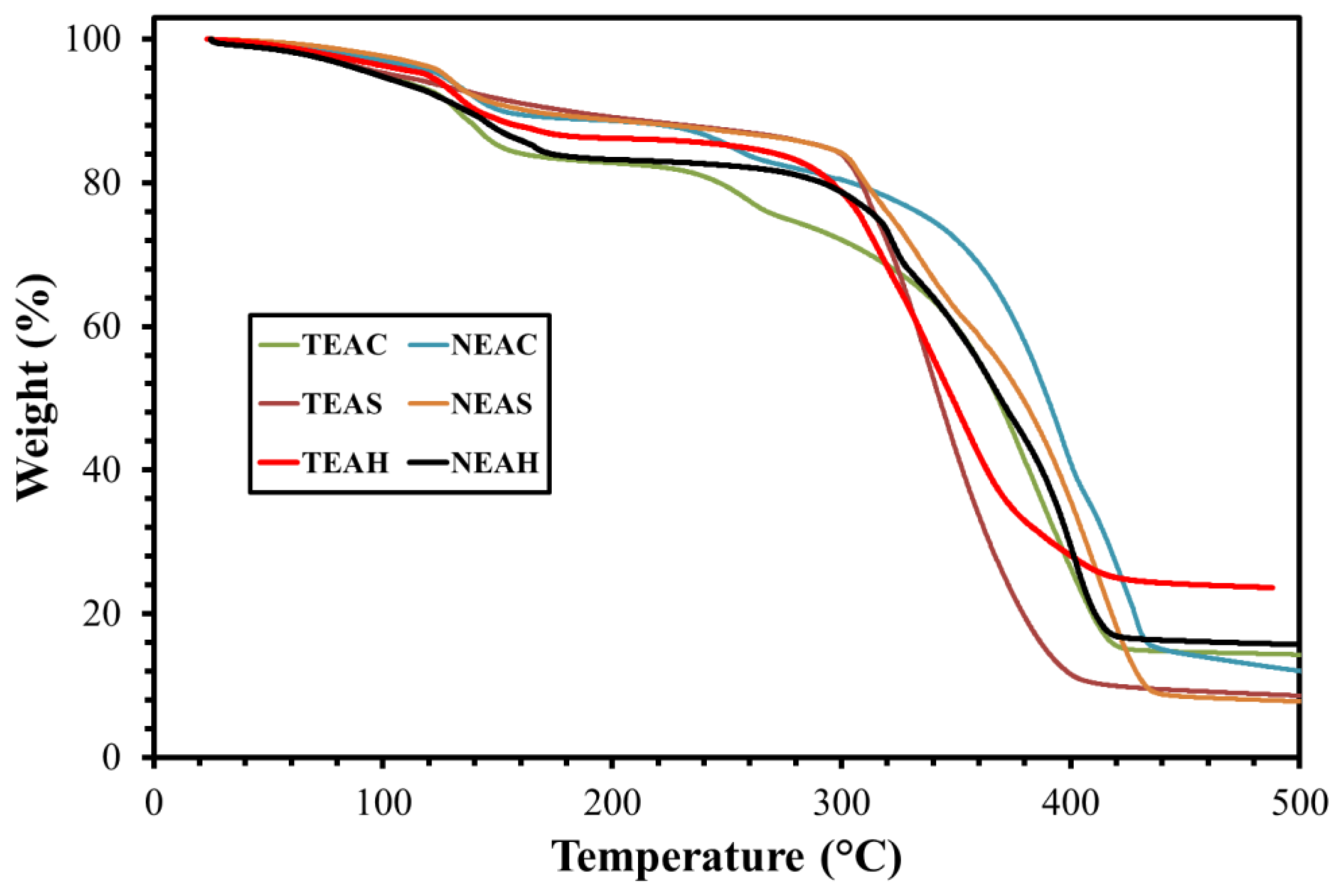
2.4. Thermal Gravimetric Analysis (TGA)
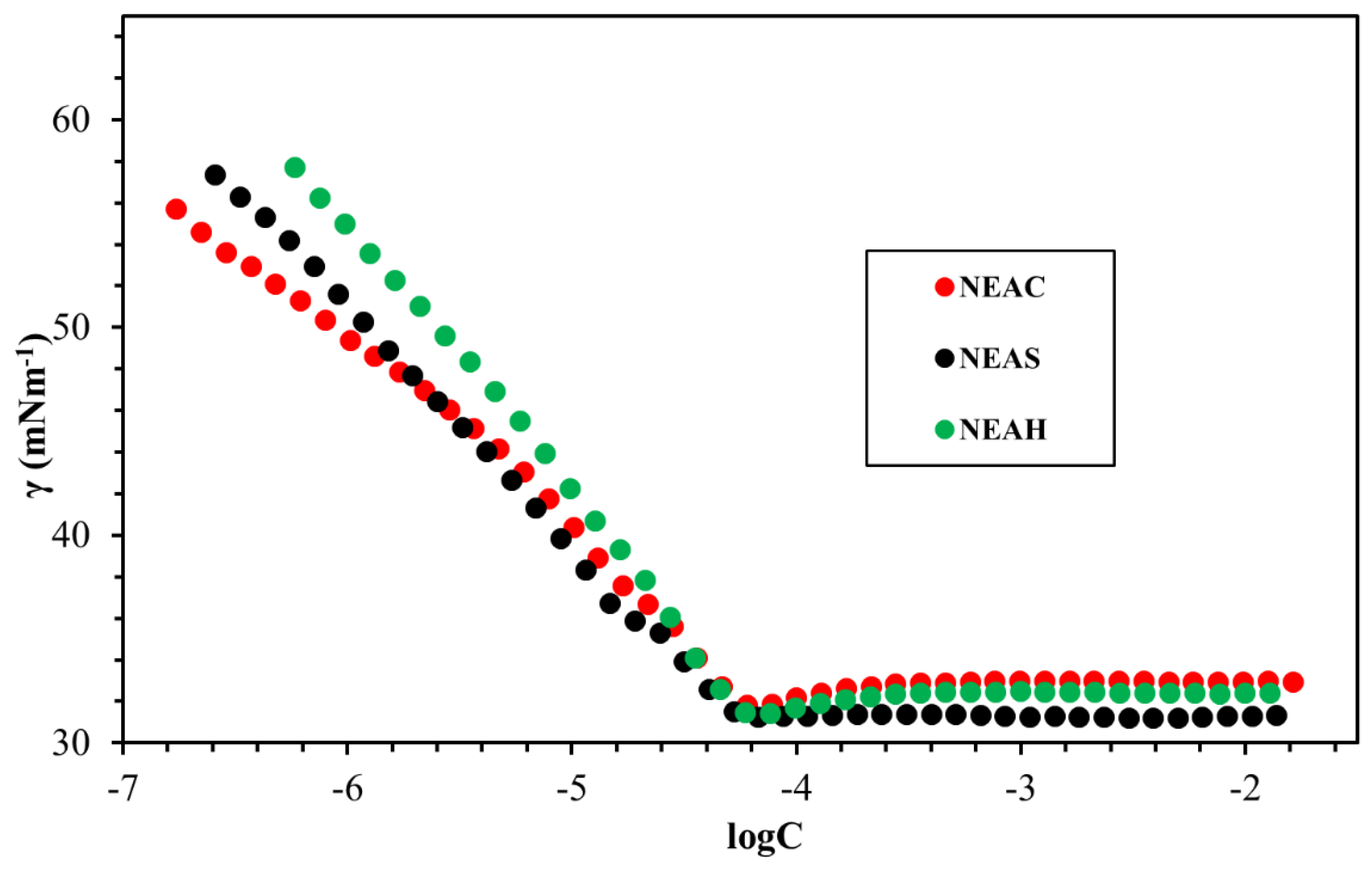
2.5. Surface Tension
2.6. Synthesis
2.6.1. Synthesis of Intermediate 5
2.6.2. Synthesis of 4-Tert-Butylphenyl Ethoxylate Amidopropyl Carboxybetaine (TEAC)
2.6.3. Synthesis of 4-Tert-Butylphenyl Ethoxylate Amidopropyl Sulfobetaine (TEAS)
2.6.4. Synthesis of 4-Tert-Butylphenyl Ethoxylate Amidopropyl Hydroxy Sulfobetaine (TEAH)
3. Results and Discussion
3.1. Structure Interpretation
3.2. Solubility and Salt Tolerance
3.3. Thermal Gravimetric Analysis (TGA)
3.4. Surface Tension
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Kurnia, I.; Zhang, G.; Han, X.; Yu, J. Zwitterionic-anionic surfactant mixture for chemical enhanced oil recovery without alkali. Fuel 2020, 259, 116236. [Google Scholar] [CrossRef]
- Malik, I.A.; Al-Mubaiyedh, U.A.; Sultan, A.S.; Kamal, M.S.; Hussein, I.A. Rheological and thermal properties of novel surfactant-polymer systems for EOR applications. Can. J. Chem. Eng. 2016, 94, 1693–1699. [Google Scholar] [CrossRef]
- Kamal, M.S.; Shakil Hussain, S.M.; Fogang, L.T. A Zwitterionic Surfactant Bearing Unsaturated Tail for Enhanced Oil Recovery in High-Temperature High-Salinity Reservoirs. J. Surfactants Deterg. 2018, 21, 165–174. [Google Scholar] [CrossRef]
- Kaczerewska, O.; Brycki, B.; Ribosa, I.; Comelles, F.; Garcia, M.T. Cationic gemini surfactants containing an O-substituted spacer and hydroxyethyl moiety in the polar heads: Self-assembly, biodegradability and aquatic toxicity. J. Ind. Eng. Chem. 2018, 59, 141–148. [Google Scholar] [CrossRef]
- Lim, J.C.; Kang, E.K.; Lee, H.; Lee, B.M. Synthesis and interfacial properties of ethoxylated cationic surfactants derived from n-dodecyl glycidyl ether. J. Ind. Eng. Chem. 2015, 22, 75–82. [Google Scholar] [CrossRef]
- Hussain, S.M.S.; Mahboob, A.; Kamal, M.S. Poly(Oxyethylene)-Amidoamine Based Gemini Cationic Surfactants for Oilfield Applications: Effect of Hydrophilicity of Spacer Group. Materials 2020, 13, 1046. [Google Scholar] [CrossRef]
- Gu, Y.; Yu, S.; Mou, J.; Wu, D.; Zheng, S. Research Progress on the Collaborative Drag Reduction Effect of Polymers and Surfactants. Materials 2020, 13, 444. [Google Scholar] [CrossRef]
- Andrunik, M.; Bajda, T. Modification of bentonite with cationic and nonionic surfactants: Structural and textural features. Materials 2019, 12, 3772. [Google Scholar] [CrossRef]
- Nowrouzi, I.; Mohammadi, A.H.; Manshad, A.K. Water-oil interfacial tension (IFT) reduction and wettability alteration in surfactant flooding process using extracted saponin from Anabasis Setifera plant. J. Pet. Sci. Eng. 2020, 189, 106901. [Google Scholar] [CrossRef]
- Olayiwola, S.O.; Dejam, M. A comprehensive review on interaction of nanoparticles with low salinity water and surfactant for enhanced oil recovery in sandstone and carbonate reservoirs. Fuel 2019, 1045–1057. [Google Scholar] [CrossRef]
- Ahmadi, M.A.; Galedarzadeh, M.; Shadizadeh, S.R. Wettability Alteration in Carbonate Rocks by Implementing New Derived Natural Surfactant: Enhanced Oil Recovery Applications. Transp. Porous Media 2015, 106, 645–667. [Google Scholar] [CrossRef]
- Rostami, P.; Mehraban, M.F.; Sharifi, M.; Dejam, M.; Ayatollahi, S. Effect of water salinity on oil/brine interfacial behaviour during low salinity waterflooding: A mechanistic study. Petroleum 2019, 5, 367–374. [Google Scholar] [CrossRef]
- Al-Sadat, W.; Nasser, M.S.; Chang, F.; Nasr-El-Din, H.A.; Hussein, I.A. Rheology of a viscoelastic zwitterionic surfactant used in acid stimulation: Effects of surfactant and electrolyte concentration. J. Pet. Sci. Eng. 2014, 124, 341–349. [Google Scholar] [CrossRef]
- Kumar, A.; Mandal, A. Critical investigation of zwitterionic surfactant for enhanced oil recovery from both sandstone and carbonate reservoirs: Adsorption, wettability alteration and imbibition studies. Chem. Eng. Sci. 2019, 209, 115222. [Google Scholar] [CrossRef]
- Zhang, W.; Mao, J.; Yang, X.; Zhang, Y.; Zhang, H.; Tian, J.; Lin, C.; Mao, J.; Zhao, J. Effect of propylene glycol substituted group on salt tolerance of a cationic viscoelastic surfactant and its application for brine-based clean fracturing fluid. Colloids Surf. A Physicochem. Eng. Asp. 2020, 584, 124043. [Google Scholar] [CrossRef]
- Zhang, W.; Mao, J.; Yang, X.; Zhang, H.; Zhao, J.; Tian, J.; Lin, C.; Mao, J. Development of a sulfonic gemini zwitterionic viscoelastic surfactant with high salt tolerance for seawater-based clean fracturing fluid. Chem. Eng. Sci. 2019, 207, 688–701. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Liu, X.; Wang, J.; Wei, L.; Feng, Y. Effect of a hydrophilic head group on krafft temperature, surface activities and rheological behaviors of erucyl amidobetaines. J. Surfactants Deterg. 2014, 17, 295–301. [Google Scholar] [CrossRef]
- Dong, S.J.; Li, Y.L.; Song, Y.B.; Zhi, L.F. Synthesis, characterization and performance of unsaturated long-chain carboxybetaine and hydroxy sulfobetaine. J. Surfactants Deterg. 2013, 16, 523–529. [Google Scholar] [CrossRef]
- Taleb, K.; Mohamed-Benkada, M.; Benhamed, N.; Saidi-Besbes, S.; Grohens, Y.; Derdour, A. Benzene ring containing cationic gemini surfactants: Synthesis, surface properties and antibacterial activity. J. Mol. Liq. 2017, 241, 81–90. [Google Scholar] [CrossRef]
- Hussain, S.M.S.; Kamal, M.S.; Fogang, L.T.; Patil, S. Effect of the number of ethylene oxide units on the properties of synthesized tailor-made cationic gemini surfactants for oilfield applications. J. Mol. Struct. 2019, 1196, 851–860. [Google Scholar] [CrossRef]
- Hoque, J.; Kumar, P.; Aswal, V.K.; Haldar, J. Aggregation properties of amide bearing cleavable gemini surfactants by small angle neutron scattering and conductivity studies. J. Phys. Chem. B 2012, 116, 9718–9726. [Google Scholar] [CrossRef] [PubMed]
- Chu, Z.; Feng, Y. A facile route towards the preparation of ultra-long-Chain amidosulfobetaine surfactants. Synlett 2009, 16, 2655–2658. [Google Scholar]
- Hussain, S.M.S.; Kamal, M.S.; Fogang, L.T. Synthesis and physicochemical investigation of betaine type polyoxyethylene zwitterionic surfactants containing different ionic headgroups. J. Mol. Struct. 2019, 1178, 83–88. [Google Scholar] [CrossRef]
- Zhang, Y.; Luo, Y.; Wang, Y.; Zhang, J.; Feng, Y. Single-component wormlike micellar system formed by a carboxylbetaine surfactant with C22 saturated tail. Colloids Surfaces A Physicochem. Eng. Asp. 2013, 436, 71–79. [Google Scholar] [CrossRef]
- Geng, X.F.; Hu, X.Q.; Xia, J.J.; Jia, X.C. Synthesis and surface activities of a novel di-hydroxyl-sulfate-betaine- type zwitterionic gemini surfactants. Appl. Surf. Sci. 2013, 271, 284–290. [Google Scholar] [CrossRef]
- Mohsin, G.F.; Schmitt, F.J.; Kanzler, C.; Dirk Epping, J.; Flemig, S.; Hornemann, A. Structural characterization of melanoidin formed from D-glucose and L-alanine at different temperatures applying FTIR, NMR, EPR, and MALDI-ToF-MS. Food Chem. 2018, 245, 761–767. [Google Scholar] [CrossRef]
- Kathel, P.; Mohanty, K.K. EOR in tight oil reservoirs through wettability alteration. In Proceedings of the Proceedings-SPE Annual Technical Conference and Exhibition, New Orleans, LA, USA, 30 September–2 October 2013; Society of Petroleum Engineers: Richardson, TX, USA, 2013; Volume 3, pp. 2433–2447. [Google Scholar]
- Shakil Hussain, S.M.; Kamal, M.S.; Murtaza, M. Synthesis of novel ethoxylated quaternary ammonium gemini surfactants for enhanced oil recovery application. Energies 2019, 12, 1731. [Google Scholar] [CrossRef]
- Chauhan, S.; Kaur, M.; Kumar, K.; Chauhan, M.S. Study of the effect of electrolyte and temperature on the critical micelle concentration of dodecyltrimethylammonium bromide in aqueous medium. J. Chem. Thermodyn. 2014, 78, 175–181. [Google Scholar] [CrossRef]
- Shakil Hussain, S.M.; Animashaun, M.A.; Kamal, M.S.; Ullah, N.; Hussein, I.A.; Sultan, A.S. Synthesis, Characterization and Surface Properties of Amidosulfobetaine Surfactants Bearing Odd-Number Hydrophobic Tail. J. Surfactants Deterg. 2016, 19, 413–420. [Google Scholar] [CrossRef]

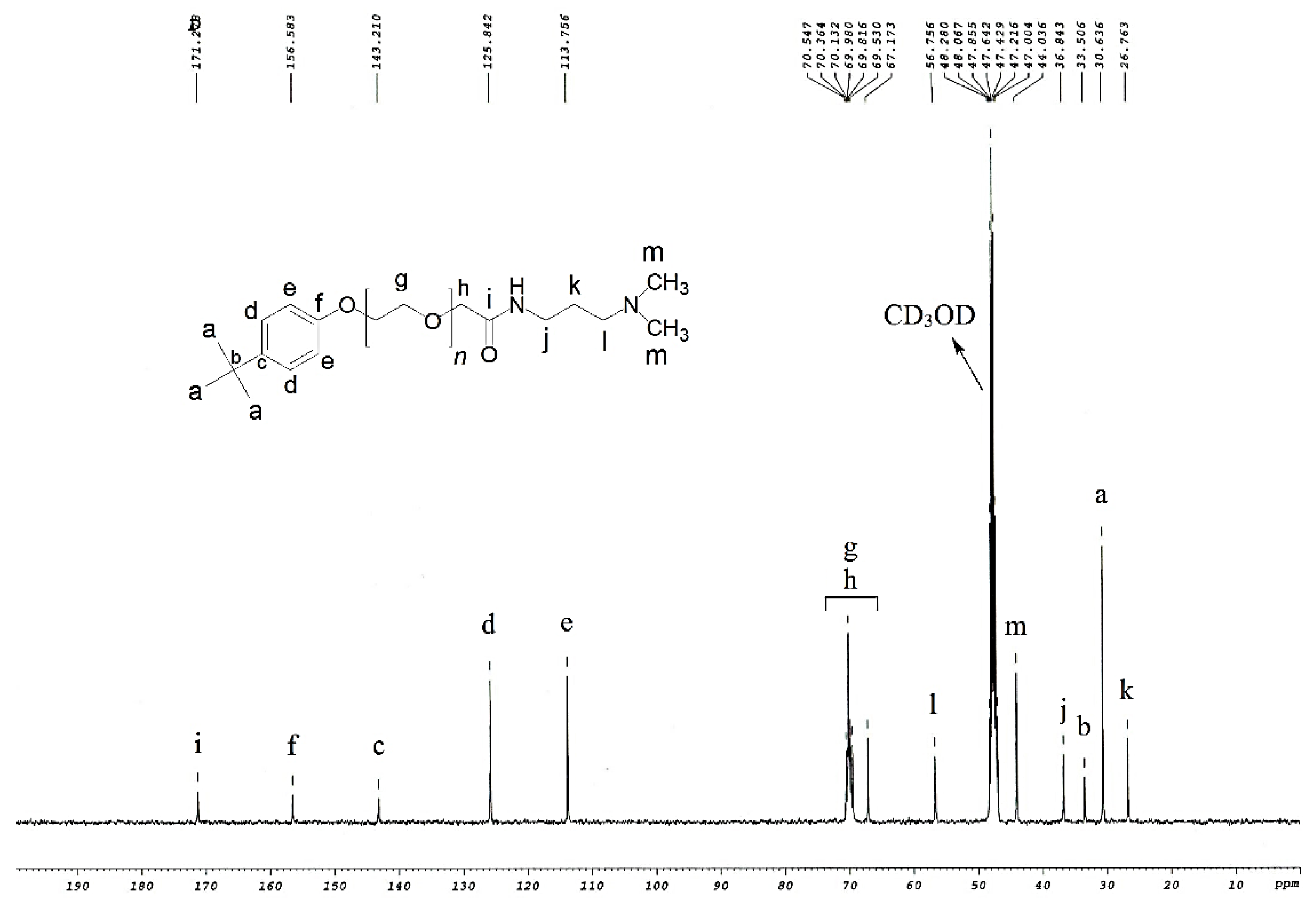
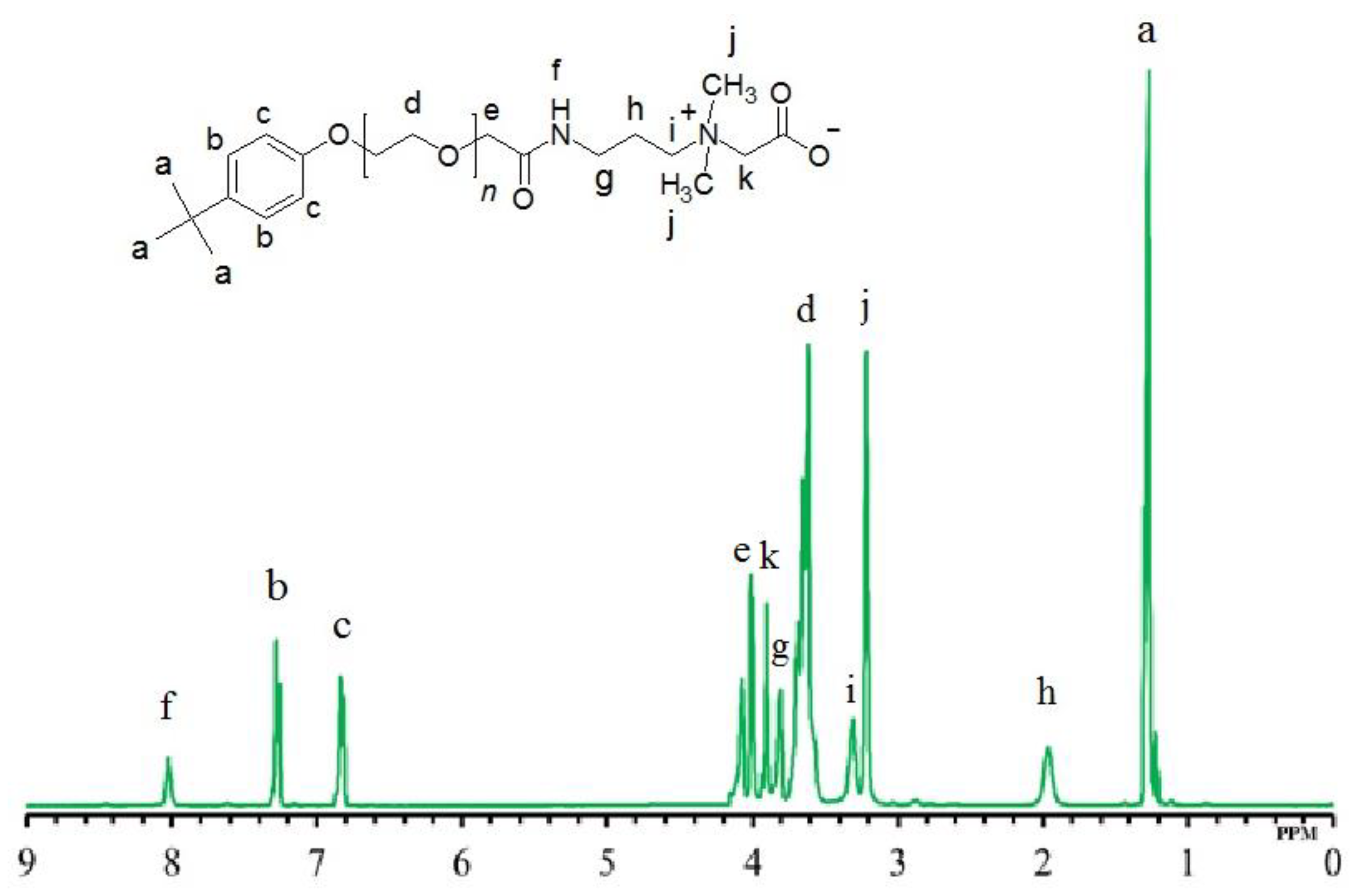
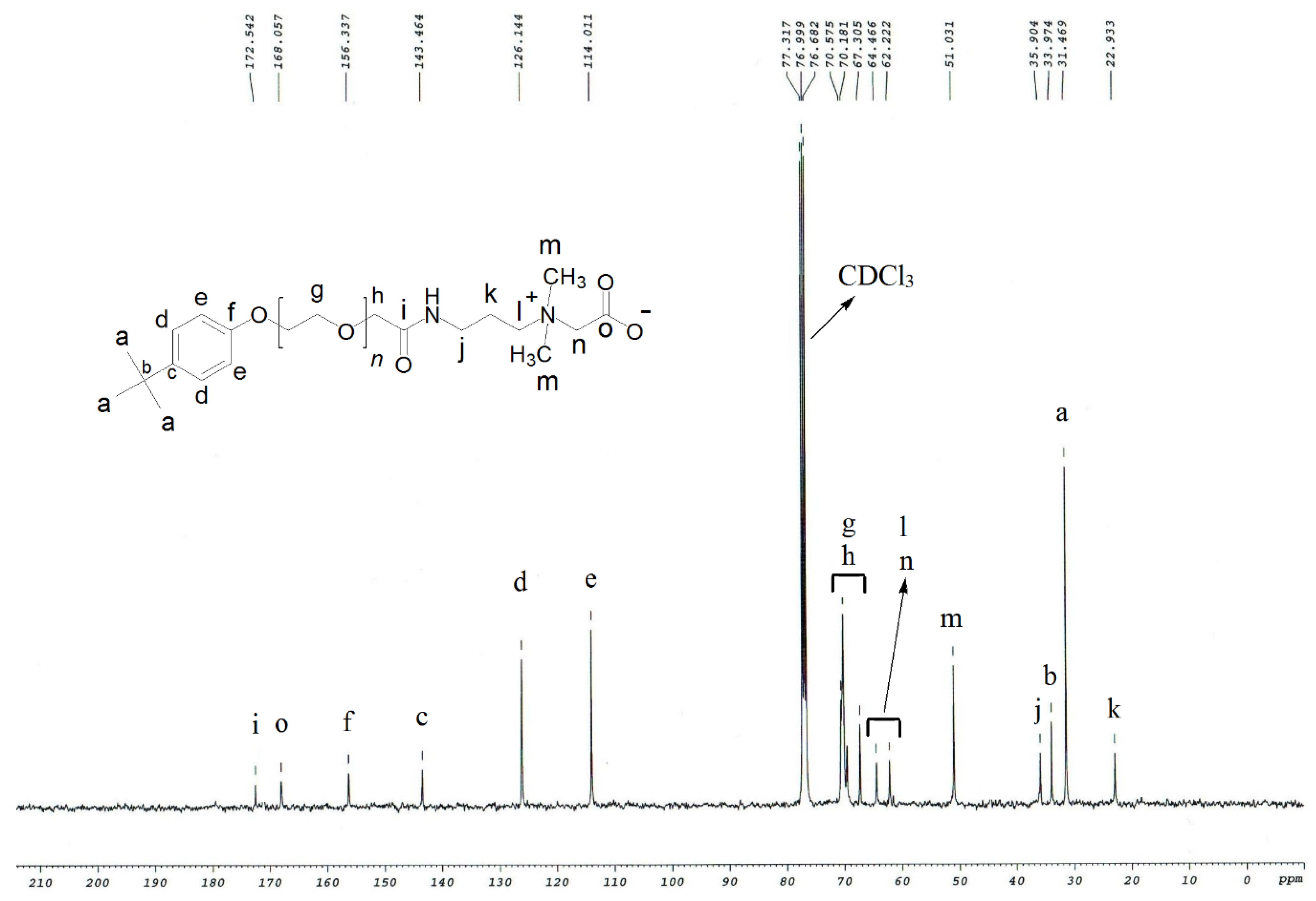
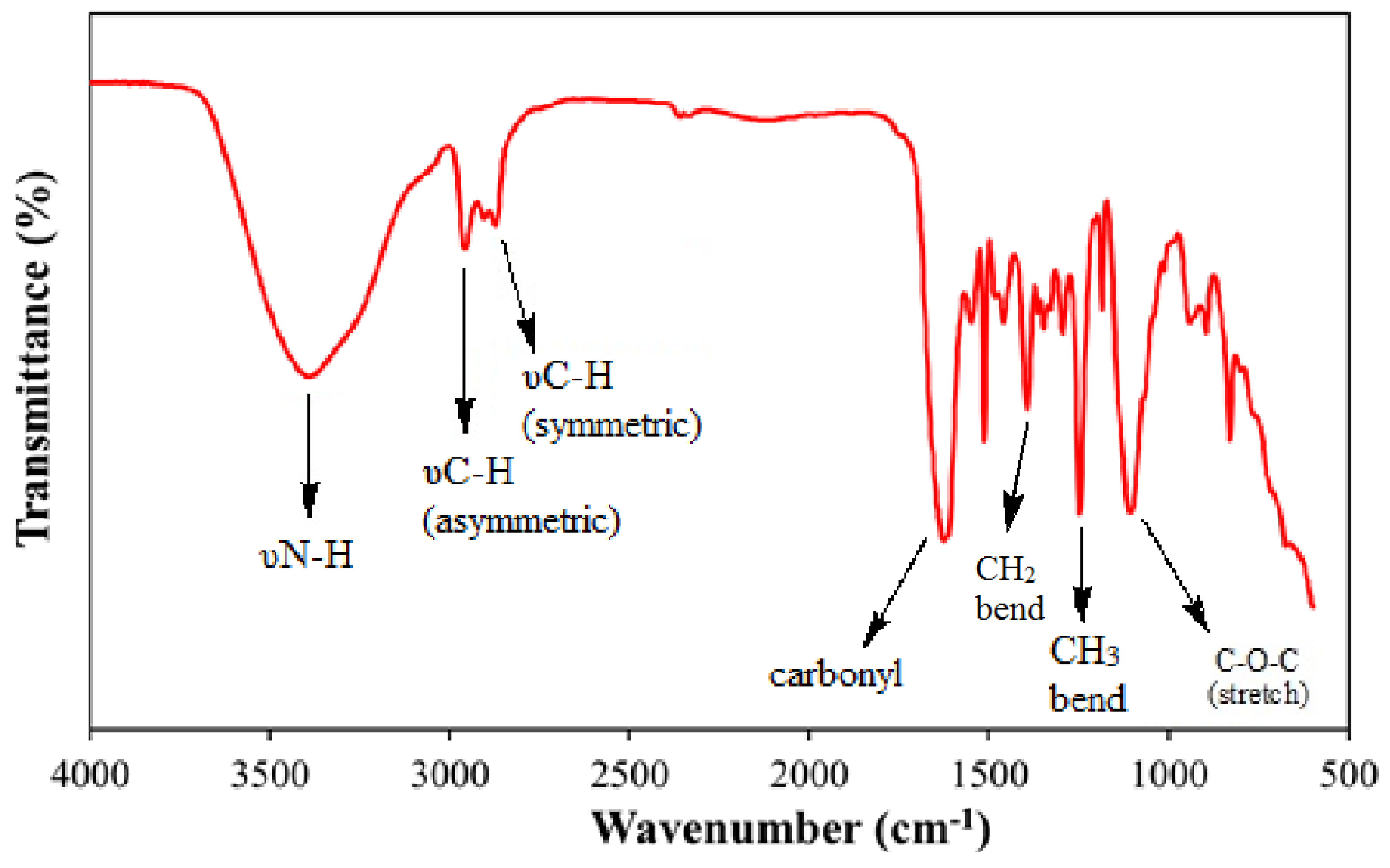






| ZwitterIonic Surfactants | 1H NMR Values (500 MHz, δ in ppm, CDCl3) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lipophilic Tail | Ethoxy Units | Acetic | Amide | Amido-Amine | Benzene Ring | |||||||||||
| 3 × CH3 | CH3CH2 | CH2 | CH2 | 2 × CH2 | CH2 | NH | 2 × CH3 | CH2 | CH2 | CH2 | CH2 | CH2 | CH2 | 2 × CH | 2 × CH | |
| TEAC | 1.27 | - | - | - | 3.64 | 4.07 | 8.02 | 3.22 | 1.96 | - | 3.30 | 3.32 | 3.61 | - | 6.82 | 7.26 |
| TEAS | 1.27 | - | - | - | 3.63 | 4.07 | 8.01 | 3.11 | 1.95 | 2.18 | 2.91 | 3.34 | 3.51 | 3.80 | 6.82 | 7.27 |
| TEAH | 1.26 | - | - | - | 3.64 | 4.06 | 7.98 | 3.20 | 2.04 | 4.67 CH | 3.15 | 3.33 | 3.52 | 3.79 | 6.81 | 7.25 |
| NEAC | - | 0.80 | 1.25 | 1.50 | 3.63 | 4.10 | 8.01 | 3.23 | 1.98 | - | 3.32 | 3.34 | 3.60 | - | 6.83 | 7.19 |
| NEAS | - | 0.77 | 1.23 | 1.53 | 3.64 | 4.09 | 8.00 | 3.10 | 1.99 | 2.17 | 2.89 | 3.34 | 3.49 | 3.82 | 6.82 | 7.20 |
| NEAH | - | 0.79 | 1.25 | 1.52 | 3.63 | 4.03 | 8.00 | 3.23 | 2.06 | 4.68 CH | 3.15 | 3.34 | 3.53 | 3.80 | 6.82 | 7.18 |
| Zwitterionic Surfactants | 13C NMR (CDCl3, δ in ppm, 125 MHz) |
|---|---|
| TEAC | 22.9, 31.5, 34.0, 35.9, 51.0, 62.2, 64.5, 67.3, 69.0–71.0, 114.0, 126.1, 143.5, 156.3, 168.1, 172.5 |
| TEAS | 18.5, 22.6, 31.4, 33.9, 35.8, 50.6, 60.5, 62.6, 67.2, 69.0–71.0, 113.9, 126.0, 143.4, 156.2, 171.3 |
| TEAH | 22.9, 31.5, 34.0, 35.9, 51.9, 55.3 63.0, 67.3, 67.5, 69.0–71.0, 114.0, 126.1, 143.4, 156.4, 171.3 |
| NEAC | 14.4, 22.9, 29.1, 35.9, 51.0, 62.2, 64.5, 67.2, 69.0–71.0, 113.8, 126.8, 156.1, 167.8, 172.3 |
| NEAS | 14.1, 22.7, 24.8, 29.2, 35.9, 50.8, 60.6, 62.7, 67.3, 69.0–71.0, 113.7, 126.9, 156.3, 171.5 |
| NEAH | 14.1, 22.6, 29.1, 35.6, 51.8, 55.3, 62.9, 67.2, 67.5, 69.0–71.0, 113.8, 126.9, 156.1, 171.3 |
| Zwitterionic Surfactants | FTIR Data (in cm−1) | |||||||
|---|---|---|---|---|---|---|---|---|
| (υN-H) | υC-H Asym. | υC-H Sym. | Amide (I) | CH2 (bend) | CH3 (bend) | Ethoxy Stretch | Asym. Stretch | |
| TEAC | 3398 | 2959 | 2872 | 1625 | 1393 | 1248 | 1106 | 941 |
| TEAS | 3406 | 2954 | 2871 | 1654 | 1393 | 1245 | 1114 | 925 |
| TEAH | 3405 | 2953 | 2871 | 1656 | 1393 | 1246 | 1104 | 926 |
| NEAC | 3405 | 2957 | 2872 | 1628 | 1393 | 1248 | 1101 | 944 |
| NEAS | 3404 | 2956 | 2872 | 1651 | 1349 | 1290 | 1104 | 947 |
| NEAH | 3405 | 2944 | 2891 | 1621 | 1371 | 1275 | 1164 | 973 |
| Zwitterionic Surfactants | Base Peak | Proposed Structure |
|---|---|---|
| TEAC | 527.3 |  |
| TEAS | 591.3 | 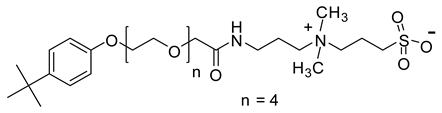 |
| TEAH | 607.3 | 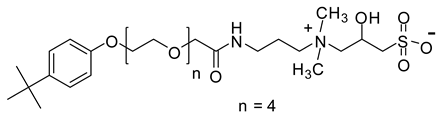 |
| NEAC | 641.4 | 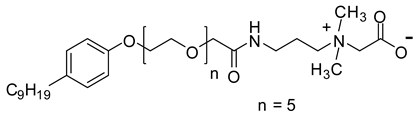 |
| NEAS | 675.4 | 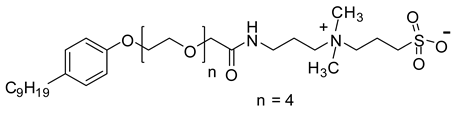 |
| NEAH | 765.5 | 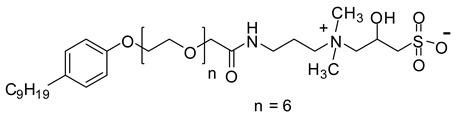 |
| Ions | FW (g L−1) | SW (g L−1) |
|---|---|---|
| Na+ | 59.5 | 18.3 |
| Ca2+ | 19.1 | 0.7 |
| Mg2+ | 2.5 | 2.1 |
| SO42− | 0.4 | 4.3 |
| Cl− | 132.1 | 32.2 |
| HCO3− | 0.4 | 0.1 |
| Total | 214 | 57.7 |
| Surfactant | Solvent | CMC (mmol L−1) | γcmc (mN m−1) | гmax × 106 (mol m−2) | Amin (nm2) |
|---|---|---|---|---|---|
| NEAC | DW | 0.1043 | 32.18 | 2.53 | 0.65 |
| NEAS | DW | 0.1045 | 30.53 | 2.35 | 0.70 |
| NEAH | DW | 0.1308 | 31.55 | 2.27 | 0.73 |
| NEAC | NaCl | 0.0507 | 32.34 | 1.95 | 0.89 |
| NEAS | NaCl | 0.0522 | 31.39 | 1.92 | 0.86 |
| NEAH | NaCl | 0.0568 | 31.89 | 2.22 | 0.74 |
| TEAC | DW | 6.142 | 30.226 | 3.02 | 0.59 |
| TEAS | DW | 6.667 | 28.98 | 2.78 | 0.59 |
| TEAH | DW | 3.828 | 31.79 | 3.46 | 0.47 |
| TEAC | NaCl | 5.792 | 31.43 | 2.35 | 0.70 |
| TEAS | NaCl | 6.010 | 29.09 | 2.43 | 0.68 |
| TEAH | NaCl | 3.163 | 32.79 | 3.63 | 0.45 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussain, S.M.S.; Mahboob, A.; Kamal, M.S. Synthesis and Evaluation of Zwitterionic Surfactants Bearing Benzene Ring in the Hydrophobic Tail. Materials 2020, 13, 1858. https://doi.org/10.3390/ma13081858
Hussain SMS, Mahboob A, Kamal MS. Synthesis and Evaluation of Zwitterionic Surfactants Bearing Benzene Ring in the Hydrophobic Tail. Materials. 2020; 13(8):1858. https://doi.org/10.3390/ma13081858
Chicago/Turabian StyleHussain, Syed Muhammad Shakil, Ahmad Mahboob, and Muhammad Shahzad Kamal. 2020. "Synthesis and Evaluation of Zwitterionic Surfactants Bearing Benzene Ring in the Hydrophobic Tail" Materials 13, no. 8: 1858. https://doi.org/10.3390/ma13081858
APA StyleHussain, S. M. S., Mahboob, A., & Kamal, M. S. (2020). Synthesis and Evaluation of Zwitterionic Surfactants Bearing Benzene Ring in the Hydrophobic Tail. Materials, 13(8), 1858. https://doi.org/10.3390/ma13081858







