Effect of PVP Coating on LiMnBO3 Cathodes for Li-Ion Batteries
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Materials
2.2. Characterization
2.3. Electrochemical Measurements
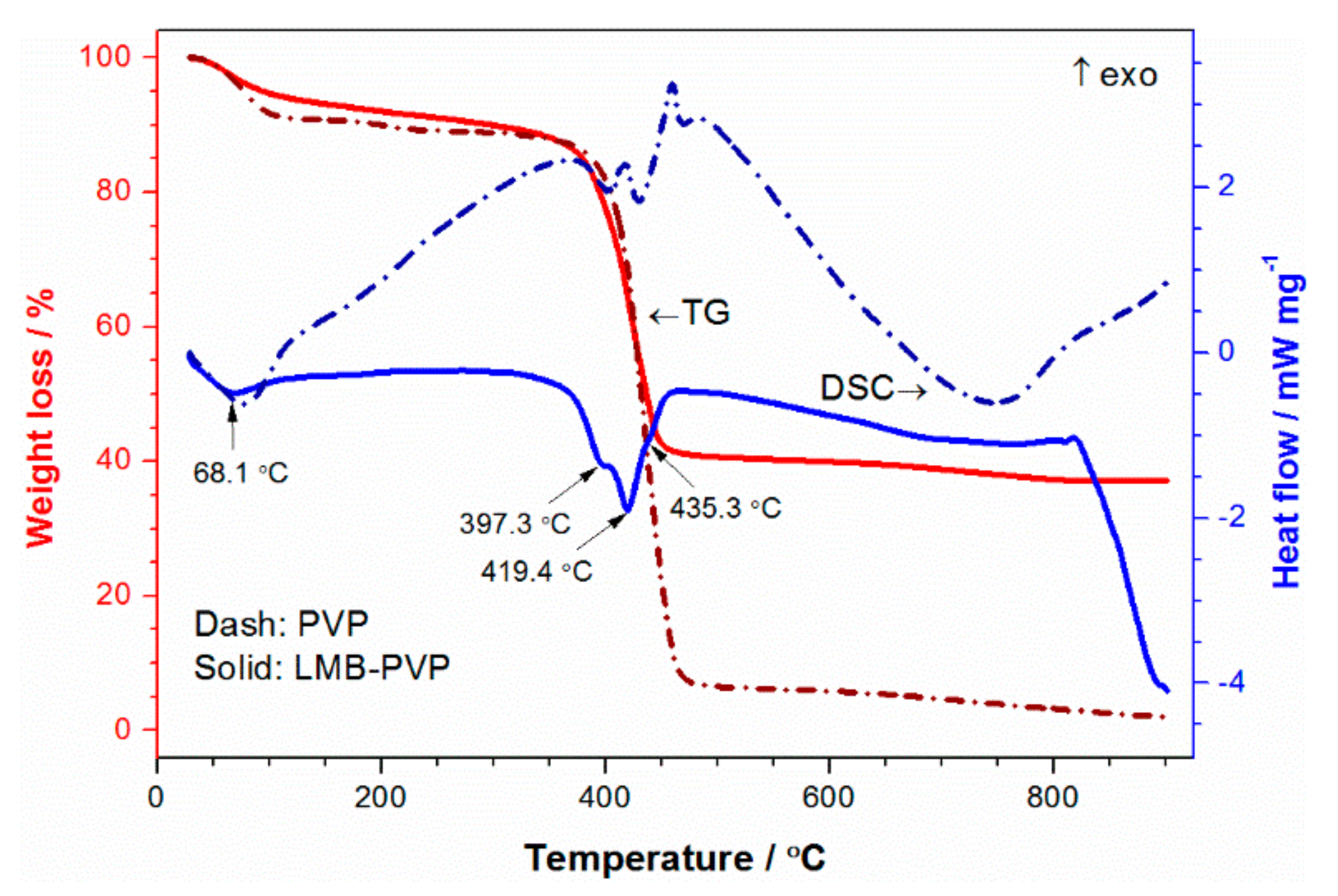
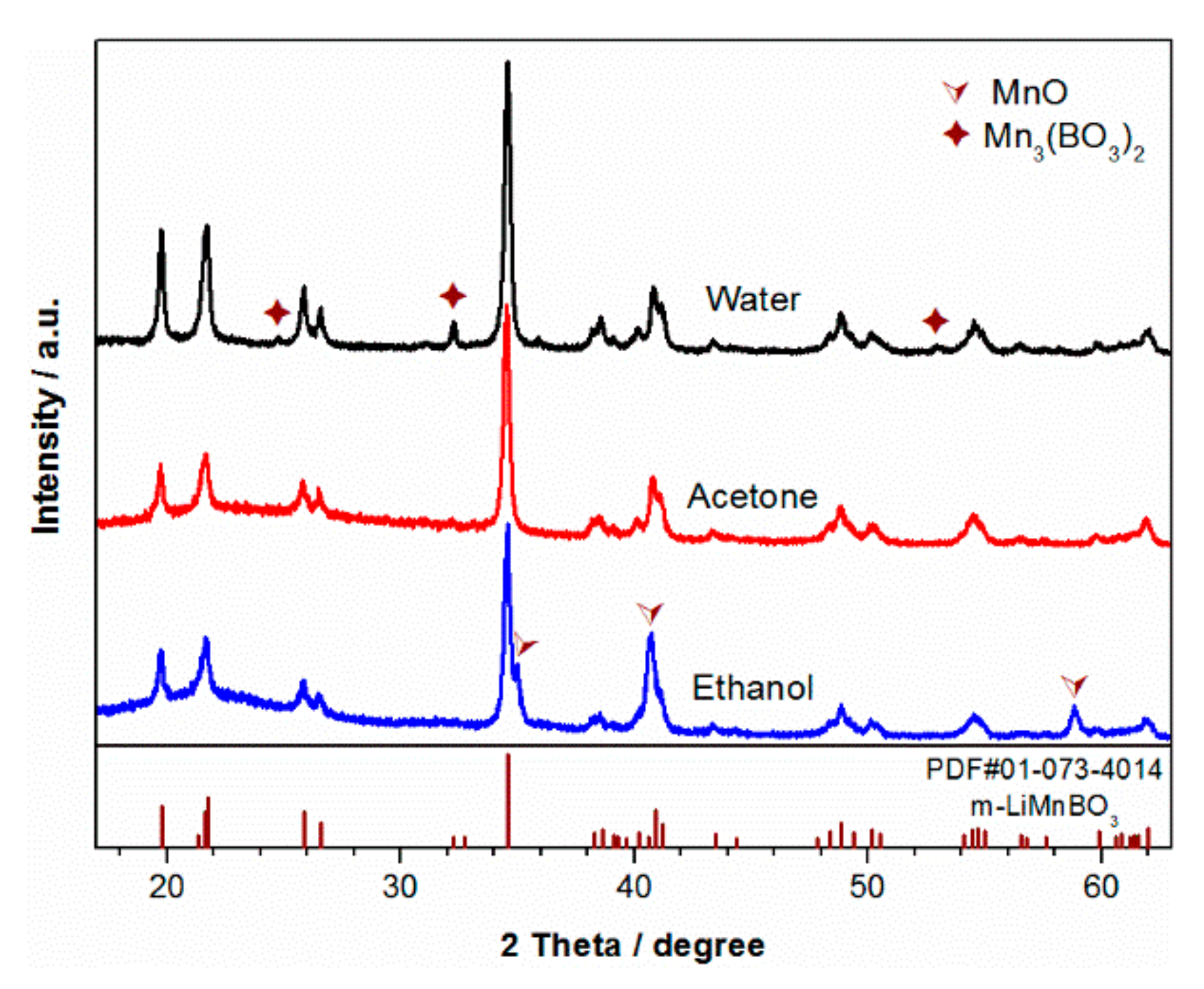
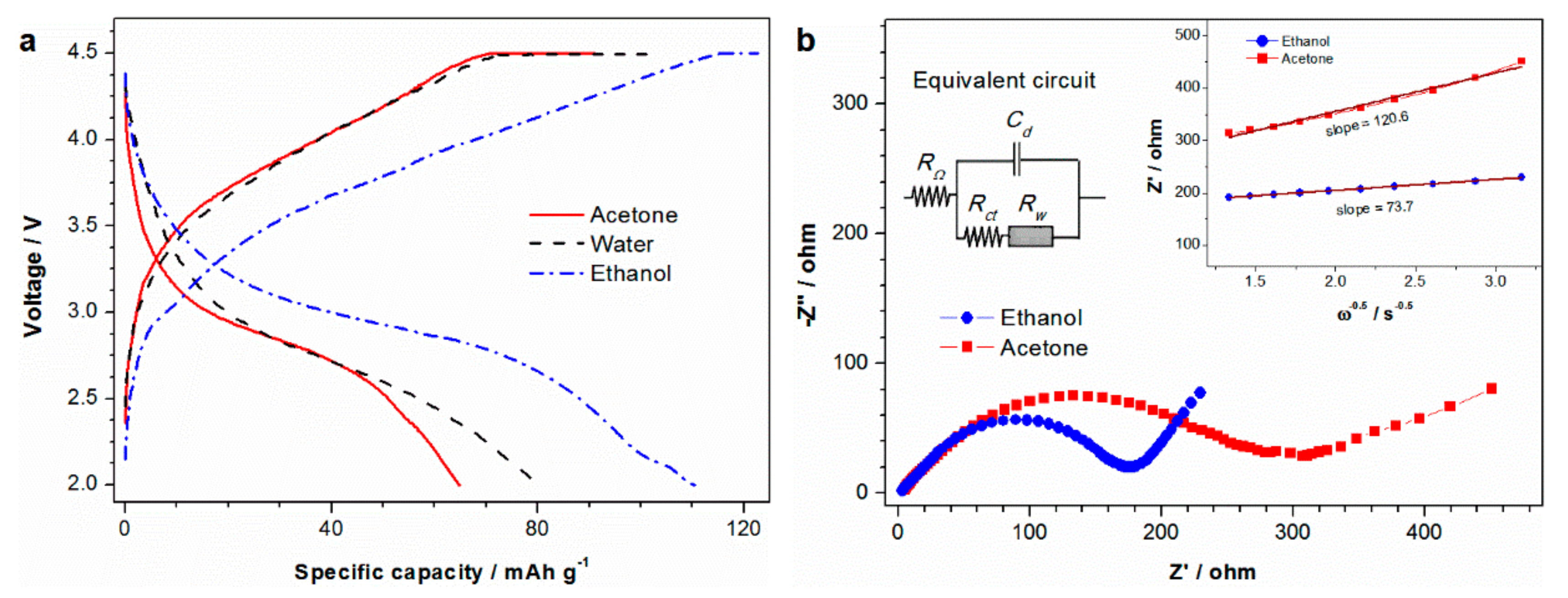
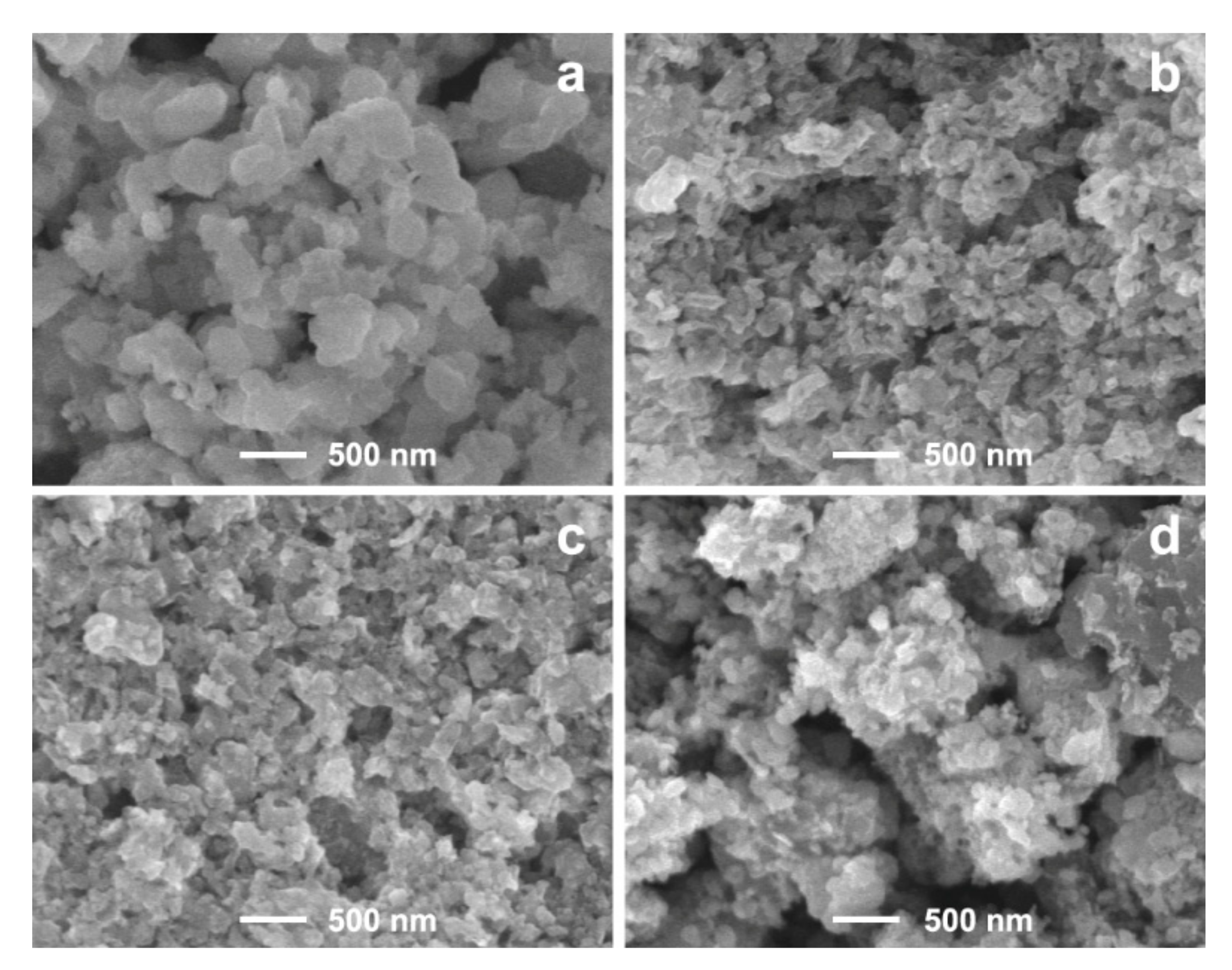
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lee, W.; Kim, J.; Yun, S.; Choi, W.; Kim, H.; Yoon, W. Multiscale factors in designing alkali-ion (Li, Na, and K) transition metal inorganic compounds for next-generation rechargeable batteries. Energy Environ. Sci. 2020. [Google Scholar] [CrossRef]
- Masquelier, C.; Croguennec, L. Polyanionic (Phosphates, Silicates, Sulfates) Frameworks as Electrode Materials for Rechargeable Li (or Na) Batteries. Chem. Rev. 2013, 113, 6552–6591. [Google Scholar] [CrossRef] [PubMed]
- Canepa, P.; Sai Gautam, G.; Hannah, D.C.; Malik, R.; Liu, M.; Gallagher, K.G.; Persson, K.A.; Ceder, G. Odyssey of Multivalent Cathode Materials: Open Questions and Future Challenges. Chem. Rev. 2017, 117, 4287–4341. [Google Scholar] [CrossRef] [PubMed]
- Kalantarian, M.M.; Hafizi-Barjini, M.; Momeni, M. Ab Initio Study of AMBO3 (A = Li, Na and M = Mn, Fe, Co, Ni) as Cathode Materials for Li-Ion and Na-Ion Batteries. ACS Omega 2020, 5, 8952–8961. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.C.; Moore, C.J.; Kang, B.; Hautier, G.; Jain, A.; Ceder, G. Synthesis and Electrochemical Properties of Monoclinic LiMnBO3 as a Li Intercalation Material. J. Electrochem. Soc. 2011, 158, A309. [Google Scholar] [CrossRef]
- Zhao, L.; Li, R.K. Study on a multifunctional crystal LiMnBO3. Mater. Res. Bull. 2013, 48, 277–280. [Google Scholar] [CrossRef]
- Yamada, A.; Iwane, N.; Nishimura, S.; Koyama, Y.; Tanaka, I. Synthesis and electrochemistry of monoclinic Li(MnxFe1-x)BO3: A combined experimental and computational study. J. Mater. Chem. 2011, 21, 10690–10696. [Google Scholar] [CrossRef]
- Lee, Y.; Lee, H. Improved lithium storage capacities of LiMnBO3/C via simple high-energy milling. Mater. Lett. 2014, 132, 401–404. [Google Scholar] [CrossRef]
- Kim, J.C.; Seo, D.; Ceder, G. Theoretical capacity achieved in a LiMn0.5Fe0.4Mg0.1BO3 cathode by using topological disorder. Energy Environ. Sci. 2015, 8, 1790–1798. [Google Scholar] [CrossRef]
- Ragupathi, V.; Safiq, M.; Panigrahi, P.; Hussain, T.; Raman, S.; Ahuja, R.; Nagarajan, G.S. Enhanced electrochemical performance of LiMnBO3 with conductive glassy phase: A prospective cathode material for lithium-ion battery. Ionics 2017, 23, 1645–1653. [Google Scholar] [CrossRef]
- Le Roux, B.; Bourbon, C.; Lebedev, O.I.; Colin, J.; Pralong, V. Synthesis and Characterization of the LiMnBO3–LiCoBO3 Solid Solution and Its Use as a Lithium-Ion Cathode Material. Inorg. Chem. 2015, 54, 5273–5279. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.C.; Li, X.; Moore, C.J.; Bo, S.; Khalifah, P.G.; Grey, C.P.; Ceder, G. Analysis of Charged State Stability for Monoclinic LiMnBO3 Cathode. Chem. Mater. 2014, 26, 4200–4206. [Google Scholar] [CrossRef]
- Bondareva, O.S.; Simonov, M.A.; Egorov-Tismenko, Y.K.; Belov, N.V. The Crystal Structures of LiZn[BO3] and LiMn[BO3]. Sov. Phys. Cryst. 1978, 23, 269–271. [Google Scholar]
- Legagneur, V.; An, Y.; Mosbah, A.; Portal, R.; Le Gal La Salle, A.; Verbaere, A.; Guyomard, D.; Piffard, Y. LiMBO3 (M=Mn, Fe, Co): Synthesis, crystal structure and lithium deinsertion/insertion properties. Solid State Ion. 2001, 139, 37–46. [Google Scholar] [CrossRef]
- Aravindan, V.; Karthikeyan, K.; Amaresh, S.; Lee, Y.S. LiMnBO3/C: A Potential Cathode Material for Lithium Batteries. Bull. Korean Chem. Soc. 2010, 31, 1506–1508. [Google Scholar] [CrossRef][Green Version]
- Tang, A.; He, D.; He, Z.; Xu, G.; Song, H.; Peng, R. Electrochemical performance of LiMnBO3/C composite synthesized by a combination of impregnation and precipitation followed by annealing. J. Power Sources 2015, 275, 888–892. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, H.; Zhang, X.; Wu, L.; Liu, J.; Liu, S.; Zhong, S. Synthesis and electrochemical performance of carbon-coated LiMnBO3 as cathode materials for lithium-ion batteries. Ionics 2018, 24, 73–81. [Google Scholar] [CrossRef]
- Li, L.; Zheng, H.; Yin, S.; Wang, S.; Feng, C.; Wang, J.; He, P. Synthesis and Electrochemical Properties of LiMnBO3 and LiMnBO3/C Composite. Sci. Adv. Mater. 2016, 8, 980–986. [Google Scholar] [CrossRef]
- Zhang, B.; Zhu, Y.; Yu, W.; Zhang, J.; An, C. Facile synthesis of carbon-encapsulated LiMnBO3 composite by the sol-gel method as a lithium-ion battery cathode material. J. Alloy. Compd. 2017, 704, 343–347. [Google Scholar] [CrossRef]
- Moradi, M.; Kim, J.C.; Qi, J.; Xu, K.; Li, X.; Ceder, G.; Belcher, A.M. A bio-facilitated synthetic route for nano-structured complex electrode materials. Green Chem. 2016, 18, 2619–2624. [Google Scholar] [CrossRef][Green Version]
- Ragupathi, V.; Srimathi, K.; Panigrahi, P.; Lee, J.W.; Nagarajan, G.S. Electrochemical Performance of Sol-Gel Derived Hexagonal LiMnBO3 Cathode Material for Lithium-Ion Batteries. Nano Hybrids Compos. 2017, 17, 106–112. [Google Scholar] [CrossRef]
- Michalski, P.P.; Gołębiewska, A.; Trébosc, J.; Lafon, O.; Pietrzak, T.K.; Ryl, J.; Nowiński, J.L.; Wasiucionek, M.; Garbarczyk, J.E. Properties of LiMnBO3 glasses and nanostructured glass-ceramics. Solid State Ion. 2019, 334, 88–94. [Google Scholar] [CrossRef]
- Afyon, S.; Kundu, D.; Krumeich, F.; Nesper, R. Nano LiMnBO3, a high-capacity cathode material for Li-ion batteries. J. Power Sources 2013, 224, 145–151. [Google Scholar] [CrossRef]
- Yamane, H.; Kawano, T.; Fukuda, K.; Suehiro, T.; Sato, T. Preparation, crystal structure and photoluminescence of lithium magnesium manganese borate solid solutions, LiMg1−xMnxBO3. J. Alloy. Compd. 2012, 512, 223–229. [Google Scholar] [CrossRef]
- Stafeeva, V.S.; Panin, R.V.; Lobanov, M.V.; Antipov, E.V. Stabilization of the LiMnBO3 monoclinic polymorph by the isovalent substitution of manganese for zinc. Russ. Chem. 2013, 62, 374–379. [Google Scholar] [CrossRef]
- Jarocka, A.; Michalski, P.P.; Ryl, J.; Wasiucionek, M.; Garbarczyk, J.E.; Pietrzak, T.K. Synthesis, thermal, structural and electrical properties of vanadium-doped lithium-manganese-borate glass and nanocomposites. Ionics 2020, 26, 1275–1283. [Google Scholar] [CrossRef]
- Chung, S.Y.; Bloking, J.T.; Chiang, Y.M. Electronically conductive phospho-olivines as lithium storage electrodes. Nat. Mater. 2002, 1, 123–128. [Google Scholar] [CrossRef]
- Gan, Q.; Qin, N.; Zhu, Y.; Huang, Z.; Zhang, F.; Gu, S.; Xie, J.; Zhang, K.; Lu, L.; Lu, Z. Polyvinylpyrrolidone-Induced Uniform Surface-Conductive Polymer Coating Endows Ni-Rich LiNi0.8Co0.1Mn0.1O2 with Enhanced Cyclability for Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2019, 11, 12594–12604. [Google Scholar] [CrossRef]
- Lee, J.; Kim, M.; Moon, S.; Kim, E.; Shin, Y.; Choi, S.; Kwon, S.; Kim, S.; Kwon, H.; Park, K. Role of polyvinylpyrrolidone in the electrochemical performance of Li2MnO3 cathode for lithium-ion batteries. RSC Adv. 2019, 9, 10297–10304. [Google Scholar] [CrossRef]
- Lee, Y.; Lee, H. Structure and electrochemical behavior of LiMnBO3 synthesized at various temperatures. Electron. Mater. Lett. 2014, 10, 253–258. [Google Scholar] [CrossRef]
- Bhide, V.G.; Dani, R.H. Electrical conductivity in oxides of manganese and related compounds. Physica 1961, 27, 821–826. [Google Scholar] [CrossRef]
- Zhan, D.; Luo, W.; Kraatz, H.; Fehse, M.; Li, Y.; Xiao, Z.; Brougham, D.F.; Simpson, A.J.; Wu, B. Facile Approach for Synthesizing High-Performance MnO/C Electrodes from Rice Husk. ACS Omega 2019, 4, 18908–18917. [Google Scholar] [CrossRef]
- Hou, C.; Tai, Z.; Zhao, L.; Zhai, Y.; Hou, Y.; Fan, Y.; Dang, F.; Wang, J.; Liu, H. High performance MnO@C microcages with a hierarchical structure and tunable carbon shell for efficient and durable lithium storage. J. Mater. Chem. A 2018, 6, 9723–9736. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods Fundamentals and Applications, 2nd ed.; John Wiley & Sons, Inc.: New York, NY, USA, 2001; pp. 376–387. [Google Scholar]
- Dinh, H.; Mho, S.; Kang, Y.; Yeo, I. Large discharge capacities at high current rates for carbon-coated LiMnPO4 nanocrystalline cathodes. J. Power Sources 2013, 244, 189–195. [Google Scholar] [CrossRef]
- Li, S.; Xu, L.; Li, G.; Wang, M.; Zhai, Y. In-situ controllable synthesis and performance investigation of carbon-coated monoclinic and hexagonal LiMnBO3 composites as cathode materials in lithium-ion batteries. J. Power Sources 2013, 236, 54–60. [Google Scholar] [CrossRef]
- Dean, J.A. Lange’s Handbook of Chemistry, 15th ed.; McGraw-Hill: New York, NY, USA, 1999; pp. 3–38. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, B.; He, X.; Yi, H.; Hu, C. Effect of PVP Coating on LiMnBO3 Cathodes for Li-Ion Batteries. Materials 2020, 13, 5528. https://doi.org/10.3390/ma13235528
Hong B, He X, Yi H, Hu C. Effect of PVP Coating on LiMnBO3 Cathodes for Li-Ion Batteries. Materials. 2020; 13(23):5528. https://doi.org/10.3390/ma13235528
Chicago/Turabian StyleHong, Bolong, Xiangming He, Huihua Yi, and Chenglin Hu. 2020. "Effect of PVP Coating on LiMnBO3 Cathodes for Li-Ion Batteries" Materials 13, no. 23: 5528. https://doi.org/10.3390/ma13235528
APA StyleHong, B., He, X., Yi, H., & Hu, C. (2020). Effect of PVP Coating on LiMnBO3 Cathodes for Li-Ion Batteries. Materials, 13(23), 5528. https://doi.org/10.3390/ma13235528






