Study of Mechanical Properties and Durability of Alkali-Activated Coal Gangue-Slag Concrete
Abstract
1. Introduction
2. Materials and Methodology
2.1. Materials
2.2. Mix Proportion and Specimen Preparation
2.3. Methods
2.3.1. Compressive Strength
2.3.2. RCM Tests
2.3.3. Freeze-Thaw Cycles
2.3.4. Microstructural Characterization of ACSC
3. Results and Discussion
3.1. Compressive Strength Analysis
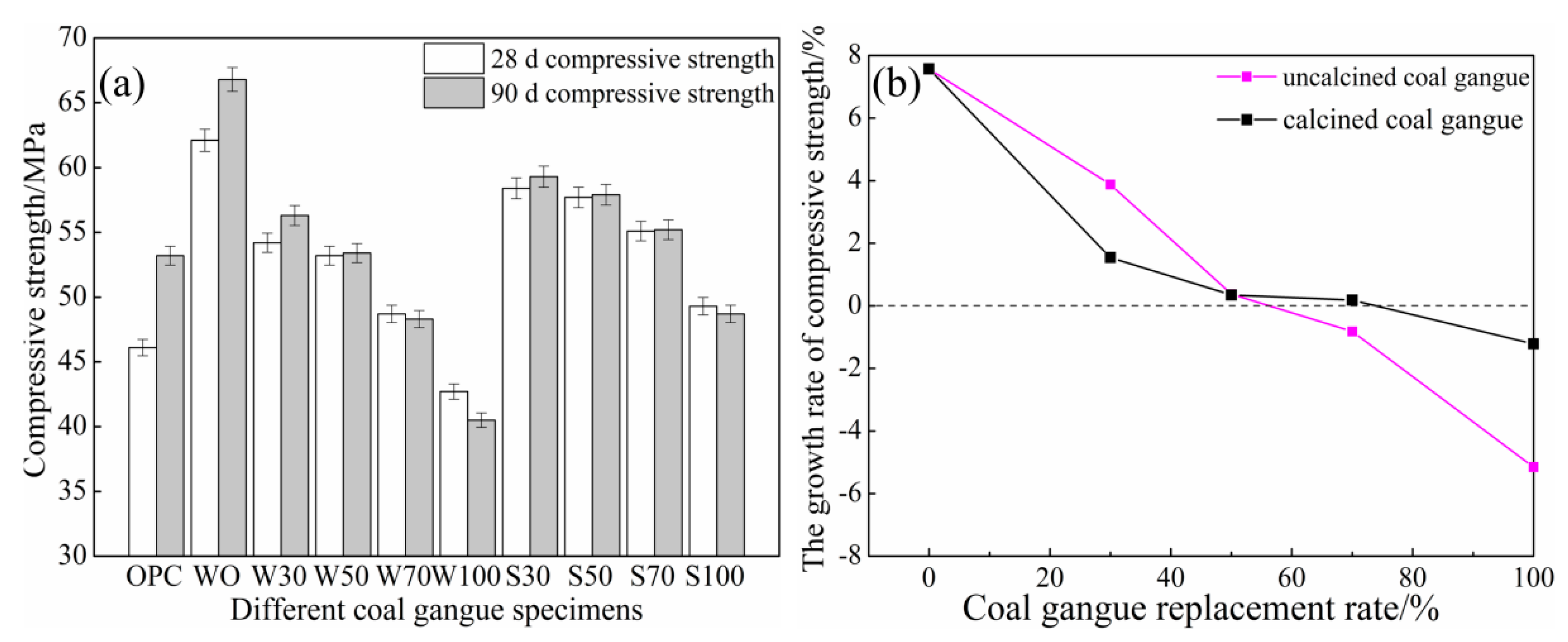
3.1.1. 28 d and 90 d Compressive Strength
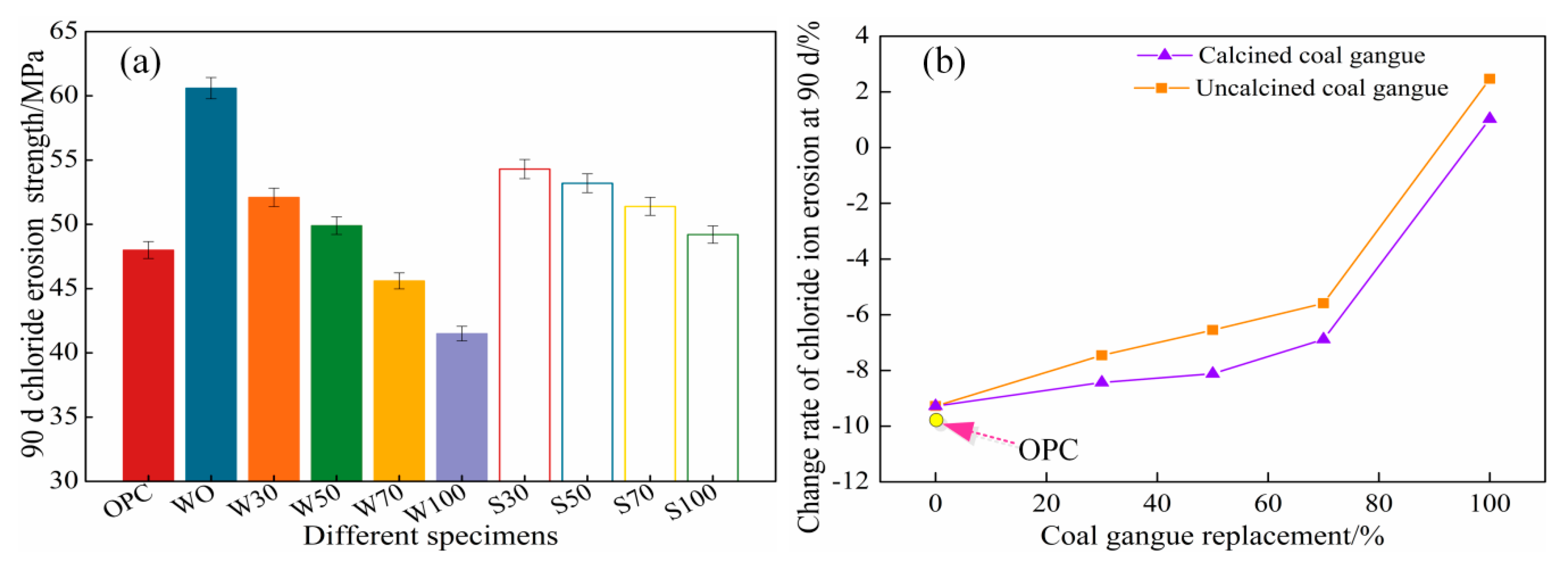
3.1.2. 90 d Chloride Salt Erosion Compressive Strength
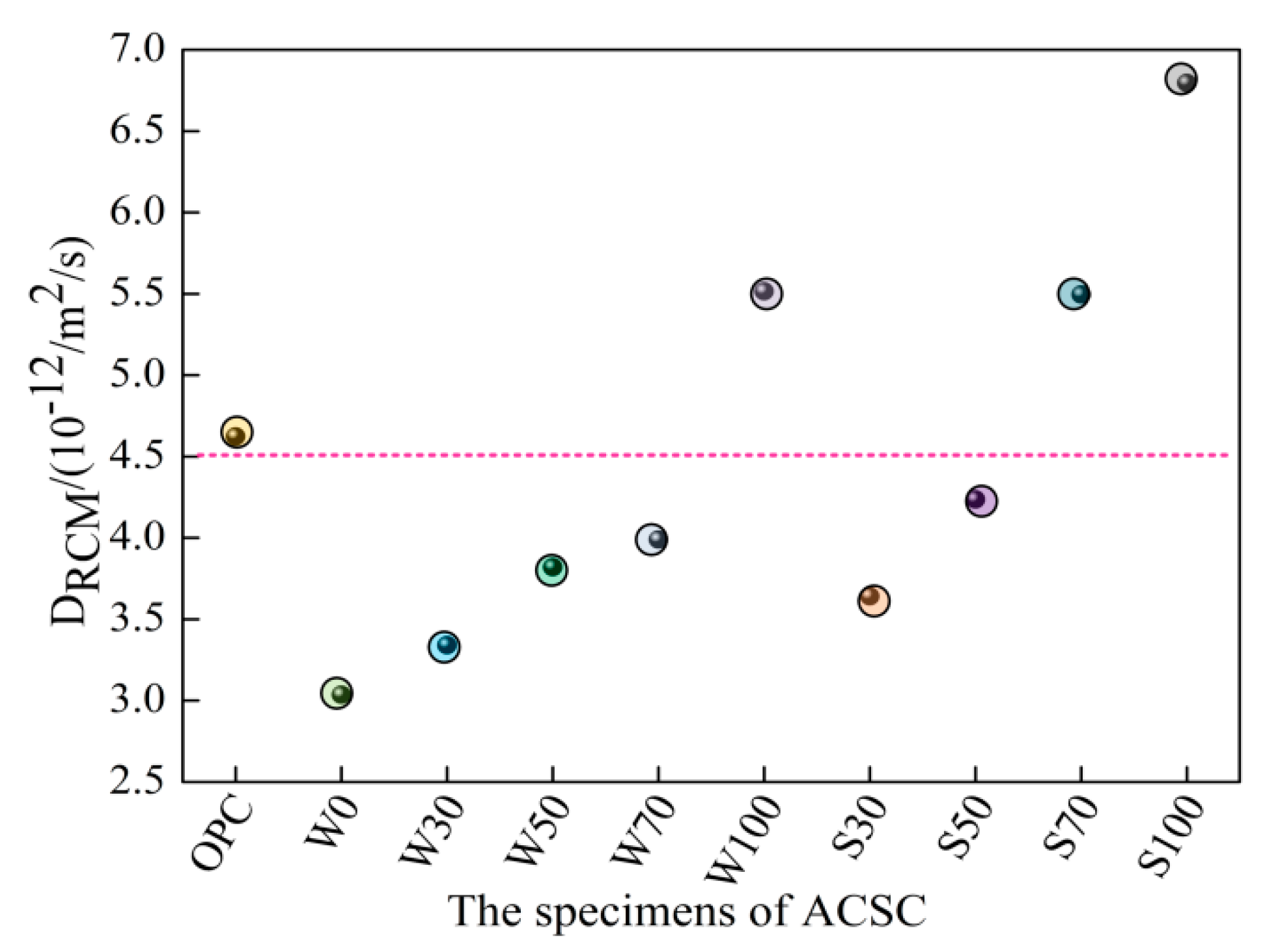
3.2. Chloride Permeation
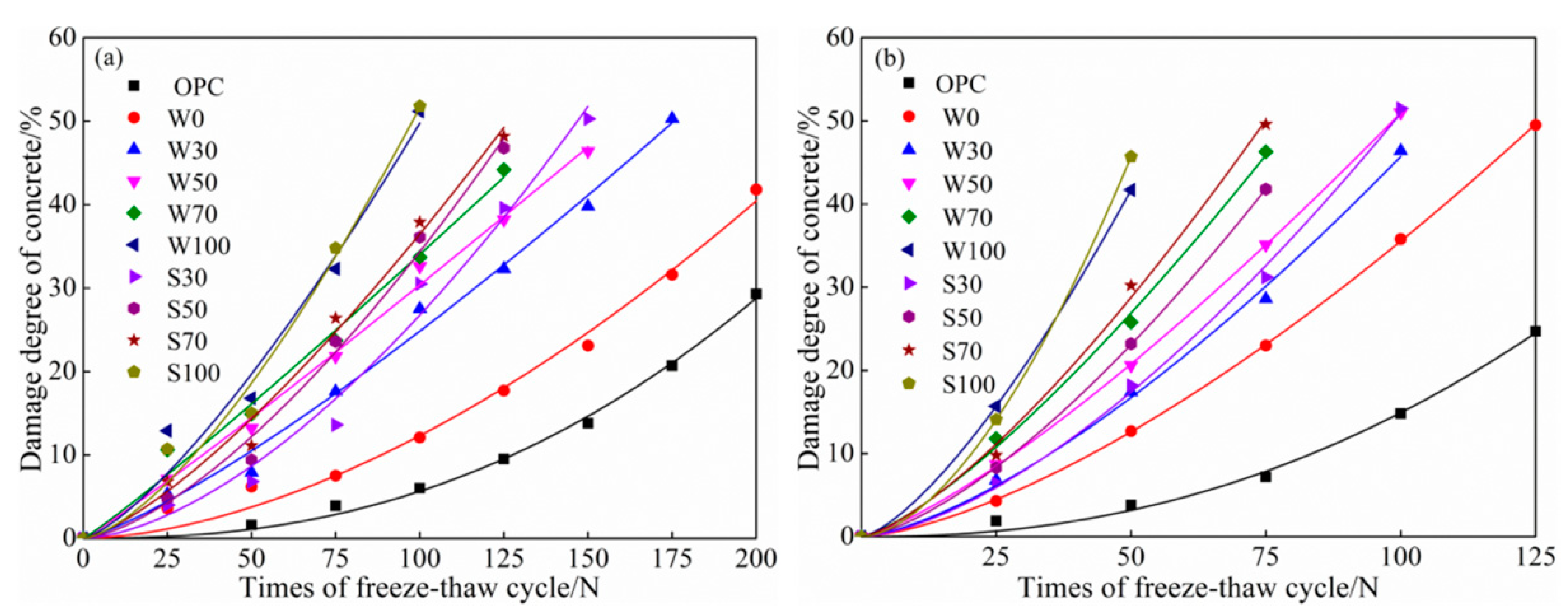
3.3. Freeze-Thaw Cycles
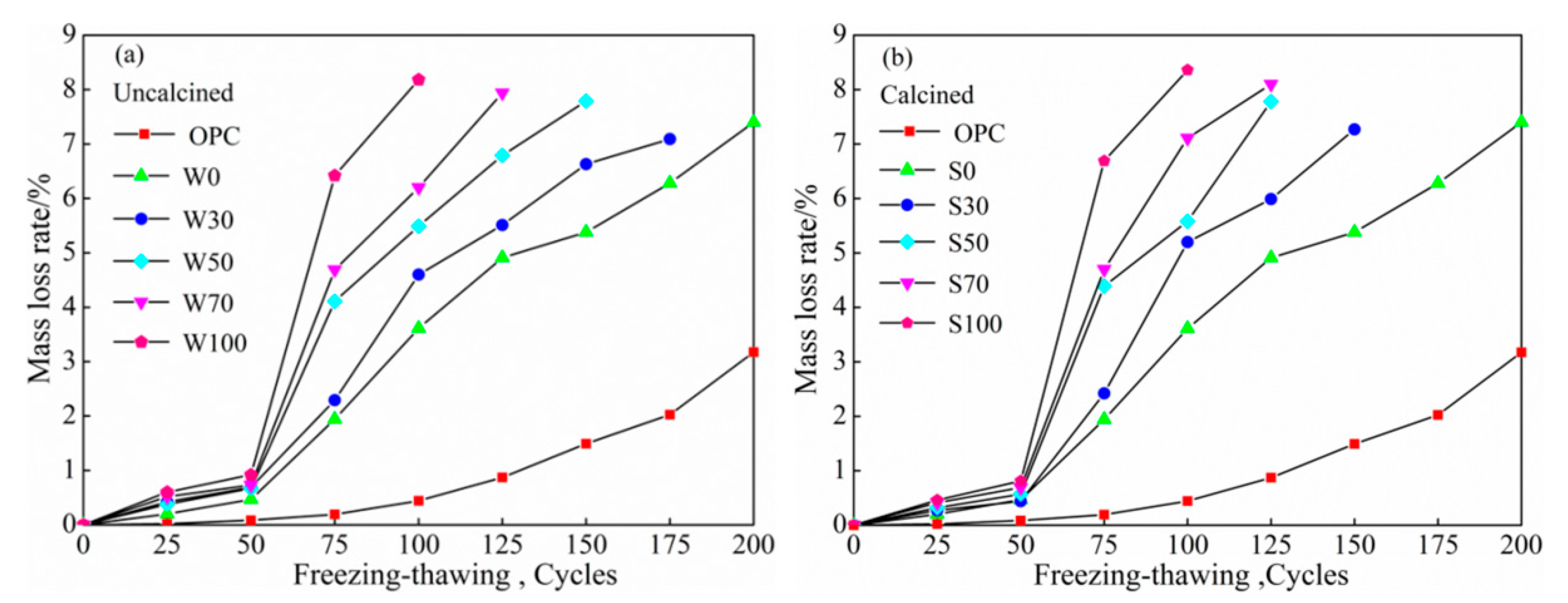
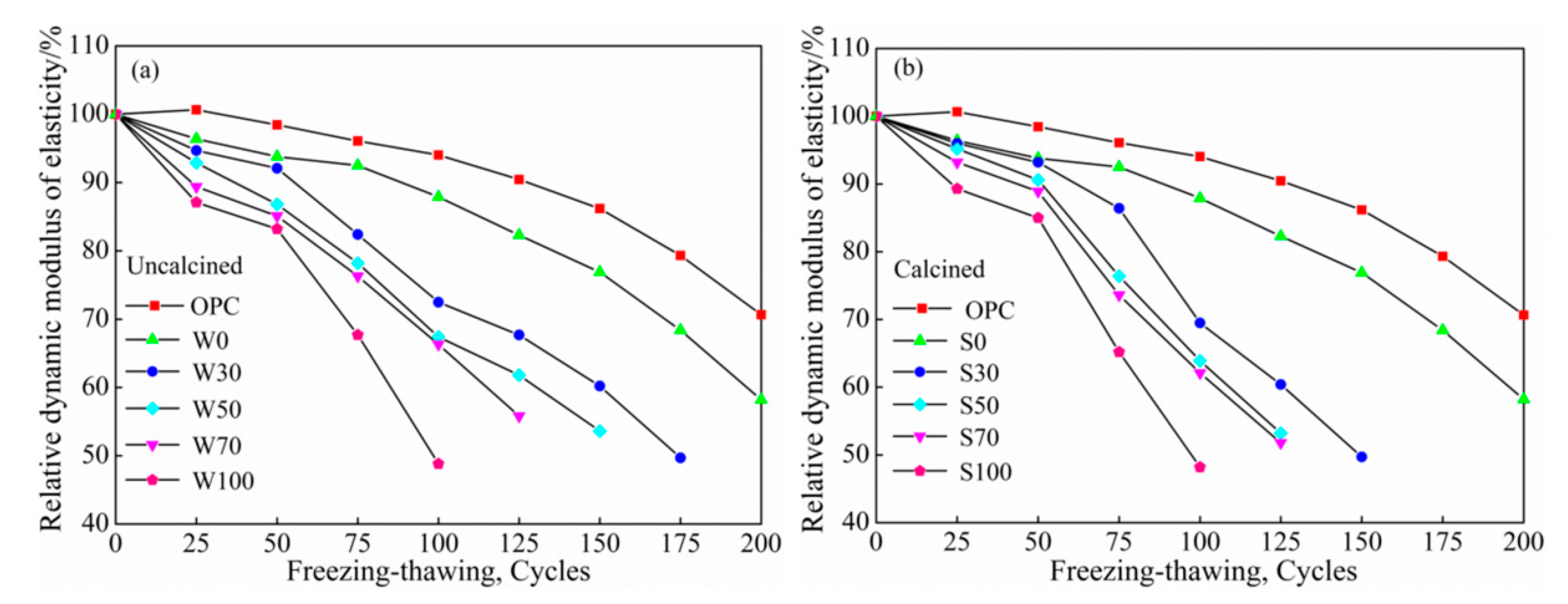
3.3.1. Water Freeze-Thaw Cycles
Mass Loss
Relative Dynamic Elasticity Modulus
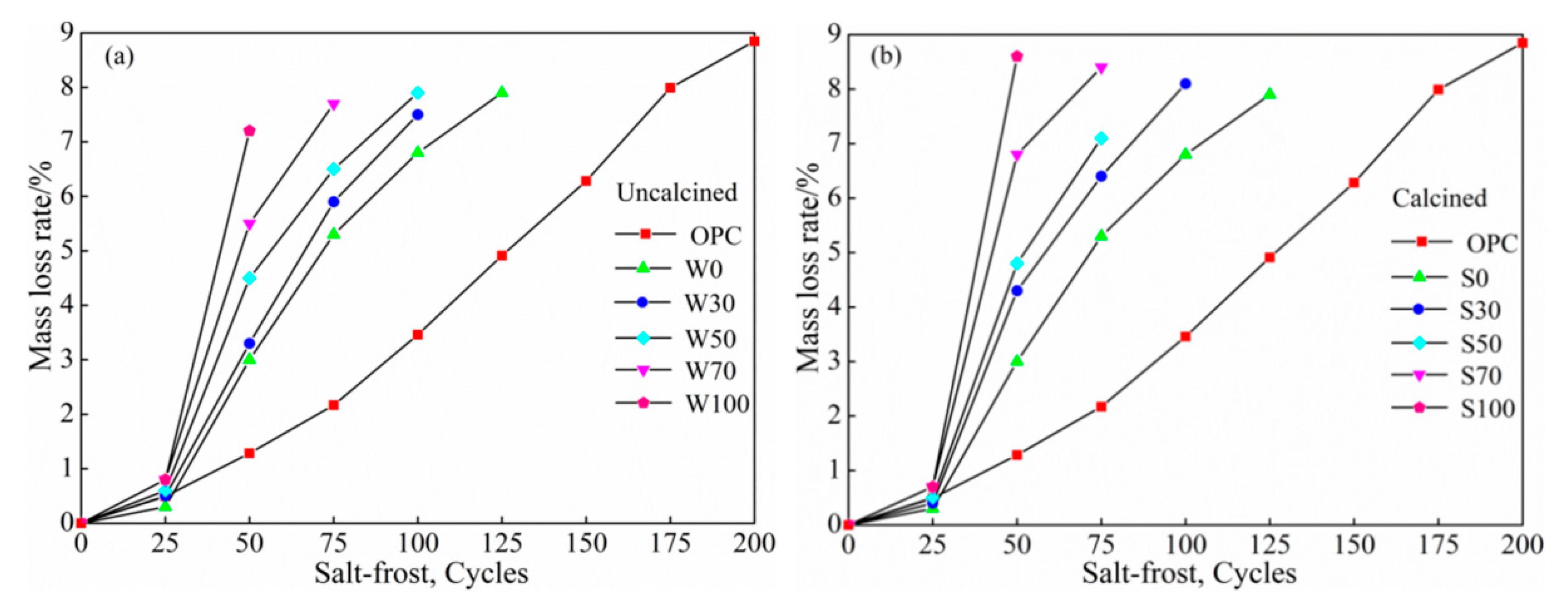
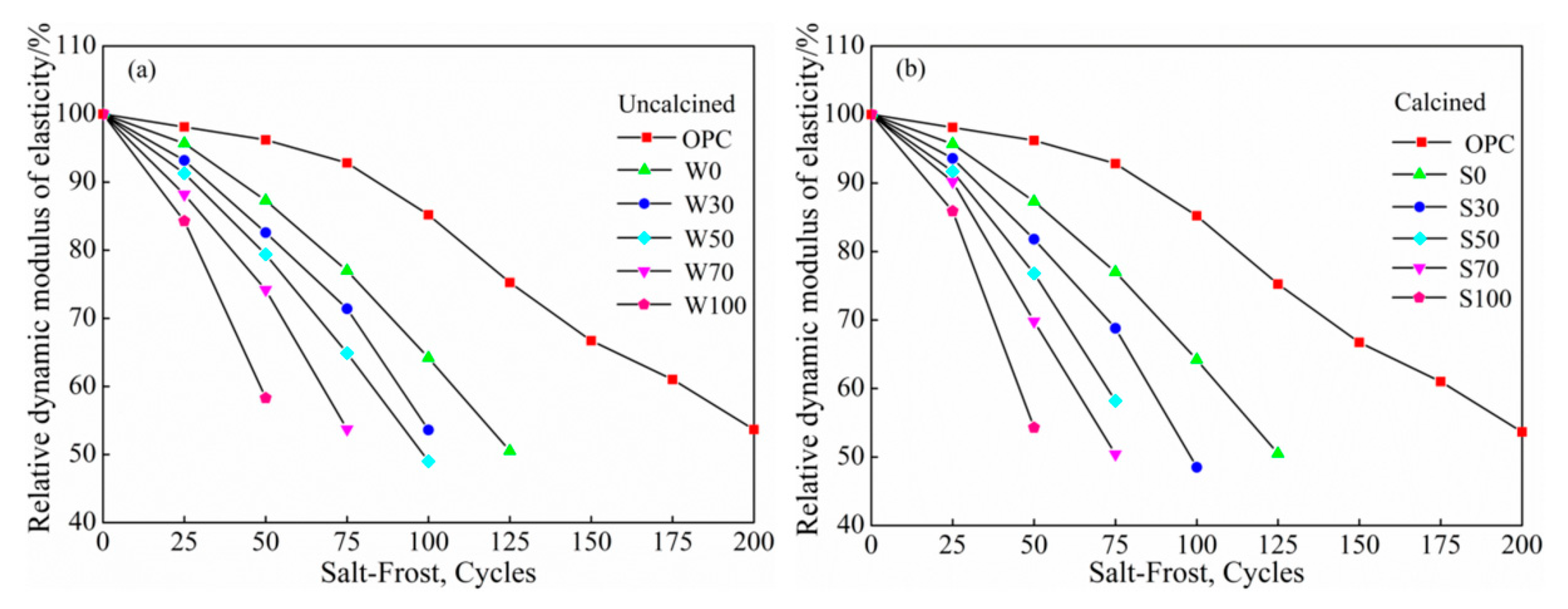
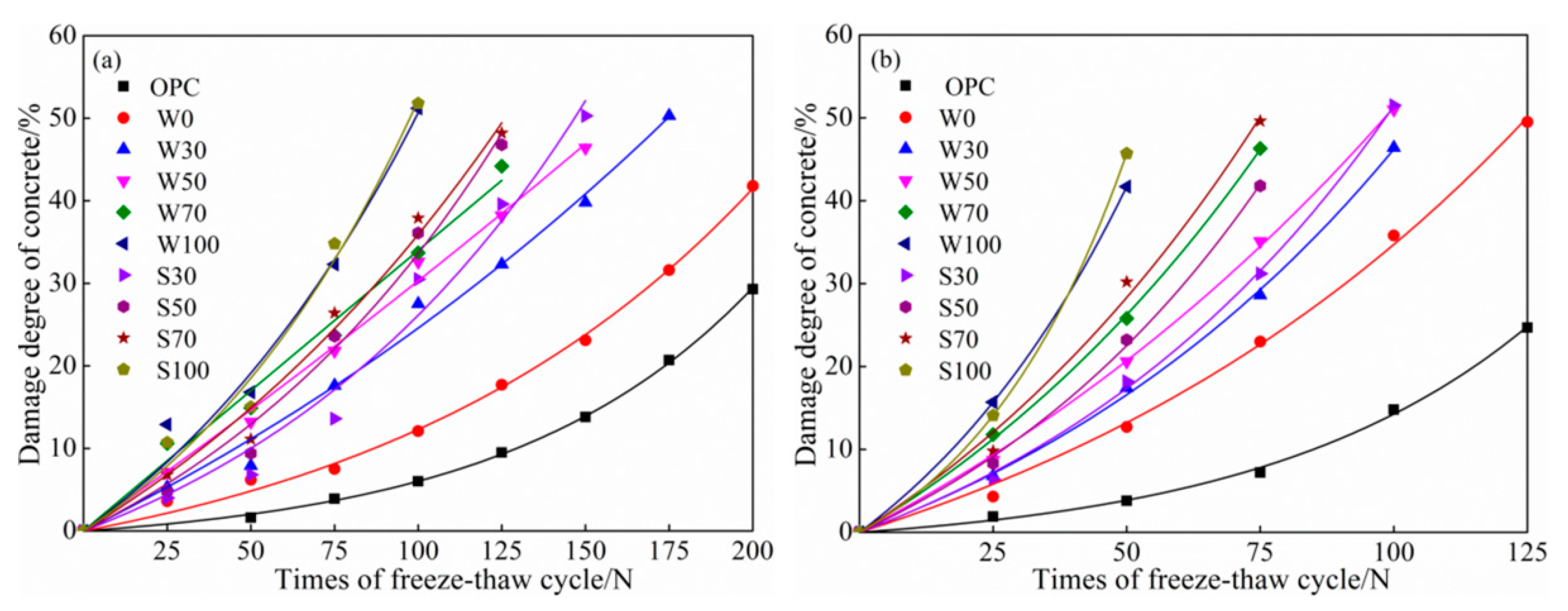
3.3.2. Salt Freezing Cycles
Mass Loss
Relative Dynamic Elasticity Modulus
3.3.3. Freeze-Thaw Damage Mechanics Models of ACSC
Freeze-Thaw Cycle Cumulative Damage Model Models
3.4. Compressive Strength and Freeze-Thaw Mechanism
3.4.1. Compressive Strength Mechanism Analysis
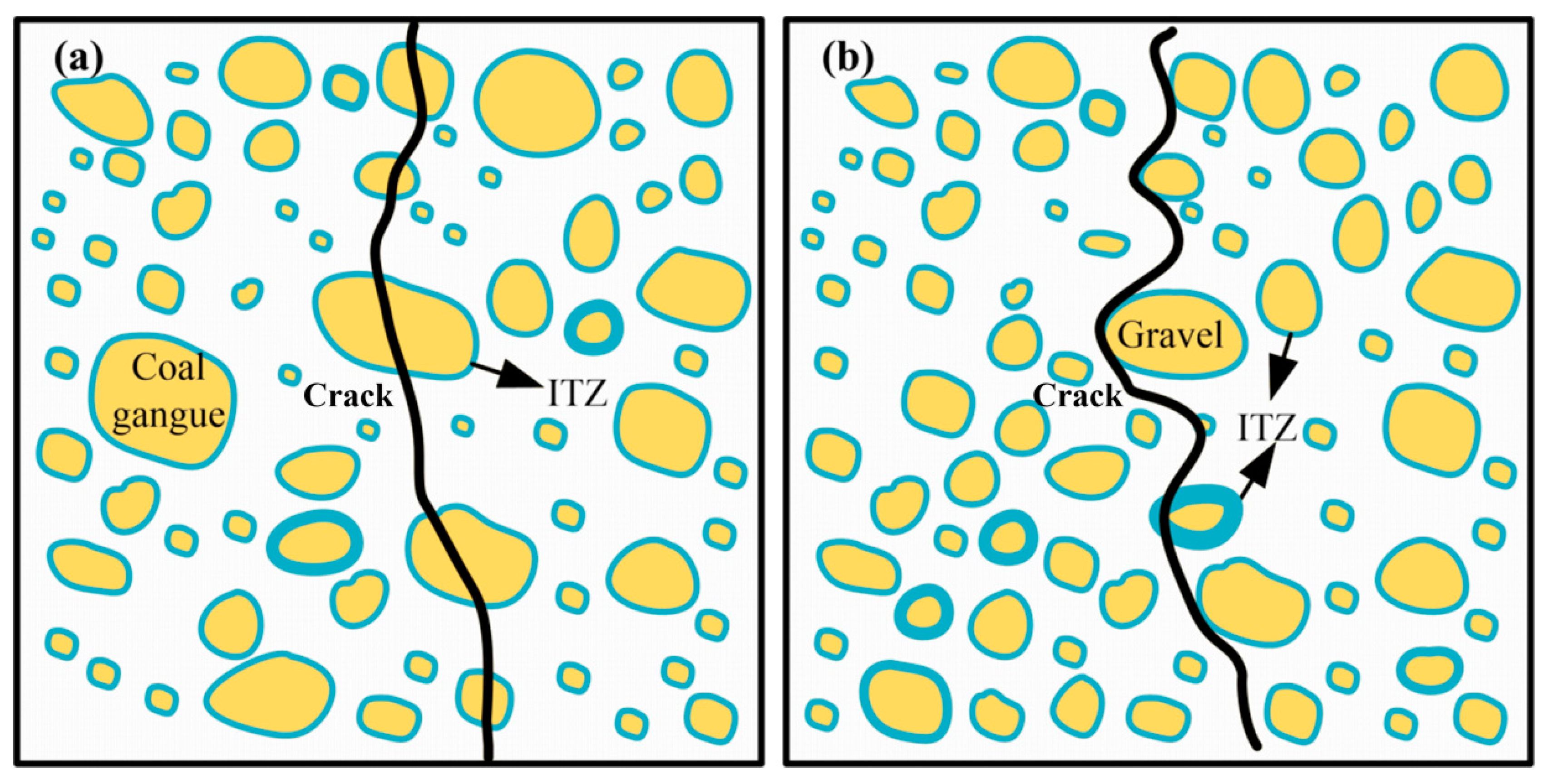
3.4.2. Freeze-Thaw Mechanism Analysis
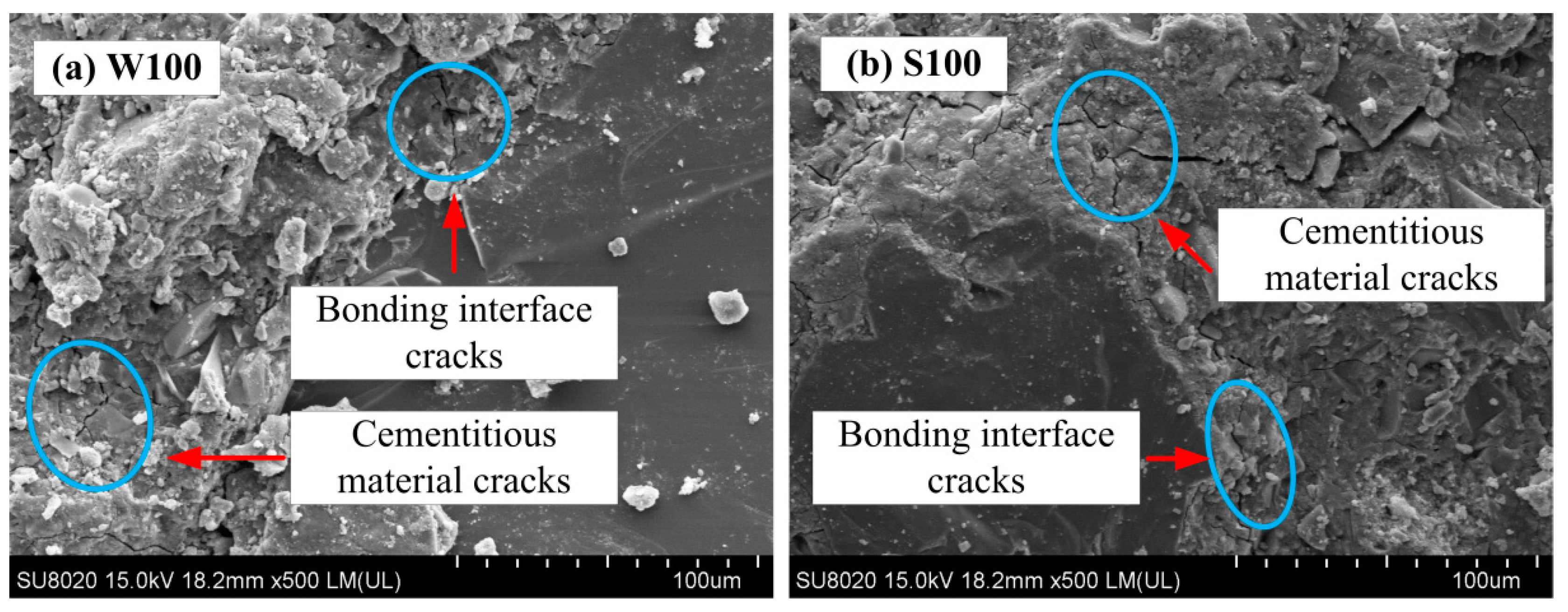
3.4.3. Cementing Mechanism
4. Conclusions
- ACSC displayed excellent early age strength compared with OPC, and the coal gangue can satisfy the compressive strength requirement of concrete aggregates. The development of the compressive strength decreased with the increase in coal gangue content, and 90 d compressive strength (compared with 28 d) reached the decrease when the coal gangue replacement rate was above 50%.
- The effect of the coal gangue content on the development of 90 d chloride corrosion compressive strength was similar to that on the development of 28 d compressive strength. The 90 d chloride ion corrosion compressive strength with coal gangue content decreased first, and then increased, and increased when the coal gangue content was 100%.
- The ACSC showed the best chloride penetration resistance under 30% uncalcined coal gangue content, which was less than 27.75% lower than that of using OPC. The DRCM values gradually increased with the coal gangue content. At the same content, the growth trend and DRCM of calcined coal gangue concrete were higher than the uncalcined form.
- At the same cycles, more was the content of coal gangue, the speed of salt–freeze cycle was faster than that of freeze–thawing, higher was the mass-loss rate, lower was the relative dynamic modulus of elasticity, damage of calcined coal gangue concrete in each group was more severe, and hence, considering the low-carbon environmental energy consumption, this is not a recommended process to calcined coal gangue.
- As for the freeze-thaw damage model with coal gangue as a variable freeze-thaw cycle, cumulative damage models based on exponential function models were superior to power function models with better precision, which can provide a reference for the quantificational design of freeze-thaw resistance and microcosmic studies on ACSC after freeze-thaw cycles reaction by mechanics methods.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Brito, J.D.; Kurda, R. The past and future of sustainable concrete: A critical review and new strategies on cement-based materials. J. Clean. Prod. 2020. [Google Scholar] [CrossRef]
- Fu, Y.W.; Cai, L.C.; Wu, Y.G. Freeze-thaw cycle test and damage mechanics models of alkali-activated slag concrete. Constr. Build. Mater. 2011, 25, 3144–3148. [Google Scholar] [CrossRef]
- Palomo, A.; Grutzeck, M.W.; Blanco, M.T. Alkali-activated fly ashes: A cement for the future. Cem. Concr. Res. 1999, 29, 1323–1329. [Google Scholar] [CrossRef]
- Zhang, J.; Shi, C.J.; Zhang, Z.H.; Zhi, H.O. Durability of alkali-activated materials in aggressive environments: A review on recent studies. Constr. Build. Mater. 2017, 152, 598–613. [Google Scholar] [CrossRef]
- Yi, C.; Ma, H.Q.; Chen, H.Y.; Wang, J.X.; Shi, J.; Li, Z.H.; Yu, M.K. Preparation and characterization of coal gangue geopolymers. Constr. Build. Mater. 2018, 187, 318–326. [Google Scholar] [CrossRef]
- Dong, Z.C.; Xia, J.W.; Fan, C.; Cao, J.C. Activity of calcined coal gangue fine aggregate and its effect on the mechanical behavior of cement mortar. Constr. Build. Mater. 2015, 100, 63–69. [Google Scholar] [CrossRef]
- Peng, B.X.; Li, X.R.; Zhao, W.H.; Yang, L. Study on the release characteristics of chlorine in coal gangue under leaching conditions of different pH values. Fuel 2018, 217, 427–433. [Google Scholar] [CrossRef]
- Li, Y.; Yao, Y.; Liu, X.M.; Sun, H.H.; Ni, W. Improvement on pozzolanic reactivity of coal gangue by integrated thermal and chemical activation. Fuel 2013, 109, 527–533. [Google Scholar] [CrossRef]
- Li, D.X.; Song, X.Y.; Gong, C.C.; Pan, Z.H. Research on cementitious behavior and mechanism of pozzolanic cement with coal gangue. Cem. Concr. Res. 2006, 36, 1752–1759. [Google Scholar] [CrossRef]
- Cao, D.W.; Ji, J.; Liu, Q.; He, Z.; Wang, H.; You, Z. Coal gangue applied to low-volume roads in China. Transp. Res. Rec. 2011, 2204, 258–266. [Google Scholar] [CrossRef]
- Qin, L.; Gao, X.J. Properties of coal gangue-Portland cement mixture with carbonation. Fuel 2019, 245, 1–12. [Google Scholar] [CrossRef]
- Chindaprasirt, P.; Homwuttiwong, S.; Sirivivatnanon, V. Influence of fly ash fineness on strength, drying shrinkage and sulfate resistance of blended cement mortar. Cem. Concr. Res. 2004, 34, 1087–1092. [Google Scholar] [CrossRef]
- Zhang, C.S.; Xue, J.P.; Fang, L.M. Mechanical properties and microstructuresof alkali activated burned coal gangue cementitious material. J. Chin. Ceram. Soc. 2004, 32, 1276–1280. [Google Scholar]
- Querol, X.; Zhuang, X.G.; Font, O.; Izquierdo, M.; Alastuey, A.; Castro, I.; van Drooge, B.L.; Moreno, T.; Grimalt, J.O.; Elvira, J.; et al. Influence of soil cover on reducing the environmental impact of spontaneous coal combustion in coal waste gobs: A review and new experimental data. Int. J. Coal Geol. 2011, 85, 2–22. [Google Scholar] [CrossRef]
- Li, Y.B.; Kang, T.H.; Wang, D. Gangue Harmful to environment and integrated control measures. In Proceedings of the International Conference on Computer Distributed Control and Intelligent Environmental Monitoring, Changsha, China, 19–20 February 2011; pp. 231–233. [Google Scholar] [CrossRef]
- Yip, C.K.; Lukey, G.C.; Provis, J.L.; van Deventer, J.S.J. Effect of calcium silicate sources on geopolymerisation. Cem. Concr. Res. 2008, 38, 554–564. [Google Scholar] [CrossRef]
- Angulo-Ramírez, D.E.; de Gutiérrez, R.M.; Puertas, F. Alkali-activated Portland blast-furnace slag cement: Mechanical properties and hydration. Constr. Build. Mater. 2017, 140, 119–128. [Google Scholar] [CrossRef]
- Shi, C.J. Strength, pore structure and permeability of alkali-activated slag mortars. Cem. Concr. Res. 1996, 26, 1789–1799. [Google Scholar] [CrossRef]
- Parthiban, K.; Mohan, K.S.R. Influence of recycled concrete aggregates on the engineering and durability properties of alkali activated slag concrete. Constr. Build. Mater. 2017, 133, 65–72. [Google Scholar] [CrossRef]
- García-Lodeiro, I.; Palomo, A.; Fernández-Jiménez, A. Alkali-aggregate reaction in activated fly ash systems. Cem. Concr. Res. 2007, 37, 175–183. [Google Scholar] [CrossRef]
- Behfarnia, K.; Rostami, M. Mechanical properties and durability of fiber reinforced alkali activated slag concrete. J. Mater. Civ. Eng. 2017, 29, 1–9. [Google Scholar] [CrossRef]
- Ranjbar, N.; Kuenzel, C.; Spangenberg, J.; Mehrali, M. Hardening evolution of geopolymers from setting to equilibrium: A review. Cem. Concr. Compos. 2020, 114, 103729. [Google Scholar] [CrossRef]
- Ma, H.Q.; Zhu, H.G.; Yi, C.; Fan, J.C.; Chen, H.Y.; Xu, X.N.; Wang, T. Preparation and reaction mechanism characterization of alkali-activated coal gangue-slag materials. Materials 2019, 12, 2250. [Google Scholar] [CrossRef]
- Liu, C.J.; Deng, X.W.; Liu, J.; Hui, D. Mechanical properties and microstructures of hypergolic and calcined coal gangue based geopolymer recycled concrete. Constr. Build. Mater. 2019, 221, 691–708. [Google Scholar] [CrossRef]
- Zuo, Y.; Nedeljković, M.; Ye, G. Pore solution composition of alkali-activated slag/fly ash pastes. Cem. Concr. Res. 2019, 115, 230–250. [Google Scholar] [CrossRef]
- Ismail, I.; Bernal, S.A.; Provis, J.L.; Nicolas, R.S.; Brice, D.G.; Kilcullen, A.R.; Hamdan, S.; Van Deventer, J.S. Influence of fly ash on the water and chloride permeability of alkali-activated slag mortars and concretes. Constr. Build. Mater. 2013, 48, 1187–1201. [Google Scholar] [CrossRef]
- Technical Specification for Lightweight Aggregate Concrete Structures; JGJ 12-2006; National Standards of the Republic of China: Beijing, China, 2006.
- Standard for Test Methods of Long-Term Performance and Durability of Ordinary Concrete; GB/T 50082-2009; National Standards of the Republic of China: Beijing, China, 2009.
- Standard for Test Method of Mechanical Properties on Ordinary Concrete; GB/T 50081-2019; National Standards of the Republic of China: Beijing, China, 2019.
- Cao, Z.; Cao, Y.D.; Dong, H.J.; Zhang, J.S.; Sun, C.B. Effect of calcination condition on the microstructure and pozzolanic activity of calcined coal gangue. Int. J. Miner. Process. 2016, 146, 23–28. [Google Scholar] [CrossRef]
- Pacheco-Torgal, F.; Castro-Gomes, J.; Jalali, S. Investigations about the effect of aggregates on strength and microstructure of geopolymeric mine waste mud binders. Cem. Concr. Res. 2007, 37, 933–941. [Google Scholar] [CrossRef]
- Shi, Z.G.; Geiker, M.R.; Weerdt, K.D.; Ostnor, T.A.; Lothenbach, B.; Winnefeld, F.; Skibsted, J. Role of calcium on chloride binding in hydrated Portland cement-metakaolin-limestone blends. Cem. Concr. Res. 2017, 95, 205–216. [Google Scholar] [CrossRef]
- Hobbs, D.W. Concrete deterioration: Causes, diagnosis, and minimising risk. Int. Mater. Rev. 2001, 46, 117–144. [Google Scholar] [CrossRef]
- Halamickova, P.; Detwiler, R.J.; Bentz, D.P.; Garboczi, E.J. Water permeability and chloride ion diffusion in portland cement mortars: Relationship to sand content and critical pore diameter. Cem. Concr. Res. 1995, 25, 790–802. [Google Scholar] [CrossRef]
- Roy, D.M.; Jiang, W.M.; Silsbee, M.R. Chloride diffusion in ordinary, blended, and alkali-activated cement pastes and its relation to other properties. Cem. Concr. Res. 2000, 30, 1879–1884. [Google Scholar] [CrossRef]
- Streicher, P.E.; Alexander, M.G. A chloride conduction test for concrete. Cem. Concr. Res. 1995, 25, 1284–1294. [Google Scholar] [CrossRef]
- Dej, J. Freezing and de-icing salt resistance of blast furnace slag concretes. Cem. Concr. Compos. 2003, 25, 357–361. [Google Scholar] [CrossRef]
- Sun, Z.H.; Scherer, G.W. Effect of air voids on salt scaling and internal freezing. Cem. Concr. Res. 2010, 40, 260–270. [Google Scholar] [CrossRef]
- Yang, Q.B. Mechanisms of deicer-frost scaling of concrete (Ι)-Capillary-uptake degree of saturation and ice-formation pressure. J. Build. Mater. 2007, 10, 522–527. (In Chinese) [Google Scholar]
- Power, T.C.; Helmuth, R.A. Theory of volume changes in hardened portland cement paste during freezing. Highw. Res. Board Proc. 1953, 32, 285–297. [Google Scholar]
- Wu, D.; Yang, B.G.; Liu, Y.C. Pressure drop in loop pipe flow of fresh cemented coal gangue–fly ash slurry: Experiment and simulation. Adv. Powder Technol. 2015, 26, 920–922. [Google Scholar] [CrossRef]
- Liu, Y.T.; Lei, S.M.; Lin, M.; Li, Y.; Ye, Z.; Fan, Y.M. Assessment of pozzolanic activity of calcined coal-series kaolin. Appl. Clay Sci. 2017, 143, 159–167. [Google Scholar] [CrossRef]
- Moghadam, M.J.; Ajalloeian, R.; Hajiannia, A. Preparation and application of alkali-activated materials based on waste glass and coal gangue: A review. Constr. Build. Mater. 2019, 221, 84–98. [Google Scholar] [CrossRef]
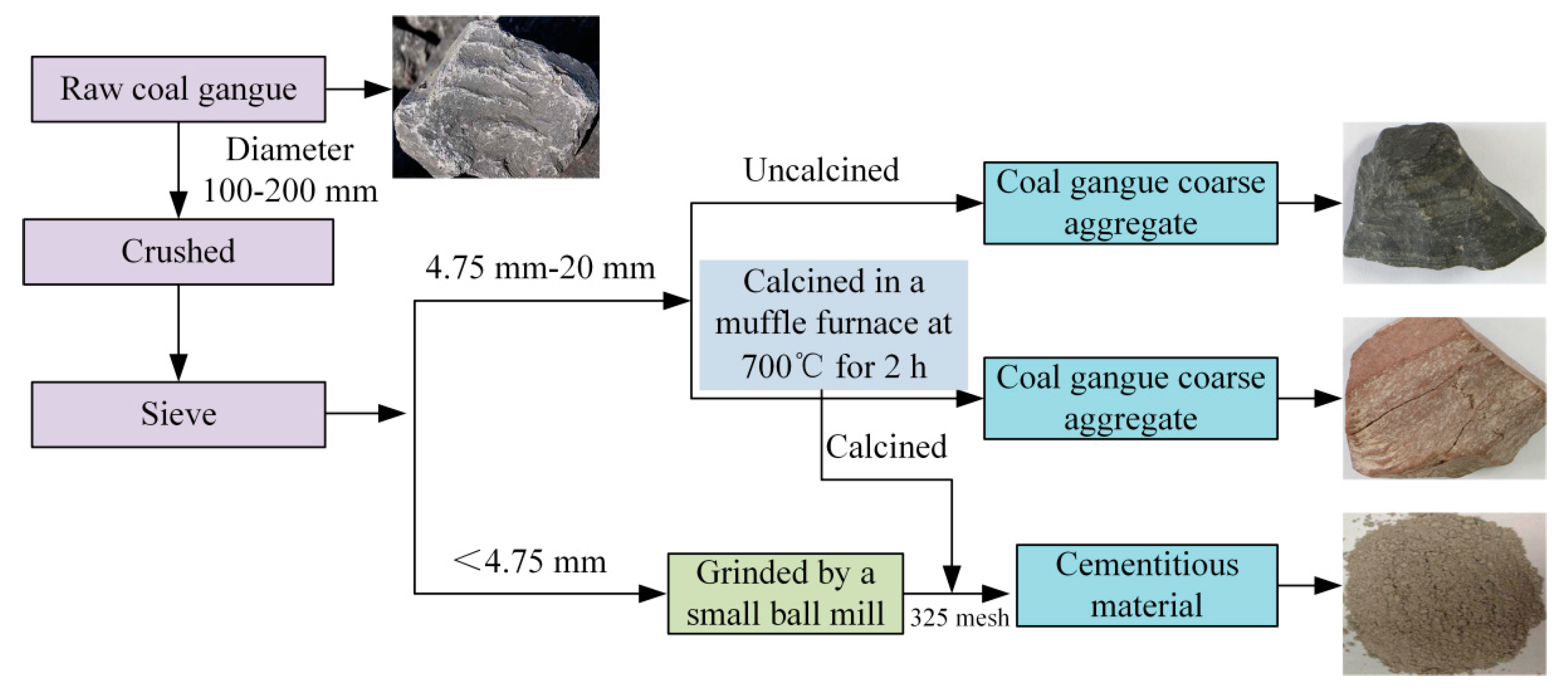


| Precursor | Al2O3 | SiO2 | CaO | Fe2O3 | Na2O | MgO | TiO2 | LOI |
|---|---|---|---|---|---|---|---|---|
| OPC | 4.65 | 21.18 | 63.25 | 3.78 | 0.18 | 3.26 | 1.55 | 2.15 |
| Slag | 14.04 | 30.58 | 38.43 | 0.35 | 0.57 | 10.57 | 1.93 | 1.17 |
| Coal Gangue | 36.78 | 56.56 | 0.62 | 1.95 | 0.42 | 0.22 | 2.10 | 1.32 |
| Types | Particle Size Range /mm | Apparent Density kg/m3 | Crushing Index Value /% | Water Absorption /% |
|---|---|---|---|---|
| Ordinary Gravel | 4.75–20 | 2680 | 16.8 | 1.51 |
| Calcined Coal Gangue | 4.75–20 | 2605 | 13.2 | 6.10 |
| Uncalcined Coal Gangue | 4.75–20 | 2678 | 13.8 | 4.09 |
| Specimens | Cementitious Materials | Water | Na2SiO3 Solution | NaOH Solution | Aggregates | Compressive Strength/MPa | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Coal Gangue | Slag | Sand | Coal Gangue | Gravel | 28 d | 90 d | ||||
| OPC | - | - | 200 | - | - | 565 | - | 1050 | 46.1 | 53.2 |
| W0 | 280 | 280 | 138.24 | 172.83 | 28.02 | 565 | 0 | 1050 | 62.1 | 66.8 |
| W30 | 280 | 280 | 138.24 | 172.83 | 28.02 | 565 | 315 | 735 | 54.2 | 56.3 |
| W50 | 280 | 280 | 138.24 | 172.83 | 28.02 | 565 | 525 | 525 | 53.2 | 53.4 |
| W70 | 280 | 280 | 138.24 | 172.83 | 28.02 | 565 | 735 | 315 | 48.7 | 48.3 |
| W100 | 280 | 280 | 138.24 | 172.83 | 28.02 | 565 | 1050 | 0 | 42.7 | 40.5 |
| S30 | 280 | 280 | 138.24 | 172.83 | 28.02 | 565 | 315 | 735 | 58.4 | 59.3 |
| S50 | 280 | 280 | 138.24 | 172.83 | 28.02 | 565 | 525 | 525 | 57.7 | 57.9 |
| S70 | 280 | 280 | 138.24 | 172.83 | 28.02 | 565 | 735 | 315 | 55.1 | 55.2 |
| S100 | 280 | 280 | 138.24 | 172.83 | 28.02 | 565 | 1050 | 0 | 49.3 | 48.7 |
| Specimens | Freeze-Thaw Damage | Salt-Frost Damage | ||||
|---|---|---|---|---|---|---|
| a | b | R2 | a | b | R2 | |
| OPC | 0.00010 | 2.35200 | 0.995 | 0.00010 | 2.22476 | 0.991 |
| W0 | 0.00454 | 1.71661 | 0.984 | 0.03655 | 1.49399 | 1.000 |
| W30 | 0.08091 | 1.24357 | 0.988 | 0.05778 | 1.44916 | 0.994 |
| W50 | 0.21719 | 1.07285 | 0.992 | 0.13139 | 1.29431 | 0.999 |
| W70 | 0.23727 | 1.07831 | 0.977 | 0.15520 | 1.31775 | 0.992 |
| W100 | 0.10563 | 1.33660 | 0.936 | – | – | – |
| S30 | 0.01485 | 1.62797 | 0.977 | 0.04073 | 1.54847 | 0.996 |
| S50 | 0.03502 | 1.49583 | 0.985 | 0.07563 | 1.46278 | 1.000 |
| S70 | 0.07412 | 1.34605 | 0.981 | 0.13653 | 1.36781 | 0.999 |
| S100 | 0.05865 | 1.47243 | 0.958 | – | – | – |
| Specimens | Freeze-Thaw Damage | Salt-Frost Damage | ||||
|---|---|---|---|---|---|---|
| a | b | R2 | a | b | R2 | |
| OPC | −2.07457 | −0.01361 | 0.996 | −2.29772 | −0.01975 | 0.998 |
| W0 | −8.93199 | −0.00866 | 0.996 | −21.13862 | −0.00972 | 0.997 |
| W30 | −53.44089 | −0.00378 | 0.990 | −21.14657 | −0.01159 | 0.999 |
| W50 | −231.07696 | −0.00123 | 0.994 | −43.44013 | −0.00779 | 0.999 |
| W70 | 199.58951 | 0.00002 | 0.985 | −34.08198 | −0.01142 | 0.999 |
| W100 | −29.42573 | −0.01001 | 0.977 | −23.93107 | −0.02018 | 1.000 |
| S30 | −16.35335 | −0.00955 | 0.974 | −18.03187 | −0.01349 | 0.999 |
| S50 | −21.70524 | −0.00935 | 0.983 | −20.09297 | −0.01504 | 0.998 |
| S70 | −36.35061 | −0.00687 | 0.984 | −35.29314 | −0.01178 | 0.991 |
| S100 | −22.60776 | −0.01197 | 0.981 | −11.36057 | −0.03228 | 1.000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, H.; Yang, S.; Li, W.; Li, Z.; Fan, J.; Shen, Z. Study of Mechanical Properties and Durability of Alkali-Activated Coal Gangue-Slag Concrete. Materials 2020, 13, 5576. https://doi.org/10.3390/ma13235576
Zhu H, Yang S, Li W, Li Z, Fan J, Shen Z. Study of Mechanical Properties and Durability of Alkali-Activated Coal Gangue-Slag Concrete. Materials. 2020; 13(23):5576. https://doi.org/10.3390/ma13235576
Chicago/Turabian StyleZhu, Hongguang, Sen Yang, Weijian Li, Zonghui Li, Jingchong Fan, and Zhengyan Shen. 2020. "Study of Mechanical Properties and Durability of Alkali-Activated Coal Gangue-Slag Concrete" Materials 13, no. 23: 5576. https://doi.org/10.3390/ma13235576
APA StyleZhu, H., Yang, S., Li, W., Li, Z., Fan, J., & Shen, Z. (2020). Study of Mechanical Properties and Durability of Alkali-Activated Coal Gangue-Slag Concrete. Materials, 13(23), 5576. https://doi.org/10.3390/ma13235576





