1. Introduction
Water phase transition in the mortar pore space has long been in the scope of interest and has been investigated by using numerous methods. Differential scanning calorimetry (DSC) seems to be a particularly well-suited technique for this purpose. Thermoporometry through the use of the DSC has been widely applied to study the influence of curing conditions [
1,
2,
3], admixtures [
4], additives [
5] and the types of cement [
6] that are used on the freezing process in cement-based materials. Thermograms and the latent heat of fusion provide information for calculating the content of ice at specific temperatures, the volume of ice in pores, and pore size distribution (only those filled with water). By investigating the amount of heat that is released/supplied to the system at a given temperature, it is possible to determine the pore size distribution, especially over the range between 2 and 50 nm. The theoretical principles of thermoporometry were thoroughly described by Brun et al. [
7].
Between the liquid and solid phases in the pore space, there is a curved interphase. The curvature of the meniscus reduces the free energy of the liquid phase and consequently lowers the temperature of the phase transition. The relationship between the phase transition temperature and the radius of the pore with the trapped liquid is described by Equation (1):
where
rK is the curvature of the solid–liquid interface;
T0 is the freezing temperature of the bulk liquid;
K,
T is the pore liquid phase transition temperature;
K,
M is the molar mass of the liquid, kg/mol;
ΔHfus is the heat of fusion, J/mol; and
ρl is the density of the liquid, kg/m
3.
The known values of surface tension and absolute free entropy allow for the obtainment of the relation between the pore radius and the phase transition temperature depression. The task of determining such a relation on the basis of available data for particular physical quantities was undertaken by Ishikiriyama [
8,
9,
10], Brun et al. [
7], Fagerlund [
11], and Landry [
12].
Thermoporometry, similarly to other pore space research methods, has its limitations. The presence of curved gas–ice and gas–liquid interphases affects the recorded thermograms [
13,
14] and their interpretation. The time and manner of soaking, as well as the occurrence of hydrophobic groups in investigated materials, may affect the degree of pore filling [
15]. In thermoporometry, the pore space needs to be fully saturated for only a water–ice interface to occur. The most common liquid that is used in this method is distilled water. Compared to other liquids, e.g., benzene [
16], it has a relatively high heat of fusion that positively influences the relation of the recorded signal to the "apparatus noise." It may be impossible to amplify the recorded signal with respect to the baseline. This problem can be solved with the use a derivative scanning calorimetry (DeSC). Derivative scanning calorimeters allow for direct access to minor thermal events that are hidden in the signal-to noise-ratio of classical DSCs [
17] due to the special design of pans and the generation of the temperature difference between the cells.
At a fixed scanning rate of calorimetric tests, the signals recorded lag behind the actual temperatures at which the processes take place. The discrepancy between the temperature inside the sample and the temperature sensor increases with an increase in the scanning rate [
12,
18]. When thermoporometry based on these signals is used to calculate the pore radius distribution for ice melting within the pore space, and the delays due to measuring system thermal inertia can significantly affect the results.
The influence of inertia can be reduced by lowering the cooling or heating rate. However, even at a small scanning rate, there is always a certain temperature shift of the recorded signals in relation to the temperatures at which the exo- or endothermic processes occur. Another approach to eliminate the influence of thermal inertia on the temperature of recorded effects is the quasi-isothermal mode (QI-MDSC) in differential scanning calorimetry [
19], which allows for the assignment of the obtained results to temperatures. This method is able to handle samples of the order of milligrams in which temperature gradients are small. It is impossible to prepare small representative samples for the examination of macroscopically heterogeneous materials such as cement mortars, concrete, and rock by QI-MDSC. In the case of measuring instruments in which there is a significant difference between the temperature of the external casing (which is responsible for maintaining the programmed temperature) and the central part of the calorimeter in which the samples (such as in the tests described in this paper) are placed, the use of this method would require a considerable extension of the time of the experiment.
An important element that is necessary to make corrections that are related to the thermal inertia of the measuring system when using constant heating or cooling rates is the knowledge of the so-called apparatus function
a(
Ei,heat). In calorimetric tests, the apparatus function allows for the determination of the value of the recorded signal over designated temperature ranges, when the signal is triggered by a single thermal pulse, which is understood as the amount of energy that is released or absorbed by the sample within a very narrow temperature range. Kozłowski [
20,
21] discussed the application of apparatus functions in the study of the phase transition of water in water–clay systems. In his works, a single apparatus function was used, regardless of the energy pulse value. The total energy of phase transition was broken down into equal components, and appropriate combinations of these components were analyzed, in particular temperature ranges. The result of the calculations was the energy distribution for which the difference between the experimentally determined curve and the curve that was obtained from the convolution of energy distribution with the apparatus function was as small as possible. The idea of Kozłowski’s solution was used to construct modified algorithm. In studies of calorimetric phase transitions of water confined in the pore space of materials such as concrete, mortar, or paste, the effects to thermal inertia of the measuring system have been omitted. Hence, the proposal of the algorithm based on consecutive approximations in which a separate apparatus function is determined for each thermal pulse.
2. Apparatus Function
The experimental determination of calorimetric curves is possible through the use of a material in which thermal effects that result from water melting inside the pore space occur within a very narrow temperature range. When this condition is met, the recorded signal is assumed to originate from a single thermal pulse. Because of the spontaneous, random nature of the initial stage of water freezing in the material and the temperature at which the process begins, the signals that are recorded during the ice melting can be used to analyze the volume and dimensions of the pores. In addition, it is known that there may be pore groups with dimensions below 50 nm in which water does not freeze according to the Gibbs–Thomson rule, though spontaneous nucleation is already complete [
22]. Only by examining the melting process can the size of the ice-filled pores be correctly assessed. Here, the apparatus functions were experimentally determined for five porous ceramic materials in which more than 97% of the pore volume was made up of pores with dimensions above 0.1 µm (using mercury intrusion porosimetry MIP).
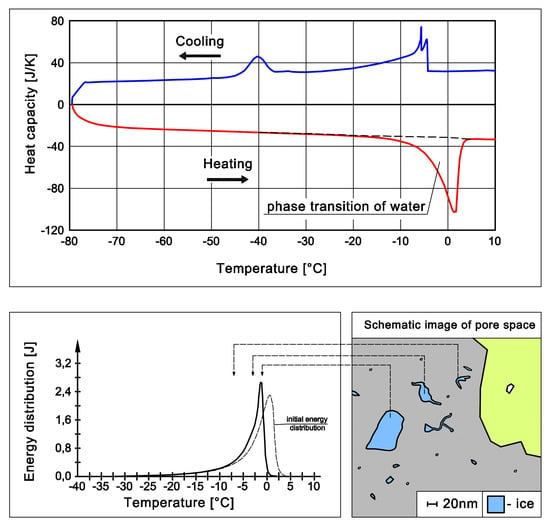
Figure 1 shows the values of the recorded thermal effects. The tests were carried out on cylindrical samples (ø13.5 × 70 mm), and which were vacuum saturated with water. The scanning rate was 0.09 C/min. Ice in the pores of the samples melted in a relatively narrow temperature range close to zero degrees Celsius. Therefore, it was possible to assume that the observed heat fluxes were the result of transition processes, which can be referred to as single thermal pulses. In the presented analyses, the temperature range corresponding to the hypothetical “single thermal pulse” was assumed to be 0.05 °C.
The curves of the thermal capacity
φ(
T) (
Figure 1) were divided into 0.05 °C-wide segments, and the values were determined in the center of each segment/interval
φi. The
φ(
T) curves provided apparatus functions according to the following equation:
where
ai is the value of apparatus function in the
i-th interval,
In this way, five apparatus functions were obtained from the φ(T) curves for SAMPLES 1–5, corresponding to their total phase transition energies E
sum (
Table 1).
For the calculations presented for cement mortar, the basic issue is the knowledge of apparatus functions that are generated by single thermal pulses with a total energy amount of less than 193.8 J (
Figure 2).
In order to obtain any apparatus function (corresponding to the thermal pulse of
Esum of less than 193.8 J) the curve of thermal capacity
φ1(
T) for SAMPLE 1 (
Figure 1) was transformed according to the equation below.
where
A is the variable responsible for the vertical translation of apparatus function,
B is the variable that is responsible for the horizontal translation of apparatus function, and
T is temperature.
With the known plot φ(T) for the given pulse energy Esum, it is possible to determine the apparatus function in accordance with Equation (1).
Variable A is the ratio of the maximum value on the
φ(
T) plot that is triggered by a given heat pulse to the maximum signal value
φ1(
T1,max) for SAMPLE 1 (
Figure 1). To determine the Variable A equation, the values of Variable A were determined for ceramic SAMPLES 1–5, and the quadratic trendline was produced (
Figure 3).
Variable B value is the ratio of the maximum peak temperature on the
φ(
T) graph, triggered by a given thermal pulse, to the maximum peak temperature
T1max of SAMPLE 1 (
Figure 1). The procedure was the same as for Variable A; the values of Variable B were determined for ceramic SAMPLES 1–5 (points B
1–B
5 in
Figure 4), and the quadratic trend line was produced for them (
Figure 4).
The quadratic equations were used for the A and B trendline formulas. The authors of this paper are aware that for the extrapolated energy area from 0 to 193.8 J, there may be another relation for Variables A and B. Nevertheless, at that stage of analysis, the formulas above were used in further calculations.
The selection of the apparatus function depends not only on the amount of energy of the given thermal impulse for which the function is defined but also on the values of neighboring thermal impulses and the "distance" between them. Whether the thermal impulses influence each other depends on their position with respect to each other on along the temperature axis, which is expressed as ΔT
sig. In the case of very close location of two peaks (ΔT
sig ≈ 0), the recorded effect is the same as in the case of a single peak whose apparatus function is selected on the basis of their cumulative energy (
Figure 5). Thus, when determining the apparatus function of a given thermal pulse, one should take into account not only the amount of energy of this peak but also a certain percentage of the value of neighboring peaks, where this value depends on ΔT
sig.
Note that the assumed width of the ΔT intervals affects the value of energy, in particular the intervals of the energy distribution plot, and thus influences both the apparatus function that is assigned to them and the resolution of the calculations (
Figure 6).
The following assumptions related to the apparatus function were made:
- Temperature functions have the same shape regardless of the temperature at which the thermal pulse is located (i.e., the only variable on the basis of which the apparatus function is selected is the value of the thermal pulse expressed in joules of energy).
- Apparatus functions that are obtained on the basis of ceramic sample tests may be used for testing other materials (thermal conductivity of the skeleton of the sample is negligible).
3. Algorithm Based on the Thermal Inertia of the Measurement System
Since the calorimetric curve depends on the value of the thermal pulse, which is unknown at the beginning of calculations, an original algorithm that involves approximations of the true distribution of thermal pulses and the corresponding apparatus functions was used.
The first step was to divide the recorded signal into segments 0.05 K in width and calculate the amount of energy in each segment (
Ei,heat-event) according to
Figure 7. As a result, a series of single thermal pulses with (
Ei,heat-event) that were assigned to specific temperatures (initial energy distribution) was obtained.
Apparatus functions
a(
Ei,heat) were determined for each single pulse (
Figure 8) according to Equation (4). Knowing that the selection of the apparatus function for a given pulse (
Ei,heat-event) depends on the values of neighboring pulses, the energy on the basis of which the apparatus function was determined is the sum of energies of the
i-th thermal pulse, its preceding pulse, and the one that followed, according to Equation (6):
Then, on the basis of the initial energy distribution, the shifted energy distribution was calculated from Equation (7), as shown in
Figure 9.
Finally, the differences between the initial and the shifted energy distributions were determined (
Figure 10).
With the energy differences and the known the shifted energy distribution, it was possible to, respectively, increase or decrease individual heat pulses according to Equation (9) and to obtain the first approximation of the energy distribution (
Figure 11).
where
w = 0.1.
In subsequent cycles, Ei,aprox was used to compute the shifted energy distribution by comparing it to the initial energy distribution.
With consecutive cycles, the values of Ei,aprox became closer to the true values, and, in this way, the effect of the thermal inertia of the measurement system could be eliminated and the effect related to water melting at individual temperatures could be separated.
4. Calculations
Appendix A describes the procedure for the phase change energy calculation from a calorimetric signal.
When the spontaneous nucleation ends, the freezing process depends on the pore neck sizes [
14]. Melting provides information about the pore internal sizes. The studies of Homeshaw et al. [
23] and Kjeldsen et al. [
18] showed that heating thermograms ensure a better assessment of the pore size distribution than cooling curves. In this paper, only some of the thermal effects that are associated with ice melting were used to investigate the pore space. To calculate the volume of pores in which ice melts at a given temperature, the latent heat
L(
T) (10) and ice density
ρice (11) must be known. In this paper, the values given by Sun and Scherer [
22] for ice melting in a cylindrical pore were used:
where
T is pore liquid phase transition temperature and
K,
T0 is the freezing temperature of the bulk liquid.
The mass of ice melting at a given temperature interval
Δm(
T) can be obtained from the following equation:
where
L(
Ti) is the latent heat value at temperature
Ti.
The volume of the melting ice at a given temperature was calculated from the formula:
The equations above, when using the known values of both the exact phase transition temperature and the amount of energy, enabled the determination of the pore size distribution. In this paper, the equation proposed by Brun et al. [
7] for ice melting (14) in a hypothetical cylindrical pore was used:
Water close to pore walls does not freeze. The thickness of molecular layer that does not solidify was assumed to be equal
δ = 0.8 nm. The volume of the pore was calculated from Equation (15):
For cylindrical pores, n = 2.
6. Results
Figure 12 shows the change in energy distribution when the algorithm (
Section 3), which took the thermal inertia of the measuring system into account, was used. Even small energy shifts over the temperature range from −2 to 0 °C affected the radius to which a given amount of energy was assigned and hence, the calculated pore size distributions. Note that a portion of the energy was still assigned to temperatures greater than 0 °C.
Assigning the recorded signals to lower temperatures caused the corresponding amounts of energy to be divided by the lower value of the latent heat, and, thus, the calculations gave a higher total mass of ice. On basis of the initial energy distribution, the mass of ice equaled 1.08 g and increased to 1.09 g after the application of the algorithm.
The second factor that influenced the difference between the total volume of pores without accounting for thermal inertia of the measuring system and after applying the algorithm was the assumed volume of a film of non-freezable water, which was strongly adsorbed on pore wall surfaces. The relative portion of unfrozen water increased with the decrease in pore size (
Figure 13).
Thus, the calculated pore volume increased proportionally to the increase in the amount of adsorbed water (
Table 2). The example of the studied cement mortar showed that by taking the apparatus function into account, the decrease in the calculated volume of pores that were larger than 20 nm nearly doubled (
Figure 14 and
Figure 15).
7. Conclusions
If the process of the reasoning underlying the calculation procedure with the algorithm that accounts for the thermal inertia of the measurement system is correct, the size of pores and their distribution in cement mortar can be described with much greater precision.
In the case of the tested CEMII/B-V mortar (w/c = 0.53), 38.6% of the total ice–water energy of phase transition Esum was recorded over the temperature range above 0 °C. This is obviously a result that diverges from physical knowledge. When the energy distribution was used without taking the proposed modified algorithm into account, the volume of the relatively largest pores was greatly overestimated, while that of the smaller pores (rp < 20 nm) was underestimated, in some cases by 70%.
The proposed calculation formula can be used to analyze pore structure in other capillary and porous materials.