Esthetic and Physical Changes of Innovative Titanium Surface Properties Obtained with Laser Technology
Abstract
1. Introduction
2. Materials and Methods
Surface Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1211–1259.
- Branemark, P.I.; Svensson, B.; van Steenberghe, D. Ten-year survival rates of fixed prostheses on four or six implants ad modum Branemark in full edentulism. Clin. Oral Implant. Res. 1995, 6, 227–231. [Google Scholar] [CrossRef] [PubMed]
- ADA Coucil on Scientific Affairs. Dental endosseous implants: An update. J. Am. Dent. Assoc. 2004, 135, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Sul, Y. The significance of the surface properties of oxidized titanium to the bone response: Special emphasis on potential biochemical bonding of oxidized titanium implant. Biomaterials 2003, 24, 3893–3907. [Google Scholar] [CrossRef]
- Yang, T.-C.; Shu, H.-Y.; Chen, H.-T.; Chung, C.-J.; He, J.-L. Interface between grown osteoblast and micro-arc oxidized bioactive layers. Surf. Coat. Technol. 2014, 259, 185–192. [Google Scholar] [CrossRef]
- El Askary, A.S.; Meffert, R.M.; Griffin, T. Why do dental implants fail? Part I. Implant. Dent. 1999, 8, 173–185. [Google Scholar] [CrossRef]
- Higginbottom, F.L. Implants as an option in the esthetic zone. J. Oral Maxillofac. Surg. 2005, 63, 33–44. [Google Scholar] [CrossRef]
- Paolantoni, G.; Marenzi, G.; Blasi, A.; Mignogna, J.; Sammartino, G. Findings of a Four-Year Randomized Controlled Clinical Trial Comparing Two-Piece and One-Piece Zirconia Abutments Supporting Single Prosthetic Restorations in Maxillary Anterior Region. Biomed. Res. Int. 2016, 2016, 8767845. [Google Scholar] [CrossRef][Green Version]
- Chang, M.; Odman, P.A.; Wennstrom, J.L.; Andersson, B. Esthetic outcome of implant supported single-tooth replacements assessed by the patient and by prosthodontists. Int. J. Prosthodont. 1999, 12, 335–341. [Google Scholar]
- Papaspyridakos, P.; Chen, C.J.; Singh, M.; Weber, H.P.; Gallucci, C.O. Success criteria in implant dentistry: A systematic review. J. Dent. Res. 2012, 91, 242–248. [Google Scholar] [CrossRef]
- Dawson, A.; Chen, S.; Buser, D. The SAC Classification in Implant Dentistry; Quintessence: Berlin, Germany, 2009. [Google Scholar]
- Fuentealba, R.; Jofrè, J. Esthetic failure in implant dentistry. Dent. Clin. N. Am. 2015, 59, 227–246. [Google Scholar] [CrossRef] [PubMed]
- Napoli, G.; Paura, M.; Vela, T.; Di Schino, A. Colouring titanium alloys by anodic oxidation. Metal. Sisak Zagreb 2018, 57, 111. [Google Scholar]
- Oleari, C. Colour in optical coatings. In Optical Thin Films and Coatings: From Materials to Applications; Piegari, A., Flory, F., Eds.; Woodhead Publishing Limited: Cambridge, UK, 2013; pp. 391–424. [Google Scholar]
- Rajab, F.H.; Liauw, C.M.; Benson, P.S.; Li, L.; Whitehead, K.A. Production of hybrid macro/micro/nano surface structures on Ti6Al4V surfaces by picosecond laser surface texturing and their antifouling characteristics. Colloids Surf. B Biointerfaces 2017, 160, 688–696. [Google Scholar] [CrossRef]
- Vanhumbeeck, J.F.; Proost, J. Current understanding of Ti anodization: Functional, morphological, chemical and mechanical aspects. Corros. Rev. 2009, 27, 117–194. [Google Scholar] [CrossRef]
- Diamanti, M.V.; Del Curto, B.; Masconale, V.; Passaro, C.; Pedeferri, M.P. Anodic colouring of titanium and its alloy for jewels production. Colour. Res. Appl. 2012, 37, 384–390. [Google Scholar] [CrossRef]
- Albrektsson, T.; Wennerberg, A. On osseointegration in relation to implant surfaces. Clin. Implant. Dent. Relat. Res. 2019, 21, 4–7. [Google Scholar] [CrossRef]
- Scarano, A.; Piattelli, A.; Quaranta, A.; Lorusso, F. Bone Response to Two Dental Implants with Different Sandblasted/Acid-Etched Implant Surfaces: A Histological and Histomorphometrical Study in Rabbits. Biomed. Res. Int. 2017, 2017, 1–8. [Google Scholar] [CrossRef]
- Rupp, F.; Liang, L.; Geis-Gerstorfer, J.; Scheideler, L.; Hüttig, F. Surface characteristics of dental implants: A review. Dent. Mater. 2018, 34, 40–57. [Google Scholar] [CrossRef]
- Tetè, S.; Mastrangelo, F.; Traini, T.; Vinci, R.; Sammartino, G.; Marenzi, G.; Gherlone, E. A macro- and nanostructure evaluation of a novel dental implant. Implant. Dent. 2008, 17, 309–320. [Google Scholar] [CrossRef]
- Wang, T.; Wang, L.; Lu, Q.; Fan, Z. Changes in the esthetic, physical, and biological properties of a titanium alloy abutment treated by anodic oxidation. J. Prosthet. Dent. 2019, 121, 156–165. [Google Scholar] [CrossRef]
- Chen, J.; Mwenifumbo, S.; Langhammer, C.; McGovern, J.P.; Li, M.; Beye, A.; Soboyejo, W.O. Cell/surface interactions and adhesion on Ti-6Al-4V: Effects of surface texture. J. Biomed. Mater. Res. B Appl. Biomater. 2007, 82, 360–373. [Google Scholar] [CrossRef] [PubMed]
- Tu, Z.M.; Zhu, Y.M.; Li, N.; Hu, H.L.; Cao, L.X. Applications and Advances on surface treatment for titanium and titanium alloy. Surf. Technol. 2009, 38, 76–77. [Google Scholar]
- Liu, J.; Alfantazi, A.; Asselin, E. A new method to improve the corrosion resistance of titanium for hydrometallurgical applications. Appl. Surf. Sci. 2015, 332, 480–487. [Google Scholar] [CrossRef]
- Lim, H.P.; Lee, K.M.; Koh, Y.I.; Park, S.W. Allergic contact stomatitis caused by a titanium nitride-coated implant abutment: A clinical report. J. Prosthetic. Dent. 2012, 108, 209–213. [Google Scholar] [CrossRef]
- Wadhwani, C.; Brindis, M.; Kattadiyil, M.; O’Brien, R.; Chung, K.H. Colourizing titanium-6aluminum-4vanadium alloy using electrochemical anodization: Developing a colour chart. J. Prosthet. Dent. 2018, 119, 26–28. [Google Scholar] [CrossRef]
- Karambakhsh, A.; Afshar, A.; Ghahramani, S.; Malekinejad, P. Pure commercial titanium colour anodizing and corrosion resistance. J. Mater. Eng. Perform. 2011, 20, 1690–1696. [Google Scholar] [CrossRef]
- Al-Nawas, B.; Hangen, U.; Duschner, H.; Krummenauer, F.; Wagner, W. Turned, machined versus double-etched dental implants in vivo. Clin. Implant. Dent. Relat. Res. 2007, 9, 71–78. [Google Scholar] [CrossRef]
- Tetè, S.; Mastrangelo, F.; Quaresima, R.; Vinci, R.; Sammartino, G.; Stuppia, L.; Gherlone, E. Influence of novel nano-titanium implant surface on human osteoblast behavior and growth. Implant. Dent. 2010, 19, 520–531. [Google Scholar] [CrossRef]
- Belser, U.C.; Schmid, B.; Higginbottom, F.; Buser, D. Outcome analysis of implant restorations located in the anterior maxilla: A review of the recent literature. Int. J. Oral Maxillofac. Implant. 2004, 19, 30–42. [Google Scholar]
- Marenzi, G.; Impero, F.; Scherillo, F.; Sammartino, J.C.; Squillace, A.; Spagnuolo, G. Effect of Different Surface Treatments on Titanium Dental Implant Micro-Morphology. Materials 2019, 12, 733. [Google Scholar] [CrossRef]

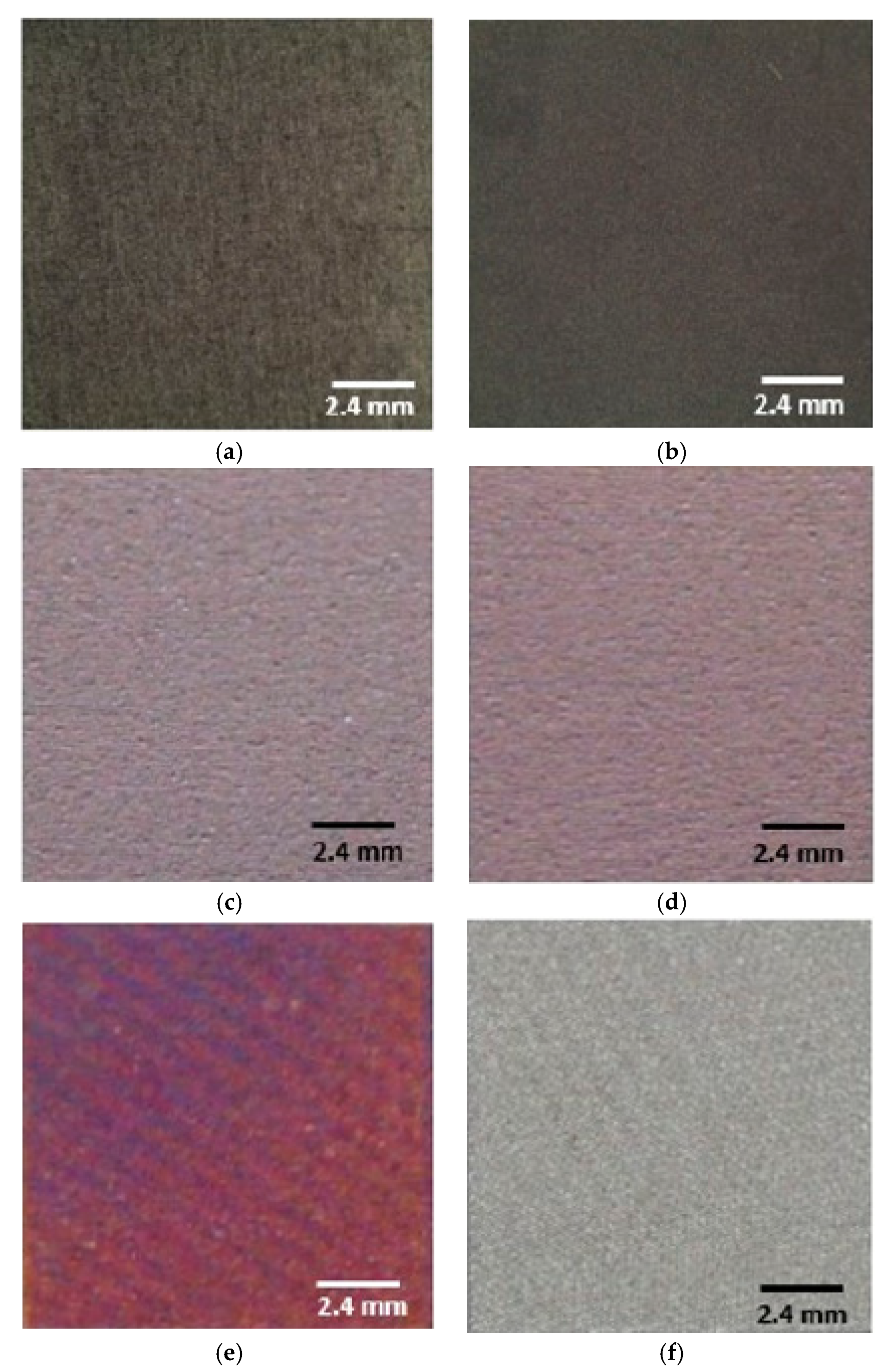

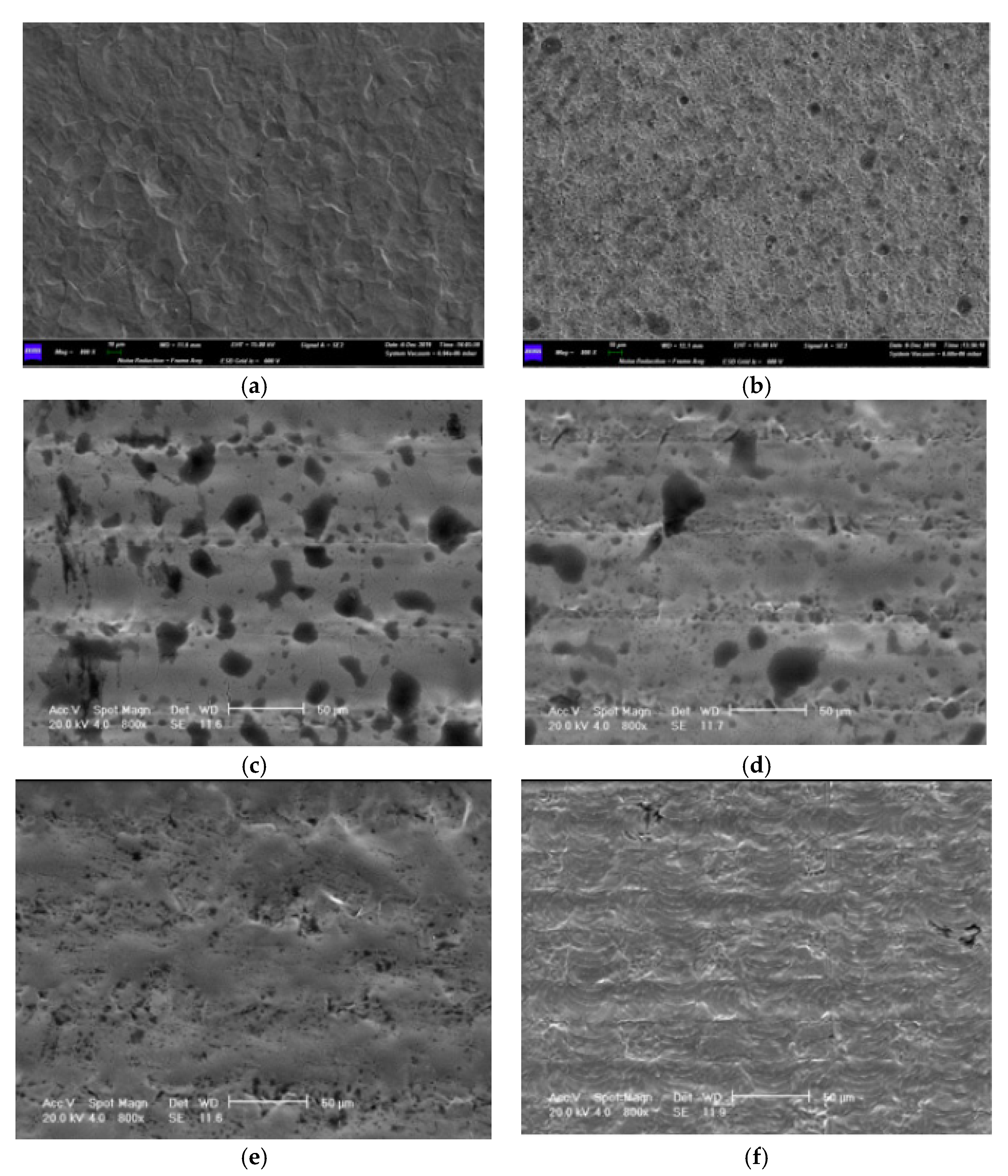
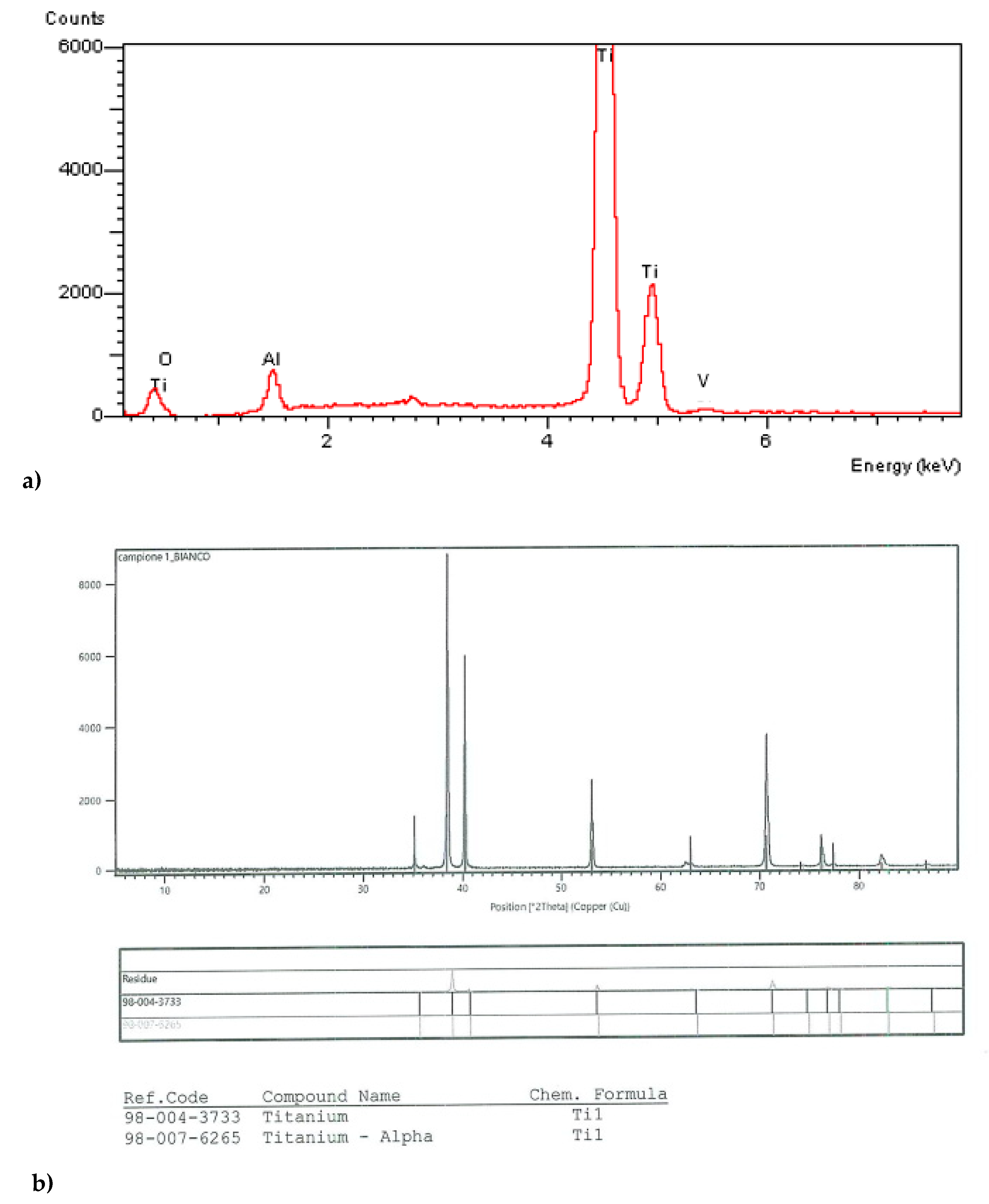
| Sample | L* | a* | b* | ΔE |
|---|---|---|---|---|
| M | 67.64 | −0.39 | −0.99 | - |
| SBAE | 65.47 | −0.49 | −0.89 | 2.17 |
| P1 | 88.42 | +45.34 | +39.21 | 64.30 |
| P2 | 90.65 | +43.72 | +37.31 | 62.75 |
| I | 34.27 | +44.53 | −21.92 | 59.80 |
| W | 96.31 | +2.45 | −10.91 | 30.69 |
| Sample | Ra | Rq | Rz | Ry | Sm |
|---|---|---|---|---|---|
| M | 0.32 (±0.02) | 0.47 (±0.03) | 2.21 (±0.19) | 2.74 (±0.15) | 53 (±4.86) |
| SBAE | 1.41 (±0.19) | 21.92 ±1.31) | 12.87 (±1.39) | 2.52 (±0.13) | 83 (±8.73) |
| P1 | 0.44 (±0.02) | 0.58 (±0.03) | 3.24 (±0.27) | 1.93 (±0.10) | 54 (±4.99) |
| P2 | 0.54 (±0.03) | 0.72 (±0.05) | 3.86 (±0.36) | 2.61 (±0.14) | 66 (±6.96) |
| I | 0.52 (±0.03) | 0.67 (±0.04) | 3.47 (±0.31) | 2.14 (±0.12) | 66 (±6. 31) |
| W | 0.32 (±0.02) | 0.41 (±0.02) | 2.21 (±0.18) | 1.21 (±0.12) | 37 (±4.01) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mastrangelo, F.; Quaresima, R.; Abundo, R.; Spagnuolo, G.; Marenzi, G. Esthetic and Physical Changes of Innovative Titanium Surface Properties Obtained with Laser Technology. Materials 2020, 13, 1066. https://doi.org/10.3390/ma13051066
Mastrangelo F, Quaresima R, Abundo R, Spagnuolo G, Marenzi G. Esthetic and Physical Changes of Innovative Titanium Surface Properties Obtained with Laser Technology. Materials. 2020; 13(5):1066. https://doi.org/10.3390/ma13051066
Chicago/Turabian StyleMastrangelo, Filiberto, Raimondo Quaresima, Roberto Abundo, Gianrico Spagnuolo, and Gaetano Marenzi. 2020. "Esthetic and Physical Changes of Innovative Titanium Surface Properties Obtained with Laser Technology" Materials 13, no. 5: 1066. https://doi.org/10.3390/ma13051066
APA StyleMastrangelo, F., Quaresima, R., Abundo, R., Spagnuolo, G., & Marenzi, G. (2020). Esthetic and Physical Changes of Innovative Titanium Surface Properties Obtained with Laser Technology. Materials, 13(5), 1066. https://doi.org/10.3390/ma13051066








