MFC/NFC-Based Foam/Aerogel for Production of Porous Materials: Preparation, Properties and Applications
Abstract
1. Introduction
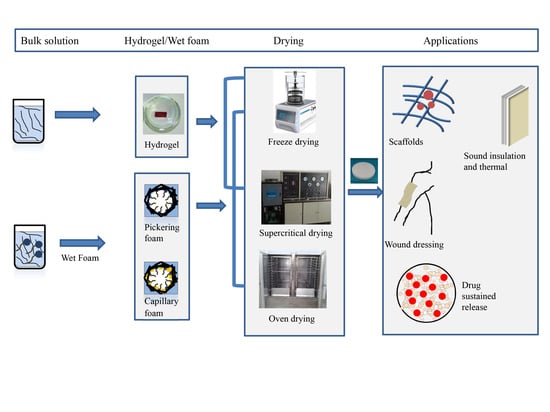
2. Preparation of Porous Foam Materials
2.1. Process from Suspension to Solid Foam
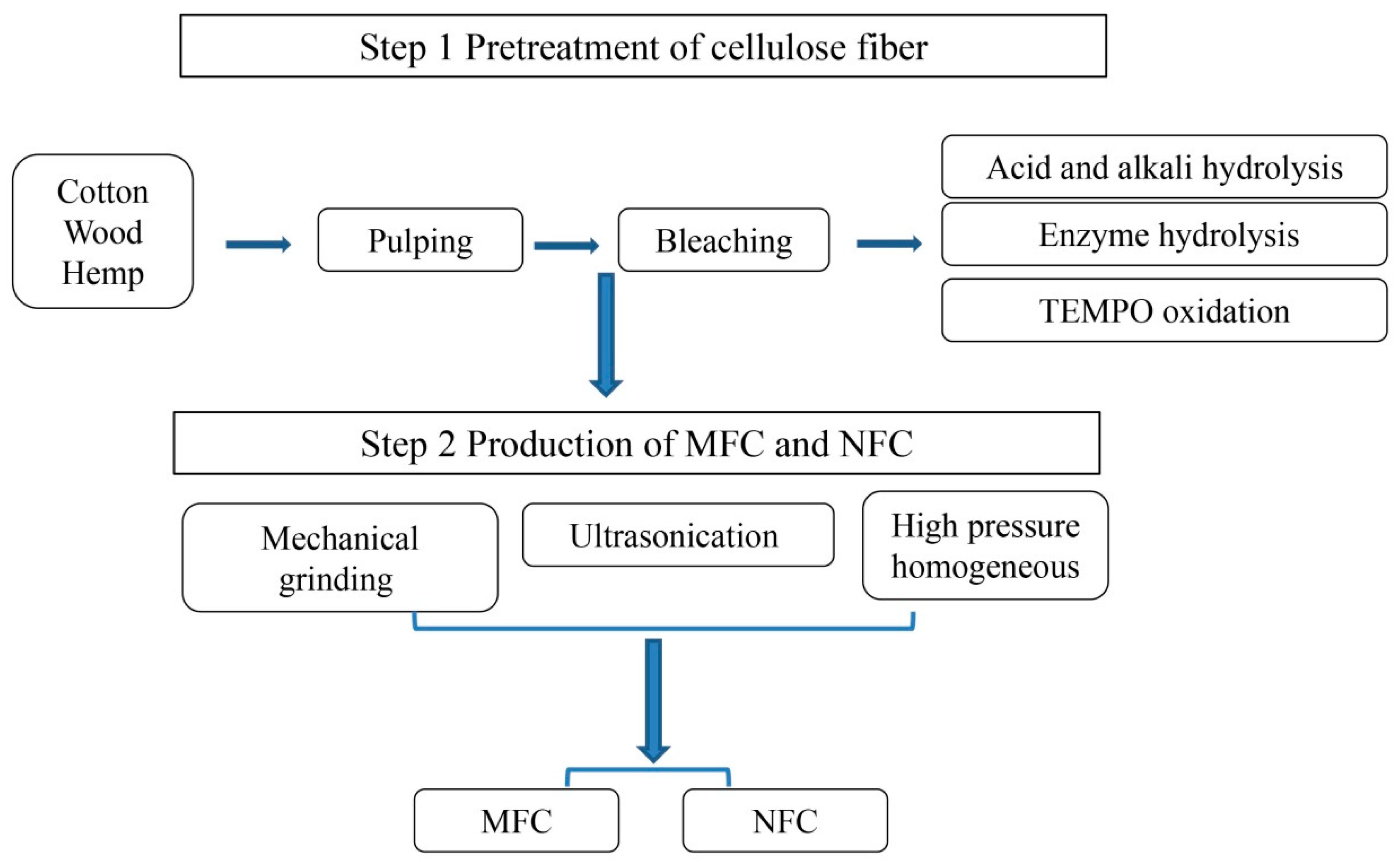
2.2. Preparation of Nanofibrillated Cellulose (NFC) and Microfibrillated Cellulose (MFC)
2.3. Preparation of Wet-Foam
2.3.1. The Processing from MFC/NFC Dispersions to Wet Foam
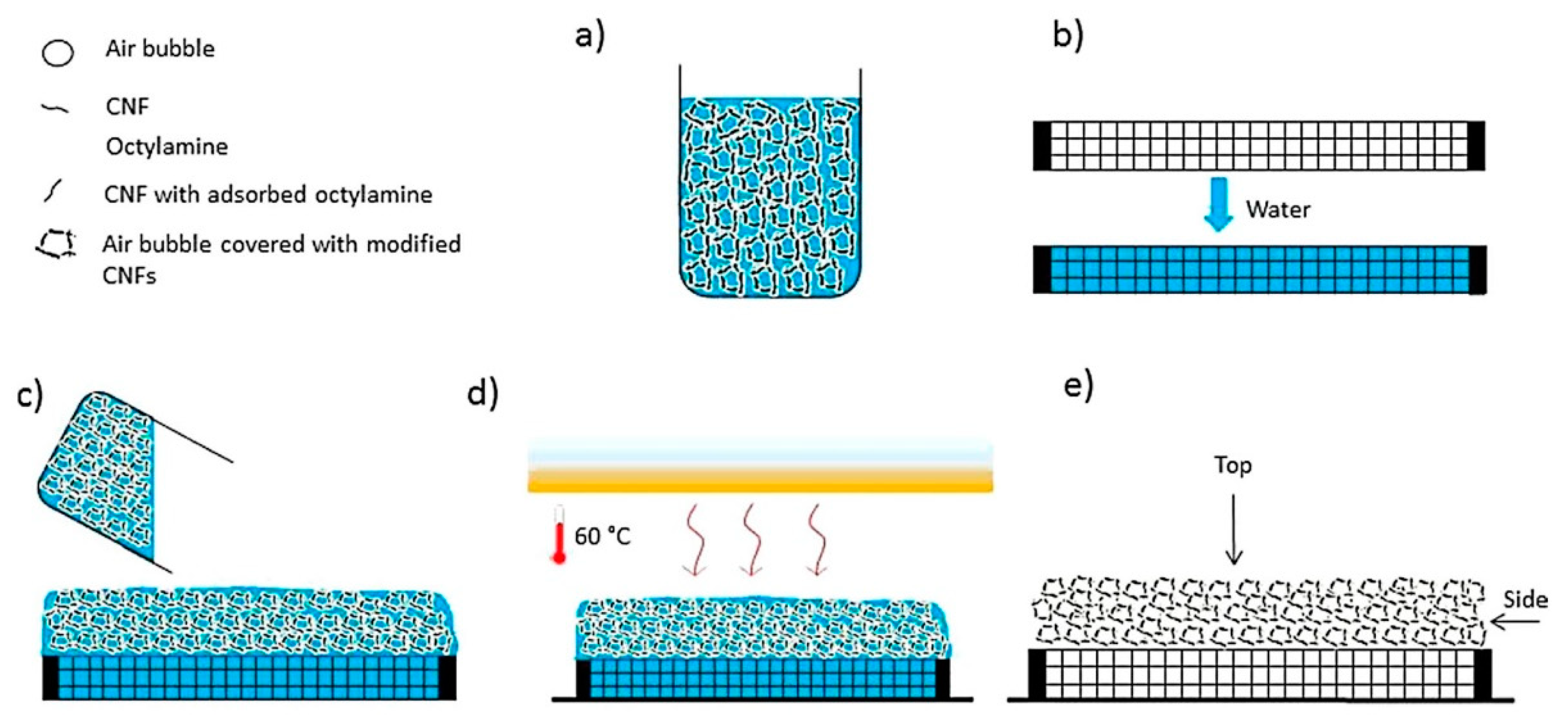
2.3.2. Wet Foam Stabilization Mechanism
2.3.3. Pickering Foam of MFC and NFC
2.3.4. Capillary Foam
2.4. Preparation of MFC-/NFC-Based Porous Solid Foam
2.4.1. Freezing Drying
2.4.2. Supercritical Drying
2.4.3. Oven Drying
3. Properties of Porous Foam Materials
3.1. Surface Area and Porosity
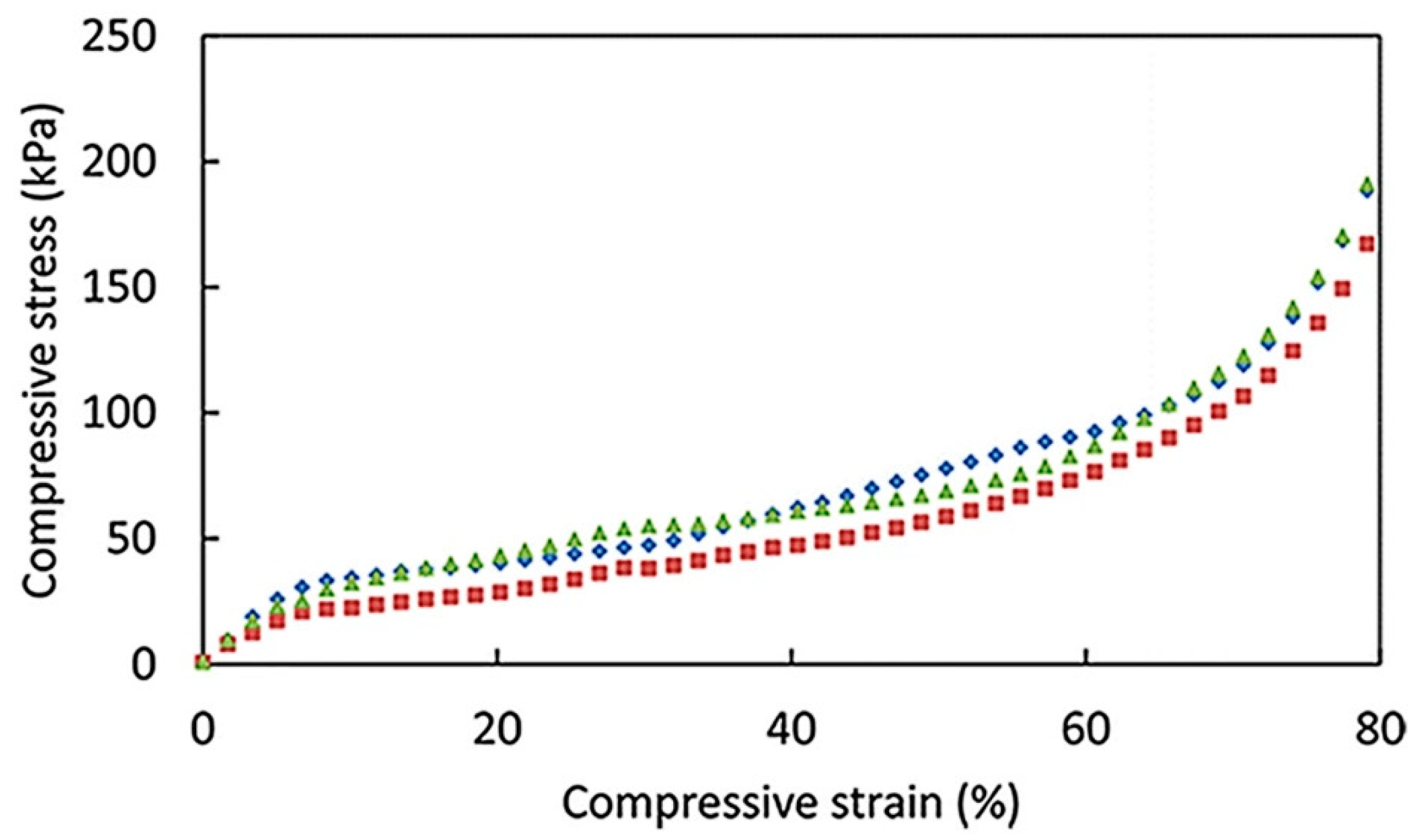
3.2. Mechanical Properties
4. Applications
4.1. Sound Insulation and Thermal
4.2. Medical Application
4.3. Packaging Materials
5. Conclusions and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Lavoine, N.; Bergstrm, L. Nanocellulose-based foams and aerogels: Processing, properties, and applications. J. Mater. Chem. A 2017, 5, 16105–16117. [Google Scholar] [CrossRef]
- Sehaqui, H.; Salajkova, M.; Zhou, Q.; Berglund, L.A. Mechanical performance tailoring of tough ultra-high porosity foams prepared from cellulose I nanofiber suspensions. Soft Matter 2010, 6, 1824–1832. [Google Scholar] [CrossRef]
- Martoia, F.; Cochereau, T.; Dumont, P.J.J.; Orgeas, L.; Terrien, M.; Belgacem, M.N. Cellulose nanofibril foams: Links between ice-templating conditions, microstructures and mechanical properties. Mater. Des. 2016, 104, 376–391. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, Y.; Jiang, Z.; Wang, H. The effect of freezing speed and hydrogel concentration on the microstructure and compressive performance of bamboo-based cellulose aerogel. J. Wood Sci. 2015, 61, 595–601. [Google Scholar] [CrossRef]
- Paakko, M.; Vapaavuori, J.; Silvennoinen, R.; Kosonen, H.; Ankerfors, M.; Lindstrom, T.; Berglund, L.; Ikkala, O. Long and entangled native cellulose I nanofibers allow flexible aerogels and hierarchically porous templates for functionalities. Soft Matter 2008, 4, 2492–2499. [Google Scholar] [CrossRef]
- Wu, T.-T.; Zeng, Z.-H.; Siqueira, G.; De France, K.; Sivaraman, D.; Schreiner, C.; Figi, R.; Zhang, Q.H.; Nystrom, G. Dual-porous cellulose nanofibril aerogels via modular drying and cross-linking. Nanoscale 2020, 12, 7383–7394. [Google Scholar] [CrossRef]
- Cervin, N.T.; Andersson, L.; Ng, J.B.S.; Olin, P.; Bergström, L.; Wågberg, L. Lightweight and Strong Cellulose Materials Made from Aqueous Foams Stabilized by Nanofibrillated Cellulose. Biomacromolecules 2013, 14, 503–511. [Google Scholar] [CrossRef]
- Liu, Y.; Kong, S.; Xiao, H.; Bai, C.Y.; Lu, P.; Wang, S.F. Comparative study of ultra-lightweight pulp foams obtained from various fibers and reinforced by MFC. Carbohyd. Polym. 2018, 182, 92–97. [Google Scholar] [CrossRef]
- Li, J.; Song, T.; Xiu, H.; Cheng, R.; Yang, X.; Liu, Q.; Zhang, X.; Kozliak, E.; Ji, Y. Control of structure and properties of cellulose nanofibrils (CNF)-based foam materials by using ethanol additives prior to freeze-drying. Wood Sci. Technol. 2019, 53, 837–854. [Google Scholar] [CrossRef]
- Cervin, N.T.; Johansson, E.; Benjamins, J.W.; Wagberg, L. Mechanisms behind the stabilizing action of cellulose nanofibrils in wet-stable cellulose foams. Biomacromolecules 2015, 16, 822–831. [Google Scholar] [CrossRef]
- Xiang, W.; Preisig, N.; Ketola, A.; Tardy, B.L.; Bai, L.; Ketoja, J.A.; Stubenrauch, C.; Rojas, O.J. How Cellulose Nanofibrils Affect Bulk, Surface, and Foam Properties of Anionic Surfactant Solutions. Biomacromolecules 2019, 20, 4361–4369. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Nishiyama, Y.; Wada, M.; Kuga, S. Nanofibrillar cellulose aerogels. Colloid Surf. A 2004, 240, 63–67. [Google Scholar] [CrossRef]
- Gavillon, R.; Budtova, T. Aerocellulose: New Highly Porous Cellulose Prepared from Cellulose? NaOH Aqueous Solutions. Biomacromolecules 2008, 9, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.-H.; Li, Q.; Chen, W.-S.; Yu, H.-P. Composite aerogels based on dialdehyde nanocellulose and collagen for potential applications as wound dressing and tissue engineering scaffold. Compos. Sci. Technol. 2014, 94, 132–138. [Google Scholar] [CrossRef]
- Fang, B.; Wan, Y.-Z.; Tang, T.-T.; Gao, C.; Dai, K.-R. Proliferation and Osteoblastic Differentiation of Human Bone Marrow Stromal Cells on Hydroxyapatite/Bacterial Cellulose Nanocomposite Scaffolds. Tissue Eng. Part A 2009, 15, 1091–1098. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Sharma, S.; Liu, W.; Mu, W.; Liu, W.; Zhang, X.; Deng, Y. Aerogel Microspheres from Natural Cellulose Nanofibrils and Their Application as Cell Culture Scaffold. Biomacromolecules 2014, 15, 2540–2547. [Google Scholar] [CrossRef]
- Liu, J.; Cheng, F.; Grenman, H.; Spoljaric, S.; Seppala, J.; Eriksson, J.E.; Willfor, S.; Xu, C.L. Development of nanocellulose scaffolds with tunable structures to support 3D cell culture. Carbohyd. Polym. 2016, 148, 259–271. [Google Scholar] [CrossRef]
- Bukhari, N.; Joseph, J.P.; Hussain, S.S.; Khan, M.A.; Sharif, N. Prevalence of Human Papilloma Virus Sub Genotypes following Head and Neck Squamous Cell Carcinomas in Asian Continent, A Systematic Review Article. Asian Pac. J. Cancer Prev. 2019, 20, 3269–3277. [Google Scholar] [CrossRef]
- Ghafari, R.; Jonoobi, M.; Amirabad, L.M.; Oksman, K.; Taheri, A.R. Fabrication and characterization of novel bilayer scaffold from nanocellulose based aerogel for skin tissue engineering applications. Int. J. Biol. Macromol. 2019, 136, 796–803. [Google Scholar] [CrossRef]
- Kolakovic, R.; Laaksonen, T.; Peltonen, L. Spray-dried nanofibrillar cellulose microparticles for sustained drug release. Int. J. Pharm. 2012, 430, 47–55. [Google Scholar] [CrossRef]
- Valo, H.; Arola, S.; Laaksonen, P.I.; Torkkeli, M.; Peltonen, L.; Linder, M.B.; Serimaa, R.; Kuga, S.; Hirvonen, J.; Laaksonen, T. Drug release from nanoparticles embedded in four different nanofibrillar cellulose aerogels. Eur. J. Pharm. Sci. 2013, 50, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Svagan, A.J.; Benjamins, J.W.; Al-Ansari, Z.; Bar Shalom, D.; Mullertz, A.; Wagberg, L.; Lobmann, K. Solid cellulose nanofiber based foams—Towards facile design of sustained drug delivery systems. J. Control Release 2016, 244, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Gonzalez, C.A.; Alnaief, M.; Smirnova, I. Polysaccharide-based aerogels-Promising biodegradable carriers for drug delivery systems. Carbohyd. Polym. 2011, 86, 1425–1438. [Google Scholar] [CrossRef]
- Bhandari, J.; Mishra, H.; Mishra, P.K.; Wimmer, R.; Ahmad, F.J.; Talegaonkar, S. Cellulose nanofiber aerogel as a promising biomaterial for customized oral drug delivery. Int. J. Nanomed. 2017, 12, 2021–2031. [Google Scholar] [CrossRef]
- Fan, X.-L.; Li, Y.-J.; Li, X.-M.; Wu, Y.-H.; Tang, K.-Y.; Liu, J.; Zheng, X.-J.; Wan, G.-M. Injectable antibacterial cellulose nanofiber/chitosan aerogel with rapid shape recovery for noncompressible hemorrhage. Int. J. Biol. Macromol. 2020, 154, 1185–1193. [Google Scholar] [CrossRef]
- De Oliveira, J.P.; Bruni, G.P.; el Halal, S.L.M.; Bertoldi, F.C.; Dias, A.R.G.; Zavareze, E.D. Cellulose nanocrystals from rice and oat husks and their application in aerogels for food packaging. Int. J. Biol. Macromol. 2019, 124, 175–184. [Google Scholar] [CrossRef]
- Jiang, F.; Hsieh, Y.L. Amphiphilic superabsorbent cellulose nanofibril aerogels. J. Mater. Chem. A 2014, 2, 6337–6342. [Google Scholar] [CrossRef]
- Cervin, N.T.; Johanson, E.; Larsson, P.A.; Wagberg, L. Strong, Water-Durable, and Wet-Resilient Cellulose Nanofibril-Stabilized Foams from Oven Drying. Acs Appl. Mater. Interfaces 2016, 8, 11682–11689. [Google Scholar] [CrossRef]
- Cervin, N.T.; Aulin, C.; Larsson, P.T.; Wagberg, L. Ultra porous nanocellulose aerogels as separation medium for mixtures of oil/water liquids. Cellulose 2012, 19, 401–410. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Lyu, S.Y.; Zhou, X.M.; Gu, H.B.; Ma, C.; Wang, C.H.; Ding, T.; Shao, Q.; Liu, H.; Guo, Z.H. Super light 3D hierarchical nanocellulose aerogel foam with superior oil adsorption. J. Colloid Interface Sci. 2019, 536, 245–251. [Google Scholar] [CrossRef]
- Carlsson, D.O.; Nyström, G.; Zhou, Q.; Berglund, L.A.; Nyholm, L.; Strømme, M. Electroactive nanofibrillated cellulose aerogel composites with tunable structural and electrochemical properties. J. Mater. Chem. 2012, 22, 19014–19024. [Google Scholar] [CrossRef]
- Wu, Z.-Y.; Li, C.; Liang, H.-W.; Chen, J.-F.; Yu, S.-H. Ultralight, flexible, and fire-resistant carbon nanofiber aerogels from bacterial cellulose. Angew. Chem. Int. Ed. Engl. 2013, 52, 2925–2929. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.-J.; Li, B.; Xu, Y.; Essawy, H.; Wu, Z.-G.; Du, G.-B. Tannin-furanic resin foam reinforced with cellulose nanofibers (CNF). Ind. Crop. Prod. 2019, 134, 107–112. [Google Scholar] [CrossRef]
- Li, J.-H.; Yan, Q.-G.; Cai, Z.-Y. Mechanical properties and characteristics of structural insulated panels with a novel cellulose nanofibril-based composite foam core. J. Sandw. Struct. Mater. 2020, 0, 1–16. [Google Scholar] [CrossRef]
- Wicklein, B.; Kocjan, A.; Salazar-Alvarez, G.; Carosio, F.; Camino, G.; Antonietti, M.; Bergström, L. Thermally insulating and fire-retardant lightweight anisotropic foams based on nanocellulose and graphene oxide. Nat. Nanotechnol. 2015, 10, 277–283. [Google Scholar] [CrossRef]
- Yildirim, N.; Shaler, S.M.; Gardner, D.J.; Rice, R.; Bousfield, D.W. Cellulose nanofibril (CNF) reinforced starch insulating foams. Cellulose 2014, 21, 4337–4347. [Google Scholar] [CrossRef]
- Poehler, T.; Jetsu, P.; Isomoisio, H. Benchmarking new wood fibre-based sound absorbing material made with a foam-forming technique. Build. Acoust. 2016, 23, 131–143. [Google Scholar] [CrossRef]
- Chen, B.; Zheng, Q.-F.; Zhu, J.-L.; Li, J.-H.; Cai, Z.-Y.; Chen, L.-G.; Gong, S.-Q. Mechanically strong fully biobased anisotropic cellulose aerogels. Rsc Adv. 2016, 6, 96518–96526. [Google Scholar] [CrossRef]
- Siro, I.; Plackett, D. Microfibrillated cellulose and new nanocomposite materials: A review. Cellulose 2010, 17, 459–494. [Google Scholar] [CrossRef]
- Tayeb, A.H.; Amini, E.; Ghasemi, S.; Tajvidi, M. Cellulose Nanomaterials-Binding Properties and Applications: A Review. Molecules 2018, 23, 2684. [Google Scholar] [CrossRef]
- Henriksson, M.; Henriksson, G.; Berglund, L.-A.; Lindström, T. An environmentally friendly method for enzyme-assisted preparation of microfibrillated cellulose (MFC) nanofiber. Eur. Polym. J. 2007, 43, 3434–3441. [Google Scholar] [CrossRef]
- Pääkkö, M.; Ankerfors, M.; Kosonen, H.; Nykänen, A.; Ahola, S.; Österberg, M.; Ruokolainen, J.; Laine, J.; Larsson, P.T.; Ikkala, O. Enzymatic hydrolysis combined with mechanical shearing and high-pressure homogenization for nanoscale cellulose fibrils and strong gels. Biomacromolecules 2007, 8, 1934–1941. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.-L.; Yang, J.-W.; Li, B.; Xiong, C.-X. Carbonized Cellulose Nanofibril_Graphene Oxide__Composite Aerogels for High-Performance Supercapacitors. Acs Appl. Energy Mater. 2020, 3, 1145–1151. [Google Scholar] [CrossRef]
- Al-Qararah, A.M.; Hjelt, T.; Koponen, A.; Harlin, A.; Ketoja, J.A. Bubble size and air content of wet fibre foams in axial mixing with macro-instabilities. Colloids Surf. A Physicochem. Eng. Asp. 2013, 436, 1130–1139. [Google Scholar] [CrossRef]
- Darpentigny, C.; Nonglaton, G.; Bras, J.; Jean, B. Highly Absorbent Cellulose Nanofibrils Aerogels Prepared by Supercritical Drying. Carbohyd. Polym. 2019, 229, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Ali, Z.M.; Gibson, L.J. The structure and mechanics of nanofibrillar cellulose foams. Soft Matter 2013, 9, 1580–1588. [Google Scholar] [CrossRef]
- Han, J.; Zhou, C.; Wu, Y.; Liu, F.; Wu, Q. Self-assembling behavior of cellulose Nanoparticles during freeze-drying: Effect of suspension concentration, particle size, crystal structure, and surface charge. Biomacromolecules 2013, 14, 1529–1540. [Google Scholar] [CrossRef]
- Hellstrom, P.; Heijnessonhulten, A.; Paulsson, M.; Hakansson, H.; Germgard, U. The effect of Fenton chemistry on the properties of microfibrillated cellulose. Cellulose 2014, 21, 1489–1503. [Google Scholar] [CrossRef]
- Masruchin, N.; Park, B.D.; Causin, V. Influence of sonication treatment on supramolecular cellulose microfibril-based hydrogels induced by ionic interaction. J. Ind. Eng. Chem. 2015, 29, 265–272. [Google Scholar] [CrossRef]
- Yue, Y.; Song, B.; Zhou, X.-F. Preparation and Characterization of Cellulose Nanofibers with High Yield Via ZnCl_2 Aqueous Solution Treatment. J. Cellul. Sci. Technol. 2015, 23, 49–54. [Google Scholar]
- Qiu, Y.; Qiu, L.; Cui, J.; Wei, Q. Bacterial cellulose and bacterial cellulose-vaccarin membranes for wound healing. Mater. Sci. Eng. C 2016, 59, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Bikerman, J.J. The unit of foaminess. Trans. Faraday Soc. 1938, 34, 634–638. [Google Scholar] [CrossRef]
- Josset, S.; Hansen, L.; Orsolini, P.; Griffa, M.; Geiger, T. Microfibrillated cellulose foams obtained by a straightforward freeze–thawing–drying procedure. Cellulose 2017, 24, 3825–3842. [Google Scholar] [CrossRef]
- Faruk, O.; Bledzki, A.K.; Matuana, L.M. Microcellular foamed wood-plastic composites by different processes: A review. Macromol. Mater. Eng. 2007, 292, 113–127. [Google Scholar] [CrossRef]
- Saint-Jalmes, A.; Peugeot, M.L.; Ferraz, H.; Langevin, D. Differences between protein and surfactant foams: Microscopic properties, stability and coarsening. Colloid Surf. A 2005, 263, 219–225. [Google Scholar] [CrossRef]
- Chen, X.; Chen, Y.; Zou, L.; Zhang, X.; Dong, Y.; Tang, J.; McClements, D.J.; Liu, W. Plant-Based Nanoparticles Prepared from Proteins and Phospholipids Consisting of a Core-Multilayer-Shell Structure: Fabrication, Stability, and Foamability. J. Agric. Food Chem. 2019, 67, 6574–6584. [Google Scholar] [CrossRef]
- Xiang, W.; Preisig, N.; Laine, C.; Hjelt, T.; Tardy, B.L.; Stubenrauch, C.; Rojas, O.J. Surface Activity and Foaming Capacity of Aggregates Formed between an Anionic Surfactant and Non-Cellulosics Leached from Wood Fibers. Biomacromolecules 2019, 20, 2286–2294. [Google Scholar] [CrossRef]
- Chin, S.F.; Binti Romainor, A.N.; Pang, S.C. Fabrication of hydrophobic and magnetic cellulose aerogel with high oil absorption capacity. Mater. Lett. 2014, 115, 241–243. [Google Scholar] [CrossRef]
- Schmitt, C.; Tania, P.D.S.; Bovay, C.; Rami-Shojaei, S.; Frossard, P.; Kolodziejczyk, E.; Leser, M.E. Effect of time on the interfacial and foaming properties of beta-lactoglobulin/acacia gum electrostatic complexes and coacervates at pH 4.2. Langmuir Acs J. Surf. Colloids 2005, 21, 7786–7795. [Google Scholar] [CrossRef]
- Tzoumaki, M.V.; Karefyllakis, D.; Moschakis, T.; Biliaderis, C.G.; Scholten, E. Aqueous foams stabilized by chitin nanocrystals. Soft Matter 2015, 11, 6245–6253. [Google Scholar] [CrossRef]
- Lesov, I.; Tcholakova, S.; Denkov, N. Drying of particle-loaded foams for production of porous materials: Mechanism and theoretical modeling. Rsc Adv. 2013, 4, 811–823. [Google Scholar] [CrossRef]
- Du, X.; Zhao, L.; Xiao, H.L.; Liang, F.; Chen, H.; Wang, X.; Wang, J.J.; Qu, W.M.; Lei, Z.X. Stability and shear thixotropy of multilayered film foam. Colloid Polym. Sci. 2014, 292, 2745–2751. [Google Scholar] [CrossRef]
- Khan, M.Y.; Samanta, A.; Ojha, K.; Mandal, A. Interaction between aqueous solutions of polymer and surfactant and its effect on physicochemical properties. Asia-Pac. J. Chem. Eng. 2008, 3, 579–585. [Google Scholar] [CrossRef]
- Tasset, S.; Cathala, B.; Bizot, H.; Capron, I. Versatile cellular foams derived from CNC-stabilized Pickering emulsions. Rsc Adv. 2014, 4, 893–898. [Google Scholar] [CrossRef]
- Gonzenbach, U.T.; Studart, A.R.; Tervoort, E.; Gauckler, L.J. Macroporous ceramics from particle-stabilized wet foams. J. Am. Ceram. Soc. 2007, 90, 16–22. [Google Scholar] [CrossRef]
- Zhang, Y.; Allen, M.C.; Zhao, R.; Deheyn, D.D.; Behrens, S.H.; Meredith, J.C. Capillary Foams: Stabilization and Functionalization of Porous Liquids and Solids. Langmuir 2015, 31, 2669–2676. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, P.; Xiao, H.-N.; Heydarifard, S.; Wang, S.F. Novel aqueous spongy foams made of three-dimensionally dispersed wood-fiber: Entrapment and stabilization with NFC/MFC within capillary foams. Cellulose 2017, 24, 241–251. [Google Scholar] [CrossRef]
- Koos, E.; Willenbacher, N. Capillary Forces in Suspension Rheology. Science 2011, 331, 897–900. [Google Scholar] [CrossRef]
- Butt, H.J. Controlling the Flow of Suspensions. Science 2011, 331, 868–869. [Google Scholar] [CrossRef]
- Koos, E. Capillary suspensions: Particle networks formed through the capillary force. Curr. Opin. Colloid 2014, 19, 575–584. [Google Scholar] [CrossRef]
- Ahsan, H.M.; Pei, Y.; Luo, X.-G.; Wang, Y.-X.; Li, Y.; Li, B.; Liu, S.-L. Novel stable pickering emulsion based solid foams efficiently stabilized by microcrystalline cellulose/chitosan complex particles. Food Hydrocolloid 2020, 108, 1–9. [Google Scholar] [CrossRef]
- Srinivasa, P.; Kulachenko, A. Analysis of the compressive response of Nano Fibrillar Cellulose foams. Mech. Mater. 2015, 80, 13–26. [Google Scholar] [CrossRef]
- Khalil, H.P.S.A.; Adnan, A.S.; Yahya, E.B.; Olaiya, N.G.; Safrida, S.; Hossain, M.S.; Balakrishnan, V.; Gopakumar, D.A.; Abdullah, C.K.; Oyekanmi, A.A.; et al. A Review on Plant Cellulose Nanofibre-Based Aerogels for Biomedical Applications. Polymers 2020, 12, 2286–2294. [Google Scholar]
- Gupta, S.; Martoïa, F.; Orgéas, L.; Dumont, P. Ice-Templated Porous Nanocellulose-Based Materials: Current Progress and Opportunities for Materials Engineering. Appl. Sci. 2018, 8, 2463. [Google Scholar] [CrossRef]
- Aulin, C.; Netrval, J.; Wagberg, L.; Lindström, T. Aerogels from nanofibrillated cellulose with tunable oleophobicity. Soft Matter 2010, 6, 3298–3305. [Google Scholar] [CrossRef]
- Chen, W.; Yu, H.; Li, Q.; Liu, Y.; Li, J. Ultralight and highly flexible aerogels with long cellulose I nanofibers. Soft Matter 2011, 7, 10360–10368. [Google Scholar] [CrossRef]
- Ras, R.H.A.; Ikkala, O.; Korhonen, J.T.; Kettunen, M. Hydrophobic nanocellulose aerogels as floating, sustainable, reusable, and recyclable oil absorbents. Acs Appl. Mater. Interfaces 2011, 3, 1813–1819. [Google Scholar]
- Sehaqui, H.; Zhou, Q.; Berglund, L.A. High-porosity aerogels of high specific surface area prepared from nanofibrillated cellulose (NFC). Compos. Sci. Technol. 2011, 71, 1593–1599. [Google Scholar] [CrossRef]
- Ciftci, D.; Ubeyitogullari, A.; Huerta, R.R.; Ciftci, O.N.; Flores, R.A.; Saldaña, M.D. Lupin hull cellulose nanofiber aerogel preparation by supercritical CO2 and freeze drying. J. Supercrit. Fluids 2017, 127, 137–145. [Google Scholar] [CrossRef]
- Guo, L.; Chen, Z.; Lyu, S.; Fu, F.; Wang, S. Highly flexible cross-linked cellulose nanofibril sponge-like aerogels with improved mechanical property and enhanced flame retardancy. Carbohydr. Polym. 2018, 179, 333–340. [Google Scholar] [CrossRef]
- Haimer, E.; Wendland, M.; Schlufter, K.; Frankenfeld, K.; Miethe, P.; Potthast, A.; Rosenau, T.; Liebner, F. Loading of Bacterial Cellulose Aerogels with Bioactive Compounds by Antisolvent Precipitation with Supercritical Carbon Dioxide. Macromol. Symp. 2010, 294, 64–74. [Google Scholar] [CrossRef]
- Nemoto, J.; Saito, T.; Isogai, A. Simple Freeze-Drying Procedure for Producing Nanocellulose Aerogel-Containing, High-Performance Air Filters. Acs Appl. Mater. Interfaces 2015, 7, 19809–19815. [Google Scholar] [CrossRef] [PubMed]
- Nissila, T.; Karhula, S.S.; Saarakkala, S.; Oksman, K. Cellulose nanofiber aerogels impregnated with bio-based epoxy using vacuum infusion: Structure, orientation and mechanical properties. Compos. Sci. Technol. 2018, 155, 64–71. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Saito, T.; Isogai, A. Aerogels with 3D ordered nanofiber skeletons of liquid-crystalline nanocellulose derivatives as tough and transparent insulators. Angew. Chem. Int. Ed. 2015, 53, 10253. [Google Scholar] [CrossRef][Green Version]
- Lee, J.; Deng, Y. The morphology and mechanical properties of layer structured cellulose microfibril foams from ice-templating methods. Soft Matter 2011, 7, 6034–6040. [Google Scholar] [CrossRef]
- Wang, P.; Aliheidari, N.; Zhang, X.; Ameli, A. Strong ultralight foams based on nanocrystalline cellulose for high-performance insulation. Carbohydr. Polym. 2019, 218, 103–111. [Google Scholar] [CrossRef]
- Arenas, J.P.; Crocker, M.J. Recent Trends in Porous Sound-Absorbing Materials. Sound Vib. 2010, 44, 12–17. [Google Scholar]
- Weng, L.; Boda, S.K.; Wang, H.J.; Teusink, M.J.; Shuler, F.D.; Xie, J.W. Novel 3D Hybrid Nanofiber Aerogels Coupled with BMP-2 Peptides for Cranial Bone Regeneration. Adv. Healthc. Mater. 2018, 7, 1–16. [Google Scholar] [CrossRef]
- Salgado, M.; Santos, F.; Rodríguez-Rojo, S.; Reis, R.L.; Duarte, A.R.C.; Cocero, M.J. Development of barley and yeast β-glucan aerogels for drug delivery by supercritical fluids. J. Co2 Util. 2017, 22, 252–269. [Google Scholar] [CrossRef]
- Svagan, A.J.; Berglund, L.A.; Jensen, P. Cellulose Nanocomposite Biopolymer Foam-Hierarchical Structure Effects on Energy Absorption. Acs Appl. Mater. Interfaces 2011, 3, 1411–1417. [Google Scholar] [CrossRef]
- Sinthu, S.; Srikulkit, K. Preparation of Transparent Cellulose/PMMA Composite Sheet from Cellulose Aerogel. Key Eng. Mater. 2018, 773, 56–61. [Google Scholar] [CrossRef]
- Mi, Q.-Y.; Ma, S.-R.; Yu, J.; He, J.-S.; Zhang, J. Flexible and Transparent Cellulose Aerogels with Uniform Nanoporous Structure by a Controlled Regeneration Process. Acs Sustain. Chem. Eng. 2016, 4, 656–660. [Google Scholar] [CrossRef]


| Materials | Name | Method | Particle | Reference |
|---|---|---|---|---|
| Kraft pulp bleached from coniferous wood | NFC | TEMPO Mechanical | D: 3.0 ± 0.3 nm L: 1.2 ± 0.4 nm | [43] |
| Bleached Kraft pulp | NFC | — | D: 35–40 nm L: 2.3 mm | [44] |
| — | NFC | TEMPO | D: 4 ± 1.4 nm L: 255 ± 104 nm | [45] |
| Kraft pulp bleached from coniferous wood | MFC | Mechanical | D: 30 um L: 2.3 mm | [37] |
| Bleached sulfite pulp from spruce | NFC | Mechanical | D: 30 um L: 2–3 mm | [46] |
| Commercial eucalyptus bleach pulp | NFC | TEMPO Homogeneous | D: 20–50 nm | [3] |
| Birch bleached Kraft pulp | NFC | Enzyme Homogeneous | D: 20 um L: 150–300 um | [3] |
| Bleached sulfite softwood cellulose | NFC | Enzyme Homogeneous | D: 5–10 nm, 100–500 nm | [5] |
| Bleached wood pulp | NFC | Acid Homogeneous | D: 16 ± 4 nm, 21 ± 7 nm L: 616 ± 200, 732 ± 208 nm | [47] |
| Bleached Kraft pulp | MFC | Fenton Mechanical | D: 10–100 um L: 0.2–7.5 mm | [48] |
| Bleached hardwood Kraft pulp and soft acid bagasse sulfite pulp | MFC | Enzyme Mechanical | D: 60 um | [49] |
| Name | Method | Particle | Advantages | Disadvantages |
|---|---|---|---|---|
| Supercritical drying | Replacing the solvent with supercritical fluid (methanol, ethanol and CO2) | Nanosize | Dimensions stay in nanosize | Expensive and complicated method |
| Freezing drying | Precooling at −4 °C and freezing in liquid nitrogen then freezing overnight in a frozen drying oven | nm to um | Establish a good network structure | Expensive |
| Oven drying | Drying in the oven at 105 °C for 24 h | um to mm | Well established for the industry | Severe structural collapse |
| Name | Method | Parameter | Advantage | Application Reference | |
|---|---|---|---|---|---|
| Aerogel | Freeze drying | 514.15 m2/g 2–10 nm | Good circulation stability | High performance super capacitors | [43] |
| Foam | Freeze drying | 35.8 m2/g | Controllable structural | Thermal insulation | [9] |
| Aerogel | Liquid nitrogen and freeze drying | 40.31 m2/g 13.66 nm | Thermal resistance and high tenacity | Personal protectable equipment | [80] |
| Foam | Oven drying | 300–500 μm 98% | Lightweight and strong | _ | [7] |
| Aerogel | Supercritical carbon dioxide drying | 200 m2/g | _ | Drug sustained release | [81] |
| Aerogel | Supercritical drying | 130–160 m2/g 0.5 ± 0.2 μm | Good hygroscopic property | wound dressing | [44] |
| Aerogel | Freeze drying | 20–30 m2/g 24.7 ± 10.4 μm | _ | _ | [44] |
| Aerogel | Supercritical carbon dioxide drying | 240–280 m2/g 0.86–0.92 μm 91–96% | Sound absorption High intensity | Office ceiling | [13] |
| Foam | Freeze drying | 93–99.5% | Porous and excellent mechanical properties | New composite material for energy absorption | [2] |
| Aerogel | Oven drying | 70–120 m2/g | Excellent mechanical properties | Green heat insulation building materials water purification material | [12] |
| Aerogel | Freeze drying | 70 m2/g 98% | High strength and deformation | Functional conductive material | [5] |
| Foam | Oven drying | 99.6% | Water resistance and wet elasticity | Water and oil separation | [28] |
| Aerogel | Liquid nitrogen and freeze drying | 99.38–99.97% | Controllable structural | _ | [48] |
| Aerogel | Liquid nitrogen and freeze drying | 11–42 m2/g 99.8–99.1% | Super hydrophobic | Water and oil separation | [29] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, C.; Yao, M.; Liu, Y.; Yang, Y.; Zong, Y.; Zhao, H. MFC/NFC-Based Foam/Aerogel for Production of Porous Materials: Preparation, Properties and Applications. Materials 2020, 13, 5568. https://doi.org/10.3390/ma13235568
Qin C, Yao M, Liu Y, Yang Y, Zong Y, Zhao H. MFC/NFC-Based Foam/Aerogel for Production of Porous Materials: Preparation, Properties and Applications. Materials. 2020; 13(23):5568. https://doi.org/10.3390/ma13235568
Chicago/Turabian StyleQin, Chenni, Mingzhu Yao, Yang Liu, Yujie Yang, Yifeng Zong, and Hui Zhao. 2020. "MFC/NFC-Based Foam/Aerogel for Production of Porous Materials: Preparation, Properties and Applications" Materials 13, no. 23: 5568. https://doi.org/10.3390/ma13235568
APA StyleQin, C., Yao, M., Liu, Y., Yang, Y., Zong, Y., & Zhao, H. (2020). MFC/NFC-Based Foam/Aerogel for Production of Porous Materials: Preparation, Properties and Applications. Materials, 13(23), 5568. https://doi.org/10.3390/ma13235568