Synthesis Based on a Preceramic Polymer and Alumina Nanoparticles via UV Lithography for High Temperature Applications
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Si Wafer Treatment
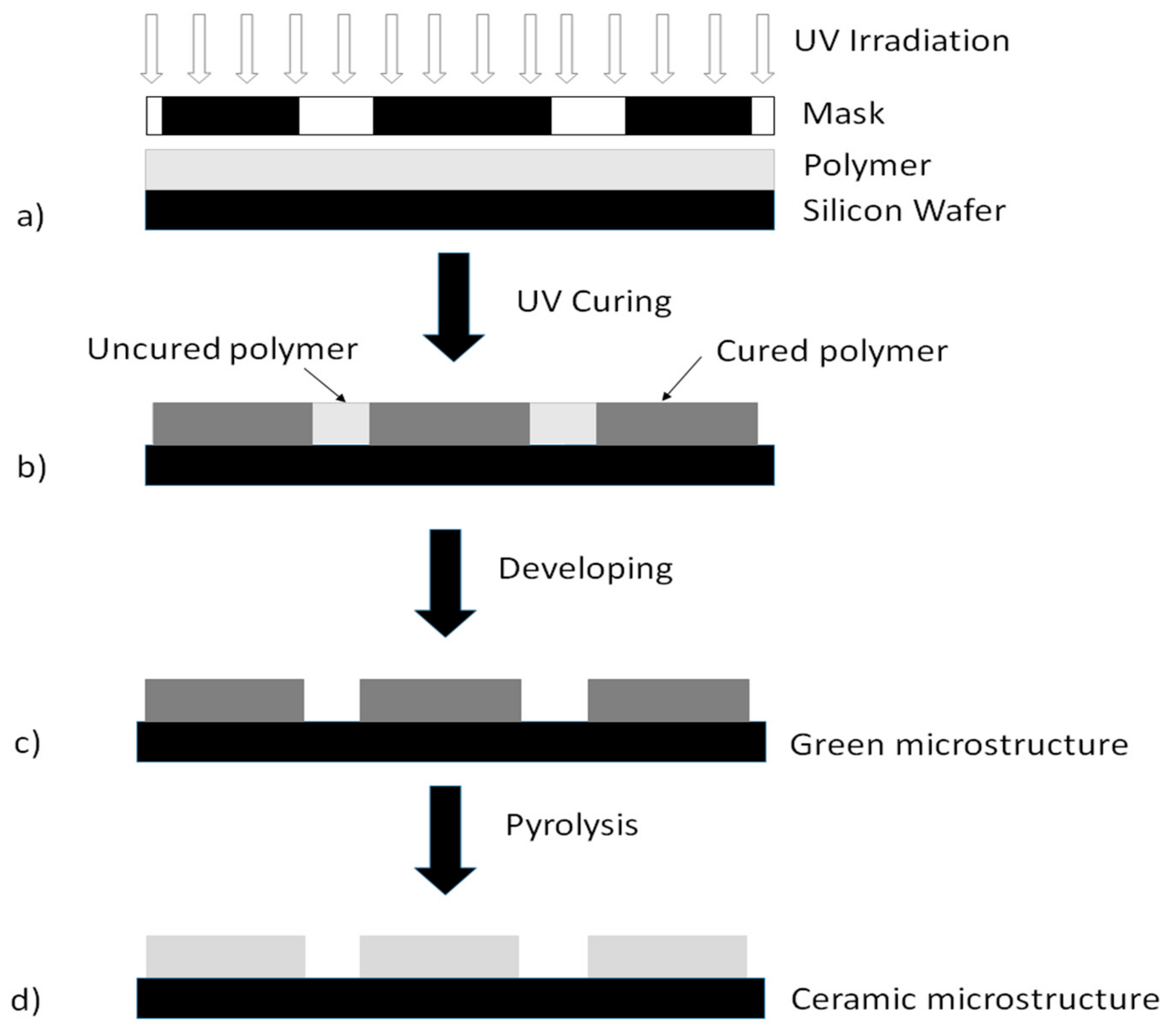
2.3. Experimental Procedure
2.4. Characterizations
2.4.1. Thickness Measurement
2.4.2. Fourier Transform Infrared (FT-IR) Spectroscopy
2.4.3. X-Ray Diffraction (XRD)
2.4.4. Thermal Analysis (TGA/DSC)
2.4.5. Scanning Electron Microscopy (SEM)
3. Results and Discussion
3.1. Spectroscopic Ellipsometry Measurement
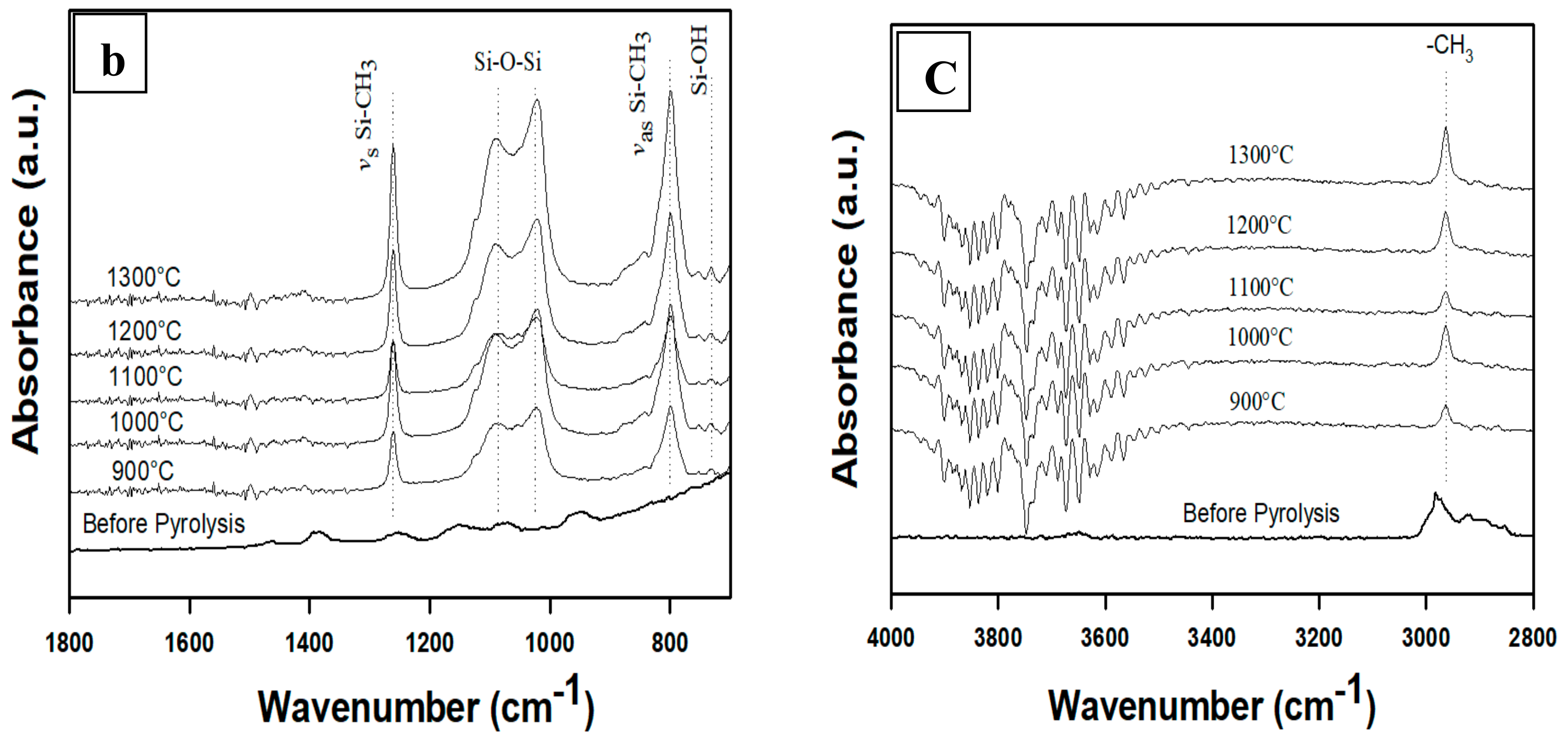
3.2. Fourier-Transformed Infrared Spectroscopy
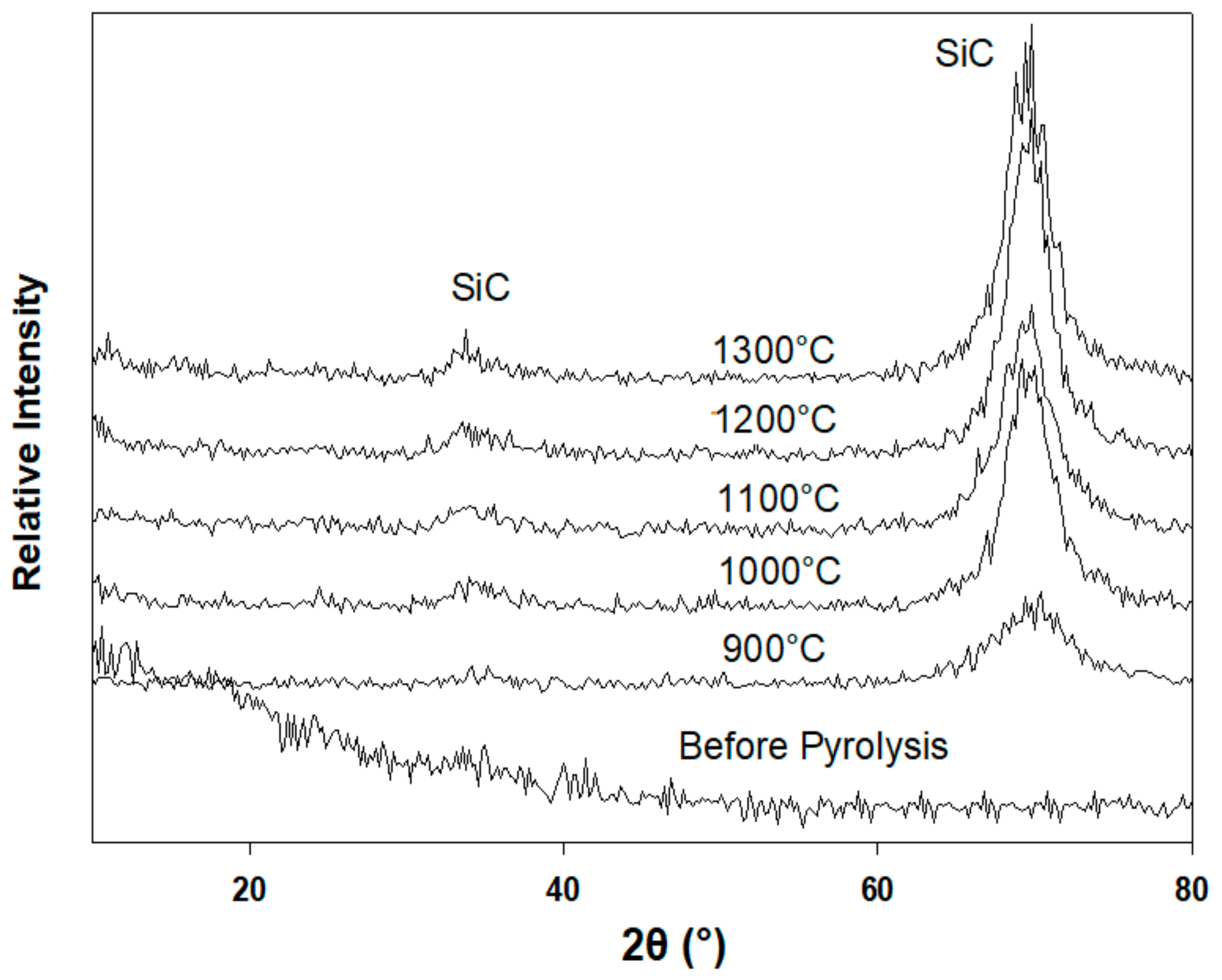
3.3. X-Ray Diffraction
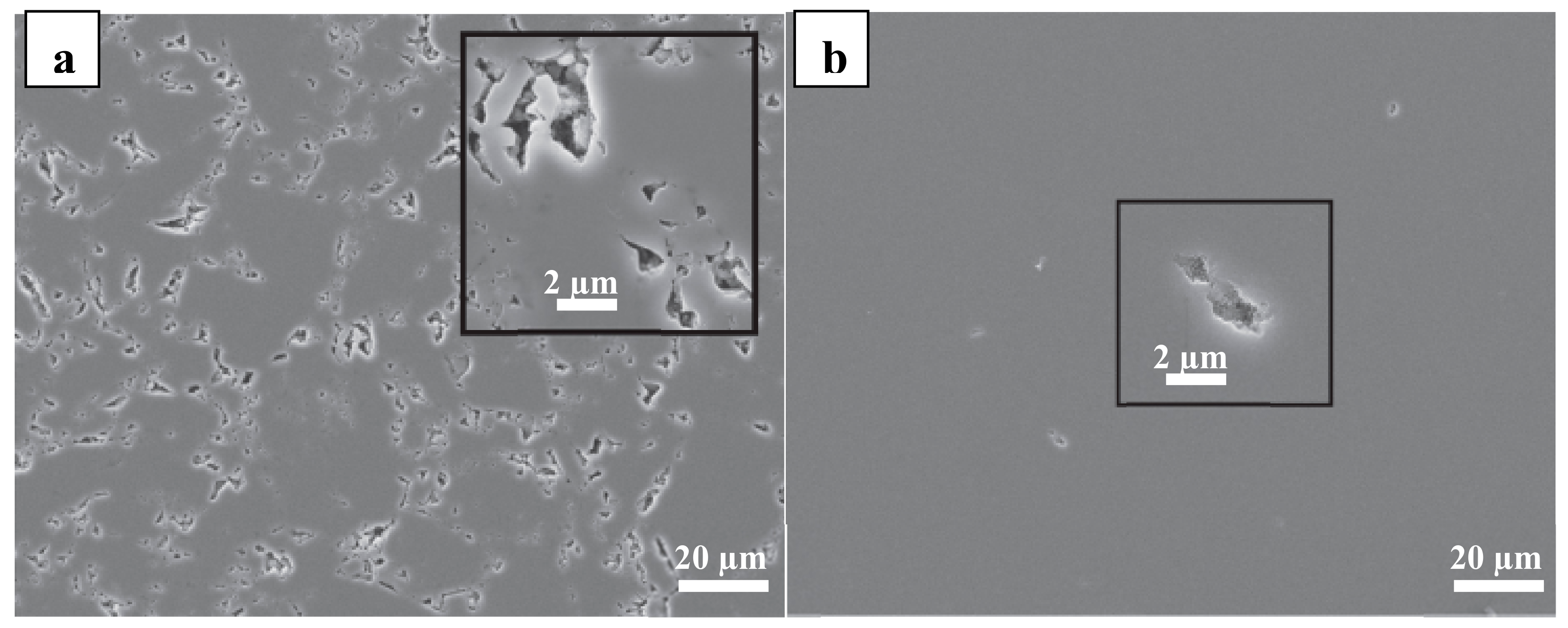
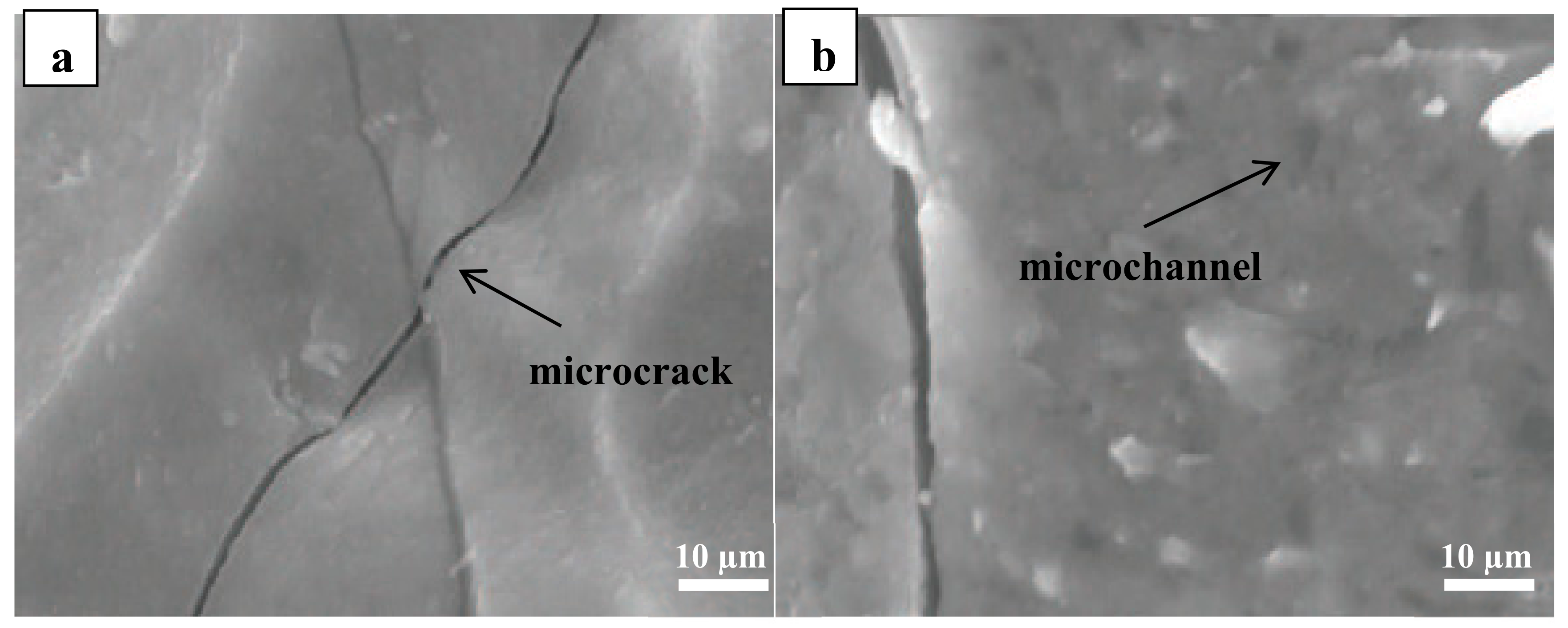
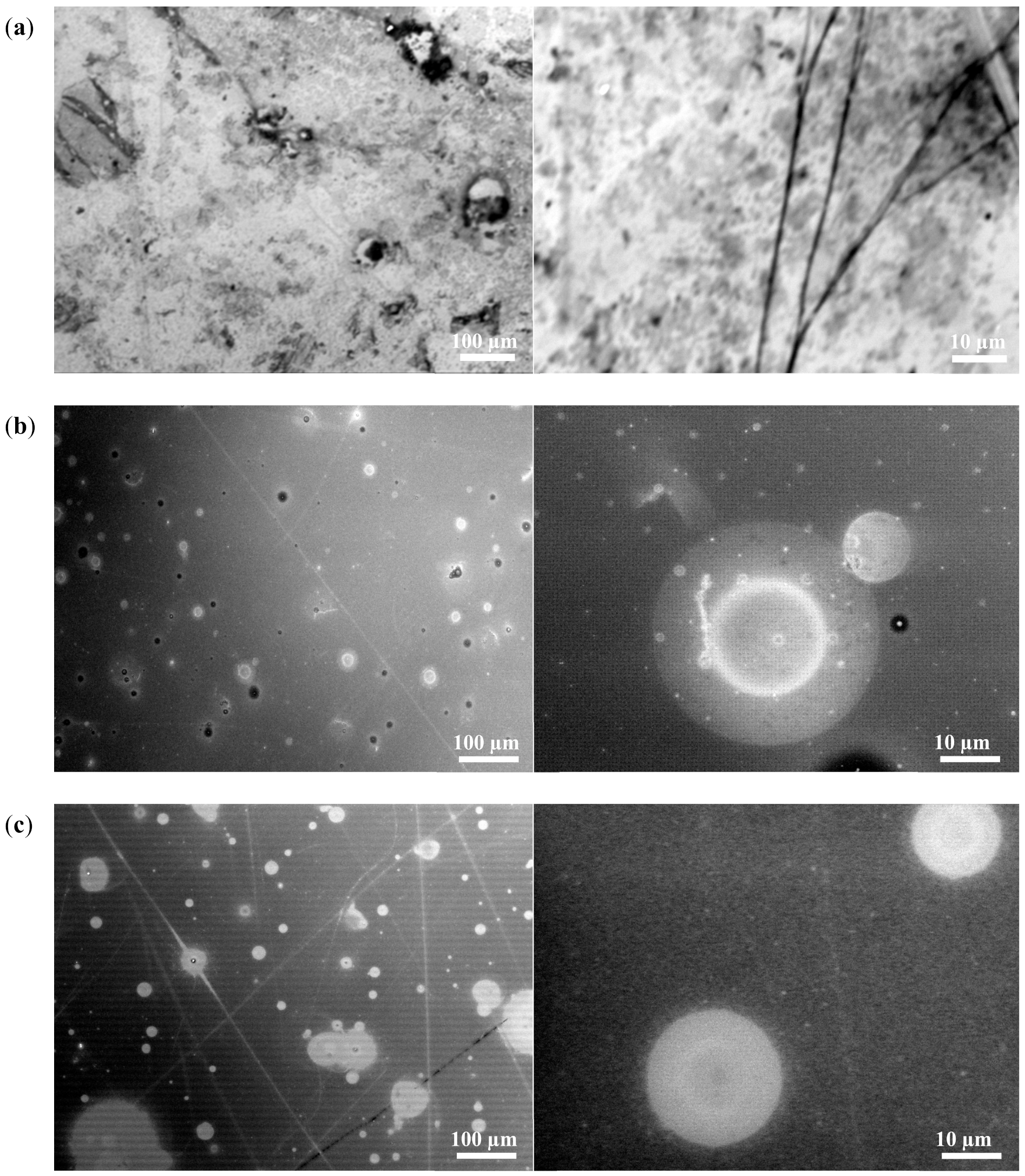
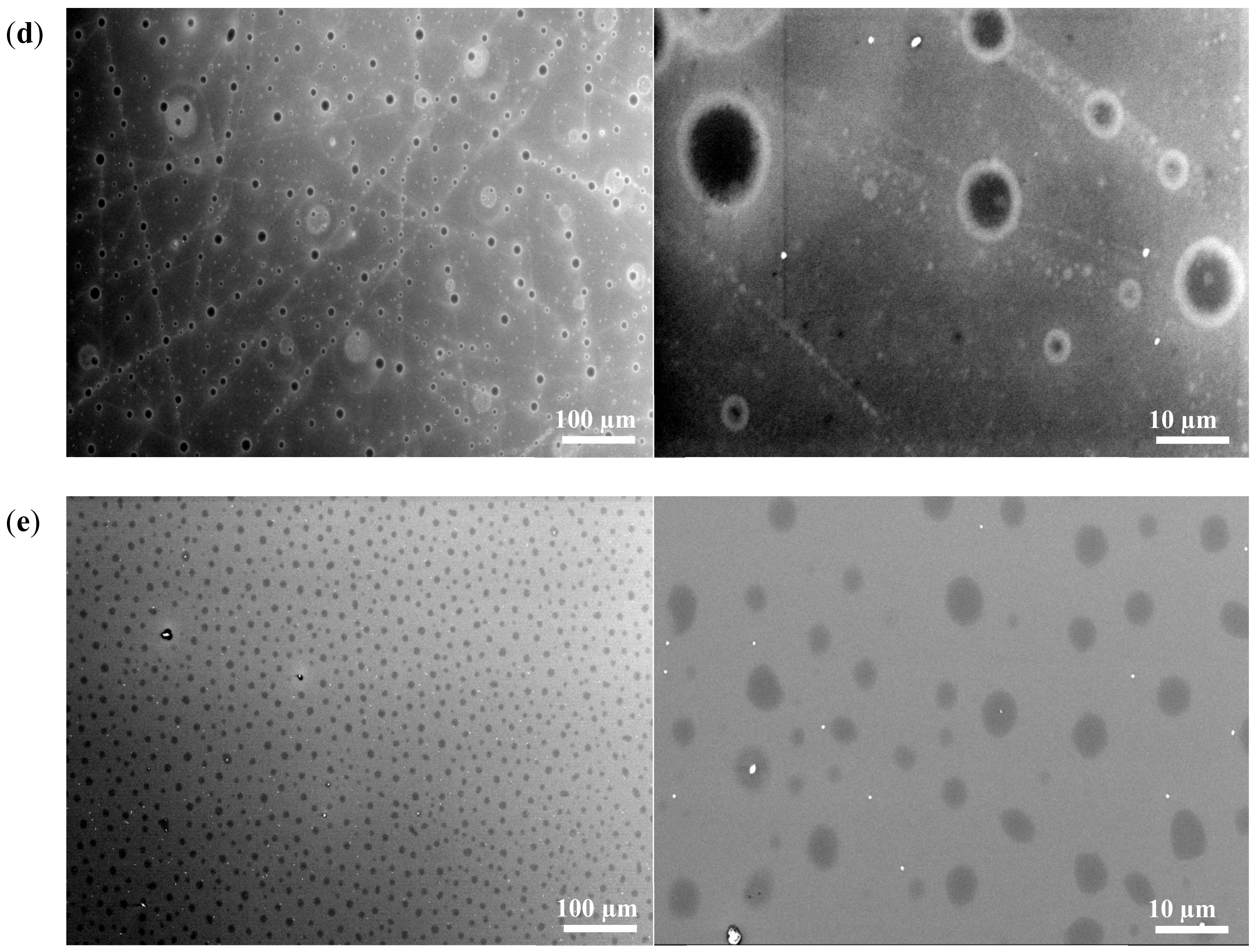
3.4. Scanning Electron Microscopy
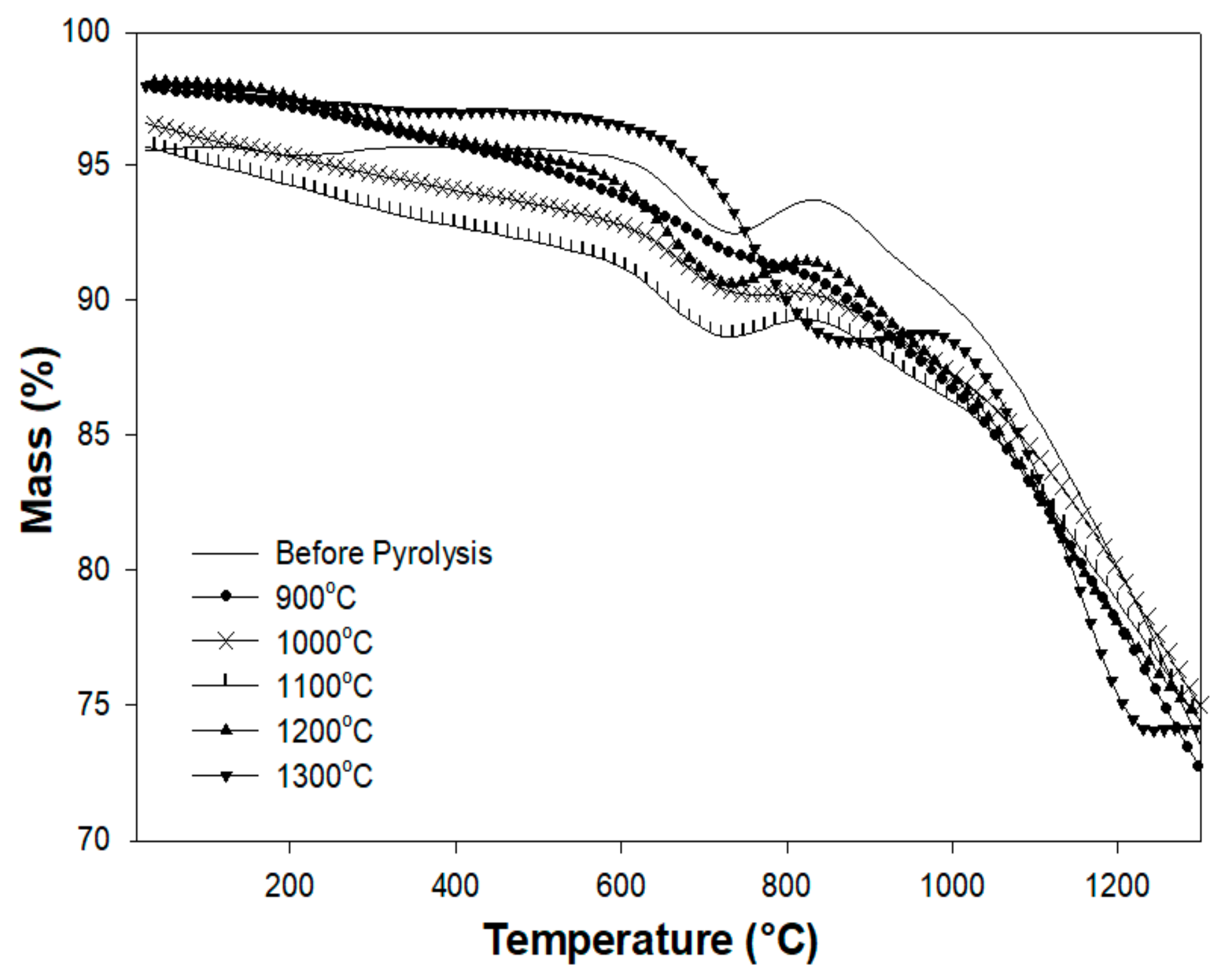
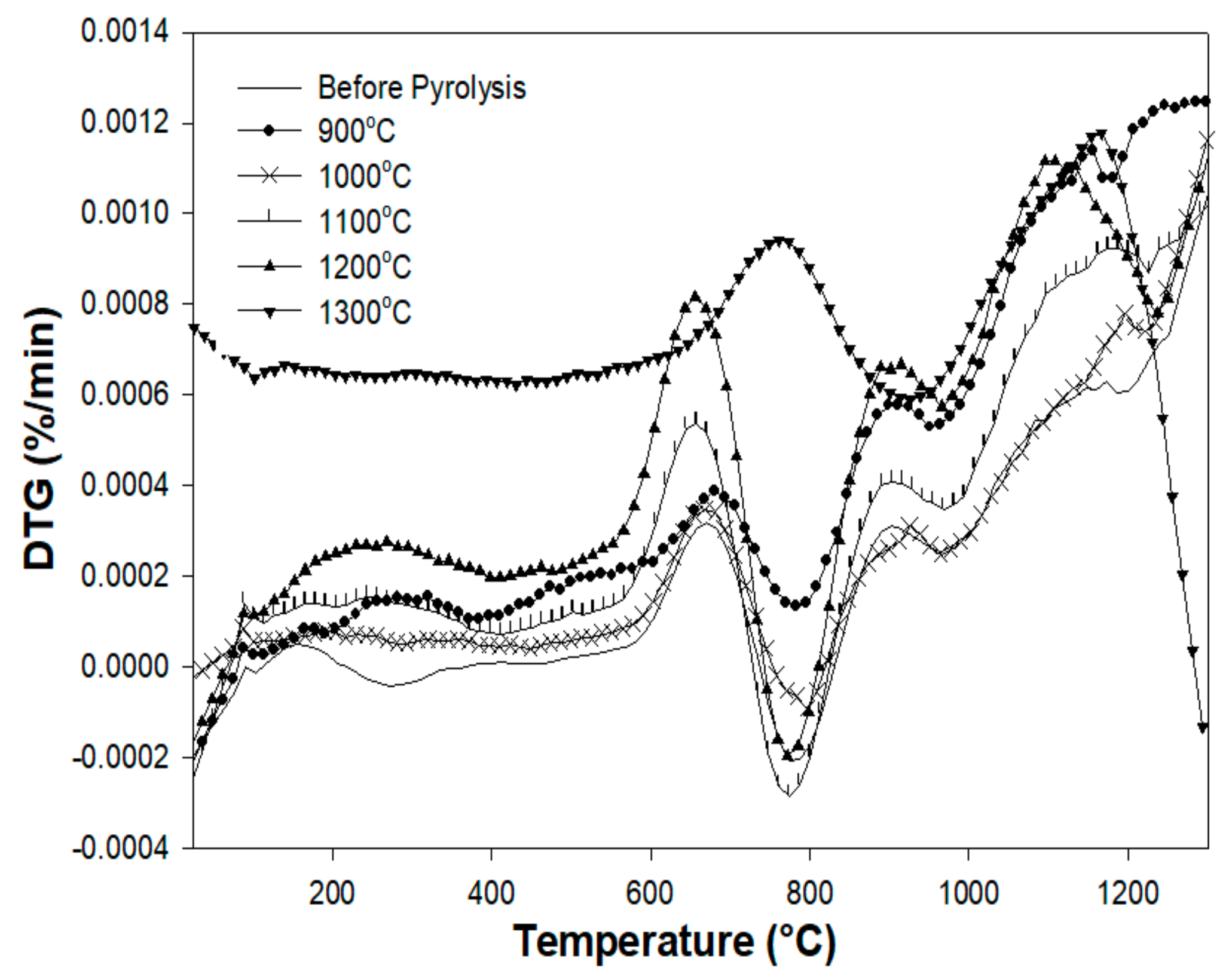
3.5. Thermogravimetric Analysis
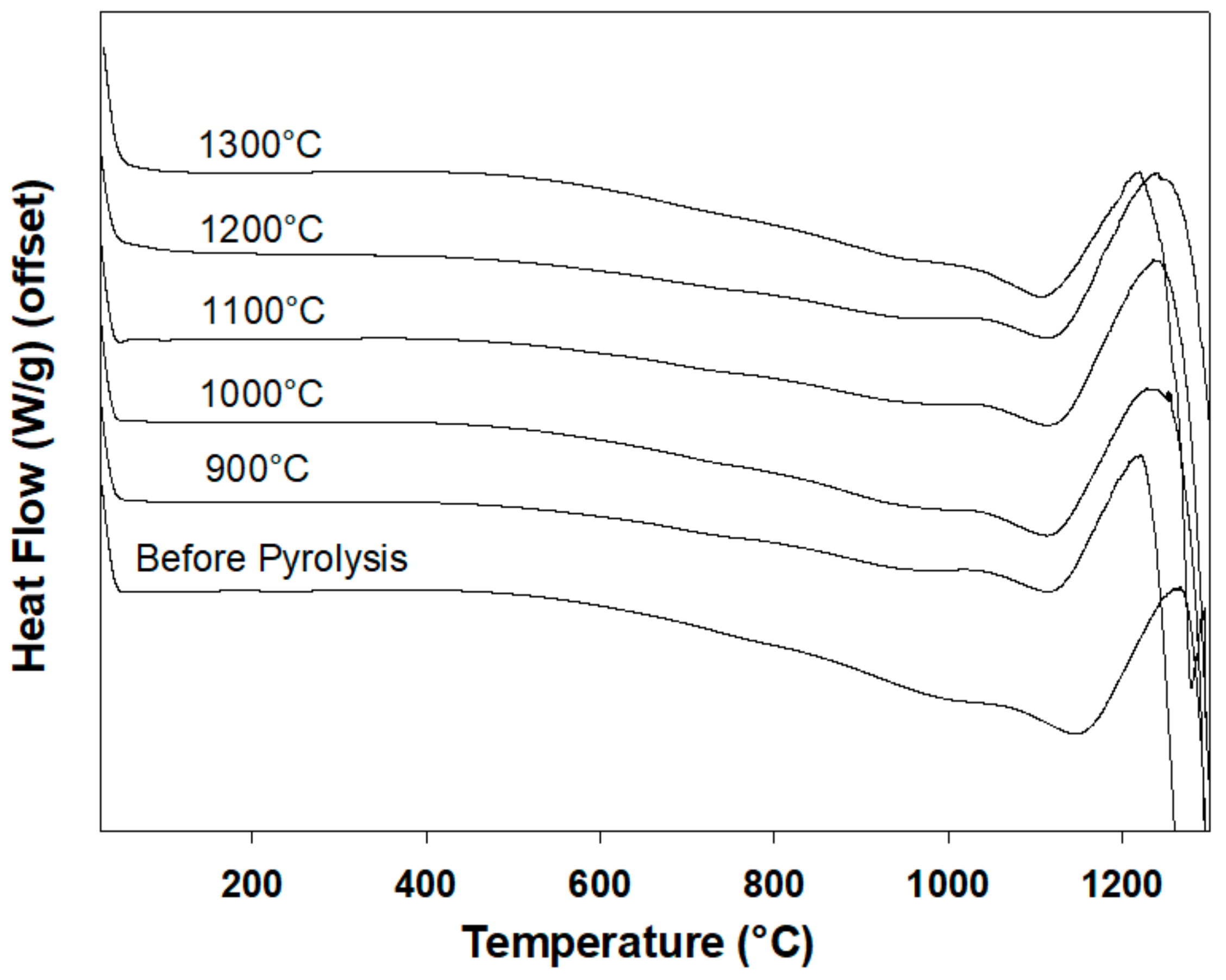
3.6. Differential Scanning Calorimetry
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Colombo, P.; Mera, G.; Riedel, R.; Soraru, D. Polymer-derived ceramics: 40 years of research and innovation in advanced ceramics. J. Am. Ceram. Soc. 2010, 93, 1805–1837. [Google Scholar] [CrossRef]
- Colombo, P.; Mera, G.; Riedel, R.; Sorarù, D. Polymer-derived ceramics: 40 years of research and innovation in advanced ceramics, Eds.; R. Riedel and I-W. Chen. Ceram. Sci. Technol. 2013, 4, 245–320. [Google Scholar]
- Riedel, R.; Mera, G.; Hauser, R.; Klonczynski, A. Silicon-based polymer-derived ceramics: Synthesis properties and applications-A review. J. Ceram. Soc. Jpn. 2006, 114, 425–444. [Google Scholar] [CrossRef]
- Riedel, R.; Toma, L.; Fasel, C.; Miehe, G. Polymer-derived mullite-SiC-based nanocomposites. J. Eur. Ceram. Soc. 2009, 29, 3079–3090. [Google Scholar] [CrossRef]
- Bernardo, E.; Colombo, P.; Pippel, E.; Woltersdorf, J. Novel mullite synthesis based on alumina nanoparticles and a preceramic polymer. J. Am. Ceram. Soc. 2006, 89, 1577–1583. [Google Scholar] [CrossRef]
- Griggio, F.; Bernardo, E.; Colombo, P.; Messing, G. Kinetic studies of mullite synthesis from alumina nanoparticles and a preceramic polymer. J. Am. Ceram. Soc. 2008, 9, 2529–2533. [Google Scholar] [CrossRef]
- Bernardo, E.; Fiocco, L.; Parcianello, G.; Storti, E.; Colombo, P. Advanced ceramics from preceramic polymers modified at the nano-scale: A review. Materials 2014, 7, 1927–1956. [Google Scholar] [CrossRef] [PubMed]
- Schulz, M. Polymer derived ceramics in MEMS/NEMS - a review on production processes and application. Adv. Appl. Ceram. 2013, 108, 454–460. [Google Scholar] [CrossRef]
- Ye, C.; Chen, A.; Colombo, P.; Martinez, C. Ceramic microparticles and capsules via microfluidic processing of a preceramic polymer. J. R. Soc. Interface 2010, 7, 461–473. [Google Scholar] [CrossRef]
- Schulz, M.; Börner, M.; Hausselt, J.; Heldele, R. Polymer derived ceramic microparts from x-ray lithography-cross-linking behavior and process optimization. J. Eur. Ceram. Soc. 2005, 25, 199–204. [Google Scholar] [CrossRef]
- del Campo, A.; Arzt, E. Fabrication approaches for generating complex micro- and nanopatterns on polymeric surfaces. Chem. Rev. 2008, 108, 911–945. [Google Scholar] [CrossRef] [PubMed]
- Rothschild, M. Projection optical lithography. Mater. Today 2005, 8, 18–24. [Google Scholar] [CrossRef]
- Martínez-Crespiera, S.; Ionescu, E.; Schlosser, M.; Flittner, K.; Mistura, G.; Riedel, R.; Schlaak, H. Fabrication of silicon oxycarbide-based microcomponents via photolithographic and soft lithography approaches. Sens. Actuators A. 2011, 169, 242–249. [Google Scholar]
- Yang, H.; Deschatelets, P.; Brittain, S.T.; Whitesides, G.M. Fabrication of high performance ceramic microstructures from a polymeric precursor using soft lithography. Adv. Mater. 2001, 13, 54–58. [Google Scholar] [CrossRef]
- Imbesi, P.M.; Finlay, J.A.; Aldred, M.J.; Eller, S.E.; Felder, K.A.; Pollack, A.T.; Lonnecker, J.E.; Raymond, M.E.; Mackay, E.A. Targeted surface nanocomplexity: Two-dimensional control over the composition, physical properties and anti-biofouling performance of hyperbranched fluoropolymer-poly(ethylene glycol) amphiphilic crosslinked networks. Polym. Chem. 2012, 3, 3121–3131. [Google Scholar] [CrossRef]
- Li, Y.L.; Kroke, R.; Riedel, C.; Fasel, G.C.; Babonneau, F. Thermal cross-linking and pyrolytic conversion of poly(ureamethylvinyl)silazanes to silicon-based ceramics. Appl. Organomet. Chem. 2001, 15, 820–832. [Google Scholar] [CrossRef]
- Parcianello, G. Advanced ceramics from preceramic polymers and fillers. Ph.D Thesis, The University of Padua, Padova, Italy, 2012. [Google Scholar]
- Renlund, M.; Prochazka, S.; Doremus, H. Silicon oxycarbide glasses: Part II. Structure and properties. J. Mater. Res. 1991, 6, 2723–2734. [Google Scholar] [CrossRef]
- Gupta, D.; Awasthy, B.; Varma, P. Infrared characterization studies of poly-crystalline silicon annealed in a nitrogen atmosphere. J. Mater. Sci. 1993, 28, 1488–1490. [Google Scholar] [CrossRef]
- Liu, Q.; Shi, W.; Babonneau, F.; Interrante, L. Synthesis of polycarbosilane/siloxane hybrid polymers and their pyrolytic conversion to silicon oxycarbide ceramics. Chem. Mater. 1997, 9, 2434–2441. [Google Scholar] [CrossRef]
- Ma, S.; Chen, H.; Zheng, W.; Hu, F. Processing and characterization of particles reinforced Si-O-C composites via pyrolysis of polysiloxane with SiC or/and Al fillers. Ceram. Int. 2005, 31, 1045–1051. [Google Scholar] [CrossRef]
- Plummer, A.; Kuznetsov, V.; Gascooke, J.; Shapter, J.; Voelcker, N. Combined thermal and FTIR analysis of porous silicon based nano-energetic films. RSC Adv. 2017, 7(12), 7338–7345. [Google Scholar] [CrossRef]
- Kaspar, J.; Graczyk-Zajac, M.; Riedel, R. Lithium insertion into carbon-rich SiOC ceramics: Influence of pyrolysis temperature on electrochemical properties. J. Power Sources 2013, 244, 450–455. [Google Scholar] [CrossRef]
- Ionescu, E.; Terzioglu, C.; Linck, C.; Kaspar, J.; Navrotsky, A.; Riedel, R. Thermodynamic control of phase composition and crystallization of metal-modified silicon oxycarbides. J. Am. Ceram. Soc. 2013, 96, 1899–1903. [Google Scholar] [CrossRef]
- Ionescu, E.; Papendorf, B.; Kleebe, J.; Poli, F.; Müller, K.; Riedel, R. Polymer-derived silicon oxycarbide/hafnia ceramic nanocomposites. Part I: Phase and microstructure evolution during the ceramization process. J. Am. Chem. Soc. 2010, 93, 1774–1782. [Google Scholar] [CrossRef]
- Li, Z.Q.; Lu, C.J.; Xia, Z.P.; Zhou, Y.; Luo, Z. X-ray diffraction patterns of graphite and turbostratic carbon. Carbon 2007, 45, 1686–1695. [Google Scholar] [CrossRef]
- Choi, J.; Yee, A.F.; Laine, R.M. Organic/inorganic hybrid composites from cubic silsesquioxanes. epoxy resins of octa(dimethylsiloxyethylcyclohexylepoxide) silsesquioxane. Macromolecules 2003, 36, 5666–5682. [Google Scholar] [CrossRef]
- Hurwitz, I.; Heimann, P.; Farmer, C.; Hembree, M. Characterization of the pyrolytic conversion of polysilsesquioxanes to silicon oxycarbides. J. Mater. Sci. 1993, 28(24), 6622–6630. [Google Scholar] [CrossRef]
- Monthioux, M.; Delverdier, O. Thermal behavior of (organosilicon) polymer-derived ceramics. V: Main facts and trends. J. Eur. Ceram. Soc. 1996, 16, 721–737. [Google Scholar] [CrossRef]




| p(PDMS-co-AMS) | Al2O3/ p(PDMS-co-AMS) | |
|---|---|---|
| Thickness (nm) | 18.1 ± 0.1 | 20.8 ± 1.4 |
| Sample | Tg (°C) | Ton (°C) | Tend (°C) | Tm (°C) |
|---|---|---|---|---|
| Before Pyrolysis | 978 | 871 | 1020 | 1146 |
| 900°C | 898 | 838 | 960 | 1116 |
| 1000°C | 922 | 834 | 987 | 1111 |
| 1100°C | 905 | 844 | 963 | 1122 |
| 1200°C | 890 | 830 | 933 | 1119 |
| 1300°C | 919 | 846 | 961 | 1114 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almeataq, M.S.; Alosime, E.M. Synthesis Based on a Preceramic Polymer and Alumina Nanoparticles via UV Lithography for High Temperature Applications. Materials 2020, 13, 1140. https://doi.org/10.3390/ma13051140
Almeataq MS, Alosime EM. Synthesis Based on a Preceramic Polymer and Alumina Nanoparticles via UV Lithography for High Temperature Applications. Materials. 2020; 13(5):1140. https://doi.org/10.3390/ma13051140
Chicago/Turabian StyleAlmeataq, Mohammed S., and Eid M. Alosime. 2020. "Synthesis Based on a Preceramic Polymer and Alumina Nanoparticles via UV Lithography for High Temperature Applications" Materials 13, no. 5: 1140. https://doi.org/10.3390/ma13051140
APA StyleAlmeataq, M. S., & Alosime, E. M. (2020). Synthesis Based on a Preceramic Polymer and Alumina Nanoparticles via UV Lithography for High Temperature Applications. Materials, 13(5), 1140. https://doi.org/10.3390/ma13051140






