Two-Dimensional Zeolite Materials: Structural and Acidity Properties
Abstract
1. Introduction
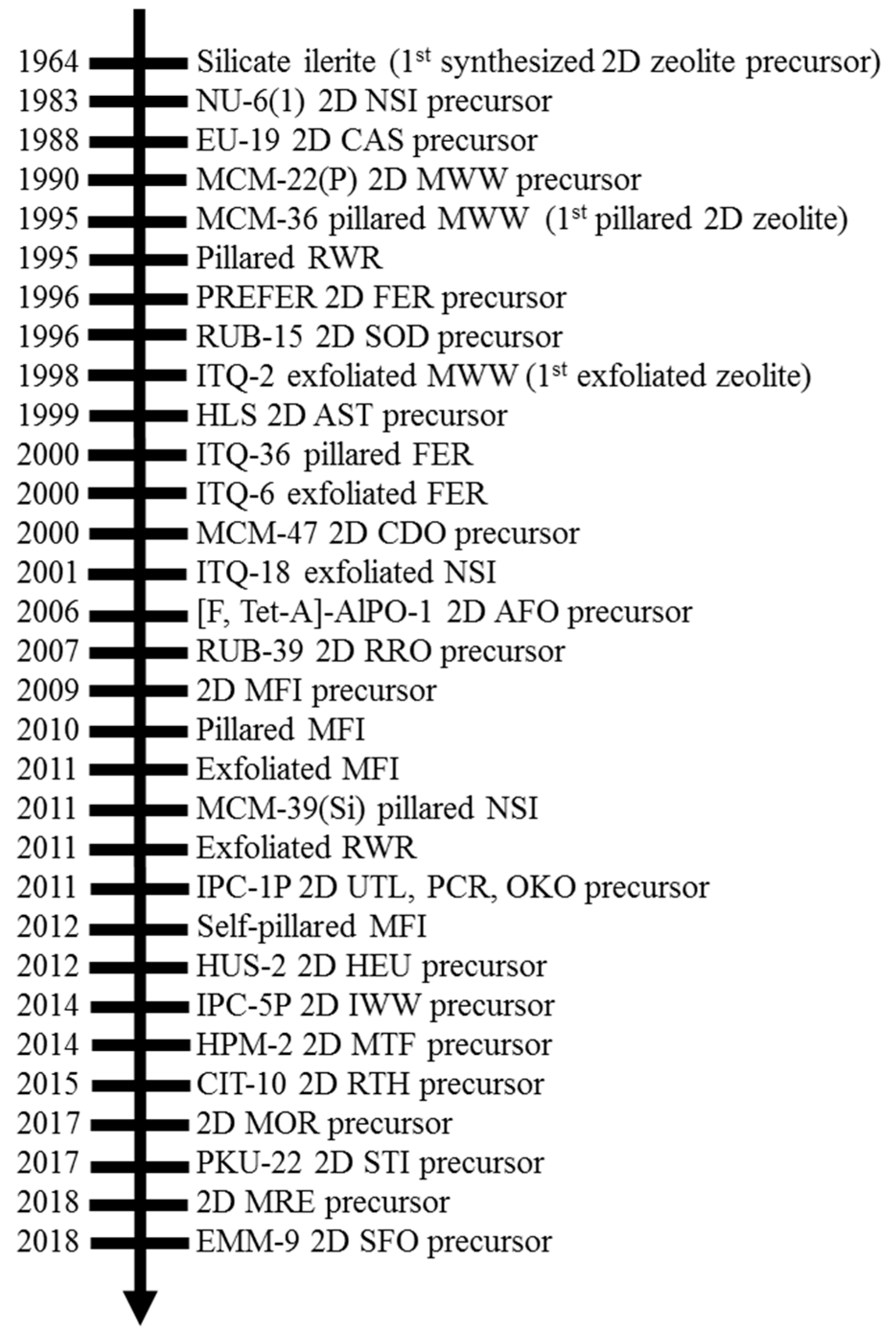
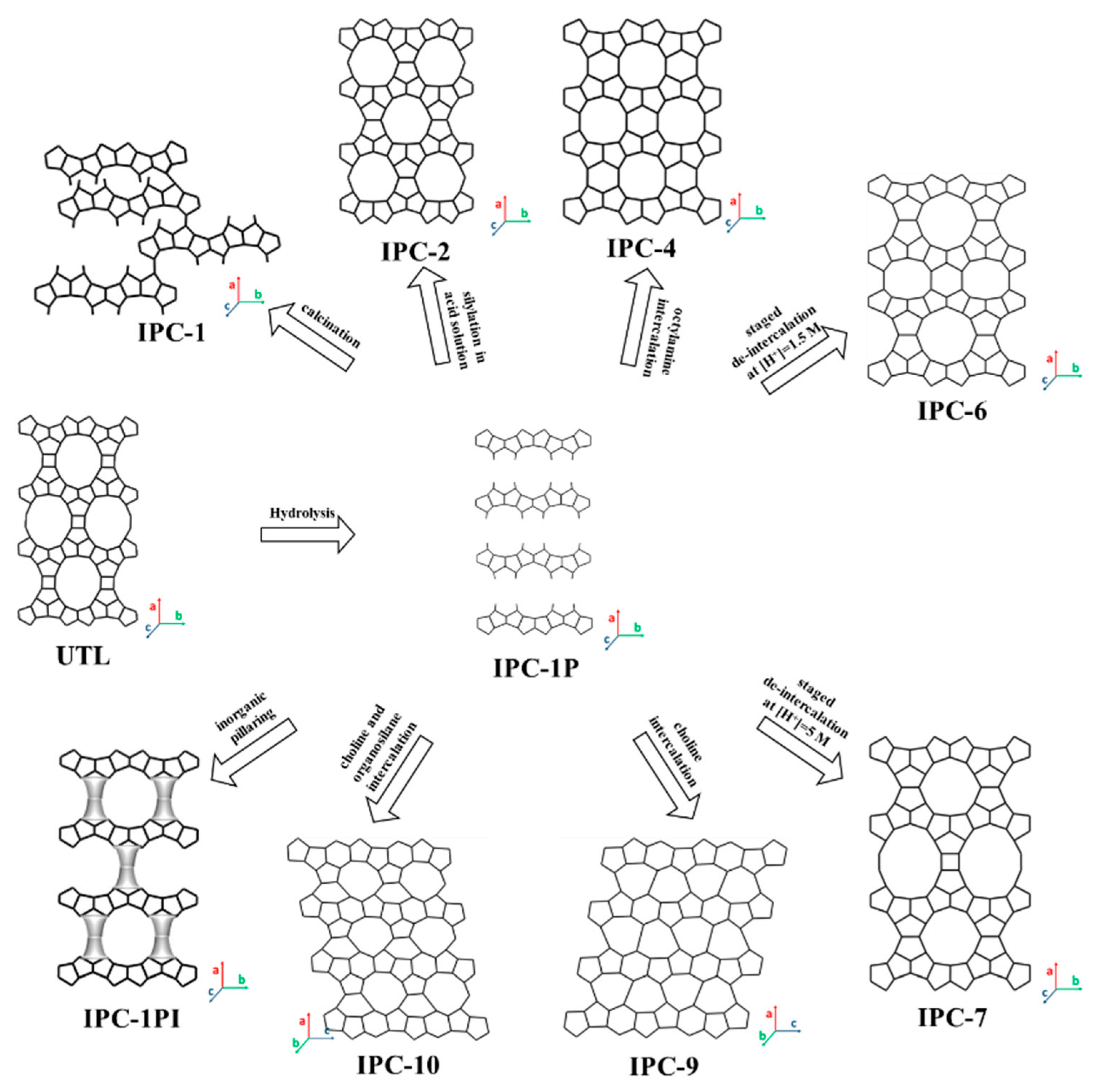
2. History of 2D Zeolite Precursors and Their Derivatives
3. Structural Properties of 2D Zeolite Materials
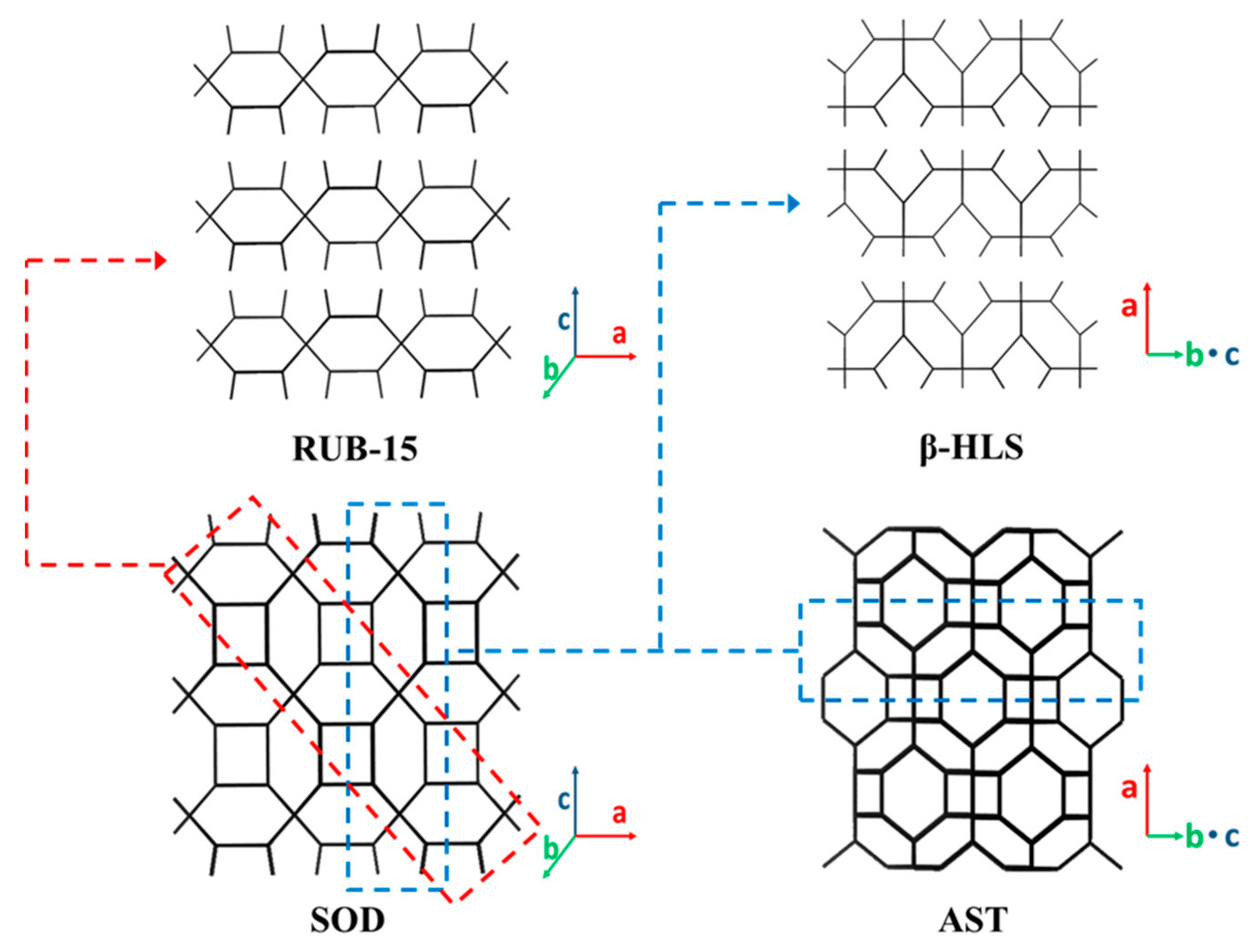
3.1. 2D Layers with 6-MR Zeolite (AST and SOD) Topology
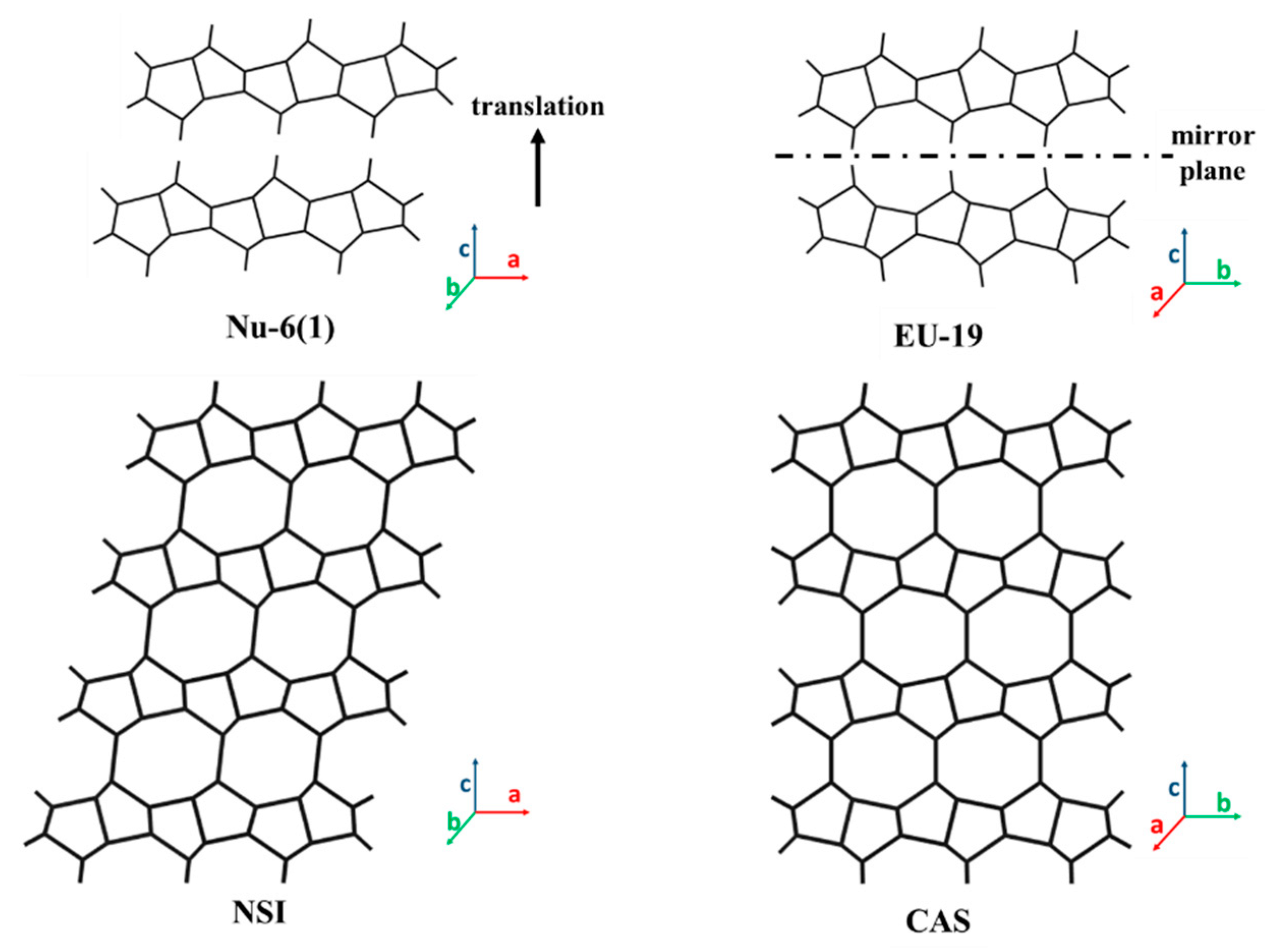
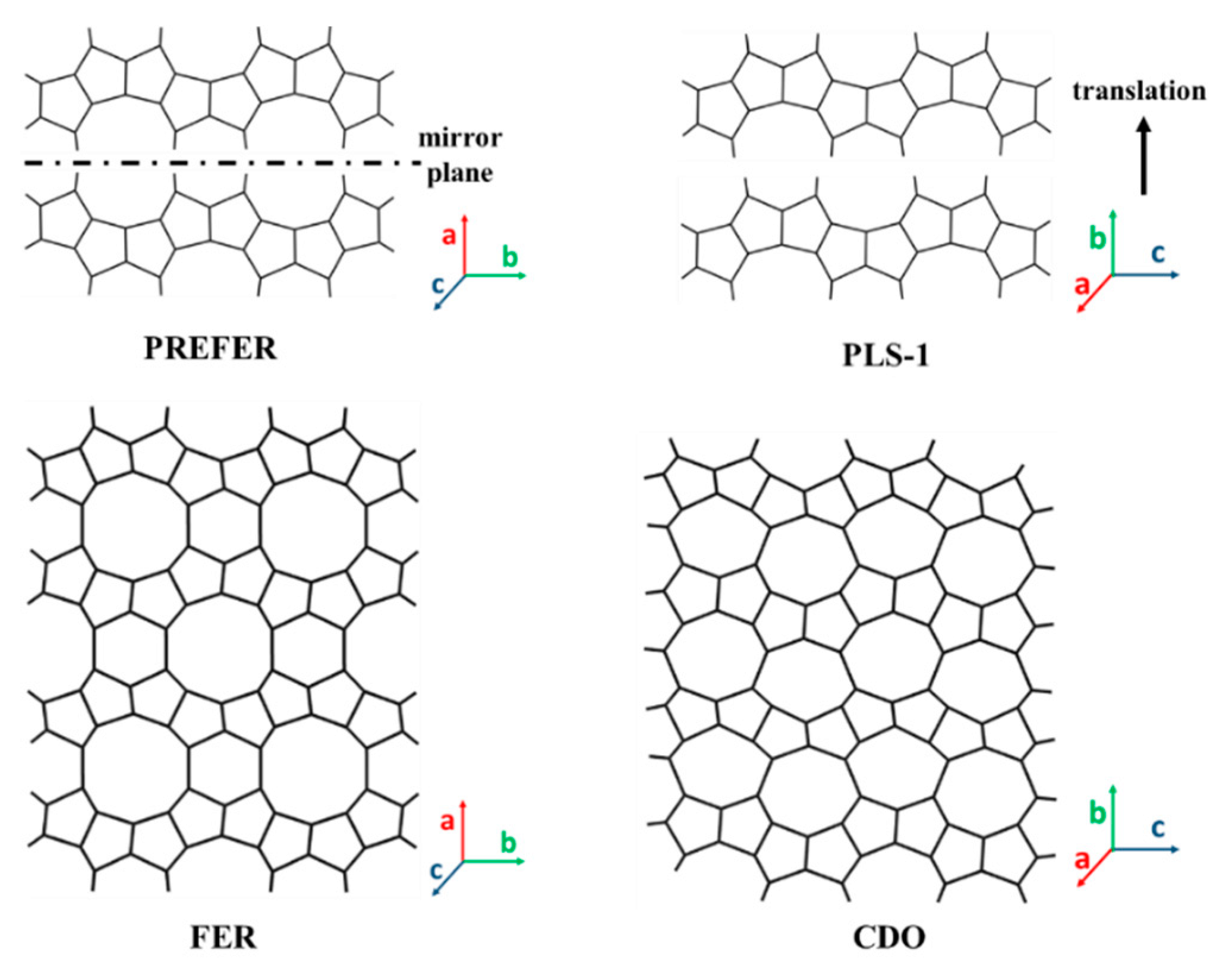
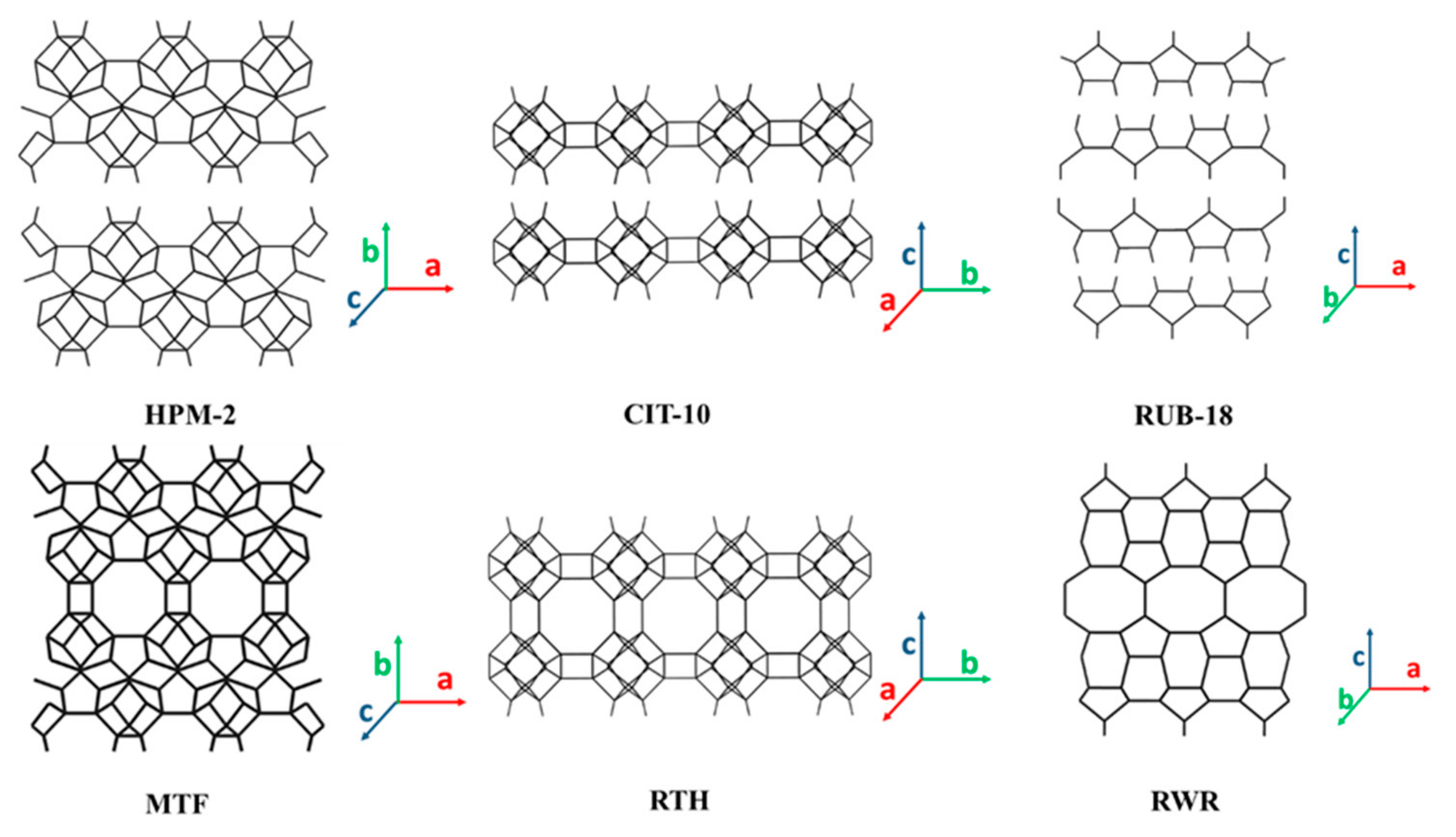
3.2. 2D Layers with 8-MR Zeolite (CAS, CDO, MTF, NSI, RTH, RWR) Topology
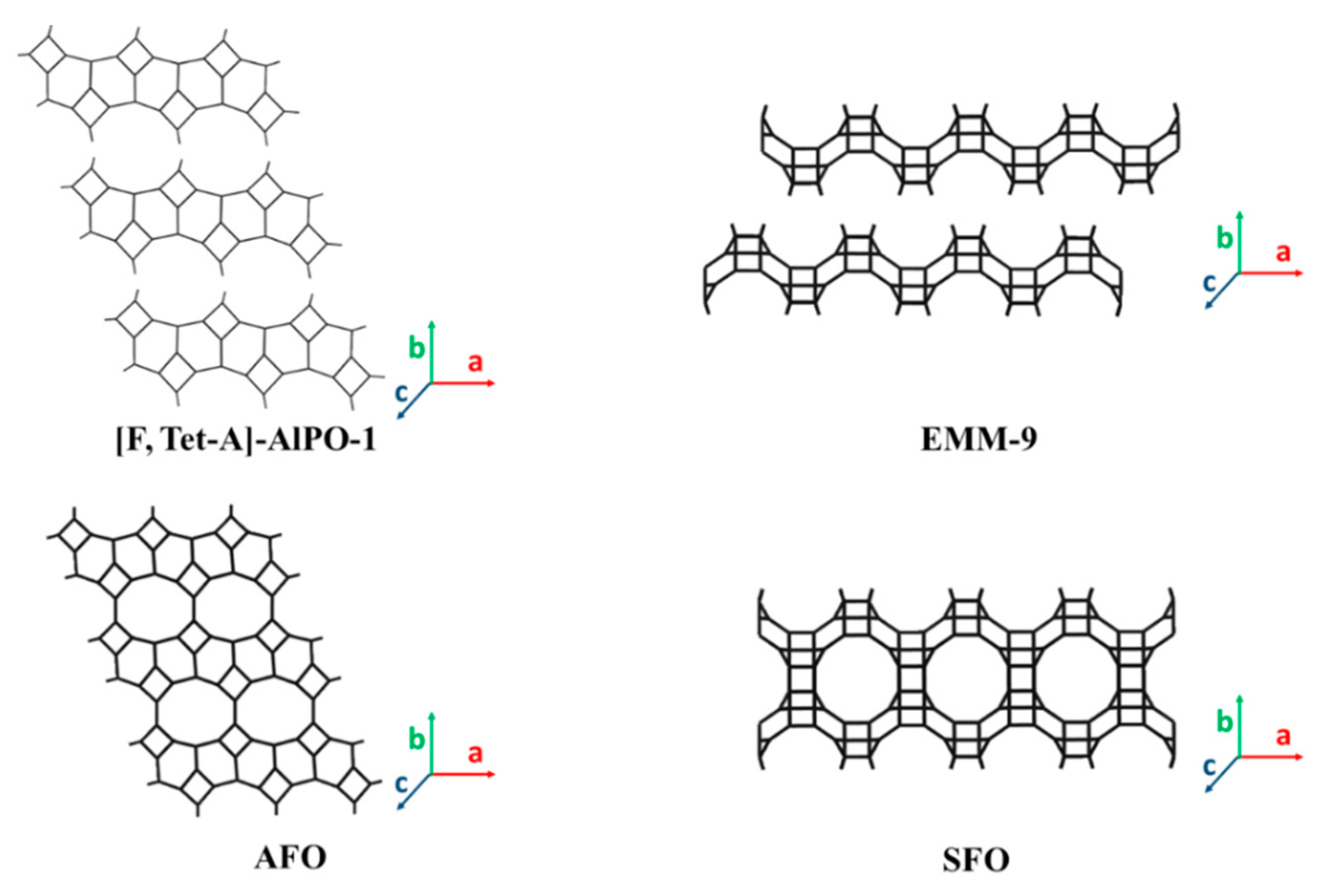
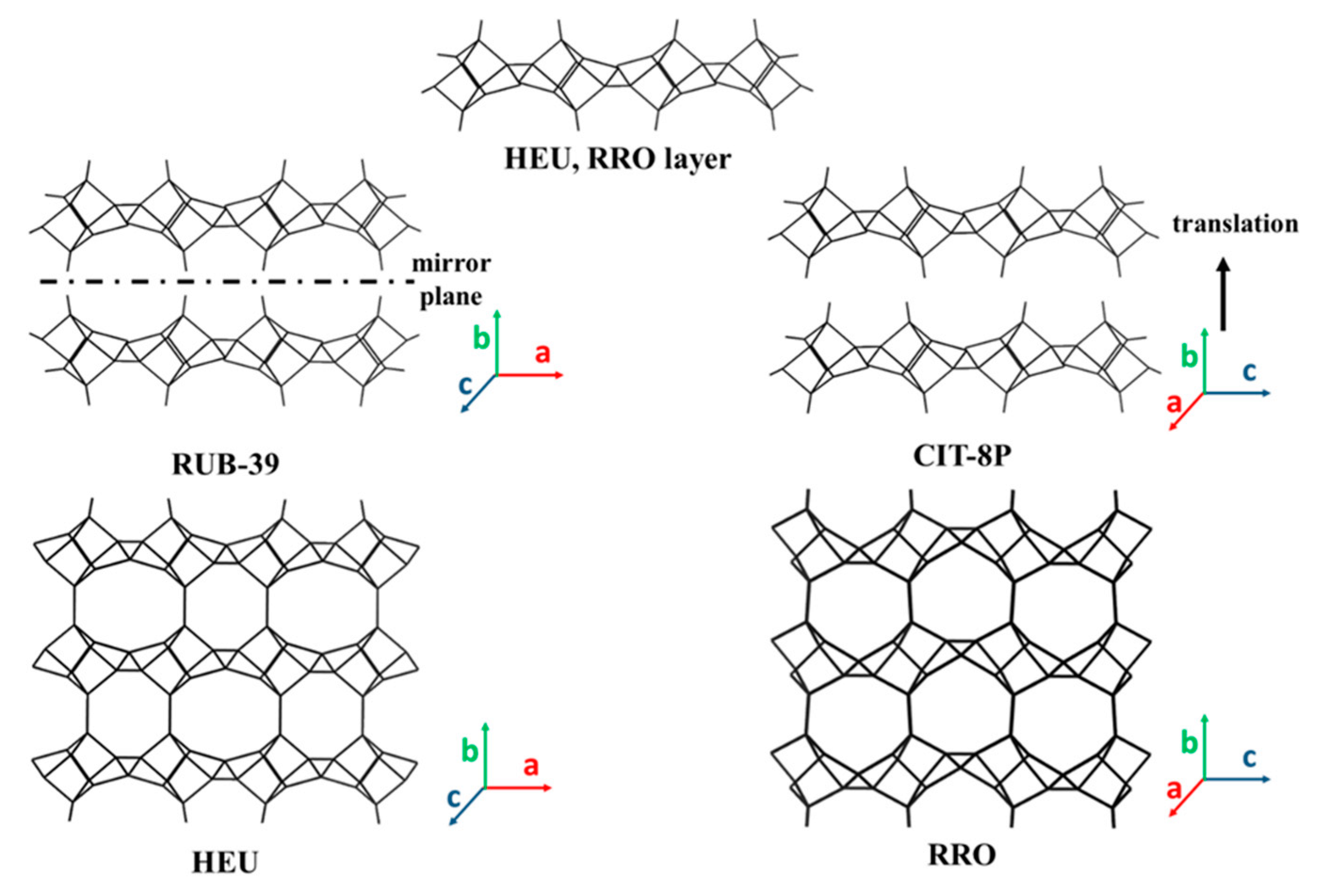
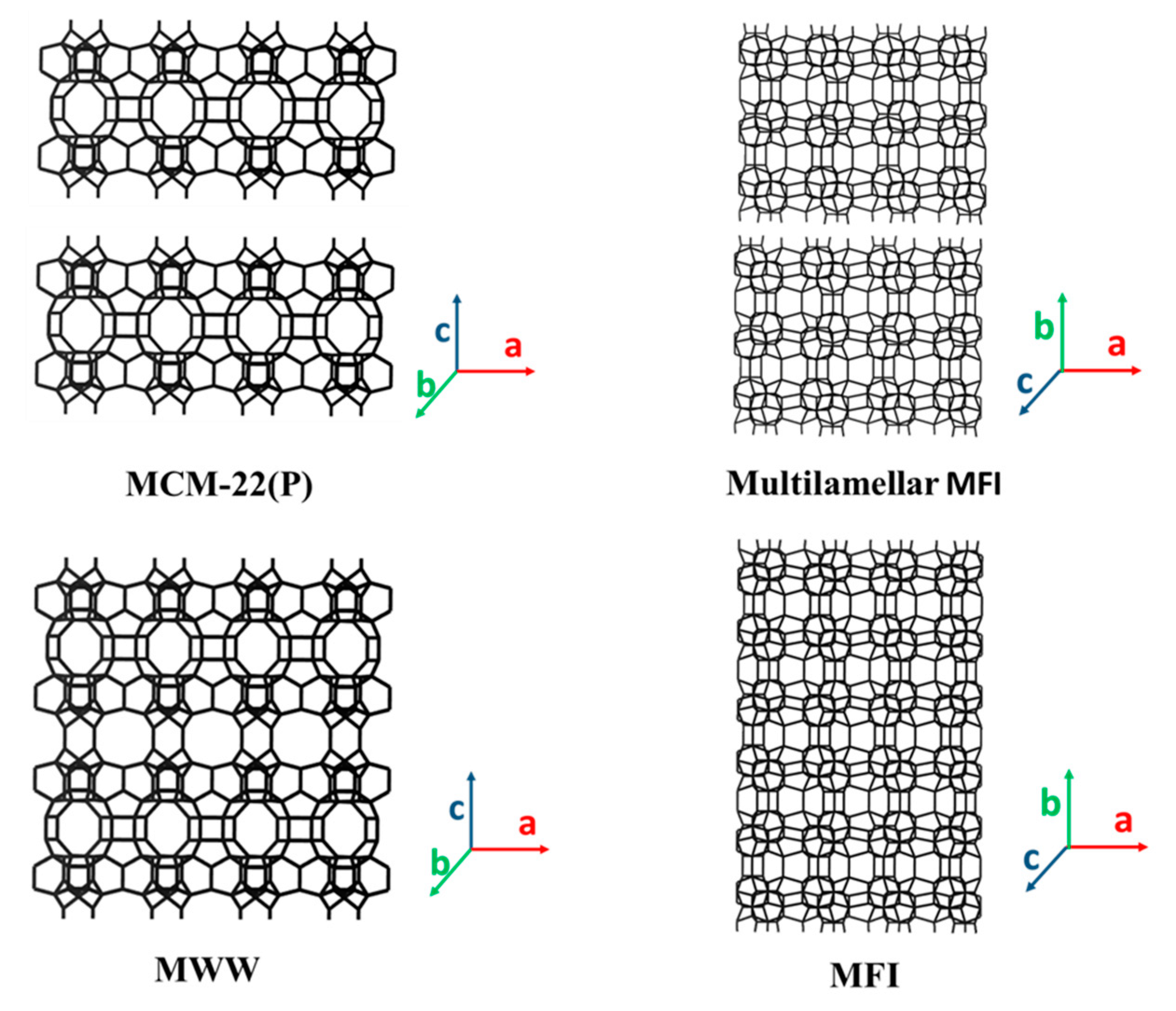
3.3. 2D Layers with 10-MR (AFO, FER, HEU, MFI, MWW, RRO) Topology
3.3.1. 2D Layers with Aluminophosphate Framework
3.3.2. 2D Layers with FER Framework Topology
3.3.3. 2D Layers with HEU Framework Topology
3.3.4. 2D Layers with MFI Framework Topology
3.3.5. 2D Layers with MWW Framework Topology
3.3.6. 2D Layers with STI and RRO Framework Topology
3.4. 2D Layers from 14- and/or 12-MR Zeolite (UTL, IWW, UOV, SAZ-1) Topology
3.5. Other Types of 2D Zeolites (MEL, FAU, MOR, MRE, TON)
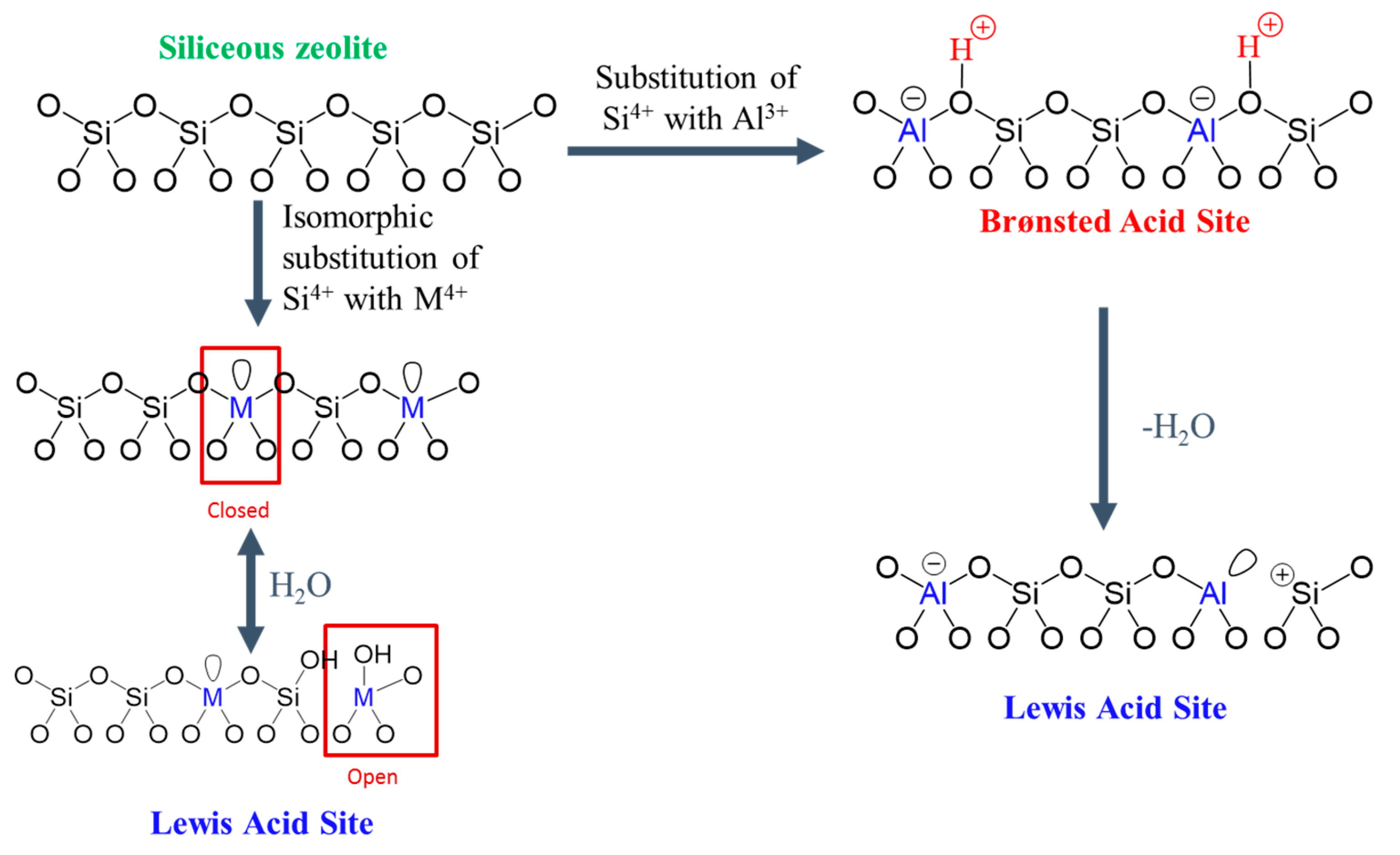
4. Acidity Properties of 2D Zeolites
4.1. Techniques for Acidity Characterization of 2D Zeolite Materials
4.2. 2D Zeolites Explored as Acid Catalysts
4.2.1. Acidity Characterization for 2D NSI, RWR and FER Zeolites
4.2.2. Acidity Characterization for 2D MFI Zeolites
4.2.3. Acidity Characterization for 2D MWW Zeolites
4.2.4. Acidity Characterization for 2D Materials Generated from UTL Zeolite
5. Summary and Outlook
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| [F, Tet-A]-AlPO-1 | [Fluoride, meso-5,7,7,12,14,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane]- Aluminium Phosphate number 1 |
| Ada-4-16 | C10H15–N+(CH3)2–C4H8–N+(CH3)2–C16H33 |
| AFO | AlPO4-41 (forty-one) (IZA code AFO) |
| AlPO4-41 | Aluminophosphate number 41 |
| APZ-1 | Atom Pillared Zeolitic material number 1 |
| AST | Aluminophosphate with sequence number SixTeen (IZA code AST) |
| ATMP | 4-Amino-2,2,6,6-tetramethylpiperidine |
| BPTn-6-0 | (C6H2)3–(O–CnH2n–N+(CH3)2–C6H12–N(CH3)2(Br−))6 |
| BTMAOH | benzyltrimethylammonium hydroxide |
| Bza-4-16 | C16H33–N+(CH3)2–C4H8–N+(CH3)2–benzylamine |
| C16-2-0 | C16H33–N+(CH3)2–C2H4–N(CH3)2Br |
| C18-6-6-18Br3 | C18H37–N+(CH3)2–C6H12–N+(CH3)2–C6H12–N+(CH3)2–C18H37(Br−)3 |
| C22-6-6Br2 | C22H45–N+(CH3)2–C6H12–N+(CH3)2–C6H13(Br−)2 |
| CNh-10-6 | C6H4–C4H3–O–C10H20–N+(CH3)2–C6H13(Br−) |
| CPh–Ph-10-6 | C6H5–C6H4–O–C10H20–N+(CH3)2–C6H13(Br−) |
| CAS | Cesium AluminoSilicate (IZA code CAS) |
| CD3CN | Acetonitrile-d3 |
| CDS-1 | Cylindrically Double Saw-Edged zeolite number 1 |
| CDO | CDS-1 (one) (IZA code CDO) |
| CIT-8P | California Institute of Technology number 8 Precursor |
| COE-3 | International Network of Centers Of Excellence number 3 |
| CTAB | cetyltrimethylammonium bromide |
| CTMA | hexadecyltrimethylammonium |
| Del-Nu-6 | Delaminated-Nu-6 |
| DEDMAOH | diethyldimethylammonium hydroxide |
| DLM-2 | Delft Layered Material number 2 |
| DMDPA | dimethyldipropylammonium |
| DMDPAOH | dimethyldipropylammonium hydroxide |
| DME | dimethyl ether |
| DMEP | (1R,2S)-dimethylephedrinium |
| DMF | dimethylformamide |
| DS-ITQ-2 | Direct Synthesis Instituto de Tecnología Química number 2 |
| DTBP | di-tert-butyl peroxide |
| ENCU-9 | East China Normal University number 9 |
| EMM-10P | ExxonMobil Material number 10 Precursor |
| ERB-1P | EniRicerche-Boralite number 1 Precursor |
| ERS-12 | EniRicerche molecular Sieve number 12 |
| ETMAOH | ethyltrimethylammonium hydroxide |
| EU-19 | Edinburgh University number 19 |
| FAU | Faujasite (IZA code FAU) |
| FER | Ferrierite (IZA code FER) |
| HEU | Heulandite (IZA code HEU) |
| HLS | Helix Layered Silicate |
| HMI | hexamethyleneimine |
| HPM-2 | Nanostructured Hybrid biohybrid and Porous Materials number 2 |
| HUS-1 | Hiroshima University Silicate number 1 |
| ICP-2 | Instituto de Catálisis y Petroleoquímica number 2 |
| IEZ | interlayer expanded zeolite |
| IM-12 | Institut Français du Pétrole and University of Mulhouse |
| IPC-1P | Institute of Physical Chemistry number 1 Precursor |
| IPC-4 | Institute of Physical Chemistry number 4 |
| ITH | Instituto de Tecnologia Quimica Valencia—thirteen (IZA code ITH) |
| ITR | Instituto de Tecnologia Quimica Valencia—thirty-four (IZA code ITR) |
| ITQ-2 | Instituto de Tecnología Química number 2 |
| IWR | Instituto de Tecnologia Quimica Valencia—twenty-four (IZA code IWR) |
| IWW | ITQ-22 (twenty-two) (IZA code IWW) |
| LDS | Lower Dimensional Silicate |
| MCM-22 | Mobil Composition of Matter number 22 |
| MCM-22(P) | Mobil Composition of Matter number 22 (Precusor) |
| MCM-22(P)-sw | Mobil Composition of Matter number 22 (Precusor) swollen |
| MEL | ZSM-11 (eleven) (IZA code MEL) |
| MFI | ZSM-5 (five) (IZA code MFI) |
| MIT-1 | Massachusetts Institute of Technology number 1 |
| MOR | Mordenite (IZA code MOR) |
| MRE | ZSM-48 (forty-eight) (IZA code MRE) |
| MTEAOH | methyltriethylammonium hydroxide |
| MTF | MCM-35 (thirty-five) (IZA code MTF) |
| MTS-2 | Multilayered Titanium Silicalite-2 |
| MWW | MCM-22 (twenty-two) (IZA code MWW) |
| MZIN | Mesoporous ZSM-5 with Intercrossed Nanosheets |
| NSI | Nu-6(2) (six) (IZA code NSI) |
| Nu-6(1) | (New (ICI, Imperial Chemical Industries) with sequence number Six (one) |
| OKO | Oppervlakte en Katalyse One (IZA code OKO) |
| PCR | IPC-4 (four) (IZA code PCR) |
| PKU-22 | Peking University number 22 |
| PLS-3 | Pentagonal-cylinder Layered Silicate number 3 |
| PREFER | Precursor of ferrierite |
| RRO | RUB-41 (forty-one) (IZA code RRO) |
| RTH | RUB-13 (thirteen) (IZA code RTH) |
| RUB-55 | Ruhr University Bochum number 55 |
| RWR | RUB-24 (twenty-four) (IZA code RWR) |
| SAZ-1 | University of St. Andrews Zeolite—one |
| SCZN-1 | Single-Crystalline mesostructured Zeolite Nanosheets number 1 |
| SFO | SSZ-51 (fifty-one) (IZA code SFO) |
| SOD | Sodalite (IZA code SOD) |
| SSZ-51 | Standard Oil Synthetic Zeolite number 51 |
| STI | Stilbite (IZA code STI) |
| TBPO | tributylphosphine oxide |
| TEAOH | tetraethylammonium hydroxide |
| TEOS | tetraethyl orthosilicate |
| Ti-YNU-1 | Yokohama National University number 1 |
| TMAOH | tetramethylammonium hydroxide |
| TMMPBr | tetramethylene bis(N-methylpyrrolidinium) dibromide |
| TMPOH | tetramethylphosphonium hydroxide |
| TMPAOH | trimethyl-isopropylammonium hydroxide |
| TON | Theta-1 (one) |
| TPAOH | tetra-n-propylammonium hydroxide |
| TPHAC | 3-(trimethoxysilyl)propyl hexadecyl dimethyl ammonium chloride |
| TPP | triphenylphosphine |
| UCB-1 | University of California at Berkeley number 1 |
| UJM-1P | Uniwersytet Jagiellonski Material #1 |
| UOV | Institut Français du Pétrole and University of Mulhouse—seventeen (one seven) (IZA code UOV) |
| UTL | IM-12, Mulhouse (twelve) (IZA code UTL) |
| UZM-13 | Universal Oil Products Zeolitic Material number 13 |
| ULS-1 | UOP Layered Silicate-1 |
| ZSM-5 | Zeolite Socony Mobil number 5 |
References
- Weitkamp, J. Zeolites and catalysis. Solid State Ion. 2000, 131, 175–188. [Google Scholar] [CrossRef]
- Čejka, J.; Wichterlová, B. Acid-catalyzed synthesis of mono- and dialkyl benzenes over zeolites: Active sites, zeolite topology, and reaction mechanisms. Catal. Rev. 2002, 44, 375–421. [Google Scholar] [CrossRef]
- Eliasova, P.; Čejka, J. Two-dimensional zeolites. In Zeolites in Catalysis: Properties and Applications; The Royal Society of Chemistry: Cambridge, UK, 2017; pp. 146–193. [Google Scholar]
- Farneth, W.E.; Gorte, R.J. Methods for characterizing zeolite acidity. Chem. Rev. 1995, 95, 615–635. [Google Scholar] [CrossRef]
- Luo, H.Y.; Lewis, J.D.; Román-Leshkov, Y. Lewis acid zeolites for biomass conversion: Perspectives and challenges on reactivity, synthesis, and stability. Annu. Rev. Chem. Biomol. 2016, 7, 663–692. [Google Scholar] [CrossRef]
- Roth, W.J.; Nachtigall, P.; Morris, R.E.; Čejka, J. Two-dimensional zeolites: Current status and perspectives. Chem. Rev. 2014, 114, 4807–4837. [Google Scholar] [CrossRef]
- Čejka, J.; Centi, G.; Perez-Pariente, J.; Roth, W.J. Zeolite-based materials for novel catalytic applications: Opportunities, perspectives and open problems. Catal. Today 2012, 179, 2–15. [Google Scholar] [CrossRef]
- Roth, W.J.; Gil, B.; Makowski, W.; Marszalek, B.; Eliasova, P. Layer like porous materials with hierarchical structure. Chem. Soc. Rev. 2016, 45, 3400–3438. [Google Scholar] [CrossRef]
- Opanasenko, M.V.; Roth, W.J.; Čejka, J. Two-dimensional zeolites in catalysis: Current status and perspectives. Catal. Sci. Technol. 2016, 6, 2467–2484. [Google Scholar] [CrossRef]
- Xu, L.; Sun, J. Recent advances in the synthesis and application of two-dimensional zeolites. Adv. Energy Mater. 2016, 6, 1600441. [Google Scholar] [CrossRef]
- Díaz, U.; Corma, A. Layered zeolitic materials: An approach to designing versatile functional solids. Dalton Trans. 2014, 43, 10292–10316. [Google Scholar] [CrossRef]
- Roth, W.J.; Gil, B.; Marszalek, B. Comprehensive system integrating 3D and 2D zeolite structures with recent new types of layered geometries. Catal. Today 2014, 227, 9–14. [Google Scholar] [CrossRef]
- Corma, A.; Fornes, V.; Pergher, S.B.; Maesen, T.L.M.; Buglass, J.G. Delaminated zeolite precursors as selective acidic catalysts. Nature 1998, 396, 353–356. [Google Scholar] [CrossRef]
- Corma, A.; Diaz, U.; Domine, M.E.; Fornés, V. AlITQ-6 and TiITQ-6: Synthesis, characterization, and catalytic activity. Angew. Chem. Int. Ed. 2000, 39, 1499–1501. [Google Scholar] [CrossRef]
- Corma, A.; Fornes, V.; Diaz, U. ITQ-18 a new delaminated stable zeolite. Chem. Commun. 2001, 2642–2643. [Google Scholar] [CrossRef]
- Osada, S.; Iribe, A.; Kuroda, K. Exfoliation of layered octosilicate by simple cation exchange with didecyldimethylammonium ions. Chem. Lett. 2013, 42, 80–82. [Google Scholar] [CrossRef]
- Gorgojo, P.; Galve, A.; Uriel, S.; Téllez, C.; Coronas, J. Direct exfoliation of layered zeolite Nu-6(1). Microporous Mesoporous Mater. 2011, 142, 122–129. [Google Scholar] [CrossRef]
- Varoon, K.; Zhang, X.; Elyassi, B.; Brewer, D.D.; Gettel, M.; Kumar, S.; Lee, J.A.; Maheshwari, S.; Mittal, A.; Sung, C.-Y.; et al. Dispersible exfoliated zeolite nanosheets and their application as a selective membrane. Science 2011, 334, 72–75. [Google Scholar] [CrossRef]
- Zhang, H.; Xiao, Q.; Guo, X.; Li, N.; Kumar, P.; Rangnekar, N.; Jeon Mi, Y.; Al-Thabaiti, S.; Narasimharao, K.; Basahel Sulaiman, N.; et al. Open-pore two-dimensional MFI zeolite nanosheets for the fabrication of hydrocarbon-isomer-selective membranes on porous polymer supports. Angew. Chem. Int. Ed. 2016, 55, 7184–7187. [Google Scholar] [CrossRef]
- Sabnis, S.; Tanna, V.A.; Li, C.; Zhu, J.; Vattipalli, V.; Nonnenmann, S.S.; Sheng, G.; Lai, Z.; Winter, H.H.; Fan, W. Exfoliation of two-dimensional zeolites in liquid polybutadienes. Chem. Commun. 2017, 53, 7011–7014. [Google Scholar] [CrossRef]
- Maheshwari, S.; Jordan, E.; Kumar, S.; Bates, F.S.; Penn, R.L.; Shantz, D.F.; Tsapatsis, M. Layer structure preservation during swelling, pillaring, and exfoliation of a zeolite precursor. J. Am. Chem. Soc. 2008, 130, 1507–1516. [Google Scholar] [CrossRef]
- Ogino, I.; Nigra, M.M.; Hwang, S.-J.; Ha, J.-M.; Rea, T.; Zones, S.I.; Katz, A. Delamination of layered zeolite precursors under mild conditions: Synthesis of UCB-1 via fluoride/chloride anion-promoted exfoliation. J. Am. Chem. Soc. 2011, 133, 3288–3291. [Google Scholar] [CrossRef]
- Ouyang, X.; Hwang, S.-J.; Runnebaum, R.C.; Xie, D.; Wanglee, Y.-J.; Rea, T.; Zones, S.I.; Katz, A. Single-step delamination of a MWW borosilicate layered zeolite precursor under mild conditions without surfactant and sonication. J. Am. Chem. Soc. 2014, 136, 1449–1461. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, N.; Hata, H.; Kuroda, K. Exfoliation of layered silicates through immobilization of imidazolium groups. Chem. Mater. 2011, 23, 266–273. [Google Scholar] [CrossRef]
- Roth, W.J.; Kresge, C.T.; Vartuli, J.C.; Leonowicz, M.E.; Fung, A.S.; McCullen, S.B. MCM-36: The first pillared molecular sieve with zeolite properties. In Studies in Surface Science and Catalysis; Beyer, H.K., Karge, H.G., Kiricsi, I., Nagy, J.B., Eds.; Elsevier: Amsterdam, The Netherlands, 1995; Volume 94, pp. 301–308. [Google Scholar]
- He, Y.J.; Nivarthy, G.S.; Eder, F.; Seshan, K.; Lercher, J.A. Synthesis, characterization and catalytic activity of the pillared molecular sieve MCM-36. Microporous Mesoporous Mater. 1998, 25, 207–224. [Google Scholar] [CrossRef]
- Barth, J.O.; Kornatowski, J.; Lercher, J.A. Synthesis of new MCM-36 derivatives pillared with alumina or magnesia–alumina. J. Mater. Chem. 2002, 12, 369–373. [Google Scholar] [CrossRef]
- Barth, J.O.; Jentys, A.; Kornatowski, J.; Lercher, J.A. Control of acid-base properties of new nanocomposite derivatives of MCM-36 by mixed oxide pillaring. Chem. Mater. 2004, 16, 724–730. [Google Scholar] [CrossRef]
- Corma, A.; Díaz, U.; García, T.; Sastre, G.; Velty, A. Multifunctional hybrid organic–inorganic catalytic materials with a hierarchical system of well-defined micro- and mesopores. J. Am. Chem. Soc. 2010, 132, 15011–15021. [Google Scholar] [CrossRef]
- Macedo, T.R.; Airoldi, C. Organofunctionalized RUB-18 from the intercalated precursor cetyltrimethylammonium cation. Microporous Mesoporous Mater. 2010, 128, 158–164. [Google Scholar] [CrossRef]
- Chlubná, P.; Roth, W.J.; Greer, H.F.; Zhou, W.; Shvets, O.; Zukal, A.; Čejka, J.; Morris, R.E. 3D to 2D routes to ultrathin and expanded zeolitic materials. Chem. Mater. 2013, 25, 542–547. [Google Scholar] [CrossRef]
- Roth, W.J.; Kresge, C.T. Intercalation chemistry of NU-6(1), the layered precursor to zeolite NSI, leading to the pillared zeolite MCM-39(Si). Microporous Mesoporous Mater. 2011, 144, 158–161. [Google Scholar] [CrossRef]
- Roth, W.J.; Gil, B.; Mayoral, A.; Grzybek, J.; Korzeniowska, A.; Kubů, M.; Makowski, W.; Čejka, J.; Olejniczak, Z.; Mazur, M. Pillaring of layered zeolite precursors with ferrierite topology leading to unusual molecular sieves on the micro/mesoporous border. Dalton Trans. 2018, 47, 3029–3037. [Google Scholar] [CrossRef]
- Lara, A.C.; Canós, A.C.; Segúi, V.F.; Morales, U.D. Acid Oxide with Micro and Mesoporous Characteristics: ITQ-36. U.S. Patent 6,555,090, 29 April 2003. [Google Scholar]
- Na, K.; Choi, M.; Park, W.; Sakamoto, Y.; Terasaki, O.; Ryoo, R. Pillared MFI zeolite nanosheets of a single-unit-cell thickness. J. Am. Chem. Soc. 2010, 132, 4169–4177. [Google Scholar] [CrossRef]
- Liu, B.; Wattanaprayoon, C.; Oh, S.C.; Emdadi, L.; Liu, D. Synthesis of organic pillared MFI zeolite as bifunctional acid–base catalyst. Chem. Mater. 2015, 27, 1479–1487. [Google Scholar] [CrossRef]
- Lee, H.W.; Park, S.H.; Jeon, J.-K.; Ryoo, R.; Kim, W.; Suh, D.J.; Park, Y.-K. Upgrading of bio-oil derived from biomass constituents over hierarchical unilamellar mesoporous MFI nanosheets. Catal. Today 2014, 232, 119–126. [Google Scholar] [CrossRef]
- Wu, Y.; Emdadi, L.; Wang, Z.; Fan, W.; Liu, D. Textural and catalytic properties of Mo loaded hierarchical meso-/microporous lamellar MFI and MWW zeolites for direct methane conversion. Appl. Catal. A 2014, 470, 344–354. [Google Scholar] [CrossRef]
- Ren, L.; Guo, Q.; Orazov, M.; Xu, D.; Politi, D.; Kumar, P.; Alhassan, S.M.; Mkhoyan, K.A.; Sidiras, D.; Davis, M.E.; et al. Pillared Sn-MWW prepared by a solid-state-exchange method and its use as a Lewis acid catalyst. ChemCatChem 2016, 8, 1274–1278. [Google Scholar] [CrossRef]
- Ren, L.; Guo, Q.; Kumar, P.; Orazov, M.; Xu, D.; Alhassan, S.M.; Mkhoyan, K.A.; Davis, M.E.; Tsapatsis, M. Self-pillared, single-unit-cell Sn-MFI zeolite nanosheets and their use for glucose and lactose isomerization. Angew. Chem. Int. Ed. 2015, 54, 10848–10851. [Google Scholar] [CrossRef]
- Lima, S.; Pillinger, M.; Valente, A.A. Dehydration of d-xylose into furfural catalysed by solid acids derived from the layered zeolite Nu-6(1). Catal. Commun. 2008, 9, 2144–2148. [Google Scholar] [CrossRef]
- Emdadi, L.; Tran, D.T.; Zhang, J.; Wu, W.; Song, H.; Gan, Q.; Liu, D. Synthesis of titanosilicate pillared MFI zeolite as an efficient photocatalyst. RSC Adv. 2017, 7, 3249–3256. [Google Scholar] [CrossRef]
- Přech, J.; Pizarro, P.; Serrano, D.P.; Čejka, J. From 3D to 2D zeolite catalytic materials. Chem. Soc. Rev. 2018, 47, 8263–8306. [Google Scholar] [CrossRef]
- Zhao, Q.; Chen, W.-H.; Huang, S.-J.; Wu, Y.-C.; Lee, H.-K.; Liu, S.-B. Discernment and quantification of internal and external acid sites on zeolites. J. Phys. Chem. B 2002, 106, 4462–4469. [Google Scholar] [CrossRef]
- Thibault-Starzyk, F.; Stan, I.; Abelló, S.; Bonilla, A.; Thomas, K.; Fernandez, C.; Gilson, J.-P.; Pérez-Ramírez, J. Quantification of enhanced acid site accessibility in hierarchical zeolites—The accessibility index. J. Catal. 2009, 264, 11–14. [Google Scholar] [CrossRef]
- Roth, W.J. Synthesis of delaminated and pillared zeolitic materials. In Studies in Surface Science and Catalysis; Čejka, J., van Bekkum, H., Corma, A., Schüth, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2007; Volume 168, pp. 221–239. [Google Scholar]
- Iler, R.K. Ion exchange properties of a crystalline hydrated silica. J. Colloid Sci. 1964, 19, 648–657. [Google Scholar] [CrossRef]
- Choi, M.; Na, K.; Kim, J.; Sakamoto, Y.; Terasaki, O.; Ryoo, R. Stable single-unit-cell nanosheets of zeolite MFI as active and long-lived catalysts. Nature 2009, 461, 246–249. [Google Scholar] [CrossRef]
- Eliášová, P.; Opanasenko, M.; Wheatley, P.S.; Shamzhy, M.; Mazur, M.; Nachtigall, P.; Roth, W.J.; Morris, R.E.; Čejka, J. The ADOR mechanism for the synthesis of new zeolites. Chem. Soc. Rev. 2015, 44, 7177–7206. [Google Scholar] [CrossRef]
- Vortmann, S.; Rius, J.; Siegmann, S.; Gies, H. Ab initio structure solution from X-ray powder data at moderate resolution: Crystal structure of a microporous layer silicate. J. Phys. Chem. B 1997, 101, 1292–1297. [Google Scholar] [CrossRef]
- Marler, B.; Stroter, N.; Gies, H. The structure of the new pure silica zeolite RUB-24, Si32O64, obtained by topotactic condensation of the intercalated layer silicate RUB-18. Microporous Mesoporous Mater. 2005, 83, 201–211. [Google Scholar] [CrossRef]
- Akiyama, Y.; Mizukami, F.; Kiyozumi, Y.; Maeda, K.; Izutsu, H.; Sakaguchi, K. A novel layered silicate with a helical morphology. Angew. Chem. Int. Ed. 1999, 38, 1420–1422. [Google Scholar] [CrossRef]
- Ikeda, T.; Akiyama, Y.; Izumi, F.; Kiyozumi, Y.; Mizukami, F.; Kodaira, T. Crystal structure of a helix layered silicate containing tetramethylammonium ions in sodalite-like cages. Chem. Mater. 2001, 13, 1286–1295. [Google Scholar] [CrossRef]
- Asakura, Y.; Takayama, R.; Shibue, T.; Kuroda, K. Topotactic conversion of β-Helix-Layered silicate into AST-type zeolite through successive interlayer modifications. Chem. Eur. J. 2014, 20, 1893–1900. [Google Scholar] [CrossRef]
- Marler, B.; Gies, H. Hydrous layer silicates as precursors for zeolites obtained through topotactic condensation: A review. Eur. J. Mineral. 2012, 24, 405–428. [Google Scholar] [CrossRef]
- Whittam, T.V. Zeolites Nu-6(1) and Nu-6(2). U.S. Patent 4,397,825, 9 August 1983. [Google Scholar]
- Leonowicz, M.E.; Lawton, J.A.; Lawton, S.L.; Rubin, M.K. MCM-22: A molecular sieve with two independent multidimensional channel systems. Science 1994, 264, 1910–1913. [Google Scholar] [CrossRef]
- Blake, A.J.; Franklin, K.R.; Lowe, B.M. Preparation and properties of piperazine silicate (EU-19) and a silica polymorph (EU-20). J. Am. Chem. Soc. Dalton Trans. 1988, 2513–2517. [Google Scholar] [CrossRef]
- Moteki, T.; Chaikittisilp, W.; Shimojima, A.; Okubo, T. Silica sodalite without occluded organic matters by topotactic conversion of lamellar precursor. J. Am. Chem. Soc. 2008, 130, 15780–15781. [Google Scholar] [CrossRef]
- Wang, Y.X.; Gies, H.; Lin, J.H. Crystal structure of the new layer silicate RUB-39 and its topotactic condensation to a microporous zeolite with framework type RRO. Chem. Mater. 2007, 19, 4181–4188. [Google Scholar] [CrossRef]
- Schreyeck, L.; Caullet, P.; Mougenel, J.C.; Guth, J.L.; Marler, B. PREFER: A new layered (alumino) silicate precursor of FER-type zeolite. Microporous Mater. 1996, 6, 259–271. [Google Scholar] [CrossRef]
- Corma, A.; Diaz, U.; Fornes, V.; Guil, J.M.; Martinez-Triguero, J.; Creyghton, E.J. Characterization and catalytic activity of MCM-22 and MCM-56 compared with ITQ-2. J. Catal. 2000, 191, 218–224. [Google Scholar] [CrossRef]
- Chica, A.; Diaz, U.; Fornes, V.; Corma, A. Changing the hydroisomerization to hydrocracking ratio of long chain alkanes by varying the level of delamination in zeolitic (ITQ-6) materials. Catal. Today 2009, 147, 179–185. [Google Scholar] [CrossRef]
- Corma, A.; Diaz, U.; Domine, M.E.; Fornés, V. New aluminosilicate and titanosilicate delaminated materials active for acid catalysis, and oxidation reactions using H2O2. J. Am. Chem. Soc. 2000, 122, 2804–2809. [Google Scholar] [CrossRef]
- Chlubna-Eliasova, P.; Tian, Y.Y.; Pinar, A.B.; Kubů, M.; Čejka, J.; Morris, R.E. The assembly-disassembly-organization-reassembly mechanism for 3D-2D-3D transformation of germanosilicate IWW zeolite. Angew. Chem. Int. Ed. 2014, 53, 7048–7052. [Google Scholar] [CrossRef]
- Roth, W.J.; Nachtigall, P.; Morris, R.E.; Wheatley, P.S.; Seymour, V.R.; Ashbrook, S.E.; Chlubna, P.; Grajciar, L.; Polozij, M.; Zukal, A.; et al. A family of zeolites with controlled pore size prepared using a top-down method. Nat. Chem. 2013, 5, 628–633. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.Y.; Michaelis, V.K.; Hodges, S.; Griffin, R.G.; Roman-Leshkov, Y. One-pot synthesis of MWW zeolite nanosheets using a rationally designed organic structure-directing agent. Chem. Sci. 2015, 6, 6320–6324. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.J.; Ren, L.; Vattipalli, V.; Yeh, Y.-H.; Gould, N.; Xu, B.; Gorte, R.J.; Lobo, R.; Dauenhauer, P.J.; Tsapatsis, M.; et al. Renewable p-xylene from 2,5-dimethylfuran and ethylene using phosphorus-containing zeolite catalysts. ChemCatChem 2017, 9, 398–402. [Google Scholar] [CrossRef]
- Chen, H.L.; Li, S.W.; Wang, Y.M. Synthesis and catalytic properties of multilayered MEL-type titanosilicate nanosheets. J. Mater. Chem. A 2015, 3, 5889–5900. [Google Scholar] [CrossRef]
- Inayat, A.; Knoke, I.; Spiecker, E.; Schwieger, W. Assemblies of mesoporous FAU-type zeolite nanosheets. Angew. Chem. Int. Ed. 2012, 51, 1962–1965. [Google Scholar] [CrossRef] [PubMed]
- Inayat, A.; Schneider, C.; Schwieger, W. Organic-free synthesis of layer-like FAU-type zeolites. Chem. Commun. 2015, 51, 279–281. [Google Scholar] [CrossRef][Green Version]
- Ferdov, S. FAU-type zeolite nanosheets from additives-free system. Microporous Mesoporous Mater. 2017, 242, 59–62. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, Z.; Jin, S.; Sun, H.; Yuan, X.; Yang, W. Synthesis and crystal growth mechanism of ZSM-22 zeolite nanosheet. CrystEngComm 2016, 18, 5611–5615. [Google Scholar] [CrossRef]
- Ma, M.; Huang, X.; Zhan, E.; Zhou, Y.; Xue, H.; Shen, W. Synthesis of mordenite nanosheets with shortened channel lengths and enhanced catalytic activity. J. Mater. Chem. A 2017, 5, 8887–8891. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, Y.; Che, S. Synthesis of lamellar mesostructured ZSM-48 nanosheets. Chem. Mater. 2018, 30, 1839–1843. [Google Scholar] [CrossRef]
- Díaz, U. Layered materials with catalytic applications: Pillared and delaminated zeolites from MWW precursors. ISRN Chem. Eng. 2012, 2012, 35. [Google Scholar] [CrossRef][Green Version]
- Tsapatsis, M. 2-Dimensional zeolites. AICHE J. 2014, 60, 2374–2381. [Google Scholar] [CrossRef]
- Burton, A.; Accardi, R.J.; Lobo, R.F.; Falcioni, M.; Deem, M.W. MCM-47: A highly crystalline silicate composed of hydrogen-bonded ferrierite layers. Chem. Mater. 2000, 12, 2936–2942. [Google Scholar] [CrossRef]
- Seo, Y.; Cho, K.; Jung, Y.; Ryoo, R. Characterization of the surface acidity of MFI zeolite nanosheets by 31P NMR of adsorbed phosphine oxides and catalytic cracking of decalin. ACS Catal. 2013, 3, 713–720. [Google Scholar] [CrossRef]
- Ikeda, T.; Oumi, Y.; Honda, K.; Sano, T.; Momma, K.; Izumi, F. Synthesis and crystal structure of a layered silicate HUS-1 with a halved sodalite-cage topology. Inorg. Chem. 2011, 50, 2294–2301. [Google Scholar] [CrossRef]
- Tsunoji, N.; Fukuda, M.; Yoshida, K.; Sasaki, Y.; Ikeda, T.; Ide, Y.; Sadakane, M.; Sano, T. Characterization of layered silicate HUS-5 and formation of novel nanoporous silica through transformation of HUS-5 ion-exchanged with alkylammonium cations. J. Mater. Chem. A 2013, 1, 9680–9688. [Google Scholar] [CrossRef]
- Marler, B.; Grünewald-Lüke, A.; Grabowski, S.; Gies, H. RUB-55, a new hydrous layer silicate with silicate layers known as motives of the sodalite and octadecasil frameworks: Synthesis and crystal structure. Z. Krist. Cryst. Mater. 2012, 227, 427–437. [Google Scholar] [CrossRef]
- Li, Z.; Marler, B.; Gies, H. A new layered silicate with structural motives of silicate zeolites: Synthesis, crystals structure, and properties. Chem. Mater. 2008, 20, 1896–1901. [Google Scholar] [CrossRef]
- Massüger, L.; Baerlocher, C.; McCusker, L.B.; Zwijnenburg, M.A. Synthesis and structure analysis of the layer silicate DLM-2. Microporous Mesoporous Mater. 2007, 105, 75–81. [Google Scholar] [CrossRef]
- Ide, Y.; Torii, M.; Tsunoji, N.; Sadakane, M.; Sano, T. Molecular recognitive adsorption of aqueous tetramethylammonium on the organic derivative of Hiroshima University Silicate-1 with a silane coupling reagent. Chem. Commun. 2012, 48, 7073–7075. [Google Scholar] [CrossRef]
- Oberhagemann, U.; Bayat, P.; Marler, B.; Gies, H.; Rius, J. A layer silicate: Synthesis and structure of the zeolite precursor RUB-15—[N(CH3)4]8[Si24O52(OH)4]·20·H2O. Angew. Chem. Int. Ed. 1996, 35, 2869–2872. [Google Scholar] [CrossRef]
- Miller, M.A.; Miller, S.R.; Broach, R.W.; Galey, M.M.; Prabhakar, S.; Lyons, B.; Nicholas, C.L.; Nicholas, C.P. Synthesis, characterization and structure solution of ULS-1 |ETMA8(H2O)20|[Si24O48(OH)8], a layered silicate composed of half-sodalite cages. Microporous Mesoporous Mater. 2015, 202, 250–258. [Google Scholar] [CrossRef]
- Marler, B.; Camblor, M.A.; Gies, H. The disordered structure of silica zeolite EU-20b, obtained by topotactic condensation of the piperazinium containing layer silicate EU-19. Microporous Mesoporous Mater. 2006, 90, 87–101. [Google Scholar] [CrossRef]
- Rollmann, L.D.; Schlenker, J.L.; Lawton, S.L.; Kennedy, C.L.; Kennedy, G.J. MCM-69, a novel layered analogue of EU-19. Microporous Mesoporous Mater. 2002, 53, 179–193. [Google Scholar] [CrossRef]
- Zanardi, S.; Alberti, A.; Cruciani, G.; Corma, A.; Fornés, V.; Brunelli, M. Crystal structure determination of zeolite Nu-6(2) and Its layered precursor Nu-6(1). Angew. Chem. Int. Ed. 2004, 43, 4933–4937. [Google Scholar] [CrossRef]
- Ikeda, T.; Kayamori, S.; Mizukami, F. Synthesis and crystal structure of layered silicate PLS-3 and PLS-4 as a topotactic zeolite precursor. J. Mater. Chem. 2009, 19, 5518–5525. [Google Scholar] [CrossRef]
- Ikeda, T.; Akiyama, Y.; Oumi, Y.; Kawai, A.; Mizukami, F. The topotactic conversion of a novel layered silicate into a new framework zeolite. Angew. Chem. Int. Ed. 2004, 43, 4892–4896. [Google Scholar] [CrossRef]
- Marler, B.; Wang, Y.; Song, J.; Gies, H. Topotactic condensation of layer silicates with ferrierite-type layers forming porous tectosilicates. Dalton Trans. 2014, 43, 10396–10416. [Google Scholar] [CrossRef]
- Dorset, D.L.; Kennedy, G.J. Crystal structure of MCM-65: An alternative linkage of ferrierite layers. J. Phys. Chem. B 2004, 108, 15216–15222. [Google Scholar] [CrossRef]
- Knight, L.M.; Miller, M.A.; Koster, S.C.; Gatter, M.G.; Benin, A.I.; Willis, R.R.; Lewis, G.J.; Broach, R.W. UZM-13, UZM-17, UZM-19 and UZM-25: Synthesis and structure of new layered precursors and a zeolite discovered via combinatorial chemistry techniques. In Studies in Surface Science and Catalysis; Xu, R., Gao, Z., Chen, J., Yan, W., Eds.; Elsevier: Amsterdam, The Netherlands, 2007; Volume 170, pp. 338–346. [Google Scholar]
- Tsunoji, N.; Ikeda, T.; Ide, Y.; Sadakane, M.; Sano, T. Synthesis and characteristics of novel layered silicates HUS-2 and HUS-3 derived from a SiO2-choline hydroxide-NaOH-H2O system. J. Mater. Chem. 2012, 22, 13682–13690. [Google Scholar] [CrossRef]
- Rubin, M.K. Crystalline borosilicate. U.S. Patent 5,063,037, 5 November 1991. [Google Scholar]
- Roth, W.J.; Gil, B.; Makowski, W.; Sławek, A.; Grzybek, J.; Kubů, M.; Čejka, J. Interconversion of the CDO layered precursor ZSM-55 between FER and CDO frameworks by controlled deswelling and reassembly. Chem. Mater. 2016, 28, 3616–3619. [Google Scholar] [CrossRef]
- Chu, P.; Herbst, J.A.; Klocke, D.J.; Vartuli, J. Crystalline aluminosilicate. U.S. Patent 4,985,223, 15 January 1991. [Google Scholar]
- Rojas, A.; Camblor, M.A. HPM-2, the layered precursor to zeolite MTF. Chem. Mater. 2014, 26, 1161–1169. [Google Scholar] [CrossRef]
- Schmidt, J.E.; Xie, D.; Davis, M.E. Synthesis of the RTH-type layer: The first small-pore, two dimensional layered zeolite precursor. Chem. Sci. 2015, 6, 5955–5963. [Google Scholar] [CrossRef]
- Brenn, U.; Ernst, H.; Freude, D.; Herrmann, R.; Jähnig, R.; Karge, H.G.; Kärger, J.; König, T.; Mädler, B.; Pingel, U.T.; et al. Synthesis and characterization of the layered sodium silicate ilerite. Microporous Mesoporous Mater. 2000, 40, 43–52. [Google Scholar] [CrossRef]
- Borowski, M.; Wolf, I.; Gies, H. Investigation of proton dynamics within the hydrogen-bond network of the layer silicate Na–RUB-18. Chem. Mater. 2002, 14, 38–43. [Google Scholar] [CrossRef]
- Kresge, C.T.; Roth, W.J. Crystalline oxide material. U.S. Patent 5,266,541, 30 November 1993. [Google Scholar]
- Zubowa, H.-L.; Schneider, M.; Schreier, E.; Eckelt, R.; Richter, M.; Fricke, R. The influence of the expanding and exfoliating conditions on the structural transformation of the layered zeolite Nu-6(1). Microporous Mesoporous Mater. 2008, 109, 317–326. [Google Scholar] [CrossRef]
- De Pietre, M.K.; Bonk, F.A.; Rettori, C.; Garcia, F.A.; Pastore, H.O. Delaminated vanadoaluminosilicate with [V,Al]-ITQ-18 structure. Microporous Mesoporous Mater. 2012, 156, 244–256. [Google Scholar] [CrossRef]
- Xu, H.; Jia, L.; Wu, H.; Yang, B.; Wu, P. Structural diversity of lamellar zeolite Nu-6(1)—Postsynthesis of delaminated analogues. Dalton Trans. 2014, 43, 10492–10500. [Google Scholar] [CrossRef]
- Jiang, J.-G.; Jia, L.; Yang, B.; Xu, H.; Wu, P. Preparation of interlayer-expanded zeolite from lamellar precursor Nu-6(1) by silylation. Chem. Mater. 2013, 25, 4710–4718. [Google Scholar] [CrossRef]
- Borbély, G.; Beyer, H.K.; Karge, H.G.; Schwieger, W.; Brandt, A.; Bergk, K.H. Chemical characterization, structural features, and thermal behavior of sodium and hydrogen octosilicate. Clays Clay Miner. 1991, 39, 490–497. [Google Scholar] [CrossRef]
- Kosuge, K.; Tsunashima, A. New silica-pillared material prepared from the layered silicic acid of ilerite. J. Am. Chem. Soc. Chem. Commun. 1995, 2427–2428. [Google Scholar] [CrossRef]
- Kim, M.; Ko, Y.; Kim, S.; Uh, Y. Vapor phase Beckmann rearrangement of cyclohexanone oxime over metal pillared ilerite. Appl. Catal. A 2001, 210, 345–353. [Google Scholar] [CrossRef]
- Kosuge, K.; Singh, P.S. Mixed-oxide pillared silicates from H-Ilerite by intercalation. Chem. Mater. 2000, 12, 421–427. [Google Scholar] [CrossRef]
- Ishii, R.; Shinohara, Y. Preparation of a microporous biphenyl-pillared layered hybrid material using organic-bridged alkoxysilane and layered silicic acid. J. Mater. Chem. 2005, 15, 551–553. [Google Scholar]
- Ishii, R.; Ikeda, T.; Mizukami, F. Preparation of a microporous layered organic–inorganic hybrid nanocomposite using p-aminotrimethoxysilane and a crystalline layered silicate, ilerite. J. Colloid Interface Sci. 2009, 331, 417–424. [Google Scholar] [CrossRef]
- Wu, P.; Ruan, J.; Wang, L.; Wu, L.; Wang, Y.; Liu, Y.; Fan, W.; He, M.; Terasaki, O.; Tatsumi, T. Methodology for synthesizing crystalline metallosilicates with expanded pore windows through molecular alkoxysilylation of zeolitic lamellar precursors. J. Am. Chem. Soc. 2008, 130, 8178–8187. [Google Scholar] [CrossRef]
- Martínez-Franco, R.; Paris, C.; Martínez-Triguero, J.; Moliner, M.; Corma, A. Direct synthesis of the aluminosilicate form of the small pore CDO zeolite with novel OSDAs and the expanded polymorphs. Microporous Mesoporous Mater. 2017, 246, 147–157. [Google Scholar] [CrossRef]
- Yilmaz, B.; Müller, U.; Feyen, M.; Zhang, H.; Xiao, F.-S.; De Baerdemaeker, T.; Tijsebaert, B.; Jacobs, P.; De Vos, D.; Zhang, W.; et al. New zeolite Al-COE-4: Reaching highly shape-selective catalytic performance through interlayer expansion. Chem. Commun. 2012, 48, 11549–11551. [Google Scholar] [CrossRef]
- De Baerdemaeker, T.; Gies, H.; Yilmaz, B.; Müller, U.; Feyen, M.; Xiao, F.-S.; Zhang, W.; Yokoi, T.; Bao, X.; De Vos, D.E. A new class of solid Lewis acid catalysts based on interlayer expansion of layered silicates of the RUB-36 type with heteroatoms. J. Mater. Chem. A 2014, 2, 9709–9717. [Google Scholar] [CrossRef]
- Inagaki, S.; Yokoi, T.; Kubota, Y.; Tatsumi, T. Unique adsorption properties of organic–inorganic hybrid zeolite IEZ-1 with dimethylsilylene moieties. Chem. Commun. 2007, 5188–5190. [Google Scholar] [CrossRef]
- Ikeda, T.; Kayamori, S.; Oumi, Y.; Mizukami, F. Structure analysis of Si-atom pillared lamellar silicates having micropore structure by powder X-ray diffraction. J. Phys. Chem. C 2010, 114, 3466–3476. [Google Scholar] [CrossRef]
- Xu, H.; Yang, B.; Jiang, J.-G.; Jia, L.; He, M.; Wu, P. Post-synthesis and adsorption properties of interlayer-expanded PLS-4 zeolite. Microporous Mesoporous Mater. 2013, 169, 88–96. [Google Scholar] [CrossRef]
- Kresge, C.T.; Casmer, S.G.; Dhingra, S. Crystalline molecular sieve composition MCM-65, its synthesis and use. WO2002042208A1, 30 May 2002. [Google Scholar]
- Komura, K.; Murase, T.; Sugi, Y.; Koketsu, M. Synthesis of boron-containing CDS-1 zeolite by topotactic dehydration–condensation of [B]-PLS-1 prepared from layered silicate H-LDS. Chem. Lett. 2010, 39, 948–949. [Google Scholar] [CrossRef]
- Murase, T.; Komura, K. Synthesis of germanosilicate type CDS-1 zeolite with CDO topology and its zeolitic layered precursor. J. Porous Mater. 2015, 23. [Google Scholar] [CrossRef]
- Gies, H.; Müller, U.; Yilmaz, B.; Feyen, M.; Tatsumi, T.; Imai, H.; Zhang, H.; Xie, B.; Xiao, F.-S.; Bao, X.; et al. Interlayer expansion of the hydrous layer silicate RUB-36 to a functionalized, microporous framework silicate: Crystal structure analysis and physical and chemical characterization. Chem. Mater. 2012, 24, 1536–1545. [Google Scholar] [CrossRef]
- Lee, G.S.; Zones, S.I. Polymethylated [4.1.1] octanes leading to zeolite SSZ-50. J. Solid State Chem. 2002, 167, 289–298. [Google Scholar] [CrossRef]
- Ikeda, T.; Oumi, Y.; Takeoka, T.; Yokoyama, T.; Sano, T.; Hanaoka, T. Preparation and crystal structure of RUB-18 modified for synthesis of zeolite RWR by topotactic conversion. Microporous Mesoporous Mater. 2008, 110, 488–500. [Google Scholar] [CrossRef]
- Ishimaru, S.I.; Togawa, M.; Ikeda, R.; Shimizu, T.; Shinohara, E.; Umemura, Y. Complex impedance and solid-state NMR studies on a novel high proton conductor Na-RUB-18. Bull. Chem. Soc. JPN. 2006, 79, 656–659. [Google Scholar] [CrossRef]
- Ide, Y.; Ozaki, G.; Ogawa, M. Swelling in water of a Layered alkali silicate, octosilicate, modified with a sulfonic acid group. Langmuir 2009, 25, 5276–5281. [Google Scholar] [CrossRef]
- Ogawa, M.; Iwata, D. Arrangements of interlayer quaternary ammonium ions in a layered silicate, octosilicate. Cryst. Growth Des. 2010, 10, 2068–2072. [Google Scholar] [CrossRef]
- Kim, S.; Jung, K.-D.; Joo, O.-S.; Kim, E.; Kang, T. Catalytic performance of metal oxide-loaded Ta-ilerite for vapor phase Beckmann rearrangement of cyclohexanone oxime. Appl. Catal. A 2004, 266, 173–180. [Google Scholar] [CrossRef]
- Kim, S.; Kim, E.; Kang, T.; Jung, K.-D.; Joo, O.-S.; Shin, C.-H. Synthesis and characterization of transition metal oxide-pillared materials with mesoporosity from layered silicate ilerite. J. Porous Mater. 2006, 13, 27–35. [Google Scholar] [CrossRef]
- Wheatley, P.S.; Love, C.J.; Morrison, J.J.; Shannon, I.J.; Morris, R.E. Synthesis of two new aluminophosphate based layered materials using Tet-A as a structure-directing agent. J. Mater. Chem. 2002, 12, 477–482. [Google Scholar] [CrossRef]
- Wheatley, P.S.; Morris, R.E. Calcination of a layered aluminofluorophosphate precursor to form the zeolitic AFO framework. J. Mater. Chem. 2006, 16, 1035–1037. [Google Scholar] [CrossRef]
- Guo, P.; Afeworki, M.; Cao, G.; Yun, Y.; Sun, J.; Su, J.; Wan, W.; Zou, X. Synthesis and structure of a layered fluoroaluminophosphate and its transformation to a three-dimensional zeotype framework. Inorg. Chem. 2018, 57, 11753–11760. [Google Scholar] [CrossRef]
- Roth, W.J.; Dorset, D.L. The role of symmetry in building up zeolite frameworks from layered zeolite precursors having ferrierite and CAS layers. Struct. Chem. 2010, 21, 385–390. [Google Scholar] [CrossRef]
- Gálvez, P.; Bernardo-Maestro, B.; Vos, E.; Díaz, I.; López-Arbeloa, F.; Pérez-Pariente, J.; Gómez-Hortigüela, L. ICP-2: A new hybrid organo-inorganic ferrierite precursor with expanded layers stabilized by π–π stacking interactions. J. Phys. Chem. C 2017, 121, 24114–24127. [Google Scholar] [CrossRef]
- Millini, R.; Carluccio, L.C.; Carati, A.; Bellussi, G.; Perego, C.; Cruciani, G.; Zanardi, S. ERS-12: A new layered tetramethylammonium silicate composed by ferrierite layers. Microporous Mesoporous Mater. 2004, 74, 59–71. [Google Scholar] [CrossRef]
- Schmidt, J.E.; Xie, D.; Davis, M.E. High-silica, heulandite-type zeolites prepared by direct synthesis and topotactic condensation. J. Mater. Chem. A 2015, 3, 12890–12897. [Google Scholar] [CrossRef]
- Tsunoji, N.; Ikeda, T.; Sadakane, M.; Sano, T. Synthesis and characteristics of novel layered silicate HUS-7 using benzyltrimethylammonium hydroxide and its unique and selective phenol adsorption behavior. J. Mater. Chem. A 2014, 2, 3372–3380. [Google Scholar] [CrossRef]
- Park, W.; Yu, D.; Na, K.; Jelfs, K.E.; Slater, B.; Sakamoto, Y.; Ryoo, R. Hierarchically structure-directing effect of multi-ammonium surfactants for the generation of MFI zeolite nanosheets. Chem. Mater. 2011, 23, 5131–5137. [Google Scholar] [CrossRef]
- Jung, J.; Jo, C.; Cho, K.; Ryoo, R. Zeolite nanosheet of a single-pore thickness generated by a zeolite-structure-directing surfactant. J. Mater. Chem. 2012, 22, 4637–4640. [Google Scholar] [CrossRef]
- Xu, D.; Ma, Y.; Jing, Z.; Han, L.; Singh, B.; Feng, J.; Shen, X.; Cao, F.; Oleynikov, P.; Sun, H.; et al. π–π interaction of aromatic groups in amphiphilic molecules directing for single-crystalline mesostructured zeolite nanosheets. Nat. Commun. 2014, 5, 4262. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Tran, D.T.; Wu, X.; Oh, S.C.; Wang, M.; Chen, H.; Emdadi, L.; Zhang, J.; Schulman, E.; Liu, D. Multilamellar and pillared titanium Silicalite-1 with long-range order of zeolite nanosheet layers: Synthesis and catalysis. Microporous Mesoporous Mater. 2019, 278, 414–422. [Google Scholar] [CrossRef]
- Rubin, M.K.; Chu, P. Composition of synthetic porous crystalline material, its synthesis and use. U.S. Patent 4,954,325, 4 September 1990. [Google Scholar]
- Roth, W.J.; Yorke, T.; Kerby, M.C.; Weston, S.C. Molecular sieve composition (EMM-10-P), its method of making, and use for hydrocarbon conversions. U.S. Patent 7,959,899, 14 June 2011. [Google Scholar]
- Roth, W.J.; Dorset, D.L.; Kennedy, G.J. Discovery of new MWW family zeolite EMM-10: Identification of EMM-10P as the missing MWW precursor with disordered layers. Microporous Mesoporous Mater. 2011, 142, 168–177. [Google Scholar] [CrossRef]
- Millini, R.; Perego, G.; Parker, W., Jr.; Bellussi, G.; Carluccio, L. Layered structure of ERB-1 microporous borosilicate precursor and its intercalation properties towards polar molecules. Microporous Mater. 1995, 4, 221–230. [Google Scholar] [CrossRef]
- Wu, P.; Tatsumi, T.; Komatsu, T.; Yashima, T. A novel titanosilicate with MWW structure. I. Hydrothermal synthesis, elimination of extraframework titanium, and characterizations. J. Phys. Chem. B 2001, 105, 2897–2905. [Google Scholar] [CrossRef]
- Fung, A.S.; Lawton, S.L.; Roth, W.J. Synthetic layered MCM-56, its synthesis and use. US5362697A, 19 April 1994. [Google Scholar]
- Juttu, G.G.; Lobo, R.F. Characterization and catalytic properties of MCM-56 and MCM-22 zeolites. Microporous Mesoporous Mater. 2000, 40, 9–23. [Google Scholar] [CrossRef]
- Qian, B.; Gao, H.X.; Yang, D.Q.; Kong, D.J. Preparation and structure characterization of UZM-8 zeolite templated by DEDMAOH. Acta Chim. Sin. 2009, 67, 2579–2584. [Google Scholar]
- Archer, R.H.; Carpenter, J.R.; Hwang, S.-J.; Burton, A.W.; Chen, C.-Y.; Zones, S.I.; Davis, M.E. Physicochemical properties and catalytic behavior of the molecular sieve SSZ-70. Chem. Mater. 2010, 22, 2563–2572. [Google Scholar] [CrossRef]
- Archer, R.H.; Zones, S.I.; Davis, M.E. Imidazolium structure directing agents in zeolite synthesis: Exploring guest/host relationships in the synthesis of SSZ-70. Microporous Mesoporous Mater. 2010, 130, 255–265. [Google Scholar] [CrossRef]
- Zones, S.I.; Burton, A.W., Jr. Molecular sieve SSZ-70 composition of matter and synthesis thereof. WO2006071354A1, 6 July 2006. [Google Scholar]
- Kubů, M.; Roth, W.J.; Greer, H.F.; Zhou, W.; Morris, R.E.; Přech, J.; Čejka, J. A new family of two-dimensional zeolites prepared from the intermediate layered precursor IPC-3P obtained during the synthesis of TUN zeolite. Chem. Eur. J. 2013, 19, 13937–13945. [Google Scholar] [CrossRef] [PubMed]
- Grzybek, J.; Roth, W.J.; Gil, B.; Korzeniowska, A.; Mazur, M.; Čejka, J.; Morris, R.E. A new layered MWW zeolite synthesized with the bifunctional surfactant template and the updated classification of layered zeolite forms obtained by direct synthesis. J. Mater. Chem. A 2019, 7, 7701–7709. [Google Scholar] [CrossRef]
- Grünewald-Lüke, A.; Gies, H.; Müller, U.; Yilmaz, B.; Imai, H.; Tatsumi, T.; Xie, B.; Xiao, F.-S.; Bao, X.; Zhang, W.; et al. Layered precursors for new zeolitic materials: Synthesis and characterization of B-RUB-39 and its condensation product B-RUB-41. Microporous Mesoporous Mater. 2012, 147, 102–109. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, S.; Wang, X.; Zhang, L.; Wu, N.; Liao, F.; Wang, Y. Synthesis and characterization of a layered silicogermanate PKU-22 and its topotactic condensation to a three-dimensional STI-type zeolite. Cryst. Growth Des. 2017, 17, 5465–5473. [Google Scholar] [CrossRef]
- Schreyeck, L.; Caullet, P.; Mougenel, J.-C.; Guth, J.-L.; Marler, B. A layered microporous aluminosilicate precursor of FER-type zeolite. J. Am. Chem. Soc. Chem. Commun. 1995, 2187–2188. [Google Scholar] [CrossRef]
- Yang, B.; Jiang, J.-G.; Xu, H.; Liu, Y.; Peng, H.; Wu, P. Selective skeletal isomerization of 1-butene over FER-type zeolites derived from PLS-3 lamellar precursors. Appl. Catal. A 2013, 455, 107–113. [Google Scholar] [CrossRef]
- Eilertsen, E.A.; Ogino, I.; Hwang, S.-J.; Rea, T.; Yeh, S.; Zones, S.I.; Katz, A. Nonaqueous fluoride/chloride anion-promoted delamination of layered zeolite precursors: Synthesis and characterization of UCB-2. Chem. Mater. 2011, 23, 5404–5408. [Google Scholar] [CrossRef]
- Yang, B.; Wu, H.; Wu, P. Synthesis, characterization, and catalytic properties of interlayer expanded aluminosilicate IEZ-PLS-3. J. Phys. Chem. C 2014, 118, 24662–24669. [Google Scholar] [CrossRef]
- Yang, B.; Jiang, J.-G.; Xu, H.; Wu, H.; He, M.; Wu, P. Synthesis of extra-large-pore zeolite ECNU-9 with intersecting 14*12-ring channels. Angew. Chem. Int. Ed. 2018, 57, 9515–9519. [Google Scholar] [CrossRef]
- Yang, B.T.; Zou, Q.Y. Post-synthesis and catalytic performance of novel interlayer expanded stanosilicate IEZ-Sn-PLS-3. Chem. Lett. 2017, 46, 1781–1784. [Google Scholar] [CrossRef]
- Tsunoji, N.; Yuki, S.; Oumi, Y.; Sekikawa, M.; Sasaki, Y.; Sadakane, M.; Sano, T. Design of microporous material HUS-10 with tunable hydrophilicity, molecular sieving, and CO2 adsorption ability derived from interlayer silylation of layered silicate HUS-2. ACS Appl. Mater. Interfaces 2015, 7, 24360–24369. [Google Scholar] [CrossRef] [PubMed]
- Tsunoji, N.; Ide, Y.; Yagenji, Y.; Sadakane, M.; Sano, T. Design of layered silicate by grafting with metal acetylacetonate for high activity and chemoselectivity in photooxidation of cyclohexane. ACS Appl. Mater. Interfaces 2014, 6, 4616–4621. [Google Scholar] [CrossRef] [PubMed]
- Gies, H.; Müller, U.; Yilmaz, B.; Tatsumi, T.; Xie, B.; Xiao, F.-S.; Bao, X.; Zhang, W.; Vos, D.D. Interlayer expansion of the layered zeolite precursor RUB-39: A universal method to synthesize functionalized microporous silicates. Chem. Mater. 2011, 23, 2545–2554. [Google Scholar] [CrossRef]
- Tijsebaert, B.; Henry, M.; Gies, H.; Xiao, F.-S.; Zhang, W.; Bao, X.; Imai, H.; Tatsumi, T.; Müller, U.; Yilmaz, B.; et al. Exploring the void structure and activity of RUB-39 based expanded materials using the hydroconversion of decane. J. Catal. 2011, 282, 47–53. [Google Scholar] [CrossRef]
- Na, K.; Park, W.; Seo, Y.; Ryoo, R. Disordered assembly of MFI zeolite nanosheets with a large volume of intersheet mesopores. Chem. Mater. 2011, 23, 1273–1279. [Google Scholar] [CrossRef]
- Jeon, M.Y.; Kim, D.; Kumar, P.; Lee, P.S.; Rangnekar, N.; Bai, P.; Shete, M.; Elyassi, B.; Lee, H.S.; Narasimharao, K.; et al. Ultra-selective high-flux membranes from directly synthesized zeolite nanosheets. Nature 2017, 543, 690–694. [Google Scholar] [CrossRef]
- Na, K.; Jo, C.; Kim, J.; Ahn, W.-S.; Ryoo, R. MFI titanosilicate nanosheets with single-unit-cell thickness as an oxidation catalyst using peroxides. ACS Catal. 2011, 1, 901–907. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, D.; Xu, D.; Asahina, S.; Cychosz, K.A.; Agrawal, K.V.; Al Wahedi, Y.; Bhan, A.; Al Hashimi, S.; Terasaki, O.; et al. Synthesis of self-pillared zeolite nanosheets by repetitive branching. Science 2012, 336, 1684–1687. [Google Scholar] [CrossRef]
- Singh, B.K.; Xu, D.; Han, L.; Ding, J.; Wang, Y.; Che, S. Synthesis of single-crystalline mesoporous ZSM-5 with three-dimensional pores via the self-assembly of a designed triply branched cationic surfactant. Chem. Mater. 2014, 26, 7183–7188. [Google Scholar] [CrossRef]
- Emdadi, L.; Wu, Y.; Zhu, G.; Chang, C.-C.; Fan, W.; Pham, T.; Lobo, R.F.; Liu, D. Dual template synthesis of meso- and microporous MFI zeolite nanosheet assemblies with tailored activity in catalytic reactions. Chem. Mater. 2014, 26, 1345–1355. [Google Scholar] [CrossRef]
- Přech, J.; Carretero Marta, A.; Čejka, J. Baeyer–Villiger oxidation of cyclic ketones by using tin–silica pillared catalysts. ChemCatChem 2017, 9, 3063–3072. [Google Scholar] [CrossRef]
- Přech, J.; Eliášová, P.; Aldhayan, D.; Kubů, M. Epoxidation of bulky organic molecules over pillared titanosilicates. Catal. Today 2015, 243, 134–140. [Google Scholar] [CrossRef]
- Galve, A.; Gorgojo, P.; Navascues, N.; Casado, C.; Tellez, C.; Coronas, J. Structural study on the Al distribution in zeolites Nu-6(1) and Nu-6(2). Microporous Mesoporous Mater. 2011, 145, 211–216. [Google Scholar] [CrossRef]
- Wei, L.; Song, K.; Wu, W.; Holdren, S.; Zhu, G.; Shulman, E.; Shang, W.; Chen, H.; Zachariah, M.R.; Liu, D. Vapor-phase strategy to pillaring of two-dimensional zeolite. J. Am. Chem. Soc. 2019, 141, 8712–8716. [Google Scholar] [CrossRef]
- Fermoso, J.; Hernando, H.; Jana, P.; Moreno, I.; Přech, J.; Ochoa-Hernández, C.; Pizarro, P.; Coronado, J.M.; Čejka, J.; Serrano, D.P. Lamellar and pillared ZSM-5 zeolites modified with MgO and ZnO for catalytic fast-pyrolysis of eucalyptus woodchips. Catal. Today 2016, 277, 171–181. [Google Scholar] [CrossRef]
- Roth, W.J. MCM-22 zeolite family and the delaminated zeolite MCM-56 obtained in one-step synthesis. In Studies in Surface Science and Catalysis; Elsevier: Amsterdam, The Netherlands, 2005; Volume 158, pp. 19–26. [Google Scholar]
- Gil, B.; Makowski, W.; Marszalek, B.; Roth, W.J.; Kubů, M.; Čejka, J.; Olejniczak, Z. High acidity unilamellar zeolite MCM-56 and its pillared and delaminated derivatives. Dalton Trans. 2014, 43, 10501–10511. [Google Scholar] [CrossRef]
- Ogino, I.; Eilertsen, E.A.; Hwang, S.-J.; Rea, T.; Xie, D.; Ouyang, X.; Zones, S.I.; Katz, A. Heteroatom-tolerant delamination of layered zeolite precursor materials. Chem. Mater. 2013, 25, 1502–1509. [Google Scholar] [CrossRef]
- Roth, W.J.; Dorset, D.L.; Kennedy, G.J.; Yorke, T.; Helton, T.E. A novel molecular sieve composition EMM-12, a method of making and a process of using the same. WO2010021795A1, 25 February 2010. [Google Scholar]
- Runnebaum, R.C.; Ouyang, X.; Edsinga, J.A.; Rea, T.; Arslan, I.; Hwang, S.-J.; Zones, S.I.; Katz, A. Role of delamination in zeolite-catalyzed aromatic alkylation: UCB-3 versus 3-D Al-SSZ-70. ACS Catal. 2014, 4, 2364–2368. [Google Scholar] [CrossRef][Green Version]
- Okrut, A.; Aigner, M.; Schöttle, C.; Grosso-Giordano, N.A.; Hwang, S.-J.; Ouyang, X.; Zones, S.; Katz, A. SSZ-70 borosilicate delamination without sonication: Effect of framework topology on olefin epoxidation catalysis. Dalton Trans. 2018, 47, 15082–15090. [Google Scholar] [CrossRef]
- Corma, A.; Fornés, V.; Guil, J.M.; Pergher, S.; Maesen, T.L.M.; Buglass, J.G. Preparation, characterisation and catalytic activity of ITQ-2, a delaminated zeolite. Microporous Mesoporous Mater. 2000, 38, 301–309. [Google Scholar] [CrossRef]
- Corma, A.; Diaz, U.; Fornes, V.L.; Jorda, J.; Domine, M.; Rey, F. Ti/ITQ-2, a new material highly active and selective for the epoxidation of olefins with organic hydroperoxides. Chem. Commun. 1999, 779–780. [Google Scholar] [CrossRef]
- Chang, C.-C.; Lee, J.-F.; Cheng, S. Highly catalytically active micro/meso-porous Ti-MCM-36 prepared by a grafting method. J. Mater. Chem. A 2017, 5, 15676–15687. [Google Scholar] [CrossRef]
- Jin, F.; Chang, C.-C.; Yang, C.-W.; Lee, J.-F.; Jang, L.-Y.; Cheng, S. New mesoporous titanosilicate MCM-36 material synthesized by pillaring layered ERB-1 precursor. J. Mater. Chem. A 2015, 3, 8715–8724. [Google Scholar] [CrossRef]
- Jin, F.; Chen, S.-Y.; Jang, L.-Y.; Lee, J.-F.; Cheng, S. New Ti-incorporated MCM-36 as an efficient epoxidation catalyst prepared by pillaring MCM-22 layers with titanosilicate. J. Catal. 2014, 319, 247–257. [Google Scholar] [CrossRef]
- Margarit, V.J.; Martinez-Armero, M.E.; Navarro, M.T.; Martinez, C.; Corma, A. Direct dual-template synthesis of MWW zeolite monolayers. Angew. Chem. Int. Ed. 2015, 54, 13724–13728. [Google Scholar] [CrossRef]
- Roth, W.J.; Gil, B.; Makowski, W.; Sławek, A.; Korzeniowska, A.; Grzybek, J.; Siwek, M.; Michorczyk, P. Framework-substituted cerium MCM-22 zeolite and its interlayer expanded derivative MWW-IEZ. Catal. Sci. Technol. 2016, 6, 2742–2753. [Google Scholar] [CrossRef]
- Fan, W.; Wu, P.; Namba, S.; Tatsumi, T. A titanosilicate that is structurally analogous to an MWW-type lamellar precursor. Angew. Chem. Int. Ed. 2004, 43, 236–240. [Google Scholar] [CrossRef]
- Ruan, J.; Wu, P.; Slater, B.; Terasaki, O. Structure elucidation of the highly active titanosilicate catalyst Ti-YNU-1. Angew. Chem. Int. Ed. 2005, 117, 6877–6881. [Google Scholar] [CrossRef]
- Wang, Y.X.; Gies, H.; Marler, B.; Müller, U. Synthesis and crystal structure of zeolite RUB-41 obtained as calcination product of a layered precursor: A systematic approach to a new synthesis route. Chem. Mater. 2005, 17, 43–49. [Google Scholar] [CrossRef]
- Yilmaz, B.; Müller, U.; Tijsebaert, B.; De Vos, D.; Xie, B.; Xiao, F.-S.; Gies, H.; Zhang, W.; Bao, X.; Imai, H.; et al. Al-RUB-41: A shape-selective zeolite catalyst from a layered silicate. Chem. Commun. 2011, 47, 1812–1814. [Google Scholar] [CrossRef] [PubMed]
- Henkelis, S.E.; Mazur, M.; Rice, C.M.; Bignami, G.P.M.; Wheatley, P.S.; Ashbrook, S.E.; Čejka, J.; Morris, R.E. A procedure for identifying possible products in the assembly–disassembly–organization–reassembly (ADOR) synthesis of zeolites. Nat. Protoc. 2019, 14, 781–794. [Google Scholar] [CrossRef] [PubMed]
- Roth, W.J.; Shvets, O.V.; Shamzhy, M.; Chlubná, P.; Kubů, M.; Nachtigall, P.; Čejka, J. Postsynthesis transformation of three-dimensional framework into a lamellar zeolite with modifiable architecture. J. Am. Chem. Soc. 2011, 133, 6130–6133. [Google Scholar] [CrossRef] [PubMed]
- Bignami, G.P.M.; Dawson, D.M.; Seymour, V.R.; Wheatley, P.S.; Morris, R.E.; Ashbrook, S.E. Synthesis, isotopic enrichment, and solid-state NMR characterization of zeolites derived from the assembly, disassembly, organization, reassembly process. J. Am. Chem. Soc. 2017, 139, 5140–5148. [Google Scholar] [CrossRef]
- Wheatley, P.S.; Chlubná-Eliášová, P.; Greer, H.; Zhou, W.; Seymour, V.R.; Dawson, D.M.; Ashbrook, S.E.; Pinar, A.B.; McCusker, L.B.; Opanasenko, M.; et al. Zeolites with continuously tuneable porosity. Angew. Chem. Int. Ed. 2014, 53, 13210–13214. [Google Scholar] [CrossRef]
- Mazur, M.; Wheatley, P.; Navarro, M.; Roth, W.; Položij, M.; Mayoral, A.; Eliášová, P.; Nachtigall, P.; Čejka, J.; Morris, R. Synthesis of ‘unfeasible’ zeolites. Nat. Chem. 2015, 8, 58–62. [Google Scholar] [CrossRef]
- Shamzhy, M.; Mazur, M.; Opanasenko, M.; Roth, W.J.; Čejka, J. Swelling and pillaring of the layered precursor IPC-1P: Tiny details determine everything. Dalton Trans. 2014, 43, 10548–10557. [Google Scholar] [CrossRef]
- Mazur, M.; Chlubná-Eliášová, P.; Roth, W.J.; Čejka, J. Intercalation chemistry of layered zeolite precursor IPC-1P. Catal. Today 2014, 227, 37–44. [Google Scholar] [CrossRef]
- Přech, J.; Čejka, J. UTL titanosilicate: An extra-large pore epoxidation catalyst with tunable textural properties. Catal. Today 2016, 277, 2–8. [Google Scholar] [CrossRef]
- Navarro, M.; Morris, S.A.; Mayoral, A.; Čejka, J.; Morris, R.E. Microwave heating and the fast ADOR process for preparing zeolites. J. Mater. Chem. A 2017, 5, 8037–8043. [Google Scholar] [CrossRef]
- Kasneryk, V.; Shamzhy, M.; Opanasenko, M.; Wheatley, P.S.; Morris, S.A.; Russell, S.E.; Mayoral, A.; Trachta, M.; Čejka, J.; Morris, R.E. Expansion of the ADOR strategy for the synthesis of zeolites: The synthesis of IPC-12 from zeolite UOV. Angew. Chem. Int. Ed. 2017, 56, 4324–4327. [Google Scholar] [CrossRef]
- Kasneryk, V.; Shamzhy, M.; Opanasenko, M.; Wheatley, P.S.; Morris, R.E.; Čejka, J. Insight into the ADOR zeolite-to-zeolite transformation: The UOV case. Dalton Trans. 2018, 47, 3084–3092. [Google Scholar] [CrossRef]
- Firth, D.S.; Morris, S.A.; Wheatley, P.S.; Russell, S.E.; Slawin, A.M.Z.; Dawson, D.M.; Mayoral, A.; Opanasenko, M.; Položij, M.; Čejka, J.; et al. Assembly–disassembly–organization–reassembly synthesis of zeolites based on cfi-type layers. Chem. Mater. 2017, 29, 5605–5611. [Google Scholar] [CrossRef]
- Shamzhy, M.; Opanasenko, M.; Tian, Y.; Konysheva, K.; Shvets, O.; Morris, R.E.; Čejka, J. Germanosilicate precursors of ADORable zeolites obtained by disassembly of ITH, ITR, and IWR zeolites. Chem. Mater. 2014, 26, 5789–5798. [Google Scholar] [CrossRef]
- Shvets, O.V.; Konysheva, K.M.; Shamzhy, M.V.; Opanasenko, M.V.; Yaremov, P.S.; Xiao, C.; Zou, X.; Čejka, J. Mordenite nanorods and nanosheets prepared in presence of gemini type surfactants. Catal. Today 2019, 324, 115–122. [Google Scholar] [CrossRef]
- Derouane, E.G.; Védrine, J.C.; Pinto, R.R.; Borges, P.M.; Costa, L.; Lemos, M.A.N.D.A.; Lemos, F.; Ribeiro, F.R. The acidity of zeolites: Concepts, measurements and relation to catalysis: A review on experimental and theoretical methods for the study of zeolite acidity. Catal. Rev. 2013, 55, 454–515. [Google Scholar] [CrossRef]
- Sandoval-Díaz, L.-E.; González-Amaya, J.-A.; Trujillo, C.-A. General aspects of zeolite acidity characterization. Microporous Mesoporous Mater. 2015, 215, 229–243. [Google Scholar] [CrossRef]
- Freitas, C.; Barrow, N.S.; Zholobenko, V. Accessibility and location of acid sites in zeolites as probed by fourier transform infrared spectroscopy and magic angle spinning nuclear magnetic resonance. Johnson Matthey Technol. Rev. 2018, 62, 279–290. [Google Scholar] [CrossRef]
- Fyfe, C.A.; Gobbi, G.C.; Hartman, J.S.; Klinowski, J.; Thomas, J.M. Solid-state magic-angle spinning. Aluminum-27 nuclear magnetic resonance studies of zeolites using a 400-MHz high-resolution spectrometer. J. Phys. Chem. 1982, 86, 1247–1250. [Google Scholar] [CrossRef]
- Majano, G.; Delmotte, L.; Valtchev, V.; Mintova, S. Al-Rich zeolite beta by seeding in the absence of organic template. Chem. Mater. 2009, 21, 4184–4191. [Google Scholar] [CrossRef]
- Gilson, J.-P.; Edwards, G.C.; Peters, A.W.; Rajagopalan, K.; Wormsbecher, R.F.; Roberie, T.G.; Shatlock, M.P. Penta-co-ordinated aluminium in zeolites and aluminosilicates. J. Am. Chem. Soc. Chem. Commun. 1987, 91–92. [Google Scholar] [CrossRef]
- Jo, C.; Cho, K.; Kim, J.; Ryoo, R. MFI zeolite nanosponges possessing uniform mesopores generated by bulk crystal seeding in the hierarchical surfactant-directed synthesis. Chem. Commun. 2014, 50, 4175–4177. [Google Scholar] [CrossRef] [PubMed]
- Lacarriere, A.; Luck, F.; Świerczyński, D.; Fajula, F.; Hulea, V. Methanol to hydrocarbons over zeolites with MWW topology: Effect of zeolite texture and acidity. Appl. Catal. A 2011, 402, 208–217. [Google Scholar] [CrossRef]
- Jiang, M.; Karge, H.G. Investigation of acid properties of dealuminated H-mordenite zeolites by low-temperature diffuse reflectance FTIR. J. Am. Chem. Soc. Faraday Trans. 1996, 92, 2641–2649. [Google Scholar] [CrossRef]
- Busca, G. Spectroscopic characterization of the acid properties of metal oxide catalysts. Catal. Today 1998, 41, 191–206. [Google Scholar] [CrossRef]
- Trombetta, M.; Busca, G. On the characterization of the external acid sites of ferrierite and other zeolites: A reply to Pieterse et al. J. Catal. 1999, 187, 521–523. [Google Scholar] [CrossRef]
- Dapsens, P.Y.; Mondelli, C.; Jagielski, J.; Hauert, R.; Pérez-Ramírez, J. Hierarchical Sn-MFI zeolites prepared by facile top-down methods for sugar isomerisation. Catal. Sci. Technol. 2014, 4, 2302–2311. [Google Scholar] [CrossRef]
- Schallmoser, S.; Ikuno, T.; Wagenhofer, M.F.; Kolvenbach, R.; Haller, G.L.; Sanchez-Sanchez, M.; Lercher, J.A. Impact of the local environment of Brønsted acid sites in ZSM-5 on the catalytic activity in n-pentane cracking. J. Catal. 2014, 316, 93–102. [Google Scholar] [CrossRef]
- Bai, Y.; Wei, L.; Yang, M.; Chen, H.; Holdren, S.; Zhu, G.; Tran, D.T.; Yao, C.; Sun, R.; Pan, Y.; et al. Three-step cascade over a single catalyst: Synthesis of 5-(ethoxymethyl)furfural from glucose over a hierarchical lamellar multi-functional zeolite catalyst. J. Mater. Chem. A 2018, 6, 7693–7705. [Google Scholar] [CrossRef]
- Laforge, S.; Ayrault, P.; Martin, D.; Guisnet, M. Acidic and catalytic properties of MCM-22 and MCM-36 zeolites synthesized from the same precursors. Appl. Catal. A 2005, 279, 79–88. [Google Scholar] [CrossRef]
- Arean, C.O.; Delgado, M.R.; Nachtigall, P.; Thang, H.V.; Rubeš, M.; Bulánek, R.; Chlubná-Eliášová, P. Measuring the brønsted acid strength of zeolites—Does it correlate with the O–H frequency shift probed by a weak base? Phys. Chem. Chem. Phys. 2014, 16, 10129–10141. [Google Scholar] [CrossRef] [PubMed]
- Nesterenko, N.S.; Thibault-Starzyk, F.; Montouillout, V.; Yuschenko, V.V.; Fernandez, C.; Gilson, J.P.; Fajula, F.; Ivanova, I.I. Accessibility of the acid sites in dealuminated small-port mordenites studied by FTIR of co-adsorbed alkylpyridines and CO. Microporous Mesoporous Mater. 2004, 71, 157–166. [Google Scholar] [CrossRef]
- Niwa, M.; Katada, N. New method for the temperature- programmed desorption (TPD) of ammonia experiment for characterization of zeolite acidity: A review. Chem. Rec. 2013, 13, 432–455. [Google Scholar] [CrossRef] [PubMed]
- Rakiewicz, E.F.; Peters, A.W.; Wormsbecher, R.F.; Sutovich, K.J.; Mueller, K.T. Characterization of acid sites in zeolitic and other inorganic systems using solid-state 31P NMR of the probe molecule trimethylphosphine oxide. J. Phys. Chem. B 1998, 102, 2890–2896. [Google Scholar] [CrossRef]
- Duan, J.; Higuchi, M.; Krishna, R.; Kiyonaga, T.; Tsutsumi, Y.; Sato, Y.; Kubota, Y.; Takata, M.; Kitagawa, S. High CO2/N2/O2/CO separation in a chemically robust porous coordination polymer with low binding energy. Chem. Sci. 2014, 5, 660–666. [Google Scholar] [CrossRef]
- Wu, Y.; Emdadi, L.; Qin, D.; Zhang, J.; Liu, D. Quantification of external surface and pore mouth acid sites in unit-cell thick pillared MFI and pillared MWW zeolites. Microporous Mesoporous Mater. 2017, 241, 43–51. [Google Scholar] [CrossRef]
- Pelmenschikov, A.G.; van Santen, R.A.; Janchen, J.; Meijer, E. Acetonitrile-d3 as a probe of Lewis and Broensted acidity of zeolites. J. Phys. Chem. 1993, 97, 11071–11074. [Google Scholar] [CrossRef]
- Pereira, C.; Gorte, R.J. Method for distinguishing Brønsted-acid sites in mixtures of H-ZSM-5, H-Y and silica-alumina. Appl. Catal. A 1992, 90, 145–157. [Google Scholar] [CrossRef]
- Góra-Marek, K.; Tarach, K.; Choi, M. 2,6-Di-tert-butylpyridine sorption approach to quantify the external acidity in hierarchical zeolites. J. Phys. Chem. C 2014, 118, 12266–12274. [Google Scholar] [CrossRef]
- Bevilacqua, M.; Busca, G. A study of the localization and accessibility of Brønsted and Lewis acid sites of H-mordenite through the FT-IR spectroscopy of adsorbed branched nitriles. Catal. Commun. 2002, 3, 497–502. [Google Scholar] [CrossRef]
- Ordomsky, V.V.; Murzin, V.Y.; Monakhova, Y.V.; Zubavichus, Y.V.; Knyazeva, E.E.; Nesterenko, N.S.; Ivanova, I.I. Nature, strength and accessibility of acid sites in micro/mesoporous catalysts obtained by recrystallization of zeolite BEA. Microporous Mesoporous Mater. 2007, 105, 101–110. [Google Scholar] [CrossRef]
- Ayrault, P.; Datka, J.; Laforge, S.; Martin, D.; Guisnet, M. Characterization of the Internal and External Acidity of H-MCM-22 Zeolites. J. Phys. Chem. B 2004, 108, 13755–13763. [Google Scholar] [CrossRef]
- Hsia Chen, C.S.; Schramm, S.E. Type and catalytic activity of surface acid sites of medium and large pore zeolites Their deactivation with bulky organophosphorus compounds. Microporous Mater. 1996, 7, 125–132. [Google Scholar] [CrossRef]
- Thang, H.V.; Rubeš, M.; Bludský, O.; Nachtigall, P. Computational investigation of the Lewis acidity in three-dimensional and corresponding two-dimensional zeolites: UTL vs. IPC-1P. J. Phys. Chem. A 2014, 118, 7526–7534. [Google Scholar] [CrossRef]
- Ramos, F.S.O.; Pastore, H.O. 2D-to-disguised 3D materials with built-in acid sites: H+-[Al]-RUB-18. Dalton Trans. 2017, 46, 11728–11737. [Google Scholar] [CrossRef] [PubMed]
- Ramli, Z.; Yusoff, N.; Hamdan, H. Delaminated zeolite, ITQ-6 as heterogeneous catalyst for Friedel Crafts alkylation. Mala. J. Anal. Sci. 2007, 11, 84–92. [Google Scholar]
- De Pietre, M.K.; Bonk, F.A.; Rettori, C.; Garcia, F.A.; Pastore, H.O. [V,Al]-ITQ-6: Novel porous material and the effect of delamination conditions on V sites and their distribution. Microporous Mesoporous Mater. 2011, 145, 108–117. [Google Scholar] [CrossRef]
- Yang, B.; Jiang, J.-G.; Xu, H.; Ji, P.; Wu, P. Sub-zeolite of FER topology derived from an interlayer modification of PLS-3 lamellar precursor. Microporous Mesoporous Mater. 2015, 203, 54–62. [Google Scholar] [CrossRef]
- Liu, D.; Bhan, A.; Tsapatsis, M.; Al Hashimi, S. Catalytic behavior of brønsted acid sites in MWW and MFI zeolites with dual meso- and microporosity. ACS Catal. 2011, 1, 7–17. [Google Scholar] [CrossRef]
- Bleken, B.-T.L.; Wragg, D.S.; Arstad, B.; Gunnæs, A.E.; Mouzon, J.; Helveg, S.; Lundegaard, L.F.; Beato, P.; Bordiga, S.; Olsbye, U.; et al. Unit cell thick nanosheets of zeolite H-ZSM-5: Structure and activity. Top. Catal. 2013, 56, 558–566. [Google Scholar] [CrossRef]
- Jo, C.; Ryoo, R.; Žilková, N.; Vitvarová, D.; Čejka, J. The effect of MFI zeolite lamellar and related mesostructures on toluene disproportionation and alkylation. Catal. Sci. Technol. 2013, 3, 2119–2129. [Google Scholar] [CrossRef]
- Wu, L.L.; Magusin, P.C.M.M.; Degirmenci, V.; Li, M.Q.; Almutairi, S.M.T.; Zhu, X.C.; Mezari, B.; Hensen, E.J.M. Acidic properties of nanolayered ZSM-5 zeolites. Microporous Mesoporous Mater. 2014, 189, 144–157. [Google Scholar] [CrossRef]
- Meng, L.; Zhu, X.; Mezari, B.; Pestman, R.; Wannapakdee, W.; Hensen, E.J.M. On the role of acidity in bulk and nanosheet [T]MFI (T = Al3+, Ga3+, Fe3+, B3+) zeolites in the methanol-to-hydrocarbons reaction. ChemCatChem 2017, 9, 3942–3954. [Google Scholar] [CrossRef]
- Bleken, B.-T.L.; Mino, L.; Giordanino, F.; Beato, P.; Svelle, S.; Lillerud, K.P.; Bordiga, S. Probing the surface of nanosheet H-ZSM-5 with FTIR spectroscopy. Phys. Chem. Chem. Phys. 2013, 15, 13363–13370. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, X.; Bhan, A.; Tsapatsis, M. Activity and selectivity differences of external Brønsted acid sites of single-unit-cell thick and conventional MFI and MWW zeolites. Microporous Mesoporous Mater. 2014, 200, 287–290. [Google Scholar] [CrossRef]
- Wu, Y.; Lu, Z.; Emdadi, L.; Oh, S.C.; Wang, J.; Lei, Y.; Chen, H.; Tran, D.T.; Lee, I.C.; Liu, D. Tuning external surface of unit-cell thick pillared MFI and MWW zeolites by atomic layer deposition and its consequences on acid-catalyzed reactions. J. Catal. 2016, 337, 177–187. [Google Scholar] [CrossRef]
- Kim, K.; Ryoo, R.; Jang, H.-D.; Choi, M. Spatial distribution, strength, and dealumination behavior of acid sites in nanocrystalline MFI zeolites and their catalytic consequences. J. Catal. 2012, 288, 115–123. [Google Scholar] [CrossRef]
- Ji, Y.; Shi, B.; Yang, H.; Yan, W. Synthesis of isomorphous MFI nanosheet zeolites for supercritical catalytic cracking of n-dodecane. Appl. Catal. A 2017, 533, 90–98. [Google Scholar] [CrossRef]
- Xu, M.; Mukarakate, C.; Iisa, K.; Budhi, S.; Menart, M.; Davidson, M.; Robichaud, D.J.; Nimlos, M.R.; Trewyn, B.G.; Richards, R.M. Deactivation of multilayered mfi nanosheet zeolite during upgrading of biomass pyrolysis vapors. ACS Sustain. Chem. Eng. 2017, 5, 5477–5484. [Google Scholar] [CrossRef]
- Kresge, C.T.; Roth, W.J.; Simmons, K.G.; Vartuli, J.C. A method of preparing a pillared layered oxide material. WO1992011935A1, 23 July 1992. [Google Scholar]
- Kresge, C.T.; Roth, W.J. Method for preparing a pillared layered oxide material. U.S. Patent 5,278,115, 11 January 1994. [Google Scholar]
- Corma, A.; Fornés, V.; Martínez-Triguero, J.; Pergher, S.B. Delaminated zeolites: Combining the benefits of zeolites and mesoporous materials for catalytic uses. J. Catal. 1999, 186, 57–63. [Google Scholar] [CrossRef]
- Min, H.-K.; Park, M.B.; Hong, S.B. Methanol-to-olefin conversion over H-MCM-22 and H-ITQ-2 zeolites. J. Catal. 2010, 271, 186–194. [Google Scholar] [CrossRef]
- Maheshwari, S.; Martínez, C.; Teresa Portilla, M.; Llopis, F.J.; Corma, A.; Tsapatsis, M. Influence of layer structure preservation on the catalytic properties of the pillared zeolite MCM-36. J. Catal. 2010, 272, 298–308. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhu, W.; Zai, S.; Jia, M.; Zhang, W.; Wang, Z. Synthesis, characterization and catalytic properties of MCM-36 pillared via the MCM-56 precursor. J. Porous Mater. 2013, 20, 531–538. [Google Scholar] [CrossRef]
- Schenkel, R.; Barth, J.O.; Kornatowski, J.; Lercher, J.A. Chemical and structural aspects of the transformation of the MCM-22 precursor into ITQ-2. In Studies in Surface Science and Catalysis; Aiello, R., Giordano, G., Testa, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2002; Volume 142, pp. 69–76. [Google Scholar]
- Onida, B.; Borello, L.; Bonelli, B.; Geobaldo, F.; Garrone, E. IR study of the acidity of ITQ-2, an “all-surface” zeolitic system. J. Catal. 2003, 214, 191–199. [Google Scholar] [CrossRef]
- Chlubná, P.; Roth, W.J.; Zukal, A.; Kubů, M.; Pavlatová, J. Pillared MWW zeolites MCM-36 prepared by swelling MCM-22P in concentrated surfactant solutions. Catal. Today 2012, 179, 35–42. [Google Scholar] [CrossRef]
- Purova, R.; Narasimharao, K.; Ahmed, N.S.I.; Al-Thabaiti, S.; Al-Shehri, A.; Mokhtar, M.; Schwieger, W. Pillared HMCM-36 zeolite catalyst for biodiesel production by esterification of palmitic acid. J. Mol. Catal. A 2015, 406, 159–167. [Google Scholar] [CrossRef]
- Carriço, C.S.; Cruz, F.T.; dos Santos, M.B.; Oliveira, D.S.; Pastore, H.O.; Andrade, H.M.C.; Mascarenhas, A.J.S. MWW-type catalysts for gas phase glycerol dehydration to acrolein. J. Catal. 2016, 334, 34–41. [Google Scholar] [CrossRef]
- Käldström, M.; Kumar, N.; Heikkilä, T.; Murzin, D.Y. Pillared H-MCM-36 mesoporous and H-MCM-22 microporous materials for conversion of levoglucosan: Influence of varying acidity. Appl. Catal. A 2011, 397, 13–21. [Google Scholar] [CrossRef]
- Rutkowska, M.; Díaz, U.; Palomares, A.E.; Chmielarz, L. Cu and Fe modified derivatives of 2D MWW-type zeolites (MCM-22, ITQ-2 and MCM-36) as new catalysts for DeNOx process. Appl. Catal. B 2015, 168–169, 531–539. [Google Scholar] [CrossRef]
- Roth, W.J.; Chlubná, P.; Kubů, M.; Vitvarová, D. Swelling of MCM-56 and MCM-22P with a new medium—Surfactant–tetramethylammonium hydroxide mixtures. Catal. Today 2013, 204, 8–14. [Google Scholar] [CrossRef]
- Hu, B.; Gay, I.D. Probing surface acidity by 31P nuclear magnetic resonance spectroscopy of arylphosphines. Langmuir 1999, 15, 477–481. [Google Scholar] [CrossRef]
- Wang, Y.; Zhuang, J.; Yang, G.; Zhou, D.; Ma, D.; Han, X.; Bao, X. Study on the external surface acidity of MCM-22 zeolite: theoretical calculation and 31P MAS NMR. J. Phys. Chem. B 2004, 108, 1386–1391. [Google Scholar] [CrossRef]
- Žilková, N.; Eliášová, P.; Al-Khattaf, S.; Morris, R.E.; Mazur, M.; Čejka, J. The effect of UTL layer connectivity in isoreticular zeolites on the catalytic performance in toluene alkylation. Catal. Today 2016, 277, 55–60. [Google Scholar] [CrossRef]
- Wan, Y.; Zhao, D. On the controllable soft-templating approach to mesoporous silicates. Chem. Rev. 2007, 107, 2821–2860. [Google Scholar] [CrossRef]
- Zhang, K.; Ostraat, M.L. Innovations in hierarchical zSeolite synthesis. Catal. Today 2016, 264, 3–15. [Google Scholar] [CrossRef]
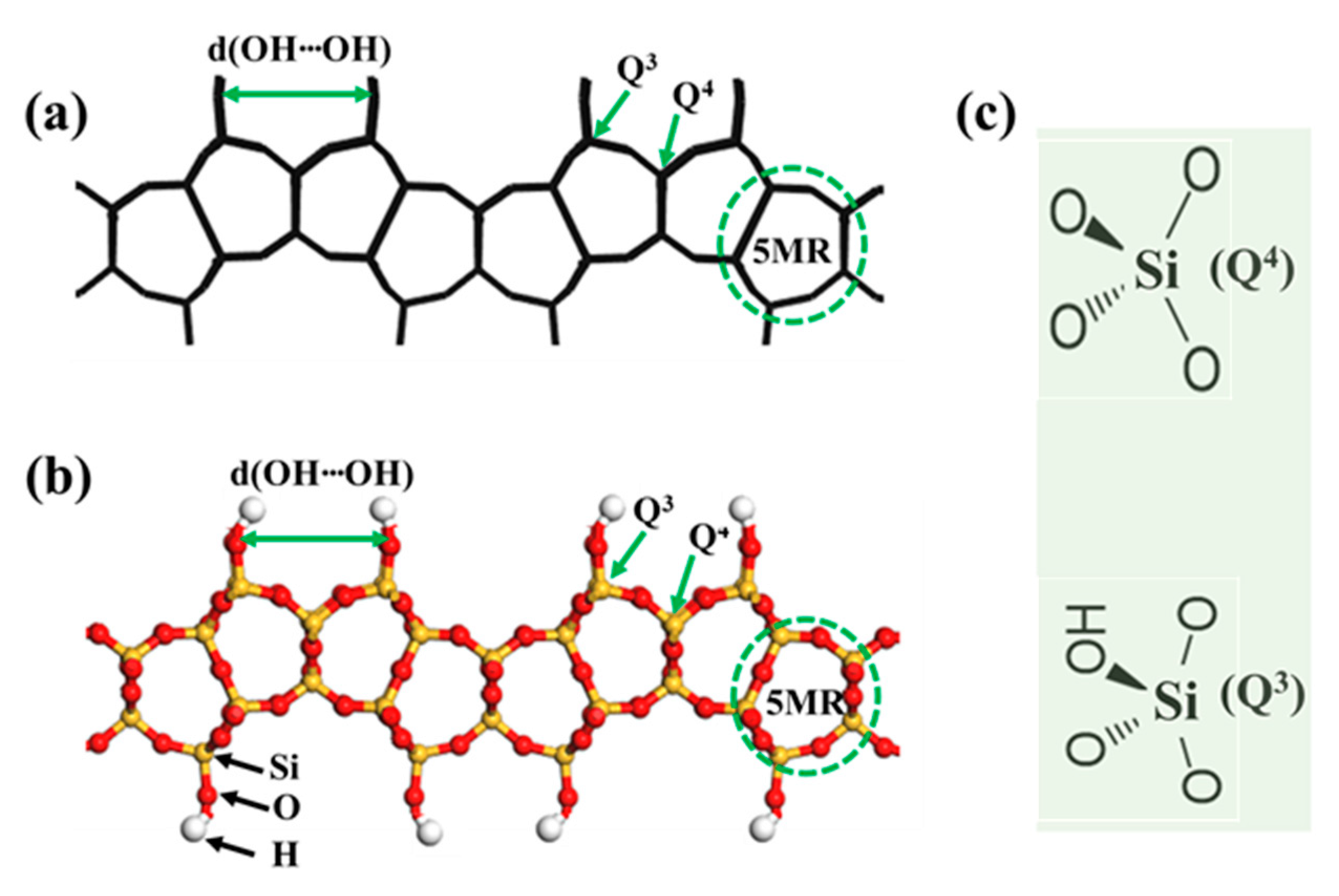
| 3D Zeolite Framework a (Pore Structure) (Å) | 2D Layer Precursor (Layer Stacking direction) b | SDA in 2D Precursor Synthesis | 2D Layer Pore Structure a (Å) | 2D Layer Property | |||
|---|---|---|---|---|---|---|---|
| Q3:Q4 Ratio c | d(OH⋯OH) d (Å) | Layer Thickness (Å) | Inter-Layer Distance (Å) | ||||
| AST (6MR: 1.7 × 2.9) | β-HLS [52,53,54] (a-axis) | TMAOH | 6MR 1.7 × 2.9 | 4.1:1 | 3.2 | 7.2 | 4.0 and 4.6 e |
| HUS-1 [80] (a-axis) | TMAOH BTMAOH | 6MR 1.7 × 2.9 | 4.3:1 | 2.6 | 7.4 | 1.5–2.6 | |
| HUS-5 [81] (a-axis) | TMAOH | 6MR 1.7 × 2.9 | 4.9:1 | - | 7.4 | 4.0 | |
| RUB-55 [82] (a-axis) | TMAOH | 6MR 1.7 × 2.9 | 3.7:1 | 2.3 | 6.9 | 7.7 or 2.9 f | |
| SOD (6MR: 2.5 × 1.8) | RUB-15 [59,86] (c-axis) | TMAOH | 6MR 2.5 × 1.8 | 2.0:1 | 2.5 | 6.3 | 7.7 |
| DLM-2 [84] (c-axis) | TMAOH | 6MR 2.5 × 1.8 | - | - | - | - | |
| RUB-51 [83] (c-axis) | BTMAOH | 6MR 2.5 × 1.8 | 2.0:1 | - | - | 8.8 | |
| ULS-1 [87] (c-axis) | ETMAOH | 6MR 2.5 × 1.8 | 2.0:1 | - | - | 8.3 | |
| 3D Zeolite Framework (Pore Structure) a (Å) | 2D Layered Precursor (Layer Stacking Direction) b | SDA in 2D Precursor Synthesis | 2D Layer Pore Structure a (Å) | 2D Layer Property | |||
|---|---|---|---|---|---|---|---|
| Q3:Q4 Ratio c | d(OH⋯OH) d (Å) | Layer Thickness (Å) | Inter-Layer Distance (Å) | ||||
| CAS (8MR: 2.4 × 4.7) | EU-19 [58,88] (c-axis) | Piperazine | 6MR 1.9 × 2.6 | 0.5:1 | 6.0 | 8.3 | 3.2 |
| MCM-69(P) [89] (c-axis) | Piperazine | 6MR 1.9 × 2.6 | 0.5:1 e | 4.9 f | - | - | |
| NSI (8MR: 2.6 × 4.5 8MR: 2.4 × 4.8) | Nu-6(1) [56,90] (c-axis) | 4,4′-bipyridine | 6MR 1.8 × 2.5 | - | - | 8.0 | 5.4 |
| CDO (8MR: 3.1 × 4.7 8MR: 2.5 × 4.2) | PLS-4 [91] (b-axis) | DEDMAOH | 5MR 1.1 × 1.5 | - | 2.2 | - | 11.1 |
| PLS-1 [92] (b-axis) | TMAOH and K+ | 5MR 1.1 × 1.5 | - | - | - | 10.5 | |
| RUB-20 [93] (b-axis) | TMAOH | 5MR 1.1 × 1.5 | 0.5:1 | 2.4 | - | 10.4 | |
| RUB-40 [93] (b-axis) | TMPOH | 5MR 1.1 × 1.5 | 0.4:1 | 2.6 | - | 10.6 | |
| RUB-36 [93] (b-axis) | DEDMAOH | 5MR 1.1 × 1.5 | 0.3:1 | 2.4 | - | 11.1 | |
| RUB-38 [93] (b-axis) | MTEAOH | 5MR 1.1 × 1.5 | 0.3:1 | 2.4 | - | 11.3 | |
| RUB-48 [93] (b-axis) | TMPAOH | 5MR 1.1 × 1.5 | 0.3:1 | 2.4 | - | 11.1 | |
| MCM-47 [78] (b-axis) | TMMPBr | 5MR 1.1 × 1.5 | 0.3:1 | 2.2 | - | 11.2 | |
| MCM-65 [94] (b-axis) | Quinuclidine and TMAOH | 5MR 1.1 × 1.5 | 1:1 | 2.7 | - | 11.3 | |
| UZM-13 [95] (b-axis) | DEDMAOH | 5MR 1.1 × 1.5 | 0.3:1 | 2.5 | - | 11.1 | |
| HUS-4 [96] (b-axis) | Choline hydroxide and Na+/K+/Rb+/Cs+ | 5MR 1.1 × 1.5 | - | - | - | - | |
| ZSM-55 [33,94,97,98] (b-axis) | choline chloride | 5MR 1.1 × 1.5 | 0.3:1 | - | - | 11.2 | |
| ZSM-52 [94,99] (b-axis) | choline chloride | 5MR 1.1 × 1.5 | - | - | - | - | |
| MTF (8MR: 3.6 × 3.9) | HPM-2 [100] (b-axis) | 2E134TMI | 6MR 1.5 × 2.9 | 0.3:1 | 2.5 | - | 17.5 g |
| RTH (8MR: 3.8 × 4.1 8MR: 2.5 × 5.6) | CIT-10 [101] (c-axis) | diquaternary imidazoles | 8MR 2.5 × 5.6 | 0.3:1 | - | - | 11.8 g |
| RWR (8MR: 2.8 × 5.0) | RUB-18/ilerite [47,50,102,103] (c-axis) | sodium | 5MR 1.1 × 1.7 | 2:1 or 1:1 h | 2.3 | 7.1 | 2.0 |
| 3D Zeolite Framework | 2D Layered Precursor | Re-Organizing Method | Derivative Structure Property | ||||
|---|---|---|---|---|---|---|---|
| 2D Zeolite Derivative | Inter-Layer Pore Formed a | Layer Heteroatom Composition | Pillar Heteroatom Composition | Inter-Layer Distance (Å) | |||
| AST | HUS-1 | silylation | DMS-HUS [85] | 8MR | - | - | 1.8 |
| CAS | MCM-69(P) | detemplated | MCM-69 [89] | - | Al | - | - |
| delaminated | [89] | - | Al | - | - | ||
| NSI | Nu-6(1) | detemplated | MCM-39 [32,104] | - | Al | - | 1.7 |
| delaminated | ITQ-18 [15,105] | - | Al | - | - | ||
| Direct exfoliated Nu-6(2) [17] | - | Al | - | - | |||
| [V,Al]-ITQ-18 [106] | - | V, Al | - | - | |||
| Del-Nu-6 [107] | - | Al | - | - | |||
| inorganic pillared | MCM-39(Si) [32] | 30 Å | Al | - | 28.8 | ||
| silylation | IEZ-Nu-6(1) [108] | 10MR 4.8 Å × 5.8 Å | Al | - | - | ||
| RWR | RUB-18/ilerite | detemplated | octosilicate [109] | - | - | - | - |
| delaminated | Ex-bim-Oct [24] | - | - | - | - | ||
| (C10)2DMA-Oct [16] | - | - | - | - | |||
| inorganic pillared | Silica- pillared [110] | 10 Å | - | - | 25.9 | ||
| Ta-, Nb-, Si- pillared [111] | mesopore | - | Ta, Nb | 12.9–18.0 | |||
| Ti-, Al- Zr- SiO2-pillared [112] | 20 Å | - | Ti, Al, Zr | 20.3–30.3 | |||
| organic pillared | B-ilerite [113] | - | - | - | 12.2 | ||
| RUB-N, RUB-2N, RUB-3N [30] | - | - | - | 11.5, 23.1, 30.9 | |||
| silylation | APhS-ilerite-2 [114] | - | - | - | - | ||
| RTH | CIT-10 | silylation | CIT-12 [101] | 10MR | - | - | - |
| CDO | MCM-47 | silylation | IEZ-CDO [115] | 10MR | - | - | - |
| PreCDO | silylation | IEZ-CDO [116] | 10MR | Al | - | - | |
| Al-RUB-36 | silylation | Al-COE-4 [117] | 10MR | Al | - | - | |
| RUB-36 | silylation | Al-COE-4/Fe [118] | 10MR | Al, Fe | - | - | |
| PLS-1 | silylation | IEZ-CDO [115] | 10MR | - | - | - | |
| IEZ-1 [119] | 10MR | - | - | - | |||
| APZ-1 [120] | 10MR | - | - | - | |||
| PLS-4 | silylation | IEZ-PLS-4 [121] | 10MR | - | - | - | |
| APZ-3 [120] | 10MR | - | - | - | |||
| ZSM-55 | inorganic pillared | [33] | mesopore | - | - | 18.0 | |
| 3D Zeolite Framework (Pore Structure) a (Å) | 2D Zeolite Precursor (Layer Stacking Direction) b | SDA in 2D Precursor Synthesis | 2D Layer Pore Structure a (Å) | 2D Layer Property | |||
|---|---|---|---|---|---|---|---|
| Q3:Q4 Ratio c | d(OH⋯OH) d (Å) | Layer Thickness (Å) | Inter-Layer Distance (Å) | ||||
| AFO (10MR: 4.1 × 5.3) | [F, Tet-A]-AlPO-1 [133,134] (b-axis) | TMAOH | 6MR 2.2 × 3.0 | - | - | - | - |
| FER (10MR: 4.2 × 5.4 8MR: 3.5 × 4.8) | PREFER [61] (a-axis) | ATMP | 5MR 1.0 × 1.8 | 0.3:1 | 5.7 | 9.5 | 3.6 |
| PLS-3 [91] (a-axis) | TEAOH | 5MR 1.0 × 1.8 | 0.3:1 | 1.9 | - | 11.7 | |
| ICP-2 [137] (a-axis) | DMEP | 5MR 1.0 × 1.8 | - | - | - | 19.8 | |
| ERS-12 [138] (a-axis) | TMAOH | 5MR 1.0 × 1.8 | - | - | - | 10.6 | |
| HEU (10MR: 3.1 × 5.5 8MR: 4.1 × 4.1) | CIT-8P [139] (b-axis) | diquaternary imidazoles | 5MR 0.9 × 2.2 | 0.7:1 e | - | - | 12.8 f |
| HUS-2 [96] (b-axis) | choline hydroxide and Na+ | 5MR 0.9 × 2.2 | 0.6:1 | 2.6 | - | 3.6 | |
| HUS-7 [140] (b-axis) | BTMAOH and biphenyl | 5MR 0.9 × 2.2 | 0.7:1 | 2.4 | - | 17.3 | |
| MFI (10MR: 5.1 × 5.5 10MR: 5.3 × 5.6) | multilamellar MFI [48,141] (b-axis) | C22-6-6Br2 | 10MR 5.1 × 5.5 10MR 5.3 × 5.6 | 0.2:1 | 2.7 | 19.7 or 34.0 g | 41.0 |
| multi-quaternary ammonium | 10MR 5.1 × 5.5 10MR 5.3 × 5.6 | - | - | 20.0–34.0 h | 20.0–60.0 i | ||
| single-pore thickness MFI [142] (b-axis) | C18-6-6-18Br3 | 10MR 5.1 × 5.5 10MR 5.3 × 5.6 | - | - | 15.0 | 34.0 | |
| SCZN-1 [143] (b-axis) | CPh–Ph-10-6/CNh-10-6 | 10MR 5.1 × 5.5 10MR 5.3 × 5.6 | - | - | - | - | |
| Multilamellar TS-1 [144] (b-axis) | C22-6-6Br2 | 10MR 5.1 × 5.5 10MR 5.3 × 5.6 | - | - | 34.0 | 12.0 | |
| MWW (10MR: 4.0 × 5.5 10MR: 4.1 × 5.1) | MCM-22(P) [57,145] (c-axis) | HMI | 12MR 7.1 × 18.2 10MR 4.1 × 5.1 | 0.5:1 | 8.3 | 25.1 | 1.9 |
| EMM-10P [146,147] (c-axis) | Diquat-C5 | 12MR 7.1 × 18.2 10MR 4.1 × 5.1 | - | - | 25.0 | >1 | |
| ERB-1 [148,149] (c-axis) | Piperidine | 12MR 7.1 × 18.2 10MR 4.1 × 5.1 | - | - | - | 1.8 | |
| MCM-56 [62,150,151] | HMI | 12MR 7.1 × 18.2 10MR 4.1 × 5.1 | - | 9.9–11.0 | 25.0 | - | |
| UZM-8 [152] | DEDMAOH | 12MR 7.1 × 18.2 10MR 4.1 × 5.1 | - | - | - | 13.4 | |
| SSZ-70 [153,154,155] | diquaternary imidazoles | 12MR 7.1 × 18.2 10MR 4.1 × 5.1 | - | - | - | 2.0 | |
| IPC-3P [156] | 1,4-MPB | 12MR 7.1 × 18.2 10MR 4.1 × 5.1 | - | - | - | 4–12.6 | |
| UJM-1P [157] | Ada-4-16 | 12MR 7.1 × 18.2 10MR 4.1 × 5.1 | - | - | - | 26 f | |
| RRO (10MR: 4.0 × 6.5 8MR: 2.7 × 5.0) | RUB-39 [60] (b-axis) | DMDPAOH | 5MR 1.1 × 1.8 | 0.3:1 | 7.0 | 7.8 | 3.0 |
| Al-, B-RUB-39 [158] (b-axis) | DMDPA | 5MR 1.1 × 1.8 | - | - | - | - | |
| STI (10MR: 4.7 × 5.0 8MR: 2.7 × 5.6) | PKU-22 [159] (b-axis) | TEAOH | 6MR 0.5 × 2.6 | - | 2.8 | - | 10.6 f |
| 3D Zeolite Framework | 2D Zeolite Precursor | Re-Organizing Method | Derivative Structure Property | ||||
|---|---|---|---|---|---|---|---|
| 2D Zeolite Derivative | Inter-Layer Pore Formed a | Layer Heteroatom Composition | Pillar Heteroatom Composition | Inter-Layer Distance (Å) | |||
| FER | PREFER | delaminated | ITQ-6 [14,63] | - | Al, Ti | - | - |
| UCB-2 [162] | - | Al | - | - | |||
| inorganic pillared | ITQ-36 [34] | mesopore | Al, Ge, Ti | Ge, Ti, Al, B, Fe, Cr, Ga | 27.5 | ||
| silylation | IEZ-FER [115,163] | 12MR | - | - | - | ||
| silylation | APZ-4 [120] | 12MR | - | - | - | ||
| ZSM-55 | inorganic pillared | Pillared FER [33] | mesopore | B | - | 25.0 | |
| PLS-3 | silylation | IEZ-Sn-PLS-3 [165] | 12MR | - | Sn | - | |
| silylation | IEZ-PLS-3 [163] | 12MR | Al | - | - | ||
| silylation | ECNU-9 [164] | 14MR | Ti | - | - | ||
| silylation | APZ-2 [120] | 12MR | - | - | - | ||
| HEU | HUS-2 | silylation | HUS-10 [166] | 12MR | - | - | - |
| Tix-HUS [167] | 12MR | - | Ti | - | |||
| RRO | RUB-39 | silylation | COE-1 [168] | 12MR | - | - | - |
| silylation | RUB-39 DCDMS/HMDS [169] | 12MR | Al | - | - | ||
| 3D Zeolite Framework | 2D Zeolite Precursor | Re-Organizing Method | Derivative Structure Property | ||||
|---|---|---|---|---|---|---|---|
| 2D Zeolite Derivative | Inter-Layer Pore Formed a | Layer Heteroatom Composition | Pillar Heteroatom Composition | Inter-Layer Distance (Å) | |||
| MFI | multilamellar MFI | delaminated | exfoliated MFI [18,19,20] | - | Al | - | - |
| inorganic pillared | pillared MFI [35] | mesopore | Al | - | 41.0 | ||
| titanosilicate pillared MFI [42] | mesopore | Al | Ti | 23.0 | |||
| tin–silica pillared MFI [176] | mesopore | - | Sn | 31.9 | |||
| organic pillared | BTEB pillared MFI [36] | - | Al | BTEB | 12.6 | ||
| multilamellar TS-1 | inorganic pillared | pillared TS-1, Ti-pillared TS-1 [177] | mesopore | Ti | Ti | 31.9 | |
| P-TS-1 with long-range order [144] | mesopore | Ti | - | 28.0 | |||
| direct synthesis | unilamellar | MFI nanosheet agglomeration [178] | - | Al | - | - | |
| MFI nanosheet [171] | - | - | - | - | |||
| TS-1 nanosheet agglomeration [172] | - | Ti | - | - | |||
| inorganic pillared | self-pillared pentasil [173] | mesopore | Al, Sn | Al | 20.0–70.0 | ||
| SCZN-2 [143] | mesopore | Al | Al | 16.7–28.2 | |||
| MZIN [174] | mesopore | Al | Al | 20.0–40.0 | |||
| 3D Zeolite Framework | 2D Zeolite Precursor | Derivative Structure Property | |||||
|---|---|---|---|---|---|---|---|
| Re-Organizing Method | 2D Zeolite Derivative | Inter-Layer Pore Formed a | Layer Heteroatom Composition | Pillar Heteroatom Composition | Inter-Layer Distance (Å) | ||
| MWW | MCM-22(P) | detemplated | [46] | - | Al | - | - |
| delaminated | ITQ-2 [13,187], Ti-ITQ-2 [188] | - | Al, Ti | - | - | ||
| UCB-1 [22] | - | Al | - | - | |||
| exfoliated MCM-22(S) [20] | - | Al | - | - | |||
| swollen MCM-22(P) [21] extrusion | - | Al | - | - | |||
| inorganic pillared | MCM-36 [25] | 30.0 Å-35.0 Å | Al | - | >24.9 | ||
| Al2O3-MCM-36, MgO-Al2O3-MCM-36, BaO-Al2O3-MCM-36 [27,28] | mesopore | Al | Al, Mg, Ba | 5.0–24.9 | |||
| Ti-MCM-36, Si/Ti-MCM-36 [189,190,191] | mesopore | Al | Ti | 14.9–18.9 | |||
| organic pillared | MCM-22(PS-RT) [21] | - | Al | - | 16.9 | ||
| MWW-BTEB [29] | - | Al | - | 15.1 | |||
| direct synthesis | unilamellar | DS-ITQ-2 [192] | - | Al | - | - | |
| MIT-1 [67] | - | Al | - | - | |||
| MCM-56 | delaminated | [182] | - | Al | - | - | |
| pillared | [182] | mesopore | Al, Sn, B | - | 45.1 b | ||
| ERB-1P | delaminated | ERB-1-del-135 [23] | - | Al | - | - | |
| inorganic pillared | Si/Ti oxide pillared MCM-36 [190] | - | Al | Ti | 45.1 b | ||
| silylation | IEZ-MWW [115,193] | 12MR | Al, Ce | - | - | ||
| Ti-YNU-1 [194,195] | 12MR | Al | - | - | |||
| SSZ-70 | delaminated | UCB-3, UCB-4 [183] | - | Al, B | - | - | |
| 3D Parent Zeolite (Pore Structure) a (Å) | 2D Zeolite Precursor (Layer Stacking Direction) b | Re-Organizing Method | Derivative Structure Property | |||||
|---|---|---|---|---|---|---|---|---|
| 2D Zeolite Derivative | Inter-Layer Connection Unit | Inter-Layer Pore Dimension | Layer Heteroatom Composition | Pillar Heteroatom Composition | d-Spacing (Å) | |||
| UTL (14MR: 9.5 × 7.1 12MR: 8.5 × 5.5) | IPC-1P [199] (c-axis) | direct calcination | IPC-1 [199,200] | oxygen c | sub-zeolite f | Ge, B | -- | 9.0 |
| silylation in acid solution | IPC-2 [199] (OKO) | s4R d | 12 and 10MR | Ge, Ti | - | 11.5 | ||
| octylamine intercalation | IPC-4 [66] (PCR) | oxygen | 10 and 8MR | Ge, Ti | - | - | ||
| staged de-intercalation | IPC-6 [201] (*PCS) | oxygen and s4R | 12, 10 and 8MR | Ge | - | - | ||
| staged de-intercalation | IPC-7 [201] | d4R e and s4R | 14, 12 and 10 MR | Ge | - | - | ||
| choline intercalation | IPC-9 [202] | oxygen | 10 and 7MR | Ge | - | - | ||
| choline and organosilane intercalation | IPC-10 [202] | s4R | 12 and 9MR | Ge | - | - | ||
| swelling | IPC-1SW [31,203,204] | organic | mesopore | Ge | - | 10.4–39.0 | ||
| inorganic pillaring | IPC-1PI [31,203] | SiO2 | mesopore | Ge, | - | 38.0 | ||
| B-IPC-1PI [31] | SiO2 | mesopore | Ge, B | - | 42.0 | |||
| Fe-IPC-1PI [31] | SiO2 | mesopore | Ge, Fe | - | 44.1 | |||
| Ti-IPC-1PISi [205] Ti-IPC-1PITi | SiO2 SiO2/TiO2 | mesopore | Ge, Ti | Ti | 37.0 | |||
| IWW (12MR: 6.0 × 6.7 10MR: 4.9 × 4.9) | IPC-5P [65] (c-axis) | direct calcination | IPC-5 [206] | d4R | 12, 10 and 8MR | Ge | - | - |
| silylation | IWW (siliceous) [65] | d4R | 12, 10 and 8MR | - | - | - | ||
| alumination with AlCl3 | IWW (Al-containing) [65] | d4R | 12, 10 and 8MR | Ge, Al | - | - | ||
| swelling | IPC-5SW [65] | organic | mesopore | Ge | - | - | ||
| UOV (12MR: 6.0 × 7.7 12MR: 5.9 × 7.1; 10MR: 4.7 × 5.9) | IPC-12P [207,208] (a-axis) | direct calcination | IPC-12 [207,208] | oxygen | 12 and 8MR | Ge | - | - |
| SAZ-1 | SAZ-1P [209] (a-axis) | octylamine intercalation | IPC-15 [209] | oxygen | - | - | - | 8.4 |
| silylation in acid solution | IPC-16 [209] | s4R | - | - | - | 10.2 | ||
| 3D Zeolite Framework (Pore Structure) a (Å) | 2D Zeolite Properties | ||||
|---|---|---|---|---|---|
| 2D Zeolite Structure (Layer Stacking Direction) b | SDA Used in Synthesis | Particle Morphology | Layer Thickness (Å) | Heteroatom Composition | |
| MEL (10MR: 5.3 × 5.4) | MTS-2 [69] | CTATos, TBAOH | olive-like nanosheet aggregates | 50–100 | Ti |
| FAU (12MR: 7.4 × 7.4) | NaX-T-cal [70,71] | TPHAC, Zn(NO3)2, Li2CO3 | ball-shaped house-of-cards nanoplate assemblies | ~70 | Al |
| MOR (12MR: 6.5 × 7.0 8MR: 4.8 × 3.4 8MR: 2.6 × 5.7) | MOR nanoplate [74] (c-axis) | C16-2-0 | nanoplate aggregates | 200–400 | Al |
| MOR nanoplate [211] (c-axis) | poly-quaternary ammonium | nanoplate aggregates | - | Al | |
| TON (10MR: 4.6 × 5.7) | ZSM-22 [73] | 1-ethylpyridinium bromide | nanoplates | 80–500 | Al |
| MRE (10MR: 5.6 × 5.6) | LMZN [75] (c-axis) | BPTn−6−0 | flower-like nanosheet agglomerates | 30 b | - |
| Organic Base | Kinetic Diameter (Å) | Accessibility to Acid Sites in Zeolites | Acidity Type |
|---|---|---|---|
| CO | 3.8 [231] | >6 MR | Brønsted; Lewis |
| DME | 4.7 [232] | >6 MR | Brønsted |
| CD3CN | 4.8 [233] | >6 MR | Brønsted; Lewis |
| IPA | 5.3 [234] | >8 MR | Brønsted |
| Pyridine | 5.4 [235] | >8 MR | Brønsted; Lewis |
| Pivalonitrile | 6.2 [236] | >10 MR | Brønsted |
| 2,6-lutidine | 6.7 [235] | >12 MR | Brønsted; Lewis |
| 2,4,6-collidine | 7.4 [235] | >12 MR | Brønsted; Lewis |
| DTBP | 7.9 [237] | >12 MR | Brønsted |
| DMQ | 8.3 [238] | >12 MR | Brønsted |
| TPP | 9.4 [239] | >12 MR | Brønsted |
| TMPO | 5.5 [230] | >8 MR | Brønsted; Lewis |
| TBPO | 8.2 [44] | >10 MR | Brønsted; Lewis |
| Zeolite Material | Extra-Framework Al (%) | External Acid Sites (%) | |||
|---|---|---|---|---|---|
| 27Al MAS NMR a | FTIR of Adsorbed Base | Base Titration | 31P MAS NMR | ||
| DTBP | Collidine g | DTBP h | TBPO j | ||
| 3D MFI | 22.0 b/6.7 c [248] | 4.0 e [235] | 6.3 b/2.5 c [248] | 3.2 i [173] | 4.7 i [79] |
| Unilamellar MFI | - | 11.3 f [247] 50.0 e [235] | - | - | 32.0 [79] |
| Multilamellar MFI | 30.0–46.5 d [248] | - | 19.0–45.9 [248] | - | - |
| Pillared MFI | 16.0 [245] | - | - | 28.7 [173] | - |
| SPP | - | - | - | 40.8 [173] | - |
| Ref. # | Zeolite Material | Si/Al Ratio | Acidity (µmol g−1) | Acid Strength | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Brønsted | Lewis | 523 K/423 K e | 623 K/423 K f | |||||||
| 423 K | 523 K | 623 K | 423 K | 523 K | 623 K | |||||
| [187,258] | MCM-22 | 50 | 39 | 24 | 15 | 23 | 15 | 14 | 0.63 | 0.47 |
| MCM-36 | - a | 7 | 5 | 3 | 7 | 6 | 6 | 0.79 | 0.64 | |
| ITQ-2 | - a | 21 | 15 | 9 | 23 | 20 | 15 | 0.80 | 0.55 | |
| [62] | MCM-22 | 50 | 57 | 48 | 33 d | 23 | 20 | 20 d | 0.85 | 0.66 g |
| MCM-56 | 9 | 64 | 59 | 35 d | 77 | 25 | 21 d | 0.60 | 0.40 g | |
| ITQ-2 | - a | 21 | 15 | 9 d | 23 | 20 | 15 d | 0.80 | 0.55 g | |
| [260] | MCM-36 | 54 b | 56 | 50 | 33 | 19 | 17 | 17 | 0.89 | 0.67 |
| MCM-36 | 23 b | 108 | 98 | 65 | 40 | 31 | 30 | 0.87 | 0.64 | |
| MCM-36 | 24 | 62 | 47 | 21 | 61 | 44 | 42 | 0.74 | 0.51 | |
| [267] | MCM-22 | 50 | - | 208 | 191 | - | 47 | 19 | - | 0.82 h |
| MCM-36 | 28 c | - | 87 | 70 | - | 61 | 32 | - | 0.69 h | |
| MCM-36 | 50 c | - | 57 | 48 | - | 40 | 23 | - | 0.73 h | |
| MCM-36 | 100 c | - | 36 | 32 | - | 31 | 15 | - | 0.70 h | |
| [268] | MCM-22 | 15 | 216 | 195 | 157 | 65 | 67 | 66 | 0.93 | 0.79 |
| MCM-36 | - a | 214 | 187 | 125 | 81 | 74 | 62 | 0.88 | 0.63 | |
| ITQ-2 | - a | 130 | 112 | 78 | 80 | 77 | 65 | 0.90 | 0.68 | |
| Zeolite Material | Extra-Framework Al (%) | External Acid Sites (%) | |||||
|---|---|---|---|---|---|---|---|
| 27Al MAS NMR b | FTIR of Adsorbed Base | Base Titration | 31P MAS NMR | ||||
| DTBP e | DTBP f | Pivalonitrile or DMQ | DTBP i | TBPO j | |||
| MCM-22 | 12.8 [28] | 1.0 [28,62] | 6.5 [187]; 5.1 [264]; 3.0 [235]; 12.1 [156]; 17.8 [269] | 29.7 g [182]; 11.7–14.6 h [226] | 8.0 [245] | 13.0 [67] | |
| ITQ-2 | - | 4.0 [258]; 1.6 [62] | 40.0 [187]; 65.0 [235] | - | - | - | |
| MCM-36 a | Al2O3 | 44.9 [28] | 1.4 [28] | - | - | - | - |
| MgO-Al2O3 | 80.7 [28] | 1.7 [28] | - | - | - | - | |
| BaO-Al2O3 | 75.6 [28] | 1.5 [28] | - | - | - | - | |
| SiO2 | 29.5 [28] | 1.5 [28] | 18.4 [264]; 30.0 [156]; 50.0 [269] | 42.9–45.7 h [226] | 67.0 [245] | - | |
| Al2O3-SiO2 | 30.4 [28] | 2.5 [28] | - | - | - | - | |
| MgO-Al2O3-SiO2 | 37.7 [28] | 2.4 [28] | - | - | - | - | |
| BaO-Al2O3-SiO2 | 33.7 [28] | 2.3 [28] | - | - | - | - | |
| MCM-56 | - | 1.3 [62] | 43.6 [269] | 58.8 g [182] | - | 40.6 [67] | |
| Pillared MCM-56 | - | - | 55.2 [269] | - | - | - | |
| Delaminated MCM-56 | - | - | - | 40.9–68.3 g [182] | - | - | |
| IPC-3 | - | - | 8.1 [156] | - | - | - | |
| Pillared IPC-3-PI | - | - | 50.8 [156] | - | - | - | |
| MIT-1 | 8.0 c [67]; 30.0 d [67] | - | - | - | - | 63.6 [67] | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schulman, E.; Wu, W.; Liu, D. Two-Dimensional Zeolite Materials: Structural and Acidity Properties. Materials 2020, 13, 1822. https://doi.org/10.3390/ma13081822
Schulman E, Wu W, Liu D. Two-Dimensional Zeolite Materials: Structural and Acidity Properties. Materials. 2020; 13(8):1822. https://doi.org/10.3390/ma13081822
Chicago/Turabian StyleSchulman, Emily, Wei Wu, and Dongxia Liu. 2020. "Two-Dimensional Zeolite Materials: Structural and Acidity Properties" Materials 13, no. 8: 1822. https://doi.org/10.3390/ma13081822
APA StyleSchulman, E., Wu, W., & Liu, D. (2020). Two-Dimensional Zeolite Materials: Structural and Acidity Properties. Materials, 13(8), 1822. https://doi.org/10.3390/ma13081822






